Abstract
Pseudomonas sp. strain U2 was isolated from oil-contaminated soil in Venezuela by selective enrichment on naphthalene as the sole carbon source. The genes for naphthalene dioxygenase were cloned from the plasmid DNA of strain U2 on an 8.3-kb BamHI fragment. The genes for the naphthalene dioxygenase genes nagAa (for ferredoxin reductase), nagAb (for ferredoxin), and nagAc and nagAd (for the large and small subunits of dioxygenase, respectively) were located by Southern hybridizations and by nucleotide sequencing. The genes for nagB (for naphthalene cis-dihydrodiol dehydrogenase) and nagF (for salicylaldehyde dehydrogenase) were inferred from subclones by their biochemical activities. Between nagAa and nagAb were two open reading frames, homologs of which have also been identified in similar locations in two nitrotoluene-using strains (J. V. Parales, A. Kumar, R. E. Parales, and D. T. Gibson, Gene 181:57–61, 1996; W.-C. Suen, B. Haigler, and J. C. Spain, J. Bacteriol. 178:4926–4934, 1996) and a naphthalene-using strain (G. J. Zylstra, E. Kim, and A. K. Goyal, Genet. Eng. 19:257–269, 1997). Recombinant Escherichia coli strains with plasmids carrying this region were able to convert salicylate to gentisate, which was identified by a combination of gas chromatography-mass spectrometry and nuclear magnetic resonance. The first open reading frame, designated nagG, encodes a protein with characteristics of a Rieske-type iron-sulfur center homologous to the large subunits of dihydroxylating dioxygenases, and the second open reading frame, designated nagH, encodes a protein with limited homology to the small subunits of the same dioxygenases. Cloned together in E. coli, nagG, nagH, and nagAb, were able to convert salicylate (2-hydroxybenzoate) into gentisate (2,5-dihydroxybenzoate) and therefore encode a salicylate 5-hydroxylase activity. Single-gene knockouts of nagG, nagH, and nagAb demonstrated their functional roles in the formation of gentisate. It is proposed that NagG and NagH are structural subunits of salicylate 5-hydroxylase linked to an electron transport chain consisting of NagAb and NagAa, although E. coli appears to be able to partially substitute for the latter. This constitutes a novel mechanism for monohydroxylation of the aromatic ring. Salicylate hydroxylase and catechol 2,3-dioxygenase in strain U2 could not be detected either by enzyme assay or by Southern hybridization. However growth on both naphthalene and salicylate caused induction of gentisate 1,2-dioxygenase, confirming this route for salicylate catabolism in strain U2. Sequence comparisons suggest that the novel gene order nagAa-nagG-nagH-nagAb-nagAc-nagAd-nagB-nagF represents the archetype for naphthalene strains which use the gentisate pathway rather than the meta cleavage pathway of catechol.
Most of the primary information about bacterial catabolism of naphthalene has been obtained from studies of Pseudomonas putida NCIB 9816 (3, 7) and PpG7 (9). They initiate metabolism of naphthalene by incorporating dioxygen into the aromatic nucleus to form cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene (cis-naphthalene dihydrodiol) (24). This reaction is catalyzed by a multicomponent enzyme system, naphthalene dioxygenase (NDO) (14). The O2-dependent hydroxylation is effected by a tetrameric iron-sulfur dioxygenase, consisting of two large Rieske-type [2Fe-2S]-containing subunits (NahAc) and two small subunits (NahAd), to which electrons are transferred from NADH via an iron-sulfur ferredoxin (NahAb) and a flavin-containing ferredoxin reductase (NahAa) (13, 19, 20, 44). The genes encoding NDO in both strains are carried on plasmids, clustered in the order nahAa-nahAb-nahAc-nahAd, and located upstream in the same operon as the other genes for the conversion of naphthalene to salicylate (2, 4, 52). In both strains a second operon encodes the enzymes for further catabolism of salicylate to central metabolites via catechol and extradiol cleavage by a catechol 2,3-dioxygenase (2, 34, 52).
Analyses of NDO genes in other bacterial strains have shown the same organization and high degrees of similarity between the analogous components (8, 10, 47, 48), indicating that these systems have evolved from a common ancestor and are widely distributed in nature. Recently, several different assemblages of the NDO and related genes have been found in naphthalene-degrading strains of Comamonas testosteroni and Sphingomonas paucimobilis (55). In particular, in C. testosteroni GZ42 the NDO genes contain two additional open reading frames, one of which encodes a protein with some homology to NahAc and which is referred to as nahAc2 (55). The gene order is nahAa-nahAc2-open reading frame-nahAb-nahAc1-nahAd. Remarkably, genes with this organization and that are highly homologous to the NDO genes of strain GZ42 have also been found in two strains, one of which is capable of growing on 2-nitrotoluene, Pseudomonas sp. strain JS42 (33), and the other of which is capable of growing on 2,4-dinitrotoluene, Burkholderia sp. strain DNT (45). In both strains, the NDO homologs initiate attack on the nitrated aromatic ring. In these strains and in C. testosteroni GZ42, the functions of the additional open reading frames are unknown and appear to have no role in the dioxygenation of the aromatic nucleus.
Pseudomonas sp. strain U2 was isolated for its ability to metabolize naphthalene from soil exposed to refinery oily sludge in the southeastern region of Venezuela (17). From the plasmid DNA in this strain, we have cloned the genes encoding the initial steps for the catabolism of naphthalene. We report here the nucleotide sequence and functional analysis of the 5.7-kb region encoding NDO in strain U2 and the identification of genes involved in the conversion of salicylate into gentisate. We have designated the genes nag (naphthalene through gentisate).
MATERIALS AND METHODS
Classification of Pseudomonas sp. strain U2.
Pseudomonas sp. strain U2 was isolated by selective enrichment from oil-contaminated soil in Venezuela (17). It was classified as a Pseudomonas sp. as a result of negative Gram staining, its motility, its catalase and oxidase positivities, and its oxidative metabolism of glucose. It was negative in tests for gelatine liquefaction, growth at 42°C, arginine dihydrolase activity, and production of fluorescent pigments.
Bacterial plasmids and strains.
The bacterial plasmids used and constructed in this study are listed in Table 1. Escherichia coli DH5α [φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argG)U169] (Life Technologies, Paisley, United Kingdom) was used routinely as a host in cloning experiments. E. coli Top 10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG] was used as the host for hybrid plasmids derived from the pCR-Blunt vector (Invitrogen B. V., Leek, The Netherlands). E. coli BL21(DE3) pLysS [F− ompT hsdSB (rB− mB−) dcm gal λ(DE3) pLysS (Cmr)] (43) was purchased from Promega and used as the host for the overexpression of genes cloned in plasmid pET-5a.
TABLE 1.
Plasmids used and constructed in this study
| Plasmid | Description | Reference or source |
|---|---|---|
| pUC18 | Vector; Apr; multiple cloning site in lacZ α subunit | 51 |
| pCR-Blunt | Vector; Kmr; blunt site disrupting lethal gene ccdB and encompassed by two EcoRI sites | Invitrogen |
| pBluescript II SK +/− | Vector; Apr; multiple cloning site in lacZ α subunit | Stratagene |
| pET-5a | Expression vector; Apr; NdeI site overlapping the initiating start codon downstream of the T7 promoter and multicloning site | 43 |
| pWWF6 | 8.3-kb BamHI fragment from strain U2 inserted into pUC18 | This study |
| pWWF6-17 | Deletion of SalI fragments from pWWF6 | This study |
| pWWF6-18 | 3.2-kb SalI fragment from pWWF6 subcloned into pUC18 | This study |
| pWWF6-21 | 2.1-kb EcoRI fragment from pWWF6 subcloned into pUC18 | This study |
| pWWF6-24 | Insertion of the 3.2-kb SalI insert from pWWF6-18 into pWWF6-17 | This study |
| pWWF6-25 | Deletion of the 4-kb HindIII fragment from pWWF6 | This study |
| pWWF6-30 | NdeI- and EcoRI-cut PCR fragment containing nagG-nagH-nagAb inserted into pET-5a | This study |
| pWWF6-31 | PCR fragment containing nagG-nagH-nagAb inserted into pUC18 | This study |
| pWWF6-32 | pWWF6-31 with modified NcoI site causing frameshift in nagH | This study |
| pWWF6-33 | pWWF6-31 in which 3′ end of nagAb has been deleted | This study |
| pWWF6-34 | pWWF6-31 in which 5′ end of nagG has been deleted | This study |
| pWWF24 | ≈10-kb XhoI fragment from U2 carrying NDO region cloned into XhoI site of pBluescript II SK +/− | This study |
| pWWF60-3032 | 4.5-kb XhoI fragment containing nahRGH from plasmid pWW60 of strain P. putida NCIB 9816 in pUC18 | 2 |
| pWWF60-1 | 2.3-kb PstI fragment containing nahG subcloned from pWW60-3032 into pUC18 | This study |
| pWWF60-2 | 2-kb SmaI fragment containing nahH from pWW60-3032 subcloned into pUC18 | This study |
Media.
Liquid Luria-Bertani (LB) medium (30) containing an appropriate antibiotic was used for the cultivation of E. coli strains. Sensitivity test agar (LabM, Bury, United Kingdom) with added ampicillin was used for the selection of recombinant strains carrying hybrid plasmids derived from pUC18. LB agar was used for the selection of strains carrying plasmids derived from pET-5a or pCR-Blunt. Minimal medium (MM) was prepared according to the method of Worsey and Williams (50). Solid LB agar and MM contained 1.5% agar (LabM). For the culture of strain U2, 10 mM succinate, 2.5 mM salicylate, or 0.5% (wt/vol) pulverized naphthalene was used, either added to liquid medium or, for naphthalene only, spread on the lids of inverted petri dishes. Ampicillin was used at 100 μg/ml, and kanamycin was used at 50 μg/ml where appropriate.
Plasmid extraction and DNA manipulation.
Plasmid DNA was extracted from strain U2 by the method of Wheatcroft and Williams (49). Restriction endonuclease digestions and ligations with T4 ligase were in accordance with the manufacturer’s instructions. E. coli DH5α was transformed by standard procedures (36). Plasmid was extracted for rapid screening by the method of Holmes and Quigley (23) and purified by CsCl centrifugation (18) or by using a QIAprep kit (Qiagen, West Sussex, United Kingdom).
Detection of strains expressing catabolic genes.
E. coli strains carrying cloned DNA fragments encoding NDO activity were identified by spreading transformants onto sensitivity test agar or LB agar plates supplemented with antibiotics and 1 mM indole. Positive colonies were detected as blue colonies, indicative of conversion of indole to indigo (15). Selection plates designed to detect salicylate hydroxylase activity contained an appropriate antibiotic and 2.5 mM salicylate. Colonies expressing this activity accumulate the brown color derived from the autoxidation of the product catechol. Detection of cis-naphthalene dihydrodiol dehydrogenase activity was performed by subculturing NDO-expressing colonies on solid medium in the presence of naphthalene vapor. Strains expressing this activity together with that of NDO are recognized by the accumulation of brown oxidation products derived from the product 1,2-dihydroxynaphthalene.
Location of genes for NDO components.
Individual genes encoding NDO were located by Southern hybridizations with the NDO genes obtained from strain NCIB 9816 by PCR. The two PCR primers used for nahAa were 5′-TCATACAGCCAAACAATC and 5′-GATAGAAGGCATCGG, those used for nahAb were 5′-ACTGTCGAGGGCAAG and 5′-ATTACGCGCAGGTTCTC, those used for nahAc were 5′-TGAGTGAATCTGGGCTG and 5′-ATCCTCGAACTCAGCC, and those used for nahAd were 5′-ATTCAAGAAGACAAGCTG and 5′-GTAATCCACGAATCGCTG. The fragments generated were purified by agarose gel electrophoresis and band extraction. Probe preparations and hybridizations were made by using the ECL direct nucleic acid labelling and detection system (Amersham International plc, Little Chalfont, Buckinghamshire, United Kingdom). Southern blotting was performed according to the method of Southern (42).
Subcloning and inactivation of nagG, nagH, and nagAb.
A DNA fragment from the region encoding NDO in strain U2 was amplified by PCR and cloned into pET-5a. It was produced by using an upstream primer (5′-GGATACCAACATATGAGTGAACCCCAAC) designed with an NdeI site (underlined) spanning the start codon of the first open reading frame (nagG) at bases 1290 to 1318 in the sequence; the downstream primer (5′-CATAAATCATGATTAATGTCTCCGTT) included the EcoRI site (underlined) downstream of nagAb (Fig. 1) and is complementary to bases 3458 to 3483 in the sequence. PCR was performed by using Vent DNA polymerase according to the instructions of the manufacturer (New England Biolabs). The fragment produced contained the complete sequences for the genes nagG, nagH, and nagAb (Fig. 1). It was purified through agarose gel electrophoresis and band extraction, digested with NdeI and EcoRI, and inserted into the similarly digested pET-5a vector. The plasmid was designated pWWF6-30 (Fig. 1). The same PCR fragment was also ligated directly into the pCR-Blunt vector, removed as an EcoRI fragment, and directly ligated to pUC18 to produce plasmid pWWF6-31 (Fig. 1). To inactivate nagH, pWWF6-31 was digested at its single NcoI site, close to the 5′ end of nagH (Fig. 1); the single-stranded termini produced were filled in with Klenow fragment polymerase, and the plasmid was religated to produce pWWF6-32 (Fig. 1). To inactivate nagAb, pWWF6-31 was digested with HpaI at the unique site within nagAb (Fig. 1) and at the downstream KpnI site in the multicloning site of pUC18. The 3′ overhang of the KpnI site was filled in with T4 polymerase, and the blunt ends were religated to produce pWWF6-33 (Fig. 1). To inactivate nagG, plasmid pWWF6-31 was digested with SmaI, which produces a 1.4-kb fragment extending from the downstream site within nagG (Fig. 1) through nagH and nagAb to the SmaI site from the pUC18 multicloning site. This fragment was purified and recloned into fresh SmaI-cut pUC18 to produce pWWF6-34, which therefore carries the complete nagH and nagAb genes but is missing the 5′ end of nagG.
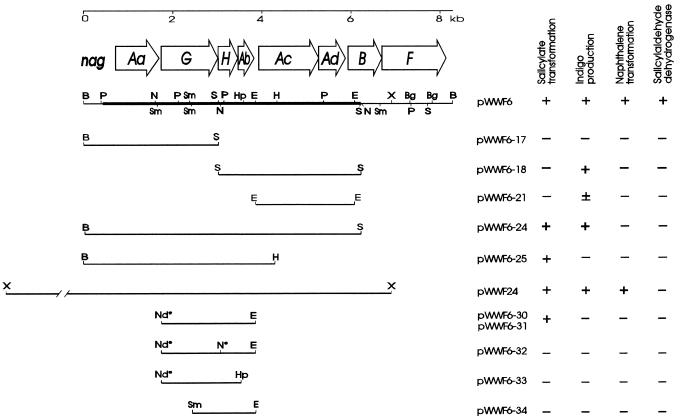
FIG. 1.
Physical map of plasmids carrying nag genes from the large catabolic plasmid of Pseudomonas sp. strain U2 together with the phenotypic properties they confer. Salicylate transformation implies the conversion of salicylate to gentisate as visualized by the production of brown oxidation products on plates or in liquid media containing salicylate. Indigo production was determined by a blue-black coloration of colonies grown on plates containing indole. Naphthalene transformation was measured by a brown coloration produced from naphthalene on agar plates and shows the expression of both NDO and naphthalene diol dehydrogenase (NagB) in the culture. Salicylaldehyde dehydrogenase was measured qualitatively in cell extracts by monitoring in a spectrophotometer the conversion of salicylaldehyde to salicylate. The thick line on the pWWF6 map shows the part of the insert which has been sequenced. The restriction sites are designated as follows: B, BamHI; Bg, BglII; E, EcoRI; H, HindIII; Hp, HpaI; N, NcoI; P, PstI; S, SalI; Sm, SmaI; and X, XhoI. Nd* represents an NdeI site which was engineered in the PCR primer used to generate the insert of pWWF6-30, and N* represents the modified NcoI site produced by filling in with Klenow fragment polymerase.
Cultivation of strains for the preparation of cell extracts.
A 5-ml overnight culture of E. coli strains, grown in LB agar supplemented with an appropriate antibiotic, was used to inoculate 200 ml of the same medium in 500-ml flasks. After incubation with shaking at 200 rpm for 2 to 3 h at 37°C, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a concentration of 0.5 mM and incubation was continued for 4 h. Cultures were chilled before the cells were harvested by centrifugation at 4°C. Cells were washed once with cold MM and stored as a pellet at −20°C until use. Pseudomonas sp. strain U2 was grown overnight in 10 ml of MM containing an appropriate carbon source (in 125-ml flasks) with shaking at 200 rpm and 30°C. The whole culture was inoculated in a 2-liter flask containing 500 ml of the same medium and incubated under the same conditions until late exponential phase (optical density at 600 nm, 1.2). The cells were harvested and stored until use as for E. coli strains.
Preparation of cell extract.
Cell extracts were prepared by resuspending the bacterial pellets in ice-cold 50 mM Na-K phosphate buffer (pH 7.4) (approximately 0.1 g [wet weight]/ml) and disrupted by sonication in an ice-water bath for three periods of 30 s with 30-s intervals, after which cell debris were removed by centrifugation at 23,000 × g for 1 h at 4°C.
Enzyme assays.
The activity of salicylate hydroxylase was determined spectrophotometrically at 340 nm by measuring the rate of oxidation of NADH (53). The activity of gentisate 1,2-dioxygenase was assayed by measuring the increase in absorbance at 334 nm due to the appearance of maleylpyruvate (5). Protein concentrations were determined by the Biuret procedure. One unit of enzyme activity is defined as the amount of enzyme required for the disappearance or production of 1 μmol of substrate or product, respectively, per min at 30°C. Specific activities are expressed as units per milligram of protein. Qualitative expression of salicylaldehyde dehydrogenase by recombinant strains was detected in cell extracts by monitoring spectrophotometrically the conversion of salicylaldehyde into salicylate as performed by Eaton and Chapman (11).
Transformation of metabolites.
Transformation of gentisate into maleylpyruvate by cell extracts of U2 was monitored spectrophotometrically under the same conditions as for the enzyme assay for gentisate 1,2-dioxygenase. In order to blank off gentisate absorbance, the incubation mix and the same amount of inactive cell extract (from strain U2 growing on succinate) were added to the reference cuvette. Transformation of salicylate was analyzed by growing the strains in 100 ml of LB medium (in 500-ml flasks) containing 1 mM salicylate and 0.5 mM IPTG. The cultures were incubated with shaking at 200 rpm and 30°C. Spectra of cleared samples were recorded immediately after inoculating and then periodically at intervals of about 1 h.
Characterization of salicylate transformation product.
Proton nuclear magnetic resonance (NMR) spectra were recorded with a Bruker AC250 spectrometer in CDCl3 with tetramethylsilane as the internal standard. Solutions for gas chromatography (GC) and GC-mass spectrometry (GC-MS) were made in diethyl ether or dichloromethane. GC was performed with a Perkin-Elmer 8410 GC fitted with a cross-bonded RTX-1701 column with a flow rate of 5 ml/min, starting a 130°C for 1 min and increasing to 195°C at a rate of 15°C/min. GC-MS was performed with a Finnigan 4500 GC fitted with a 30-m, 0.25-mm-internal-diameter decyl butyl siloxane (DBS) liquid-phase column, with helium as the carrier gas, at a 70-eV ionization potential. Mass spectra were determined with a Finnigan 8200 GC with a 70-eV ionization potential. Methylated derivatives of hydroxy-salicylic acids were prepared by stirring 50 mg in a suspension of sodium hydride (100 mg, 4.2 mmol) and dry dimethylformamide (DMF) (3 ml) in a 50-ml reaction vessel. The suspension was stirred for 15 min, followed by the dropwise addition of methyl iodide (0.5 ml, 8 mmol). This mixture was then sealed and stirred for 12 h, quenched with diluted HCl (10 ml), and extracted twice with diethyl ether (10 ml). The yellow organic layer was washed sequentially with water (two times with 20 ml), with sodium thiosulfate (20 ml) until colorless, and again with water (two times with 20 ml), and then dried with anhydrous magnesium sulfate. The resulting solution was evaporated to dryness without heating on a rotary evaporator.
Sequence determination and analyses.
Nucleotide sequences were determined by Alta Bioscience (University of Birmingham, Birmingham, United Kingdom). Sequence analysis was done by using the Lasergene software package (DNASTAR). Multiple alignments were produced by CLUSTAL V (22). BLASTP and BLASTX (1) were used for the deduced amino acid identity searches.
Nucleotide sequence accession number.
The DNA sequence of 5,749 bp carrying the complete nagAa, nagG, nagH, nagAb, nagAC, and nagAd genes and the 5′ end of nagB has been submitted to GenBank under accession no. AF036940.
RESULTS
Cloning and preliminary locations of naphthalene catabolic genes.
A previous study (17) based on the correlation between conjugational transfer and plasmid content suggested that the genes encoding naphthalene catabolism in strain U2 are carried on a large plasmid, probably >250 kbp in size. Plasmid DNA was digested with BamHI or HindIII and ligated with the similarly digested plasmid vector pUC18. Transformants in E. coli DH5α were selected for the expression of NDO activity by screening for the production of indigo from indole. We identified several indigo-producing colonies, each of which contained an 8.3-kb BamHI insert. Two intensities of indigo production were apparent among the colonies. Restriction digests of the plasmids revealed that this was due to opposite orientations of the insert. We selected plasmid pWWF6 (Fig. 1), that conferred high intensity of indigo production and carried the NDO genes under the control of the vector promoter, as demonstrated by induction with IPTG. Subsequently, a larger overlapping XhoI fragment of about 10 kb with indigo-producing activity was also ligated into the XhoI site of pBluescript II SK +/− to produce plasmid pWWF24 (Fig. 1). Indigo production was also apparent with subclones of pWWF6: pWWF6-24 and pWWF6-18 were strong producers, whereas pWWF6-21 was a very weak producer (Fig. 1). The NDO genes therefore lie upstream of the SalI site at 6.1 kb (Fig. 1).
The presence of naphthalene cis-dihydrodiol dehydrogenase (NagB) in E. coli (pWWF6) was identified after growth on LB agar plates in the presence of naphthalene vapor. The expression of both NDO and NagB caused accumulation of 1,2-dihydroxynaphthalene, which is apparent from the accumulation of brown oxidation products (4): similar activity was found with pWWF24. In combination with sequence analysis (see below), this finding locates nagB downstream of NDO but upstream of the XhoI site. Salicylaldehyde dehydrogenase (NagF) activity was detected by monitoring in a spectrophotometer the conversion of salicylaldehyde into salicylate by cell extracts (11) of recombinant strains carrying plasmid pWWF6 but not pWWF24 (Fig. 1), thus locating the gene downstream of nagB and showing the same order for the genes of NDO, NagB, and NagF as in the archetypal plasmid NAH7 (10, 41). This order was confirmed by analysis of the region encoding NDO by Southern hybridizations, with PCR-generated probes carrying single NDO genes from strain NCIB 9816 (data not shown).
An additional phenotype of E. coli (pWWF6) was the accumulation of a soluble brown product when the organism grew in the presence of salicylate. This brown product is often due to accumulation of a catechol and therefore suggests the presence of a homolog of nahG, the gene for salicylate hydroxylase (4). Two results showed that the product was not catechol: the absorption spectra of culture supernatants showed a peak with a maximum wavelength (λmax) of 320 nm, whereas catechol’s λmax is 275 nm, and addition to the supernatants of cell extracts of an E. coli expressing high catechol 2,3-dioxygenase activity failed to produce any yellow color, indicative of the presence of 2-hydroxymuconic semialdehyde (λmax = 375 nm), the product of extradiol cleavage of catechol (6).
Nucleotide sequence analysis of the region encoding NDO.
The sources of DNA for nucleotide sequencing were pWWF6-18 and pWWF6-25 (Fig. 1). Inserts in these plasmids span approximately 6 kb and include all NDO genes as determined by indigo production and Southern hybridizations (Fig. 1). Examination of the sequence indicated that it contained six complete open reading frames. Their homology with the classical NDO components (NahAa, NahAb, NahAc, and NahAd) (41) was determined through database searches, the presence of highly conserved regions described previously in NDO components (31), and deduced amino acid sequence alignments. As a consequence, nagAa, nagAb, nagAc, and nagAd were identified (Fig. 1). Their deduced protein sizes are as follows: for NagAa, 328 residues (Mr = 35,089); for NagAb, 104 residues (Mr = 11,566); for NagAc, 447 residues (Mr = 49,576); and for NagAd, 194 residues (Mr = 23,015).
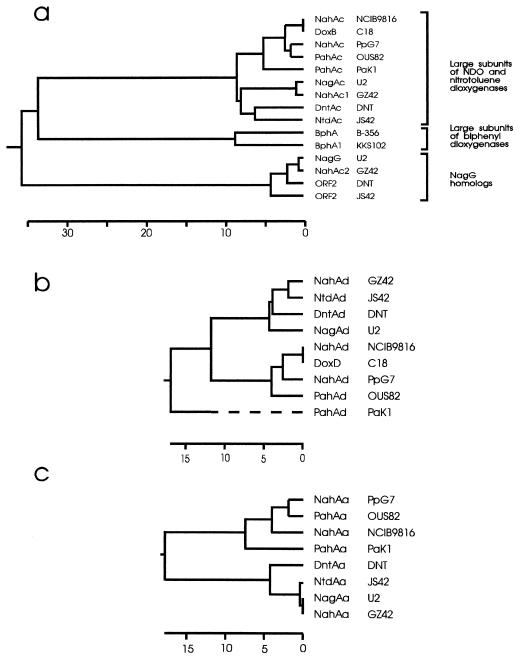
Between nagAa and nagAb are two additional open reading frames. The first, designated nagG, encodes a 423-residue protein (Mr = 48,799) that is 95 and 98% similar to those encoded by open reading frame 2 of the dnt (2,4-dinitrotoluene) genes from Burkholderia sp. strain DNT (45) and the truncated open reading frame 2 of the ntd (2-nitrotoluene) cluster from Pseudomonas sp. strain JS42 (33), respectively. nagG is virtually identical, with only one amino acid difference, to nahAc2 in the NDO cluster of C. testosteroni GZ42 (54). The proteins encoded by these genes show limited similarity to the large-subunit NahAc of the oxygenase component of NDO systems and contain the two conserved cysteines and histidines at the N-terminal region as part of the motif CXHX17CX2H, which constitutes a Rieske-type [2Fe-2S] cluster (31). A dendrogram comparing NagG and its homologs with large subunits from NDO and biphenyl dioxygenase (Fig. 2a) shows three main subgroups: (i) NDO large subunits (NahAc homologs); (ii) analogous subunits in the biphenyl dioxygenases from two typical biphenyl-degrading strains, KKS102 (25) and C. testosteroni B-356 (46); and (iii) a more distant cluster including NahG from U2 and the reading frames of unknown function from strains GZ42 (NahAc2), DNT, and JS42. The plot also includes the large subunits of the nitrotoluene dioxygenases, which, although clearly related to the NahAc proteins, form a distinct subgroup within the first-mentioned subgroup, which also includes NagAc from U2 and NahAc1 from GZ42. Directly downstream of nagG is a second open reading frame (designated nagH), homologs of which are also present in the same position in Burkholderia sp. strain DNT (45) and C. testosteroni GZ42 (54, 55). This sequence elicits no significant matches when it is run through the BLASTX program but does show some similarities with the small subunits of the aromatic ring-hydroxylating dioxygenases, albeit at a very low level (54).
FIG. 2.
Dendrograms of large α subunits of dihydroxylating aromatic dioxygenases and of NagG homologs (a), small β subunits of naphthalene dioxygenases (b), and ferredoxin reductase components of NDOs (c). The dendrograms were constructed by using the published amino acid sequences processed through the CLUSTAL procedure in DNASTAR with the default parameters. The horizontal scale in each panel denotes the percentage of divergence of the sequences. The sequences were taken from (reference; GenBank accession no.) P. putida NCIB 9816 nah genes (41; M83950), Pseudomonas sp. strain C18 dox genes (8; M60405), P. putida PpG7 nah genes (41; M83949), P. putida OUS82 pah genes (47; D16629), Pseudomonas aeruginosa PaK1 pah genes (48; D84146), Pseudomonas sp. strain U2 nag genes (this paper; AF036940), C. testosteroni GZ42 nah genes (54), Burkholderia sp. strain DNT dnt genes (45; U62430), Pseudomonas sp. strain JS42 ntd genes (33; U49504), C. testosteroni B-356 bph genes (46; U47637), and Pseudomonas sp. strain KKS102 bph genes (25; D17319).
Ten and 22 bases downstream from the TGA stop codon of nagAd are two in-frame start codons which initiate an open reading frame (of 97 or 93 codons) which continues without a stop codon to the end of the determined sequence. Database comparisons show this open reading frame to have very high homology with five naphthalene cis-dihydrodiol dehydrogenase genes, and we have therefore identified two possible alternative start sites for nagB, thus confirming its location as being that deduced from naphthalene transformation.
Identification of gentisate as the product of salicylate transformation.
The UV spectrum of the cleared culture of E. coli (pWWF6-31) grown in the presence of salicylate was compared with those of all possible hydroxylated salicylates but was similar only to that of 2,5-dihydroxybenzoate (gentisate). To confirm the identity of this compound, fully methylated derivatives were prepared from salicylate, 2,3-dihydroxybenzoate, 2,4-dihydroxybenzoate, gentisate, and 2,6-dihydroxybenzoate and from the freeze-dried cleared culture by using a standard Williamson ether synthesis procedure, so as to allow comparison by gas-liquid chromatography-MS. Gas liquid chromatography-MS was performed in two ways, by using both internal and external standards, and all but two of the possible acids were eliminated: gentisate and 2,6-dihydroxybenzoate (with retention times of 3.9 and 4.2 min, respectively) could not be adequately resolved from the methylated metabolite. However, comparison by NMR of the characteristic coupling patterns of the methylated derivatives in the aromatic region proved that the metabolite was gentisate. The proton NMR spectrum of gentisate was identical to that of the metabolite, with signals as follows: δ 6.9 (1H, d, J = 9.0 Hz), 7.0 (1H, dd, J = 3.1 and 9.0 Hz), and 7.3 (1H, d, J = 3.1 Hz). By contrast 2,6-dihydroxybenzoate has a very different coupling pattern in the proton NMR: δ 6.5 (2H, d, J = 8.4 Hz) and one proton triplet at δ 7.3 (1H, t, J = 8.4 Hz).
Determining the genes involved in salicylate transformation.
pWWF6-18 (Fig. 1) confers the ability to transform indole into indigo but not the ability to transform salicylate into gentisate. Sequence analysis confirmed that it carried the genes for both dioxygenase (NagAc and NagAd) and the ferredoxin (NagAb) components of NDO. Plasmid pWWF6-25 (Fig. 1), which carries only the 5′ end of the nagAc gene but all of the remaining upstream genes (nagAa nagG, nagH, and nagAb) of U2 NDO, did cause the transformation of salicylate, indicating that NagAc and NagAd were not responsible for this phenotype.
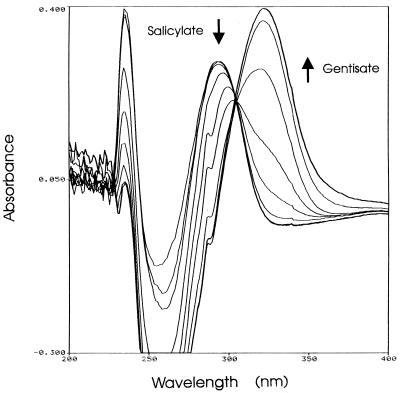
To determine which of the genes of the U2 NDO cluster present on pWWF6-25 were involved in the transformation of salicylate, we synthesized a PCR fragment containing the complete reading frames of nagG, nagH, and nagAb. When this fragment was ligated into the expression vector pET-5a as plasmid pWWF6-30 (Fig. 1), overexpression of recombinant proteins under the control of the vector T7 promoter resulted in very unhealthy host cells and low salicylate-transforming activity compared with those of strains carrying pWWF6, in which the entire U2 NDO gene cluster is present in pUC18. The same PCR fragment, when ligated into pUC18 as plasmid pWWF6-31 (Table 1), had a far less deleterious effect upon its E. coli hosts and caused better salicylate transformation. Figure 3 shows the transformation of salicylate by E. coli (pWWF6-31), which was in a few hours able to transform virtually all the salicylate to gentisate, as was determined by its λmax of 320 nm.
FIG. 3.
Transformation of salicylate into gentisate by E. coli DH5α (pWWF6-31) growing in LB medium containing 1 mM salicylate, 100 μg of ampicillin per ml, and 0.5 mM IPTG. The culture was incubated with shaking at 200 rpm and 30°C. Spectra of 1-ml cleared samples were recorded immediately after inoculation and then after 1.0, 2.0, 4.6, 5.9, 6.9, 7.9, 9.2, and 10 h.
Plasmids with gene knockouts in each of their three cloned genes were constructed from pWWF6-31. In pWWF6-32, a frameshift was introduced into the 5′ end of nagH at its NcoI site (Fig. 1). In plasmid pWWF6-33, a deletion of the 3′ end of nagAb was made between the unique HpaI site (Fig. 1) and the KpnI site of the pUC18 multicloning site. Plasmid pWWF6-34 was constructed from pWWF6-31 with a deletion of the 5′ end of nagG from the start site of the insert up to the second SmaI site within the gene (Fig. 1). In E. coli containing either pWWF6-32 (nagH mutant) or pWWF6-34 (nagG mutant), no accumulation of the brown oxidation products of gentisate could be detected on agar plates or in liquid culture. With pWWF6-33 (nagAb mutant), there was a drastic reduction in the rate of product accumulation compared with that of pWWF6-31 and, after 3 days, only a very pale brownish coloration could just be seen on plates containing salicylate. We attempted to quantitate the comparative rates of production of gentisate in liquid culture with pWWF6-33 and pWWF6-31. Whereas E. coli (pWWF6-31) converted about 60 to 70% of salicylate to gentisate during 7 h of growth at 30°C, under identical conditions E. coli (pWWF6-33) converted no detectable salicylate to gentisate.
Catabolism of salicylate by strain U2.
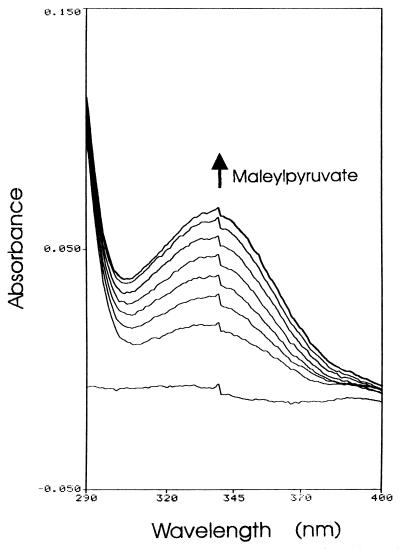
In strains PpG7 and NCIB 9816, salicylate is converted to catechol by salicylate hydroxylase and then dissimilated to central metabolites through a meta cleavage pathway. The genes encoding the pathway for salicylate catabolism are organized in a single operon, the promoter of which overlaps that of the divergent regulatory protein NahR (37, 38, 52). We searched for the genes encoding homologs of salicylate hydroxylase (nahG) and catechol 2,3-dioxygenase (nahH) in strain U2 by Southern blot analyses. The individual genes from P. putida NCIB 9816, subcloned from plasmid pWW60-3032 (2), were used to probe genomic and plasmid DNAs from strain U2. No homology with these genes was detected in U2 DNA, suggesting that an alternative pathway operates in strain U2 for the catabolism of salicylate. Further circumstantial evidence for the absence of a catechol 2,3-dioxygenase gene in U2 was obtained from cloning experiments in which the transformants were sprayed with catechol to detect extradiol ring-cleavage activity. Such experiments were performed on numerous occasions, and the only gene obtained was that of 1,2-dihydroxynaphthalene dioxygenase (nagC), which has overlapping but distinguishable specificity with those of catechol 2,3-dioxygenases and which we have located downstream of nagF (data not presented). To determine by which pathway strain U2 uses salicylate, we assayed for salicylate hydroxylase and gentisate 1,2-dioxygenase activities in cell extracts from cultures grown with succinate, naphthalene, or salicylate as the sole carbon source. Whereas no salicylate hydroxylase activity was detected in any of these extracts, gentisate 1,2-dioxygenase activity was detected in cell extracts from the cultures grown on either naphthalene (0.078 μmol/min/mg of protein) or salicylate (0.010 μmol/min/mg of protein) but was undetectable in cells grown on succinate. Figure 4 shows the formation of maleylpyruvate from gentisate by induced cell extract. This data shows that both naphthalene and salicylate are metabolized in strain U2 through gentisate and not through catechol.
FIG. 4.
Formation of maleylpyruvate from gentisate by cell extract of strain U2 grown with salicylate. Sample and reference cuvettes contained 100 mM Na-K phosphate buffer (pH 7.4) and 0.15 μmol of gentisate in 1-ml volumes. Spectra were recorded before and 0.33, 0.7, 1.0, 1.5, 2.0, 3.0, and 5.0 min following the addition of cell extract (50 μl containing 0.34 mg of protein) prepared from a salicylate-grown culture. Cell extract added to the reference cuvette, in an equivalent amount, was prepared from a succinate-grown culture which was shown to lack gentisate 1,2-dioxygenase activity. The ε334 for maleylpyruvate is 10,800 M−1 cm−1.
DISCUSSION
A number of DNA sequences encoding NDO activities have been reported for Pseudomonas strains isolated from different geographical regions whose functional genes have shown the same organization and significant identity (10, 27, 41, 47, 48). However, recent reports have shown a distinctively different organization, which is similar to what we have found in Pseudomonas sp. strain U2. In only one of these reports does the strain described, C. testosteroni GZ42, also use naphthalene as a growth substrate (55). In Burkholderia sp. strain DNT, the genes encode the dioxygenative denitration of 2,4-nitrotoluene (45). In both strains the genes for the dioxygenase components, equivalent to nahAa, nahAb, nahAc, and nahAd, are interrupted between nahAa and nahAb by a sequence encoding two open reading frames, the deletion of which has no effect on the dioxygenative attack. In a third strain, the 2-nitrotoluene user Pseudomonas sp. strain JS42 (33), there is an insertion in the same location but it encodes only a truncated variant of the first of the two open reading frames. Sequence analysis of the region encoding NDO activity in strain U2 revealed two genes, designated nagG and nagH, which encode 48.8- and 18.8-kDa polypeptides. In this paper we have shown that both are required for a salicylate 5-hydroxylase activity that converts salicylate to gentisate, the DNA for which has not knowingly been cloned or sequenced before. This activity also requires the presence of NagAb, the ferredoxin component of NDO for full activity: inactivation of nagAb reduces gentisate formation to a negligible rate. The residual rate is possibly because the E. coli host has a ferredoxin which is very poorly able to complement NagAb.
Because NagG aligns with the large subunits of both NDO and biphenyl dioxygenases (Fig. 2a), contains the residues indicative of a Rieske-type [2Fe-2S] protein, and requires the additional presence of a ferredoxin, it is reasonable to suppose that it has a mode of action similar to those of related dioxygenases. On the evidence presented, we cannot be certain of the role of NagH, but its limited homology with the small subunits of the two-subunit dioxygenases leads to the most reasonable hypothesis, namely, that it too is a small subunit of the salicylate 5-hydroxylase. If this hypothesis is correct, the hydroxylation of salicylate occurs through NagGH acting as a monooxygenase, with an associated electron transport chain consisting of a ferredoxin and a ferredoxin reductase supplying the electrons from a reduced nicotinamide cofactor (Fig. 5). From the proximity of the nagG and nagH genes to the genes for both NagAa and NagAb, it seems possible, and in our opinion likely, that both of these proteins form the electron transport chain for salicylate 5-hydroxylase in addition to fulfilling identical roles for NDO. E. coli (pWWF6-25) carrying all four genes nagAa, nagG, nagH, and nagAb converts salicylate to gentisate. However, this transformation also takes place in strains carrying plasmid pWWF6-31 with only nagG, nagH, and nagAb. Plasmids pWWF6-18 and pWWF6-21 also lack nagAa (Fig. 1) but are able to direct conversion of indole to indigo, an activity which requires a functional NDO. NDO action in E. coli in the absence of NahAa has been previously reported (41), confirming that E. coli contains one or more ferredoxin reductase proteins which can substitute for the NDO protein.
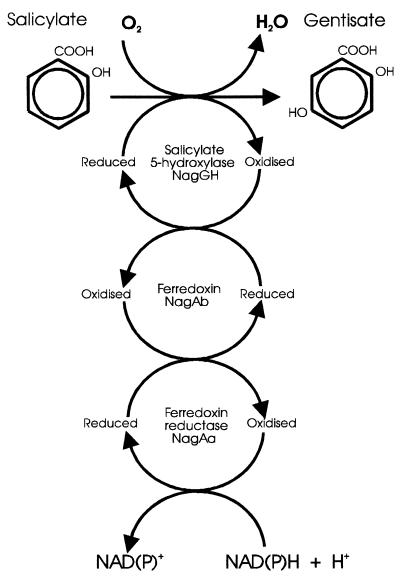
FIG. 5.
Proposed model for the conversion of salicylate to gentisate by salicylate 5-hydroxylase.
If our hypothesis is correct, salicylate 5-hydroxylase represents a novel protein combination for a hydroxylating monooxygenase. Enzymes of this class make up a very heterogeneous group (21) and vary from single- or double-subunit flavoproteins, typified by 4-hydroxybenzoate hydroxylase (16, 40) or 4-hydroxyphenylacetate 3-hydroxylase (35), to six-component proteins, such as the phenol hydroxylase from P. putida CF600 (32). Salicylate 5-hydroxylase partially resembles two hydroxylases. The alkane hydroxylase from the P. putida OCT plasmid has a single hydroxylase component (AlkB), which is not a Rieske-type protein, linked to a two-component electron transport chain consisting of a rubredoxin and a rubredoxin reductase (12, 26). 4-Toluene sulfonate methyl monooxygenase has a single hydroxylase component which contains a Rieske-type center, but its electron transport chain consists of a single protein (28).
All four strains with this novel gene arrangement for NDO-like dioxygenase genes form a subcluster when the protein sequences of the oxygenase large subunits (Fig. 2a), the small subunits (Fig. 2b), and the ferredoxin reductases (Fig. 2c) are compared. This homology separates them from the standard NDO genes exemplified by strains PpG7 and NCIB 9816 and suggests that they represent a separate divergent lineage. Only the four ferredoxins do not cluster separately (data not shown), which may be the result of a less constrained selective pressure. The close evolutionary relationship of the gene cluster in U2 and the two nitrotoluene users is also confirmed by the noncoding DNA upstream of the start codon of nagAa (Fig. 6). Compared with the nucleotide sequence of U2 over the region of 211 bases upstream, the corresponding sequences of DNT and JS42 are 94 and 99% identical, respectively, yet they show only from 23 to 31% identity to the four other naphthalene users (exemplified by PpG7 in Fig. 6). Eaton (10) has noted a region which is directly repeated both upstream and downstream of the operon in PpG7, and there is no similarity between U2 and PpG7 in the region where the upstream sequences of these repeats are found in PpG7. However, all four upstream sequences do show homology with each other at the sites identified as regulatory sequences: (i) the 15-bp binding site for NahR, TATTCAnGnTGnTGA (37), and, to a lesser extent, (ii) two overlapping −10 and −35 promoter sequences, one of which was identified as the functional promoter in PpG7 (39) and the other of which is homologous to the TOL promoter sequence identified by Mermod et al. (29) and was suggested to be the promoter region in Burkholderia sp. strain DNT (45). This homology indicates that all these sequences have a common mechanism of regulation.
FIG. 6.
Alignment of DNA sequence upstream of nagAa with corresponding upstream regions of nitrotoluene-degrading strains of Pseudomonas sp. strain JS42 (33), Burkholderia sp. strain DNT (45), and naphthalene-degrading strain P. putida PpG7 (41). The 15-bp binding site for NahR on PpG7 identified by Schell and Poser (39) is overmarked with asterisks. The possible promoter sequences which show homology with Pseudomonas consensus promoters and which were identified for strain DNT (45) are underlined, and the promoter sequences shown in PpG7 (37, 38) are in boldface type.
In the absence of direct evidence, it is possible only to hypothesize about the catabolic pathway for salicylate use in C. testosteroni GZ42, the only other naphthalene user in this group. However, the virtual identity of the amino acid sequence of the NagG and NagH homologs in GZ42 to that in U2 suggests very strongly that it too encodes a salicylate 5-hydroxylase and initiates catabolism of the salicylate through gentisate. Downstream of the NDO cluster in GZ42 is a glutathione S-transferase gene of unknown function (55). We have recently cloned a gene for the ring-cleavage enzyme gentisate 1,2-dioxygenase (NagI) from a region downstream of the U2 NDO, and preliminary analysis shows that there is an adjacent gene which shows strong homology with other glutathione S-transferases (data not presented). The two operons of U2 and GZ42 may well therefore be the archetypes of naphthalene operons metabolizing salicylate through gentisate.
The sequence evidence suggests that the pathway of the type found in U2 diverged from the pathway of the PpG7 type and that a recombination event or events led to the acquisition of a gentisate pathway in the U2 lineage with an insertion between nagAa and nagAb of the nagG and nagH genes, enabling their gene products to share the electron transport chain of the NDO. However, there remains between the two divergent lines similarities both in the regulatory upstream sequences and in the order of the nagAa, nagAb, nagAc, nagAd, nagB, and nagF genes (which, if the same order holds for the latter part of the U2 cluster as for the GZ42 cluster, extends through nagCQED [55]). The two nitrotoluene operons (33, 45) have both clearly evolved from NDO precursors, perhaps in recent times, given the novelty of nitrotoluenes in the environment, to dihydroxylate the aromatic ring and thereby eliminate one of the nitro substituents. The presence of complete NagG and NagH homologs in strain DNT and of a truncated NagG in strain JS42 suggests that they have evolved from nag-like genes, but there has been a deletion in the 3′ end of the nagGH region in strain JS42. This poses the question of whether there is any reason why the evolution of both of these strains should take place from a nag (naphthalene through gentisate) precursor rather than from a nah (naphthalene through catechol) precursor. Is it due to the diverged nature of the dioxygenase components NagAc and NagAd, making them more adaptable to the change in substrate specificity, or is it due to some advantage or disadvantage of possessing the subsequent gentisate-catechol route?
ACKNOWLEDGMENTS
S.L.F. carried out this work with a studentship from the National Council for Scientific and Technological Research (CONICIT), Caracas, Venezuela.
We thank Gerben Zylstra for releasing his protein sequences and for open exchange of data and ideas.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Assinder S J, Williams P A. Comparison of the meta-pathway operons on NAH plasmid pWW60-22 and TOL plasmid pWW53-4 and its evolutionary significance. J Gen Microbiol. 1988;234:2769–2778. doi: 10.1099/00221287-134-10-2769. [DOI] [PubMed] [Google Scholar]
- 3.Cane P A, Williams P A. The plasmid-coded metabolism of naphthalene and 2-methylnaphthalene in Pseudomonas strains: phenotypic changes correlated with structural modification of the plasmid pWW60-1. J Gen Microbiol. 1982;128:2281–2290. [Google Scholar]
- 4.Cane P A, Williams P A. A restriction map of naphthalene catabolic plasmid pWW60-1 and the location of some of its catabolic genes. J Gen Microbiol. 1986;132:2919–2929. [Google Scholar]
- 5.Crawford R L, Hutton S W, Chapman P J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella oslensis. J Bacteriol. 1975;121:794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagley S, Evans W C, Ribbons D W. New pathways in the oxidative metabolism of aromatic compounds by micro-organisms. Nature. 1960;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- 7.Davies J I, Evans W C. Oxidative metabolism of naphthalene by soil pseudomonads. Biochem J. 1964;91:251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denome S A, Stanley D C, Olson E, Young K D. Metabolism of dibenzothiophene and naphthalene in Pseudomonas strains: complete DNA sequence of an upper naphthalene catabolic pathway. J Bacteriol. 1993;175:6890–6901. doi: 10.1128/jb.175.21.6890-6901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn N W, Gunsalus I C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973;114:974–976. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton R W. Organization and evolution of naphthalene catabolic pathways: sequence of the DNA encoding 2-hydroxychromene-2-carboxylate isomerase and trans-o-hydroxybenzylidenepyruvate hydratase-aldolase from the NAH7 plasmid. J Bacteriol. 1994;176:7757–7762. doi: 10.1128/jb.176.24.7757-7762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton R W, Chapman P J. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J Bacteriol. 1992;174:7542–7554. doi: 10.1128/jb.174.23.7542-7554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggink G, Engel H, Vriend G, Terpstra P, Witholt B. Rubredoxin reductase of Pseudomonas oleovorans. Structural relationships to other flavoprotein oxidoreductases based on NAD and two FAD fingerprints. J Mol Biol. 1990;212:135–142. doi: 10.1016/0022-2836(90)90310-I. [DOI] [PubMed] [Google Scholar]
- 13.Ensley B D, Gibson D T. Naphthalene dioxygenase: purification and properties of a terminal oxygenase component. J Bacteriol. 1983;155:505–511. doi: 10.1128/jb.155.2.505-511.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ensley B D, Gibson D T, Laborde A L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982;149:948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ensley B D, Ratzkin B J, Osslund T D, Simon M J. Expression of naphthalene oxidation genes in E. coli results in the biosynthesis of indigo. Science. 1983;222:167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- 16.Entsch B, Palfrey B A, Ballou D P, Massey V. Catalytic function of tyrosine residues in p-hydroxybenzoate hydroxylase as determined by the study of site-directed mutants. J Biol Chem. 1991;266:17341–17349. [PubMed] [Google Scholar]
- 17.Fuenmayor S L, Rodriguez-Lemoine V. Characterization of polycyclic aromatic hydrocarbons degradative soil Pseudomonas. Acta Cient Venez. 1992;43:349–354. [PubMed] [Google Scholar]
- 18.Guerry P, LeBlanc D J, Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973;116:1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haigler B E, Gibson D T. Purification of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990;172:457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haigler B E, Gibson D T. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990;172:465–468. doi: 10.1128/jb.172.1.465-468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harayama S, Kok M. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- 22.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 23.Holmes D S, Quigley N. A rapid boiling method for preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 24.Jeffrey A M, Yeh H J C, Jerina D M, Patel T R, Davey J F, Gibson D T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975;14:575–583. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- 25.Kimbara K, Hashimoto T, Fukuda M, Koana T, Takagi M, Oishi M, Yano K. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J Bacteriol. 1989;171:2740–2747. doi: 10.1128/jb.171.5.2740-2747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kok M, Oldenhuis R, van der Linden M P, Meulenberg C H, Kingma J. Pseudomonas oleovorans alkABC operon encodes two structurally related rubredoxins and an aldehyde dehydrogenase. J Biol Chem. 1989;264:5442–5451. [PubMed] [Google Scholar]
- 27.Kurkela S, Lehväslaiho H, Palva E T, Teeri T H. Cloning, nucleotide sequence and characterization of genes encoding naphthalene dioxygenase of Pseudomonas putida strain NCIB 9816. Gene. 1988;73:355–362. doi: 10.1016/0378-1119(88)90500-8. [DOI] [PubMed] [Google Scholar]
- 28.Locher H H, Leisinger T, Cook A M. 4-Toluene sulfonate methyl-monooxygenase from Comamonas testosteroni T-2: purification and some properties of the oxygenase component. J Bacteriol. 1991;173:3741–3748. doi: 10.1128/jb.173.12.3741-3748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mermod N, Lehrbach P R, Reineke W, Timmis K N. Transcription of the TOL plasmid toluate catabolic pathway operon of Pseudomonas putida is determined by a pair of coordinately and positively regulated overlapping promoters. EMBO J. 1984;3:2461–2466. doi: 10.1002/j.1460-2075.1984.tb02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 31.Neidle E L, Harnett C, Ornston N, Bairoch A, Rekik M, Harayama S. Nucleotide sequences of the Acinetobacter calcoaceticus benABC genes for benzoate 1,2-dioxygenase reveal evolutionary relationships among multicomponent oxygenases. J Bacteriol. 1991;173:5385–5395. doi: 10.1128/jb.173.17.5385-5395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordlund I, Powlowski J, Shingler V. Complete nucleotide sequence and polypeptide analysis of multicomponent phenol hydroxylase from Pseudomonas sp. strain CF600. J Bacteriol. 1990;172:6826–6833. doi: 10.1128/jb.172.12.6826-6833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parales J V, Kumar A, Parales R E, Gibson D T. Cloning and sequencing of the genes encoding 2-nitrotoluene dioxygenase from Pseudomonas sp. JS42. Gene. 1996;181:57–61. doi: 10.1016/s0378-1119(96)00462-3. [DOI] [PubMed] [Google Scholar]
- 34.Platt A, Shingler V, Taylor S C, Williams P A. The 4-hydroxy-2-oxovalerate aldolase and acetaldehyde dehydrogenase (acylating) encoded by the nahM and nahO genes of the naphthalene catabolic plasmid pWW60-22 provide further evidence of conservation of meta-cleavage pathway gene sequences. Microbiology. 1995;141:2223–2233. doi: 10.1099/13500872-141-9-2223. [DOI] [PubMed] [Google Scholar]
- 35.Prieto M A, Garcia J L. Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli: a 2-protein component enzyme. J Biol Chem. 1994;269:22823–22829. [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Schell M A. Homology between nucleotide sequences of promoter regions of nah and sal operons of NAH7 plasmid of Pseudomonas putida. Proc Natl Acad Sci USA. 1986;83:369–373. doi: 10.1073/pnas.83.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schell M A. Regulation of the naphthalene degradation genes of plasmid NAH7: example of a generalized positive control system in Pseudomonas and related bacteria. In: Silver S, Chakrabarty A M, Iglewski B, Kaplan S, editors. Pseudomonas biotransformations, pathogenesis, and evolving biotechnology. Washington, D.C: American Society for Microbiology; 1990. pp. 165–176. [Google Scholar]
- 39.Schell M A, Poser E F. Demonstration, characterization, and mutational analysis of NahR protein binding to nah and sal promoters. J Bacteriol. 1989;171:837–846. doi: 10.1128/jb.171.2.837-846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreuder H A, Hol W G J, Drenth J. Analysis of the active site of flavoprotein p-hydroxybenzoate hydroxylase and some ideas with respect to its reaction mechanism. Biochemistry. 1990;29:3101–3108. doi: 10.1021/bi00464a029. [DOI] [PubMed] [Google Scholar]
- 41.Simon M J, Osslund T D, Sounders R, Ensley B D, Suggs S, Harcourt A, Suen W-C, Cruden D L, Gibson D T, Zylstra G J. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene. 1993;127:31–37. doi: 10.1016/0378-1119(93)90613-8. [DOI] [PubMed] [Google Scholar]
- 42.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 43.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 44.Suen W-C, Gibson D T. Isolation and preliminary characterization of the subunits of the terminal component of naphthalene dioxygenase from Pseudomonas putida NCIB 9816-4. J Bacteriol. 1993;175:5877–5881. doi: 10.1128/jb.175.18.5877-5881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suen W-C, Haigler B, Spain J C. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J Bacteriol. 1996;178:4926–4934. doi: 10.1128/jb.178.16.4926-4934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sylvestre M, Sirois M, Hurtubise Y, Bergeron J, Ahmad D, Shareck F, Barriault D, Guillemette I, Juteau J M. Sequencing of Comamonas testosteroni strain B-356 biphenyl/chlorobiphenyl dioxygenase genes: evolutionary relationship among Gram negative bacterial biphenyl dioxygenases. Gene. 1996;174:195–202. doi: 10.1016/0378-1119(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 47.Takizawa N, Kaida N, Torigoe S, Moritani T, Sawada T, Satoh S, Kiyohara H. Identification and characterization of genes encoding polycyclic aromatic hydrocarbon dihydrodiol dehydrogenase in Pseudomonas putida OUS82. J Bacteriol. 1994;176:2444–2449. doi: 10.1128/jb.176.8.2444-2449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takizawa, N., T. Iida, K. Yamauchi, S. Satoh, Y. Wang, M. Fukuda, and H. Kiyohara. 1996. GenBank accession no. D84146.
- 49.Wheatcroft R, Williams P A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981;124:433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]
- 50.Worsey M J, Williams P A. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 52.Yen K-M, Gunsalus I C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci USA. 1982;79:874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.You I-S, Murray R I, Jollie D, Gunsalus I C. Purification and characterization of salicylate hydroxylase from Pseudomonas putida PpG7. Biochem Biophys Res Commun. 1990;169:1049–1054. doi: 10.1016/0006-291x(90)92000-p. [DOI] [PubMed] [Google Scholar]
- 54.Zylstra, G. J. Personal communication.
- 55.Zylstra G J, Kim E, Goyal A K. Comparative molecular analysis of genes for polycyclic aromatic hydrocarbon degradation. Genet Eng. 1997;19:257–269. doi: 10.1007/978-1-4615-5925-2_14. [DOI] [PubMed] [Google Scholar]