Abstract
Background and Aims
Atrial fibrillation (AF) is the most common sustained arrhythmia in adults. Investigations of risk factor profiles for AF according to age and genetic risk groups are essential to promote individualized strategies for the prevention and control of AF.
Methods
A total of 409 661 participants (mean age, 56 years; 46% men) free of AF at baseline and with complete information about risk factors were included from the UK Biobank cohort. The hazard ratios and population-attributable risk (PAR) percentages of incident AF associated with 23 risk factors were examined, including 3 social factors, 7 health behaviours, 6 cardiometabolic factors, 6 clinical comorbidities, and the genetic risk score (GRS), across 3 age groups (40–49, 50–59, and 60–69 years) and 3 genetic risk groups (low, moderate, and high GRS).
Results
After a follow-up of 5 027 587 person-years, 23 847 participants developed AF. Most cardiometabolic factors and clinical comorbidities showed a significant interaction with age, whereby the associations were generally strengthened in younger groups (Pinteraction < .002). However, only low LDL cholesterol, renal dysfunction, and cardiovascular disease showed a significant interaction with genetic risk, and the associations with these factors were stronger in lower genetic risk groups (Pinteraction < .002). Cardiometabolic factors consistently accounted for the largest number of incident AF cases across all age groups (PAR: 36.2%–38.9%) and genetic risk groups (34.0%–41.9%), with hypertension and overweight/obesity being the two leading modifiable factors. Health behaviours (PAR: 11.5% vs. 8.7%) and genetic risk factors (19.1% vs. 14.3%) contributed to more AF cases in the 40–49 years group than in the 60–69 years group, while the contribution of clinical comorbidities remained relatively stable across different age groups. The AF risk attributable to overall cardiometabolic factors (PAR: 41.9% in the low genetic risk group and 34.0% in the high genetic risk group) and clinical comorbidities (24.7% and 15.9%) decreased with increasing genetic risk. The impact of social factors on AF was relatively low across the groups by age and genetic risk.
Conclusions
This study provided comprehensive information about age- and genetic predisposition-related risk factor profiles for AF in a cohort of UK adults. Prioritizing risk factors according to age and genetic risk stratifications may help to achieve precise and efficient prevention of AF.
Keywords: Atrial fibrillation, Age, Genetic risk, Risk profile, UK Biobank
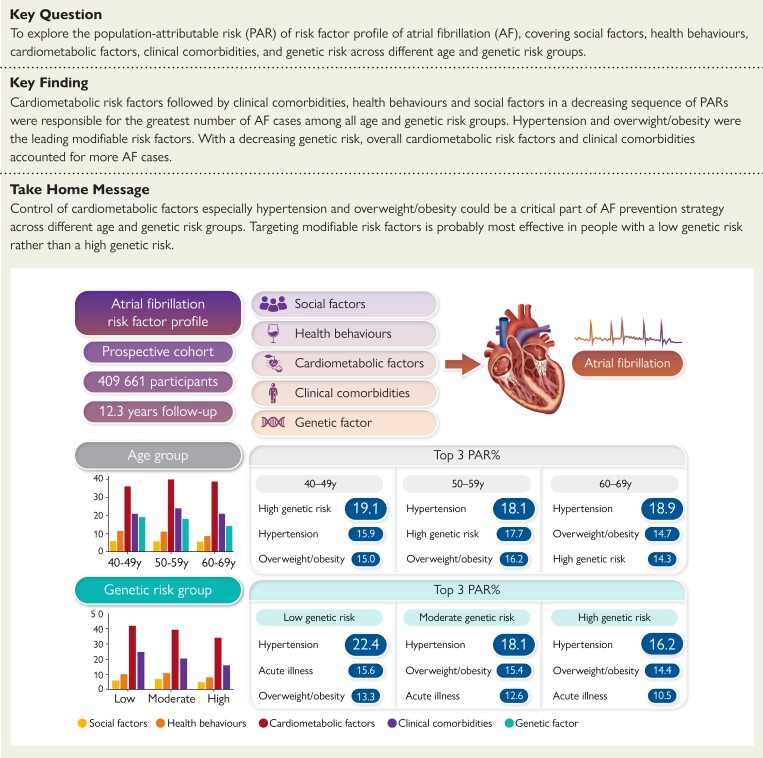
Structured Graphical Abstract
Structured Graphical Abstract.
Age-specific and genetic predisposition-specific associations of risk factor profiles with atrial fibrillation (AF). Population-attributable risk percentages of AF risk factors were presented for different age and genetic risk population groups.
See the editorial comment for this article ‘Prevention of atrial fibrillation: a call to action’, by A.D. Elliott et al., https://doi.org/10.1093/eurheartj/ehad738.
Introduction
Atrial fibrillation (AF), as the most common sustained arrhythmia, imposes a huge burden on individuals and public health worldwide.1 In the 2019 global statistics, there were ∼59.7 million people with AF/flutter.2 With the continuous improvement of lifespan and medical standards, the population with AF in the European Union will double in the next 40 years, and the lifetime risk will be one in three individuals of European ancestry at an index age of 55.3,4 Atrial fibrillation is an independent risk factor for heart failure and myocardial infarction5,6 and is responsible for one in five strokes.7 Even worse, people with AF have twice the risk of premature death.8
The pathophysiological mechanism and risk factors for AF are complex, multifactorial, and interacting, making prevention challenging.9,10 Previous studies have found that many social, lifestyle, and cardiometabolic factors and comorbidities are associated with the occurrence and development of AF.11–14 It has also been reported that interventions for some risk factors, such as alcohol reduction and weight management, are beneficial in reducing the AF burden, recurrence, and symptoms.15–17 Thus, the observed impact of risk factor burden/multiple comorbidities suggests that an early intervention addressing risk factors is urgently needed to reduce the number of new AF cases.
However, current evidence comes mainly from the general population, and considering that age and genetic predisposition are critical fixed risk factors for AF,12,18,19 it remains unclear whether there is a varied impact of those individual factors on the AF risk by age and genetic risk group and whether age and genetic risk-specific strategies of primary prevention should be adopted. Although the number of people with AF increases significantly with aging,14 a diagnosis of AF at a younger age or early onset AF poses a greater burden on health.20 Identifying the contribution of various risk factors to incident AF in different age and genetic risk groups is of great significance for the prevention of AF.
In this study, we aimed to explore the associations of 23 risk factors with incident AF and to present the ranking order of these factors by partial population-attributable risk (PAR) percentages among three consecutive age groups (40–49, 50–59, and 60–69 years) and different genetic risk groups (low, moderate, and high) in a large prospective UK cohort.
Methods
Study sample
The UK Biobank (UKB) is a prospective cohort study including >500 000 community-dwelling adults aged 40–69 years across the UK (https://www.ukbiobank.ac.uk/).21 Participants were recruited through 22 assessment centres between 2006 and 2010. We declare that all data are publicly available in the UKB repository.21 The UKB received ethical approval from the UK National Health Service, National Research Ethics Service North West, the National Information Governance Board for Health and Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland. All participants provided written informed consent. This study was approved by the UKB (application number 777407740).
A total of 502 411 participants were included in our study. We excluded 8412 participants with AF at baseline, and 82 490 with missing data on any risk factor mentioned below were excluded from our cohort, as well as 1848 out of range 40–69 years. Therefore, 409 661 participants were included in the present study (see Supplementary data online, Figure S1).
Definition of risk factors
At baseline, participants provided information, such as demographic characteristics, lifestyle behaviours, and medical information, by completing a comprehensive and standardized touchscreen questionnaire and verbal interview. In addition to self-reported information, the diagnosis of clinical comorbidities was also obtained using linkages with primary care and hospital inpatient records. In the assessment centre, height, weight, and blood pressure were measured at baseline using standard methods and devices, and body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in metres. In addition, a range of key biochemistry markers [e.g. lipid profile, C-reactive protein (CRP), and cystatin C] were measured in the blood sample collected at recruitment.
The 2020 ESC guidelines for the diagnosis and management of AF developed in collaboration with the European Association for Cardio-Thoracic Surgery summarized 46 modifiable and nonmodifiable risk factors for incident AF.12 In summary, nine factors were excluded due to unavailable/inadequate data (competitive or athlete-level endurance sports, coronary artery calcification, carotid intima-media thickness, carotid plaque, PR interval prolongation, family history of AF, short QT syndrome, fibrinogen, other biomarkers, and HDL), age was the stratification variable, and sex and ethnicity were adjusted in the model. Then, the other 34 factors were combined into 23 factors. Detailed information is presented in Supplementary data online, Tables S1 and S2.
The 23 factors covered 5 aspects. The first aspect consisted of social factors, including less education (less than high school), socioeconomic deprivation (Townsend index ≥ 1.34, the highest quintile), and severe air pollution (local air concentration of nitrogen dioxide ≥ 32.49 µg/m3 or nitrogen oxides ≥ 53.22 µg/m3). The second aspect consisted of health behaviours, including current smoking, excessive alcohol intake (alcohol intake ≥24 g/day22), physical inactivity (<150 min/week of moderate intensity, <75 min/week of vigorous intensity, or an equivalent combination23,24), excessive physical activity (>55 metabolic equivalent task-hours/week25), no coffee intake, loneliness (‘YES’ to ‘Do you often feel lonely?’), and broad depression (‘YES’ to ‘Have you ever seen a psychiatrist for nerves, anxiety, tension or depression?’26). The third aspect consisted of cardiometabolic factors, including hypertension (history of hypertension, regularly taking anti-hypertension medications, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg), low LDL-cholesterol (<2.81 mmol/L, the lowest quintile), low triglycerides (<.986 mmol/L, the lowest quintile), diabetes mellitus/prediabetes (history of diabetes, taking antidiabetic medications, or HbA1c ≥5.7%), overweight/obesity (BMI ≥25 kg/m2), and elevated CRP (≥3.12 mg/L, the highest quintile). The fourth aspect consisted of clinical comorbidities [cardiovascular disease (CVD), sleep apnoea, chronic obstructive pulmonary disease (COPD), renal dysfunction (chronic renal disease or estimated glomerular filtration rate <60 mL/min/1.73 m2), immune-mediated disease (hypo- and hyperthyroidism, coeliac disease, rheumatoid arthritis, and psoriasis), and acute illness (history of sepsis, major operations, and pneumonia)]. The last aspect was high genetic risk [genetic risk score (GRS) >6.72, the highest quintile (ascending sequence)]. More detailed definitions and references are provided in Supplementary data online, Table S2.
Furthermore, we established weighted risk scores in four aspects (social factors, health behaviours, cardiometabolic factors, and clinical comorbidities) by summing all risk factors within each aspect while considering magnitudes of their relative risks. Risk score = (β1 × factor 1 + β2 × factor 2 + L + βn × factor n) × (n/sum of the β coefficients).27 β was driven from the effect estimate of each risk factor on AF in adjusted regression models. A higher score indicated a higher risk of the corresponding aspect.
Genetic risk for atrial fibrillation
A total of 104 single nucleotide polymorphisms (SNPs) were found to be distinctly associated with AF at the genome-wide association significance level in a multi-ethnic genome-wide association study.28 The weighted GRS for each participant was calculated by summing the product of the AF-increasing allele and its effect size.29 Individual SNPs were coded as 0, 1, and 2 according to the number of risk alleles. The effect size of each SNP was obtained from the genome-wide association study mentioned before,28 which is listed in Supplementary data online, Table S3.
Ascertainment of incident atrial fibrillation
Incident AF cases (both primary and secondary diagnoses) were identified from the ‘first occurrence of health outcomes defined by a 3-character ICD 10th Revision code’ (category ID in UKB 1712) (I48). The diagnosis of individual AF was obtained using linkages with the death register, primary care, and hospital inpatient records. Detailed information regarding the linkage procedure is available online (https://biobank.ctsu.ox.ac.uk/crystal/exinfo.cgi?src=diag_xtabs_HES).
Statistical analyses
Participants were divided into 40–49, 50–59, and 60–69 years old groups. The genetic risk groups included low (lowest quintile of GRS), moderate (mid-three quintiles of GRS), and high (highest quintile of GRS) genetic risk groups.
Baseline characteristics are presented as the means with standard deviations (SDs) for continuous variables or numbers with percentages for categorical variables. COLLIN option in regression model and Kendall’s tau coefficient were used to test collinearity among risk factors, and the results showed an absence of collinearity (see Supplementary data online, Table S4). The end event was the first report of incident AF. The follow-up time was from the date of recruitment to the date of first occurrence or to the death date or latest censoring date (December 2021), whichever came first. We used Cox proportional hazards models with age as time scale to examine the associations between risk factors and incident AF. In each age group and genetic risk group, the model was adjusted for sex, age, ethnicity, and the use of lipid-lowering medication and mutually adjusted for individual risk factors. The associations of risk scores with incident AF were examined in Cox regression models adjusting for sex, age, ethnicity, the use of lipid-lowering medication, and individual risk factors that were not included in the risk score. We tested whether age and genetic risk modified the associations of risk factors and risk scores with the probability of AF using a log-likelihood ratio test to compare models with and without cross-product interaction terms.
We calculated partial PAR percentages of AF for each risk factor using the previously well-established method of multivariable-adjusted PAR30 for the effects of the same set of variables as the hazard ratio (HR) calculation. Population-attributable risk percentages can be interpreted as a population proportion that would avoid suffering from the disease if no one was exposed to a certain risk factor.31 The negative PAR% of a risk factor was not included in the models and was truncated at the limit of 0, as this is the lowest threshold to determine an association with increased risk.
In the secondary analyses, we also measured similar HRs and PAR% values among all participants, men, and women, with further adjustments for age groups. We performed several sensitivity analyses. First, we tested the three-way interaction among risk factors, age group, and genetic risk group on incident AF. Second, we used waist circumference (men ≥90 cm, women ≥80 cm) in place of BMI to define central obesity. Third, to shed light on those who already had diabetes and hypertension but were under reasonable control with medication, we used only blood pressure and glycated haemoglobin (HbA1c) to define high blood pressure (systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg) and high blood glucose (HbA1c ≥5.7%), with further adjustments for the use of anti-hypertension and antidiabetic medications. Fourth, competing risk analysis was performed using proportional subdistribution hazards regression models,32 in which non-AF death was regarded as a competing outcome event to AF. Fifth, to address bias from complete case analysis, we used multiple imputation by chained equations (MICE)33 to impute baseline missing data for risk factors.
A two-sided P-value <.05 was considered statistically significant. However, to account for multiple comparisons for the analysis of the 23 risk factors and 4 risk scores, Bonferroni’s correction was used. P < .002 (.05/23) and P < .013 (.05/4) were considered statistically significant. SAS software, version 9.4 and R software, version 4.1.2 were used for analyses. We estimated the HR for risk factors with the Survival R package. We used a competing risk model with the CMPRSK R package. The macro of PAR% was published in a previous article.30 We implemented multiple imputation with the MICE R package.
Results
Participant characteristics
Baseline characteristics by age and genetic risk groups are shown in Table 1. The mean age of the cohort was 56 (SD 8) years old, and 46% were men. Participants in the 40–49 years age group were more educated and socioeconomically deprived, had a higher proportion of current smokers, greater physical activity, and reported loneliness compared with those in the 60–69 years age group. Cardiometabolic factors were more common in elderly participants. The prevalence of clinical comorbidities increased with age. Moreover, most of the baseline characteristics were rather comparable among the different genetic risk groups. Participants excluded due to missing data seemed to have worse health behaviours than those who were included (see Supplementary data online, Table S5).
Table 1.
Baseline characteristics of participants by age groups and genetic risk groups
| 40–49 years | 50–59 years | 60–69 years | Low genetic risk | Moderate genetic risk | High genetic risk | |
|---|---|---|---|---|---|---|
| Participants, n | 98 839 | 138 553 | 172 269 | 81 956 | 246 685 | 81 020 |
| Incidence of AF | ||||||
| Case (percentage) | 1251 (1.3) | 5229 (3.8) | 17 367 (10.1) | 2880 (3.5) | 13 532 (5.5) | 7435 (9.2) |
| Events per 1000 person-years | 1.0 | 3.0 | 8.5 | 2.8 | 4.5 | 7.6 |
| Age, years | 45.0 ± 2.7 | 54.8 ± 2.9 | 64.0 ± 2.8 | 56.2 ± 8.1 | 56.0 ± 8.0 | 56.3 ± 8.0 |
| Sex | ||||||
| Male | 44 914 (45.4) | 61 237 (44.2) | 81 999 (47.6) | 38 002 (46.4) | 113 481 (46.0) | 36 667 (45.3) |
| Female | 53 925 (54.5) | 77 316 (55.8) | 90 270 (52.5) | 43 954 (53.6) | 133 204 (54.0) | 44 353 (54.7) |
| Ethnic groups | ||||||
| White | 86 662 (87.7) | 125 959 (90.9) | 160 958 (93.4) | 72 960 (89.0) | 226 022 (91.6) | 74 597 (92.1) |
| Mixed | 4611 (4.7) | 5040 (3.6) | 4795 (2.8) | 3802 (4.6) | 8262 (3.3) | 2382 (2.9) |
| Asian or Asian British | 4708 (4.8) | 4828 (3.5) | 4480 (2.6) | 3129 (3.8) | 8266 (3.4) | 2621 (3.2) |
| Black or Black British | 824 (.8) | 700 (.5) | 525 (.3) | 680 (.8) | 1071 (.4) | 298 (.4) |
| Others | 1765 (1.8) | 1645 (1.2) | 965 (.6) | 1124 (1.4) | 2363 (1.0) | 888 (1.1) |
| Social factors | ||||||
| Less education | 45 189 (45.7) | 69 586 (50.2) | 109 621 (63.6) | 44 872 (54.8) | 135 013 (54.7) | 44 511 (54.9) |
| Socioeconomic deprivation | 22 509 (22.8) | 25 504 (18.4) | 27 542 (16) | 16 300 (19.9) | 44 752 (18.1) | 14 503 (17.9) |
| Severe air pollution | 27 500 (27.8) | 33 083 (23.9) | 35 616 (20.7) | 20 379 (24.9) | 57 213 (23.2) | 18 607 (23) |
| Health behaviours | ||||||
| Current smoking | 9358 (9.5) | 11 144 (8) | 10 156 (5.9) | 5916 (7.2) | 18 545 (7.5) | 6197 (7.6) |
| Excessive alcohol intake | 30 750 (31.1) | 46 582 (33.6) | 54 872 (31.9) | 25 935 (31.6) | 80 012 (32.4) | 26 257 (32.4) |
| Physical inactivity | 35 967 (36.4) | 54 971 (39.7) | 58 452 (33.9) | 29 869 (36.4) | 90 003 (36.5) | 29 518 (36.4) |
| Excessive physical activity | 23 244 (23.5) | 30 531 (22) | 43 271 (25.1) | 19 584 (23.9) | 58 250 (23.6) | 19 212 (23.7) |
| No coffee intake | 32 726 (33.1) | 41 633 (30) | 44 108 (25.6) | 24 647 (30.1) | 70 613 (28.6) | 23 207 (28.6) |
| Loneliness | 20 406 (20.6) | 26 568 (19.2) | 26 489 (15.4) | 15 056 (18.4) | 43 935 (17.8) | 14 472 (17.9) |
| Broad depression | 11 382 (11.5) | 15 947 (11.5) | 18 118 (10.5) | 9030 (11) | 27 289 (11.1) | 9128 (11.3) |
| Cardiometabolic factors | ||||||
| Hypertension | 35 995 (36.4) | 75 920 (54.8) | 124 642 (72.4) | 47 372 (57.8) | 142 759 (57.9) | 46 426 (57.3) |
| Low LDL-cholesterol | 21 674 (21.9) | 21 684 (15.7) | 35 671 (20.7) | 16 263 (19.8) | 47 252 (19.2) | 15 514 (19.1) |
| Low triglycerides | 28 982 (29.3) | 28 314 (20.4) | 26 056 (15.1) | 17 214 (21) | 49 816 (20.2) | 16 322 (20.1) |
| Diabetes/prediabetes | 8341 (8.4) | 23 115 (16.7) | 42 900 (24.9) | 15 668 (19.1) | 44 516 (18) | 14 172 (17.5) |
| Overweight/obesity | 60 475 (61.2) | 91 435 (66) | 119 527 (69.4) | 54 586 (66.6) | 163 388 (66.2) | 53 463 (66) |
| Elevated C-reactive protein | 16 068 (16.3) | 26 223 (18.9) | 36 628 (21.3) | 15 936 (19.4) | 47 445 (19.2) | 15 538 (19.2) |
| Clinical comorbidities | ||||||
| Sleep apnoea | 302 (.3) | 710 (.5) | 914 (.5) | 386 (.5) | 1172 (.5) | 368 (.5) |
| COPD | 632 (.6) | 1920 (1.4) | 4621 (2.7) | 1396 (1.7) | 4373 (1.8) | 1404 (1.7) |
| Renal dysfunction | 713 (.7) | 3614 (2.6) | 15 013 (8.7) | 4004 (4.9) | 11 649 (4.7) | 3687 (4.6) |
| Cardiovascular disease | 1002 (1) | 3829 (2.8) | 11 288 (6.6) | 3290 (4) | 9698 (3.9) | 3131 (3.9) |
| Immune-mediated disease | 6117 (6.2) | 12 328 (8.9) | 18 257 (10.6) | 7107 (8.7) | 22 124 (9) | 7471 (9.2) |
| Acute illness | 55 322 (56) | 88 568 (63.9) | 121 958 (70.8) | 52 564 (64.1) | 160 474 (65.1) | 52 810 (65.2) |
| High genetic risk score | 19 545 (19.8) | 27 554 (19.9) | 33 921 (19.7) | - | - | - |
Values are expressed as N (%) or mean ± SD.
COPD, chronic obstructive pulmonary disease.
Age-specific association of risk factors and scores with atrial fibrillation
After a follow-up of 5 027 587 person-years (median 12.3 years, interquartile range 11.9–13.5 years, range 1–15 years), 23 847 (5.8%) of participants developed AF (4.7 per 1000 person-years, 3.2 and 6.6 per 1000 person-years in men and women). The incidence rate of AF increased from the younger group to the older group (40–49 years: 1.0 per 1000 person-years; 50–59 years: 3.0 per 1000 person-years; 60–69 years: 8.5 per 1000 person-years). In all participants, the risk of incident AF was significantly associated with all selected risk factors except severe air pollution, physical inactivity, excessive physical activity, and no coffee intake (see Supplementary data online, Table S6).
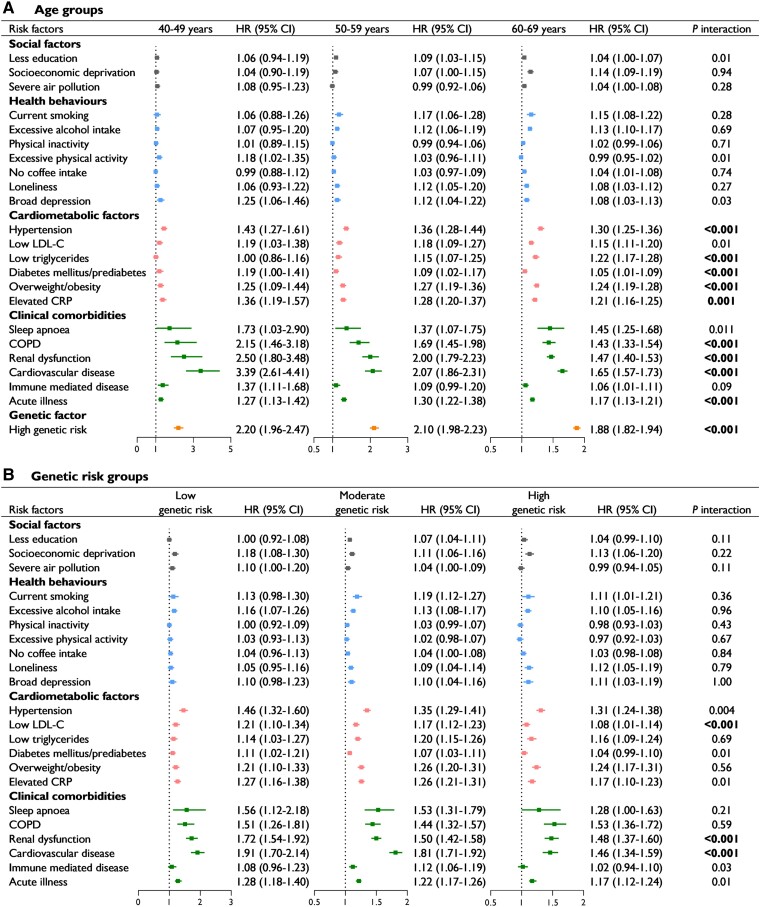
The associations between risk factors and AF by age group are shown in Figure 1A (absolute rate shown in Supplementary data online, Table S7). The associations between AF and all cardiometabolic factors, except low LDL-C, were strengthened in younger groups (Pinteraction < .002). Among the comorbidities, COPD, renal dysfunction, CVD, and acute illness had significant interactions with age groups regarding AF risk, and these interactions were also stronger in younger groups (Pinteraction < .001). Notably, the association with CVD was particularly strengthened in younger groups {HR 3.39 [95% confidence interval (CI) 2.61–4.41] among 40–49 years group vs. 1.65 [1.57–1.73] among 60–69 years group}. Additionally, a high genetic risk was more strongly associated with AF in the younger groups (Pinteraction < .001). The social factors and health behaviours showed no significant interactions. When further stratified by sex, the profiles of some specific risk factors across age groups were different between men and women (see Supplementary data online, Table S8). For example, the association with elevated CRP was strengthened in younger groups in women [1.46 (1.15–1.85) vs. 1.25 (1.18–1.32)] but not in men. Conversely, the HR of high genetic risk significantly decreased with age in men only [3.03 (2.23–4.12) vs. 1.56 (1.47–1.65)].
Figure 1.
Multivariable-adjusted hazard ratios (95% confidence intervals) of incident atrial fibrillation associated with risk factors in different age groups (A) and genetic risk groups (B). Models were adjusted for sex, age, ethnicity, and the use of lipid-lowering medication and were mutually adjusted for individual risk factors. Statistical significance was defined as a Bonferroni-corrected threshold of P < .002 (.05/23). LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease
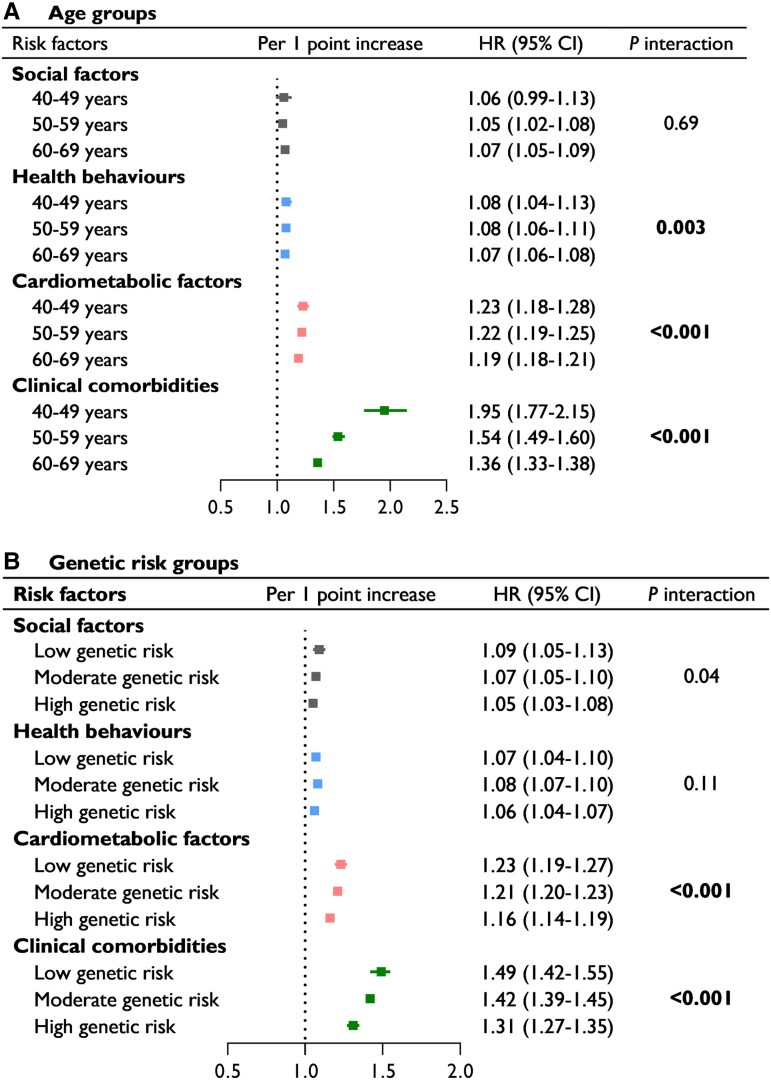
The associations between risk scores and AF by age group are shown in Figure 2A. The strength of the association was strongest for the GRS, followed by the clinical comorbidity and cardiometabolic risk scores. All risk scores interacted significantly with age group (all Pinteraction < .01), except for the social risk score. Each one-point increment in the clinical comorbidity risk score was associated with a 95% [HR 1.95 (95% CI 1.77–2.15)] and 36% [1.36 (1.33–1.38)] higher risk of AF in the 40–49 years group and 60–69 years group, respectively. These results were also observed for cardiometabolic risk [1.23 (1.18–1.28) vs. 1.19 (1.18–1.21)] and GRSs [2.20 (1.96–2.47) vs. 1.88 (1.82–1.94)]. Consistent significant interactions of the three risk factor categories with age group on AF were found in both men and women (see Supplementary data online, Table S9).
Figure 2.
Multivariable-adjusted hazard ratios (95% confidence intervals) of incident atrial fibrillation associated with risk scores in different age groups (A) and genetic risk groups (B). Models were adjusted for sex, age, ethnicity, the use of lipid-lowering medication and individual risk factors that were not included in the risk score. Statistical significance was defined using Bonferroni-corrected thresholds of P < .013 (.05/4)
Genetic risk-specific association of risk factors and scores with atrial fibrillation
Figure 1B presents the associations between risk factors and AF by genetic risk group. Only 3 of the 22 risk factors showed a significant interaction with genetic risk (Pinteraction < .002). One cardiometabolic factor was low LDL-C [HR 1.21 (95% CI 1.10–1.34) among the low genetic risk group vs. 1.08 (1.01–1.14) among the high genetic risk group]. The associations of renal dysfunction [1.72 (1.54–1.92) vs. 1.48 (1.37–1.60)] and CVD [1.91 (1.70–2.14) vs. 1.46 (1.34–1.59)] were also significantly strengthened in the low genetic risk group.
The associations between risk scores and AF by genetic risk groups are shown in Figure 2B. There were significant interactions of cardiometabolic and clinical morbidity risk scores with genetic risk for AF (Pinteraction < .01). The risk of AF was stronger for the clinical morbidity and cardiometabolic risk scores in the low genetic risk group than in the high genetic risk group.
Age and genetic risk-specific population-attributable risk of risk factors and scores
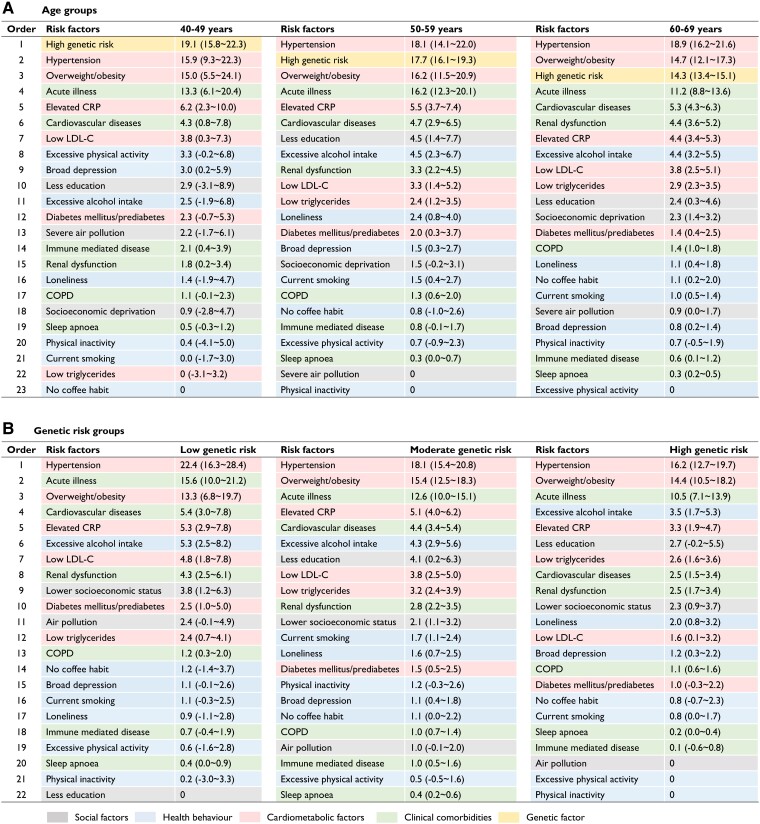
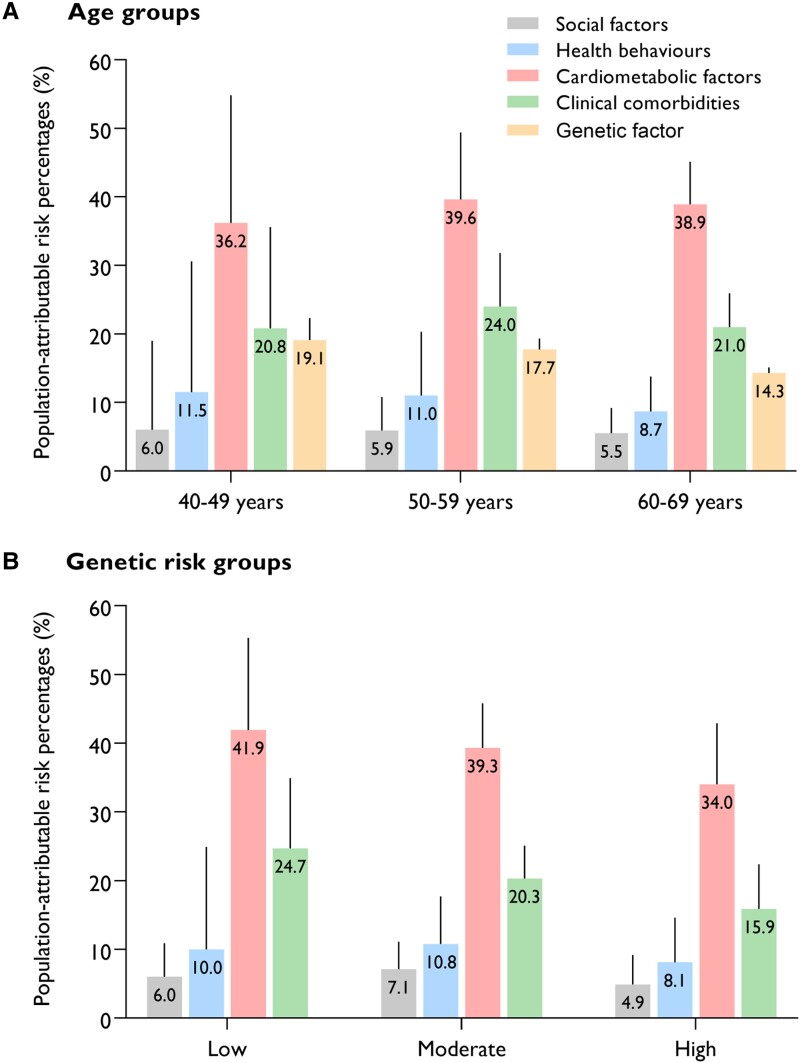
After stratification by age, hypertension was the top modifiable risk factor in all age groups, with a PAR% of 15.9% (95% CI 9.3–22.3) in the 40- to 49-year-old group and 18.9% (16.2–21.6) in the 60- to 69-year-old group (Figure 3). High genetic risk accounted for a PAR% of 19.1% (15.8–22.3) and 14.3% (13.4–15.1) for AF cases in the 40–49- and 60–69-year-old groups, respectively. Overweight/obesity and acute illness were two other major contributors in all three age groups [PAR%: 15.0% (95% CI 5.5–24.1) and 13.3% (6.1–20.4) in the 40–49 years group, 14.7% (12.1–17.3) and 11.2% (8.8–13.6) in the 60–69 years group]. When considered jointly, the single predominant contributor was the combination of cardiometabolic factors, and the PAR% ranged from 36.2% in the 40–49 years group to 38.9% in the 60–69 years group (Figure 4). The AF risk attributable to health behaviours declined from 11.5% in the 40–49 years group to 8.7% in the 60–69 years group. The lowest PAR% was observed for social factors in all three groups.
Figure 3.
Partial population-attributable risk percentages for incident atrial fibrillation associated with risk factors in different age groups (A) and genetic risk groups (B). Values are expressed as population-attributable risk percentages (95% confidence intervals). Models were adjusted for sex, age, ethnicity, and the use of lipid-lowering medication and were mutually adjusted for individual risk factors. Since this threshold for determining an increase in risk is 0, the risk factors with negative population-attributable risk percentages were excluded from the models and were truncated at the limit of 0. LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease
Figure 4.
Partial population-attributable risk percentages for incident atrial fibrillation associated with combinations of risk factors by age group (A) and genetic risk group (B). Models were adjusted for sex, age, ethnicity, the use of lipid-lowering medication and individual risk factors that were not included in the risk score
Hypertension, overweight/obesity, and acute illness were the top three contributors in each genetic risk group (Figure 3). The PAR% of most risk factors decreased with increasing genetic risk. However, the PAR% of feeling lonely increased with increasing genetic risk. When examined jointly, cardiometabolic factors overall were still the single predominant contributor to AF, and the PAR% ranged from 41.9% in the low genetic risk group to 34.0% in the high genetic risk group (Figure 4). The AF risk attributable to clinical comorbidities declined from 24.7% in the low genetic risk group to 15.9% in the high genetic risk group. Similarly, the PAR% of social factors remained the lowest in all three groups by genetic risk.
Sensitivity analysis
We found similar results in the model considering death as a competing risk (see Supplementary data online, Tables S10 and S11). Furthermore, the difference in age-related changes in the risk of incident AF with each risk factor was nonsignificant among genetic risk groups (see Supplementary data online, Table S12). Using waist circumference to define central obesity, the PAR of central obesity increased compared with overweight/obesity by the BMI definition. When the use of anti-hypertension and anti-diabetes medications was among the adjustment covariates, the PARs of high blood pressure and glucose defined by measured blood pressure and HbA1c decreased compared with the PARs of hypertension and diabetes mellitus/prediabetes (see Supplementary data online, Table S13). Furthermore, we repeated the analyses after multiple imputation for the missing exposures (see Supplementary data online, Tables S14–S17) and found no obvious difference in the primary results. For example, the leading risk factors (hypertension, overweight/obesity, high genetic risk, acute illness) that explained >10% of the onset of AF were the same. The relative contribution of the four risk factor categories to the development of AF remained consistent with primary results.
Discussion
Using data from the large, prospective UKB cohort, this study provides novel and comprehensive information about age- and genetic predisposition-specific risk of new-onset AF attributable to risk factor profiles. Cardiometabolic factors were responsible for the greatest number of AF cases among all age and genetic risk groups, with hypertension and overweight/obesity being the leading risk factors. Health behaviours and genetic risk factors accounted for more AF cases in the 40- to 49-year-old group than in the 60- to 69-year-old group, while the contribution of clinical comorbidities remained stable. The AF risk attributable to overall cardiometabolic factors and clinical comorbidities decreased significantly with increasing genetic risk. Moreover, most cardiometabolic factors and clinical comorbidities showed a significant interaction with age, while only low LDL-C, renal dysfunction, and CVD showed significant interactions with genetic risk (Structured Graphical Abstract). These findings could help to shed light on the potential causes of AF and, more importantly, provide evidence to facilitate precision strategies for the prevention and management of AF.
It is notable that overall cardiometabolic factors contributed the most to incident AF cases, among all age groups. Most previous studies of the PAR% of AF have focused on hypertension in the general population.34 Rodriguez et al.35 suggested that hypertension was the largest contributor to AF, followed by BMI, diabetes, and smoking, but only a significant PAR% for hypertension regarding new-onset AF. Our results showed that hypertension was the top contributor to AF in participants aged 50–59 years and 60–69 years old, which is consistent with a previous study,36 but not in those aged 40–49 years old. Moreover, hypertension explained more AF with increasing age, mostly because of a higher prevalence of hypertension in elderly individuals. Cardiometabolic factors, especially hypertension, have a strongly intrinsic association with AF. Atrial fibrosis and dysfunction of the autonomic nervous system, which can be caused by hypertension, usually trigger the development of AF.9 Overweight/obesity ranked second among cardiometabolic factors, accounting for a similar proportion of AF as in previous studies.36,37 The PARs of overweight/obesity were rather similar among the age groups. Haemodynamic alteration caused by obesity is a central mechanism for the structural abnormalities of the ventricles that ultimately leading to AF.38 These findings prioritize the monitoring and intervention approaches targeting hypertension and overweight/obesity across all age groups for AF prevention.
Clinical comorbidities overall were another major contributor to incident AF in all age groups. Age-related differences in clinical comorbidities for AF were less mentioned previously.39,40 We found that the strength of the association between clinical comorbidities and AF decreased with age. Interestingly, the PAR% for acute disease ranked highest among clinical comorbidities and explained >10% of the AF cases in all age groups, and the largest proportion was observed in the 50- to 59-year-old age group (PAR% of 16.2%). This outcome might be related to more than half of the participants aged 50–59 years old having experienced acute illness or major surgery. Similarly, Lowres et al.41 found that 1.4% of patients who were admitted for noncardiac surgery or acute illness developed AF during their hospital stay, and one-third of those with new-onset AF had a recurrence of AF within 9 days of discharge. This finding draws special attention to clinical signs of AF in patients admitted for acute illness or surgery and professional guidance for early detection of AF after discharge.
Maintaining healthy behaviours has been recognized as the fundamental measure that not only improves the prognosis after AF treatment but also plays an important role in AF prevention.42 The PAR% for AF attributable to healthy behaviours in the 40- to 49-year-old group was nearly twice as high as that in the 60- to 69-year-old group. The PAR% of individual behavioural risk factors was generally low, which might partly have occurred because these behavioural risk factors often coexist with several other cardiometabolic factors or clinical comorbidities. For example, alcohol can trigger AF by constricting blood vessels to raise blood pressure or indirectly by affecting autonomic function.43 As a result, the contribution of behaviour risk factors was diluted after considering cardiometabolic factors and comorbidities. In addition, we noted that the impact of social factors on AF burden was rather limited across all age groups.
Notably, instead of cardiometabolic factors, the PAR% of high genetic risk ranked the first in the 40–49 years group, which has not been mentioned in previous studies.36,37,44 High genetic risk also ranked highly (second and third) in the other two age groups. Genetic predisposition accounts for a certain amount of AF onset. A previous cohort study reported that 15% of patients with AF had a family history.45 Thus far, >100 genetic loci28 have been identified to be significantly associated with AF. Our results confirmed the existing evidence that early genetic screening could help to increase personalized prevention and control of AF,46 particularly among younger adults.
In addition to the relationship between genetic predisposition and AF incidence, investigations on risk factor profiles according to genetic risk stratification might also be useful to develop efficient prevention strategies. Overall, there are some commonalities for risk profiles across different genetic risk subgroups. For example, cardiometabolic factors and clinical comorbidities were consistently the leading factors accounting for AF onset although the PAR% decreased with higher genetic risk. In detail, hypertension, overweight/obesity, and acute illness remained the three major contributors to AF onset across the genetic risk groups, similar to the age subgroups. Conversely, the PARs% of hypertension and acute illness were nearly half higher in the low genetic risk group than in the high genetic risk group, indicating the need for equivalent or even more rigorous control of cardiometabolic factors and clinical comorbidities among individuals with low genetic risk.
At the same time, we observed other differences in risk profiles among different genetic risk groups. The PAR% for AF attributable to CVD and renal dysfunction was twice as high in the low genetic risk group as in the high genetic risk group. In contrast, the PAR% of less education was twice as high in the high genetic risk group as in the low genetic risk group. A possible explanation is that people with less education tended to have unhealthier lifestyles and receive fewer medical resources,47 which might be more strongly associated with AF incidence based on high genetic susceptibility to AF. Less education is a powerful socioeconomic determinant for CVD and mortality.48 It is difficult to improve academic qualifications in later stages of life, but education about health knowledge and awareness of regular monitoring could still help to reduce lifetime risk and extend disease-free survival time.
Although the risk profiles for AF according to age and genetic predisposition stratification were largely comparable, the relative contribution of certain risk factors to some extent varied. Cardiometabolic factors were the dominant factors for AF onset independent of age and genetic predisposition, but the variations in PAR% seemed to be greater across genetic risk stratification than across age stratification. There were more pronounced differences in PAR% associated with health behaviours among different age groups, while more obvious differences for clinical comorbidities, including acute disease, CVD, and renal dysfunction, were found among different genetic risk groups. Thus, in addition to prioritizing target risk factors by age, determination of genetic risk as a complementary means could yield additional evidence to guide AF prevention on an individual level.
To our knowledge, this study is the first to quantify age and genetic risk-related disparities in AF risk attributable to risk factor profiles. We included almost all risk factors reported to be associated with the development of AF. However, several limitations apply to our findings. Unfortunately, the UKB database did not recruit participants older than 70 years old, which might partly reduce the ability to detect more age-related differences in risk factor profiles associated with AF. The potential bias might be caused by removing the population with missing information. Given that excluded individuals were more likely to have worse health profiles, the effects of risk factors might potentially be larger in more representative populations because of this selection bias. Secondly, all risk factors were extracted from baseline data, and their changes over time could not be accounted for in this study. The data of some risk factors were self-reported and might have been prone to recall bias. Additionally, several risk factors were discarded due to unavailable/inadequate data, such as HDL cholesterol, coronary artery calcification, family history of AF, short QT syndrome, PR interval prolongation, carotid intima-media thickness, and carotid plaque. However, most of these factors might not significantly alter the PAR results. For example, coronary artery calcification, carotid intima-media thickness, and carotid plaque could largely overlap with CVD, and the prevalence of short QT syndrome could be very low. We included the AF GRS as a proxy for the family history of AF. Third, participants in the UKB cohort are more likely to represent a ‘healthy volunteer’ population. Consequently, the strength of the observed associations in our study would have been underestimated. Moreover, the UKB participants were mostly White British, and whether our findings could be generalized to other populations requires further research. Lastly, there was a possibility of missed AF cases from medical records because of asymptomatic paroxysmal episodes before diagnosis.
Public health and clinical implications
The European Heart Rhythm Association has recently issued updated guidelines that listed >20 risk factors for AF.12 The expansion of the interactions between age and common risk factors for AF in our study provides an opportunity to further advance targeted screening initiatives, modifications, and interventions. Beyond the similarity of cardiometabolic factors and related comorbidities as the predominant contributors across all age groups, the decreasing PAR attributable to lifestyle risk factors with age emphasizes the priority for intervention efforts targeting lifestyle behaviours in younger adults in preventing or delaying the onset of AF. By comparison, regarding genetic risk stratification, cardiometabolic factors, and clinical comorbidities played more dominant roles in people with a lower genetic predisposition to AF, suggesting that improving the management of cardiometabolic factors and clinical comorbidities might yield more benefit in reducing AF cases in people with low genetic risk than in those with high genetic risk. Our findings of age- and genetics-related risk factor profiles for AF could not only help to improve future risk evaluation but also provide evidence to facilitate precise and efficient prevention and control of AF.
Conclusions
This large, prospective cohort study provided comprehensive information about age- and genetic predisposition-related risk factor profiles for AF in a cohort of UK adults. These findings emphasize the importance of individualized approaches in accordance with age- and genetic risk-specific risk profiles for effective prevention of AF.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors declare no conflict of interest for this contribution.
Supplementary Material
Contributor Information
Ningjian Wang, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Yuefeng Yu, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Ying Sun, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Haojie Zhang, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Yuying Wang, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Chi Chen, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Xiao Tan, School of Public Health, Zhejiang University, Hangzhou, China; Department of Medical Sciences, Uppsala University, Uppsala, Sweden.
Bin Wang, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Yingli Lu, Institute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, No. 639 Zhizaoju Road, Huangpu District, Shanghai 200011, China.
Data Availability
This research has been conducted using the UK Biobank Resource under Application Number 77740. The data underlying this article are available in UK Biobank at https://www.ukbiobank.ac.uk/.
Funding
This study was supported by Shanghai Municipal Health Commission (2022XD017), Shanghai Municipal Hospital Development Center (SHDC2020CR4006), Shanghai Municipal Human Resources and Social Security Bureau (2020074), and Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZDCX20212501). The funders played no role in the design or conduction of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the article.
Ethical Approval
The North West Multi-Centre Research Ethics Committee Study approved the UKB study, and all participants provided written informed consent. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Pre-registered Clinical Trial Number
Not applicable.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. 10.1161/cir.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–51. 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018;361:k1453. 10.1136/bmj.k1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107–14. 10.1001/jamainternmed.2013.11912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol 2015;66:1000–7. 10.1016/j.jacc.2015.06.1314 [DOI] [PubMed] [Google Scholar]
- 7. Lacoin L, Lumley M, Ridha E, Pereira M, McDonald L, Ramagopalan S, et al. Evolving landscape of stroke prevention in atrial fibrillation within the UK between 2012 and 2016: a cross-sectional analysis study using CPRD. BMJ Open 2017;7:e015363. 10.1136/bmjopen-2016-015363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. 10.1161/01.cir.98.10.946 [DOI] [PubMed] [Google Scholar]
- 9. Lau DH, Schotten U, Mahajan R, Antic NA, Hatem SN, Pathak RK, et al. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J 2016;37:1573–81. 10.1093/eurheartj/ehv375 [DOI] [PubMed] [Google Scholar]
- 10. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501–17. 10.1161/circresaha.117.309732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu Z, Geurts S, Aribas E, De Groot N, Kavousi M. Women-specific risk factors and risk of incident atrial fibrillation in UK Biobank. Eur J Prev Cardiol 2022;29:i210. 10.1093/eurjpc/zwac056.146 [DOI] [PubMed] [Google Scholar]
- 12. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 13. Wang N, Sun Y, Zhang H, Wang B, Chen C, Wang Y, et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur Heart J 2021;42:4180–8. 10.1093/eurheartj/ehab505 [DOI] [PubMed] [Google Scholar]
- 14. Allan V, Honarbakhsh S, Casas JP, Wallace J, Hunter R, Schilling R, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost 2017;117:837–50. 10.1160/th16-11-0825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stewart W, Hunting K. Mortality odds ratio, proportionate mortality ratio, and healthy worker effect. Am J Ind Med 1988;14:345–53. 10.1002/ajim.4700140312 [DOI] [PubMed] [Google Scholar]
- 16. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 17. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–60. 10.1001/jama.2013.280521 [DOI] [PubMed] [Google Scholar]
- 18. Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank study. JAMA Cardiol 2018;3:693–702. 10.1001/jamacardio.2018.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options–a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14:8–27. 10.1093/europace/eur241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De With RR, Marcos EG, Van Gelder IC, Rienstra M. Atrial fibrillation progression and outcome in patients with young-onset atrial fibrillation. Europace 2018;20:1750–7. 10.1093/europace/euy028 [DOI] [PubMed] [Google Scholar]
- 21. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallagher C, Hendriks JML, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, et al. Alcohol and incident atrial fibrillation—a systematic review and meta-analysis. Int J Cardiol 2017;246:46–52. 10.1016/j.ijcard.2017.05.133 [DOI] [PubMed] [Google Scholar]
- 23. Azarbal F, Stefanick ML, Salmoirago-Blotcher E, Manson JE, Albert CM, LaMonte MJ, et al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc 2014;3:e001127. 10.1161/jaha.114.001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Keefe JH, Patil HR, Lavie CJ, Magalski A, Vogel RA, McCullough PA. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc 2012;87:587–95. 10.1016/j.mayocp.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Keefe EL, Torres-Acosta N, O'Keefe JH, Lavie CJ. Training for longevity: the reverse J-curve for exercise. Mo Med 2020;117:355–61. [PMC free article] [PubMed] [Google Scholar]
- 26. Baselmans BML, Jansen R, Ip HF, van Dongen J, Abdellaoui A, van de Weijer MP, et al. Multivariate genome-wide analyses of the well-being spectrum. Nat Genet 2019;51:445–51. 10.1038/s41588-018-0320-8 [DOI] [PubMed] [Google Scholar]
- 27. Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J 2020;41:1182–9. 10.1093/eurheartj/ehz849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet 2018;50:1225–33. 10.1038/s41588-018-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang N, Lu M, Chen C, Xia F, Han B, Li Q, et al. Adiposity genetic risk score modifies the association between blood lead level and body mass index. J Clin Endocrinol Metab 2018;103:4005–13. 10.1210/jc.2018-00472 [DOI] [PubMed] [Google Scholar]
- 30. Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control 2007;18:571–9. 10.1007/s10552-006-0090-y [DOI] [PubMed] [Google Scholar]
- 31. Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol 1994;140:303–9. 10.1093/oxfordjournals.aje.a117252 [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 33. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–9. 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lau DH, Shenasa HA, Shenasa M. Hypertension, prehypertension, hypertensive heart disease, and atrial fibrillation. Card Electrophysiol Clin 2021;13:37–45. 10.1016/j.ccep.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol 2015;25:71–6, 76.e71. 10.1016/j.annepidem.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501–8. 10.1161/circulationaha.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morseth B, Geelhoed B, Linneberg A, Johansson L, Kuulasmaa K, Salomaa V, et al. Age-specific atrial fibrillation incidence, attributable risk factors and risk of stroke and mortality: results from the MORGAM consortium. Open Heart 2021;8:e001624. 10.1136/openhrt-2021-001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol 2017;70:2022–35. 10.1016/j.jacc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 39. Roberts JD, Vittinghoff E, Lu AT, Alonso A, Wang B, Sitlani CM, et al. Epigenetic age and the risk of incident atrial fibrillation. Circulation 2021;144:1899–911. 10.1161/circulationaha.121.056456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim IS, Choi YJ, Choi EY, Min PK, Yoon YW, Lee BK, et al. Comparison of risk profiles for new-onset atrial fibrillation between patients aged <60 and ≥60 years. PLoS One 2021;16:e0258770. 10.1371/journal.pone.0258770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lowres N, Hillis GS, Gladman MA, Kol M, Rogers J, Chow V, et al. Self-monitoring for recurrence of secondary atrial fibrillation following non-cardiac surgery or acute illness: a pilot study. Int J Cardiol Heart Vasc 2020;29:100566. 10.1016/j.ijcha.2020.100566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Middeldorp ME, Ariyaratnam J, Lau D, Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart 2020;106:325–32. 10.1136/heartjnl-2019-315327 [DOI] [PubMed] [Google Scholar]
- 43. Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol 2016;68:2567–76. 10.1016/j.jacc.2016.08.074 [DOI] [PubMed] [Google Scholar]
- 44. Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njølstad I, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017;136:1588–97. 10.1161/circulationaha.117.028981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol 2003;41:2185–92. 10.1016/s0735-1097(03)00465-0 [DOI] [PubMed] [Google Scholar]
- 46. Yoneda ZT, Anderson KC, Quintana JA, O'Neill MJ, Sims RA, Glazer AM, et al. Early-onset atrial fibrillation and the prevalence of rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol 2021;6:1371–9. 10.1001/jamacardio.2021.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raghupathi V, Raghupathi W. The influence of education on health: an empirical assessment of OECD countries for the period 1995–2015. Arch Public Health 2020;78:20. 10.1186/s13690-020-00402-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;395:795–808. 10.1016/s0140-6736(19)32008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research has been conducted using the UK Biobank Resource under Application Number 77740. The data underlying this article are available in UK Biobank at https://www.ukbiobank.ac.uk/.