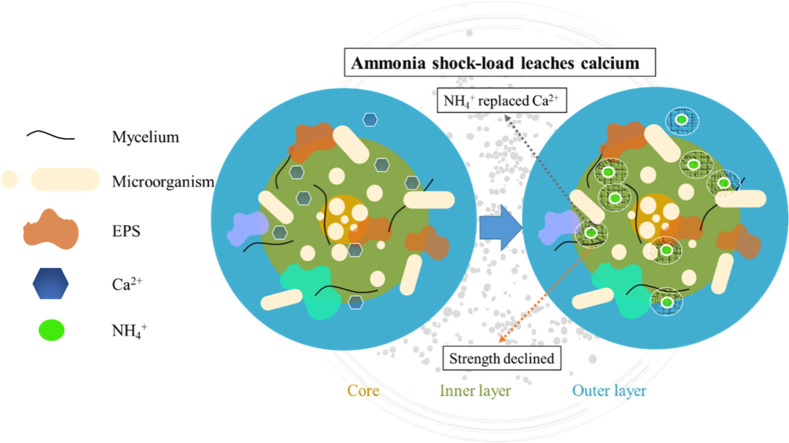
Highlights
-
•
Ca2+ was rapidly leached from anaerobic granular sludge by low pH and high NH4+.
-
•
The residual Ca2+ declined while the bound Ca2+ increased in granular sludge.
-
•
NH4+ shock drastically weakened granule structure.
-
•
Ca2+ leaching effects by high NH4+ should not be overshadowed by its biotoxicity.
Keywords: Granular sludge, NH4+ shock-loads, Ca2+ leaching, pH
Abstract
Previous researches have primarily emphasized the deleterious impacts of NH4+ on anaerobic granular sludge due to its biotoxicity. Despite this, the role of NH4+ as a monovalent cation in leaching multivalent Ca2+, thereby hindering granule formation and undermining its stability, remains underappreciated. This study investigated the potential of NH4+ to leach Ca2+ from anaerobic granular sludges. The results indicated that a shock loading of NH4+ at a concentration of 900 mg/L caused a Ca2+ leaching of 57.1 mg/L at pH 7.0. In an acidified environment (pH 5.0), the shock loading resulted in a Ca2+ release of 127.3 mg/L, a magnitude 5.24 times greater than the control group. The leaching process modestly affected granular sludge activity and size but markedly compromised granular strength due to calcium loss. Subsequent to the NH4+ shock, the granular strength manifested a significant reduction, as evidenced by a 15-fold increase in protein release from the granules compared to the intact ones. Additionally, NH4+ shock altered the calcium partitioning within the granular sludge, resulting in a decrease in residual calcium and a concomitant increase in bound calcium, further affecting granular strength. This study underscores the overlooked significant phenomenon of NH4+ shock-leaching Ca2+ in anaerobic granular sludge, which warrants significant attention given to its rapid and deleterious effects on granular strength and the shift in calcium state.
Graphic abstract
Introduction
Anaerobic granular sludge technology, which includes processes such as up-flow anaerobic sludge bed and expanded granular sludge bed, has gained significant attention in biological wastewater treatment due to its superior settling characteristics and bioactivity (van Loosdrecht and Brdjanovic, 2014). Representing over 90 % of industrial wastewater treatment processes, it is increasingly considered to be a promising wastewater treatment technology (van Lier et al., 2015). Granular sludge plays a crucial role in this technology as it forms densely packed microbial aggregates through self-assembly by physical, chemical, and biological forces (Ran et al., 2022).
Ca2+ ions significantly influence the granules by acting as coagulative nuclei (Liu et al., 2002; Wang et al., 2018), providing structural bridges (de Graaff et al., 2011; Ren et al., 2008; Zhang et al., 2018), and enhancing cell hydrophobicity through surface dehydration (Pevere et al., 2007). It is crucial to maintain an appropriate concentration of Ca2+ to ensure the proper functioning of the reactor. An inadequate concentration of Ca2+ can hinder granulation and lead to granule disintegration (Grotenhuis et al., 1991), while an excessively high concentration of Ca2+ can severely suppress microbial activity and result in sludge mineralization (Wang et al., 2018; Xia et al., 2016). Therefore, maintaining a stable Ca2+ concentration in anaerobic granular sludge is crucial for efficient reactor operation (Morais et al., 2018; Zhang et al., 2021a, 2021b).
Ammonia, while essential for bacterial growth, can inhibit methanogenesis when present in high concentrations, necessitating careful management to ensure optimal functionality (Yenigün and Demirel, 2013). Within the wastewater treatment, where normal ammonium concentrations can range from 50 to 1000 mg/L, maintaining the right balance is particularly critical (Wu et al., 2022). Ammonia nitrogen in wastewater predominantly exists as free ammonia (NH3·H2O) and ammonium ions (NH4+), depending on pH and temperature (Hansen et al., 1998). Considering that bioreactors typically operate within the pH range of 5.0–7.0 and temperature range of 25–35 °C, ammonium ions (NH4+) are the predominant form of ammonia species. It is recognized that monovalent cations, e.g., K+ and Na+, can leach bivalent cations like Ca2+ (Pevere et al., 2007), posing potential risks to granular sludge stability (Jeison et al., 2008). Some studies have used granular sludge to absorb NH4+ without accounting for potential calcium leaching (Bassin et al., 2011; Yan et al., 2016). While previous research in wastewater treatment has emphasized ammonium removal and its biotoxicity (Liu and Sung, 2002), the role of ammonium as a monovalent cation and its leaching effects have been largely overlooked. In other fields like hydrometallurgical processes, ammonium is commonly used as an efficient leaching agent due to its low environmental toxicity and cost, rapid recovery, and superior selective metal recovery (Chao et al., 2017; Chen et al., 2016; Xiao et al., 2016). Therefore, NH4+ in wastewater has the potential to disintegrate granules, leading to deteriorated treatment processes by replacing Ca2+. This gap in understanding Ca2+ leaching by NH4+ might be a significant reason to explain why high-strength ammonia impedes granules (Vavilin et al., 1995). By leaching Ca2+, NH4+ might further exert significant impacts on extracellular polymeric substances (EPS), which are integral to granular formation and settling. Proteins, in particular, have been identified as the primary component of EPS, and they exhibit properties closely correlated with settling, hydrophobicity, cell aggregation and granular strength (McSwain et al., 2005). The loss of protein from granular sludge would weaken the structural stability of them (Kobayashi et al., 2015; Wan et al., 2013).
This work aimed to explore the Ca2+ leaching from granular sludge under NH4+ shocks at different pH levels. The fractions of calcium involved in the leaching, and the impacts to the granular activity and strength were also explored. This study could provide valuable insights into the potential shock-leaching effects of NH4+ on Ca2+ and offer a new perspective on maintaining the stable operation of granular sludge systems.
Results
NH4+ shock loading leached Ca2+
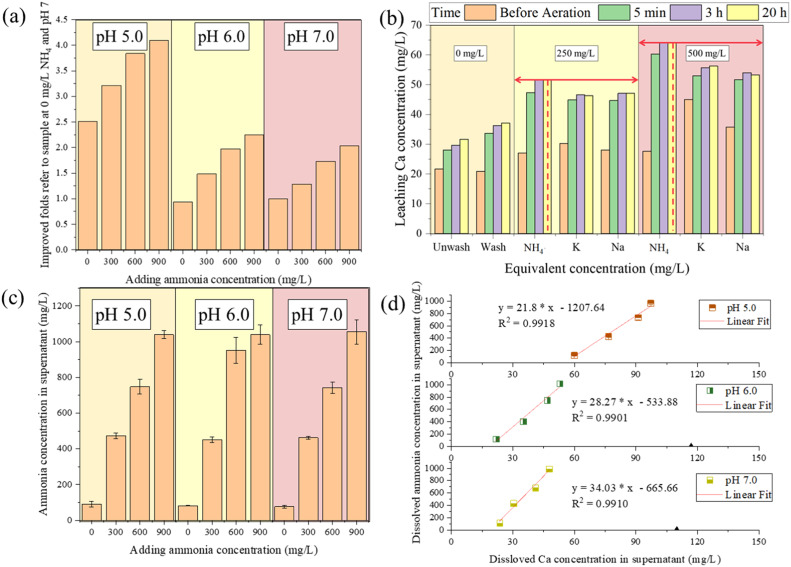
As shown in Figs. 1a and S1, the findings align with previous research on NH4+ leaching Ca2+ from soil and ore (Chao et al., 2017; Xiao et al., 2016), highlighting an enhancement of Ca2+ leaching under high NH4+ shock loading. Simultaneously, Ca2+ dissolution was notably expedited at lower pH levels, which can be attributed to a shift in equilibrium between Ca2+ dissolution and precipitation owing to an increased concentration of protons (Tang et al., 2020). Under neutral conditions (pH 7.0), 900 mg/L NH4+ concentration instigated a Ca2+ release of 57.1 mg/L, approximately 2.35 times higher than the control group. This effect was amplified under acidic conditions (pH 5.0), where the Ca2+ release reached 127.3 mg/L, a staggering 5.24 times higher than the control group. Notably, this significant leaching effect was observed within a short time (Table 1), with a peak in Ca2+ leaching within 5 min, followed by a slow decline over the subsequent 12 h. Most NH4+ existed in supernatant but not in granular sludge (Fig. 1c), and the Ca2+ leaching and NH4+ concentration revealed a linear correlation (Figs. 1d and S2).
Table 1.
Ca2+ leaching in the different groups at 5 min and 1 h.
| 5 min |
1 h |
||||||
|---|---|---|---|---|---|---|---|
| pH | NH4+ (mg/L) | Ca2+@@@@(mg/L) | Improved folds vs. control group of 0 mg/L NH4+ at pH 7.0 | pH | NH4+ (mg/L) | Ca2+@@@@(mg/L) | Improved folds vs. control group of 0 mg/L NH4+ at pH 7.0 |
| 5.0 | 0 | 78.0 | 321% | 5.0 | 0 | 80.1 | 305% |
| 5.0 | 300 | 103.8 | 427% | 5.0 | 300 | 96.9 | 369% |
| 5.0 | 600 | 117.3 | 483% | 5.0 | 600 | 110.0 | 419% |
| 5.0 | 900 | 127.3 | 524% | 5.0 | 900 | 106.2 | 405% |
| 6.0 | 0 | 25.5 | 105% | 6.0 | 0 | 25.5 | 97% |
| 6.0 | 300 | 43.6 | 180% | 6.0 | 300 | 41.4 | 158% |
| 6.0 | 600 | 58.3 | 240% | 6.0 | 600 | 53.3 | 203% |
| 6.0 | 900 | 62.6 | 258% | 6.0 | 900 | 54.0 | 206% |
| 7.0 | 0 | 24.3 | 100% | 7.0 | 0 | 26.2 | 100% |
| 7.0 | 300 | 36.5 | 150% | 7.0 | 300 | 34.0 | 129% |
| 7.0 | 600 | 52.4 | 216% | 7.0 | 600 | 44.3 | 169% |
| 7.0 | 900 | 57.1 | 235% | 7.0 | 900 | 47.3 | 180% |
Fig. 1.
Ca2+ leaching under NH4+ shocking at different pH. (a) Different improved folds of Ca2+ dissolution vs. control group at 0 mg/L NH4+ and pH 7.0. (b) Ca2+ leaching under wash and aeration operation and different equivalent concentrations of monovalent cations. The red dashed line and two-way arrow indicated the dissolved Ca2+ concentration at 20 h in the group with NH4+ addition. (c) Average NH4+ concentration in supernatant of each group. (d) Relationships and linear fit between dissolved NH4+ concentration and dissolved Ca2+ concentration.
Comparative analysis of Ca2+ leaching by other monovalent cations, such as K+ and Na+, showed a weaker but similar leaching effect (Fig. 1b). Hence, it could be concluded that monovalent cations, and particularly NH4+, can effectively leach Ca2+ from granular sludge. The strong leaching effects of NH4+ may be owing to the small hydration radius (Chen et al., 2020; Jalali, 2008).
Distribution and forms of Ca in granular sludge
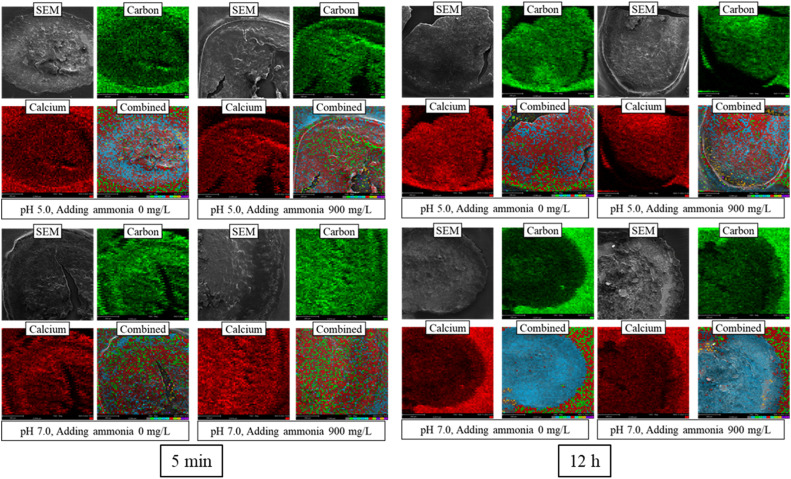
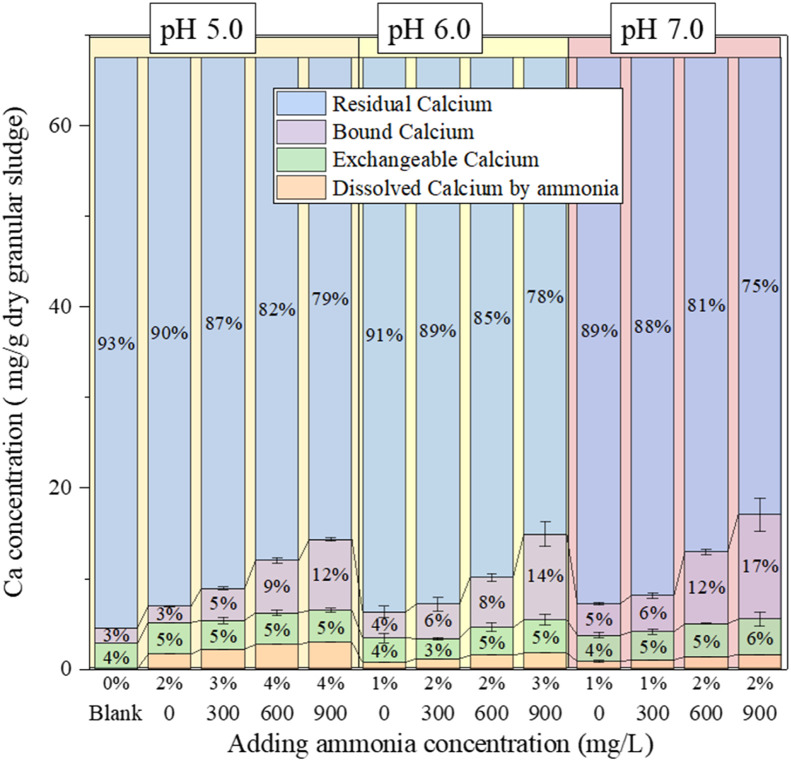
The spatial distribution of divalent Ca within the granules from four distinct groups (0 and 900 mg-NH4+/L at pH 5.0, 0 and 900 mg-NH4+/L at pH 7.0) were sliced and observed by SEM‒EDS (Figs. 2, S3 and S4). The Ca distribution across granules corroborates its pivotal role as a stabilizing agent for granular sludge, both in its exterior and interior layers (van Langerak et al., 1998). The modified Tessier graded extraction was applied to fractionate the Ca in the granules (Tan et al., 2017). This technique classified Ca into three different states: exchangeable, bound (potentially associated with phosphate and carbonate), and residual (primarily organic and other components) forms (Fig. 3). The residual form emerged as the dominant form, accounting for approximately 80 % of total Ca, while the bound and exchangeable forms constituted around 10 % and 5 %, respectively. Interestingly, high NH4+ concentration and low pH reduced residual Ca content in granular sludge, possibly due to enhanced Ca2+ dissolution (Dauer and Perakis, 2013). Meanwhile, an increased concentration of NH4+ corresponded to elevated contents of bound Ca. A lower pH seemed to diminish these bound Ca contents, possibly due to the instability of these forms under acidic conditions. Conversely, the exchangeable Ca content demonstrated relative stability, irrespective of fluctuations in pH and NH4+ shocks.
Fig. 2.
SEM‒EDS photographs of sliced granular sludge at 5 min and 12 h of different groups.
Fig. 3.
Calcium fraction in granular sludge at 12 h under NH4+ shock loading.
Correlation between functional groups and Ca2+ leaching
Raman spectra of the functional groups in the bulk liquid are outlined in Figs. S5, S6, and S7. A correlation matrix between Ca2+ leaching concentration and functional groups was also established to elucidate their relationship (Table S1). In the initial 5 min, the Raman spectra of functional groups exhibited an erratic correlation with Ca2+ release due to the weak peak intensity. Subsequently, the corresponding biomolecule peaks i.e., ν(CΝ) and ν(C = O), ν(CN) and ν(NH) and ν(Ring), increased in bulk liquids (van Langerak et al., 2000). This indicated an elevation in granules disintegration in the supernatant. After 3 h, the Ca2+ concentration demonstrated a pronounced negative correlation with phosphate, carbonate, and acyl concentrations, with an absolute value of the coefficient nearing 0.8. This correlation diminished after 12 h, with the absolute value of the coefficient dropped to approximately 0.45.
Impact of Ca2+ leaching on granular sludge
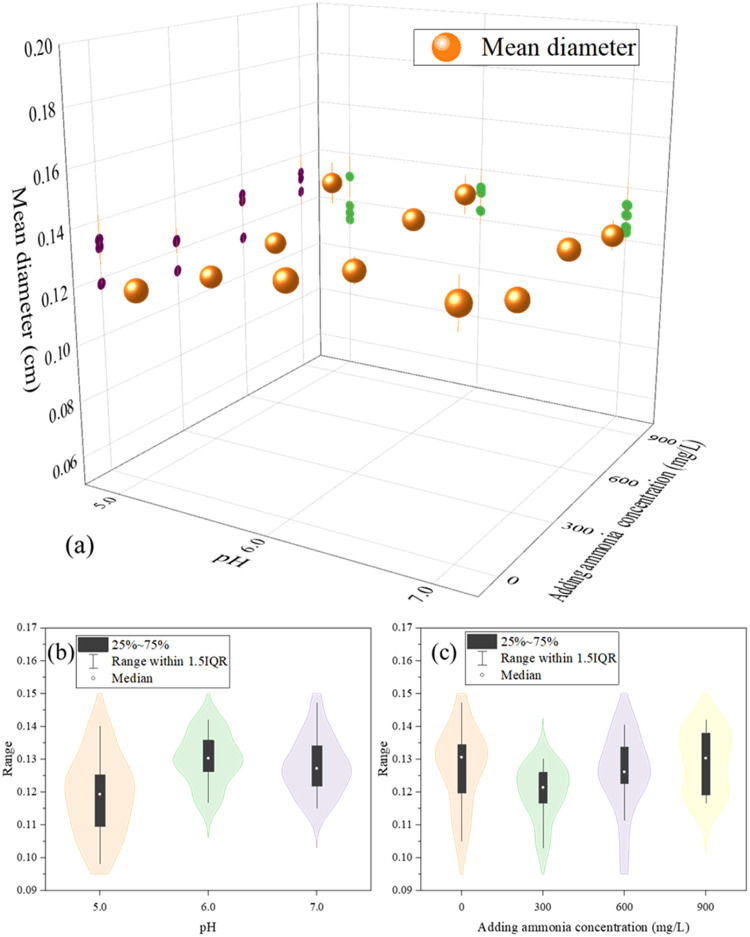
A comparative analysis of granular sludge size before and after NH4+ shock loading revealed negligible differences in the average granule diameter (Figs. 4 and S8). The mean diameter of the granules in each group consistently maintained around 0.12 cm. The minute variation in granule size with Ca2+ leaching could be ascribed to swelling and strength reduction due to the loss of repulsive electrostatic forces resulting from the substitution of Ca2+ by NH4+ (Ismail et al., 2010).
Fig. 4.
Mean diameter (cm) of granular sludge at 12 h after different treatments (a). The violin plot depicted the relationship between mean diameter of granular sludge with pH (b) and NH4+ concentration (c).
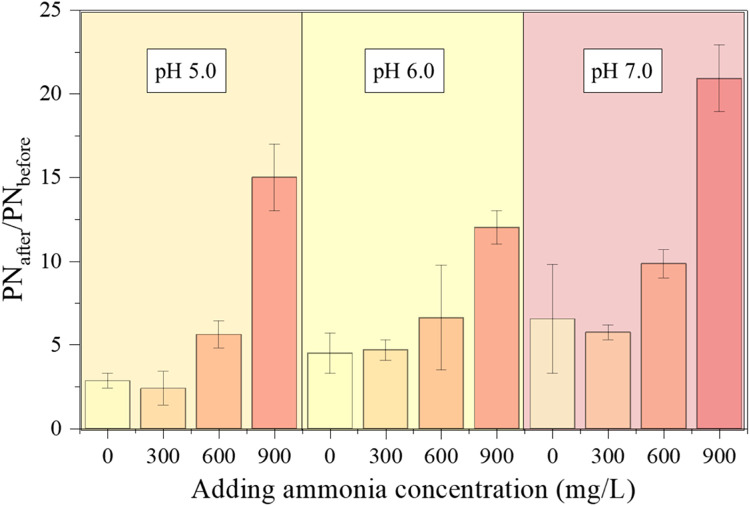
NH4+ shock loading significantly impaired the structural integrity of granular sludge (Fig. 5). A higher NH4+ concentration led to further destabilization of the granular sludge structure. For instance, an ammonium loading of 900 mg/L triggered nearly a 15-fold increase in protein release from the granules, indicating a severe decline in granular strength. On the other hand, the pH displayed a relatively minor effect on granular sludge strength, with slightly higher protein release at pH 7.0 compared to pH 5.0 (Jeison et al., 2008).
Fig. 5.
Ratio of protein release from the 12 h-granules after shaking (PNafter) to that before shaking (PNbefore) in the tests for the granular strength.
The biological activity of granular sludge post NH4+ shock loading was found to be comparable to the initial granules. The tested groups showed similar substrate degradation rates and total methane production (Figs. S9 and S10). Each group accomplished nearly 94 % substrate degradation, and their methane production reached nearly 65 L/kg COD.
Discussion
NH4+ shock loading is recognized to elicit rapid calcium leaching in granular sludge, a phenomenon documented in related domains like hydrometallurgical processes and soil remediation. However, the impacts of NH4+ leaching on granular sludge in wastewater treatments have often been underestimated. In this study, we have quantified the process of calcium leaching from granular sludge. While inhibitory effects of NH4+, such as a decline in methanogenic community abundance and slowed granulation, typically appear after several days or months (Vavilin et al., 1995). Interestingly, this study did not observe an obvious difference among methane production levels, a finding that was inconsistent with some previous research (Yenigün and Demirel, 2013). Under varying ammonia and pH conditions, the groups demonstrated similar methane production rates (about 65 L/kg COD) within a 12 h timeframe. There are two potential reasons for these observed phenomena: First, the threshold for ammonia inhibition can widely differ among various microorganisms and operational conditions. In this case, the chosen concentration might have been within the tolerable limits for the specific microbial community involved (Yenigün and Demirel, 2013). Second, the granular sludge's activity might not have altered significantly within the test timeframe (Zhang et al., 2021a, 2021b), and a longer exposure to high ammonia levels might have yielded different results. In contrast to the consistent methane production activity, calcium leaching occurred rapidly, leading to a marked decrease in granular strength. Previous researches focusing on long-term adverse effects of high NH4+ loading has overlooked this swift leaching effect. Overlooking the instability instigated by calcium leaching could pose challenges to biotechnological applications, hindering the development of strategies to mitigate acute calcium leaching.
NH4+ demonstrated a stronger leaching impact than other monovalent cations, potentially due to its small hydration radius that facilitates its penetration into the granules (Chen et al., 2020; Jalali, 2008). This Ca leaching by NH4+ occurred in all granular depths, resulting in a dramatic decrease in granular strength. After NH4+ shock loading, calcium distribution amongst various forms changed, with residual calcium decreasing while bound calcium increased. Insights from studies on NH4+ leaching in soil and hydrometallurgical processes suggest that an NH4+ shock might alter the form of metal ions, facilitating their dissolution (Dauer and Perakis, 2013; Xiao et al., 2016). The decrease in residual calcium, typically tightly bound form, partially accounts for the granular structure weakening under NH4+ shock loading. Besides, high concentrations of ammonium have been reported to stimulate the secretion of extracellular polymeric substances, which could further affect the form of calcium within the granules (Wang et al., 2022). However, given the dearth of information regarding the contribution of different calcium fractions to granular strength, there is a clear need for additional research. It is crucial to elucidate the relationship between the observed shifts in the proportions of Ca forms and the corresponding changes in granular strength.
In anaerobic wastewater treatment, high NH4+ concentrations and low pH levels are commonly encountered, leading to frequent occurrences of Ca leaching (Srisowmeya et al., 2020). Historically, the instability of granular sludge under high NH4+ loading has been mainly attributed to biological toxicity. However, this study offers a fresh perspective by investigating NH4+-induced instability of granular sludge from a physicochemical standpoint, thereby highlighting the need for further attention to this mechanism. Within the anaerobic digestion process, granular sludge often encountered high-strength NH4+ and low pH wastewater like landfill leachate (Jung et al., 2017). As such, the potential effects of NH4+ leaching must be acknowledged as a source of system instability (Dapena-Mora et al., 2004; Gonzalez-Gil et al., 2015). Research employing granular sludge as an adsorbent for ammonium has often overlooked the potential loss of calcium and the release of substances from granules, factors that could diminish NH4+ adsorption efficiency and introduce new contaminants into the wastewater. Above all, future research should explore targeted remediation strategies such as pH control and Ca replenishment to mitigate the acute Ca loss instigated by NH4+ shock loading.
Conclusions
This study provides critical insights into the phenomenon of Ca2+ leaching in granular sludge, induced by a high concentration of NH4+. Intriguingly, the loss of Ca2+ occurred within a short time and was found to be expedited by low pH. The NH4+ shock loading instigated a shift in the forms of calcium within the granules. Alongside the loss of calcium, a noticeable transition in the proportion of residual to bound calcium was evident, potentially contributing to the diminishment of granular strength. Although the bioactivity of the granules was not significantly affected by the NH4+ shock loading, the structural strength of the granules greatly declined, as evidenced by the remarkable increase in protein release. This work highlighted the overlooked leaching effects by high concentration of NH4+, effects that have frequently been eclipsed by the ammonium's biotoxicity. Therefore, the study recommends more attention should be allocated to the Ca2+ leaching induced by NH4+ shock loading and new strategies should be devised to promptly mitigate such leaching. These findings are valuable for the advancement of granular sludge technology and provide a new perspective on the NH4+-induced instability in bioreactors.
Materials and methods
Batch tests
Granular sludge was collected from a UASB operated for over one year. The synthetic wastewater consisted of CH3COONa, NH4Cl and KH2PO4 as sources of chemical oxygen demand (COD), nitrogen (N), and phosphorus (P), respectively. The COD: N: P ratio was maintained at 2000 (mg/L): 50 (mg/L): 10 (mg/L) to ensure the sufficient nutrients for granular activity. The concentration of CH3COONa was converted into COD unit according to the Equation 1, i.e., 1 mg CH3COONa is equivalent to 0.78 mg COD (Aboutalebi et al., 2012). The characteristics of the granules are given in the supplementary materials (SM). The experimental medium used in the batch tests was prepared by adjusting pH and adding extra ammonium (in the form of NH4Cl) to the synthetic wastewater (Table S2). According to the pH range within which UASB reactors are commonly operated, the batch tests were carried out at the pH range of 5.0 to 7.0 (Leitão et al., 2006). To compare the leaching effect of NH4+ with that of other typical monovalent cations, the Na+ and K+ induced Ca2+ leaching was also tested at pH 7.0. In the tests, equivalent concentration of KCl or NaCl, instead of NH4Cl, was added into the medium. Two levels of concentration of KCl, NaCl and NH4Cl, i.e., 200 and 500 mg/L, were examined for their capability of leaching.
| CH3COONa + 2 O2 = 2 CO2 + NaOH +H2O | (1) |
The batch tests were performed in serum bottles. The granular sludge was washed with deionized water, after which 10 g wet-weight granular sludge and 40 mL experimental medium were introduced into a 125 mL serum bottle. Anaerobic conditions were maintained by purging with high-purity N2 for 5 min and sealing the bottles with butyl rubber stoppers. Thereafter, the bottles were incubated at 30 °C without agitation to eliminate any impact of shaking (Nancharaiah and Venugopalan, 2011).
Biogas was collected at different time intervals (5 min, 1 h, 2 h, 3 h, 6 h, and 12 h) and analyzed for gas volume and methane content. Concurrently, the supernatant in the serum bottles were drawn to determine the concentrations of Ca2+, NH4+, and Raman spectra. The gas volume was measured using a glass syringe with graduations that was lubricated with Vaseline to minimize resistance and maintain a proper seal. Methane was analyzed by a gas chromatography (GC) (GC7920, CEAuLight Beijing, China) (Zuo et al., 2023). Ca2+ concentration was measured by inductively coupled plasma optic emission spectrometry (ICP-OES, Opmita 7300 DV, PerkinElmer Corp., U.S.). Acetate and NH4+ concentrations were analyzed by an Agilent GC (7890A, Agilent CA. USA) (Wen et al., 2020) and Nessler's reagent (Al-Thubaiti and Khan, 2020), respectively.
Evaluating Ca distribution in granular sludge
To assess the effect of pH and NH4+ on Ca distribution in granular sludge, two parallel tests were carried out (see Table S2 for details). Samples of granular sludge from each group were collected at various time points (5 min, 1 h, 2 h, 3 h, 6 h, and 12 h), fixed in 4 % paraformaldehyde at 4 °C for 12 h, and then sliced into 10 µm thick sections using a M1950 cryostat (LeiCa Microsystems GmbH, Germany) at −20 °C. Next, the Ca distribution in granules were determined by scanning electron microscopy combined with energy dispersive spectrometry (SEM‒EDS, Phenom ProX, the Netherlands) operated at 15 kV in mapping detection mode.
Ca fractionation in granular sludge
The forms of bivalent Ca state were fractionated by a modified version of the Tessier graded extraction (Tan et al., 2017). Granular sludge samples were taken from each group after 12 h, dried at 105 °C to constant weight, and then treated with solutions of 1 mol/L MgCl2 (pH 7.0) and 1 mol/L CH3COONa (pH 5.0). These solutions extracted exchangeable Ca and bound Ca (which might be phosphate and carbonate), respectively. The residual bivalent Ca primarily of organic and other fractions. The extraction process details can be found in Table S3.
Identification of functional groups associated with Ca2+ release
Raman spectroscopy was employed to identify functional groups of molecules that could potentially influence Ca2+ leaching. The functional groups were identified from three types of molecules: medium components including phosphate, ammonium, and acetate; molecules that might combine with Ca, such as carbonate; and biological molecules, like acyl and benzene rings (Cui et al., 2022; Drapanauskaite et al., 2021). Details are given in SM.
Measurement of granular size and strength
The granular sludge from each group at 12 h was collected and imaged. Image-Pro-Plus (version 6.0.0.260, Media Cybernetics Co., USA) was used to calculate the mean diameter of granules. Granular strength was evaluated by comparing the dissolved protein in the bulk liquid before and after agitation (Chen et al., 2021; Xiao et al., 2008). A sample of 1 g wet-biomass granular sludge from the 12 h group was placed into a 10 mL centrifuge tube and shaken at 200 rpm for 5 min. Granular sludge strength was evaluated by the increased folds of dissolved protein during shake, which showed a negative relationship with granular sludge strength (Qian et al., 2021). Instability was measured by comparing the amounts of released protein after shaking to that before shaking. The dissolved protein concentration was detected by a BCA acid kit (C503061–1250, Sangon Biotech Co., China).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the National Science Foundation of China (52200075, 51821006, 52192684 and 52170057), Key Project of Anhui Province (2021d07050001) and the China Postdoctoral Science Foundation Funded Project (2022M713041) for supporting this work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.wroa.2023.100200.
Contributor Information
Yu-Sheng Li, Email: liyus@ustc.edu.cn.
Han-Qing Yu, Email: hqyu@ustc.edu.cn.
Appendix. Supplementary materials
Data availability
Data will be made available on request
References
- Aboutalebi H., Sathasivan A., Kuan M.S. Application of air-cathode pipe reactor to simultaneously suppress sulphate reduction and accelerate COD oxidation in synthetic wastewater. Bioresour. Technol. 2012;113:276–279. doi: 10.1016/j.biortech.2012.01.118. [DOI] [PubMed] [Google Scholar]
- Al-Thubaiti K.S., Khan Z. Trimetallic nanocatalysts to enhanced hydrogen production from hydrous hydrazine: the role of metal centers. Int. J. Hydrogen Energy. 2020;45(27):13960–13974. [Google Scholar]
- Bassin J.P., Pronk M., Kraan R., Kleerebezem R., van Loosdrecht M.C.M. Ammonium adsorption in aerobic granular sludge, activated sludge and anammox granules. Water Res. 2011;45(16):5257–5265. doi: 10.1016/j.watres.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Chao S., Changli L., Guilin H. Impact of fertilization with irrigation on carbonate weathering in an agricultural soil in northern China: a column experiment. Geochem. J. 2017;51:143–155. [Google Scholar]
- Chen C., Jiang Y., Liu J., Adams M., Chang Y., Guo M., Xie J., Xie J. The structure of anammox granular sludge under varying long-term organic matter stress: performance, physiochemical and microbial community. J. Clean. Prod. 2021;323 [Google Scholar]
- Chen W., Zhang L., Peng J., Yin S., Ma A., Yang K., Li S., Xie F. Effects of roasting pretreatment on zinc leaching from complicated zinc ores. Green Process. Synth. 2016;5(1):41–47. [Google Scholar]
- Chen Z., Zhang Z., Chi R. Leaching process of weathered crust elution-deposited rare earth ore with formate salts. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.598752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D., Kong L., Wang Y., Zhu Y., Zhang C. In situ identification of environmental microorganisms with Raman spectroscopy. Environ. Sci. Ecotechnol. 2022;11 doi: 10.1016/j.ese.2022.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapena-Mora A., Campos J.L., Mosquera-Corral A., Jetten M.S.M., Méndez R. Stability of the ANAMMOX process in a gas-lift reactor and a SBR. J. Biotechnol. 2004;110(2):159–170. doi: 10.1016/j.jbiotec.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Dauer J.M., Perakis S.S. Contribution of calcium oxalate to soil-exchangeable calcium. Soil Sci. 2013;178(12):671–678. [Google Scholar]
- de Graaff M.S., Temmink H., Zeeman G., van Loosdrecht M.C.M., Buisman C.J.N. Autotrophic nitrogen removal from black water: calcium addition as a requirement for settleability. Water Res. 2011;45(1):63–74. doi: 10.1016/j.watres.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Drapanauskaite D., Buneviciene K., Silva M., Slepetiene A., Baltrusaitis J. Phosphate removal from simulated wastewater using industrial calcium-containing solid waste. J. Environ. Chem. Eng. 2021;9(6) [Google Scholar]
- Gonzalez-Gil G., Sougrat R., Behzad A.R., Lens P.N.L., Saikaly P.E. Microbial community composition and ultrastructure of granules from a full-scale anammox reactor. Microb. Ecol. 2015;70(1):118–131. doi: 10.1007/s00248-014-0546-7. [DOI] [PubMed] [Google Scholar]
- Grotenhuis J.T.C., van Lier J.B., Plugge C.M., Stams A.J.M., Zehnder A.J.B. Effect of ethylene glycol-bis(β-aminoethyl ether)-N,N-tetraacetic acid (EGTA) on stability and activity of methanogenic granular sludge. Appl. Microbiol. Biotechnol. 1991;36(1):109–114. [Google Scholar]
- Hansen K.H., Angelidaki I., Ahring B.K. Anaerobic digestion of swine manure: inhibition by ammonia. Water Res. 1998;32(1):5–12. [Google Scholar]
- Ismail S.B., de La Parra C.J., Temmink H., van Lier J.B. Extracellular polymeric substances (EPS) in upflow anaerobic sludge blanket (UASB) reactors operated under high salinity conditions. Water Res. 2010;44(6):1909–1917. doi: 10.1016/j.watres.2009.11.039. [DOI] [PubMed] [Google Scholar]
- Jalali M. Effect of sodium and magnesium on kinetics of potassium release in some calcareous soils of western Iran. Geoderma. 2008;145(3):207–215. [Google Scholar]
- Jeison D., Del Rio A., Van Lier J.B. Impact of high saline wastewaters on anaerobic granular sludge functionalities. Water Sci. Technol. 2008;57(6):815–819. doi: 10.2166/wst.2008.098. [DOI] [PubMed] [Google Scholar]
- Jung C., Deng Y., Zhao R., Torrens K. Chemical oxidation for mitigation of UV-quenching substances (UVQS) from municipal landfill leachate: fenton process versus ozonation. Water Res. 2017;108:260–270. doi: 10.1016/j.watres.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Xu K.Q., Chiku H. Release of extracellular polymeric substance and disintegration of anaerobic granular sludge under reduced sulfur compounds-rich conditions. Energies. 2015;8(8):7968–7985. [Google Scholar]
- Leitão R.C., van Haandel A.C., Zeeman G., Lettinga G. The effects of operational and environmental variations on anaerobic wastewater treatment systems: a review. Bioresour. Technol. 2006;97(9):1105–1118. doi: 10.1016/j.biortech.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Liu T., Sung S. Ammonia inhibition on thermophilic aceticlastic methanogens. Water Sci. Technol. 2002;45(10):113–120. [PubMed] [Google Scholar]
- Liu Y., Xu H.L., Show K.Y., Tay J.H. Anaerobic granulation technology for wastewater treatment. World J. Microbiol. Biotechnol. 2002;18(2):99–113. [Google Scholar]
- McSwain B.S., Irvine R.L., Hausner M., Wilderer P.A. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Appl. Environ. Microb. 2005;71:1051–1057. doi: 10.1128/AEM.71.2.1051-1057.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais I., Silva C., Zanuncio J., Zanuncio A. Structural stabilization of granular sludge by addition of calcium ions into aerobic bioreactors. Bioresources. 2018;13:176–191. [Google Scholar]
- Nancharaiah Y.V., Venugopalan V.P. Denitrification of synthetic concentrated nitrate wastes by aerobic granular sludge under anoxic conditions. Chemosphere. 2011;85(4):683–688. doi: 10.1016/j.chemosphere.2011.06.077. [DOI] [PubMed] [Google Scholar]
- Pevere A., Guibaud G., van Hullebusch E.D., Boughzala W., Lens P.N.L. Effect of Na+ and Ca2+ on the aggregation properties of sieved anaerobic granular sludge. Colloids Surf. Physicochem. Eng. Aspects. 2007;306(1):142–149. [Google Scholar]
- Qian J., Bai L., Zhang M., Chen L., Yan X., Sun R., Zhang M., Chen G.H., Wu D. Achieving rapid thiosulfate-driven denitrification (TDD) in a granular sludge system. Water Res. 2021;190 doi: 10.1016/j.watres.2020.116716. [DOI] [PubMed] [Google Scholar]
- Ran X., Zhou M., Wang T., Wang W., Kumari S., Wang Y. Multidisciplinary characterization of nitrogen-removal granular sludge: a review of advances and technologies. Water Res. 2022;214 doi: 10.1016/j.watres.2022.118214. [DOI] [PubMed] [Google Scholar]
- Ren T.T., Liu L., Sheng G.P., Liu X.W., Yu H.Q., Zhang M.C., Zhu J.R. Calcium spatial distribution in aerobic granules and its effects on granule structure, strength and bioactivity. Water Res. 2008;42(13):3343–3352. doi: 10.1016/j.watres.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Srisowmeya G., Chakravarthy M., Nandhini Devi G. Critical considerations in two-stage anaerobic digestion of food waste—a review. Renew. Sustain. Energy Rev. 2020;119 [Google Scholar]
- Tan W., Liang T., Du Y.P., Zhai H. The distribution and species of Ca2+ and subcellular localization of Ca2+ and Ca2+-ATPase in grape leaves of plants treated with fluoroglycofen. Pestic. Biochem. Phys. 2017;143:207–213. doi: 10.1016/j.pestbp.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Tang C.S., Yin L.Y., Jiang N.J., Zhu C., Zeng H., Li H., Shi B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: a review. Environ. Earth Sci. 2020;79(5):94. [Google Scholar]
- van Langerak E.P.A., Gonzalez-Gil G., van Aelst A., van Lier J.B., Hamelers H.V.M., Lettinga G. Effects of high calcium concentrations on the development of methanogenic sludge in upflow anaerobic sludge bed (UASB) reactors. Water Res. 1998;32(4):1255–1263. [Google Scholar]
- van Langerak E.P.A., Ramaekers H., Wiechers J., Veeken A.H.M., Hamelers H.V.M., Lettinga G. Impact of location of CaCO3 precipitation on the development of intact anaerobic sludge. Water Res. 2000;34(2):437–446. [Google Scholar]
- van Lier J.B., van der Zee F.P., Frijters C.T.M.J., Ersahin M.E. Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev. Environ. Sci. Biotechnol. 2015;14(4):681–702. [Google Scholar]
- van Loosdrecht M.C.M., Brdjanovic D. Anticipating the next century of wastewater treatment. Science. 2014;344(6191):1452–1453. doi: 10.1126/science.1255183. [DOI] [PubMed] [Google Scholar]
- Vavilin V.A., Vasiliev V.B., Rytov S.V., Ponomarev A.V. Modeling ammonia and hydrogen sulfide inhibition in anaerobic digestion. Water Res. 1995;29(3):827–835. [Google Scholar]
- Wan C., Zhang P., Lee D.J., Yang X., Liu X., Sun S., Pan X. Disintegration of aerobic granules: role of second messenger cyclic di-GMP. Bioresour. Technol. 2013;146:330–335. doi: 10.1016/j.biortech.2013.07.073. [DOI] [PubMed] [Google Scholar]
- Wang N., Feng Y., Li Y., Zhang L., Liu J., Li N., He W. Effects of ammonia on electrochemical active biofilm in microbial electrolysis cells for synthetic swine wastewater treatment. Water Res. 2022;219 doi: 10.1016/j.watres.2022.118570. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhu C., Min Y.K., Yang Z., Feng Q., Wang S. Effect of the Ca2+ concentration on anaerobic digestion and microbial communities of granular sludge. Bioresources. 2018;13(3):6062–6076. [Google Scholar]
- Wen H.Q., Ren H.Y., Xie G.J., Cao G.L., Xing D.F., Ren N.Q., Liu B.F. Synthesized effects of proteomic and extracellular polymeric substance (EPS) revealing the enhanced hydrogen production by formed biofilm of photo-fermentative bacteria. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105683. [DOI] [PubMed] [Google Scholar]
- Wu P., Chen J., Garlapati V.K., Zhang X., Wani Victor Jenario F., Li X., Liu W., Chen C., Aminabhavi T.M., Zhang X. Novel insights into Anammox-based processes: a critical review. Chem. Eng. J. 2022;444 [Google Scholar]
- Xia Y., He P.J., Pu H.X., Lü F., Shao L.M., Zhang H. Inhibition effects of high calcium concentration on anaerobic biological treatment of MSW leachate. Environ. Sci. Pollut. Res. 2016;23(8):7942–7948. doi: 10.1007/s11356-016-6052-3. [DOI] [PubMed] [Google Scholar]
- Xiao F., Yang S.F., Li X.Y. Physical and hydrodynamic properties of aerobic granules produced in sequencing batch reactors. Sep. Purif. Technol. 2008;63(3):634–641. [Google Scholar]
- Xiao Y., Feng Z., Huang X., Huang L., Chen Y., Liu X., Wang L., Long Z. Recovery of rare earth from the ion-adsorption type rare earths ore: II. Compound leaching. Hydrometallurgy. 2016;163:83–90. [Google Scholar]
- Yan L., Zhang X., Hao G., Guo Y., Ren Y., Yu L., Bao X., Zhang Y. Insight into the roles of tightly and loosely bound extracellular polymeric substances on a granular sludge in ammonium nitrogen removal. Bioresour. Technol. 2016;222:408–412. doi: 10.1016/j.biortech.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Yenigün O., Demirel B. Ammonia inhibition in anaerobic digestion: a review. Process Biochem. 2013;48(5):901–911. [Google Scholar]
- Zhang H., Wang L., Wang H., Yang F., Chen L., Hao F., Lv X., Du H., Xu Y. Effects of initial temperature on microbial community succession rate and volatile flavors during Baijiu fermentation process. Food Res. Int. 2021;141 doi: 10.1016/j.foodres.2020.109887. [DOI] [PubMed] [Google Scholar]
- Zhang J., Pan J.Q., Zhao S., Gan P., Qin C.R., Wang Z.W., Chen Y.L., Liu X., Lu L.H., Wang S.F. Calcium migration inside anaerobic granular sludge: evidence from calcium carbonate precipitation pattern. Colloids Surf. Physicochem. Eng. Aspects. 2021;625 [Google Scholar]
- Zhang M., Hong H., Lin H., Shen L., Yu H., Ma G., Chen J., Liao B.Q. Mechanistic insights into alginate fouling caused by calcium ions based on terahertz time-domain spectra analyses and DFT calculations. Water Res. 2018;129:337–346. doi: 10.1016/j.watres.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Xing Y., Duan H., Ren D., Zheng M., Liu Y., Huang X. Reducing sulfide and methane production in gravity sewer sediments through urine separation, collection and intermittent dosing. Water Res. 2023;234 doi: 10.1016/j.watres.2023.119820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request