Abstract
Introduction
The kidney failure risk equation (KFRE) estimates a person’s risk of kidney failure and has great potential utility in clinical care.
Methods
We used mixed methods to explore implementation of the KFRE in nephrology clinics.
Results
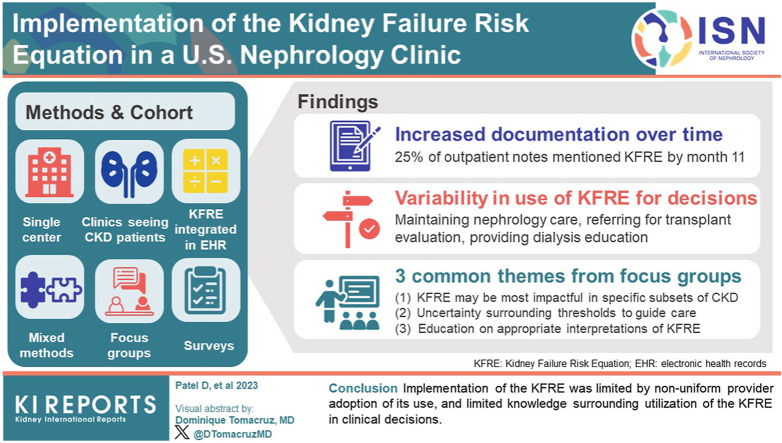
KFRE scores were integrated into the electronic health record at Johns Hopkins Medicine and were displayed to nephrology providers. Documentation of KFRE scores increased over time, reaching 25% of eligible outpatient nephrology clinic notes at month 11. Three providers documented KFRE scores in >75% of notes, whereas 25 documented scores in <10% of notes. Surveys and focus groups of nephrology providers were conducted to probe provider views on the KFRE. Survey respondents (n = 25) reported variability in use of KFRE for decisions such as maintaining nephrology care, referring for transplant evaluation, or providing dialysis modality education. Provider perspectives on the use of KFRE, assessed in 2 focus groups of 4 providers each, included 3 common themes as follows: (i) KFRE scores may be most impactful in the care of specific subsets of people with chronic kidney disease (CKD); (ii) there is uncertainty about KFRE risk-based thresholds to guide clinical care; and (iii) education of patients, nephrology providers, and non-nephrology providers on appropriate interpretations of KFRE scores may help maximize their utility.
Conclusion
Implementation of the KFRE was limited by non-uniform provider adoption of its use, and limited knowledge about utilization of the KFRE in clinical decisions.
Keywords: kidney failure risk equation (KFRE), provider perspectives
Graphical abstract
CKD affects more than 10% of the global population.1 Although nephrologists aim to identify CKD early, prevent its progression, and (in cases of progression) facilitate optimal transitions to kidney replacement therapy (KRT), resource and time constraints limit success. In the United States, 30% of people with CKD G4 and 50% of people with CKD G5 were not seen by a nephrologist in 2020.2 Over 83% of individuals starting dialysis in 2020 did so suboptimally with a central venous catheter.2 Many of the opportunities to improve nephrology care stem from a need to target patients at highest risk of progression to kidney failure.
The KFRE is an internationally validated tool which calculates a person’s risk of progression to kidney failure.3 The 4-variable KFRE incorporates an individual’s age, sex, estimated glomerular filtration rate (eGFR), and albuminuria to provide 2-year and 5-year percent risks of kidney failure, for patients with an eGFR <60 ml/min per 1.73 m2. Prognostication is calibrated by region,4 and can be further refined by incorporating additional data (serum phosphorus, albumin, bicarbonate, and calcium) into the 8-variable KFRE.3 KFRE scores have been proposed as a method to institute risk-based thresholds for referrals to nephrology care5,6 or multidisciplinary nephrology clinics,7,8 and to guide KRT planning.9 Some guidelines suggest referral to nephrology care when 5-year KFRE scores are >5%, and referral for dialysis access and/or transplantation when 2-year KFRE scores are >20% to 40%.10
Although the KFRE may be impactful, a roadmap to implement its use in clinical care is not available. We recently integrated KFRE scores into electronic health records of patients with CKD being seen in nephrology clinics. In this study, we quantified KFRE score documentation in outpatient nephrology clinic notes, surveyed nephrology providers to assess use of the KFRE, and conducted 2 focus groups of nephrology providers to identify common themes influencing provider perspectives on the KFRE. Our data looks to inform future efforts to implement the KFRE in nephrology and non-nephrology care.
Methods
Setting
This study was conducted at Johns Hopkins University and the affiliated Johns Hopkins Medicine outpatient clinics. Nephrologists working in this practice care for a variety of patients with acute kidney disease and CKD, who are referred from primary care physicians, other specialty providers, and/or self-referred. A multidisciplinary nephrology care clinic is not available, and nephrologists continue to care for patients as they transition to late stages of CKD and begin preparations for possible KRT.
KFRE Score Calculations
Using data available in Epic electronic medical records (Epic Systems Corporation), estimated risks are calculated for the 4-variable 2-year and 5-year KFRE scores.3,4 Scores were reported for patients seen in nephrology clinic with an eGFR <60 ml/min per 1.73 m2 and albuminuria data available. KFRE score calculations use the most recent outpatient serum creatinine within 3 years using the 2021 CKD Epidemiology Collaboration creatinine-based equation.11 Albuminuria data utilized inpatient and outpatient urine albumin-to-creatinine measurements (spot or 24-hour; UACR, mg/g), urine protein-to-creatinine ratio (spot or 24 hour; UPCR, mg/g), or urine protein dipstick measurements within 3 years of the eGFR measurement. The algorithm selected the albuminuria measurement closest to the time of eGFR measurement with preference given to UACR or UPCR. The most recent urine protein dipstick value was used if there were no UACR or UPCR measurements within 3 years of the eGFR measurement. UPCR and dipstick values were converted to UACR prior to inclusion in the KFRE score calculation.12 Some laboratories obtained by external laboratories were not used in score calculations.
KFRE Score Reporting Tools
KFRE scores were integrated into Epic at Johns Hopkins Medicine in January 2022, and made accessible to nephrology providers in 3 ways (Supplementary Figure S1). The first is a display of KFRE scores in the “Storyboard” feature of Epic, which displays 2-year and 5-year KFRE scores upon hovering over, or clicking, the KFRE hyperlink. The second is a “.KFREscore” dotphrase, which allows providers to pull 2-year and 5-year KFRE scores into clinic notes. The third is “Patient Insight”, which is a visual aid displaying KFRE scores, 2-year risk of cardiovascular disease events for patients with eGFR <30 ml/min per 1.73 m2,13 prescription of therapies known to be renoprotective (renin-angiotensin system inhibitors, sodium/glucose cotransporter-2 inhibitors, mineralocorticoid receptor antagonists, and glucagon-like peptide 1 receptor agonists), and trends in eGFR and albuminuria. KFRE on Storyboard and the “.KFREscore” dotphrase were available after January 12, 2022, and Patient Insight was available after January 22, 2022. Score reporting tools display the date of laboratory data being used in the calculated KFRE scores. Unless entered into clinic notes, KFRE scores are not shown directly to patients.
Dissemination Into Nephrology Clinic
Availability of KFRE score reporting tools was first advertised to nephrology providers in January 2022 through conferences and division-wide email communications. Two additional information sessions (1 during an in-person educational conference for fellows, and 1 during a web-based meeting for faculty) were held in April 2022. Six new trainees joined our practice in July 2022 and were provided with educational information via an in-person conference and subsequent email communication about the KFRE in August 2022.
Quantification of KFRE Score Documentation
We used text string searches of outpatient nephrology clinic notes to quantify documentation of KFRE scores from February to December 2022, as assessed by inclusion of the text string “2-year KFRE” in note text, for patients with KFRE scores available in Epic. Data was collected longitudinally and summarized using descriptive statistics, stratified by CKD stage and by nephrology provider (using masked provider identities). Results were reported as a percentage of eligible outpatient notes.
Nephrology Provider Perspectives
An online survey (Supplementary Item S1) was distributed to Johns Hopkins nephrology providers (all current providers, plus nephrology fellows who had graduated in July 2022) from September–October 2022, probing individual perspectives on clinical use of the KFRE. Respondents were prompted to provide open-ended feedback and were asked about potential interest in focus group participation. Survey data was collected using Qualtrics XM software, using descriptive statistics to summarize data.
To further explore provider perspectives on KFRE use, we conducted 2 focus groups in October 2022 (1 with nephrology faculty and 1 with nephrology fellows) guided by semi-structured interview questions (Supplementary Item S2). Topics of discussion included use of KFRE scores as decision support aids, thresholds of KFRE to determine if patients could be “discharged” from nephrology care, and potential suggestions to improve reporting of KFRE scores. Questions were not pilot tested, and field notes were not taken. Interview guides and techniques, with a detailed study protocol, were pre-emptively discussed among the investigators (DMP, CRP, and DCC) to ensure credibility and dependability of data collection.
Focus groups were led by DMP who is a member of the nephrology faculty with experience in qualitative research with no conflicts of interest regarding KFRE. Focus groups were held through online video conferencing and were 1 hour each in duration. Participants were assigned a participant number to state prior to any response, such that responses were linked to a participant number only and not to names. Though video cameras were on for the duration of focus groups, only audio recordings were sent to Ubiqus Translation Services, which transcribed verbatim and provided redacted transcripts. Transcripts were not offered to participants for review.
Qualitative Data Analysis
Three authors (DMP, BMC, and DCC) independently reviewed transcripts to ensure data confirmability, using thematic content analysis14 to code and analyze focus group transcripts. MAXQDA software was used to manage qualitative data. The coding tree was discussed by the 3 authors until consensus was reached. After transcript data was organized using the coding tree, the 3 authors reviewed data in aggregate and inductively derived themes influencing provider perspectives on the KFRE, until consensus was reached.
Participation in surveys and focus groups was not mandated, and consent was obtained in all cases. Remuneration was not offered.
Ethical Approval
The study was approved by the Johns Hopkins University Institutional Review Board (IRB00339347, PI: D. Patel), using data within the Johns Hopkins Kidney Precision Medicine Center of Excellence (IRB00234653, PI: C. Parikh).
Results
Quantification of KFRE Documentation
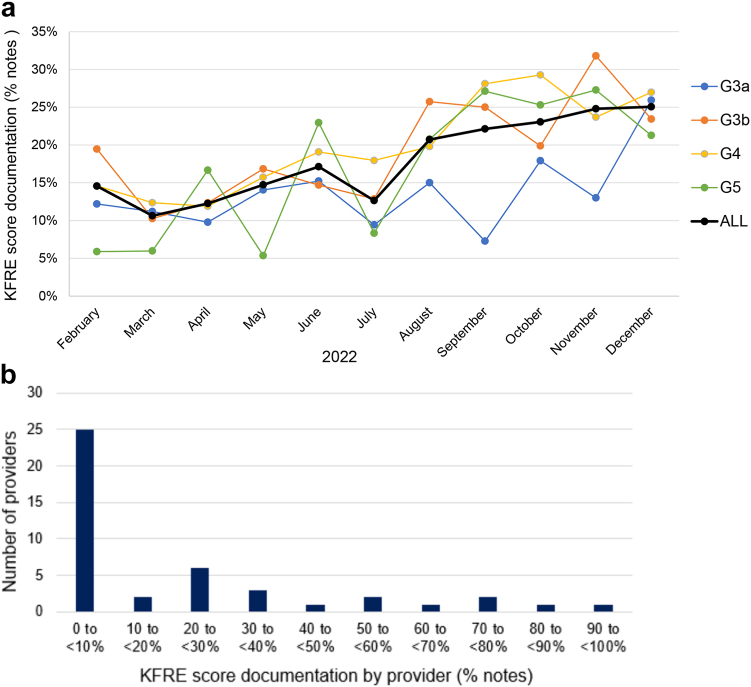
We evaluated documentation of KFRE scores, expressed as a percentage of outpatient nephrology clinic notes containing “2-year KFRE,” for people with CKD stages G3a–G5 (Figure 1a). Documentation of KFRE scores increased over time, reaching 25% of clinic notes by December 2022. In total, KFRE scores were documented in 18% of outpatient nephrology clinic notes from February to December 2022 (CKD G3a: 14%; CKD G3b: 19%; CKD G4: 20%; and CKD G5: 17%).
Figure 1.
KFRE score utilization. (a) Rates of KFRE score documentation in outpatient nephrology clinic notes per month, stratified by CKD stage. (b) Rates of KFRE score documentation in outpatient nephrology clinic notes during the study period, grouped by provider into categories of documentation frequency.
Adoption of KFRE documentation in clinic notes was variable across providers (Figure 1b). Among 44 providers with outpatient nephrology clinic notes during the study period, 3 documented KFRE scores in >75% of clinic notes, whereas 25 documented KFRE scores in <10% of clinic notes.
Provider Surveys
We surveyed nephrology providers in our practice to ask their views on KFRE use in nephrology clinic. From a group of 27 nephrology faculty and 20 nephrology fellows who were sent the survey, 25 providers (53%; 13 faculty and 12 fellows) provided complete responses (Supplementary Table S1). Responses were anonymous and additional demographic information was not collected. The length of nephrology clinic experience varied, with 5 respondents (19%) having <3 months’ experience and 8 (31%) having >5 years’ experience.
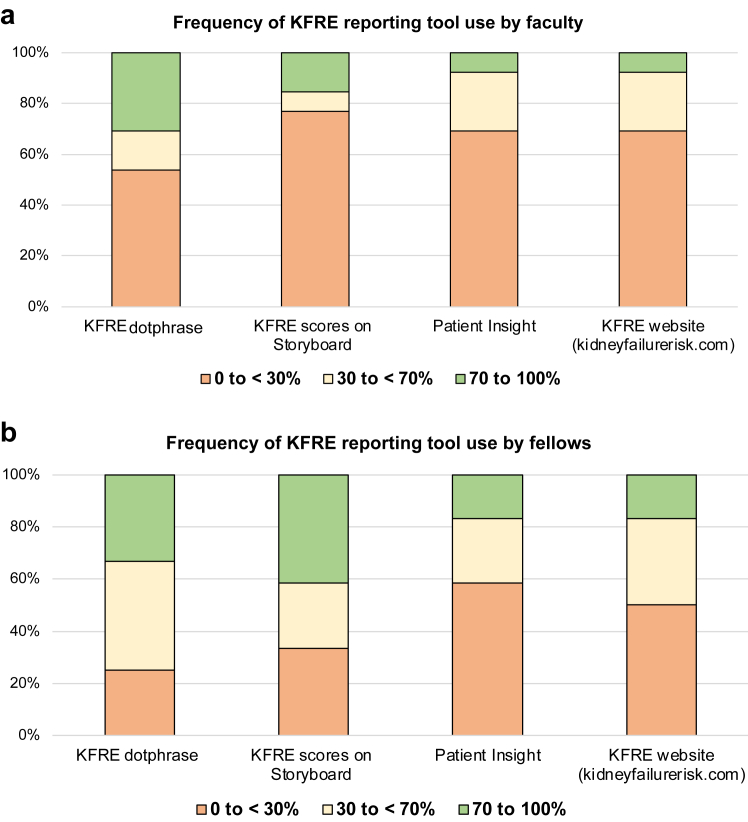
Providers estimated their frequency of using KFRE score reporting tools (Figure 2). Among survey respondents, 31% of faculty and 33% of fellows reported use of the KFRE dotphrase for 70% to 100% of clinic visits, and 15% of faculty and 42% of fellows reported use of Storyboard for 70% to 100% of clinic visits. One faculty member and 2 fellows reported ongoing use of the external KFRE website (kidneyfailurerisk.com) in 70% to 100% of clinic visits. Fellows reported higher awareness of KFRE score reporting tools, as compared to faculty (Supplementary Figure S2).
Figure 2.
Self-reported frequency of using KFRE score reporting tools. Providers were asked to quantify the proportion of patients seen in clinic for which each KFRE reporting tool was used (0% to <30%, 30% to <70%, or 70%–100%). Responses are shown for each KFRE score reporting tool, by provider type (n = 13 faculty and 12 fellows for all questions; no missing cases).
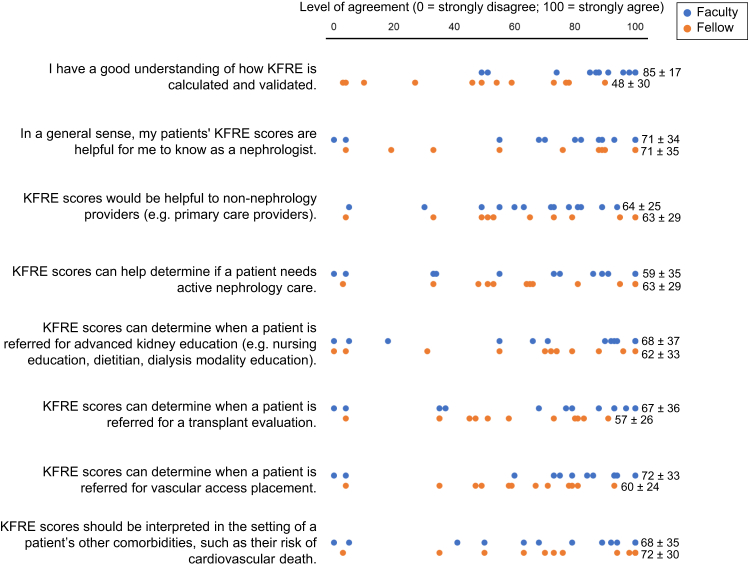
Providers were then asked to rate their level of agreement with several potential uses of KFRE scores (Figure 3). On a scale of 0 to 100 (100 = strongly agree), providers on average rated KFRE scores as being “useful to know as a nephrologist” as 71 (SD 33), and KFRE scores as being “useful to non-nephrology providers” as 63 (SD 26). There was a range of agreement levels regarding use of KFRE scores as a tool to guide clinical care, including maintenance of active nephrology care (61, SD 32), referral for advanced CKD education (65, SD 35), transplant evaluation (62, SD 31), or access placement (66, SD 29). Faculty reported a higher level of understanding of KFRE calculation and validation (85, SD 17) compared to fellows (48, SD 30).
Figure 3.
Provider-rated level of agreement with specific aspects of KFRE score utilization Providers (n = 13 faculty and 12 fellows for all questions; no missing cases) were asked to use a sliding scale (0–100) to rate their level of agreement with each statement (reported as mean, SD).
Select open-ended comments written by survey respondents are provided in Supplementary Table S2.
Qualitative Assessment of Provider Perspectives
Characteristics of participants for focus groups (1 held for faculty and 1 for fellows) are provided in Supplementary Table S3. Transcripts from focus groups were coded, using thematic content analysis to inductively derive a coding tree (Supplementary Figure S3) and to identify experiences and themes influencing provider perspectives on KFRE use in a nephrology clinic. Illustrative quotations from participants are presented as Table 1.
Table 1.
Illustrative quotations from focus group transcripts. Quotations are identified by the role of the participant (faculty or fellow) and participant number.
| Provider perspectives on the KFRE itself | |
| Applicability of the KFRE to subsets of people with CKD | “I also find myself using the age and adding 5 years when I'm talking to patients above a certain age because it helps to put that in perspective. So, if I'm talking to a patient who's in their 50s, for some reason, I don't tend to do that thing where I say, Okay, so by the time you're 55 we're looking at a, you know, 30% risk of [kidney failure]. But in a patient who's 75, I do find myself doing that way more and saying, so basically by the time you turn 80, because I think those ages seem to mean a little bit, a little bit more to the patients and then also to me as a provider somehow.” (Fellow #4) “I don't think the 5-year KFRE for the 30-year old when I'm just glancing up this would… change me saying that this is somebody who would be getting some closer follow up in the clinic.” (Faculty #8) “I have a mix of patients in my clinic. So, if I was seeing…somebody who was coming in for high-risk pregnancy, that wouldn't be part of the discussion unless they had preexisting kidney disease. If I was seeing…someone who was already coming in as a second opinion who's stage 5, already prepped for dialysis, that wasn't a relevant part of the discussion.” (Faculty #8) |
| Knowledge about meaning of KFRE scores | “…it's a snapshot in time” (Faculty #1) “I think if, if we're using it in patients who aren't at steady state…then misinterpretation can be a problem.” (Faculty #1) “…as this gets rolled out and as it's more universally accepted and used in other clinics, I think that's one thing to keep in mind…Using it…not in the setting of an acute illness where patients or providers may get the false sense of…concern…But, you know, I think it, it's all about more education.” (Faculty #1) “I've had patients who are clearly seeking grade 5…The risk that you clinically expect of being on dialysis sort of doesn't correlate as much. So, it's [important] to know those situations. And sometimes, you know, if you don't, then you may see the risk is 30% …Well, the risk may actually be higher just because there's certain factors, right?” (Fellow #2) “I use…the change in the KFRE score, you know, from say a previous visit or…the slope or…the GFR change in addition to the KFRE score more for dialysis education.” (Fellow #2) |
| Views on KFRE reporting tools | |
| Positive impact of KFRE integration with EHR | “I was using KFRE prior to the scores automatically being calculated….it was cumbersome, it required me to use the Kidney Failure Risk Equation website, putting in the numbers there…and calculating what the kidney failure risk score would be at 2 years and 5 years.” (Faculty #3) “I was initially skeptical that the KFRE would be useful for us as…nephrologist…and I have come around to it, I think that it is a nice way to summarize…all the information that we have into a nice number that predicts risk….the availability for nephrologists and the ease of use in nephrologists…could really be…enhanced by a more widespread use of the KFRE.” (Faculty #3) “…it does give you a good idea and it's easy for patients to understand and kind of…see where their numbers are, and what it means for them, and what, how rapidly it has progressed or not…that's how I use Patient Insight. And then…the Dot Phrase, I do use it to just for clinical recommendation.” (Fellow #3) |
| Opportunities to improve KFRE score reporting within EHR | “anywhere between one-fourth to one-half of my clinic has labs done elsewhere and they have to be scanned in…so the most recent labs here, might be from 6 months ago, whereas I have more recent months from last month. So that's, that's probably the biggest challenge.” (Faculty #4) “I think for people who are using the Dot Phrase, it's 3 lines of…numbers, so that it just adds disorganization to…the note.” (Fellow #2) “…just because the way it's formatted…it just shows up as numbers and the date that it was appearing. So, for people reading my note, it's not clear what that is. So I tend to phrase it as the risk of [kidney failure]…in 2 years is this and this based on labs obtained on this day, is the way I use it.” (Fellow #2) |
| Utilization of KFRE as a decision support aid | |
| KFRE as a guide to intensity of nephrology care | “There's a lot of the other physicians involved with the patient's care often don't want us to step back. And I, I think I find it challenging to sort of navigate that.” (Faculty #4) “I think, you know, it could be used as, as a tool…you know, quote, unquote, discharging from nephrology care, but you know, in the right context and, and for the right patient, right? So, for obviously the patient with a GN who's in remission? Right, whose KFRE may be…less than 3%...you'd still wanna see them yearly or whatever it may be...but if it's somebody who, you know, previously had really poorly controlled diabetes…who…I know their primary care…they're getting good care endocrinology and they're on an SGLT2 and the A1C is now 6 and a half and…their KFRE…is…appropriately less than, you know, 3% or 5%, then yeah…it could be used as another tool.” (Faculty #1) “I think the time that I have done it, was when the patient's elderly and 5-year…risk is low…it's just at that point, are they going, you know, are the kidney's gonna outlive them? And if the answer is yes, they probably don't need to be seeing another provider.” (Faculty #8) “I think there could be some benefit, but I also feel that, or at least CKD prevention management is the most important part of, you know, preventing progression to [kidney failure], that it has to be taken in consideration with the comorbidities which don't go into the equation and the potential for having other events contributing to…you know…the social situation or other factors that could cause a lot more rapid progression than not, which do not necessarily go into the equation.” (Fellow #2) |
| KFRE as a guide to kidney replacement therapy planning | “…someone whose KFRE is at 40% is probably a little more of a time-sensitive one to sort of say…we really do need you to meet with the dialysis educator, we do need to start looking at some of the material about renal replacement therapy. Whereas if the KFRE was 1.5%, you could kind of give them a little more time…you know, before your next visit. Take some time to look at this material, decide what you were, you know…what you're interested in, and then we can kind of have a continue, you know, an ongoing discussion about it.” (Faculty #8) “… for access placement…because there's so many other factors that go into it and at that time I feel like personally when someone's already at that CKD 3, 4/5…edge, the KFRE score is going to be high anyway…so that for me…the GFR sort of seems to be the primary thing that I go for. And the KFRE supports education to guide that access placement, but not for referral.” (Fellow #2) |
| Utilization of KFRE as a method of communication | |
| Impact of relaying KFRE scores to patient | “…when you start the discussion about dialysis…their first question is, okay…when am I gonna go on dialysis? Or what is my risk at this point? And then that helps sort of, for patients that actually prefer numbers, it helps give them also these numbers to say, okay, well this is how it is.” (Faculty #4) “…in new patient visits when I would have people come in for a brand new evaluation who didn't know about kidney disease before and were really, really concerned to hear that their GFR was…45% or something like that. And for those patients, I think it's really, really illustrative when you can show them that even though you're operating with less than half of your kidney function, that actually doesn't translate to a really acute risk of progression.” (Fellow #4) “I learn my lesson very early on in fellowship, you're in the COVID era…we had a lot of patients that…they would leave, they would have a stable GFR, get admitted for COVID, and then come back with…a creatinine of 4, and just then thereafter end up on dialysis. And they're like, well, you know, we were told that our risk for dialysis in 5 years was next to nothing. So, after that, I think I got a little more diligent about, um, sort of throwing in that disclaimer at the end as well.” (Faculty #4) “I think the challenge will be if people put the score on there without a real, on any paperwork for a patient without really a, a discussion and a disclaimer. You know? This is based on point in time, assuming everything else relatively stays the same and there's no big major other health event that might…alter…the scoring. And so, you know, you don't want someone walking away from this going, oh, well, they told me…my chance of going on dialysis in 5 years is less than 10%. But that assumes nothing else is going to, you know, no major of their health event. And to make sure that they understand how we're using those scores, that they're not necessarily guarantees.” (Faculty #8) |
| Utility of relaying KFRE scores to other providers | “I think it helps them sort of plan for different things when you're speaking with oncology. You know? What is the potential risk for someone progressing to end-stage kidney disease…when you're talking with some of the surgery subspecialties…is this somebody who really we need to look at dialysis options before they go through this major procedure?” (Faculty #8) “…if the KFRE is used by other providers beyond nephrologists, it does have the potential…that if someone's not in steady state, they might have one value that suggests they're at a high-risk state of progression…and that might generate earlier nephrology appointments.” (Faculty #3) “…it may be a little bit more risky if there's a lack of communication.” (Fellow #2) |
CKD, chronic kidney disease; EHR, electronic health record; KFRE, kidney failure risk equation.
KFRE Scores May Be Most Impactful in the Care of Specific Subsets of People With CKD
Generally, KFRE score reporting tools were viewed positively, given the rapid availability of KFRE scores, with updated calculations based on the most recent laboratory data. Patient Insight provided a quick method for providers to review eGFR and albuminuria trends over time. The KFRE was noted to be particularly helpful for specific low-risk subsets of patients with CKD.
“…when you have patients who show up who really aren't likely to [progress to] kidney failure…this is really helpful and it's also helpful for other people who [have] really severe disease to just…emphasize…the pace at which we need to move in order to prepare for dialysis or transplantation. Having those numbers can be helpful.” (Fellow #1)
Although low 5-year KFRE scores were felt to be impactful in the management of older adults with CKD, participants felt that the utility of 5-year KFRE scores in younger people with CKD was less certain, given the inability to account for unexpected medical or other life events that could take place over the next several years. High KFRE scores were reported by some providers to be less useful in management of people with known advanced progressive kidney disease. Participants noted the inability of the KFRE to distinguish steady state from acute illness, which they believed could result in falsely high KFRE scores.
There Is Uncertainty About KFRE Risk-Based Thresholds to Guide Clinical Care
Some providers commented that KFRE scores could help determine the need for new and/or continued outpatient nephrology care.
“…there are more patients with kidney disease or that want to see us…than we have the manpower to be able to…it's difficult to…discharge patients from our practice…I think the KFRE has the potential to be one of those tools.” (Faculty #3)
However, others reported uncertainty of risk-based thresholds for nephrology care from the perspective of primary care providers, who were anticipated to feel less comfortable in situations in which patients were “discharged” from nephrology clinic. Focus group participants commented that select patient subsets, including young people with CKD but low KFRE scores, or people with known glomerular disease in remission, should still maintain nephrology care regardless of KFRE scores.
Providers reported several approaches to the use of KFRE risk-based thresholds in the care of people with advanced CKD. Some commented that KFRE scores influenced the timeline of KRT planning, whereas the eGFR still dictated the need for referral for services such as vascular access placement. The need to follow traditional eGFR cutoffs was highlighted in regard to transplant evaluation.
“…although that might [play] a role in me thinking about a patient needing a transplant sooner rather than later, the actual referral and evaluation process is just guided by…a concrete target…I think dialysis education, I just am, I tend to be a little more proactive with giving that out…maybe I start that a little bit sooner…than I would looking at access.” (Fellow #4)
Education of Patients, Nephrology Providers, and Non-nephrology Providers on Appropriate Interpretations of KFRE Scores May Help Maximize their Utility
Although participants noted the potential benefits of using KFRE scores to provide a risk estimate directly to patients or other non-nephrology providers, some participants had concerns about providing these data without appropriate discussions of the inability of KFRE scores to account for future events which might impact kidney failure risk. They noted that lack of education about these topics could impair physician-patient relationships.
“I have had some people who really tend to…anchor on those numbers and get stuck on those. And then…if the course of their kidney disease doesn’t really follow what the KFRE would’ve suggested, then there, I think maybe there could be room for…mistrust or…a lack of faith in, in medicine and…science and equations and these sorts of predictors.” (Fellow #4)
Focus group participants also commented on potential drawbacks of having KFRE scores included in clinical documentation, without an explanation of what the scores meant and their potential caveats. Explanation of KFRE scores in clinical documentation was not uniformly reported by providers.
“…in my notes to [other] providers…I won’t just leave the table in there, I’ll put in kind of, you know, based on these parameters, this is our estimated risk…this is why we’re gonna go forward with this thing or not, or we’re gonna refer someone for transplant…more as an explanation as opposed to just putting the actual table or…copy of the results in there.” (Faculty #8)
We also asked focus group participants to engage in thought exercises, commenting on how KFRE scores for theoretical patients might influence subsequent clinical care (Table 2).
Table 2.
Illustrative excerpts from focus group thought exercises. Quotations are identified by the role of the participant (faculty or fellow).
| Pair 1: 30-year old female with eGFR 45 ml/min per 1.73 m2 and 2-year KFRE 1.5% 80-year old female with eGFR 45 ml/min per 1.73 m2 and 2-year KFRE 1.5% “The 30-year old, I think even if the KFRE was on the lower side…being 30 years old and already having a GFR of 45 is a little worrisome. Because ultimately, even if it's not at the 5-year mark, this is somebody who's potentially gonna get into a lot, you know, will get into trouble during their lifetime….” (Faculty #8) “I think I'll start off with the 80 year old female and seeing the KFRE of 1.5% is reassuring in an 80 year old female with a GFR 45 and sort of helps guide the decision of saying that, you know, your risk of requiring potential dialysis or transplant is lower…at that age. And it affects potentially how frequently I can get labs and even, you know, follow up as well in the clinic…” (Fellow #2) |
| Pair 2: 65-year old male with eGFR 20 ml/min per 1.73 m2 and 2-year KFRE 1.5% 65-year old male with eGFR 20 ml/min per 1.73 m2 and 2-year KFRE 40% “…both individuals are going to be getting into some type of trouble in terms of…progressive kidney disease…I would almost take this more as how much of a timeframe that we give someone to look at options.” (Faculty #8) “…both of them I would refer for transplant just so that they can start accruing time. Both of them I would provide dialysis education materials to…casually like the, the videos that they can watch on their terms or maybe some flyers or something like that. But the second patient with the KFRE of 40%, I would probably have them talk to…an actual dialysis educator like we have access to here who can really spend some one-on-one time and go into detail. I would probably nudge them a lot more to make a decision about the modality and then probably refer them for access pretty soon…highly unlikely that unless you have a living donor that you're gonna get transplanted before you end up on dialysis likely with, with that, KFRE that high.” (Fellow #4) |
| Pair 3: 70-year old male with 2-year KFRE 40% and 2-year CVD risk score 10% 70-year old male with 2-year KFRE 40% and 2-year CVD risk score 60% “I think if anything, maybe it's more of a discussion with primary care…seeing if we can optimize various other things…I don't know how much it would change what I do from a kidney standpoint outside of maybe just being more aware of in general how much sicker the patient is.” (Faculty #1) “I think it can be used from the standpoint of…counseling individuals about renal replacement therapy…sort of best-case scenario, worst-case scenario in terms of how they may do. Also, I think I almost take it to the fact of, you know, they're much higher risk of having some cardiovascular event, which is going to potentially alter their trajectory of their kidney disease.” (Faculty #8) “I think with the second patient, I would focus a lot more on what their necessary goals of care are and you know, focus on…whether dialysis would even be beneficial going forward and you know, even discuss potential other options like medical management just given that already existing…mortality without being on dialysis.” (Fellow #2) |
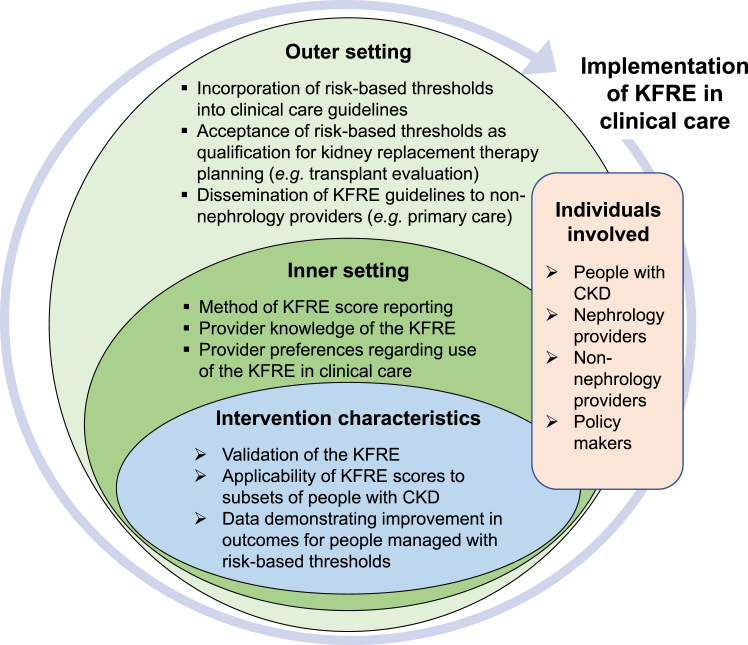
Our data are summarized into key considerations to guide KFRE implementation (Figure 4).
Figure 4.
Implementation of the KFRE in clinical care, as modeled by the Consolidated Framework for Implementation Research (CFIR).15,16
Discussion
In this mixed methods study, which described our institution’s experiences with KFRE implementation in nephrology care, we found that KFRE score documentation increased over time, with variability in adoption by providers. Provider-reported utilization was influenced by knowledge of KFRE interpretation, perspectives on applicability of KFRE scores to subsets of patients, and views on the use of the KFRE as a decision support aid. We highlight some targetable issues to consider when optimizing KFRE implementation in clinical care.
First, our data suggest that knowledge about the KFRE influences provider perspectives on its use. Fewer than 50% of fellows who responded to our survey reported having a self-reported “good understanding” of KFRE calculation and validation. Faculty reported higher awareness of KFRE calculation and validation, though knowledge was not uniform. Focus group participants were aware that KFRE scores reflect a single point in time, do not account for external factors such as acute illnesses or social determinants of health, and may need to be reassessed after significant changes in health. Still, some focus group participants alluded to trending changes in KFRE, which has not been validated. Implementation of high-yield guides providing education on appropriate interpretations and applications of the KFRE, designed for nephrology providers, non-nephrology providers, and patients, is an area of potential future research. It is plausible that through this education, providers and patients will better recognize the need for albuminuria testing,10 which has historically been low.17, 18, 19
Similarly, our data allude to a need to better identify the most effective method to disseminate KFRE scores to providers and/or patients. Providers reported use of KFRE on Storyboard or as a dotphrase more often than Patient Insight. Some providers continued to use the external KFRE website, citing the need to incorporate external data or a preference to calculate 8-variable KFRE scores.3 Other clinical centers may display KFRE scores differently: at least 1 center reports 2-year KFRE scores on clinic schedule lists, such that providers can view scores when opening a clinic encounter (personal communication with M.E. Grams). Provider input on methods of score reporting, including desired accompanying clinical information, may help implement the KFRE as a tool to assist clinicians in providing person-centered care. We also anticipate that a KFRE “champion” within each health care setting could lead iterative review of score reporting tools and provide continued education to providers, which may improve utilization.
KFRE risk-based thresholds have been proposed as a method to guide several aspects of nephrology care.5, 6, 7, 8, 9, 10 Several studies have predicted that using KFRE scores as a criterion for nephrology referral will result in a reduction in unnecessary referrals, and an increase in earlier referrals for patients at high risk of progression to kidney failure.5,20, 21, 22 In one care setting in Canada, KFRE scores dictate the urgency of nephrology referral.6 However, a pragmatic clinical trial in which primary care physicians were provided 5-year KFRE scores with a decision support aid demonstrated low rates of use and no improvement in clinical monitoring.23 An ongoing trial of KFRE and decision support aid delivery (including patient-facing) to primary care clinics in Canada24 will assess subsequent management of patients in addition to provider satisfaction, costs of care, and patient health literacy.
In our study, providers reported some degree of uncertainty about the use of KFRE scores as a decision support aid. For instance, although providers reported ease of using KFRE scores to guide clinical care of older patients with low risk scores, there was no consensus on whether or not to “discharge” this subset of patients from clinic. Similarly, although very high risk scores were reported to be beneficial in raising awareness or altering the timeline of KRT planning, there was no uniformity in using scores to determine when a person would be referred for steps such as dialysis access placement. To our knowledge, there are no published trials assessing outcomes of KFRE-based thresholds (as opposed to eGFR-based thresholds) for referral to nephrology, or referral from nephrology to transplantation, dialysis education, or vascular access placement. With such data, and through dissemination and implementation of KFRE-based care guidelines, we anticipate that KFRE scores could improve care for patients with CKD. KFRE score use might help avoid unneeded dialysis access placement in patients at low risk of progression to kidney failure within their lifetime, and could enable targeted outreach to people at highest risk requiring more immediate attention. Person-centered outcomes including patient activation and patient-physician relationships are additional metrics that could improve through the use of KFRE scores in CKD clinic.
Finally, during implementation in clinical care, patient-specific factors may influence provider perspectives on applicability of the KFRE. KFRE has been validated in several large international patient cohorts3,13,25, 26, 27 and has been incorporated into clinical care guidelines in the United Kingdom,28 though some studies have raised the possibility of variability across geographic regions,4 age,29 or disease etiology.30,31 KFRE may overestimate risk in older people and in those with high burden of comorbidities due to a competing risk of death.25,32 Data on calibration of the KFRE in transplant recipients has been mixed,33,34 and the KFRE may underestimate risk in patients with prior episodes of acute kidney injury.35 Although 2-year KFRE has outperformed patients’ and physicians’ abilities to predict the onset of kidney failure,36 the 2-year KFRE underestimated risk in a cohort of high-risk patients.30 Additional data on clinical outcomes of subsets of patients with CKD, based on KFRE scores, may increase provider-rated acceptability of utilizing KFRE in clinical care.
We note several considerations in the interpretation of our data. KFRE score documentation was quantified conservatively: use of Patient Insight was not reported, and we did not capture events in which providers looked at KFRE scores on Storyboard but did not document them in notes. We were not able to capture specific use of the KFRE dotphrase and instead used a text string search for “2-year KFRE”, acknowledging limitations that if the provider edited score reporting in any way (e.g., “2-yr KFRE”), or documented “5-year KFRE,” our queries would not have captured this use of scores. Analysis was done at the note level, not at the patient level, and would not account for variation in documentation on a provider-level, or variation in documentation during type of visit (e.g., providers may only document KFRE scores the first time a patient is seen in clinic). KFRE score reporting tools did not use some external laboratories. Provider surveys, however, were used as an additional method to assess provider use of the KFRE. As a single-center study, we describe perspectives of a single group of nephrology providers, and additional testing would be needed to determine if this clinical tool could feasibly be implemented in community-based and smaller practices. Focus group and survey participants were self-selected and may have had greater knowledge and/or awareness of the KFRE compared to other internal and external providers. Further qualitative analysis with additional nephrology providers, internal and external to our practice, would increase transferability of our qualitative data. We did not assess the impact of KFRE on patient discussions or clinical care; including albuminuria testing; medication management; or optimal dialysis initiation, including placement of vascular access. Surveys and interviews of nephrology providers in multiple health care settings, in addition to input from stakeholders including patients with CKD,37 could greatly inform guidelines for KFRE implementation. The overarching impact of provider characteristics (e.g., length of clinic experience), clinical setting (e.g., private practice or academics), and patient-specific factors (e.g., age, disease etiology, and personal preferences) was not explored.
The strengths of our study include it being among the first to describe nephrology provider utilization and perspectives on the use of the KFRE in routine nephrology care, and to report implementation outcomes of the KFRE in a United States nephrology clinic. We also describe our institution’s method of implementing the KFRE in a widely used electronic health system, and our experiences can inform future expansion of the KFRE to non-nephrology providers within our institution, and to additional health care settings nationally and globally.
In conclusion, we implemented KFRE at our institution and found that KFRE documentation increased over time, with variability in adoption and perspectives on its use among providers. Additional data describing clinical outcomes of people managed with KFRE risk-based thresholds, improvements in knowledge about the KFRE, and clarification for provider preferences on KFRE score reporting, can be pursued in an effort to implement the KFRE in clinical care. Studies of KFRE uptake and utilization in additional clinical centers may clarify the best methods of dissemination. Ultimately, weighing evidence along with stakeholder input (nephrology and non-nephrology providers, people with CKD, and policy makers) would help define a standard for implementation of the KFRE in clinical care.
Disclosure
CRP is a member of the advisory board and owns equity in RenalytixAI. CRP also serves as a consultant for Genfit. All the other authors have declared no competing interests.
Acknowledgments
The authors acknowledge members of the Johns Hopkins Precision Medicine Center of Excellence team (Jack Bitzel, Ken Harkness, John Scott, and Aparna Natarajan) and Core for Clinical Research Data Acquisition (Kerry Smith) for contributing to data acquisition and design of KFRE score reporting tools. The authors thank survey and focus group participants.
MEG and DCC were supported, in part, by grants K24 HL155861 (MEG) and K24 HL148181 (DCC) from the National Heart, Lung and Blood Institute, National Institutes of Health (NIH). CRP is supported by NIH grants R01HL085757, U01DK114866, U01DK106962, and U01DK129984. This work was supported by the Kidney Precision Medicine Center of Excellence at John Hopkins University.
Data Sharing Statement
Where not available within the article and supplementary materials, the authors agree to make additional results available upon reasonable request, and upon completion of a data use agreement with Johns Hopkins University.
Footnotes
Item S1. Survey distributed to providers.
Item S2. Semi-structured interview questions for focus groups.
Figure S1. KFRE score reporting tools.
Figure S2. Provider-rated awareness of KFRE score reporting tools.
Figure S3. Coding tree for thematic analysis of focus group transcripts.
Table S1. Survey respondent characteristics.
Table S2. Open-ended comments provided by survey respondents.
Table S3. Focus group participant characteristics.
COREQ Checklist.
Supplementary Materials
Item S1. Survey distributed to providers.
Item S2. Semi-structured interview questions for focus groups.
Figure S1. KFRE score reporting tools.
Figure S2. Provider-rated awareness of KFRE score reporting tools.
Figure S3. Coding tree for thematic analysis of focus group transcripts.
Table S1. Survey respondent characteristics.
Table S2. Open-ended comments provided by survey respondents.
Table S3. Focus group participant characteristics.
COREQ Checklist.
References
- 1.Kovesdy C.P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022;12:7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2022. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 3.Tangri N., Stevens L.A., Griffith J., et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 4.Tangri N., Grams M.E., Levey A.S., et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhachu H.K., Cockwell P., Subramanian A., et al. Impact of using risk-based stratification on referral of patients with chronic kidney disease from primary care to specialist care in the United Kingdom. Kidney Int Rep. 2021;6:2189–2199. doi: 10.1016/j.ekir.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hingwala J., Wojciechowski P., Hiebert B., et al. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117722782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Che M., Iliescu E., Thanabalasingam S., Day A.G., White C.A. Death and dialysis following discharge from chronic kidney disease clinic: a retrospective cohort study. Can J Kidney Health Dis. 2022;9 doi: 10.1177/20543581221118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smekal M.D., Tam-Tham H., Finlay J., et al. Perceived benefits and challenges of a risk-based approach to multidisciplinary chronic kidney disease care: a qualitative descriptive study. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358118763809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuningas K., Stringer S., Cockwell P., Khawaja A., Inston N. Is there a role of the kidney failure risk equation in optimizing timing of vascular access creation in pre-dialysis patients? J Vasc Access. 2022 doi: 10.1177/11297298221084799. [DOI] [PubMed] [Google Scholar]
- 10.Oliva-Damaso N., Tangri N., Delanaye P., Glassock R.J. Bridging the gap of referral to nephrology care. Nat Rev Nephrol. 2023;19:275–276. doi: 10.1038/s41581-023-00693-1. [DOI] [PubMed] [Google Scholar]
- 11.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumida K., Nadkarni G.N., Grams M.E., et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis : an individual participant-based meta-analysis. Ann Intern Med. 2020;173:426–435. doi: 10.7326/M20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grams M.E., Sang Y., Ballew S.H., et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int. 2018;93:1442–1451. doi: 10.1016/j.kint.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun V., Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 15.Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damschroder L.J., Reardon C.M., Opra Widerquist M.A., Lowery J. Conceptualizing outcomes for use with the Consolidated Framework for Implementation Research (CFIR): the CFIR Outcomes Addendum. Implement Sci. 2022;17:7. doi: 10.1186/s13012-021-01181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu C.D., Powe N.R., Shlipak M.G., et al. Albuminuria testing and nephrology care among insured US adults with chronic kidney disease: a missed opportunity. BMC Prim Care. 2022;23:299. doi: 10.1186/s12875-022-01910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Chu C., Guzman D., et al. Albuminuria testing by race and ethnicity among patients with hypertension with and without diabetes. Am J Nephrol. 2019;50:48–54. doi: 10.1159/000500706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin J.I., Chang A.R., Grams M.E., et al. Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension. 2021;78:1042–1052. doi: 10.1161/HYPERTENSIONAHA.121.17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duggal V., Montez-Rath M.E., Thomas I.C., Goldstein M.K., Tamura M.K. Nephrology referral based on laboratory values, kidney failure risk, or both: a study using Veterans Affairs health system data. Am J Kidney Dis. 2022;79:347–353. doi: 10.1053/j.ajkd.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Major R.W., Shepherd D., Medcalf J.F., Xu G., Gray L.J., Brunskill N.J. The Kidney Failure Risk Equation for prediction of end stage renal disease in UK primary care: an external validation and clinical impact projection cohort study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh K., Waikar S.S., Samal L. Evaluating the feasibility of the KDIGO CKD referral recommendations. BMC Nephrol. 2017;18:223. doi: 10.1186/s12882-017-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samal L., D’Amore J.D., Gannon M.P., et al. Impact of kidney failure risk prediction clinical decision support on monitoring and referral in primary care management of CKD: a randomized pragmatic clinical trial. Kidney Med. 2022;4 doi: 10.1016/j.xkme.2022.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harasemiw O., Drummond N., Singer A., et al. Integrating risk-based care for patients with chronic kidney disease in the community: study protocol for a cluster randomized trial. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119841611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramspek C.L., Evans M., Wanner C., et al. Kidney failure prediction models: a comprehensive external validation study in patients with advanced CKD. J Am Soc Nephrol. 2021;32:1174–1186. doi: 10.1681/ASN.2020071077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tangri N., Inker L.A., Hiebert B., et al. A dynamic predictive model for progression of CKD. Am J Kidney Dis. 2017;69:514–520. doi: 10.1053/j.ajkd.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Thanabalasingam S.J., Iliescu E.A., Norman P.A., et al. Independent external validation and comparison of death and kidney replacement therapy prediction models in advanced CKD. Kidney Med. 2022;4 doi: 10.1016/j.xkme.2022.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence (NICE) National Institute for Health and Care Excellence (NICE); 2021. Chronic kidney disease: assessment and management. [PubMed] [Google Scholar]
- 29.Hundemer G.L., Tangri N., Sood M.M., et al. The effect of age on performance of the kidney failure risk equation in advanced CKD. Kidney Int Rep. 2021;6:2993–3001. doi: 10.1016/j.ekir.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali I., Donne R.L., Kalra P.A. A validation study of the kidney failure risk equation in advanced chronic kidney disease according to disease aetiology with evaluation of discrimination, calibration and clinical utility. BMC Nephrol. 2021;22:194. doi: 10.1186/s12882-021-02402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hundemer G.L., Tangri N., Sood M.M., et al. Performance of the kidney failure risk equation by disease etiology in advanced CKD. Clin J Am Soc Nephrol. 2020;15:1424–1432. doi: 10.2215/CJN.03940320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravani P., Fiocco M., Liu P., et al. Influence of mortality on estimating the risk of kidney failure in people with Stage 4 CKD. J Am Soc Nephrol. 2019;30:2219–2227. doi: 10.1681/ASN.2019060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali I., Kalra P.A. A validation study of the 4-variable and 8-variable kidney failure risk equation in transplant recipients in the United Kingdom. BMC Nephrol. 2021;22:57. doi: 10.1186/s12882-021-02259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tangri N., Ferguson T.W., Wiebe C., et al. Validation of the kidney failure risk equation in kidney transplant recipients. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120922627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawhney S., Beaulieu M., Black C., et al. Predicting kidney failure risk after acute kidney injury among people receiving nephrology clinic care. Nephrol Dial Transplant. 2020;35:836–845. doi: 10.1093/ndt/gfy294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potok O.A., Nguyen H.A., Abdelmalek J.A., Beben T., Woodell T.B., Rifkin D.E. Patients,’ Nephrologists,’ and Predicted Estimations of ESKD Risk Compared with 2-Year Incidence of ESKD. Clin J Am Soc Nephrol. 2019;14:206–212. doi: 10.2215/CJN.07970718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Horst D.E.M., Engels N., Hendrikx J., et al. Predicting outcomes in chronic kidney disease: needs and preferences of patients and nephrologists. BMC Nephrol. 2023;24:66. doi: 10.1186/s12882-023-03115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.