Abstract
Background
The majority of studies assessing executive function in attention deficit disorder (ADD) have shown deficits in attentional set shifting using either the Wisconsin card sorting task or the intra-dimensional/extra-dimensional set-shifting task (ID/ED). Damage to the prefrontal cortex in humans, primates, and rodents impairs extra-dimensional (ED) shifts. Noradrenergic depletion of the medial prefrontal cortex in rats is sufficient to impair attentional set shifting. Atomoxetine, a selective norepinephrine (NE) reuptake inhibitor, is hypothesized to produce beneficial effects in patient with ADD by augmenting NE release in prefrontal cortex.
Materials and methods
We assessed the effects of systemic administration of atomoxetine (0.0, 0.1, 0.3, and 0.9 mg/kg/ml) in normal and noradrenergically lesioned (NE-LX) rats on attentional-set shifts. We replicated findings showing NE-LX rats are selectively impaired on the ED shifts but not reversals or other discriminations.
Results
Atomoxetine remediated the attentional set-shifting impairments in NE-LX rats but impaired ED performance of non-lesioned rats.
Discussion
Though atomoxetine is neurochemically selective, it is not wholly specific at doses >0.3 mg/kg. All doses of the drug were similar in their efficacy in reversing the ED deficit, but the effectiveness of the 0.1 mg/kg dose supports the hypothesis that increases in prefrontal NE alone are sufficient to improve attention in NE-LX rats. Moreover, the detrimental effects of the drug in non-lesioned rats support the hypothesis that optimal levels of NE in prefrontal cortex are critical to attentional set shifting with both supra- and sub-optimal levels producing attentional impairments.
Keywords: Executive function, ADHD, Attentional set shift, Infralimbic cortex, Prelimbic cortex
Introduction
Imbalances in catecholamine systems are hypothesized to underlie cognitive deficits in attention deficit disorder (ADD) while many studies support a role for dopaminergic dysfunction (Barkley 1997; Sagvolden and Sergeant 1998; Castellanos and Tannock 2002; Viggiano et al. 2004b), and recent data suggest that dysfunction in norepinephrine (NE) may also contribute to cognitive impairments in ADD (Arnsten 1997; Arnsten 1998; Viggiano et al. 2004a; Arnsten 2006). Psychostimulants used to alleviate these impairments increase both dopamine and NE levels (Solanto 1984; Mehta et al. 2004; Wilens 2006). Recent data from Berridge and colleagues show that low doses of psychostimulants increase prefrontal catecholamine efflux but have less effect on catecholamine levels in the nucleus accumbens (Berridge et al. 2006). It is hypothesized that increases in accumbens dopamine levels underlie the addictive properties of stimulants (Koob and LeMoal 1997), so decreasing the addiction liability may require compounds that produce minimal changes in subcortical dopamine.
Atomoxetine (tomoxetine, LY 139603) is a newer generation, non-stimulant drug used to treat ADD (Caballero and Nahata 2003; Kratochvil et al. 2003; Christman et al. 2004; Kratochvil et al. 2006) with minimal addictive liability. Atomoxetine augments prefrontal NE levels with little effect on cortical dopamine and in the absence of increases in catecholamine increases in nucleus accumbens (Bymaster et al. 2002; Tzavara et al. 2007). This profile is hypothesized to remediate cognitive impairments in patients with ADD and minimize addiction liability (Wong et al. 1982; Bymaster et al. 2002). In addition to a low liability for abuse, it has fewer side effects relative to the psychostimulants also used to treat ADD (Caballero and Nahata 2003; Kratochvil et al. 2003; Christman et al. 2004; Kratochvil et al. 2006). Based on its utility for treating ADD and fewer adverse effects, it is important to understand the cognitive effects of atomoxetine as it relates to the treatment of ADD. Previous studies in humans have assessed the effects of atomoxetine on impulsivity (Robinson et al. 2007) and measures of daily functioning in ADD (Caballero and Nahata 2003; Kratochvil et al. 2003; Christman et al. 2004; Kratochvil et al. 2006). The administration of atomoxetine to normal rats and humans has been shown to decrease impulsivity (Chamberlain et al. 2006; Blondeau and Dellu-Hagedorn 2007; Robinson et al. 2007) but less is known about the effects of this compound in attentional set shifting (Spencer et al. 1998; Chamberlain et al. 2007).
Norepinehrine has been shown to be critical to selective attention (Devauges and Sara 1990; Aston-Jones et al. 1994; Aston-Jones et al. 2000; Dalley et al. 2001; Dalley et al. 2004; Aston-Jones and Cohen 2005) and attentional set shifting (Lapiz and Morilak 2006; Lapiz et al. 2007; Tait et al. 2007). In the intra-dimensional/extra-dimensional (ID/ED) test of attentional set shifting, subjects are reinforced for focusing attention on one perceptual attribute while disregarding all other attributes. During the extra-dimensional shift, a previously irrelevant attribute predicts reward requiring subjects to disengage attention from the previously relevant attribute and learn that a new attribute predicts reinforcement (Owen et al. 1991, 1993; Dias et al. 1996a, b; Birrell and Brown 2000). Noradrenergic deafferentation of prefrontal cortex selectively impairs performance on the extra-dimensional shift (McGaughy et al. 2008; Tait et al. 2007) in a manner similar to pharmacological manipulations that decrease cortical NE (Lapiz and Morilak 2006).
Performance in the ID/ED task is impaired in children with ADD and can be remediated by methylphenidate that increases both dopamine and NE levels (Mehta et al. 2004). In the present study, we investigate the efficacy of atomoxetine to selective increase NE and facilitate attentional set shifting in both sham-lesioned (SHAM-LX) and noradrenergically lesioned (NE-LX) rats. Based on the ability of this compound to improve other aspects of executive function in normal subjects, we hypothesized that atomoxetine would facilitate performance in SHAM-LX rats. Additionally, we predicted that NE-LX rats would have greater deficits in attentional set shifting and require higher doses of the drug than SHAM-LX rats.
Materials and methods
Animals
Forty-eight Long Evans hooded rats (Harlan Indianapolis, IN, USA) were housed individually and maintained on a 12-h light/dark cycle with lights on at 7:00 A.M. with testing performed during the light cycle. Rats were moderately food restricted to maintain a weight at 90% of age-matched controls and were given water ad libitum. Prior to the initiation of any behavioral testing, rats were handled for 5 min each day. All procedures were implemented in a manner consistent with NIH guidelines for animal care and use and approved by the Institutional Care and Use Committee of the University of New Hampshire.
Surgery
Rats were anesthetized with an intra-muscular injection of ketamine (85 mg/kg/ml) and xylazine (8.5 mg/kg/ml) then placed in a stereotaxic frame using atraumatic ear bars. Rats received either lesions of the noradrenergic afferents to the prefrontal cortex using a solution of 0.01 μg/μl dopamine beta-hydroxylase saporin (DBH-SAP) in a sterile phosphate buffer or sham lesions produced by infusing sterile phosphate buffer into the medial prefrontal cortex. All infusions were made at the following coordinates (in mm)—toothbar: −3.3; anteroposterior: bregma +2.8; mediolateral: bregma ±0.6; dorsoventral: skull −5.2 using a 26-gauge, 10-μl microsyringe attached to an electronic infusion pump (Micro 4™ Microsyringe Pump Controller, World Precision Instruments, Sarasota, FL, USA). To prevent unwanted diffusion, the toxin or its vehicle was infused at a rate of 125 nl/min with the needle left in place for 4 min before and after infusion. Post-surgery animals were given 7 days of recovery time to allow retrograde transport of the toxin and apoptotic cell death. During recovery, rats were given ad libitum food and water. After the recovery period, animals were food restricted and allowed 18 g of chow per day with ad libitum water. Following 3 days of food restriction, animals began training to dig for reinforcement.
Apparatus
Rats were trained to dig terra-cotta pots with a height of 10 cm and an internal diameter of 10.2 cm. The outer surface of the pots were covered with textures, and the inside of the pots were filled with digging media which were scented using diluted aromatherapy oils (essential oils diluted 1:100 in vegetable oil; see Table 1). Training and testing was performed in a plastic testing box (91.44 × 45.72 × 25.40 cm, L × W × H) which was divided lengthwise into three sections. Test stimuli were placed in either end of the testing box with access to these sections prevented by a divider while stimuli were placed in the distal end of the box. Reinforcement for both training and testing was 1/6 of a Frosted Cheerio™ cereal (General Mills, Minneapolis, MN, USA). For all stages of training and testing, the unbaited pot contained an equal, but crushed, amount of reinforcer to prevent the rats from using the scent of cereal to identify the correct stimuli.
Table 1.
Examples of stimuli used
| Task | Testing pair 1 | Testing pair 2 | Never relevant attribute |
|---|---|---|---|
|
| |||
| SD | Cinnamon/light shapes+ vs. patchouli/light shapes | Cinnamon/dark shapes+ vs. patchouli/dark shapes | Tan fake fur |
| CD | Cinnamon/light shapes+ vs. patchouli/dark shapes | Cinnamon/dark shapes+ vs. patchouli/light shapes | Fake fur |
| Rev1 | Patchouli/light shapes+ vs. cinnamon/dark shapes | Patchouli/dark shapes+ vs. cinnamon/light shapes | Fake fur |
| ID | Lilac/gold buttons+ vs. rose/black buttons | Lilac/black buttons+ vs. rose/gold buttons | Terry cloth |
| Rev2 | Rose/Gold buttons+ vs. lilac/black buttons | Rose/black buttons+ vs. lilac/gold buttons | Terry cloth |
| ED | Metal beads/gardenia+ vs. plastic beads/jasmine | Metal beads/jasmine+ vs. plastic beads/gardenia | Ribbed-side corduroy |
| Rev3 | Plastic beads/gardenia+ vs. metal beads/jasmine | Plastic beads/jasmine+ vs. metal beads/gardenia | Ribbed-side corduroy |
| Learned irrelevance | Plastic beads/gardenia+ vs. metal beads/jasmine | Plastic beads/jasmine+ vs. metal beads/gardenia | Smooth side corduroy |
Post-surgical training
Day 1: shaping
All training and testing was conducted from 9 A.M. to 12 P.M. Rats were placed in the testing box (88 × 42 × 30 cm, L × W × H; Sterilite Corp., Townsend, MA, USA) with an unscented terra-cotta pot filled with pine chip bedding. A rat was placed behind a divider while the pot was placed in the distal end of the box (Fig. 1). This procedure allowed the experimenter to control the rat’s access to a stimulus. A stopwatch was begun after removal of the divider and response latency recorded after the animal displaced digging media in the pot using either its paw or nose. A piece of cereal (reinforcer) was initially placed on top of the bedding and the rat was allowed 90 s to retrieve it. After the expiration of the limited hold or retrieval of the reward by the rat, the pot was removed from the box. In between trials, the rat was again placed behind the divider as the pot was placed in the testing box. The position of the pot was varied randomly from right to left within a testing area to prevent the development of response bias. After the animal readily retrieved ten unburied rewards, the reinforcer was buried at increasing depths until the rat completed ten consecutive successful trials recovering a fully buried reward with his forepaw.
Fig. 1.
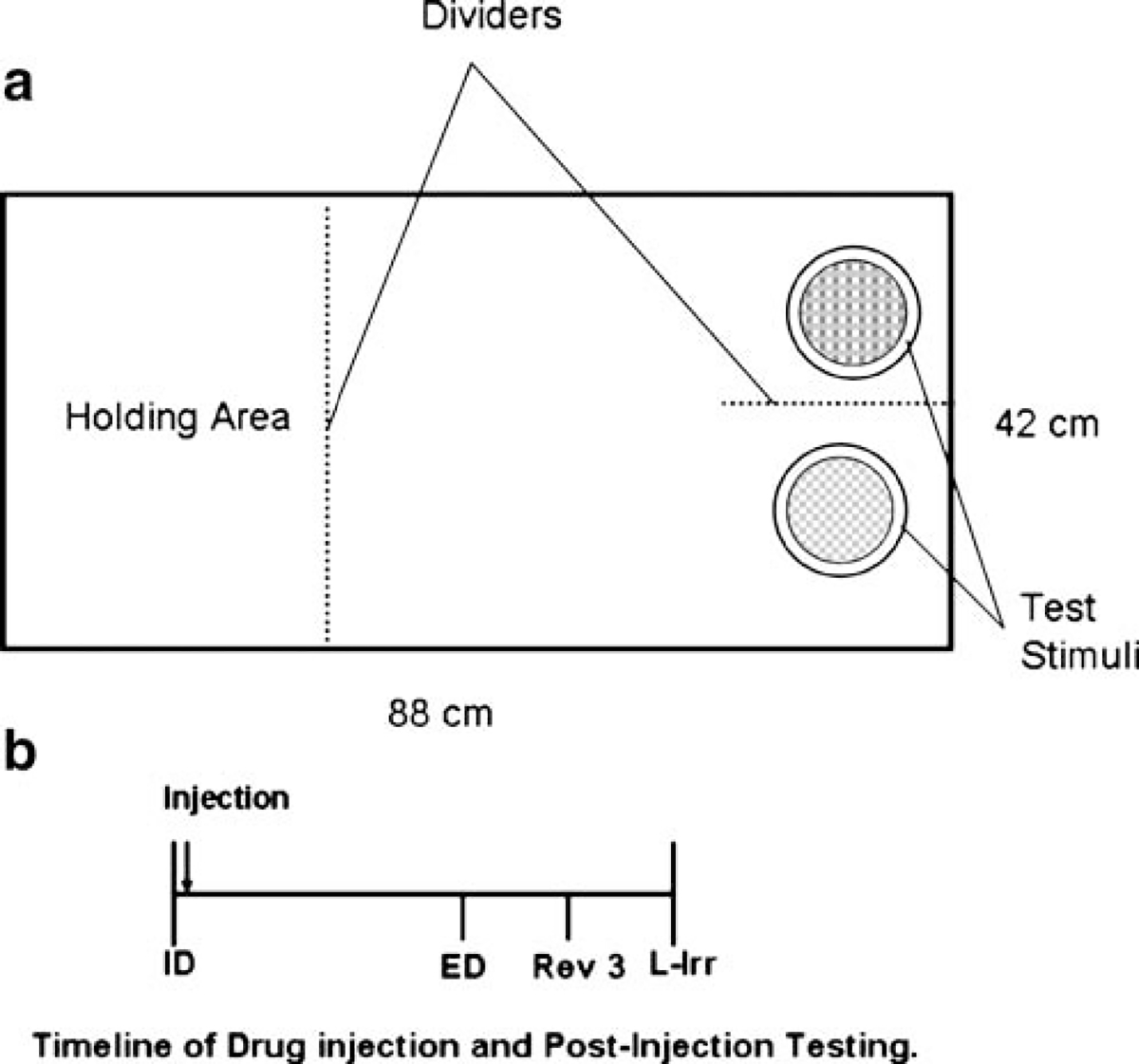
a Schematic diagram of the testing box. Rats were tested in a white box (88 × 42 × 30 cm, L × W × H). A removable divider was inserted into the box 29 cm from one end of the box, and the rat was placed in this holding area prior to the initiation of a trial. A single bedding-filled pot was placed in the center of the testing area while rats were shaped to dig. During exemplar training and subsequent testing, the experimenter placed two stimulus pots in the distal end of the box approximately 20 cm apart and segregated these pots by insertion of a second removable divider. When the test stimuli were ready, the divider separating the holding area from the testing area was removed, and the rat was allowed to investigate the test pots. Response latencies were recorded from the time this divider was removed until the rat displaced digging media in one of the pots. The first four trials of any discrimination were discovery trials designed to facilitate learning. On these trials, rats were given a 90-s limited hold to make a response. Additionally, if the subject made an incorrect first choice, the barrier between pots was removed and he was allowed to explore the second, correct pot to retrieve the reinforcer before test stimuli were removed from the box. On subsequent trials, the limited hold was abbreviated to 60 s. In the case of an incorrect choice on these trials, both pots were immediately removed from the testing box and the animal received no reinforcement. After the removal of the test stimuli, a divider was inserted into the box so the test area became the holding area for the subsequent trial. A subject remained here until test stimuli were readied in the distal end of the box and the next trial began. The side of reinforcement was randomly assigned to either the left or right side of the testing area. b A timeline of drug injection and post-injection testing are shown. Upon completion of the ID, rats were injected with atomoxetine. ED testing began 30 min later. Upon completion of ED testing, Rev3 was performed. The test of learned irrelevance (L-IRR) was complete immediately after Rev3. Rats required 30.6±1.2 min (mean ± SEM) to complete all post-injection tests
Day 2: exemplar training
After training to dig for reward, rats were given a series of conditional discriminations that exposed the animals to the types of stimuli that would be tested in the ID/ED task: odor, digging media, and texture. Behavioral procedures were identical to those used in shaping to dig except that the single, bedding-filled pot was replaced by two stimulus pots (Fig. 1). In these trials, only one dimension was presented on the two pots, e.g., digging media. Two different exemplars of each dimension were presented, and the rat was consistently reinforced for digging in one exemplar. Exemplars for each dimension were, digging media: shredded green tissue paper vs. shredded white tissue paper; texture: black fake fur with 2.5 cm pile vs. the reverse side of the fur; odor: pine vs. black cherry. In the case of odor and texture discriminations, both pots were filled with shredded manilla folder to hide the reinforcer. In all trials, an equal amount of crushed reinforcer was placed in the non-baited pot to prevent rats from using the scent of the reward to cue responding. These exemplars were not used again.
Behavioral testing
Day 3: intra-dimensional/extra-dimensional set shifting task
After exemplar training, rats began testing in the ID/ED task which consisted of the following subtests: the simple discrimination (SD), compound discrimination (CD), the intra-dimensional (ID) and extra-dimensional (ED) tests, and three reinforcement reversals that followed the CD, ID, and ED. For each test, the animal was allowed four discovery trials, where the rat had 90 s to explore both pots. If the animal dug in the incorrect pot during these discovery trials, an incorrect response and latency was recorded but the rat was allowed to search in the correct pot to receive reinforcement prior to the expiration of the limited hold to facilitate learning. Response latencies were recorded as the amount of time between the removal of the divider and when the rat displaced digging media with his forepaw. The limited hold was abbreviated to 60 s in non-discovery trials. If the rat dug in the unbaited pot during a non-discovery trial, a divider was placed between the pots to prevent access to the correct pot, and the trial was discontinued. An incorrect choice and response latency were recorded. If the animal failed to respond within the limited hold, the trial was marked an omission. Trials were continued for each test until the criterion level of six consecutive correct responses was made. The stimuli were removed after a response or expiration of the limited hold.
The simple discrimination tested conditional discrimination between pots that differed on only one of the three dimensions (odor, digging media, or texture). For example, in a test of odor discrimination a pot scented with cinnamon+ was baited with an intact reinforcer and presented simultaneously with a pot scented with patchouli with an equal amount of crushed reinforcer. Digging media and texture were the same for both pots. The plus sign indicates the reinforced stimulus. Alternate pairs of pots were tested where one dimension, e.g., digging media varied between pairs so that tests of cinnamon/light foam shapes+ vs. patchouli/light foam shapes would alternate with cinnamon/dark foam shapes+ vs. patchouli/dark foam shapes but the reinforced odor was the same regardless of digging media. A table is provided with examples of stimuli used (Table 1).
In the compound discrimination, the rewarded attribute remained consistent, e.g., odor, but the pots differed on two dimensions rather than one. All test stimuli had one dimension that was present and remained constant across a set of test stimuli (e.g., texture). Alternate testing pairs would be cinnamon/light foam shapes+ vs. patchouli/dark foam shapes and cinnamon/dark foam shapes+ vs. patchouli/light foam shapes. These alternating pairs trained rats to attend to one dimension, e.g., odor and disregard another dimension, e.g., digging media. During the reversals (Rev1, Rev2, and Rev3) the rat was reinforced for responding to previously unrewarded exemplar of a dimension, e.g., patchouli.
Using a total changeover design, a new set of stimuli was introduced in the ID but the same dimension that predicted reward in previous tests (SD, CD, Rev1) was still rewarded (e.g., odor). Testing with novel sets of stimuli was hypothesized to facilitate the formation of an attentional set, e.g., focus on odor, when presented with new stimuli. After Rev2, an additional novel set of stimuli was used for the extra-dimensional shift. In the ED, the previously unrewarded but variable attribute is rewarded, e.g., digging media, so that rats were required to inhibit responding to the attentional set and learn a previously irrelevant attribute predicted reward. Subjects were tested with one of six possible patterns of shifts between the relevant dimension in the ID and ED (texture to digging media, texture to odor, odor to digging media, odor to texture, odor to digging media, texture to digging media).
After the ED, rats were tested in a third reversal. During the third reversal, the previously unreinforced exemplar of a stimulus pair was reinforced as in Rev1 and Rev2. The final test examined learned irrelevance or the ability of a subject to disregard the never reinforced stimulus dimension. In the previous example, texture was constant for a set of stimuli but never predicted reward. The first set of pots used to test the SD, CD, and Rev1 was covered in tan-colored fake fur (1.0 cm pile). Testing pots for the ID and Rev2 were covered in terry cloth. Finally, the pots used in the ED and Rev3 were covered in corduroy fabric with the ribbed side facing out. As this dimension never predicted reward, it is hypothesized that rats learn to ignore it (learned irrelevance). This hypothesis was tested by substituting pots that are identical in both odor and digging media to those tested in the ED and Rev3 but changing the irrelevant dimension. In the previous example, pots covered with the ribbed side of corduroy facing outward are replaced with a set covered with corduroy fabric with the smooth side facing out. As stimulus–response rules are unchanged, performance should be unaffected by this switch in animals that successfully disregard this dimension. The test of learned irrelevance was counterbalanced so that each dimension, odor, digging media, and texture, served as the wholly irrelevant dimension in two shifts.
Rats were given intra-peritoneal injections of atomoxetine hydrochloride (Tocris Cookson, Ellisville, MO, USA) 30 min prior to the onset of the ED in order to test the hypothesis that it would improve the ability of NE-LX rats to shift attentional set (Fig. 1). Additionally, we hypothesized that atomoxetine would have no effect on performance in other cognitive tests namely Rev3 and the test of learned irrelevance. The doses of atomoxetine, 0.0, 0.1, 0.3, and 0.9 mg/kg/ml, were counterbalanced across subjects. This resulted in eight groups of six rats for each dose of the drug. All post-injection testing was completed within 30 min of drug administration. Though there is some variability in reports of the duration of increased prefrontal NE with similar doses of the drug, we hypothesize that drug levels were similar during all behavioral tests as the overall time from injection to completion of testing was approximately 1 h (Bymaster et al. 2002; Tzavara et al. 2007).
Histology
After all testing was completed, animals were given an injection of ketamine and xylazine (90 kg/mg ketamine, 9 kg/mg xylazine). The rats were perfused transcardially with a 0.9% saline solution with a flow rate of 35 ml/min for 5–7 min followed by 4% paraformaldehyde, at the same flow rate for an additional 5–7 min. The brains are post fixed for 1 h and then placed in a 30% sucrose solution until they sank (usually 24–48 h). Using a microtome, 50-μm coronal sections were processed for either dopamine beta hydroxylase to identify noradrenergic fibers in the cortex or nissl bodies using thionin staining.
Histological procedures for DBH staining
To prevent uneven staining, all rinses and incubations were performed on an orbital shaker. Sections were initially placed in a solution of 1% H2O2 and 3% normal goat serum in phosphate-buffered saline (PBS). Without rinsing, sections were transferred to the primary antibody solution (1:1,000 rabbit anti-DBH, Chemicon; Temecula, CA, USA) in PBS with 0.2% triton X (TxPBS) and incubated overnight. Subsequent to 3 × 10 min rinses in PBS, sections were incubated in biotinylated secondary antibody (goat anti-rabbit, Vector Labs, Burlingame, CA, USA) for 2 h. After rinsing 3 × 10 min in PBS, sections were incubated in the avidin biotin complex solution (ABC; Vector Labs, Burlingame, CA, USA) for 1.5 h. Subsequent to rinsing, 3 × 10 min PBS, visualization was accomplished with a solution of nickel enhanced 3,3 diaminobenzidine (Vector Labs, Burlingame, CA, USA) using an incubation time of 5 min. Finally, sections were rinsed with PBS (3 × 10 min) prior to mounting on gelatin-coated slides. Sections were dried overnight in a 37°C oven prior to dehydration, defatting, and coverslipping.
Histological analyses
DBH-positive fibers were quantified in cortical areas in a manner modified from that described by McGaughy et al. (1996). Sections were analyzed using a 60 × objective on an Olympus Bx51 microscope attached to a Nikon DXM 1200 camera in conjunction with Image Pro Plus v. 6.0.0.260. DBH-positive fibers were counted in a 300 × 300-μm area using the number of fibers that crossed a grid imposed over the perimeter of the area. Counts were obtained for both hemispheres of the medial prefrontal cortex in the area of the IL/PL at the levels of bregma: +4.7, +3.7, +2.7, and +2.5; the anterior cingulate cortex at bregma: +3.7, +2.5, and +0.7; and the lateral orbitofrontal cortex at bregma: +4.7, +3.7, and +2.7 and as noted in Paxinos and Watson (1986).
Statistical analyses
All statistical analyses were performed with SPSS v. 14.0 (SPSS, Chicago, IL, USA). Histological data were analyzed using a mixed-factor analysis of variance (ANOVA) for each cortical subregion with the between-subjects factor of Lesion (two) and the within-subjects factor of Rostral to Caudal (four levels in counts from IL/PL; three levels in counts from cingulate or orbitofrontal cortex). As subtests of the ID/ED assess dissociable cognitive functions that recruit distinct subregions of the frontal cortex, we analyzed related subtests in separate ANOVAs. The number of trials needed to reach criterion performance in the ID was compared to the ED in a mixed-factors ANOVA with the within-subjects factor Test and between-subject factors of Lesion and Dose, Test (two) × Dose (four) × Lesion (two). An assessment of the effects of prefrontal lesions and atomoxetine on Rev3 and learned irrelevance was done in separate, univariate ANOVAs with the between-subjects factors of Dose (four) × Lesion (two). Each stage of the ID/ED was terminated after a subject emitted six consecutive correct responses, rather than after a set period of time. We recorded the amount of time in minutes between the injection of vehicle or drug until the completion of the final testing stage to determine if lesions or atomoxetine influenced the amount of total time required to complete all behavioral stages. These latencies were analyzed in a univariate ANOVA with the between-subjects factors of Lesion (two) × Dose (four). Though the drug administration was given prior to the ED, we assessed the effects of Group on pre-drug measures to ensure that no baseline differences existed prior to the administration of atomoxetine and differentiate this from tests assessed after drug administration by using the factor Planned Dose in the tests prior to ED. The number of trials to reach criteria performance in initial acquisition of the task was analyzed in a separate ANOVA with Test (two levels, SD, CD) as a within-subjects factor and Lesion (two levels), Shift (six levels), and Planned Dose (4) as between-subject factors. Note that six levels of the variable Shift represents the possible modality shifts over the course of testing with one stimulus dimension the focus of attention prior to the ED and another after the ED, e.g., texture to digging media (full description above in behavioral testing). In the second ANOVA, the effects of lesion on reversals prior to the administration of atomoxetine (Rev1) and (Rev2) were analyzed, Reversal (two) × Shift (six) × Lesion (two) × Planned Dose (four) mixed-factors ANOVA.
Results
Histological results
Intra-cortical infusion of DBH-saporin produced restricted depletions of the IL/PL (Lesion: F(1,46)=383.08; p<0.001; Fig. 2). The average loss of fibers in the IL/PL of NE-LX rats was 56%. The mean ± SEM fiber counts of IL/PL for NE-LX was 28.35±1.2 and 63.2±0.84 for SHAM-LX. The toxin did not produce significant damage to either the cingulate (Lesion: F(1,46)=3.56; p=0.07) or orbitofrontal cortex (Lesion: F(1,46)=0.69; p=0.41) though there was a consistent mean loss of 5% of the fiber in cingulate cortex. The effects of the toxin did not vary along the rostro-caudal plane in any region (all interactions p>0.51).
Fig. 2.
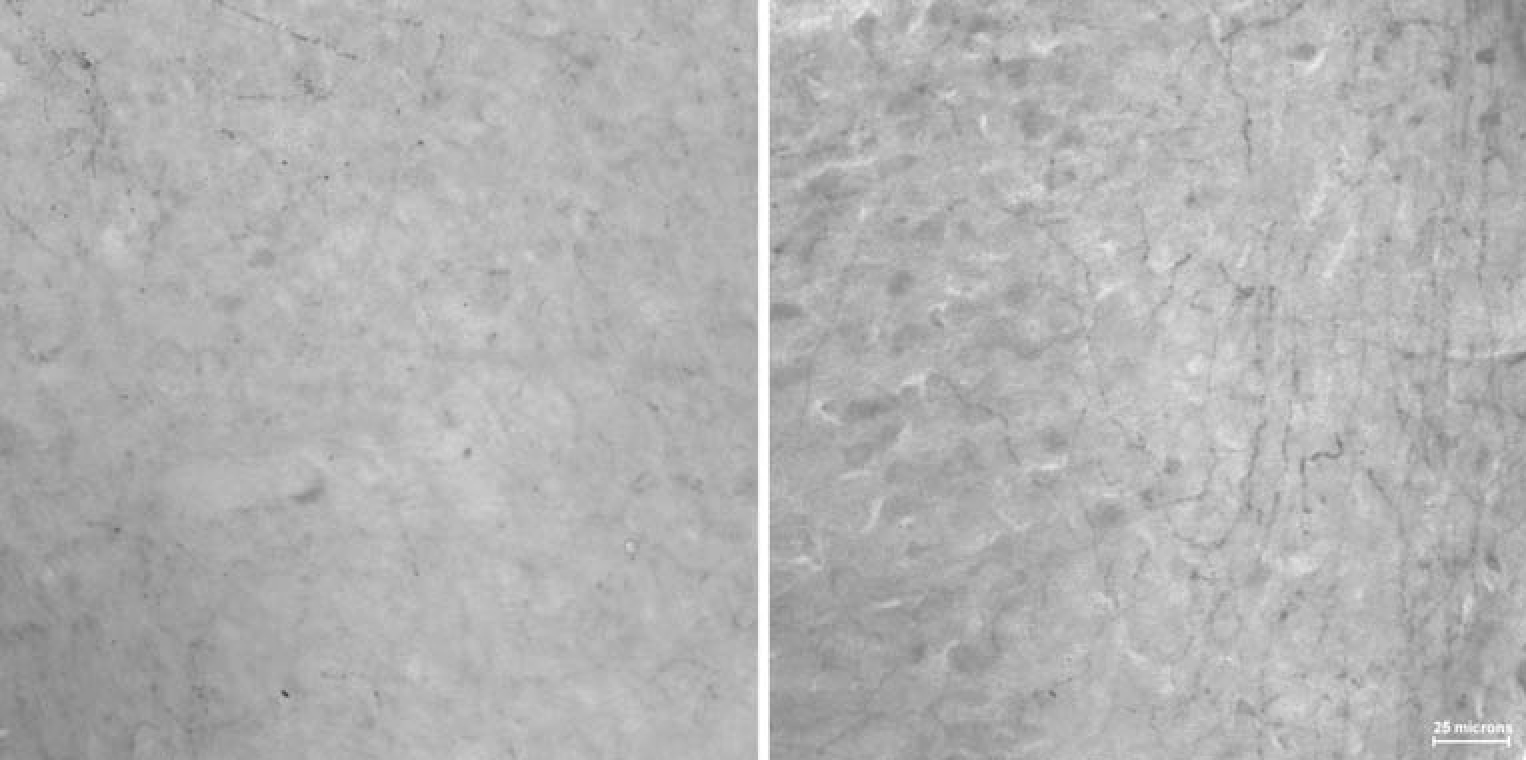
Noradrenergic fibers stained for dopamine beta-hydroxylase are show in the region of injection in the medial, frontal cortex for an NE-LX rat (left side) and SHAM-LX rat. The mean percentage of fiber loss in this region was 56% in NE-LX rats relative to SHAM-LX rats. There was no significant loss in the adjacent cingulate cortex or the orbitofrontal cortex
Behavioral results
Attentional set shift
All subjects required more trials to reach criterion performance on the ED relative to the ID (Test: F(1,40)=27.79; p<0.001) but this discrepancy was greater in NE-LX rats. Planned t tests compared the number of trials to criterion on ID vs. ED and confirmed that both groups took more trials to reach criterion on the ED (SHAM-LX: t(5)=−3.08, p=0.03; NE-LX rats (t(5)=−3.80, p=0.01). A between-groups comparison of the number of trials to criterion on the ED revealed that NE-LX rats required more trials than SHAM-LX rat (t(10)=−3.03; p=0.01; Fig. 3). Atomoxetine reversed this deficit in NE-LX but impaired the ED in SHAM-LX rats (Test × Dose × Lesion: F(3,40)=3.86, p=0.02; Fig. 3). Planned comparisons confirmed that all doses of atomoxetine were effective in NE-LX rats and decreased the number of trials to criterion for the ED relative to vehicle (all p<0.01). Following the administration of the 0.3 mg/kg dose, SHAM-LX rats performed significantly worse than NE-LX rats (t(10)=2.89; p<0.02). SHAM-LX rats’ ED performance after the 0.3 mg/kg dose showed a trend to be significantly worse than ED performance after vehicle injections (t(5)=−2.21; p=0.052). No other effects were found on accuracy (all p>0.05). There was no difference between the response latency of NE-LX and SHAM-LX rats (all p>0.05) and atomoxetine produced no effects on response latency (all main effects and interactions: p>0.05).
Fig. 3.
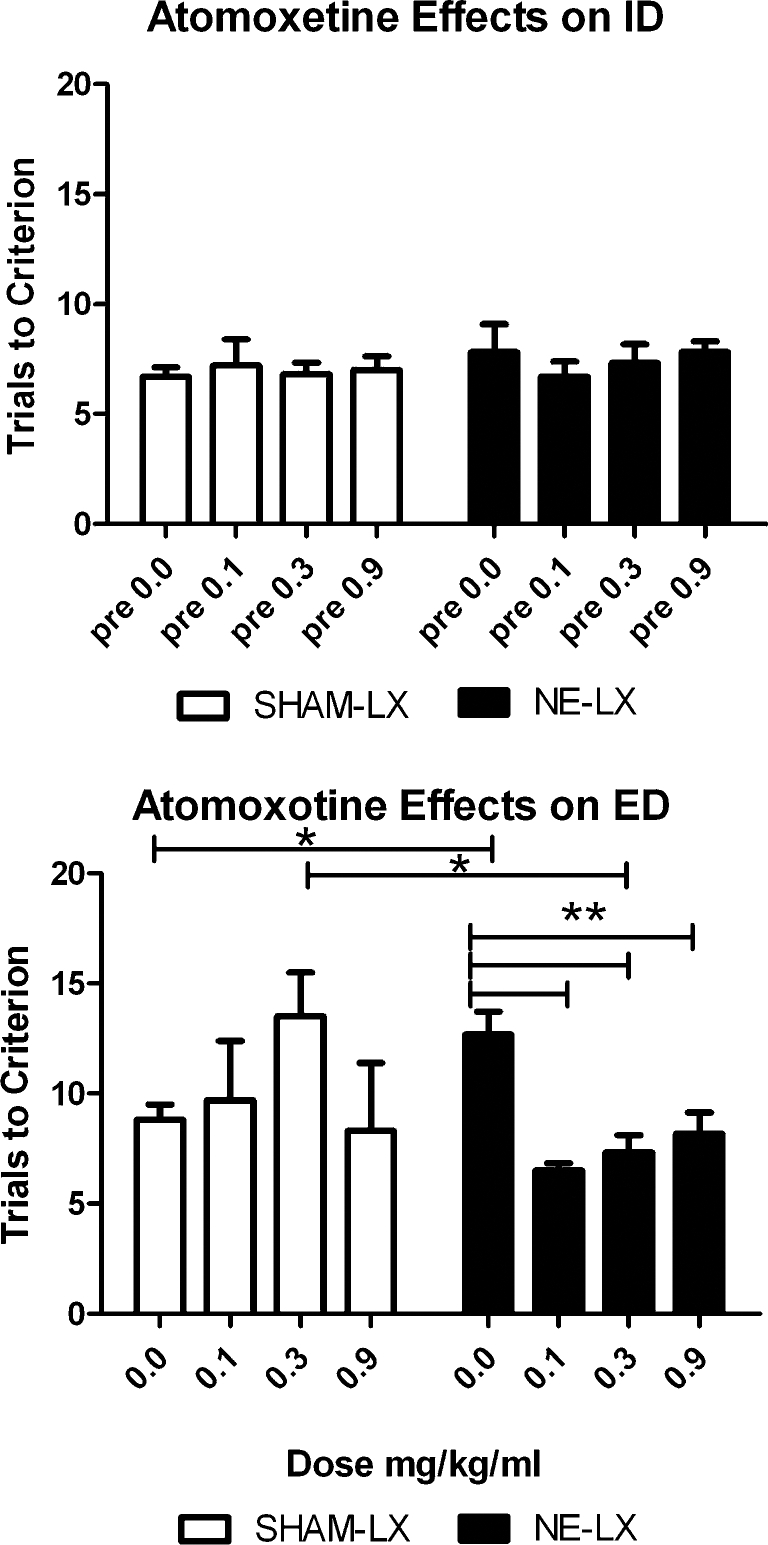
The effects of atomoxetine on performance of the ID and ED in SHAM-LX (white bars) and NE-LX (black bars) rats, (means + SEM). There was no difference between the groups on the intra-dimensional shift regardless of which dose group they had been assigned (top panel). All rats required more trials to reach criterion performance on the ED than the ID. Moreover, NE-LX rats required more trials to reach criterion performance on the ED than SHAM-LX rats (p<0.05). Every dose of atomoxetine significantly reduced the number of trials to reach criterion on the ED relative to vehicle in NE-LX rats (all p<0.01). The 0.3 mg/kg dose of drug significantly impaired performance on the test of the ED in SHAM-LX rats
Effects of atomoxetine on reversal 3 and learned irrelevance
There was no effect of the drug on accuracy or response latencies during reversal learning or the test of learned irrelevance (all p>0.05; Fig. 4). The effects of the drug did not differ between NE-LX and SHAM-LX rats in the analyses of either accuracy or response latency (all interactions p>0.05).
Fig. 4.
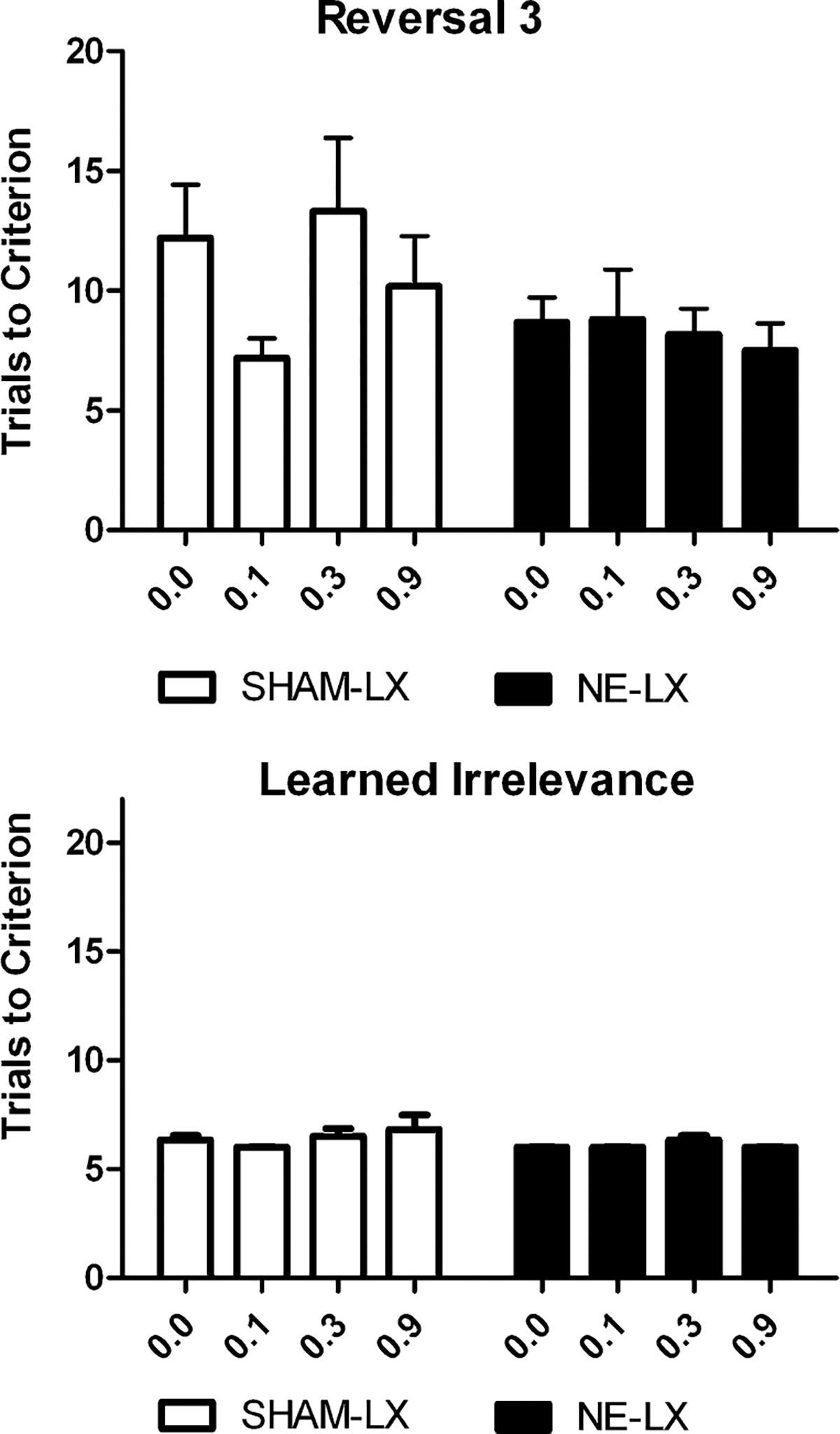
There was no difference in the number of trials required to reach criterion performance on Rev3 or the test of learned irrelevance regardless of the dose of atomoxetine these rats received. These data show the selective benefit of these doses of atomoxetine on attentional set shifting without changes in other aspects of cognitive testing
Simple and compound discrimination
There were no effects of the NE-LX on task acquisition accuracy (Fig. 5) or response latency (all p>0.05). As there were no group differences in animals assigned to receive any dose of the drug prior to the ED, data are shown collapsed over dose (all p>0.6). Similarly, performance was the same regardless the dimension tested (e.g., odor, digging media, or texture) in the analyses of accuracy and latency (all p>0.05).
Fig. 5.
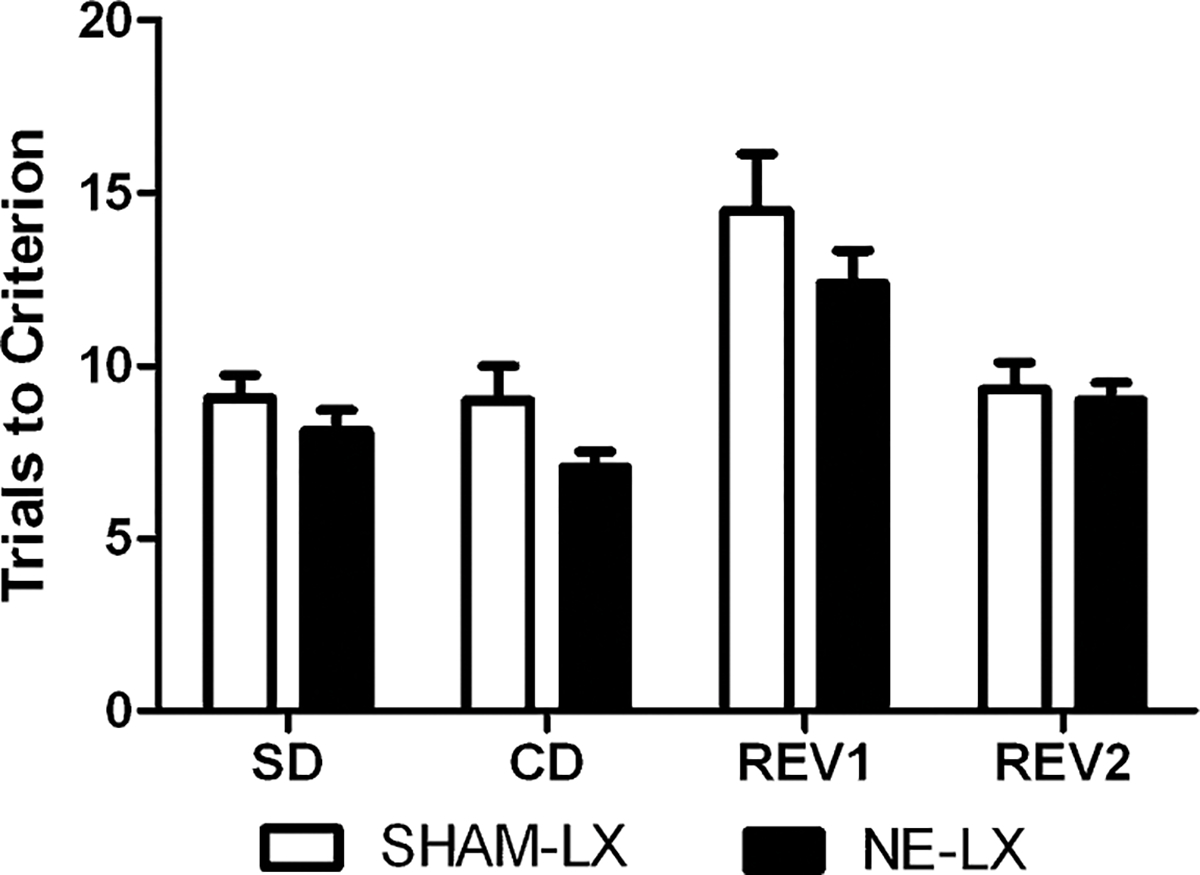
There was no difference in the number of trials required to reach criterion performance in any other subtest of the ID/ED task when the performance of NE-LX rats (black bars) was compared to SHAM-LX rats. Rats were grouped based on expected drug dose prior to the ED but these groups did not differ on pre-injection measures so data are shown collapsed over these groups for SHAM-LX and NE-LX rats
Reversals prior to ED
There were no effects of noradrenergic deafferentation on reinforcement reversals (all p>0.2; Fig. 5). All rats performed the same on these reversals regardless of which dose of drug they were scheduled to receive prior to the ED (all p>0.2). Additionally, reversal performance was similar regardless of the relevant dimension (odor, digging media, or texture; all p>0.05). All rats required less trials to meet criterion performance on Rev2 than Rev1 (F(1,26)=19.44; p<0.001). There were no other significant main effects or interactions in the analyses of accuracy and response latency.
Discussion
The present study is the first to our knowledge to show the beneficial effects of atomoxetine on attentional set shifting. Specifically, NE-LX rats showed facilitated ED shifting, but not reversal learning or learned irrelevance, after atomoxetine administration. In contrast to NE-LX rats, ED performance of SHAM-LX was worse after the 0.3 mg/kg dose relative to baseline. After the 0.3 mg/kg dose, SHAM-LX rats were significantly worse on ED performance than NE-LX rats given the same dose. The present data replicate previous work from our lab and others showing that decreased levels of NE in the prefrontal cortex impair extra-dimensional shifting while sparing all other aspects of task performance (McGaughy et al. 2008; Lapiz and Morilak 2006; Tait et al. 2007). These data suggest that NE levels must be maintained at an optimal level to perform shifts of attentional set. These data differ from findings that doses of atomoxetine facilitate other aspects of executive function in intact subjects (Chamberlain et al. 2006; Blondeau and Dellu-Hagedorn 2007; Robinson et al. 2007). The basis for this discrepancy is discussed in detail below.
All doses of drug were equally effective in remediating the attentional deficits of NE-LX rats. The 0.1 mg/kg dose is hypothesized to increase NE without concomitant increases in other neurochemicals in the prefrontal cortex. The inclusion of this dose was based on previous work showing that attentional testing is more sensitive than other behavioral tests to the effects of drugs (McGaughy et al. 1994; McGaughy and Sarter 1995) and is equal to doses used in other animal studies (Wee and Woolverton 2004) The current data suggest it may be as effective as higher doses at increasing cortical NE levels but concurrent microdialysis is required to elucidate the neurochemical effects of this dose. Previous work has shown that the middle and high dose used in our study produce comparable increases in prefrontal NE. The middle, but not high, dose produces this augmentation in the absence of increases in prefrontal dopamine (Bymaster et al. 2002). The efficacy of a 0.1- and 0.3-mg/kg dose in remediating attentional set-shifting effects suggests that increasing NE is sufficient to improve attention, and increased cortical dopamine is not necessary for this effect.
In addition to augmenting cortical dopamine, 0.3 mg/kg and higher doses of atomoxetine increase prefrontal cholinergic efflux (Tzavara et al. 2007). As acetylcholine has been consistently shown to be critical to certain types of attention (Chiba et al. 1995; Bucci et al. 1998; Sarter and Bruno 1998; Sarter and Bruno 1999; Robbins 2000; Sarter and Bruno 2000), it may be hypothesized that this increase in cortical acetylcholine is critical to the attentional improvements shown in the present study. However, previous work from our lab and others have found that cholinergic lesions produce no effect on accuracy during the ED shift (Roberts et al. 1992; Tait et al. 2002; McGaughy et al. 2008). The 0.3 mg/kg dose produces only small increases in cortical acetylcholine and we hypothesize the 0.1 mg/kg dose would produce less These data suggest it is unlikely that the beneficial effect of the 0.3 mg/kg dose in NE-LX or the detrimental effects in SHAM-LX rats depend on increases in cholinergic efflux.
The impairment on the ED for NE-LX rats was smaller than in our previous study and is hypothesized to be related to the size of the lesions. In our previous work, there was an average loss of 65% of DBH-positive fiber staining in the medial prefrontal cortex, while in the current study our average loss was 56%. Similar to our initial study, lesions of dorsal noradrenergic bundle produce a loss of 60–70% of cortical NE corresponding to larger behavioral impairments (Tait et al. 2007). Collectively, these data suggest that losses of >50% are sufficient to impair attentional performance with larger behavioral deficits shown in rats with larger lesions. As the atomoxetine acts to increase NE by blocking reuptake, we tested its effects in animals with lesions large enough to impair attentional performance but small enough to provide a residual pool of fibers as a substrate of action for the drug. We hypothesize that subjects with substantial depletions of noradrenergic fibers would not benefit from atomoxetine as there would be insufficient residual NE fibers for reuptake blockade to be useful. Though neurochemical compensation may occur after any lesion, converging evidence suggests that sensitive behavioral measures reveal attentional deficits with relatively small noradrenergic lesions (McGaughy et al. 2008; Tait et al. 2007) similar to findings with lesions of the cholinergic innervation to the cortex where sub-total depletions are sufficient to produce attentional impairments (McGaughy and Sarter 1998; McGaughy et al. 1999, 2000, 2002; Chudasama et al. 2004). Moreover, previous studies of noradrenergic depletions have found that lesions of 50% produce a system unable to maintain sufficient neurochemical compensation when challenged (Abercrombie and Zigmond 1989). These data are similar to work on functional compensation in the cholinergic system showing that attentional tests tax the system beyond the capacity of compensatory increases found before testing begins (McGaughy et al. 2002). Recent data support the most parsimonious explanation that attentional deficits after noradrenergic depletions are due to decreases in noradrenergic cortical release (Milstein et al. 2007; Tait et al. 2007). The beneficial effect of atomoxetine, a compound that is consistently found to increase cortical NE levels, is in line with this explanation that noradrenergic levels are decreased by lesioning, and the cognitive effects of these lesions are reversed by increasing NE levels.
Our results differ slightly from the previous work by Morilak and colleagues that found increasing NE in normal rats improved ED shift (Lapiz and Morilak 2006; Lapiz et al. 2007). The rats in the present study were less impaired on the ED than subjects in Morilak’s or other studies (McGaughy et al. 2008; Lapiz and Morilak 2006; Tait et al. 2007). Higher baseline performance has been shown to be less responsive to facilitation by pharmacological manipulation (Turchi et al. 1995; Granon et al. 2000). Though the basis of the better baseline performance on the ED is unknown, we hypothesize that brief handling and injection prior to testing the ED may produce a slight increase in cortical NE and facilitated performance on the ED in all rats (McGaughy et al. 2008; Lapiz and Morilak 2006; Tait et al. 2007).
As previous studies have shown noradrenergic lesions produce impairments specific to the ED (McGaughy et al. 2008; Tait et al. 2007), we injected atomoxetine immediately prior to the ED to assess the efficacy of this compound in remediating the attentional deficit. This choice allowed a greater degree of control over the time between injection and the test of the ED and also increased the likelihood that NE levels remained consistent throughout testing. Both groups required more trials to reach criterion performance on the ED relative to the ID, and NE-LX rats were significantly worse on the ED than SHAM-LX rats. NE-LX rats benefited from further increases in NE produced by atomoxetine, while increased NE in SHAM-LX rats impaired ED performance. We hypothesize that NE function in the SHAM-LX rats is at a nearly optimal level for attentional set shifting so increasing NE beyond this optimal range impairs performance. A similar finding has been found in tests of sustained attention where increasing acetylcholine levels in intact animals impairs performance (Holley et al. 1995).
Our data also differ from other studies in failing to show that increased NE facilitates reversal learning (Lapiz and Morilak 2006; Lapiz et al. 2007). Reversal learning has consistently been shown to depend on the integrity of orbitofrontal, not medial, frontal cortex (Dias et al. 1996a, b; Schoenbaum 2000; McAlonan and Brown 2003). It is presently unknown how atomoxetine changes neurochemical levels in orbitofrontal cortex so it may be that this compound does not augment NE efflux in that region or that the increases produced by our doses of atomoxetine were insufficient to improve reversal learning. Additionally, it may be that higher levels of NE facilitate response inhibition and improve performance in both the ED and reversal trials.
Similar to reversal learning, response inhibition has been shown to be dependent on areas outside the medial prefrontal cortex (Eagle and Robbins 2003; Eagle et al. 2007). Studies in rats have shown that atomoxetine decreases impulsivity in normal rats at doses of 0.6 mg/kg or higher (Robinson et al. 2007). The improvement shown in normal rats suggests that higher levels of NE are required to alter response inhibition relative to the levels needed to improve attentional set shifts. Though atomoxetine is highly selective for the NE transporter, the 0.6 mg/kg dose increases the levels of NE and dopamine in frontal cortex, and this simultaneous release may be necessary to show beneficial effects on response inhibition (Bymaster et al. 2002). The administration of atomoxetine to adult patients with ADD has also been shown to improve response inhibition (Spencer et al. 1998; Chamberlain et al. 2007). Though both Spencer and Chamberlain assessed executive function using the Wisconsin card sorting and ID/ED task respectively, no impairments in either task were reported at baseline. In contrast to work in adults, Sergeant and colleagues showed that a majority of studies using tests of executive function consistently differentiated the performance of children with ADD from control subjects (Sergeant et al. 2002). Impairments in executive control in children with ADD have also been shown to respond to treatment with methylphenidate (Mehta et al. 2004). While atomoxetine is used successfully to treat many of the core symptoms of ADD (Caballero and Nahata 2003; Kratochvil et al. 2003; Christman et al. 2004; Kratochvil et al. 2006) and improves impulsivity in patients with ADD (Chamberlain et al. 2007), its effects on executive function are still largely unexplored. Future studies may be aimed at determining if the deficits in executive functions found in children persist in adulthood and if atomoxetine can alleviate these impairments. Converging lines of data suggest that NE dysfunction underlies the symptoms of ADD (Friedman 1999; Viggiano et al. 2004a; Arnsten and Li 2005; Arnsten 2006; Faraone and Khan 2006), and our data support the hypothesis that atomoxetine can remediate cognitive deficits associated with noradrenergic dysfunction beyond impairments in response inhibition.
The present study confirms that decreasing cortical NE is sufficient to impair executive function, and this impairment can be reversed by the administration of atomoxetine. Moreover, in contrast to other studies, the present data show that increasing NE in intact subjects impairs some aspects of executive function. We hypothesize that the beneficial effects of atomoxetine in the present study result from selective increases in NE in the medial, frontal cortex and are not reliant on increasing levels of either dopamine or acetylcholine. Future studies may be aimed at testing this hypothesis by assessing the effects of the co-administration of atomoxetine and dopaminergic or cholinergic antagonists on performance of the ID/ED task.
Acknowledgments
The authors thank Nicolas Adams and David Creer for excellent technical assistance.
References
- Abercrombie ED, Zigmond MJ (1989) Partial injury to central noradrenergic neurons: reduction of tissue norepinephrine content is greater than reduction of extracellular norepinephrine measured by microdialysis. J Neurosci 9:4062–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (1997) Catecholamine regulation of the prefrontal cortex. J Psychopharmacol 11:151–162 [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (1998) Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci 2:436–447 [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (2006) Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry 67:4–9 [PubMed] [Google Scholar]
- Arnsten AFT, Li B-M (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377–1384 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T (1994) Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci 14:4467–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J (2000) Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res 126:165–182 [DOI] [PubMed] [Google Scholar]
- Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, Hamilton C, Spencer RC (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60:1111–1120 [DOI] [PubMed] [Google Scholar]
- Birrell J, Brown V (2000) Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20:4320–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F (2007) Dimensional Analysis of ADHD subtypes in rats. Biol Psychiatry 61:1340–1350 [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M (1998) Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci 18:8038–8046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gelhert DR, Perry KW (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27:699–711 [DOI] [PubMed] [Google Scholar]
- Caballero J, Nahata MC (2003) Atomoxetine hydrochloride for the treatment of attention-deficit/hyperactivity disorder. Clin Ther 25:3065–3083 [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R (2002) Neuroscience of attention deficit/hyperactivity disorder: the search for endophenotypes. Neuroscience 3:617–628 [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311:861–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, del Campo N, Dowson J, Müller U, Clark L, Robbins TW, Sahakian BJ (2007) Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 62(9):977–984 [DOI] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M (1995) Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci 15:7315–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman AK, Fermo JD, Markowitz JS (2004) Atomoxetine, a novel treatment for attention-deficit–hyperactivity disorder. Pharmacotherapy 24:1020–1036 [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW (2004) Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192 IgG-saporin lesions and intra-prefrontal infusions of scopolamine. Learn Memory 11:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O’Connell MT, Cardinal RN, Levita L, Robbins TW (2001) Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and non-contingent performance of a visual attentional task. J Neurosci 21:4908–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784 [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ (1990) Activation of noradrenergic system facilitates an attentional shift in the rat. Behav Brain Res 39:19–28 [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins T, Roberts A (1996a) Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380:69–72 [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins T, Roberts A (1996b) Primate analogue of the Wisconsin card sorting test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110:872–886 [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW (2003) Inhibitory control in rats performing a stop-signal reaction time task: effects of lesion of the medial striatum and d-amphetamine. Behav Neurosci 117:1302–1317 [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW (2008) Stop-signal reaction time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex 18 (1):977–984 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Khan SA (2006) Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry 67:10–17 [PubMed] [Google Scholar]
- Friedman JI, Adler DN, Davis KL (1999) The role of norepinephrine in pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and alzheimer’s disease. Biol Psychiatry 46:1243–1253 [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW (2000) Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci 20:1208–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley LA, Turchi J, Sarter M (1995) Dissociation between the attentional effects of infusions of a benzodiazepine receptor agonist and an inverse agonist into the basal forebrain. Psychopharmacology 120:99–108 [DOI] [PubMed] [Google Scholar]
- Koob G, LeMoal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–57 [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ, Vaughn BS, Harrington MJ, Burke WJ (2003) Atomoxetine: a selective noradrenergic reuptake inhibitor for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Pharmacother 4:1165–1174 [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ, Wilens TE, Greenhill LL, Gao H, Baker KD, Feldman PD, Gelowitz DL (2006) Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psych 45:919–927 [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA (2006) Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137:1039–1049 [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Bondi CO, Morilak DA (2007) Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology 32 (5):1000–1010 [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ (2003) Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146:97–103 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M (1995) Effects of chlordiazepoxide and scopolamine but not aging on the detection and identification of conditional visual stimuli. J Gerontol: Biol Sci 50:B90–96 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M (1998) Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav Neurosci 112:1519–1525 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M (1996) Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Beh Neuro 110:247–265 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Turchi J, Sarter M (1994) Crossmodal divided attention in rats: effects of chlordiazepoxide and scopolamine. Psychopharmacology 115:213–220 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M (1999) Enhancement of sustained attention performance by the nicotinic receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology 144:175–182 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H (2008) Noradrenergic, but not cholinergic deafferentation of prefrontal cortex impairs atentional set-shifting. Neuroscience. doi: 10.1016/j.physletb.2003.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Everitt BJ, Robbins TW, Sarter M (2000) The role of cortical cholinergic afferent projections in cognition: impact of new selective immunotoxins. Behav Brain Res 115:251–263 [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW (2002) Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a 5 choice serial reaction time task. J Neurosci 22:1905–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ (2004) Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry 45:293–305 [DOI] [PubMed] [Google Scholar]
- Milstein JA, Lehmann O, Theobald DEH, Dalley JW, Robbins TW (2007) Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology 190:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW (1991) Extra-dimensional versus intra-dimensional set-shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalohippocampectomy in man. Neuropsychologia 29:993–1006 [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW (1993) Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain 116:1159–1175 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Second Edition. New York: Academic Press [Google Scholar]
- Robbins TW (2000) Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res 133:130–138 [DOI] [PubMed] [Google Scholar]
- Roberts A, Robbins TW, Everitt BJ, Muir JL (1992) A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience 47:251–264 [DOI] [PubMed] [Google Scholar]
- Robinson ESJ, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW (2007) Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301487 [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Sergeant J (1998) Attention deficit/hyperactivity disorder—from branin dysfuncitons to behaviour. Behav Brain Res 94:1–10 [PubMed] [Google Scholar]
- Sarter M, Bruno JP (1998) Cortical acetylcholine, reality distortion, schizophrenia, and lewy body dementia: too much or too little cortical acetylcholine. Brain Cogn 38:297–316 [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP (1999) Abnormal regulation of corticopetal cholinergic neurons and impaired information processing in neuropsychiatric disorders. Trends Neurosci 22:67–74 [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP (2000) Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telecephalic and brainstem afferents. Neuroscience 95:933–952 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M (2000) Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci 20:5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant JA, Guerts H, Oosterlaan J (2002) How specific is a deficit of executive function for attention-deficit/hyperactivity disorder? Behav Brain Res 130:3–28 [DOI] [PubMed] [Google Scholar]
- Solanto MV (1984) Neuropharmacological basis of stimulant drug action in attention deficit disorder with hyperactivity: a review and synthesis. Psychol Bull 95:387–409 [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, Harding M, Faraone SV, Seidman L (1998) Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatr 155:693–695 [DOI] [PubMed] [Google Scholar]
- Tait D, McGaughy J, Latimer MP, Brown V (2002) 192 IgG-saporin lesions of the cholinergic basal forebrain do not impair attentional set-shifting, but do increase latency to dig. Proceedings of the Society for Neuroscience, New Orleans, LA [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW (2007) Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci 25:3719–3724 [DOI] [PubMed] [Google Scholar]
- Turchi J, Holley LA, Sarter M (1995) Effects of nicotinic acetylcholine receptor ligands on behavioral vigilance in rats. Psychopharmacology 118:195–205 [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Overshiner CD, Davis RJ, Perry KW, Wolff M, McKinzie DL, Witkin JM, Nomikos GG (2007) Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol Psychiatry 11:187–195 [DOI] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Arcieri S, Sadile AG (2004a) Involvement of norepinephrine in the control of activity and attentive processes in animal models of attention deficit hyperactivity disorder. Neural Plast 11:133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D, Vallone D, Sadile A (2004b) Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast 11:97–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Woolverton WL (2004) Evaluation of the reinforcing effects of atomoxetine in monkeys: comparison to methylphenidate and desipramine. Drug Alcohol Depend 75:271–276 [DOI] [PubMed] [Google Scholar]
- Wilens TE (2006) Mechanisms of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry 67:29–34 [PubMed] [Google Scholar]
- Wong DT, Threlkeld PG, Best KL, Bymaster FP (1982) A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther 222:61–65 [PubMed] [Google Scholar]


