Introduction
Focal segmental glomerulosclerosis (FSGS), a leading glomerular cause of kidney failure, is a histological pattern characterized by podocyte injury and depletion.1 FSGS is found in 20–30% of adults with nephrotic syndrome and up to 20% of patients receiving dialysis.2,S1 FSGS can be broadly categorized into 4 causative classifications: primary (idiopathic), secondary (adaptive), familial (genetic), and of unknown cause.1,3,S2 Depending on patient selection and region, genetic variants have been reported in up to 14% of individuals with adult-onset FSGS.4,S3
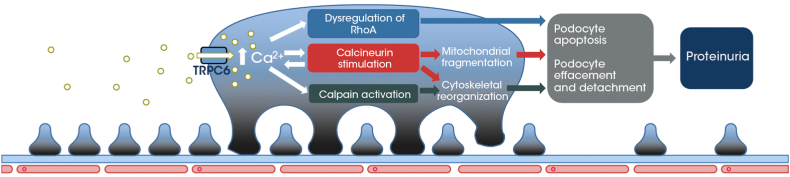
Familial FSGS has been associated with gain-of-function variants of the transient receptor potential cation channel, subfamily C, member 6 (TRPC6) gene (e.g., E897K, M132T, P112Q, and R895C),5,S1,S4–S8 with the resulting overexpression and/or overactivity of TRPC6 leading to podocyte injury.6,S4, S7 Specifically, TRPC6-mediated increases in podocyte intracellular calcium concentrations may result in cytoskeletal reorganization in podocytes, detachment of podocyte foot processes from the glomerular basement membrane, podocyte loss and depletion, and disturbance of the glomerular filtration barrier, resulting in proteinuria (Figure 1).6,S4,S9,S10 Data are now accruing to suggest that pharmacological inhibition of TRPC6 may reduce the severity of chronic kidney disease and related FSGS.5,S11
Figure 1.
Mechanism of action of TRPC6 overexpression/activity in podocytes. TRPC6 overexpression or increase in function can lead to uncontrolled Ca2+ influx into podocytes. This induces cytoskeletal reorganization, detachment of podocyte foot processes from the glomerular basement membrane, and podocyte depletion, resulting in proteinuria. Ca2+, calcium; TRPC6, transient receptor potential cation channel, subfamily C, member 6.
BI 764198 is a novel, potent, selective oral TRPC6 inhibitor. In 4 phase 1 studies (NCT03854552, NCT04102462, NCT04656288, and NCT04176536), BI 764198 was generally well tolerated at single and multiple rising doses administered once daily for up to 14 days. BI 764198 warrants further investigation to determine whether it can prevent or attenuate podocyte injury in patients with FSGS, and consequently reduce proteinuria in the context of this glomerulopathy.
This paper describes the study design and rationale for a phase 2 trial to investigate the potential effect of BI 764198, as a TRPC6 inhibitor, to lower proteinuria and act as a renoprotective agent in patients with primary FSGS or FSGS resulting from TRPC6 gene variants (TRPC6 monogenic FSGS). The trial registration numbers are as follows: EudraCT Number: 2020-000384-23; and ClinicalTrials.gov identifier: NCT05213624.
Trial Design
Study Design and Sites
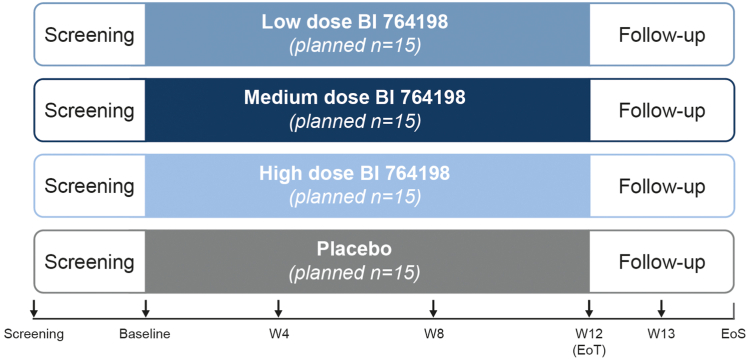
This multicenter, multinational, randomized, double-blind, parallel-group, placebo-controlled trial will assess the efficacy, safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of BI 764198 administered for 12 weeks in patients with primary or TRPC6 monogenic FSGS (NCT05213624; Figure 2). The trial will be conducted in approximately 60 sites across 11 countries.
Figure 2.
Study design. Patients will be randomized to receive 1 of 3 doses of BI 764198 (low, medium, or high) or placebo for 12 weeks. Patients will enter a follow-up period at the end of the treatment period, with follow-up visits at days 7 (week 13) and 30 (EoS) after the end of treatment. Colored boxes represent the treatment period and arrows indicate visits where endpoint measurements will be taken. EoS, end of study; EoT, end of treatment; W, week.
Study Participants
Adults aged 18–75 years with biopsy-proven primary or TRPC6 monogenic FSGS are eligible to be enrolled in this study. Key inclusion and exclusion criteria are provided in Table 1.
Table 1.
Key inclusion and exclusion criteria
| Inclusion Criteria | |
|---|---|
| All patients |
|
|
|
| |
| |
| Corticosteroid therapy |
|
| ACEi/ARB/finerenone/ASI/SGLT2i treatment |
|
| Exclusion criteria | |
| Diagnosis |
|
|
|
| |
| |
| |
| |
| |
| |
| |
| |
| Concomitant therapy |
|
|
|
| |
| |
| Safety laboratory findings |
|
|
|
ACEi, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; ASI, aldosterone synthase inhibitor; AST, aspartate aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; IgA, immunoglobulin A; SGLT2i, sodium-glucose cotransporter-2 inhibitor; TRPC6, transient receptor potential cation channel, subfamily C, member 6; ULN, upper limit of normal; UPCR, urinary protein:creatinine ratio.
Treatment
Following completion of all screening procedures (Supplementary Table S1), eligible patients will be randomized 1:1:1:1 to receive 1 of 3 doses of BI 764198 (low, medium, or high) or placebo daily over a 12-week treatment period.
Study sites in selected countries will have the option of integrating a decentralized clinical trial model, in which investigators can recruit patients from where they are licensed to practice. Decentralized study visits from screening to follow-up can be conducted outside of a dedicated healthcare or research facility. This will enable patients to participate in the trial remotely and complete the trial in their own homes or residences via telemedicine and a smartphone device.
Endpoints and Assessments
The primary endpoint of the trial is the proportion of patients reaching a ≥25% decrease in 24-hour urinary protein:creatinine ratio from baseline at week 12. Secondary endpoints comprise change in 24-hour urinary protein:creatinine ratio from baseline at weeks 12 and 13, change in 24-hour urinary protein excretion from baseline at week 12, and steady-state trough BI 764198 concentration at weeks 4 and 12. Additional efficacy endpoints comprise change in estimated glomerular filtration rate from baseline (visit 2) at weeks 12 and 13; change in urinary albumin:creatinine ratio from baseline at weeks 4, 8, 12, and 13; and change in urinary protein:creatinine ratio from spot urine over time compared with baseline. The pharmacodynamics and pharmacokinetics endpoints are provided in the Supplementary Methods. Safety endpoints comprise incidence of adverse events, serious adverse events, and adverse events leading to treatment discontinuation; vital signs; laboratory test results; 12-lead electrocardiogram findings; and ophthalmological assessments.
Randomization and Blinding
Patients will be randomized in blocks, stratified by the use of corticosteroids, to double-blind treatment with BI 764198 or placebo via interactive response technology using a pseudorandom number generator, arranged by the trial sponsor.
Statistical Analysis
A total of 60 patients are planned to be randomized, 15 per study arm, because this is sufficient to detect differences between the treatment groups and placebo, with respect to the primary endpoint. The primary endpoint will be analyzed by descriptive statistics. In addition, an analysis of variance model for the change from baseline at week 12 will be used to assess the differences across treatment groups. Further methodological detail is provided in the Supplementary Methods.
Discussion
Current treatments for FSGS are generally non-specific and focus on the reduction of blood pressure and proteinuria.7 Through its unique mechanism of action as a selective inhibitor of TRPC6, BI 764198 is expected to improve podocyte function and survival in patients with proteinuric glomerular diseases, such as FSGS. This trial in adults with primary or TRPC6 monogenic FSGS aims to show proof of clinical concept for BI 764198 and establish TRPC6 inhibition as a potential mechanism to reduce excessive proteinuria in patients. This would support further evaluation in phase 3 development. In addition, data on the reduction of proteinuria/albuminuria and stabilization of estimated glomerular filtration rate from the current study in FSGS could provide a basis for the investigation of BI 764198 and TRPC6 inhibition in other proteinuric diseases.
Several US study centers of the present clinical trial are part of the Nephrotic Syndrome Study Network (NEPTUNE) observational cohort study, a research consortium of physicians at 26 sites in North America, that partners with the patient advocacy group, NephCure Kidney International and the Halpin Foundation.8 Participants in the NEPTUNE cohort study are offered the opportunity to join NEPTUNE Match (NCT04571658), in which research data are used to match individual patients to clinical trials based on biomarker assessment.8 Considering that the current phase 2 trial is a NEPTUNE Match trial, patients with a TRPC6 aligned biomarker profile will be provided a trial matching report to help inform their decision regarding trial participation. The current study will therefore help generate innovative data to support precision-medicine approaches for the care of patients with FSGS and inform strategies to target patient populations in future trials of BI 764198 in FSGS and other proteinuric diseases.
Patient-centric approaches, such as the decentralized clinical trial model in this trial, offer potential benefits to patient recruitment and retention by making it easier for individuals to participate. Allowing investigators to recruit participants from where they are licensed to practice reduces geographical barriers to enrollment, and conducting remote visits minimizes the cost and time commitments required from participants and care providers. Overall, this may increase the number of potential participants, which is particularly important in rare diseases, such as FSGS.9,S12
The small sample size and short treatment duration are limiting factors for any conclusive assessment of potential benefits and risks of BI 764198 in patients with primary or TRPC6 monogenic FSGS. However, data from this trial will provide insight into the short-term antiproteinuric activity of BI 764198 and provide supportive evidence for phase 3 development of the compound. Recruitment for the study began on March 10, 2022, and the study is estimated to complete in 2025.
Disclosure
WC, RCM, and NS are employees of Boehringer Ingelheim. HT is a consultant to Aclipse, Boehringer Ingelheim, Travere Therapeutics, Otsuka, Walden, and PhaseV Technologies; is a member of the Board of the Kidney Health Initiative and participates in the Pediatric IgAN, the FSGS-proteinuria, and the C3G Projects; and is on the editorial board for Pediatr Nephrol and Glomerular Dis. MK has received research support on behalf of the University of Michigan from Boehringer Ingelheim, Novo Nordisk, Certa, Poxel, Astellas, and Janssen; has received research funding from NIH, JDRF, Chan Zuckerburg Initiative, amfAR, AstraZeneca, Boehringer Ingelheim, Elpidera, Gilead, Goldfinch Bio, Eli Lilly, Angion Biomedica, Certa, Novo Nordisk, Janssen, Chinook, RenalytixAI, Regeneron Pharmaceuticals, Travere Therapeutics, and Ionis Pharmaceuticals; is on the editorial boards for J Am Soc Nephrology, Kidney Int, and Kidney Dis; and is on an advisory board for NephCure Kidney International. HED has received research support through the University of Michigan from Boehringer Ingelheim, NIH, FDA, Travere Therapeutics, Certa, and Roche.
Acknowledgments
Susie Eaton, MBio, of Callisto, OPEN Health Communications (London, UK) provided writing, editorial, and formatting support, which was contracted and funded by Boehringer Ingelheim. The authors also thank Dr. Debbie Gipson for her work as the previous trial coordinating investigator; Dr. Fabia Licarião Rocha for reviewing the manuscript; and the clinical trial leads, Kristen Daniels and Christy Schroeder, for their operational oversight of the study. This work was funded by Boehringer Ingelheim.
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fufill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript and secondary analyses in peer-reviewed journals and regulatory and reimbursement activities are completed, normally within 1 year after the marketing application has been granted by major Regulatory Authorities. Researchers should use the https://vivli.org link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Footnotes
Supplementary Methods.
Supplementary Reference.
Table S1. Study endpoints and assessments.
Supplementary Material
Supplementary Methods.
Supplementary Reference.
Table S1. Study endpoints and assessments.
References
- 1.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shabaka A., Tato Ribera A., Fernández-Juárez G. Focal segmental glomerulosclerosis: state-of-the-art and clinical perspective. Nephron. 2020;144:413–427. doi: 10.1159/000508099. [DOI] [PubMed] [Google Scholar]
- 3.De Vriese A.S., Sethi S., Nath K.A., Glassock R.J., Fervenza F.C. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29:759–774. doi: 10.1681/asn.2017090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santin S., Bullich G., Tazon-Vega B., et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2011;6:1139–1148. doi: 10.2215/CJN.05260610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukerji N., Damodaran T.V., Winn M.P. TRPC6 and FSGS: the latest TRP channelopathy. Biochim Biophys Acta. 2007;1772:859–868. doi: 10.1016/j.bbadis.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Jiang L., Ding J., Tsai H., et al. Over-expressing transient receptor potential cation channel 6 in podocytes induces cytoskeleton rearrangement through increases of intracellular Ca2+ and RhoA activation. Exp Biol Med (Maywood) 2011;236:184–193. doi: 10.1258/ebm.2010.010237. [DOI] [PubMed] [Google Scholar]
- 7.Trachtman H. Emerging drugs for treatment of focal segmental glomerulosclerosis. Expert Opin Emerg Drugs. 2020;25:367–375. doi: 10.1080/14728214.2020.1803276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NEPTUNE, About Neptune. https://www.neptune-study.org/about
- 9.Moore J., Goodson N., Wicks P., Reites J. What role can decentralized trial designs play to improve rare disease studies? Orphanet J Rare Dis. 2022;17:240. doi: 10.1186/s13023-022-02388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fufill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript and secondary analyses in peer-reviewed journals and regulatory and reimbursement activities are completed, normally within 1 year after the marketing application has been granted by major Regulatory Authorities. Researchers should use the https://vivli.org link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.