Abstract
Adaptive motor behavior requires efficient error detection and correction. The posterior parietal cortex is critical for on-line control of reach-to-grasp movements. Here we show a causal relationship between disruption of cortical activity within the anterior intraparietal sulcus (aIPS) by transcranial magnetic stimulation (TMS) and disruption of goal-directed prehensile actions (either grip size or forearm rotation, depending on the task goal, with reaching preserved in either case). Deficits were elicited by applying TMS within 65 ms after object perturbation, which attributes a rapid control process on the basis of visual feedback to aIPS. No aperture deficits were produced when TMS was applied to a more caudal region within the intraparietal sulcus, to the parieto-occipital complex (putative V6, V6A) or to the hand area of primary motor cortex. We contend that aIPS is critical for dynamic error detection during goal-dependent reach-to-grasp action that is visually guided.
Everyday behaviors such as reaching for and grasping an object (reach-to-grasp movements) can be produced effortlessly despite a gauntlet of potential sources of error. Error may arise from uncompensated limb dynamics, from contextual changes in the extrinsic environment or from noise within the sensory and motor systems. The minimization of such error depends on a rapid on-line comparison between the issued motor command, current sensory information and the goal of the action. At present, little is known about the specific brain mechanisms participating in on-line adaptive control that minimize error during reach-to-grasp movements. Here we define a neural correlate that is critical for adaptive control of the human hand when grasping a visual object that is undergoing unexpected perturbations in size or orientation.
Reaching and grasping seem to involve dissociable processes1. In macaques, the reach component is mediated by a parieto-frontal circuit comprised of the superior parietal lobule and the dorsal premotor cortex2. Grasp is controlled by a more ventral circuit that involves the anterior inferior parietal lobule and the inferior frontal cortex3. The present study focused exclusively on the contribution of the anterior inferior parietal lobule to adaptive control of grasp.
Although the anatomy of the grasping circuit is well delineated in macaques4,5, an understanding of the functional contributions of nodes residing within this circuit is just beginning to emerge. In particular, electrophysiological recordings in nonhuman primates implicate a region along the anterior-lateral bank of the intraparietal sulcus (area AIP) in grasp-related control6–8. Neurons in AIP are recruited for visual and/or tactile object discrimination and are preferentially activated for various hand configurations during grasping of differently shaped objects7–11. Further, the causal involvement of AIP in grasp is demonstrated through pharmacological inactivation of this area with subsequent disruptions of hand preshaping during grasping12. Thus, it is postulated that AIP may furnish area F5 with visual signals of objects to aid in the selection of grasp configurations that are appropriate for their intrinsic attributes (shapes, sizes and orientations)7,8.
In humans, a grasp-specific region within the aIPS is proposed as the putative homolog to macaque area AIP. Patients with circumscribed lesions to the aIPS show marked deficits in hand preshaping during visually guided reach-to-grasp movements, whereas reaching remains relatively intact13. Several functional neuroimaging studies indicate that focal activation within the aIPS of the healthy brain occurs in association with visually guided grasping14–16. Together, these studies implicate aIPS in visually guided grasping but leave open the question of precisely what computations it implements.
One possibility is that the aIPS is involved in detecting and/or correcting errors in ongoing grasping movements on the basis of visual feedback in a manner similar to what more posterior parietal cortex does for reach-to-point movements. Indeed, patients with posterior parietal lesions are not able to readjust their hand path to a peri-saccade target jump, although they are able to reach-to-point to stationary targets perfectly well17,18. More direct evidence is provided by the application of TMS to a region just caudal to the aIPS, disrupting the ability to correct reach-to-point movements in response to abrupt changes in target location19. To test our hypothesis, subjects reached for and pincer-grasped a rectangular block whose long dimension was reoriented horizontally or vertically from an initial horizontal orientation by a motor (Fig. 1). In Experiment 1, subjects grasped the object under the constraint of always keeping the index finger and thumb oriented along an imaginary vertical axis (by appropriately scaling the aperture between the index finger and thumb). In Experiment 2, the subjects repeated the first experiment under the constraint of only grasping the narrow dimension (by appropriately rotating their forearm). This allowed us to investigate whether computations carried out within aIPS are exclusive to grasp alone or apply to other grasp-related processes, such as grasp orientation. Magnetic resonance imaging (MRI)-guided TMS was applied either early (at movement onset) or late (at the time of peak grip aperture) in the movement in an effort to disrupt neural activity either in the contralateral aIPS or in one of three control sites, while hand kinematics were recorded. We hypothesized that disruption of neural activity in the aIPS, but not in the other sites, would interfere selectively with adjustments of grasp that normally occur during perturbed trials—even on the first exposure to the perturbation.
Figure 1.

Reach-to-grasp adjustments required in Experiments 1 and 2. In most trials, the object rotated 180° (unperturbed), leaving the grasp aperture or grasp orientation unperturbed (left column). In a minority of trials, the object rotated 90° (perturbed), necessitating either an increase in grasp aperture (Experiment 1) or a change in grasp orientation (Experiment 2). Also shown are placements of the infrared light–emitting diodes, which allow tracking of hand position.
As predicted, TMS to the aIPS, but not to the other cortical sites, selectively disrupted adjustments in grasp only during those trials in which the object was perturbed. Notably, the deficits were specific to the goal of the task such that adjustment in aperture size was disrupted when that was the goal (Experiment 1) and adjustment in grip orientation was disrupted when that constituted the goal (Experiment 2). Finally, we show that this disruption can be elicited only by applying TMS over the aIPS within 65 ms after object perturbation, which indicates that it may be responsible for error-detection processes.
RESULTS
Experiment 1a
In support of our hypothesis, the effect of TMS was observed only during stimulation over aIPS but not when TMS was delivered to the other cortical sites (Fig. 2). Qualitatively, the aperture profiles of the early- and late-TMS conditions diverged from each other in the size-perturbed condition, but only when TMS was delivered to aIPS (Fig. 2). That this pattern is observed systematically in each of the nine subjects (remaining subjects shown in Fig. 3) underscores the critical role of the aIPS in correcting grasping movements in accordance with visual feedback. Stimulation of aIPS caused both enlargements (subject S2) and reductions (other subjects) of grip scaling in response to the object size perturbation, which is indicative of a general effect on planning aperture size.
Figure 2.
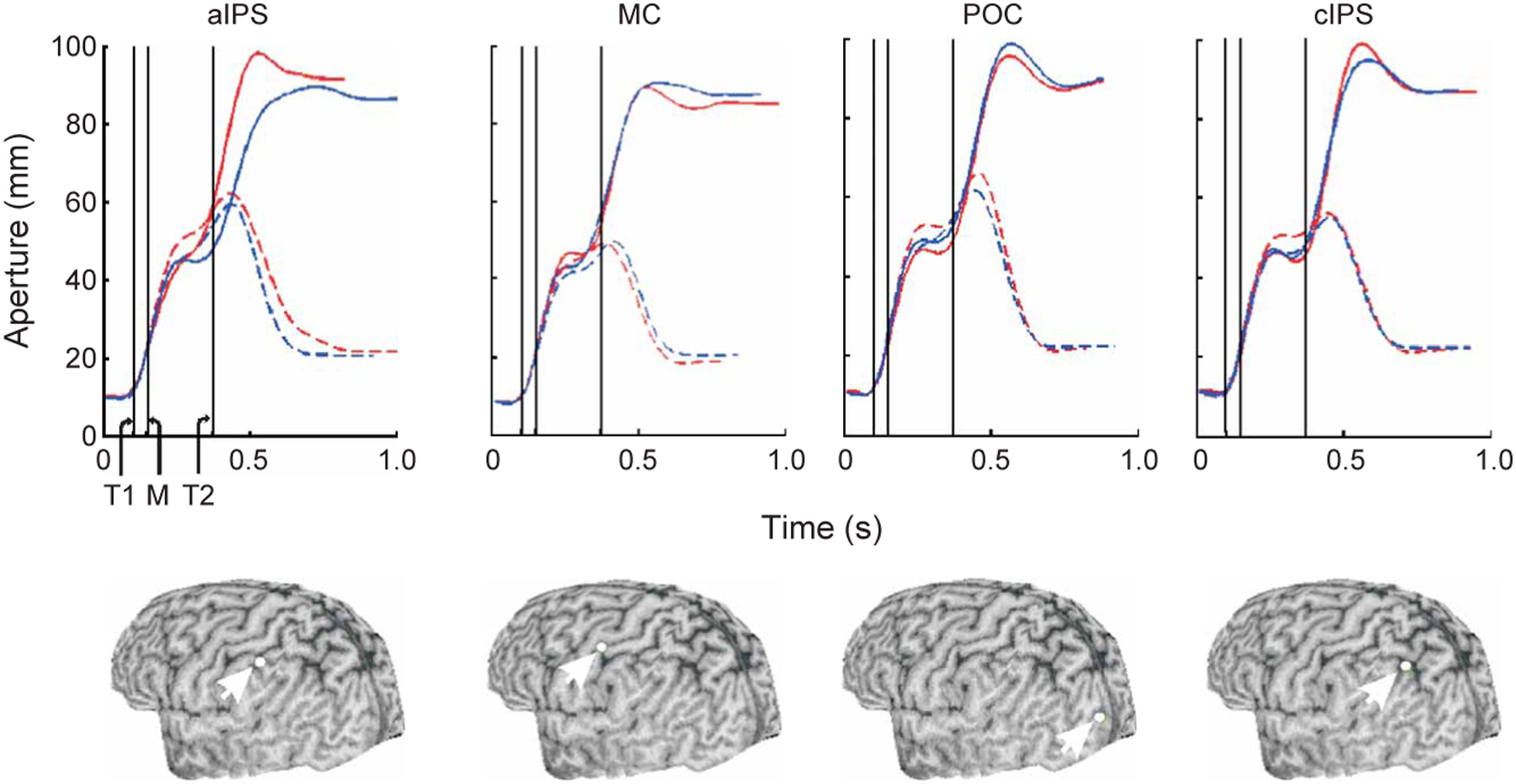
Mean grasp aperture profiles for one subject in Experiment 1. Below each panel is the reconstructed three-dimensional image of the subject’s brain and the target site (white dot) over which the TMS coil was positioned. Profiles in the unperturbed and perturbed conditions are plotted as hatched and solid lines, respectively. Trials in which TMS was delivered early and late are plotted in blue and red, respectively. Vertical lines: T1 and T2, time of the early and late TMS pulse; M, time of the motor rotation. MC, primary motor cortex. POC, parieto-occipital complex. cIPS, caudal to aIPS near apex of IPS.
Figure 3.
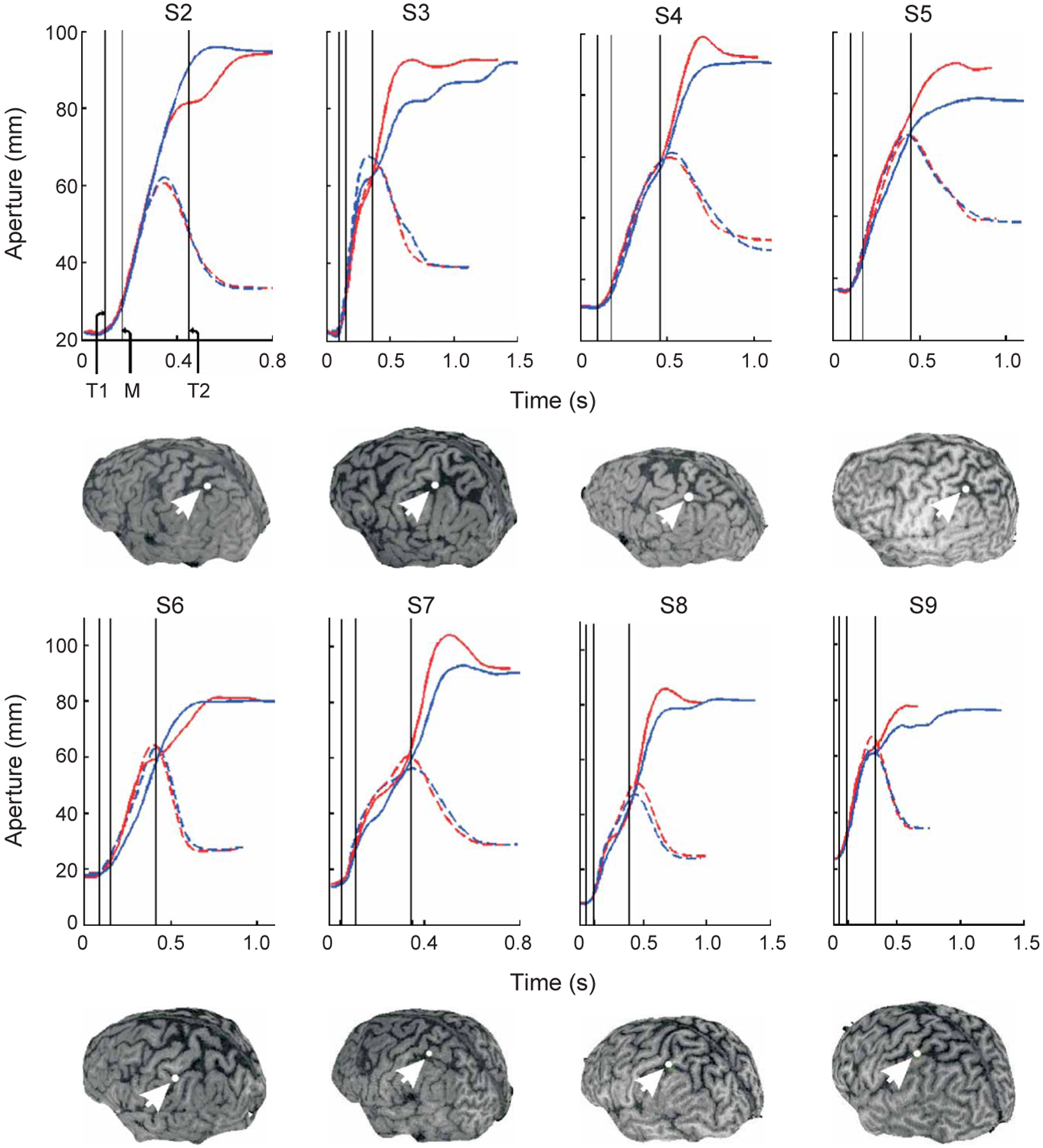
Mean aperture profiles for the remaining eight subjects (shown only for aIPS). Insets show the respective subject’s reconstructed three-dimensional brain image and the target site (white dot) over which the TMS coil was positioned. Profiles in the unperturbed and perturbed conditions are plotted as hatched and solid lines, respectively. Trials in which TMS was delivered early and late are plotted in blue and red, respectively. Vertical lines: T1 and T2, time of the early and late TMS pulse; M, time of the motor rotation.
The contribution of the aIPS to the adaptive control of hand posture was particularly susceptible to early versus late disruption. First, time to attain peak grip aperture (TPA) was affected differentially by early versus late TMS stimulation, but only when TMS was applied to aIPS (Fig. 4a; brain site × perturbation condition × TMS time interaction: F1,8 = 13.4, P < 0.01, mean square error (m.s.e.) = 8.1; perturbation condition × TMS time interaction for aIPS: F1,8 = 17.5, P < 0.01, m.s.e. = 9.9; P > 0.05 for all other brain sites). More precisely, delivery of an early versus late TMS pulse to aIPS induced a significant (group mean, 88 ms) delay in TPA in the object-perturbed condition (t8 = 4.9, P < 0.01) and a nonsignificant (group mean, 1 ms) delay in the unperturbed condition (P = 0.351). Similarly, the variance of TPA was differentially affected (brain site × perturbation condition × TMS time interaction: F1,8 = 11.6, P < 0.01, m.s.e. = 11.9; perturbation condition × TMS time interaction for aIPS: F1,8 = 20, P < 0.01; P > 0.05 for all other brain sites). Specifically, the difference in TPA variance between the early and late TMS conditions in the object size–perturbed condition (Fig. 4b; group mean, 87 ms; t8 = 4.1; P < 0.01) was 12 times greater than that in the size-unperturbed trials (group mean, 7 ms; P > 0.05). The time required to grasp the object (grasp movement time) was affected differentially by early versus late TMS stimulation, but only when TMS was applied to aIPS (Fig. 4c; brain site × perturbation condition × TMS time interaction: F1,8 = 23.3, P < 0.01, m.s.e. = 13.6; perturbation condition × TMS time interaction for aIPS: F1,8 = 26.9, P < 0.001, m.s.e. = 12.3; P > 0.05 for all other brain sites). Delivery of an early versus late TMS pulse to aIPS significantly prolonged grasp movement time by a group mean of 146 ms in the object size–perturbed condition (t8 = 6.3; P < 0.001), although it had a nonsignificant effect in the size-unperturbed condition (difference between group means, 25 ms; P > 0.05). Notably, the time taken to transport the hand to the object (reach movement time; see Methods for clarification between reach and grasp movement time) was not significantly affected (Fig. 4d; brain site × object perturbation condition × TMS time interaction, P > 0.05). Early TMS to aIPS thus led to general errors in grip sizing and specific delays in the timing of grasp aperture formation but not to deficits in reach. Furthermore, the observed deficits were present only in trials in which the object size was perturbed.
Figure 4.

Group mean (± s.e.m.) for grasp- and reach-related kinematics. (a–d) Shown is (a) the time to peak aperture, (b) the variance (s.d.) of the time to peak aperture, (c) the grasp movement time and (d) the reach movement time. Perturbed and unperturbed conditions are indicated by vertical and horizontal object orientations, respectively. The time of TMS delivery is indicated as either early or late. MC, primary motor cortex.
We analyzed the reliability of the observed effects of TMS to aIPS by carrying out preplanned comparisons for grasp movement time on an individual subject-by-subject basis. In seven of nine subjects (78%), early relative to late TMS delivery led to statistically significant delays in grasp movement time but only in the size-perturbed condition (S1: t = 4.9, P < 0.001; S2: P > 0.05; S3: t = 2.4, P = 0.02; S4: t = = 5.3, P < 0.0001; S5: t = 4.5, < 0.01; S6: P > 0.05; S7: t = 3.4, P < 0.01; S8: t = 2.2, P = 0.04; S9: t 2.4, P = 0.02). The frequency of TMS-induced effects in our study and in a previous study19 indicates that TMS may be an effective tool to investigate rapid adaptive behavior.
To summarize, we report a spatial and temporal specificity to the TMS-induced deficits. Specifically, only early TMS delivered to aIPS led to deficient adaptive responses in grip configuration in response to error arising from changes in context. These findings lend support to our hypothesis that aIPS is involved specifically in dynamic and context-dependent updating of grasp (see Discussion).
Experiment 1b
Having established that early TMS to aIPS disrupts context-dependent updating of grasp, we attempted to localize the precise time window within which this effect is reproducible. We delivered TMS to the aIPS of three participants from Experiment 1 (S1, S3 and S8) at 30-, 65-, 80- and 95-ms delays after completion of the object’s rotation. Significantly deleterious effects on grasp movement time could be produced only by TMS delivered 30 ms after object perturbation (Fig. 5; S1: t = 2.9, P = 0.02; S3: t = 2.6, P = 0.03; S8: t = 3.8, P < 0.01; P > 0.05 for all other conditions). This result implies that aIPS is capable of determining that visual cues necessitate a change in the action and of offloading any pertinent information to target brain sites in under 65 ms.
Figure 5.

Mean (± s.d.) grasp movement times in Experiment 1a. Subjects S1, S3 and S8 received TMS to aIPS either 30, 65, 80 or 95 ms after the end of object rotation. Movement was significantly prolonged only when TMS was delivered at the 30 ms time point but not thereafter, suggesting that processing within aIPS may have been completed by 65 ms after object perturbation. Note the absence of this effect in the object–non-perturbed condition.
Experiment 2
We next asked whether the aforementioned deficits that are elicited with stimulation over aIPS are specific to the adjustment of grip size or more generally affect the aspect of the reach-to-grasp action that must be reprogrammed on the basis of sensory feedback to achieve the actor’s intended goal. To this end, eight of nine participants from Experiment 1 were asked to reach-to-grasp the same rectangular object. In this experiment, however, they were told always to grasp its narrow dimension between the index finger and thumb by appropriately rotating the forearm (supinating the hand; Fig. 1). When this new goal was imposed, the trajectory profiles of forearm orientation differed notably between the early and late TMS conditions, but only in the object-perturbed case (Fig. 6a). Quantitatively, the time required to rotate the forearm accurately to the perturbed object orientation (rotation time) was significantly prolonged, but only when TMS was delivered early in the movement (mean ± s.d.: early TMS, 940 ± 70; late TMS, 780 ± 68 ms; Fig. 6b; perturbation condition × TMS time interaction: F1,7 = 44.2, P < 0.01, m.s.e. = 15.5; orientation-perturbed early TMS versus late TMS: t7 = 8.5, P < 0.0001). Forearm rotation time did not differ significantly between early and late TMS conditions in trials with unperturbed objects (mean ± s.d., 679 ± 58 versus 704 ± 65 ms, respectively; P > 0.05). Grasp movement time was also differentially affected across conditions. It was prolonged for early as compared to late TMS delivery, but only in the orientation-perturbed condition (mean ± s.d. of early versus late TMS: perturbed, 849 ± 77 versus 709 ± 78 ms; unperturbed, 710 ± 105 versus 717 ± 122 ms; perturbation condition × TMS time interaction: F1,7 = 29.7, P < 0.01, m.s.e. = 14.5; object-perturbed early TMS versus late TMS: t7 = 7.2, P < 0.001; orientation unperturbed, P > 0.05). The observation that grasp movement time was affected along with rotation movement time did not surprise us because grasp of the object could not be achieved until the forearm was properly oriented. As in Experiment 1a, the reach movement time was not significantly affected across conditions (all main effects and interactions, P > 0.05). Taken together with the results of Experiment 1a, this experiment underscores the goal-dependent nature of processing within aIPS during reach-to-grasp movements.
Figure 6.
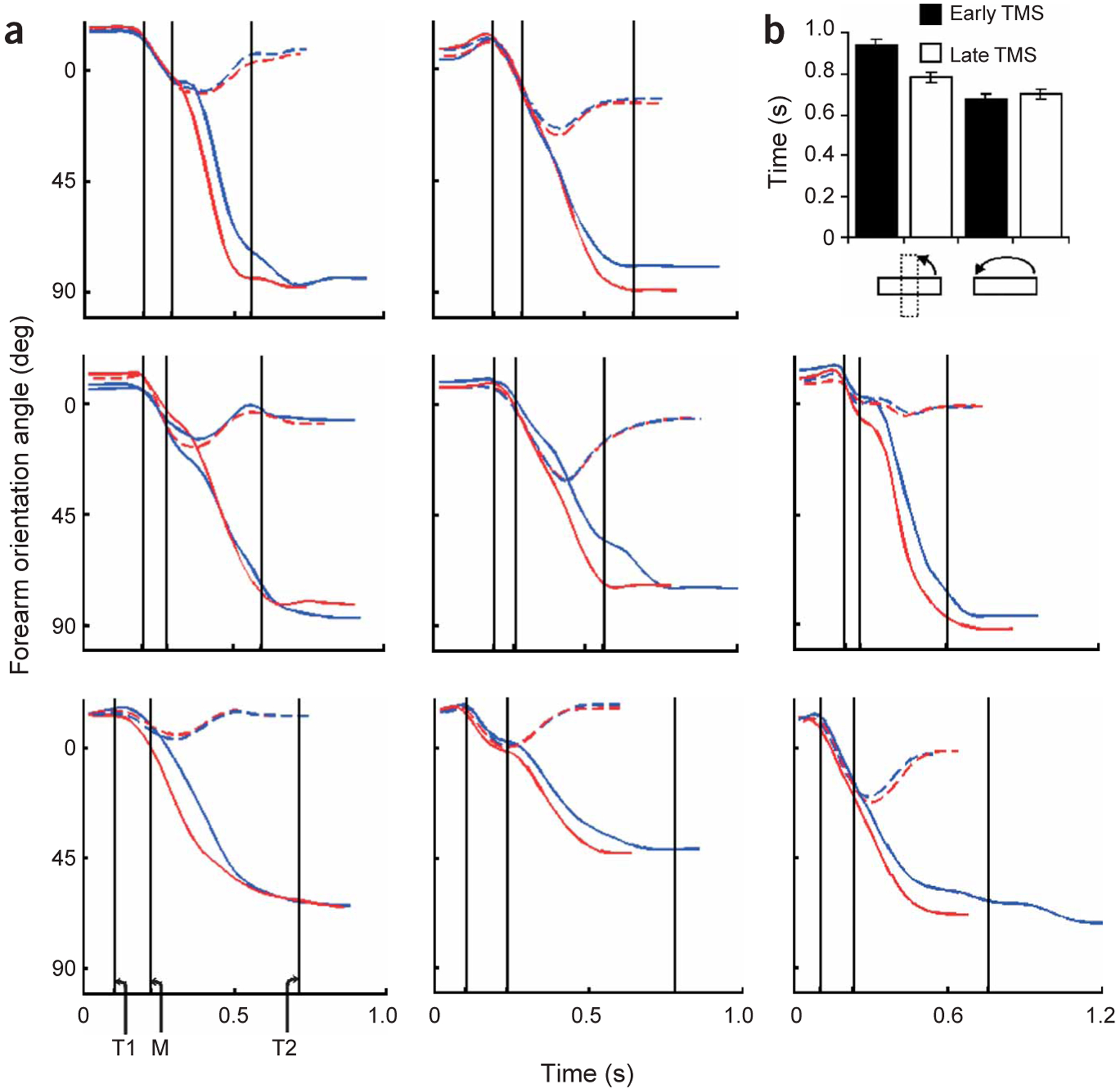
Movement kinematics for Experiment 2. (a) Mean profiles of the forearm (hand) rotation angle for eight subjects. The starting orientation is approximately 0°. The targeted orientation either remains 0° (in the unperturbed condition) or changes to about 90° (in the perturbed condition). Profiles in the unperturbed and perturbed conditions are plotted as hatched and solid lines, respectively. Trials in which TMS was delivered early and late are plotted in blue and red, respectively. Vertical lines: T1 and T2, time of the early and late TMS pulse; M, time of the motor rotation. (b) The mean time ± s.e.m. (n = 8) to rotate the forearm from the starting orientation (~0°) to match the final object orientation (~0° in the unperturbed case and ~90° in the perturbed case).
DISCUSSION
Here we show that in the context of a visual perturbation of a target object, disruption of processing within human aIPS caused significant deficiencies in adaptive reach-to-grasp kinematics. Further, the elicited deficits were goal dependent such that aperture-related deficits were produced if adjustment of grip size was the goal and forearm-related deficits were produced if adjustment of forearm orientation was the goal. Notably, aperture deficits were only apparent after magnetic stimulation over aIPS within 65 ms of object perturbation. These findings indicate that aIPS is causally involved in rapid, goal-dependent updating of reach-to-grasp actions.
Our findings in human subjects elaborate on an animal model in which the putative homolog of aIPS (AIP in macaques) furnishes premotor area F5 with visual signals to aid in the selection of grasp configurations and F5 reciprocates with an efference copy of the commanded signal7,8. This model limits AIP predominantly to a preplanning role and to one that is effector specific (such as the muscles and joints of the hand). Instead, our findings in humans indicate that information that is relevant to the reach-to-grasp movement may be fed back to aIPS (the putative homolog of AIP) iteratively during the evolution of the grasping movement. In such a feedback circuit, aIPS may be conceived of as a node that is responsible for integrating the efference copy of the motor command with incoming sensory input, either for detecting or correcting errors between these signals. Because deficient grasp kinematics could be elicited only by TMS delivery within 65 ms postperturbation, we contend that disruption of aIPS interfered with error detection as opposed to error correction. Consistent with the temporal specificity of our effect are electroencephalo-graphic recordings in humans20–22 and electrophysiological recordings in awake monkeys23 that show stimuli-elicited parietal activations with similar latencies, especially when attention to the task is maximized24. In this regard, it is notable that error correction for grasp is observable on a behavioral level after much longer latencies (>300 ms after object perturbation)25–28. Our results indicate, therefore, that error detection processes may be extremely rapid, and thus error correction processes may occupy the bulk of the latencies that are observed in grasp-related compensatory responses. This error signal, which is potentially a gain representing the magnitude of incongruence between efferent and sensory signals, may be essential for computations carried out by downstream centers for the on-line adjustment of action in changing environments. With respect to this, tracing studies have shown basal ganglia and cerebellar inputs to monkey AIP29. Taken with recent behavioral data that implicate the basal ganglia in addition to the cerebellum in on-line control30–33, it may be that these structures use the error signal provided by aIPS for error correction processes.
A second implication of our results is that processing within aIPS is likely to be goal dependent rather than limited to a particular subcomponent of the reach-to-grasp action (such as aperture adjustment only). This conclusion is supported by our findings that TMS delivery to aIPS selectively disrupted the kinematics related to either grip size or grip orientation depending on the aspect of the task that had to be reprogrammed to achieve the intended action. Notably, other regions along the IPS have been implicated distinctly in the learning and adjustment of hand path during reach-to-point movements19,34, adjustment of aperture size35 and goal-dependent hand (forearm) orientation (ref. 36; also see ref. 1). Against this backdrop, our findings indicate that current goals related to reach-to-grasp and reach-to-point movements may both be represented within and/or near aIPS and that the generated error signal may additionally contain a diagnostic component that describes a context-specific source to the error (in our case, aperture adjustment versus forearm rotation). In support of this, neurophysiological recordings in adjacent IPS regions of nonhuman primates show that neurons can have dynamic representations that are modulated by behaviorally relevant cues. For example, despite modest neural responses to auditory or color stimuli within the lateral intraparietal area, neurons of this area can represent these stimuli if the stimuli are behaviorally related to the goal of an eye movement37.
Whether goal-directed processes within aIPS generalize to other aspects of grasping remains open to discussion. It remains to be determined whether disruption within aIPS would interfere with grip force selection when a subject is confronted with unpredictably perturbed object weight and/or size. Thus far, neurophysiological recordings have been obtained only from somatosensory, premotor and cerebellar cortices during predictable perturbations of weighted objects; it has primarily been the dorsal anterior interpositus nucleus of the cerebellum that has shown significant anticipatory-related changes in firing patterns38. Given the goal-dependent nature of processing and the conglomeration of both visual and somatosensory neurons within aIPS, it would be interesting to investigate whether the function of this region is limited to visually driven error detection processes, such as those required in our study, or whether it encompasses a more-general error detection function that is related to grasp and that includes proprioceptively driven error detection (as would be required to overcome unpredictable changes in object weight).
In summary, we show a causal relationship between aIPS and dynamic, goal-dependent updating of reach-to-grasp actions. In this regard, aIPS may iteratively carry out rapid comparisons between the current motor command and sensory input, in the context of a given goal, during the evolution of the movement, continuously providing a diagnostic error signal that may be used by downstream structures to implement corrective actions.
METHODS
Subjects.
Nine healthy, right hand–dominant subjects (five females, four males; mean age ± s.d., 25 ± 1.3 years old) participated after providing written informed consents.
Procedure.
Seated subjects reached to grasp a rectangular object (1 cm × 1 cm × 5 cm) mounted on the shaft of a motor (Kollmorgen model no. S6MH4) and positioned on a table at wrist length along a sagittal axis from the subject’s right shoulder. The object was oriented horizontally at the start of each trial. Each trial began with a tone (440 Hz, 400 ms) cueing the subject to initiate movement. Subsequently, the release of a start button that was held by the subject between trials triggered two devices at predefined delays: the motor that perturbed the object orientation and a transcranial magnetic stimulator (see below). The object was to be pincer-grasped either with the index finger and thumb oriented along a vertical dimension such that the object’s narrow side would be grasped if its long axis was oriented horizontally and the wider side would be grasped if the long axis was oriented vertically (Experiment 1) or such that the object was always grasped along its narrow side (Experiment 2; Fig. 1).
The experiment consisted of three blocks. From the first block (15 trials), we obtained a normative TPA value for that subject (mean of the last 10 trials) to be used in setting the timing of the late TMS pulse and the motor rotation (100% and 20% of TPA, respectively). The second block (30 trials) was included to familiarize the subjects with the ‘default’ 180° object rotation (time for motor to complete rotation: 50 ms). No TMS was administered in the first two blocks, and the resulting data were not analyzed because their only purpose was parameter specification and familiarization—the perturbed condition (see below) was not introduced until the third block. In the third block (60 trials), the motor was used to manipulate the size (Experiment 1) or orientation (Experiment 2) of the graspable dimension of the object. For two-thirds of pseudorandomly selected trials, the object was rotated 180°, its final orientation remaining horizontal. For the remaining trials, the object was rapidly rotated 90° to a vertical orientation. This block was repeated for each brain site receiving TMS (counterbalanced across subjects; Experiment 1a only). Although the object was rotated both in 180° and 90° conditions, the task goal (size in Experiment 1; orientation in Experiment 2) was perturbed only in the 90° condition. Therefore, we refer to 180° rotations as ‘unperturbed’ trials and to 90° rotations as ‘perturbed’ trials.
Selection of control brain sites.
We chose three neural control sites to contrast against aIPS: (i) a region caudal to area aIPS near the apex of the IPS (cIPS), (ii) an extrastriate area that occupies the ventral part of the anterior bank of the parieto-occipital sulcus and the caudal part of the mesial precuneate cortex comprising areas V6 and V6A (parieto-occipital complex or POC) and (iii) the hand area of the primary motor cortex. Several studies using various methodological approaches (functional MRI, electrophysiological recordings and lesion studies) have linked the cIPS and POC sites to a variety of (mostly percept-based) functions related to grasping (cIPS9,10,39–42, POC43–46). Inclusion of these sites allowed us to rule out purely percept-based or motor deficits. Further, the spatial proximity between cIPS and aIPS serves as robust evidence for the specificity of TMS-induced effects. In Experiment 1, TMS was administered to primary motor cortex and aIPS in all nine subjects. These results are presented in the text. Additionally, six of the subjects received TMS to cIPS and POC. Given that these results remained consistent with those of the nine subjects, the results for the six subjects receiving TMS to cIPS and POC are presented in tabular form (Table 1). Because the specificity of the TMS-induced deficit was restricted to aIPS, Experiments 1a and 2 were conducted with TMS applied only to this cortical site.
Table 1.
Statistical group results of Experiment 1a for cortical sites aIPS, primary motor cortex, cIPS and POC
| Brain site × perturbation condition × TMS time | Perturbation condition × TMS time (aIPS) | Early-late TMS, size perturbed (aIPS) | Early-late TMS, size unperturbed (aIPS) | |
|---|---|---|---|---|
| TPA | F3,15 = 4.3, P = 0.02, m.s.e. = 8.3 | F1,5 = 8.5, P = 0.03, m.s.e. = 13.7 | t5 = −3, P = 0.03 | P > 0.05 |
| TPA variance | F3,15 = 3.8, P = 0.03, m.s.e. = 10 | F1,5 = 7.6, P = 0.04, m.s.e. = 13.4 | t5 = 2.7, P = 0.04 | P > 0.05 |
| Grasp movement time | F3,15 = 6.9, P < 0.01, m.s.e. = 12.6 | F1,5 = 13.6, P = 0.01, m.s.e. = 14.9 | t5 = −4.4, P < 0.01 | P > 0.05 |
| Reach movement time | P > 0.05 | n.a. | n.a. | n.a. |
Summary of statistical results (Experiment 1a) for six subjects to whom TMS was delivered to aIPS, primary motor cortex, cIPS and POC. Two-way interactions were significant only for the aIPS site (column 3). Columns 4 and 5 show results for subsequent preplanned comparisons for the aIPS site.
Localization of brain sites and TMS.
A high-resolution three-dimensional volumetric structural MRI (General Electric Horizon whole-body 1.5T MRI scanner with a standard birdcage head coil) was obtained for each subject, and the cortical surface was displayed as a three-dimensional representation using Brainsite Frameless stereotaxic software (Rogue-Research). Each targeted cortical site was demarcated on the three-dimensional image using the same software. The position of the coil and the subject’s head were monitored using a Polaris Optical Tracking System (Northern Digital, Inc.). Positional data for both rigid bodies were registered in real time to a common frame of reference and were superimposed on the reconstructed three-dimensional MRI image of the subject using the Brainsite software. Thus, the center of the coil (stimulation locus) was continuously monitored to be over the site of interest. To minimize head movement, a chin rest supported the subject’s head.
A Neotonus PNS stimulator (model no. N-0233-A-110V) with an air-cooled iron-core butterfly-shaped coil was used to administer single-pulsed magnetic fields. Pulse duration for this stimulator and head coil is 180 μs (at 100% of operating power). The interstimulus (that is, intertrial interval) was about 10 s. The motor threshold of TMS was determined as the intensity required to produce a visible contraction of the intrinsic hand muscles 50% of the time with the coil positioned over the hand area of the primary motor cortex. The intensity used during the experiment was 110% of this threshold. The onset of stimulation was coincident with the button release (early TMS) or with the time at which peak aperture was reached (late TMS). Thus, on any given trial, the subject received one (either early or late) TMS pulse to the targeted site. Depending on whether the motor rotated 180° or 90°, the early and late TMS conditions had 20 or 10 trials, respectively. Early- and late-TMS trials, and motor rotation conditions were randomly assigned and counterbalanced over the course of a block (that is, brain site).
Analysis and statistics.
Kinematic data were obtained by localizing the three-dimensional position of infrared light–emitting diodes taped to the index fingertip, thumb and the first metacarpophalangeal joint (m.c.p.; Optotrak 3020, Northern Digital, Inc.; sampling rate, 100 Hz). Off-line, missing samples were interpolated and the data were low-pass filtered (10 Hz) and were analyzed using custom-written Matlab (Mathworks) and Labview (National Instruments) software. Grasp aperture was defined as the three-dimensional distance between the index and thumb markers. Positional data for each marker, as well as the aperture, were differentiated to compute the index, thumb, m.c.p. and aperture velocity profiles. The onset of reach and grasp movements was determined as the moment when the m.c.p. marker and the aperture velocities exceeded 5% of the respective peak velocity. Movement offset for the reach was defined as the point at which the tangential velocity of the m.c.p. marker fell and remained below 5% of peak velocity. Movement offset for the grasp was defined as the moment at which the object was grasped by both fingers (aperture velocity was zero). Movement time for the reach and grasp components, therefore, was obtained by subtracting the movement onset from the respective movement offset. To analyze forearm (hand) rotation (Experiment 2), we computed the angle between the lateral direction and the frontal projection of the normal to a plane defined by the index, thumb and m.c.p. markers. From this, angular velocity was derived, movement onset and offset were identified based on a 5% peak threshold and the time required to rotate the forearm was computed by subtracting the onset from the offset.
Each dependent variable was subjected to either a three-way (factors: brain site, perturbation condition, TMS time) or a two-way (perturbation condition, TMS time) repeated-measures analysis of variance. Preplanned t-tests were used for subsequent analysis. The threshold for statistical significance was set at 0.05. For conciseness, only significant findings are reported.
ACKNOWLEDGMENTS
S.H. Frey was formerly S.H. Johnson. P. Schmitt provided critical technical assistance. This work was supported by Public Health Service grant NS044393 to S.T.G.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Johnson SH & Grafton ST From ‘acting on’ to ‘acting with’: the functional anatomy of object-oriented action schemata. Prog. Brain Res 142, 127–139 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Marconi B et al. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb. Cortex 11, 513–527 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Rizzolatti G & Matelli M Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res 153, 146–157 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Luppino G, Murata A, Govoni P & Matelli M Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp. Brain Res 128, 181–187 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Rizzolatti G & Luppino G The cortical motor system. Neuron 31, 889–901 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Taira M, Mine S, Georgopoulos AP, Murata A & Sakata H Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp. Brain Res 83, 29–36 (1990). [DOI] [PubMed] [Google Scholar]
- 7.Sakata H, Taira M, Murata A & Mine S Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb. Cortex 5, 429–438 (1995). [DOI] [PubMed] [Google Scholar]
- 8.Murata A, Gallese V, Luppino G, Kaseda M & Sakata H Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J. Neurophysiol 83, 2580–2601 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Sakata H et al. Neural coding of 3D features of objects for hand action in the parietal cortex of the monkey. Phil. Trans. R. Soc. Lond. B Biol. Sci 353, 1363–1373 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakata H et al. Neural representation of three-dimensional feature of manipulation of objects with stereopsis. Exp. Brain Res 128, 160–169 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Murata A, Gallese V, Kaseda M & Sakata H Parietal neurons related to memory-guided hand manipulation. J. Neurophysiol 75, 2180–2186 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Gallese V, Murata A, Kaseda M, Niki N & Sakata H Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport 5, 1525–1529 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Binkofski F et al. Human anterior intraparietal area subserves prehension. Neurology 50, 1253–1259 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Binkofski F et al. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur. J. Neurosci 11, 3276–3286 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Culham JC et al. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp. Brain Res 153, 180–189 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Frey S, Vinton D, Newman-Norlund R & Grafton ST Cortical topography of human anterior intraparietal cortex active during visually-guided grasping. Brain Res. Cogn. Brain Res (in the press). [DOI] [PubMed] [Google Scholar]
- 17.Pisella L et al. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat. Neurosci 3, 729–736 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Gréa H et al. A lesion of the posterior parietal cortex disrupts on-line adjustments during aiming movements. Neuropsychologia 40, 2471–2480 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Desmurget M et al. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat. Neurosci 2, 563–567 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Morand S et al. Electrophysiological evidence for fast visual processing through the human koniocellular pathway when stimuli move. Cereb. Cortex 10, 817–825 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Foxe JJ & Simpson GV Flow of activation from V1 to frontal cortex in humans: A framework for defining “early” visual processing. Exp. Brain Res 142, 139–150 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Fornells A, Kurzbuch AR & Münte TF Time course of error detection and correction in humans: Neurophysiological evidence. J. Neurosci 22, 9990–9996 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder CE, Mehta AD & Givre SJ A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb. Cortex 8, 575–592 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Martinez A et al. Putting spatial attention on the map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Res. 41, 1437–1457 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Paulignan Y, Jeannerod M, MacKenzie C & Marteniuk R Selective perturbation of visual input during prehension movements. 2. The effects of changing object size. Exp. Brain Res 87, 407–420 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Castiello U, Bennett KM & Stelmach GE Reach to grasp: the natural response to perturbation of object size. Exp. Brain Res 94, 163–178 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Jeannerod M Coordination mechanisms in prehension movements. in Tutorials in Motor Behavior II. Advances in Psychology Vol. 87 (eds. Stelmach GE & Recquin J) 265–286 (Elsevier, New York, 1992). [Google Scholar]
- 28.MacKenzie CL & Iberall T The grasping hand. in Advances in Psychology Vol. 104 (eds. Stelmach G & Vroon P) 162–166 (Elsevier, New York, 1994). [Google Scholar]
- 29.Clower DM, Dum RP & Strick PL Basal ganglia and cerebellar inputs to ‘AIP’. Cereb. Cortex advance online publication, 30 September 2004. (doi: 10.1093/cercor/bhh190). [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Brandt J & Shadmehr R Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature 403, 544–549 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desmurget M et al. On-line motor control in patients with Parkinson’s disease. Brain 127, 1755–1773 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Tunik E, Adamovich SV, Poizner H & Feldman AG Deficits in rapid adjustments of movements according to task constraints in Parkinson’s disease. Mov. Disord 19, 897–906 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Tunik E, Poizner H, Adamovich SV, Levin MF & Feldman AG Deficits in adaptive upper limb control in response to trunk perturbations in Parkinson’s disease. Exp. Brain Res 159, 23–32 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Della-Maggiore V, Malfait N, Ostry DJ & Paus T Stimulation of the posterior parietal cortex interferes with arm trajectory adjustments during the learning of new dynamics. J. Neurosci 24, 9971–9976 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glover S, Miall RC & Rushworth MF Parietal rTMS disrupts the initiation but not the execution of on-line adjustments to a perturbation of object size. J. Cogn. Neurosci 17, 124–136 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Johnson SH et al. Selective activation of a parieto-frontal circuit during implicitly imagined prehension. Neuroimage 17, 1693–1704 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Anderson RA & Buneo CA Sensorimotor integration in posterior parietal cortex. in Advances in Neurology. The Parietal Lobes Vol. 93 (eds. Siegel AM, Anderson RA, Freund H-J & Spencer DD) 159–177 (Lippincott Williams & Wilkins, New York, 2003). [PubMed] [Google Scholar]
- 38.Monzee J & Smith AM Responses of cerebellar interpositus neurons to predictable perturbations applied to an object held in a precision grip. J. Neurophysiol 91, 1230–1239 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Shikata E et al. Surface orientation discrimination activates caudal and anterior intraparietal sulcus in humans: an event-related fMRI study. J. Neurophysiol 85, 1309–1314 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Shikata E et al. functional properties and interaction of the anterior and posterior intraparietal areas in humans. Eur. J. Neurosci 17, 1105–1110 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Saito DN, Okada T, Morita Y, Yonekura Y & Sadato N Tactile-visual cross-modal shape matching: a functional MRI study. Brain Res. Cogn. Brain Res 17, 14–25 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Sakata H, Taira M, Kusunoki M, Murata A & Tanaka Y The TINS Lecture. The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci. 20, 350–357 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Galletti C, Fattori P, Kutz DF & Battaglini PP Arm movement-related neurons in the visual area V6A of the macaque superior parietal lobule. Eur. J. Neurosci 9, 410–413 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Galletti C, Kutz DF, Gamberini M, Breveglieri R & Fattori P Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp. Brain Res 153, 158–170 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Zangaladze A, Epstein CM, Grafton ST & Sathian K Involvement of visual cortex in tactile discrimination of orientation. Nature 401, 587–590 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Battaglini PP et al. Effects of lesions to area V6A in monkeys. Exp. Brain Res 144, 419–422 (2002). [DOI] [PubMed] [Google Scholar]


