Abstract
Belatacept is the first costimulatory blockade agent clinically approved for transplant immunosuppression. Although more than 10 years of study have demonstrated that belatacept offers superior long-term renal allograft and patient survival compared to conventional calcineurin inhibitor (CNI)-based immunosuppression regimens, the clinical adoption of belatacept has continued to lag because of concerns of an early risk of acute cellular rejection (ACR) and various logistical barriers to its administration. In this review, the history of the clinical development of belatacept is examined, along with the findings of the seminal BENEFIT and BENEFIT-EXT trials culminating in the clinical approval of belatacept. Recent efforts to incorporate belatacept into novel CNI-free immunosuppression regimens are reviewed, as well as the experience of the Emory Transplant Center in using a tapered course of low-dose tacrolimus in belatacept-treated renal allograft patients to garner the long-term outcome benefits of belatacept without the short-term increased risks of ACR. Potential avenues to increase the clinical adoption of belatacept in the future are explored, including surmounting the logistical barriers of belatacept administration through subcutaneous administration or more infrequent belatacept dosing. In addition, belatacept conversion strategies and potential expanded clinical indications of belatacept are discussed for pediatric transplant recipients, extrarenal transplant recipients, treatment of antibody-mediated rejection (AMR), and in patients with failed renal allografts. Finally, we discuss the novel immunosuppressive drugs currently in the development pipeline that may aid in the expansion of costimulation blockade utilization.
Keywords: abatacept, alloantibodies, belatacept, costimulation, immunosuppression, kidney transplantation
CNIs, such as cyclosporine and tacrolimus, have served as the backbone of transplant immunosuppression for the vast majority of renal transplant recipients over the past 40 years. The advent of CNI therapy undoubtedly ushered in the modern era of transplantation, establishing renal transplant as the gold-standard therapy for end-stage renal failure. However, although CNI therapy has secured exceptional short-term outcomes with transplantation, progress in improving long-term renal allograft survival has stagnated for many years,1 spurring interest in other immunosuppressive strategies. The nephrotoxicity and well-defined adverse metabolic effects of CNIs have also driven a desire to explore alternative immunosuppressive agents.2, 3, 4
Costimulation blockade emerged as a promising immunosuppression strategy based on seminal work in the 1990s demonstrating that blockade of critical costimulatory pathways (such as CD28-CD80/86 and CD40-CD154 interaction) could substantially prolong allograft survival in murine transplant models.5, 6, 7 Whereas the clinical development of anti-CD154 was initially halted because of the development of unexpected thrombotic complications (later attributed to cross-linkage of platelet-expressed CD154),8 the pursuit of drugs to disrupt CD28-B7 interaction culminated in the development and clinical trials of belatacept, a potent CD28 antagonist.9 Subsequent clinical trials have validated the promise of belatacept, demonstrating superior long-term outcomes of graft and patient survival compared to renal transplant recipients treated with cyclosporine-based immunosuppression.10,11 However, persistent fears about early ACR and logistical challenges associated with chronic intravenous infusion requirement have limited the widespread adoption of this drug. In this review, we explore the history of costimulation blockade in renal transplantation, the present use of belatacept in transplant recipients, and the future of costimulatory blockade in transplantation.
The Past
Clinical Development of Belatacept
The “three signal hypothesis” of T cell activation emerged from careful experimentation in the 1980s and 1990s.12 This paradigm established that activation of naïve T cells required antigen recognition via T cell receptor interaction with cognate MHC-antigen complex, costimulatory signals, and cytokine-mediated differentiation/expansion. A variety of costimulation receptors were identified to mediate the second signal required for T cell activation, including CD28-CD80/86, CD40-CD154, OX40-OX40L (CD134-CD252), and ICOS-ICOSL (CD278/CD275). Many of these costimulatory receptors were also found to significantly contribute to alloreactive T cell activation.13,14 Indeed, murine transplant models revealed that alloreactive T cell activation is critically dependent on the interaction between CD28 (expressed on naïve alloreactive T cells) and CD80/86 (expressed on antigen presenting cells).5
Abatacept, a fusion protein composed of the extracellular domain of CTLA-4 fused to the Fc region of IgG1, emerged as one of the earliest drugs to target this interaction through competitive inhibition, given that CTLA-4 binds to CD80/86 with higher avidity than CD28 binding to CD80/86.15 However, though abatacept has proven to be a clinically effective therapeutic for autoimmune disorders such as rheumatoid and psoriatic arthritis,16 it demonstrated limited ability to prolong allograft survival in nonhuman primates.17,18 This shortcoming spurred the development of belatacept, which has 2 point sequence variations of the abatacept backbone which significantly enhance avidity for CD80/CD86, enabling belatacept to better sequester the CD80/86 ligand required for CD28 engagement.9 Unlike abatacept, belatacept more effectively prolonged renal allograft survival in nonhuman primate transplant systems.9 Prompted by these promising preclinical results, the clinical development of belatacept ensued. The initial phase II trial of belatacept in human renal transplant recipients generated much enthusiasm in that it found similar rates of 6 month biopsy-proven ACR between patients treated with belatacept and those treated with cyclosporine-based immunosuppression, but a marked improvement in glomerular filtration rate at 12 months associated with belatacept use (estimated glomerular filtration rate [eGFR] 66.3 vs. 53.5 ml/min per 1.73 m2).19
The BENEFIT and BENEFIT-EXT Clinical Trials
The further clinical development of belatacept was underpinned by the phase 3 Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression (BENEFIT) trial, which remains the largest clinical trial of transplant immunosuppression ever conducted.11 The BENEFIT trial tested 2 different doses of belatacept (a more intensive regimen and a less intensive regimen, which ultimately would be the regimen to gain clinical approval) against a control group of renal transplant recipients treated with conventional cyclosporine-based immunosuppression. This trial, along with the similar BENEFIT-EXT trial of belatacept use in recipients of extended criteria donor kidneys,20 demonstrated that renal transplant recipients treated with belatacept demonstrated superior measured glomerular filtration rates at 12 months posttransplant compared to recipients treated with conventional cyclosporine-based immunosuppression (63 vs. 50 ml/min, P < 0.001). Interestingly, there was also a reduction in the prevalence of biopsy-proven chronic allograft nephropathy at month 12 in the belatacept-treated groups compared to CNI in the BENEFIT study (18% for more intensive regimen belatacept vs. 32% for cyclosporine)11; however, this difference in chronic allograft nephropathy rates was not observed in the BENEFIT-EXT trial.21 The reasons for this are unclear; however this discrepancy could perhaps be due to the relatively early 12 month timepoint of the protocol biopsies in these trials, when the early improvement in eGFR could be primarily due to prerenal hemodynamic benefits of belatacept compared to CNI rather than a reduction in interstitial fibrosis, which would be expected to occur at later time points. It is also possible that any early benefits in chronic allograft nephropathy were masked by the use of more marginal allografts in the BENEFIT-EXT study as compared to the living and standard criteria deceased donors used in the BENEFIT study. The original BENEFIT trial found that this improved graft function was at the cost of a higher incidence and severity of ACR in recipients treated with belatacept (17% vs. 7% incidence of biopsy-proven rejection at 12 months).11 This increased risk of acute rejection was confined to the early posttransplant period, because the 3 year follow-up of the BENEFIT trial found no additional rejection episodes in the belatacept group after year 2.22 Interestingly, neither the initial or follow-up BENEFIT-EXT reports found any acute rejection signature with belatacept.20,21,23 In addition, a higher incidence of posttransplant lymphoproliferative disease was observed in belatacept-treated renal transplant recipients who were naïve for prior Epstein-Barr virus infection at the time of transplant (5/369 patients on belatacept vs. 0/184 patients on cyclosporine), resulting in a black-box warning to be placed on the use of belatacept in this subpopulation of transplant recipients. The BENEFIT and BENEFIT-EXT trials culminated in clinical approval of belatacept for renal transplant recipients by the US Food and Drug Administration and the European Medicines Agency in 2011.24
The 7-year follow-up study of the BENEFIT trial participants confirmed that renal transplant recipients treated with belatacept maintained improved eGFR and experienced enhanced survival compared to those treated with cyclosporine-based regimens. A 43% reduction in risk of death or graft loss as a composite end point was observed for the belatacept-treated patients compared to cyclosporine-treated patients, with a similar marked difference in eGFR (70.4 vs. 44.9 ml/min per 1.73 m2, P < 0.001).10 The benefit in patient and graft survival with belatacept was significant in this long-term follow-up study, and the sustained improvement in eGFR associated with belatacept may be just as (if not more) meaningful, given that the vast majority of trial participants were alive with a functioning graft by the end of study and the percentage of reduced risk might predict more impressive changes in long-term allograft losses should belatacept have wider clinical adoption. Ten-year follow-up of the original phase II trial evaluating belatacept versus cyclosporine for de novo immunosuppression in renal transplant recipients also found that (similar to the BENEFIT trial) belatacept was associated with improved long-term eGFR of renal allografts counterbalanced by an increased incidence of early biopsy-proven ACR.25
Impact of Belatacept on Cardiovascular Risk and Glucose Metabolism
Other potential advantages of belatacept emerged from the BENEFIT trial. Studies of physical health-related quality of life surveys in the BENEFIT and BENEFIT-EXT patient populations showed improved patient-reported physical health 12 months and 36 months posttransplant in belatacept-treated compared to CNI-treated patients.26 A post hoc analysis of the patients in the BENEFIT and BENEFIT-EXT trial also revealed that compared to the cyclosporine-treated control group, the belatacept-treated group had lower mean systolic blood pressure (6–9 mm Hg), lower mean diastolic blood pressure (3–4 mm Hg), lower non-HDL cholesterol, lower serum triglycerides, and a lower rate of new-onset diabetes after transplant (19% vs. 25%).27 These advantages in glucose metabolism were validated in a subsequent retrospective trial that used oral glucose tolerance tests to show that renal transplant recipients treated with belatacept had a 93% lower odds of developing diabetes or prediabetes compared to recipients on tacrolimus-based immunosuppression.28 One study using a validated risk calculator of major adverse cardiac events in renal transplant recipients used the BENEFIT and BENEFIT-EXT data sets to predict that belatacept use may result in a >20% reduction in major adverse cardiac events compared to CNI treatment, as well as an approximately 18% to 30% reduction in cardiovascular mortality.29 This promising data recently spurred a prospective, randomized, open-label international multicenter trial to assess the impact of belatacept conversion on cardiovascular risk in renal transplant patients. A total of 105 renal transplant patients in Denmark, the Netherlands, Norway, and Sweden with stable graft function were randomized to either remain on CNI-based immunosuppression, or to convert to belatacept. The patients were 3 to 60 months posttransplant (mean 25.3 months) and 96% were on tacrolimus-based immunosuppression prior to randomization. After 1 year of treatment, the previously mentioned validated 7-year cardiovascular risk calculator was applied. Although belatacept use was associated with a significant reduction in diastolic blood pressure and a trend to reduction in systolic blood pressure, there was no difference in the calculated 7-year risk of major adverse cardiac events between the conversion group and the patients remaining on CNI.30 However, this result was predominantly due to the fact that the belatacept conversion group had no improvement in eGFR, which runs counter to the findings of almost every other belatacept conversion trial. In addition, given that this trial converted patients up to 60 months posttransplant, it is possible that the cardiovascular benefits of belatacept conversion were underestimated, because the adverse metabolic changes of initial CNI use may have been irreversible at these late time points. Therefore, further research into the cardiovascular and diabetic risk reduction associated with belatacept is needed.
Impact of Belatacept on Donor-Specific Antibody
An increasingly recognized advantage of belatacept is its ability to prevent and reduce alloantibodies.31 Post hoc analysis of the long-term extensions of these clinical trials revealed a lower incidence of de novo donor-specific antibody (DSA) production in the patients treated with low-intensity belatacept versus cyclosporine (after 7 years of follow-up, the rates of de novo DSA were 3.5% vs. 12.1% in the BENEFIT trial, and 1.1% vs. 11.2% in the BENEFIT-EXT trial).32 Mechanistic studies in renal transplant recipients shows that belatacept may control humoral alloresponses by inhibiting plasmablast differentiation, reducing the expression of the major transcription factor involved in plasma cell function (Blimp-1), and blocking activation of T follicular helper cells.33 There is even some evidence that belatacept use could reduce the mean fluorescent intensity of preexisting DSA levels after transplantation compared to cyclosporine therapy.34 Support for this hypothesis was also provided by the BELACOR pilot study, which tested the de novo use of belatacept in renal transplant recipients with low-level preformed DSA (mean fluorescent intensity 500–3000). No recipients in this BELACOR group developed acute AMR, and a significantly higher number of patients in the belatacept-treated group displayed a complete disappearance of class II DSA compared to a retrospective control group treated with CNI-based immunosuppression.35
Comparisons of Belatacept to Tacrolimus for De Novo Immunosuppression
One common criticism of the BENEFIT and BENEFIT-EXT trials is that the comparator group of patients received cyclosporine-based rather than tacrolimus-based immunosuppression, because tacrolimus is associated with lower rates of acute rejection and possibly less toxicity than cyclosporine.36 Although this comparator group was chosen for regulatory reasons impacting the potential clinical approval of belatacept, it did raise the question of whether the clinical benefits of belatacept therapy would persist compared to patients treated with tacrolimus-based immunosuppression. In one small study, de Graav et al.37 performed a randomized study of tacrolimus versus belatacept for de novo renal transplant immunosuppression. Unlike the BENEFIT trial, this randomized controlled trial (RCT) found no difference in 12 month GFR between the belatacept and tacrolimus-treated groups; however, there was a substantial increase in the incidence of biopsy-proven ACR associated with belatacept use (55% vs. 10%, P = 0.006), resulting in 3 graft losses in the belatacept group. A substantial limitation of this study, however, was that it was very small (40 total patients) and neither designed nor powered to truly assess differences in eGFR between treatment groups. In addition, as discussed more comprehensively later, the real-world published experience with several hundred patients at Emory demonstrates notable long-term benefits in eGFR in patients receiving belatacept-based maintenance immunosuppression compared to historical controls receiving tacrolimus-based maintenance.38
The Present
Limited Clinical Adoption of Belatacept
Belatacept remains a promising, but underutilized, option for transplant immunosuppression. No immunosuppressive agent has demonstrated as profound an improvement in patient and renal allograft survival as belatacept has since the introduction of CNIs 4 decades ago; however, its adoption has been stymied by logistical barriers as well as concerns of increased short-term incidence of ACR. An analysis of the Scientific Registry of Transplant Recipients revealed that from 2011 to 2016, the number of patients receiving de novo belatacept-based immunosuppression increased from 0.74% to 3.11%.39 However, less than half of US transplant centers used any de novo belatacept in renal transplant patients, and only 3% of transplant centers used it in over 50% of their patients, highlighting the limited adoption of belatacept in recent years.39 Even in the most recent Scientific Registry of Transplant Recipients Annual Report, only 5.52% of de novo renal transplant recipients in 2020 received a non-tacrolimus–based initial maintenance immunosuppression regimen and only a portion of these patients will have received belatacept.40
Novel Belatacept-Based Immunosuppression Regimens
The higher rate of biopsy-proven ACR associated with belatacept use is undoubtedly one of its chief barriers to wider adoption, and a number of randomized clinical trials and studies have sought to establish the ideal way to mitigate this concern with belatacept.41 Experimental approaches have included employing more aggressive depletional induction methods (often coupled with mTOR inhibitor-based and/or steroid-free CNI-sparing maintenance regimens) or utilizing belatacept with a short-term taper of low-dose CNI to achieve long-term CNI-free immunosuppression.
Depletional Induction, mTOR Inhibition, and Early Steroid Withdrawal
Several trials have sought to develop a CNI-sparing and steroid-sparing immunosuppression regimen based on coupling belatacept with depleting induction agents such as rabbit anti-thymocyte globulin (rATG) or alemtuzumab. In one early trial conducted at the Ohio State College of Medicine, 89 patients were randomized to receive belatacept-mycophenolate mofetil (MMF), belatacept-sirolimus or tacrolimus-MMF42 as de novo immunosuppression after kidney transplant. All these patients received induction with rATG and a short course of rapidly-tapered corticosteroids. The authors found a higher risk of rejection in the belatacept-MMF group compared to either of the 2other groups; however, two-thirds of patients in the belatacept groups remained on CNI-free and steroid-free regimens 1 year posttransplant, and the belatacept groups had a calculated GFR that was 8–10 ml/min higher than the patients in the tacrolimus/MMF group. Another pilot trial of CNI-sparing and corticosteroid-sparing immunosuppression conducted by Kirk et al.43 treated living donor kidney transplant recipients with belatacept, daily sirolimus, and alemtuzumab induction; patients were also randomized 1:1 to either receive or not receive unfractionated donor bone marrow. Of 20 patients transplanted, weaning of all oral immunosuppression (to maintain patients on belatacept monotherapy) was attempted in 10 patients and successfully achieved in 7 patients. Although there were no episodes of ACR in the first year post-transplant (regardless of donor bone marrow infusion), protocol biopsies revealed subclinical rejection in 10% of recipients. Five year follow-up of this patient cohort revealed a patient and graft survival rate of 100% and 95%, respectively, with 12 of 20 patients ultimately successful in weaning to belatacept monotherapy.44 The early success of these 2 trials spurred 2 separate Clinical Trials in Organ Transplant trials.
The Clinical Trials in Organ Transplant-10 trial was a randomized trial of CNI and corticosteroid avoidance using belatacept and alemtuzumab induction. However, this trial was aborted early because of the higher incidence of biopsy-proven rejection and vascular thrombosis rates in the alemtuzumab/belatacept experimental group compared to the alemtuzumab/tacrolimus experimental group.45 The subsequent Clinical Trials in Organ Transplant-16 trial also demonstrated the potential perils of attempting steroid-sparing immunosuppression regimens based on belatacept. This trial compared a control arm receiving rATG, rapid steroid taper, maintenance MMF, and tacrolimus with 2 different belatacept regimens; one involving rATG induction, rapid steroid taper, MMF, and belatacept and the other regimen involving basiliximab induction, rapid steroid taper, a 5-month course of tacrolimus, and maintenance therapy with MMF and belatacept. Although this study did not encounter the same high rate of vascular thromboses seen in the Clinical Trials in Organ Transplant-10 study, it was also terminated because of the high rate of ACR in the belatacept therapy arms of the study.46
Another recent trial of belatacept-based CNI-sparing and corticosteroid-sparing immunosuppression was the BEST trial, a 2-year prospective, randomized multicenter trial of rapid steroid withdrawal immunosuppression that randomized renal transplant recipients to receive belatacept with alemtuzumab induction, belatacept with rATG, or tacrolimus with rATG induction.47 Whereas 2-year eGFR <45ml/min per 1.73 m2 was observed in only 8% of both the belatacept/alemtuzumab and belatacept/rATG groups, it was observed in 19% of the tacrolimus/rATG group (P < 0.05). However, this was counterbalanced with a higher rate of biopsy-proven acute rejection in the belatacept/alemtuzumab (19%) and belatacept/rATG (25%) groups compared to the tacrolimus/rATG group (7%, P < 0.006).48
The most recent trial of belatacept-based CNI-sparing and maintenance corticosteroid-sparing immunosuppression was a multicenter phase 2 study that used rATG induction and a rapidly tapered steroid induction (weaning off by postop day 7), and compared renal transplant recipients receiving belatacept and daily everolimus versus control group recipients treated with conventional tacrolimus and MMF immunosuppression. This study showed similar biopsy-proven rejection rates at 6 months (7.7% vs. 9.4%) and 24 months (16.0% vs. 15.2%) in the belatacept + everolimus and tacrolimus + MMF groups, though there was no identified benefit in 24-month mean unadjusted eGFRs between these regimens (71.8 vs. 68.7 ml/min per 1.73 m2).49 Although promising based on similar rejection rates between groups, the lack of improvement in renal function by coupling belatacept with both depleting induction and mTOR inhibitor use may limit enthusiasm for this regimen.
Transient CNI-Minimization and Early Withdrawal (The Emory Protocol)
At Emory, belatacept was adopted as the standard of care for all renal transplant recipients upon US Food and Drug Administration approval in 2011, except for patients who were EBV-seronegative, HIV+ or had a history of prior blood malignancy. When we first adopted belatacept using the same completely CNI-sparing regimen as the BENEFIT trial, we observed a sharp increase in ACR (50.5% vs. 20.5% in a historical cohort of Emory renal transplant recipients treated with a tacrolimus-based protocol). Most of these rejection episodes occurred within the first 6 months of transplant, leading us to adopt a strategy of coupling belatacept with a transient low-dose course of tacrolimus to protect the renal allograft from early episodes of ACR. The addition of a transient course of tacrolimus that was weaned off from 9 to 11 months posttransplant resulted in a more acceptable 16% incidence of ACR,38 consistent with historical results using tacrolimus-based immunosuppression. In our data, all belatacept-treated patients (with or without transient tacrolimus therapy) who remained on a belatacept-based maintenance immunosuppression regimen demonstrated superior 4-year posttransplant eGFR compared to the historical cohort of tacrolimus-only patients (63.8 vs. 46.2 ml/min per 1.73 m2, P < 0.0001).
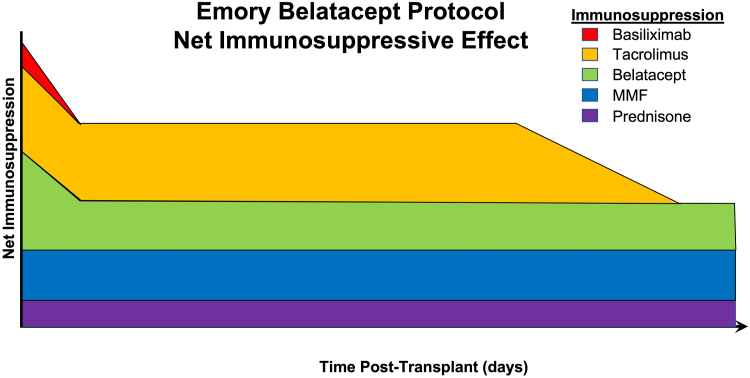
Since our initial published experience, the Emory belatacept protocol has continued to evolve (Figure 1). Currently, qualifying renal transplant recipients receive tacrolimus (4 mg) and MMF (1000 mg) preoperatively, and then receive solumedrol (500 mg IV), basiliximab induction (20mg IV) and belatacept (10 mg/kg) intraoperatively. MMF is continued, and corticosteroids are weaned to a maintenance dose of 5 mg oral prednisone by posttransplant day 3. No further basiliximab is administered, and belatacept is administered at 5 mg/kg beginning at month 1 and monthly thereafter. Tacrolimus is titrated to a goal trough level of 5 to 8 ng/ml for months 0 to 1 posttransplant, and 3 to 5 ng/ml for months 1 to 9 posttransplant. Tacrolimus taper begins at month 9 posttransplant with a decrease in tacrolimus dose by one-third, with a further decrease in tacrolimus dose by another one-third at month 10, and a discontinuation of tacrolimus 11 months posttransplant.
Figure 1.
Relative contributions of immunosuppression drugs in the current Emory University belatacept protocol. The protocol consists of basiliximab induction and an early low-dose bridge of tacrolimus to minimize early rejections that is weaned off starting at 9 months post-transplant, leaving patients on calcineurin inhibitor-free prednisone, MMF and monthly belatacept maintenance therapy long-term. MMF, mycophenolate mofetil.
Overall, these novel approaches have certainly improved upon prohibitively high initial acute rejection rates observed with the US Food and Drug Administration-approved (package insert) belatacept-based protocol, enabling the achievement of acceptable rejection rates of 15% to 20%. Ongoing efforts to optimize current regimens, identify immunosuppressive agents that may synergize with belatacept to prevent “belatacept-resistant” rejection, and the development of biomarkers to identify patients at higher or lower risk of rejection on belatacept should help elucidate the best path forward to enhance the clinical efficacy of belatacept.
Defining Recipient Populations at Risk
Beyond considerations of how belatacept can be incorporated into immunosuppression regimens that provide the long-term benefit of improved eGFR and graft survival with an acceptably low rate of ACR, further clinical use of belatacept has highlighted unique recipient populations that might not be ideal candidates for belatacept-based immunosuppression.
Patient Populations at Risk
As previously discussed, the BENEFIT and BENEFIT-EXT trials highlighted that belatacept should not be used in EBV-seronegative recipients due to a concern of high rates of posttransplant lymphoproliferative disease in these patients.50 Because of the risk of post-transplant lymphoproliferative disease associated with belatacept, we also avoid administration of belatacept to any patient with a history of hematologic malignancy. Based on their higher risk of allograft rejection in general and the exclusion of these patients from the BENEFIT and BENEFIT-EXT trials, some centers have historically avoided administration of belatacept de novo to any patient with a history of HIV infection. Nonetheless, the use of belatacept in HIV+ recipients has been reported51, 52, 53 and its use in this unique population remains to be established.
In addition, there is emerging evidence that belatacept should be administered with caution to de novo recipients who are at a high-risk for cytomegalovirus (CMV), namely CMV seronegative recipients of kidneys from CMV seropositive donors, given associated risks of greater CMV-related morbidity. In one study of 308 CMV seronegative transplant recipients (including 203 belatacept-treated patients and 168 CMV high-risk patients), belatacept use in CMV high-risk patients was associated with a higher incidence of CMV viremia at 2 years posttransplant (50% vs. 34.4%, P = 0.047), a higher rate of CMV resistance to ganciclovir (22.1% vs. 1.6%, P = 0.001), and a longer time to virus clearance compared to recipients treated with tacrolimus.54 While there was a trend to lower graft survival in high-risk patients with CMV treated with belatacept, it was not significant. A subsequent multistate Markov model confirmed these findings, demonstrating that among CMV high-risk renal transplant recipients, belatacept-treated recipients exhibited a significantly higher probability of entering moderate viremia compared to tacrolimus-treated recipients, and they persisted in moderate viremia for significantly longer than did tacrolimus-treated recipients (128 vs. 70 days).55
Experience with belatacept administration in rare instances when recipients require posttransplant plasmapheresis (such as recurrent primary focal segmental glomerulosclerosis or thrombotic microangiopathy) is limited and alternative immunosuppression should be considered, because the plasmapheresis usually used to treat this disease would also eliminate circulating belatacept.56 Finally, there is some indication that belatacept-based immunosuppression may be a risky choice for recipients who have not been vaccinated against SARS-CoV-2 prior to transplant, given that transplant recipients treated with belatacept had markedly worse antibody and cellular immune responses to SARS-CoV-2 vaccine doses administered posttransplant, even after up to 5 doses of vaccine were administered.57, 58, 59, 60, 61, 62, 63
Biomarkers
Some groups have defined some biomarkers that can identify additional patients that might by at higher risk of belatacept-resistant ACR. Using a nonhuman primate renal transplant model, Mathews et al.64 found that pretransplant frequencies of CD28+ CD8+ TEMRA cells (CD28+CD95+CD45RA+CCR7−CD8 T cells) greater than 3% was highly correlated with eventual allograft rejection on belatacept therapy, whereas a similar signature was not observed in recipients treated with tacrolimus-based immunosuppression. Similarly, another group using banked samples from human renal allograft recipients found that patients with a higher frequency of CD28+ CD4+ TEM cells were more vulnerable to subsequent rejection on belatacept therapy, again highlighting the potential role of CD28+ memory T cells in the pathogenesis of costimulatory blockade-resistant transplant rejection.65 Higher pretransplant frequencies of CD57+PD1- CD4 T cells have also been implicated in belatacept-resistant transplant rejection in human renal transplant recipients; these cells are also enriched in rejecting kidney allografts and exhibit cytolytic properties.66 Though promising, these subsets require further clinical validation and have yet to be implemented clinically for prospective use to guide belatacept usage. Other emerging technologies such as cell-free donor-derived DNA67,68 and eplet HLA mismatching69 may also ultimately prove useful in risk-stratifying patients for de novo or conversion use of belatacept, although further studies are certainly needed to validate these biomarkers in patients receiving belatacept therapy. Interestingly, an examination of predicted indirectly recognizable HLA epitopes (PIRCHE-II) by our group suggests that belatacept may modify the immune event risk for acute rejection, DSA and AMR based on risk PIRCHE-II scores that could serve to guide immunosuppression management (unpublished data).
Conversion Trials of Belatacept
Accruing evidence that belatacept may be associated with superior long-term allograft outcomes has also spurred the development of several conversion trials, switching patients from conventional CNI-based immunosuppression regimens to belatacept. These conversions have occurred both “per protocol” (i.e., at specified intervals in defined patient populations) and for cause as “rescue therapy,” initiated due to either the development of chronic histologic lesions of the allograft accompanied by poor allograft function, or due to the development of acute CNI intolerance (e.g., the development of thrombotic microangiopathy,70 neurologic intolerance of CNI, or prolonged CNI-induced delayed graft function71).
Per Protocol Conversion
The opportunity to garner the long-term renal function benefits of belatacept and avoid the early rejection risk window drove interest in protocol conversion trials of CNIs to belatacept. The initial phase 2 trial studied the conversion of renal transplant recipients maintained on CNI-based immunosuppression to belatacept with a primary outcome of change in eGFR from baseline to 12 months postrandomization.72 Low immunologic risk patients were randomized 6 to 36 months after transplant to either remain on CNI-based immunosuppression (56% on tacrolimus) or switch to belatacept-based immunosuppression. At 12 months postconversion, patients converted to belatacept had significantly improved renal function without increased death or graft loss and 7% rejection occurring within the first 6 months. Between 12 and 24 months postconversion, no additional rejection episodes occurred in the belatacept group (vs. 3 episodes in those on CNI) with maintenance of superiority in renal function.73 The 3-year follow-up of this study demonstrated no statistically significant increase in serious adverse events, infections, malignancies, or ACR episodes.74 The belatacept conversion group did maintain a significantly greater gain in mean eGFR (1.9 vs. 0.07 ml/min per 1.73 m2 per year) compared to the group randomized to maintain on CNI-based immunosuppression.74 A subsequent phase 3b RCT conducted by Budde et al.75 randomized stable adult kidney transplant recipients 6 to 60 months posttransplant to either continue on CNI-based immunosuppression (with 90% of these patients being on tacrolimus) or switch to belatacept. Whereas 24-month graft survival was identical in these 2 groups, eGFR was significantly higher in the belatacept group (55.5 vs. 48.5 ml/min per 1.73 m2) with lower rates of de novo DSAs (1% vs. 7%), albeit at a cost of a higher incidence of biopsy-proven ACR (8% vs. 4%).
For Cause (Rescue) Conversion
By far, the most common indication for belatacept conversion rescue therapy is allograft dysfunction resulting from either CNI toxicity, interstitial fibrosis/tubular atrophy, or development of de novo DSAs.76, 77, 78, 79, 80, 81, 82, 83, 84 The vast majority of these studies found that belatacept conversion rescue therapy stabilizes or even improves eGFR in this patient population with allograft dysfunction. One pilot study even found improvements in eGFR associated with very late posttransplant conversion (mean 11.9 years posttransplant) from CNI to belatacept in renal transplant recipients with chronic CNI nephrotoxicity changes.85 Another study of relatively late (median 44 months posttransplant) conversion from tacrolimus to belatacept in renal transplant patients with biopsy-proven chronic active mediated rejection found that conversion resulted in a progressive improvement in eGFR compared to a propensity-matched control cohort of patients remaining on tacrolimus.79 A recently published retrospective cohort study of 139 renal transplant recipients with chronic vascular lesions (Banff cv score >2) and impaired graft function (eGFR ≤40 ml/min per 1.73 m2) affirmed these earlier findings, demonstrating that conversion to belatacept was associated with a significant improvement in death-censored graft survival (at 3 years, 84% vs. 65.1% in the control group, P = 0.001), with less de novo DSA formation and no increase in the rate of biopsy-proven ACR.80 However, the role of belatacept conversion in this patient population still needs further study, given that a recent retrospective single-center study of 48 patients with chronic AMR failed to show any improvement in mean GFR or de novo DSA incidence in patients converted to belatacept versus maintained on tacrolimus/MMF/prednisone immunosuppression.86 Future studies may help identify patients likely to benefit most from belatacept conversion rescue therapy depending on offending etiology and preexisting degree of renal allograft dysfunction and injury. At least 1 study suggesting that patients with higher levels of proteinuria at time of conversion may be less likely to benefit from belatacept treatment supports the possibility that certain instances of allograft injury may be irreversible and beyond benefiting from belatacept conversion.84
The Future
Reducing the Logistical Barriers to Belatacept Adoption
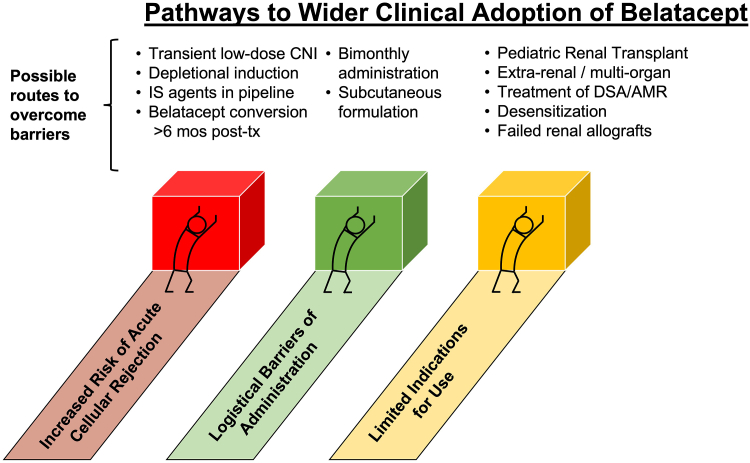
The future of costimulatory blockade immunosuppression in transplantation will likely proceed along 2 fronts: progress in surmounting barriers to wider clinical adoption of belatacept, as well as expansion into new clinical indications (Figure 2). Several promising developments offer hope of addressing the logistical barriers to the wider clinical utilization of belatacept.
Figure 2.
Current obstacles to the widespread clinical adoption of belatacept, and potential avenues to surmount these obstacles and expand belatacept clinical use. AMR, antibody-mediated rejection; CNI, calcineurin inhibitor; DSA, donor-specific antibody.
Every-2 Month Dosing
Although some transplant centers have struggled to provide a clinical infrastructure for monthly intravenous infusions of transplant patients with belatacept, this might be partially addressed if bimonthly rather than monthly infusions of belatacept maintenance therapy were possible. Several RCTs have compared monthly infusion of belatacept to infusion every 2 months. The phase 2 IM103-100 study randomized renal transplant recipients at 3 to 6 months posttransplant to receive maintenance belatacept either every 4 weeks or every 8 weeks. Estimated mean eGFR values at year 10 were identical for the monthly versus bimonthly belatacept maintenance groups (and significantly better than groups treated with cyclosporine-based immunosuppression), even though there was a nonsignificant trend toward increased rates of biopsy-proven rejection with patients administered belatacept only every 8 weeks.25 However, all these rejections occurred early (within the first year) and it was unclear if the heightened risk of rejection could really be attributed to the interval of belatacept administration due to the double randomization trial design and lack of sufficient power. An additional RCT compared monthly versus every 2 months infusion of belatacept in immunologically low-risk transplant recipients at least 1 year posttransplant, finding that patient and allograft survival with bimonthly therapy was noninferior to monthly treatment in this patient population, although there was a higher incidence of acute rejection associated with medical nonadherence in the group infused every 2 months.87 Promising 3-year follow-up data demonstrated continued maintenance of renal function without significant additional immunologic events in the patients administered belatacept every 2 months.88 Further studies are needed to better define which transplant recipients might be good candidates for this lower dose-intensive belatacept maintenance therapy, as well as to characterize the ideal timing to start spacing out belatacept infusions and the optimal monitoring of graft function in patients treated with bimonthly belatacept maintenance.
Subcutaneous Administration
Another central logistical barrier to wider utilization of costimulation blockade is that currently, belatacept requires intravenous infusion, which few transplant centers have the clinical infrastructure to provide at a large scale. However, whereas no subcutaneous formulations of belatacept exist, its precursor drug, abatacept (humanized CTLA-4-Ig) is available in subcutaneous formulations for use in rheumatoid arthritis.89 Several investigators have explored whether subcutaneous abatacept may be effective for long-term maintenance transplant immunosuppression, obviating the need for frequent intravenous infusions of belatacept. We reported a series of 9 CNI-intolerant renal transplant recipients who were converted early after transplant to abatacept as rescue immunosuppression during periods of belatacept unavailability. There was 100% patient and graft survival (median 115 months posttransplant follow-up) after conversion to abatacept.90 Pandemic-related limitations on monthly infusion therapy also led some transplant centers to attempt subcutaneous abatacept rescue therapy in kidney transplant recipients, with similar good results.91 The efficacy of subcutaneous abatacept maintenance immunosuppression was also demonstrated in a phase 2 trial of abatacept to prevent graft-versus-host disease in hematopoietic cell transplant recipients,92 a trial that culminated in an US Food and Drug Administration–approved indication for abatacept in this setting. We are currently recruiting for a randomized controlled phase 2b conversion trial, studying whether patients receiving monthly intravenous belatacept infusions after renal transplant can be converted to subcutaneous injections of abatacept (ClinicalTrials.gov NCT04955366).
Expanded Clinical Indications for Belatacept
Pediatric Transplantation
Although only approved currently for isolated adult renal transplant recipients, several case series illustrate a potential to expand the clinical indications of belatacept in transplantation. Several centers have published case reports of the use of belatacept in adolescent and pediatric renal transplant recipients.93, 94, 95, 96 Belatacept offers several unique benefits in this patient population. First, the benefits of belatacept on long-term eGFR may be augmented in a pediatric population due to the longer period of time they would otherwise be subjected to nephrotoxic CNI drugs compared to adult recipients. Second, adherence to immunosuppression is a notorious challenge in pediatric populations, and a drug such as belatacept requiring supervised monthly infusion may offer distinct adherence benefits compared to CNI, which require daily administration. However, the requirement that belatacept recipients be EBV-seropositive to mitigate risks of posttransplant lymphoproliferative disease may limit the application of belatacept in younger children, given that only 54% of children between the ages of 6 and 8 years are estimated to be EBV-seropositive versus 83% of children aged 18 to 19 years.97
Extrarenal and Multiorgan Transplantation
In addition to its potential indications in pediatric patients, belatacept may have some exciting potential in multiorgan and extrarenal transplant recipients. Although the initial multicenter trial of belatacept in liver transplant patients was terminated due to an increased risk of the composite outcome (rejection, graft loss, or death) within 6 months of transplant,98 we reported the Emory experience with 8 patients who underwent kidney-after-liver transplantation who were treated with belatacept-based immunosuppression (in conjunction with transient CNI therapy).99 No episodes of rejection, major systemic infection, or graft loss were observed, and all these patients demonstrated preserved liver and excellent renal allograft function, with 3 of the patients weaned completely off CNI and another 3 poised to transition off CNI at time of publication. Successful use of belatacept has also been published for kidney-after-heart transplantation,100 isolated heart transplant,101 and conversion trials in lung transplant recipients who developed renal insufficiency or hemolytic uremic syndrome on tacrolimus.102, 103, 104 Although 1 report of successful use of belatacept in 2 pancreas transplant patients was published,105 a later phase 2 multicenter RCT of simultaneous pancreas kidney recipients randomized to tacrolimus-based therapy or an investigational arm with low-dose CNI plus belatacept with intended CNI withdrawal was prematurely terminated due to a high rate of pancreas allograft rejection in the investigational arm following CNI withdrawal.106 A pilot study of de novo belatacept use in lung transplantation was also terminated early, after 5 of 13 patients in the belatacept-arm died compared to none in the control arm with conventional tacrolimus-based immunosuppression.107 Therefore, the optimal use of belatacept in extrarenal or multiorgan transplant recipients remains poorly defined. Although it may have a role in select patients who demonstrate intolerance of CNI or mTOR inhibitors, the use of belatacept as de novo immunosuppression in these patients should be approached with caution and may be better utilized initially as conversion therapy.
AMR
Belatacept may also have a role in treating renal transplant patients with early AMR. Based on promising data in murine transplant models, Jain and colleagues reported a pilot investigation of 6 renal transplant recipients with active AMR refractory to conventional therapy (plasmapheresis, steroids, and IVIG) who were treated with a combination of belatacept and bortezomib, a protease inhibitor thought to target plasma cells. They found that all 6 of these patients resolved their AMR and had sustained disappearance of circulating DSA for ≤30 months.108 Some preliminary evidence suggests that belatacept may also have efficacy in reducing HLA class I antibodies in highly sensitized recipients, suggesting that pretransplant treatment of highly sensitized transplant candidates with belatacept may potentially improve their pretransplant compatibility with organ donors. In a study of 72 highly-sensitized kidney transplant candidates (cPRA 98%–100%) who underwent transplant, 60 patients were maintained on belatacept immunosuppression for at least 6 months, and these patients showed a significant reduction in third-party HLA class I antibodies by FlowPRA compared to those recipients not treated with belatacept (P < 0.0009).109 Luminex single-antigen bead analysis was conducted on pretransplant and posttransplant sera from these highly-sensitized belatacept-treated renal transplant recipients, and whereas the pretransplant cPRA values ranged from 98% to 100% in all patients, 50% of the posttransplant cPRA were <98%, and 18% of patients had cPRA values <90.109 Nonhuman primate transplant models have also demonstrated the potential of desensitization with combined belatacept and proteasome inhibitors such as bortezomib110 and carfilzomib,111 supporting a phase 1/2 pilot trial (ADAPT Study, Clinicaltrials.gov NCT05017545) evaluating the ability of carfilzomib and belatacept to desensitize highly sensitized kidney transplant waitlist candidates to facilitate HLA compatible transplantation. Several additional clinical trials testing similar belatacept-based desensitization protocols with daratumumab (anti-CD38 plasma cell depletion) and bortezomib (a proteosome inhibitor) are also ongoing and recruiting (Clinicaltrials.gov NCT04827979, NCT05345717).
Failed Renal Allografts
Another potential expanded clinical indication for belatacept includes treatment of patients with failed renal allografts to prevent the development of de novo DSA that would preclude retransplant. The BENEFIT and BENEFIT-EXT trials both demonstrated the efficacy of belatacept in preventing the establishment of de novo DSA in patients with working renal allografts.32 This observation spurred a pilot randomized clinical trial at the Emory Transplant Center, comparing patients with failed renal allografts who were randomized to either immunosuppression discontinuation or to belatacept monotherapy at the time of dialysis reinitiation.112 After 36 months follow-up, there was no difference in the number of patients who developed DSA in both groups; however, belatacept monotherapy was associated with a delay in the onset of DSA (median 2.5 vs. 13.5 months, P = 0.0067) and complete prevention of the development of third-party alloantibody that often precludes retransplantation. Although larger studies are needed, these results highlight a potential role of belatacept maintenance in patients with failed renal allografts who are otherwise potential candidates for retransplantation.
Optimization of Belatacept Conversion Strategies
Overall, whether per protocol or as rescue therapy, conversion to belatacept has consistently resulted in improved renal function compared to maintenance on CNI therapy and is associated with an acceptably low risk of rejection, especially at later time-points post-transplant. The ideal strategy of belatacept conversion from CNI is currently unclear; indeed, one recent report found 13 distinct belatacept conversion protocols in place just within the 8 transplant centers affiliated with Kaiser Permanente Southern California.113 No current consensus exists to dictate the timing of belatacept conversions (early or late posttransplant), the ideal “loading” dose and frequency of belatacept administration, or the rapidity of tacrolimus taper.
The ideal timing of belatacept conversion has been examined by at least 2 large retrospective studies, comparing “early” (<3 month posttransplant) to “late” (>3 month posttransplant) conversions.114,115 Although one European multicenter study of 219 belatacept conversion patients found that early conversion was associated with a more significant increase in eGFR than later conversion, this was counterbalanced by a markedly higher rate of ACR in early conversions (33.3% vs. 4.3%, P < 0.0001).114 A nonsignificant increase in acute rejection with early belatacept conversions was also found in the other study (20% vs. 9.8%, P = 0.14).115 A randomized trial of early protocol belatacept conversion in patients with early steroid withdrawal also found a prohibitive rate of ACR associated with early belatacept conversion, resulting in the early closure of that treatment arm (interestingly, the group receiving belatacept and low-dose tacrolimus had no episodes of rejection and an 8.8 ml/min per 1.73 m2 increase in eGFR compared to a −0.38 ml/min per 1.73 m2 drop in the group maintained on tacrolimus + MMF).116 Therefore, the timing of belatacept conversion likely must balance the risk of a higher ACR rate associated with early conversions against the risks of late conversion, namely that more time on CNI risks the development of irreversible histologic changes in the kidney. Based on our published experience at Emory with de novo belatacept and transient CNI therapy,38 we have observed that early CNI withdrawal (less than 6 months posttransplant) is associated with prohibitively higher rates of ACR; thus, we begin to wean off the low-dose tacrolimus we provide our belatacept-treated patients at 9 months posttransplant. The phase 2 and 3 conversion trials, published literature, and the Emory experience indicate protocol conversions should ideally occur no earlier than 6 months posttransplant to minimize rejection risk yet maintain long-term renal function benefits.
Both published and practiced belatacept conversion protocols differ significantly in both the initial “loading” of belatacept and the rapidity of CNI withdrawal.113 Many centers practice the protocol outlined by Budde et al.75 in their large randomized clinical trial of belatacept conversion, which called for administration of belatacept (5 mg/kg) every 2 weeks for the first 8 weeks, and then monthly thereafter as a maintenance regimen. In this study, the CNI dose was tapered to 40% to 60% by day 15 and 20% to 30% by day 22, and it was discontinued by 29 ± 3 days postrandomization. In their analysis of 13 different belatacept protocols practiced in Southern California, Yazdi and colleagues found that the dose and duration of belatacept “induction” was rather irrelevant on transplant outcome; however, that there was a trend toward higher rates of rejection in patients with lower tacrolimus exposure. They therefore advocated for a conversion protocol of 5 mg/kg belatacept induction given every 2 weeks for only 1 month, with a slow tacrolimus taper over at least 2 months.113 At Emory, we immediately stop CNI and dose belatacept per package insert for indications necessitating immediate conversion and discontinuation of CNI. For protocol conversions or those that require less urgent discontinuation of CNI, we dose belatacept monthly and wean CNI over at least 3 months or until at least 6 months posttransplant. Emerging evidence is also suggesting that serial immune monitoring with biomarkers such as urinary chemokine CXCL9117 or donor-derived cell-free DNA68 may help facilitate the safe transition from CNI to belatacept by identifying patients at higher risk of ACR on belatacept, although the results of these pilot studies remain to be validated in larger patient cohorts.
Novel Immunosuppression in the Development Pipeline
Selective CD28 Blockade
Besides the logistical barriers of belatacept administration, concerns regarding short-term increased risks of ACR have also constrained the clinical growth of costimulation blockade-based immunosuppression strategies. Numerous “third-generation” costimulation blockade drugs are currently in the clinical pipeline with hopes of addressing these concerns. Many of these new agents offer “selective” costimulation blockade, antagonizing CD28-CD80/86 interaction while leaving inhibitory signaling mediated by CTLA-4 interaction with CD80/86 unimpaired (unlike belatacept, which blocks both the activating and inhibitory pathways).13 Preclinical animal models support enhanced suppression of cellular and humoral alloimmunity by these selective costimulation blockade agents.118, 119, 120 FR104 is a novel antagonistic pegylated anti-CD28 Fab antibody fragment that selectively blocks CD28-CD80/86 interaction. It has been shown to have efficacy in nonhuman primate renal allograft transplant models,121 and it currently is in phase 1/2 studies in renal transplant patients at Nantes University Hospital in France (ClinicalTrials.gov NCT04837092). Lulizumab, a pegylated domain antibody against human CD28, has also shown efficacy in preclinical nonhuman primate transplant models122,123 and has undergone successful initial pharmacokinetic, pharmacodynamic, and safety profile analysis in human subjects.124 Whether selective CD28 blockade improves the efficacy of current costimulation blockade with belatacept remains to be determined in human transplant recipients.
CD40/CD154 Blockade
Beyond the CD28-CD80/86 costimulatory pathways, other therapeutics targeting the CD40-CD154 costimulatory pathway are also in preclinical study and have shown special promise as the cornerstone of immunosuppression in several preclinical xenotransplant models,125 and early phase efforts in kidney allotransplantation persist with Fc modified agents that do not precipitate platelet aggregation or thrombosis (ClinicalTrials.gov NCT05027906). The most notable of these studies is a phase 2a trial evaluating dual costimulation blockade with dazodalibep (Fc silent CD40L protein antagonist, VIB4920 or HZN4920) and belatacept with thymoglobulin induction for prophylaxis of kidney allograft rejection (NCT04046549).
Anti-CD2 Depletion With Siplizumab
Novel more selective induction therapies may also enhance the clinical efficacy of belatacept-based immunosuppression, limiting the development of costimulatory blockade-resistant rejection. One promising candidate is siplizumab, a humanized anti-CD2 monoclonal that has been shown to selectively expand alloreactive regulatory T cells while depleting effector memory T cells,126 which are thought to be prime mediators of costimulatory blockade-resistant rejection.127 Siplizumab combination therapy with belatacept or abatacept broadly inhibited human T cell alloreactivity128 and was found to be safe in initial phase 1 dosing studies in renal transplant recipients.129 Future trials combining siplizumab induction with costimulatory blockade maintenance therapy are underway and may introduce a promising approach to achieve de novo CNI-free immunosuppression with improved acute rejection rates (ClinicalTrials.gov NCT05669001).
Conclusion
More than 10 years of clinical experience with belatacept in transplant recipients have yielded many insights into the potential of costimulatory blockade-based immunosuppression to secure improved long-term allograft function and patient outcomes. Belatacept offers one of the most promising opportunities to achieve the transplant community’s goal of “one transplant for life,” securing superior long-term allograft function through CNI avoidance and prevention of de novo DSA formation. Although limitations of belatacept, namely an increased incidence and severity of early ACR, are now fully evident, experience with belatacept has highlighted strategies to incorporate belatacept safely into transplant immunosuppression regimens while mitigating this short-term risk of ACR. The limited clinical adoption of belatacept to date (despite the accruing evidence of its superior long-term efficacy) is also likely driven by logistical barriers of belatacept administration. New approaches to addressing these barriers may promote increased adoption of belatacept in the future, both for de novo use and for conversion in renal transplant recipients experiencing allograft dysfunction and the negative sequelae of long-term CNI usage. As these optimization strategies continue to evolve, increased use of belatacept in clinical practice might also be facilitated by resumption of manufacturer support by way of reintroduction of clinical specialists and investigator-initiated studies to reinvigorate the development of strategies focused on optimizing belatacept. Improved synergy between the pharmaceutical industry and transplant community leadership will be necessary to achieve the ultimate goal of improving long-term outcomes in transplantation.
Disclosure
WHK has declared no conflicting interest. CPL has received grant support from Bristol Myers Squibb; and consulting fees from Eledon Pharmaceuticals; and has served on the CareDx Scientific Advisory Board. IRB has received grant support from Bristol Myers Squibb and consulting fees from Veloxis Pharmaceuticals.
References
- 1.Lamb K.E., Lodhi S., Meier-Kriesche H.U. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 2.Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–2243. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Malat G., Culkin C. The ABCs of immunosuppression: a primer for primary care physicians. Med Clin North Am. 2016;100:505–518. doi: 10.1016/j.mcna.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Naesens M., Kuypers D.R., Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 5.Larsen C.P., Elwood E.T., Alexander D.Z., et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 6.Lin H., Bolling S.F., Linsley P.S., et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson T.C., Alexander D.Z., Winn K.J., Linsley P.S., Lowry R.P., Larsen C.P. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. doi: 10.1097/00007890-199457120-00002. [DOI] [PubMed] [Google Scholar]
- 8.Koyama I., Kawai T., Andrews D., et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 9.Larsen C.P., Pearson T.C., Adams A.B., et al. Rational development of LEA29Y (Belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F., Rostaing L., Grinyo J., et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 11.Vincenti F., Charpentier B., Vanrenterghem Y., et al. A phase III study of Belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 12.Curtsinger J.M., Mescher M.F. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford M.L., Adams A.B., Pearson T.C. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10:14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magee C.N., Boenisch O., Najafian N. The role of costimulatory molecules in directing the functional differentiation of alloreactive T helper cells. Am J Transplant. 2012;12:2588–2600. doi: 10.1111/j.1600-6143.2012.04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dall’Era M., CTLA D.J. CTLA4Ig: a novel inhibitor of costimulation. Lupus. 2004;13:372–376. doi: 10.1191/0961203303lu1029oa. [DOI] [PubMed] [Google Scholar]
- 16.Adams A.B., Ford M.L., Larsen C.P. Costimulation blockade in autoimmunity and transplantation: the CD28 pathway. J Immunol. 2016;197:2045–2050. doi: 10.4049/jimmunol.1601135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levisetti M.G., Padrid P.A., Szot G.L., et al. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159:5187–5191. doi: 10.4049/jimmunol.159.11.5187. [DOI] [PubMed] [Google Scholar]
- 18.Kirk A.D., Harlan D.M., Armstrong N.N., et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincenti F., Larsen C., Durrbach A., et al. Costimulation blockade with Belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 20.Pestana J.O., Grinyo J.M., Vanrenterghem Y., et al. Three-year outcomes from BENEFIT-EXT: a phase III study of Belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012;12:630–639. doi: 10.1111/j.1600-6143.2011.03914.x. [DOI] [PubMed] [Google Scholar]
- 21.Durrbach A., Pestana J.M., Pearson T., et al. A phase III study of Belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10:547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 22.Vincenti F., Larsen C.P., Alberu J., et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 23.Durrbach A., Pestana J.M., Florman S., et al. Long-term outcomes in Belatacept- versus cyclosporine-treated recipients of extended criteria donor kidneys: final results from BENEFIT-EXT, a Phase III randomized study. Am J Transplant. 2016;16:3192–3201. doi: 10.1111/ajt.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archdeacon P., Dixon C., Belen O., Albrecht R., Meyer J. Summary of the US FDA approval of Belatacept. Am J Transplant. 2012;12:554–562. doi: 10.1111/j.1600-6143.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- 25.Vincenti F., Blancho G., Durrbach A., et al. Ten-year outcomes in a randomized phase II study of kidney transplant recipients administered Belatacept 4-weekly or 8-weekly. Am J Transplant. 2017;17:3219–3227. doi: 10.1111/ajt.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbels F., Wong S., Min Y., Sam J., Kalsekar A. Beneficial effect of Belatacept on health-related quality of life and perceived side effects: results from the BENEFIT and BENEFIT-EXT trials. Transplantation. 2014;98:960–968. doi: 10.1097/TP.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 27.Vanrenterghem Y., Bresnahan B., Campistol J., et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 28.Muller M.M., Schwaiger E., Kurnikowski A., et al. Glucose metabolism after kidney transplantation: insulin release and sensitivity with tacrolimus- versus Belatacept-based immunosuppression. Am J Kidney Dis. 2021;77:462–464. doi: 10.1053/j.ajkd.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Soveri I., Snyder J., Holdaas H., et al. The external validation of the cardiovascular risk equation for renal transplant recipients: applications to BENEFIT and BENEFIT-EXT trials. Transplantation. 2013;95:142–147. doi: 10.1097/TP.0b013e31827722c9. [DOI] [PubMed] [Google Scholar]
- 30.Bredewold O.W., Chan J., Svensson M., et al. Cardiovascular risk following conversion to Belatacept from a calcineurin inhibitor in kidney transplant recipients: a randomized clinical trial. Kidney Med. 2023;5 doi: 10.1016/j.xkme.2022.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons R.F., Larsen C.P., Pearson T.C., Badell I.R. Belatacept and CD28 costimulation blockade: preventing and reducing alloantibodies over the long term. Curr Transplant Rep. 2019;6:277–284. doi: 10.1007/s40472-019-00260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bray R.A., Gebel H.M., Townsend R., et al. De novo donor-specific antibodies in Belatacept-treated vs cyclosporine-treated kidney-transplant recipients: post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. 2018;18:1783–1789. doi: 10.1111/ajt.14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leibler C., Thiolat A., Henique C., et al. Control of humoral response in renal transplantation by Belatacept depends on a direct effect on B cells and impaired T follicular helper-B cell crosstalk. J Am Soc Nephrol. 2018;29:1049–1062. doi: 10.1681/ASN.2017060679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bray R.A., Gebel H.M., Townsend R., et al. Posttransplant reduction in preexisting donor-specific antibody levels after Belatacept- versus cyclosporine-based immunosuppression: post hoc analyses of BENEFIT and BENEFIT-EXT. Am J Transplant. 2018;18:1774–1782. doi: 10.1111/ajt.14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leibler C., Matignon M., Moktefi A., et al. Belatacept in renal transplant recipient with mild immunologic risk factor: a pilot prospective study (Belacor) Am J Transplant. 2019;19:894–906. doi: 10.1111/ajt.15229. [DOI] [PubMed] [Google Scholar]
- 36.Mayer A.D., Dmitrewski J., Squifflet J.P., et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64:436–443. doi: 10.1097/00007890-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 37.de Graav G.N., Baan C.C., Clahsen-van Groningen M.C., et al. A randomized controlled clinical trial comparing Belatacept with tacrolimus after de novo kidney transplantation. Transplantation. 2017;101:2571–2581. doi: 10.1097/TP.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 38.Adams A.B., Goldstein J., Garrett C., et al. Belatacept combined with transient calcineurin inhibitor therapy prevents rejection and promotes improved long-term renal allograft function. Am J Transplant. 2017;17:2922–2936. doi: 10.1111/ajt.14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karadkhele G., Duneton C., Garro R., et al. Temporal trends and current use of de novo Belatacept in kidney transplant recipients in the United States. Clin Transpl. 2022;36 doi: 10.1111/ctr.14531. [DOI] [PubMed] [Google Scholar]
- 40.Lentine K.L., Smith J.M., Hart A., et al. OPTN/SRTR 2020 annual data report: kidney. Am J Transplant. 2022;22(suppl 2):21–136. doi: 10.1111/ajt.16982. [DOI] [PubMed] [Google Scholar]
- 41.Kirk A.D., Adams A.B., Durrbach A., et al. Optimization of de novo Belatacept-based immunosuppression administered to renal transplant recipients. Am J Transplant. 2021;21:1691–1698. doi: 10.1111/ajt.16386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferguson R., Grinyo J., Vincenti F., et al. Immunosuppression with Belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11:66–76. doi: 10.1111/j.1600-6143.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 43.Kirk A.D., Guasch A., Xu H., et al. Renal transplantation using Belatacept without maintenance steroids or calcineurin inhibitors. Am J Transplant. 2014;14:1142–1151. doi: 10.1111/ajt.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitz R., Fitch Z.W., Xu H., et al. Kidney transplantation using alemtuzumab, Belatacept, and sirolimus: five-year follow-up. Am J Transplant. 2020;20:3609–3619. doi: 10.1111/ajt.16121. [DOI] [PubMed] [Google Scholar]
- 45.Newell K.A., Mehta A.K., Larsen C.P., et al. Lessons learned: early termination of a randomized trial of calcineurin inhibitor and corticosteroid avoidance using Belatacept. Am J Transplant. 2017;17:2712–2719. doi: 10.1111/ajt.14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mannon R.B., Armstrong B., Stock P.G., et al. Avoidance of CNI and steroids using Belatacept-Results of the Clinical Trials in Organ Transplantation 16 trial. Am J Transplant. 2020;20:3599–3608. doi: 10.1111/ajt.16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodle E.S., Kaufman D.B., Shields A.R., et al. Belatacept-based immunosuppression with simultaneous calcineurin inhibitor avoidance and early corticosteroid withdrawal: a prospective, randomized multicenter trial. Am J Transplant. 2020;20:1039–1055. doi: 10.1111/ajt.15688. [DOI] [PubMed] [Google Scholar]
- 48.Kaufman D.B., Woodle E.S., Shields A.R., et al. Belatacept for simultaneous calcineurin inhibitor and chronic corticosteroid immunosuppression avoidance: two-year results of a prospective, randomized multicenter trial. Clin J Am Soc Nephrol. 2021;16:1387–1397. doi: 10.2215/CJN.13100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peddi V.R., Marder B., Gaite L., et al. Treatment of de novo renal transplant recipients with calcineurin inhibitor-free, Belatacept plus everolimus-based immunosuppression. Transplant Direct. 2023;9:e1419. doi: 10.1097/TXD.0000000000001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin S.T., Powell J.T., Patel M., Tsapepas D. Risk of posttransplant lymphoproliferative disorder associated with use of Belatacept. Am J Health Syst Pharm. 2013;70:1977–1983. doi: 10.2146/ajhp120770. [DOI] [PubMed] [Google Scholar]
- 51.Cohen E.A., Mulligan D., Kulkarni S., Tichy E.M. De novo Belatacept in a human immunodeficiency virus-positive kidney transplant recipient. Am J Transplant. 2016;16:2753–2757. doi: 10.1111/ajt.13852. [DOI] [PubMed] [Google Scholar]
- 52.El Sakhawi K., Melica G., Scemla A., et al. Belatacept-based immunosuppressive regimen in HIV-positive kidney transplant recipients. Clin Kidney J. 2021;14:1908–1914. doi: 10.1093/ckj/sfaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santeusanio A., Bhansali A., De Boccardo G., et al. Conversion to Belatacept maintenance immunosuppression in HIV-positive kidney transplant recipients. Clin Transpl. 2020;34 doi: 10.1111/ctr.14041. [DOI] [PubMed] [Google Scholar]
- 54.Karadkhele G., Hogan J., Magua W., et al. CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with Belatacept. Am J Transplant. 2021;21:208–221. doi: 10.1111/ajt.16132. [DOI] [PubMed] [Google Scholar]
- 55.Magua W., Johnson A.C., Karadkhele G.M., et al. Impact of Belatacept and tacrolimus on cytomegalovirus viral load control and relapse in moderate and high-risk cytomegalovirus serostatus kidney transplant recipients. Transpl Infect Dis. 2022;24 doi: 10.1111/tid.13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain A., Xu R., Venkataramanan R., et al. Plasmapheresis decreases Belatacept exposure: requires consideration for dose and frequency adjustments. Transplantation. 2021;105:e152–e153. doi: 10.1097/TP.0000000000003840. [DOI] [PubMed] [Google Scholar]
- 57.Chavarot N., Ouedrani A., Marion O., et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with Belatacept. Transplantation. 2021;105:e94–e95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- 58.Chavarot N., Morel A., Leruez-Ville M., et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with Belatacept. Am J Transplant. 2021;21:4043–4051. doi: 10.1111/ajt.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell J., Kim J., Alejo J.L., et al. Humoral and cellular immune response to a third dose of SARS-CoV-2 vaccine in kidney transplant recipients taking Belatacept. Transplantation. 2022;106:e264–e265. doi: 10.1097/TP.0000000000004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noble J., Langello A., Bouchut W., Lupo J., Lombardo D., Rostaing L. Immune response post-SARS-CoV-2 mRNA vaccination in kidney transplant recipients receiving Belatacept. Transplantation. 2021;105:e259–e260. doi: 10.1097/TP.0000000000003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ou M.T., Boyarsky B.J., Chiang T.P.Y., et al. Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking Belatacept. Transplantation. 2021;105:2119–2123. doi: 10.1097/TP.0000000000003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osmanodja B., Ronicke S., Budde K., et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J Clin Med. 2022;11:2565. doi: 10.3390/jcm11092565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey A.J.M., Maganti H.B., Cheng W., Shorr R., Arianne Buchan C., Allan D.S. Humoral and cellular response of transplant recipients to a third dose of mRNA SARS-CoV-2 vaccine: a systematic review and meta-analysis. Transplantation. 2023;107:204–215. doi: 10.1097/TP.0000000000004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathews D.V., Wakwe W.C., Kim S.C., et al. Belatacept-resistant rejection is associated with CD28(+) memory CD8 T cells. Am J Transplant. 2017;17:2285–2299. doi: 10.1111/ajt.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cortes-Cerisuelo M., Laurie S.J., Mathews D.V., et al. Increased pretransplant frequency of CD28(+) CD4(+) T(EM) predicts Belatacept-resistant rejection in human renal transplant recipients. Am J Transplant. 2017;17:2350–2362. doi: 10.1111/ajt.14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinosa J., Herr F., Tharp G., et al. CD57(+) CD4 T cells underlie Belatacept-resistant allograft rejection. Am J Transplant. 2016;16:1102–1112. doi: 10.1111/ajt.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oellerich M., Sherwood K., Keown P., et al. Liquid biopsies: donor-derived cell-free DNA for the detection of kidney allograft injury. Nat Rev Nephrol. 2021;17:591–603. doi: 10.1038/s41581-021-00428-0. [DOI] [PubMed] [Google Scholar]
- 68.Osmanodja B., Akifova A., Oellerich M., et al. Donor-derived cell-free DNA for kidney allograft surveillance after conversion to Belatacept: prospective pilot study. J Clin Med. 2023;12:2437. doi: 10.3390/jcm12062437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiebe C., Nickerson P.W. More precise donor-recipient matching: the role of eplet matching. Curr Opin Nephrol Hypertens. 2020;29:630–635. doi: 10.1097/MNH.0000000000000649. [DOI] [PubMed] [Google Scholar]
- 70.Koppula S., Yost S.E., Sussman A., Bracamonte E.R., Kaplan B. Successful conversion to Belatacept after thrombotic microangiopathy in kidney transplant patients. Clin Transpl. 2013;27:591–597. doi: 10.1111/ctr.12170. [DOI] [PubMed] [Google Scholar]
- 71.Wojciechowski D., Chandran S., Vincenti F. Early post-transplant conversion from tacrolimus to Belatacept for prolonged delayed graft function improves renal function in kidney transplant recipients. Clin Transpl. 2017;31 doi: 10.1111/ctr.12930. [DOI] [PubMed] [Google Scholar]
- 72.Rostaing L., Massari P., Garcia V.D., et al. Switching from calcineurin inhibitor-based regimens to a Belatacept-based regimen in renal transplant recipients: a randomized phase II study. Clin J Am Soc Nephrol. 2011;6:430–439. doi: 10.2215/CJN.05840710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grinyo J., Alberu J., Contieri F.L., et al. Improvement in renal function in kidney transplant recipients switched from cyclosporine or tacrolimus to Belatacept: 2-year results from the long-term extension of a phase II study. Transpl Int. 2012;25:1059–1064. doi: 10.1111/j.1432-2277.2012.01535.x. [DOI] [PubMed] [Google Scholar]
- 74.Grinyo J.M., Del Carmen Rial M., Alberu J., et al. Safety and efficacy outcomes 3 years after switching to Belatacept from a calcineurin inhibitor in kidney transplant recipients: results from a phase 2 randomized trial. Am J Kidney Dis. 2017;69:587–594. doi: 10.1053/j.ajkd.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Budde K., Prashar R., Haller H., et al. Conversion from calcineurin Inhibitor to Belatacept-based Maintenance immunosuppression in Renal Transplant Recipients: a Randomized Phase 3b Trial. J Am Soc Nephrol. 2021;32:3252–3264. doi: 10.1681/ASN.2021050628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Hennawy H., Safar O., Al Faifi A.S., et al. Belatacept rescue therapy of CNI-induced nephrotoxicity, meta-analysis. Transplant Rev (Orlando) 2021;35 doi: 10.1016/j.trre.2021.100653. [DOI] [PubMed] [Google Scholar]
- 77.Gupta G., Regmi A., Kumar D., et al. Safe conversion from tacrolimus to Belatacept in high immunologic risk kidney transplant recipients with allograft dysfunction. Am J Transplant. 2015;15:2726–2731. doi: 10.1111/ajt.13322. [DOI] [PubMed] [Google Scholar]
- 78.Gupta G., Raynaud M., Kumar D., et al. Impact of Belatacept conversion on kidney transplant function, histology, and gene expression-a single-center study. Transpl Int. 2020;33:1458–1471. doi: 10.1111/tri.13718. [DOI] [PubMed] [Google Scholar]
- 79.Kumar D., Raynaud M., Chang J., et al. Impact of Belatacept conversion on renal function, histology, and gene expression in kidney transplant patients with chronic active antibody-mediated rejection. Transplantation. 2021;105:660–667. doi: 10.1097/TP.0000000000003278. [DOI] [PubMed] [Google Scholar]
- 80.Bertrand D., Matignon M., Morel A., et al. Belatacept rescue conversion in kidney transplant recipients with vascular lesions (Banff cv score >2): a retrospective cohort study. Nephrol Dial Transplant. 2023;38:481–490. doi: 10.1093/ndt/gfac178. [DOI] [PubMed] [Google Scholar]
- 81.Perez-Saez M.J., Yu B., Uffing A., et al. Conversion from tacrolimus to Belatacept improves renal function in kidney transplant patients with chronic vascular lesions in allograft biopsy. Clin Kidney J. 2019;12:586–591. doi: 10.1093/ckj/sfy115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulte K., Vollmer C., Klasen V., et al. Late conversion from tacrolimus to a Belatacept-based immuno-suppression regime in kidney transplant recipients improves renal function, acid-base derangement and mineral-bone metabolism. J Nephrol. 2017;30:607–615. doi: 10.1007/s40620-017-0411-0. [DOI] [PubMed] [Google Scholar]
- 83.Choi M., Bachmann F., Wu K., et al. Microvascular inflammation is a risk factor in kidney transplant recipients with very late conversion from calcineurin inhibitor-based regimens to Belatacept. BMC Nephrol. 2020;21:354. doi: 10.1186/s12882-020-01992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durr M., Lachmann N., Zukunft B., Schmidt D., Budde K., Brakemeier S. Late conversion to Belatacept after kidney transplantation: outcome and prognostic factors. Transplant Proc. 2017;49:1747–1756 e1741. doi: 10.1016/j.transproceed.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 85.Gupta S., Rosales I., Wojciechowski D. Pilot analysis of late conversion to Belatacept in kidney transplant recipients for biopsy-proven chronic tacrolimus toxicity. J Transplant. 2018;2018 doi: 10.1155/2018/1968029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moein M., Gao S.X., Martin S.J., et al. Conversion to Belatacept in kidney transplant recipients with chronic antibody-mediated rejection (CAMR) Transpl Immunol. 2022;76 doi: 10.1016/j.trim.2022.101737. [DOI] [PubMed] [Google Scholar]
- 87.Badell I.R., Parsons R.F., Karadkhele G., et al. Every 2-month Belatacept maintenance therapy in kidney transplant recipients greater than 1-year posttransplant: a randomized, noninferiority trial. Am J Transplant. 2021;21:3066–3076. doi: 10.1111/ajt.16538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson A.C., Karadkhele G.M., Shenvi N., Easley K.A., Larsen C.P., Badell I.R. Three-year Outcomes after Conversion from Monthly to Every 2-month Belatacept Maintenance Therapy in Kidney Transplant Recipients: results from a randomized controlled trial. Transplant Direct. 2023;9:e1449. doi: 10.1097/TXD.0000000000001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schiff M. Subcutaneous abatacept for the treatment of rheumatoid arthritis. Rheumatol (Oxf Engl) 2013;52:986–997. doi: 10.1093/rheumatology/ket018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Badell I.R., Karadkhele G.M., Vasanth P., Farris A.B., Robertson J.M., Larsen C.P. Abatacept as rescue immunosuppression after calcineurin inhibitor treatment failure in renal transplantation. Am J Transplant. 2019;19:2342–2349. doi: 10.1111/ajt.15319. [DOI] [PubMed] [Google Scholar]
- 91.Uro-Coste C., Atenza A., Heng A.E., Rouzaire P.O., Garrouste C. Abatacept rescue therapy in kidney transplant recipients: a case series of five patients. Transpl Int. 2022;35 doi: 10.3389/ti.2022.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watkins B., Qayed M., McCracken C., et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021;39:1865–1877. doi: 10.1200/JCO.20.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta C., Petyak C., Sgambat K., Moudgil A. Belatacept conversion in adolescent and young adult kidney transplant recipients. Clin Transpl. 2022;37 doi: 10.1111/ctr.14882. [DOI] [PubMed] [Google Scholar]
- 94.Lerch C., Kanzelmeyer N.K., Ahlenstiel-Grunow T., et al. Belatacept after kidney transplantation in adolescents: a retrospective study. Transpl Int. 2017;30:494–501. doi: 10.1111/tri.12932. [DOI] [PubMed] [Google Scholar]
- 95.Blew K.H., Chua A., Foreman J., et al. Tailored use of Belatacept in adolescent kidney transplantation. Am J Transplant. 2020;20:884–888. doi: 10.1111/ajt.15611. [DOI] [PubMed] [Google Scholar]
- 96.Soraru J., Sheriff D., Rajakaruna R., Larkins N. Belatacept in the treatment of acute T-cell mediated rejection and maintenance immunosuppression in a paediatric kidney transplant recipient. Nephrol (Carlton) 2022;27:641–642. doi: 10.1111/nep.14036. [DOI] [PubMed] [Google Scholar]
- 97.Dowd J.B., Palermo T., Brite J., McDade T.W., Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6-19, 2003-2010. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klintmalm G.B., Feng S., Lake J.R., et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. 2014;14:1817–1827. doi: 10.1111/ajt.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cristea O., Karadkhele G., Kitchens W.H., Vasanth P., Larsen C.P., Badell I.R. Belatacept conversion in kidney after liver transplantation. Transplant Direct. 2021;7 doi: 10.1097/TXD.0000000000001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schenk A.D., Anderson D.J., Cole R.T., Badell I.R., Larsen C.P. De novo Belatacept in a kidney-after-heart transplant recipient. Transplant Direct. 2020;6:e515. doi: 10.1097/TXD.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Launay M., Guitard J., Dorent R., et al. Belatacept-based immunosuppression: a calcineurin inhibitor-sparing regimen in heart transplant recipients. Am J Transplant. 2020;20:553–563. doi: 10.1111/ajt.15584. [DOI] [PubMed] [Google Scholar]
- 102.Timofte I., Terrin M., Barr E., et al. Belatacept for renal rescue in lung transplant patients. Transpl Int. 2016;29:453–463. doi: 10.1111/tri.12731. [DOI] [PubMed] [Google Scholar]
- 103.Hui C., Kern R., Wojciechowski D., et al. Belatacept for maintenance immunosuppression in lung transplantation. J Investig Med High Impact Case Rep. 2014;2 doi: 10.1177/2324709614546866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iasella C.J., Winstead R.J., Moore C.A., et al. Maintenance Belatacept-based immunosuppression in lung transplantation recipients who failed calcineurin inhibitors. Transplantation. 2018;102:171–177. doi: 10.1097/TP.0000000000001873. [DOI] [PubMed] [Google Scholar]
- 105.Mujtaba M.A., Sharfuddin A.A., Taber T., et al. Conversion from tacrolimus to Belatacept to prevent the progression of chronic kidney disease in pancreas transplantation: case report of two patients. Am J Transplant. 2014;14:2657–2661. doi: 10.1111/ajt.12863. [DOI] [PubMed] [Google Scholar]
- 106.Stock P.G., Mannon R.B., Armstrong B., et al. Challenges of calcineurin inhibitor withdrawal following combined pancreas and kidney transplantation: results of a prospective, randomized clinical trial. Am J Transplant. 2020;20:1668–1678. doi: 10.1111/ajt.15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang H.J., Schechtman K., Askar M., et al. A pilot randomized controlled trial of de novo Belatacept-based immunosuppression following anti-thymocyte globulin induction in lung transplantation. Am J Transplant. 2022;22:1884–1892. doi: 10.1111/ajt.17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jain D., Rajab A., Young J.S., et al. Reversing donor-specific antibody responses and antibody-mediated rejection with bortezomib and Belatacept in mice and kidney transplant recipients. Am J Transplant. 2020;20:2675–2685. doi: 10.1111/ajt.15881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parsons R.F., Zahid A., Bumb S., et al. The impact of Belatacept on third-party HLA alloantibodies in highly sensitized kidney transplant recipients. Am J Transplant. 2020;20:573–581. doi: 10.1111/ajt.15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burghuber C.K., Manook M., Ezekian B., et al. Dual targeting: combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant. 2019;19:724–736. doi: 10.1111/ajt.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ezekian B., Schroder P.M., Mulvihill M.S., et al. Pretransplant desensitization with costimulation blockade and proteasome inhibitor reduces DSA and delays antibody-mediated rejection in highly sensitized nonhuman primate kidney transplant recipients. J Am Soc Nephrol. 2019;30:2399–2411. doi: 10.1681/ASN.2019030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Badell I.R., Bray R.A., Elbein R., et al. Belatacept in kidney transplant recipients with failed allografts for the prevention of humoral sensitization: a pilot randomized controlled trial. Transplantation. 2021;105:e395–e396. doi: 10.1097/TP.0000000000003852. [DOI] [PubMed] [Google Scholar]
- 113.Yazdi M., Kahwaji J.M., Meguerditchian S., Lee R. Belatacept conversion protocols and outcomes in kidney transplant recipients. Transplant Proc. 2021;53:976–983. doi: 10.1016/j.transproceed.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 114.Darres A., Ulloa C., Brakemeier S., et al. Conversion to Belatacept in maintenance kidney transplant patients: a retrospective multicenter European study. Transplantation. 2018;102:1545–1552. doi: 10.1097/TP.0000000000002192. [DOI] [PubMed] [Google Scholar]
- 115.Morel A., Hoisnard L., Dudreuilh C., et al. Three-year outcomes in kidney transplant recipients switched from calcineurin inhibitor-based regimens to Belatacept as a rescue therapy. Transpl Int. 2022;35 doi: 10.3389/ti.2022.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tawhari I., Hallak P., Bin S., et al. Early calcineurin-inhibitor to Belatacept conversion in steroid-free kidney transplant recipients. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1096881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malvezzi P., Fischman C., Rigault G., et al. Switching renal transplant recipients to Belatacept therapy: results of a real-life gradual conversion protocol. Transpl Immunol. 2019;56 doi: 10.1016/j.trim.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 118.La Muraglia G.M., 2nd, Zeng S., Crichton E.S., Wagener M.E., Ford M.L., Badell I.R. Superior inhibition of alloantibody responses with selective CD28 blockade is CTLA-4 dependent and T follicular helper cell specific. Am J Transplant. 2021;21:73–86. doi: 10.1111/ajt.16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu D., Badell I.R., Ford M.L. Selective CD28 blockade attenuates CTLA-4-dependent CD8+ memory T cell effector function and prolongs graft survival. JCI Insight. 2018;3 doi: 10.1172/jci.insight.96378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Badell I.R., La Muraglia G.M., Liu D., Wagener M.E., Ding G., Ford M.L. Selective CD28 blockade results in superior inhibition of donor-specific T follicular helper cell and antibody responses relative to CTLA4-Ig. Am J Transplant. 2018;18:89–101. doi: 10.1111/ajt.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poirier N., Dilek N., Mary C., et al. FR104, an antagonist anti-CD28 monovalent Fab' antibody, prevents alloimmunization and allows calcineurin inhibitor minimization in nonhuman primate renal allograft. Am J Transplant. 2015;15:88–100. doi: 10.1111/ajt.12964. [DOI] [PubMed] [Google Scholar]
- 122.Schroder P.M., Schmitz R., Fitch Z.W., et al. Preoperative carfilzomib and lulizumab based desensitization prolongs graft survival in a sensitized non-human primate model. Kidney Int. 2021;99:161–172. doi: 10.1016/j.kint.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lovasik BP, Kim SC, Higginbotham L, et al. CD28-selective inhibition prolongs non-human primate kidney transplant survival. Preprint. Posted online May 5, 2023. bioRxiv. https://doi.org/10.1101/2023.05.03.539333
- 124.Shi R., Honczarenko M., Zhang S., et al. Pharmacokinetic, pharmacodynamic, and safety profile of a novel anti-CD28 domain antibody antagonist in healthy subjects. J Clin Pharmacol. 2017;57:161–172. doi: 10.1002/jcph.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh A.K., Goerlich C.E., Zhang T., Lewis B.G.T., Hershfeld A., Mohiuddin M.M. CD40-CD40L blockade: update on novel investigational therapeutics for transplantation. Transplantation. 2022;107:1472–1481. doi: 10.1097/TP.0000000000004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Podesta M.A., Binder C., Sellberg F., et al. Siplizumab selectively depletes effector memory T cells and promotes a relative expansion of alloreactive regulatory T cells in vitro. Am J Transplant. 2020;20:88–100. doi: 10.1111/ajt.15533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitchens W.H., Haridas D., Wagener M.E., et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. 2012;12:69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cvetkovski F., Razavi R., Sellberg F., Berglund E., Berglund D. Siplizumab combination therapy with Belatacept or abatacept broadly inhibits human T cell alloreactivity in vitro. Am J Transplant. 2023;23:1603–1611. doi: 10.1016/j.ajt.2023.05.032. [DOI] [PubMed] [Google Scholar]
- 129.Pruett T.L., McGory R.W., Wright F.H., Pescovitz M.D., Yang H., McClain J.B. Safety profile, pharmacokinetics, and pharmacodynamics of siplizumab, a humanized anti-CD2 monoclonal antibody, in renal allograft recipients. Transplant Proc. 2009;41:3655–3661. doi: 10.1016/j.transproceed.2009.06.226. [DOI] [PubMed] [Google Scholar]