Abstract
Introduction:
A neurodevelopmental disorder, autism is typically identified with three primary behavioral consequences, such as social impairment, communication problems, and limited or stereotypical behavior. Because of its co-morbidity and lack of therapeutic options, autism is a global economic burden. A short chain of fatty acid, propionic acid is formed biologically by the gut microbiome. Propionic acid levels that are too high can cause leaky intestines, which can lead to autism-like symptoms.
Methods:
To induce autism, male Albino Wistar rats were given propionic acid (250 mg/kg/po on the 21st, 22nd, and 23rd postnatal days). Rats also received a ryanodine receptor antagonist (Ruthenium red: 3 mg/kg/po; postnatal 21st to 50th day) to see what influence it had on propionic acid-induced autism. Anxiety, social behavior, and repeated behaviors were all assessed, as well as oxidative stress, inflammatory indicators, neuro signaling proteins, and blood-brain barrier permeability.
Results:
Ruthenium red was found to counter the propionic acid-induced increases in anxiety, repetitive behavior prefrontal cortex levels of IL-6, TNF-α, TBARS, Evans blue leakage, and water content along with decreases in social behavior, IL-10, and GSH followed by hippocampus CREB and BDNF levels.
Conclusion:
Ryanodine receptor antagonists presented a neuroprotective effect in propionic acid-induced conditions like autism by modulatory effects on social and repetitive behavior, oxidative stress, neuroinflammation, and neuroprotein changes. Ryanodine receptors can be further explored in depth to manage autism as a condition.
Highlights
Ruthenium red can reduce the propionic acid-induced anxiety of rats with autism.
Ruthenium red can improve the propionic acid-induced changes in repetitive behavior of rats with autism.
Ruthenium red can reduce the propionic acid-induced social behavior dysfunction in rats with autism.
Plain Language Summary
Autism is a complex heterogeneous neurodevelopmental disorder mainly diagnosed with social behavior dysfunction, communication problems, and repetitive behavior. Due to high comorbidity and multiple unknown factors involvement, its exact etiology remains unclear, and so no successful treatment is available. Among the environmentally produced models of autism in rats, the most common is created by propionic acid (PPA). With short-chain type fatty acid, PPA is one of the mediators for the cycle of cell metabolism. This study attempted to study the effect of a ryano-dine receptor antagonist (Ruthenium red) on PPA-induced Anxiety, social behavior dysfunction, and repeated behaviors in rats with autism. The results showed the modulatory effects of Ruthenium red PPA-induced conditions including social and repetitive behavior, oxidative stress, neuroinflammation, and neuroprotein changes in rats with autism.
Keywords: BDNF, Ryanodine, Interleukins-6, Mitochondria, Blood brain barrier, ASST
1. Introduction
Autism is a complex heterogeneous neuro-developmental disorder mainly diagnosed with social behavior dysfunction, communication problems, and repetitive behavior. It is reported that 1 out of 59 children suffers from autism. Autism is four times more frequent in men than in women, and many girls with autism spectrum disorder (ASD) have less apparent symptoms than boys. Autism is a disorder that lasts for life ( Maenner et al., 2020). Due to high comorbidity and multiple unknown factors involvement, its exact etiology remains unclear, and so no successful treatment is available.
Among the environmentally produced models of autism in rats, the most common is created by propionic or valproic acid, either prenatally or postnatally. With short-chain type fatty acid, propionic acid (PPA) is one of the mediators for the cycle of cell metabolism. PPA is also a byproduct of enterobacteria in human intestines and a popular food preservative ( Choi et al., 2020). Increased PPA and propionic acidemia is associated with autism ( Al-Owain et al., 2013; Cotrina et al., 2020) its toxic derivatives, and ammonia. The disease causes multiorgan damage, especially in heart, pancreas, and brain; seizures and intellectual disability are often described. Some PA children also show autism spectrum disorders (ASD. While PPA has a beneficial function, its accumulation has been documented to cause neurotoxicity ( Choi et al., 2020; El-Ansary et al., 2012). Enormous amounts of fatty acid with short chains in the rat intestinal tract, as well as peripheral PPA injection, have all been strongly correlated to autism-related behavior problems. PPA, in neurotoxic amounts, may contribute to autism-like symptoms in animals, according to researchers ( Choi et al., 2020; El-Ansary et al., 2012; MacFabe et al., 2011). PPA treatment resulted in increased neuro-inflammation, oxidative stress, poor energy metabolism, aberrant neurotransmission, and a pro-apoptotic effect in rats ( El-Ansary et al., 2012). In addition to our prior findings, several other researchers concurred that PPA treatment in rats during the early postnatal day may generate behavioral abnormalities similar to ASD ( Choi et al., 2020; El-Ansary et al., 2012; MacFabe et al., 2011; Mirza & Sharma, 2018).
IIncreased cytosolic calcium concentration stimulates neurotransmitter release at synaptic junctions, leads to synaptic function, and regulates behavior pattern modifications in neural function and cell proliferation. ( Berridge et al., 1998; Ludwig et al., 2016). Calcium homeostasis is generally regulated by various kinds of calcium channels and their receptors. Ryanodine receptor is one of the calcium Ruthenium red that is known as a selective antagonist of intracellular ryanodine receptor (RyR). Antagonism of the RyR has shown to be useful in the treatment of numerous brain illnesses, including Huntington's disease and Alzheimer's disease ( Baker et al., 2013; Calì et al., 2012; Del Prete et al., 2014; Suzuki et al., 2012). It has been documented that the antagonism of the ryanodine receptor exerts a neuro-protective effect along with decreased infarct volume and lower intracellular calcium in the brain ( Phillips et al., 2013). Previous research has also found that ruthenium red has beneficial effects on brain inflammation ( Cisneros-Mejorado et al., 2018; Poole et al., 2013) and oxidative stress ( Schilling & Eder, 2009). The antagonistic role of ryanodine receptors in ASD has not been explored yet. This manuscript explored the novel job of ryanodine receptors antagonist; ruthenium red in PPA-administered autistic phenotypes in rodents.
2. Materials and Methods
Experimental rat
Pregnant albino Wistar females and their offspring were housed and kept in polypropylene cages at Amity University (Reg No. 1327/PO/ReBi/S/10/CPCSEA). All the conditions and parameter sticky followed as per IAEC/CPCSEA, at a temperature of 25±2°C with a relative humidity of 50±5%. Typical laboratory pellet chow food and water have always been available to rats (Ashirwad Industries, Punjab, India). Animals were exposed to the natural light/dark cycle (dark starting at 19:00 hrs. and ending at 07:00 hrs.).
Chemicals, drugs, and reagents
Chemicals and reagents of analytical and laboratory grade were employed in this study. Propionic acid was taken from Lab Sales Corporation India. Evans blue, egtazic acid was purchased from SRL Pvt. Ltd, India. Lowry’s reagent, N-naphthylethylenediamine, and 5, 5`-dithiobis (2-nitrobenzoic acid) (Ellman’s reagent) were obtained from Sigma-Aldrich, India. H2O2 and C5H5N were provided by Rankem Ltd. India.
ASD induced by PPA
To develop autism-like phenotypes, rats were given 250 mg/kg propionic acid orally on three consecutive postnatal days, the 21st, 22nd, and 23rd. PPA was prepared by mixing with 0.2M phosphate buffer saline. ( Bukhari et al., 2020; El-Ansary et al., 2012; Mirza & Sharma, 2018).
Protocol design
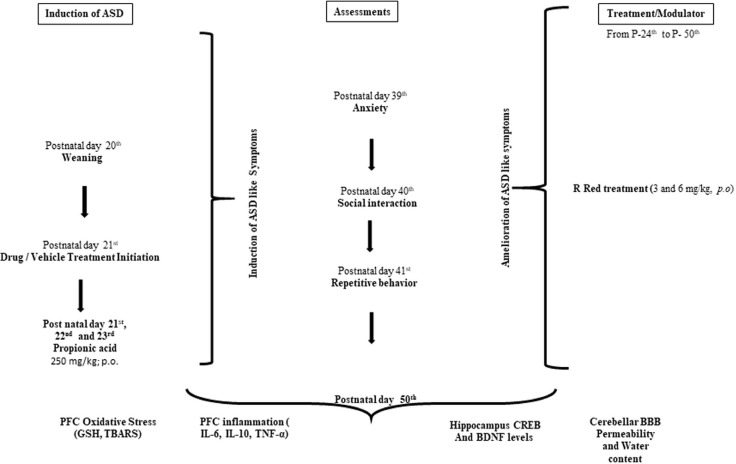
A total 48 rats (n=8) divided into 6 groups, were utilized for the protocol of the study. The animals were chosen based on previous research, an experimental ASD-like condition was successfully modeled using albino Wistar rats ( Mirza & Sharma, 2018). The timeline, groups, and parameters assessed for the present study are seen in Figure 1.
Figure 1.
Diagram of the experimental technique
Abbreviations: E: Embryonic day; PFC: Prefrontal cortex; P: Postnatal day; PPA: Propionic acid; R Red: Ruthenium Red; D1: Dose 1; D2: Dose 2.
Group I—Control group- Male rats received 0.2M phosphate buffer saline (5 ml/kg, i.p) on post-natal 21st day, 22nd day and 23rd day; Group II and III — Ruthenium Red (R Red) per se: Rats were given R Red D1 (3 mg/kg, i.p.) and R Red D2 (6 mg/kg, i.p.) from the 24th post-natal day to the end of the study; Group IV—Propionic acid (PPA) group: Rats received three consecutive dose of propionic acid (250 mg/kg, p.o) on post-natal 21st day, 22nd day and 23rd day; Group V and VI—PPA+ R Red D1 and D2 group: Rats received propionic acid on post-natal 21st day, 22nd day and 23rd day followed R Red D1 (3 mg/kg, i.p.) and R Red D2 (6 mg/kg, i.p.) from post-natal 24th day to till of the study (Figure 1).
Anxiety
We assessed anxiety on postnatal day 39th. Anxiety-related behavior changes have been reported in PPA-induced autism. ( Kumar & Sharma, 2016b; Mirza & Sharma, 2018). The EPM equipment was built of wood and had four arms that were 90 degrees apart. A 40-centimeter-high wall surrounds two closed arms and two open arms measuring 50×10 cm with a 50-centimeter-high wall above its base. All experimental rats were held in a pre-test arena for 5 minutes apiece to allow them to explore the maze. The animals were quickly transported and released in the maze’s center, pointing to the open arm. For 5 minutes, each arm’s entries and time taken time were carefully recorded. The tension between both two maze components, namely the open arms, which are aversive, bright, and exposed, and the closed arms, which are covered, dark, and protected, forms the basis for this test. The count of close and open arm entries, as well as time taken in close and open arm, were all measured to determine the % entries and time taken in open arm with the below Equation 1.
| 1. |
Social interaction assessment
On a postnatal day 40th, social interaction was evaluated by three-chamber sociability and novelty test apparatus (30 cm long and 70 cm wide divided into three identical chambers) for the rat. The test arena was divided into three equal chambers, each with an entrance point, and measured 76×30×35 cm. Animals were granted free access to all three chambers, and each trial started with the animal being placed in the apparatus’s middle chamber. Animals that were to be placed beneath a wire cage spent 30 minutes under the wire cage for acclimation 24 hours before the commencement of the sociability phase. To stimulate exploration of the side chambers, all rats were assimilated to the equipment for 5 minutes before the start of the test trial. The rats were subjected to a 10-minute sociability test after the habituation phase was over. During the sociability phase, a stranger rat was placed under a wired cage on either the right side or left side chamber, while a vacant cage was held on the opposite side. The arrangement of wired cages was randomized to eliminate side preferences, and the stranger animal chamber and the empty cage were dubbed stranger chamber and empty chamber, respectively. Time taken in empty and stranger chambers by rats was noted during the sociability phase trial. Following the sociability phase was completed, the social preference phase began after the last animal trial. During the social preference phase, each animal was given 10 minutes to examine the entire arena. During this phase, the rat that had previously been considered a stranger was now made familiar, and now another novel rat stayed in the paradigm alongside the familiar rat. The two chambers are now known as the familiar and novel chambers. The test rat’s time spent in novel and familiar chambers was recorded. The Equation 2 below was used to calculate the sociability index (SI) and social preference index (SPI) ( Kumar et al., 2015; Kumar & Sharma, 2016a, b).
| 2. |
Repetitive behavior assessment
Repetitive behavior is measured through the y maze by observing the percentage of spontaneous alternations ( Kumar et al., 2015; Mirza & Sharma, 2018). A decrease in spontaneous alternations reported an indication of increased stereotype or repetitive behavior. The rats were exposed to the starting arm during an 8-minute trial with free exploration in the three arms of the maze. The number of spontaneous alternations made was calculated using the succession of arm entries. As the rat entered each of the three independent arms, the alternation was counted. The number of % spontaneous alternations was determined as follows (Equation 3):
| 3. |
Preparation for biochemical assessments
On postnatal day 50th, a high dose of thiopental sodium (90 mg/kg; i.p) was used to isolate the PFC tissue by anaesthetization. The homogenate supernatant was collected for various biochemical estimates as defined in the procedures below. Absorption was taken with a spectrophotometer (PerkinElmer). The key brain parts engaged in ASD etiology and neurochemical abnormalities include the prefrontal cortex, hippocampus, and cerebellum ( Chauhan et al., 2011; Kumar & Sharma, 2016 b). As a result, we chose the cerebellum and prefrontal cortex brain parts for this investigation.
Oxidative stress assessments
As oxidative stress markers, gutathione (GSH) and lipid peroxidation (TBAR) were assessed in the prefrontal cortex in this investigation.
Glutathione (GSH)
The quantitative estimation of the reduced form of GSH was assessed using a spectrophotometer (PerkinElmer) at 412 nm ( Kumar et al., 2015). In a test tube, PFC supernatant and trichloroacetic acid (10 percent w/v) were combined at 1:1 proportion and the test tubes were centrifuged (10 min;1000 g at 4°C) allowing the supernatant (0.5 mL) to mix with 0.3 M Na2HPO4 (2 mL). The spectrophotometric absorbance was taken at 412 nm after adding 25 mL of freshly produced Ellman’s reagent (Ellman’s reagent dissolved in 1 percent w/v sodium citrate). The reference curve was drawn using 10-100 M of the glutathione (reduced form). To express GSH, micro-moles/mg protein units were used.
Lipid peroxidation
Lipid peroxidation was assessed as close to our earlier investigations ( Kumar et al., 2015). The brain part (PFC) supernatant sample (0.2 mL) was taken in a test tube. In addition, 8.1 percent CH3(CH2)11SO4Na (0.2 mL), 30 percent CH3COOH at pH 3.5 (1.5 ml), and 0.8 percent C4H4N2O2S (1.5 mL) were added, followed by volume built up to 4 mL with water and incubation at 95°C for 1 hour. The liquid was cooled and then distil water (1 mL) was added, followed by the n-butanol-pyridine combination (15:1 v/v; 5 mL). The centrifuge tubes were handled at 4000 g for 10 min. CH2(CH(OCH3)2)2(1-10 nM) was used for plotting of standard calibration curve. The results were demonstrated using nanomoles per milligram of protein.
Neuroinflammation and neurotrophin
Elisa method for tissue necrosis factor-α (TNF-α), iterleukin-6 (IL-6), interleukin-10 (IL-10), cAMP response element-binding protein (CREB) and brain-derived neurotrophic factor (BDNF)
These were assessed by the enzyme-linked immune-sorbent assay-sandwich method in the PFC and hippocampus area of the brain. The rat ELISA kits (Ray-Bio®) were used to measure the levels of TNF-, IL-6, IL-10, CREB, and BDNF. The procedure mentioned in the product information leaflet was completely followed and samples were run in triplicate for optical density measurement, average optical density was considered for the final calculation of concentration. Briefly, IL-6, IL-10, TNF-α, BDNF, and CREB quantitation was made in PFC supernatant at 450 nm on 96-well percolated to specific antibody plates. Supernatant and standard were taken out into the well. The immobilized antibody was bound with TNF-α, IL-6, IL-10, CREB, and BDNF present in the sample, respectively. The tubes were washed, and the plates were coated with biotinylated anti-Rat TNF-α, IL-6, IL-10, CREB, and BDNF antibodies. The well was washed to eliminate unbound biotinylated antibodies. The wells were filled with HRP-conjugated streptavidin. After washing, the wells were treated with a TMB substratum solution. TNF-α, IL-10, IL-6, CREB, and BDNF were all linked in a blue color creation in the sample. When the stop solution was pipette into the wells, the color changed to yellow. Results were expressed in pg/mL and ng/mL ( Mirza and Sharma, 2018).
Permeability of the blood-brain barrier (BBB) and water content
As stated earlier, the permeability of BBB and water content were estimated using the levels of Evans blue in the cerebellum ( Manaenko et al., 2011; Kumar et al., 2015b). In a brief, the rats were administered 4% dye/Evans blue (4 mL/kg; intra-peritoneal) and left to circulate for 2 hours. Before collecting the samples, the rats (anesthetized) were trans-cordially perfused with the saline to excrete out the remaining dye in the vessels. Weighed cerebellum for quantitative spectroscopic calculation. In short, the cerebellum was precisely measured. The dye was extracted from the cerebellum using a homogenizer at pH 7.4 in 3.5 mL of 0.1 mol/L phosphate buffer saline. Then 6 mL of 60% trichloroacetic acid was added for protein precipitation. After cooling for half an hour, the processed cerebellum was vortexed for two minutes. The cerebellar tissue was processed for 40 minutes through a centrifuge at 4000 rpm to obtain pellets. Using a spec- trophotometer, the dye levels were determined at 610 nm. The results were estimated through an Evans blue standardized curve of μg of Evans blue/g of cerebellar tissue. For 48 hours wet-weighted cerebellum tissue was put in the oven at 105°C weighed out after 48 hours of dry cerebellum. The wet-dry method was used to calculate the volume of water in the cerebellum ( Manaenko et al., 2011). The water content was estimated as follows (Equation 4):
| 4. |
Statistical analysis
For statistical analysis, Sigma statistical software, version 3.5 was employed. The results were presented in the form of a Mean±SD. All variables’ statistics were examined with a two-way analysis of variance (ANOVA) and a Bonferroni post-test. The results were scrutinized as statistically significant at P<0.05.
3. Results
Anxiety
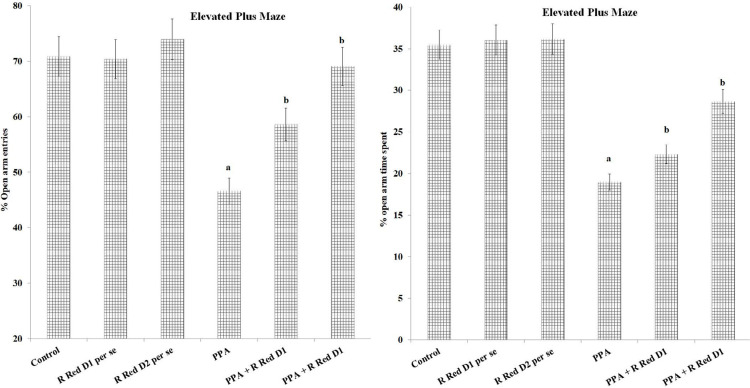
PPA recorded a substantial decrease in open arm entries (F(1, 42)=693.858, P<0.05) and time spent (F(1, 42)=218.038, P<0.05) relative to control animals. Treatment with R Red substantially decreased PPA administered drop in open arms entries (F(2, 42)=123.074, P<0.05) and time spent (F(2, 42)=159.8, P<0.05) (Figure 2).
Figure 2.
%open arm entry and %open art time spent on elevated plus maze
The statistics is presented with Mean±SD, two-way ANOVA with Bonferroni’s post-test. aP<0.05 vs control rats, bP<0.05 vs PPA treated rats.
Abbreviations: PPA: Propionic acid; R Red: Ruthenium Red; D1: Dose 1; D2: Dose 2.
Social behavior
Sociability, sociability index, social preference, and social preference Index
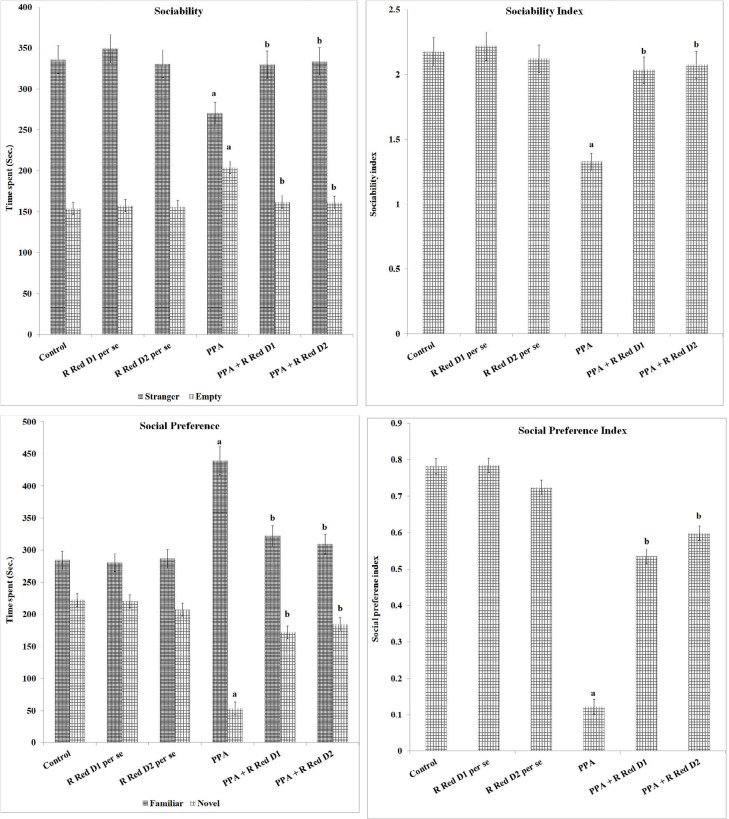
PPA has decreased time went through in stranger chamber (F(1, 42)=106.277, P<0.05) and expanded time went through in empty chamber (F(1, 42)=228.979, P<0.05), this reveals that PPA-treated rats are less sociable. The use of R Red in PPA-exposed animals highly reduced the time went through in the stranger chamber (F(2, 42)=31.543, P<0.05) followed by an increase in time went through in the empty chamber (F(2, 42)=68.632, P<0.05). PPA-treated rats have shown a lower sociability index (F(1, 42)=502.727, P<0.05) when compared to control rats, which was markedly attenuated by R Red (F(2, 42)=131.026, P<0.05) (Figure 3).
Figure 3.
Sociability, sociability index social preference and social preference index on three-chamber sociability and Novelty test apparatus
The statistics is presented with Mean±SD, two-way ANOVA followed by Bonferroni’s post-test. aP<0.05 vs control rats, bP<0.05 vs PPA treated rats.
Abbreviations: PPA: Propionic acid; R Red: Ruthenium Red; D1: Dose 1; D2: Dose 2.
PPA administration has decreased time went through the novel chamber (F(1, 42)=1477.037, P<0.05) and expand time went through the familiar side of the chamber (F(1, 42)=502.086, P<0.05), in contrast to control rats, it reveals that PPA-treated rats have a decreased social preference. The use of R Red has significantly mitigated PPA associated reduction of time went through the novel chamber (F(2, 42)=421.444, P<0.05) and increased time went through the familiar chamber (F(2, 42)=60.493, P<0.05) (Figure 3). PPA-treated rats have shown a lower social preference index (F(1, 42)=2585.419, P<0.05) in contrast to control rats, which was significantly mitigated by R Red (F(2, 42)=444.524, P<0.05).
Repetitive behavior
PPA exposure has considerably reduced the % spontaneous alteration in rats (F(1, 42)=623.665, P<0.05). This shows the repetitive behavior of PPA-exposed rats. Treatment with R Red suggestively mitigates PPA associated decreased % spontaneous alteration (F(2, 42)=274.682, P<0.05) (Figure 4), which implies a decrease in PPA-induced repetitive behavior.
Figure 4.
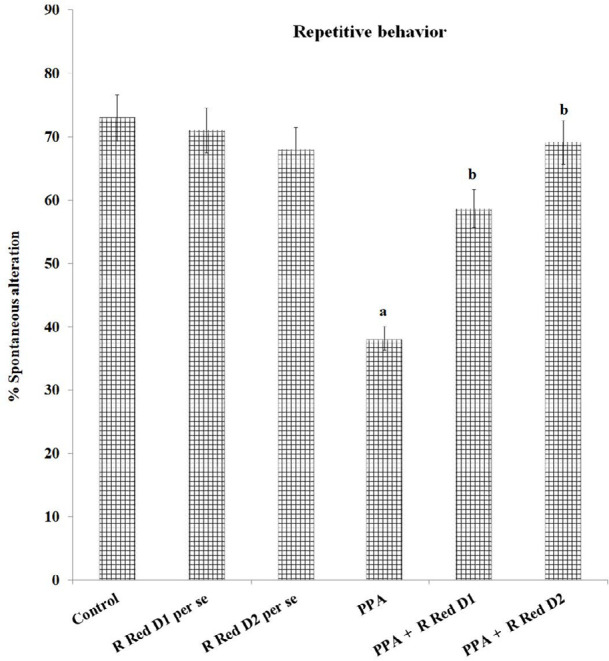
Repetitive behavior measured as % spontaneous alteration on Y maze
The statistics is presented with Mean±SD, two-way ANOVA with Bonferroni’s post-test.
aP<0.05 vs control rats, bP<0.05 vs PPA treated rats.
PPA - Propionic acid; R Red – Ruthenium Red; D1- dose 1; D2- dose 2.
PFC Neuro-inflammation, oxidative stress, and hippocampus CREB and BDNF
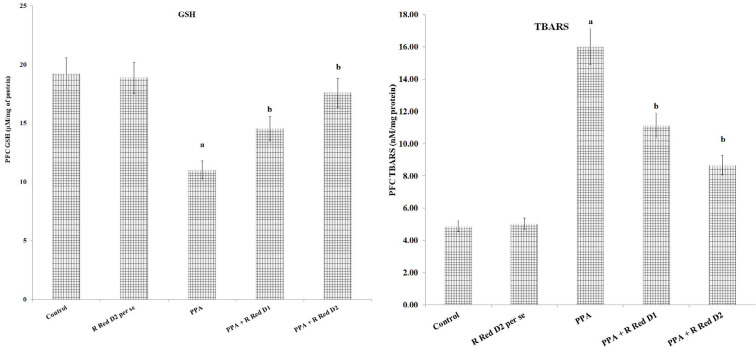
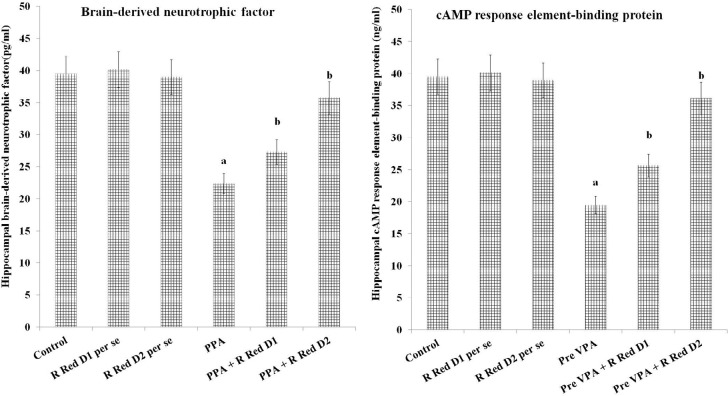
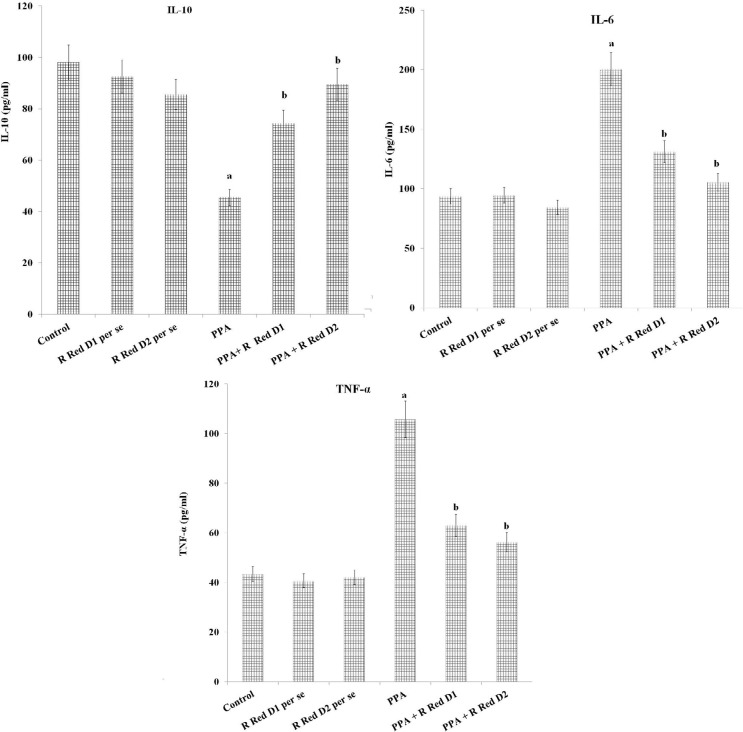
PPA has increased PFC inflammation [IL-6 levels (F(1, 18)=973.189, P<0.05), TNF-α levels (F(1, 18)=1105.281, P<0.05) and decreased IL-10 levels (F(1, 18)=219.928, P<0.05)], oxidative stress [decrease GSH levels (F (1, 18)=163.265, P<0.05) and increased TBARS levels (F(1, 18)=1257.099, P<0.05)] along with decreased hippocampus CREB (F(1, 18)=476.678, P<0.05) and BDNF (F(1, 18)=199.643, P<0.05). R Red treatment has significantly mitigated PPA induced increased, inflammation [IL-6 levels (F(2, 18)=84.89, P<0.05), TNF-α levels (F(2, 18)=364.755, P<0.05) and decreased IL-10 levels (F(2, 18)=138.116, P<0.05), oxidative stress [decrease GSH levels (F(2, 18)=685.007, P<0.05) and increased TBARS levels (F(2, 18)=175.039, P<0.05) along with decreased hippocampus CREB (F(2, 18)=29.509, P<0.05) and BDNF (F(2, 18)=78.896, P<0.05) (Figure 5, Figure 6, and Figure 7).
Figure 5.
Prefrontal cortex oxidative stress and effect of various agents
The data is presented with Mean±SD, two-way ANOVA followed by Bonferroni’s post-test. aP<0.05 vs control rats, bP<0.05 vs PPA treated rats.
Abbreviations: PPA: Propionic acid; R Red: Ruthenium Red; D1: Dose 1; D2: Dose 2.
Figure 6.
Hippocampal brain-derived neurotrophic factor (BDNF) and cAMP response element-binding protein (CREB) and effect of various agents
The data is presented with Mean±SD, two-way ANOVA followed by Bonferroni’s post-test.
aP<0.05 vs control rats, bP<0.05 vs PPA treated rats.
PPA - Propionic acid; R Red – Ruthenium Red; D1- dose 1; D2- dose 2.
Figure 7.
Prefrontal cortex inflammation (IL- 6, IL-10 and TNF-α levels) and effect of various agents The data is presented with Mean±SD, two-way ANOVA followed by Bonferroni's post-test.
aP<0.05 vs control rats, bP<0.05 vs PPA treated rats.
Abbreviations: PPA: Propionic acid; R Red: Ruthenium Red; D1: Dose 1; D2: Dose 2, IL-6: Interleukins 6; IL-10: Interleukins10; TNFα: Tumor necrosis factor-α.
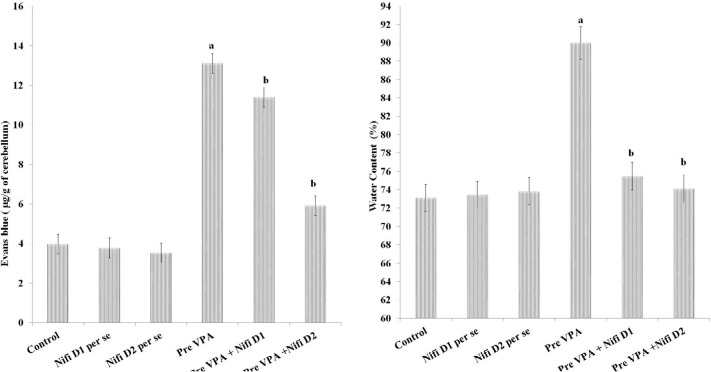
Permeability of BBB and water content
A considerably higher Evans blue concentration (F(1, 18)=971.701, P<0.05) and water content (F(1, 18)=181.92, P<0.05) were present in the cerebellum of PPA-exposed rats in contrast to control rats. Treatment with R Red expressively mitigates PPA-induced increased amount of Evans blue (F(2, 18)=163.993, P<0.05) and water content in the cerebellar tissue (F(2, 18)=94.856, P<0.05) (Figure 8), which suggest amelioration in PPA induced BBB dysfunction.
Figure 8.
Permeability of BBB assessed by Evans blue concentration and water content in cerebellum and effects of drugs. The results are representing with Mean±SD, two-way ANOVA followed by Bonferroni’s post-test. aP<0.05 vs control rats, bP<0.05 vs PPA treated rats. The cerebellum of PPA-exposed rats showed more staining than control rats, whereas the cerebellum of R Red-exposed rats showed less staining than PPA rats. PPA: Propionic acid; R Red: Ruthenium Red; D1: Dose 1; D2: Dose 2; BBB: Blood Brain Barrier.
4. Discussion
In the current investigation, we used PPA to generate autism-like symptoms in rats. PPA-exposed rats resulted in social behavior dysfunction, anxiety, and repetitive behavior. Also, PPA rats showed an increase in PFC TBARS, IL-6, TNF-α, Cerebellum Evans blue concentration, and water content. On the other hand, PPA rats demonstrated a decrease in IL-10, GSH, CREB, and BDNF levels. We also assessed the impact of ruthenium red in PPA-exposed rats. Ruthenium Red was found to mitigate the effect of PPA in rats.
Calcium-stimulated second messenger pathways can trigger the CREB, which downstream to BDNF but calcium excitotoxicity could reverse this pathway. The CREB/BDNF signaling pathway is critical in synaptic plasticity, morpho-genesis, and behavioral conduct ( Chen et al., 2012; Wang et al., 2014). Phospho-CREB controls the transcription of neuroprotective proteins like BDNF. In the brain, BDNF governs synapse development and synaptic strengthening. Reduction in BDNF level causes abnormal plasticity, learning, and memory formation ( Tye & Bolton, 2013; Uutela et al., 2012) Fmr1 knockout (KO. According to research, lowering p-CREB levels can contribute to spatial memory impairment ( Bourtchuladze et al., 1994) a cellular model of memory in Aplysia. Our studies with fear conditioning and with the water maze show that mice with a targeted disruption of the α and δ isoforms of CREB are profoundly deficient in long-term memory. In contrast, short-term memory, lasting between 30 and 60 min, is normal. Consistent with models claiming a role for long-term potentiation (LTP. Double corten, dendritic growth, production of the neuronal microtubule-associated protein, and BDNF have all been linked to CREB activity. Autistic traits such as poor synaptic plasticity and neuron survival could emerge as a result of this ( Jagasia et al., 2009). We found a significant drop in CREB and BDNF concentration in the hippocampus tissue of PPA-exposed rats, which was consistent with other studies ( Wu et al., 2017). Ryanodine receptors (RyR) regulate calcium-linked calcium release, which in turn regulates activity-dependent calcium inflow. Calcium signals in hippocampus neurons’ postsynaptic pathways drive learning and memory, and synaptic plasticity. R Red treatment also alleviated behavioral impairment in PPA rats, as well as raising p-CREB and BDNF levels.
PPA contains anti-inflammatory and pro-inflammatory cytokine modulatory effects, however IL-6, IL-10, and TNF- are connected to ASD, neuroinflammation, and other disorders ( Abdelli et al., 2019). In rats treated with PPA, we discovered higher levels of IL-6, TNF-, and lower levels of IL-10, suggesting neuroinflammation in rats with PPA-induced ASD. PPA treatment boosts glial cell proliferation at the initial of stem cell development, similar to the neuro-inflammation reported in post-partum autism brains ( Abdelli et al., 2019). PPA treatment triggered microgliosis, astrogliosis, and the release of cytokines like TNF- and IL-6 ( MacFabe et al., 2011). PPA modulates PTEN/AKT pathway followed by results in neuroinflammation and disturbed neuro-circuitry in autism ( Abdelli et al., 2019). In PPA-induced ASD symptoms, the specific process concerning PPA-induced alterations in IL-6, IL-10, and TNF- remains unknown. In contrast, the relationship between these neuroinflammatory indicators and ASD behaviors has already been extensively researched in a variety of autism studies ( Choi et al., 2020; El-Ansary et al., 2012; MacFabe et al., 2011). According to Wei and colleagues, IL-6 increases the creation of excited synapses in the cerebellum of autistic subjects and rodents. In mice and autistic patients, higher IL-6 levels affect the excitatory and inhibitory (E / I) balance by increasing glutamate excitatory neurotransmission ( Wei et al., 2011 Wei et al., 2012). Anxiety, social deficiencies, repeated behavior, and hyperactivity have all been associated with E/I imbalances ( Kim et al., 2014; Kumar et al., 2015; Wei et al., 2012). In rodents, neuroinflammation of the prefrontal cortex has resulted in elevated anxiety and decreased social behavior ( Codagnone et al., 2015) and ameliorated using anti-IL-6 antibodies treatment ( Smith et al., 2007). Clinically, low anti-inflammatory marker (IL-10) levels along with higher pro-inflammatory marker levels (IL-6 & TNF-α) are correlated with key autism symptoms such as repetitive behavior and social deficits ( Ross et al., 2013). The IL-10 induces down-regulation of microglial pro-inflammatory pathways followed by anti-inflammatory action. While one of the main inflammatory features related to reduced IL-10 is increased inflammatory tracts of the microglia ( Norden et al., 2014). As a result, PPA-related autistic symptoms may be connected to neuroinflammation caused by elevated TNF-α and IL-6 levels in the brain ( El-Ansary et al., 2012; MacFabe et al., 2007 MacFabe et al., 2008). Neuro-inflammation impairs the plasticity of dendritic spines and morphology responsible for cognitive function ( Zou et al., 2016). Few studies have shown that ryanodine receptor antagonists decrease inflammatory markers such as interleukin 2, and TNF-α and increase the anti-inflammatory markers such as IL-10 ( Dadsetan et al., 2008; López-Neblina et al., 2007) including the inflammatory response after ischemia and reperfusion. The pharmacological mechanism of dantrolene is associated with the inhibition of the release of Ca2+ from the skeletal muscle sarcoplasmic reticulum (SR. Alternatively, ryanodine receptor antagonists efficiently prevent cell death by blocking calcium mobilization ( Luciani et al., 2009). Previously, it was discovered that ryanodine antagonists limit calcium inflow through the NMDA receptors and reverse glutamate-induced excitability ( Mody, 1995; Makarewicz et al., 2003). Impaired BBB permeability was believed to be essential for cognitively impaired vascular function ( Taheri et al., 2011). As a result, we investigated the BBB’s permeability using Evans Blue. BBB dysfunction is caused by the release of ryanodine-connected intracellular calcium via the endoplasmic reticulum ( Kuhlmann et al., 2009). Higher levels of neuroinflammatory markers may modulate social interaction, repetitive behavior, and anxiety, as well as BBB function, in rats with PPA-mediated autism, according to the above lines.
In both clinical and experimental investigations, oxidative stress is one of the causes of autistic characteristics ( Fry et. al., 2011; Nadeem et al., 2018; Rossignol & Frye, 2014). Glutathione deficiency, as well as other linked antioxidant mechanisms like the redox system, the methionine cycle, and transculturation, have been found in autistic individuals ( James et al., 2004). The cerebellar damage along with abnormal glutathione redox status is associated with communication problems and social deficits in rodents and autistic subjects ( Frye et al., 2013; Morakotsriwan et al., 2016) most individuals with ASD and MD do not have a specific genetic mutation to explain the MD, raising the possibility of that MD may be acquired, at least in a subgroup of children with ASD. Acquired MD has been demonstrated in a rodent ASD model in which propionic acid (PPA. Oxidative homeostasis dysregulation induced by Wnt/-catenin pathway stimulation was observed to affect sociability, repetitive behavior, and anxiety in rats ( Zhang et al., 2012). Ryanodine antagonist reverses increased oxidative stress signals in different brain conditions ( Crouzin et al., 2007; Singh & Sharma, 2016) we have studied whether this \”hyposensitivity\” to oxidative stress could be linked to an altered Ca2+ homeostasis. Fe2+ ions triggered a Ca2+ entry which was required for Fe2+ ion-induced toxicity. This influx was sensitive to blockers of TRP-like nonspecific Ca2+ channels, including Ruthenium Red, La3+, and Gd3+ ions which also prevented the Fe2+ ion-induced toxicity and oxidative stress as revealed by protein carbonylation status. The pretreatment with α-tocopherol resulted in a reduction of the Ca2+ increase induced by Fe2+ ions and masked the blocking effect of La3+ ions. Moreover, such a pre-treatment reduced the capacitive Ca2+ entries (CCE. Endogenous oxidative stress markers are reversed by ryanodine receptor antagonists, followed by lipid peroxidation product content (TBARS), and therefore oxidative stress is alleviated ( Jain & Sharma, 2016) we have reported the induction of vascular dementia by experimental diabetes. This study investigates the efficacy of a ruthenium red, a ryanodine receptor antagonist and pioglitazone in the pharmacological interdiction of pancreatectomy diabetes (PaD. The ryanodine receptor’s antagonistic activity has been found to reduce the production of reactive oxygen species caused by glycation end products ( Kuhlmann et al., 2009). The above line of discussion that may conclude, ruthenium red possibly modulates the endogenous antioxidant pathways and results in decreased oxidative stress in PPA-exposed rats.
Autism has been linked to BBB disruption, which reinforces the gut barrier concept. Postmortem of the cerebellum of ASD altered gene expression responsible for BBB integrity ( Fiorentino et al., 2016). Interestingly, in our previous research, Pre- VPA exposed rats were found with increased BBB permeability measured by Evans blue leakage ( Kumar et al., 2015b). Altered cytokines levels, endogenous redox mechanism, mitochondrial function, and elevated neurodermatitis levels may contribute to BBB dysfunction ( Dong et al., 2019; Sorby-Adams et al., 2017) encompassing traumatic brain injury (TBI. Increased oxidative stress may be responsible for the increased BBB permeability ( Lochhead et al., 2010). The intra-cellular tight junctions between neighboring cells determine BBB permeability and both low and high amounts of Ca+ have been found to have a deleterious effect on these endothelial junctions. Collective intracellular Ca+ levels have been shown to affect in tight junction structures through apoptosis and protein kinase C-alpha ( Rakkar & Bayraktutan, 2016). PPA can modify the BBB permeability via changes in the neuroinflammatory and oxidative stress pathways. Ruthenium red modulates water homeostasis and blood-brain barrier permeability possibly via maintaining Ca+ homeostasis within the brain.
Based on the preceding discussion, ruthenium red, followed by GSH, CREB, and BDNF, could alleviate PPA-induced significant decreases in social behavior. Furthermore, ruthenium red was found to counter PPA-associated increases in anxiety, PFC TNF-α, IL-6, IL-10, and TBARS. PPA increased BBB permeability and water content, which was ameliorated by ruthenium red treatment in rats. There is a need for more research into the role of ryanodine receptor antagonists in autism-like conditions.
Ethical Considerations
Compliance with ethical guidelines
The Institutional Animal Ethics Committee of Amity University, has approved all experiments (Code: CPCSEA / IAEC/AIP/2019/01/019).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
Nirmal Singh, Faculty of Medicine, Punjab University, Patiala (Punjab), has provided us with helpful advice.
References
- Abdelli L. S., Samsam A., Naser S. A. (2019). Propionic acid induces gliosis and neuro-inflammation through modulation of PTEN/AKT pathway in autism spectrum disorder. Scientific Reports, 9(1), 8824. [DOI: 10.1038/s41598-019-45348-z] [PMID https://www.ncbi.nlm.nih.gov/pubmed/31217543] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6584527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Owain M., Kaya N., Al-Shamrani H., Al-Bakheet A., Qari A., Al-Muaigl S., et al. (2013). Autism spectrum disorder in a child with propionic acidemia. JIMD Reports, 7, 63–66. [DOI: 10.1007/8904_2012_143] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23430497] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3573175] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. D., Edwards T. M., Rickard N. S. (2013). The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neuroscience and Biobehavioral Reviews, 37(7), 1211–1239. [DOI: 10.1016/j.neubiorev.2013.04.011] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23639769] [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Bootman M. D., Lipp P. (1998). Calcium-a life and death signal. Nature, 395(6703), 645–648. [DOI: 10.1038/27094] [PMID https://www.ncbi.nlm.nih.gov/pubmed/9790183] [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A. J. (1994). Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell, 79(1), 59–68. [DOI: 10.1016/0092-8674(94)90400-6] [PMID https://www.ncbi.nlm.nih.gov/pubmed/7923378] [DOI] [PubMed] [Google Scholar]
- Bukhari S. I., Alfawaz H., Al-Dbass A., Bhat R. S., Moubayed N. M., Bukhari W., et al. (2020). Efficacy of novavit in ameliorating the neurotoxicity of propionic acid. Translational Neuroscience, 11(1), 134–146. [DOI: 10.1515/tnsci-2020-0103] [PMID https://www.ncbi.nlm.nih.gov/pubmed/33312719] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC7705989] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T., Ottolini D., Brini M. (2012). Mitochondrial Ca(2+) and neurodegeneration. Cell Calcium, 52(1), 73–85. [DOI: 10.1016/j.ceca.2012.04.015] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22608276] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3396847] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A., Gu F., Essa M. M., Wegiel J., Kaur K., Brown W. T., et al. (2011). Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. Journal of Neurochemistry, 117(2), 209–220.[DOI: 10.1111/j.1471-4159.2011.07189.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21250997] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4839269] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Y., Bambah-Mukku D., Pollonini G., Alberini C. M. (2012). Glucocorticoid receptors recruit the CaMKIIα-BDNFCREB pathways to mediate memory consolidation. Nature Neuroscience, 15(12), 1707–1714. [DOI: 10.1038/nn.3266] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23160045] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3509234] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H., Kim I. S., Mun J. Y. (2020). Propionic acid induces dendritic spine loss by MAPK/ERK signaling and dysregulation of autophagic flux. Molecular Brain, 13(1), 86.[DOI: 10.1186/s13041-020-00626-0] [PMID https://www.ncbi.nlm.nih.gov/pubmed/32487196] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC7268420] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros-Mejorado A., Gottlieb M., Ruiz A., Chara J. C., Pérez-Samartín A., Marambaud P., et al. (2018). Blockade and knock-out of CALHM1 channels attenuate ischemic brain damage. Journal of Cerebral Blood Flow and Metabolism, 38(6), 1060–1069. [DOI: 10.1177/0271678X17713587] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28597712] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5999001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codagnone M. G., Podestá M. F., Uccelli N. A., Reinés A. (2015). Differential local connectivity and neuroinflammation profiles in the medial prefrontal cortex and hippocampus in the valproic acid rat model of autism. Developmental Neuroscience, 37(3), 215–231. [DOI: 10.1159/000375489] [PMID https://www.ncbi.nlm.nih.gov/pubmed/25895486] [DOI] [PubMed] [Google Scholar]
- Cotrina M. L., Ferreiras S., Schneider P. (2019). High prevalence of self-reported autism spectrum disorder in the Propionic Acidemia Registry. JIMD Reports, 51(1), 70–75. [DOI: 10.1002/jmd2.12083] [PMID https://www.ncbi.nlm.nih.gov/pubmed/32071841] [PMCID https://www.ncbi.nlm.nih.gov/pubmed/32071841] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzin N., de Jesus Ferreira M. C., Cohen-Solal C., Aimar R. F., Vignes M., Guiramand J. (2007). Alpha-tocopherol-mediated long-lasting protection against oxidative damage involves an attenuation of calcium entry through TRP-like channels in cultured hippocampal neurons. Free Radical Biology & Medicine, 42(9), 1326–1337. [DOI: 10.1016/j.freeradbiomed.2007.01.032] [PMID https://www.ncbi.nlm.nih.gov/pubmed/17395006] [DOI] [PubMed] [Google Scholar]
- Dadsetan S., Zakharova L., Molinski T. F., Fomina A. F. (2008). Store-operated Ca2+ influx causes Ca2+ release from the intracellular Ca2+ channels that is required for T cell activation. The Journal of Biological Chemistry, 283(18), 12512–12519. [DOI: 10.1074/jbc.M709330200] [PMID https://www.ncbi.nlm.nih.gov/pubmed/18316371] [DOI] [PubMed] [Google Scholar]
- Del Prete D., Checler F., Chami M. (2014). Ryanodine receptors: Physiological function and deregulation in Alzheimer disease. Molecular Neurodegeneration, 9(1), 21. [DOI: 10.1186/1750-1326-9-21] [PMID https://www.ncbi.nlm.nih.gov/pubmed/24902695] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4063224] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Yang Y., Zhang Z., Xie K., Su L., Yu Y. (2019). Hemopexin alleviates cognitive dysfunction after focal cerebral ischemia-reperfusion injury in rats. BMC Anesthesiology, 19(1), 13. [DOI: 10.1186/s12871-019-0681-2] [PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/30646866] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6334464] [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary A. K., Ben Bacha A., Kotb M. (2012). Etiology of autistic features: The persisting neurotoxic effects of propionic acid. Journal of Neuroinflammation, 9, 74. [DOI: 10.1186/1742-2094-9-74] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22531301] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3425128] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino M., Sapone A., Senger S., Camhi S. S., Kadzielski S. M., Buie T. M., et al. (2016). Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Molecular Autism, 7(1), 49. [DOI: 10.1186/s13229-016-0110-z] [PMID https://www.ncbi.nlm.nih.gov/pubmed/27957319] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5129651] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R. E., Melnyk S., Macfabe D. F. (2013). Unique acylcarnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Translational Psychiatry, 3(1), e220. [DOI: 10.1038/tp.2012.143] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23340503] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3566723] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R. E., Rossignol D. A. (2011). Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatric Research, 69(5 Pt 2), 41R–7R. [DOI: 10.1203/PDR.0b013e318212f16b] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21289536] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3179978] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R., Steib K., Englberger E., Herold S., Faus-Kessler T., Saxe M., Gage F. H., Song H., Lie D. C. (2009). GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. The Journal of Neuroscience, 29(25), 7966–7977. [DOI: 10.1523/JNEUROSCI.1054-09.2009] [PMID https://pubmed.ncbi.nlm.nih.gov/19553437/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Sharma B. (2016). Effect of ruthenium red, a ryano-dine receptor antagonist in experimental diabetes induced vascular endothelial dysfunction and associated dementia in rats. Physiology & Behavior, 164(Pt A), 140–150. [DOI: 10.1016/j.physbeh.2016.05.052] [PMID https://www.ncbi.nlm.nih.gov/pubmed/27262216] [DOI] [PubMed] [Google Scholar]
- James S. J., Cutler P., Melnyk S., Jernigan S., Janak L., Gaylor D. W., et al. (2004). Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. The American Journal of Clinical Nutrition, 80(6), 1611–1617. [DOI: 10.1093/ajcn/80.6.1611] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15585776] [DOI] [PubMed] [Google Scholar]
- Kim K. C., Lee D. K., Go H. S., Kim P., Choi C. S., Kim J. W., et al. (2014). Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Molecular Neurobiology, 49(1), 512–528. [DOI: 10.1007/s12035-013-8535-2] [PMID https://www.ncbi.nlm.nih.gov/pubmed/24030726] [DOI] [PubMed] [Google Scholar]
- Kuhlmann C. R., Zehendner C. M., Gerigk M., Closhen D., Bender B., Friedl P., et al. (2009). MK801 blocks hypoxic blood-brain-barrier disruption and leukocyte adhesion. Neuroscience Letters, 449(3), 168–172. [DOI: 10.1016/j.neulet.2008.10.096] [PMID https://www.ncbi.nlm.nih.gov/pubmed/18996441] [DOI] [PubMed] [Google Scholar]
- Kumar H., Ranjan R. K., Yadav S., Kumar A., Ramanathan A. (2015). Hydrogeochemistry and arsenic distribution in the gorakhpur district in the middle gangetic plain, India. In Ramanathan A., Johnston S., Mukherjee A., Nath B. (Eds.) Safe and sustainable use of arsenic-contaminated aquifers in the gangetic plain (pp. 97–107). Berlin: Springer. [DOI: 10.1007/978-3-319-16124-2_7] [DOI] [Google Scholar]
- Kumar H., Sharma B. (2016). Memantine ameliorates autistic behavior, biochemistry & blood brain barrier impairments in rats. Brain Research Bulletin, 124, 27–39. [DOI: 10.1016/j.brainresbull.2016.03.013] [PMID https://www.ncbi.nlm.nih.gov/pubmed/27034117] [DOI] [PubMed] [Google Scholar]
- Kumar H., Sharma B. (2016). Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Research, 1630, 83–97. [DOI: 10.1016/j.brainres.2015.10.052] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26551768] [DOI] [PubMed] [Google Scholar]
- Kumar H., Sharma B. M., Sharma B. (2015). Benefits of agomelatine in behavioral, neurochemical and blood brain barrier alterations in prenatal valproic acid induced autism spectrum disorder. Neurochemistry International, 91, 34–45. [DOI: 10.1016/j.neuint.2015.10.007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26498253] [DOI] [PubMed] [Google Scholar]
- Lochhead J. J., McCaffrey G., Quigley C. E., Finch J., DeMarco K. M., Nametz N., et al. (2010). Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. Journal of Cerebral Blood Flow and Metabolism, 30(9), 1625–1636. [DOI: 10.1038/jcbfm.2010.29] [PMID https://www.ncbi.nlm.nih.gov/pubmed/20234382] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2949263] [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Neblina F., Toledo-Pereyra L. H., Toledo A. H., Walsh J. (2007). Ryanodine receptor antagonism protects the ischemic liver and modulates TNF-alpha and IL-10. The Journal of Surgical Research, 140(1), 121–128. [DOI: 10.1016/j.jss.2006.12.003] [PMID https://www.ncbi.nlm.nih.gov/pubmed/17359999] [DOI] [PubMed] [Google Scholar]
- Luciani D. S., Gwiazda K. S., Yang T. L., Kalynyak T. B., Bychkivska Y., Frey M. H., et al. (2009). Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes, 58(2), 422–432. [DOI: 10.2337/db07-1762] [PMID https://www.ncbi.nlm.nih.gov/pubmed/19033399] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2628616] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M., Apps D., Menzies J., Patel J. C., Rice M. E. (2016). Dendritic release of neurotransmitters. Comprehensive Physiology, 7(1), 235–252. [DOI: 10.1002/cphy.c160007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28135005] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5381730] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFabe D. F., Cain D. P., Rodriguez-Capote K., Franklin A. E., Hoffman J. E., Boon F., et al. (2007). Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behavioural Brain Research, 176(1), 149–169. [DOI: 10.1016/j.bbr.2006.07.025] [PMID https://www.ncbi.nlm.nih.gov/pubmed/20937326] [DOI] [PubMed] [Google Scholar]
- MacFabe D. F., Cain N. E., Boon F., Ossenkopp K. P., Cain D. P. (2011). Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behavioural Brain Research, 217(1), 47–54. [DOI: 10.1016/j.bbr.2010.10.005] [PMID https://www.ncbi.nlm.nih.gov/pubmed/20937326] [DOI] [PubMed] [Google Scholar]
- MacFabe D. F., Rodríguez-Capote K., Hoffman J. E., Franklin A. E., Mohammad-Asef Y., Taylor A. R., et al. (2008). A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuro-inflammation and oxidative stress in discrete regions of adult rat brain. American Journal of Biochemistry and Biotechnology, 4(2), 146-166. [DOI: 10.3844/ajbbsp.2008.146.166] [DOI] [Google Scholar]
- Maenner M. J., Shaw K. A., Baio J. EdS1, Washington A., Patrick M., DiRienzo M., et al. (2020). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2016. Morbidity and Mortality Weekly Report. Surveillance Summaries, 69(4), 1–12. [DOI: 10.15585/mmwr.ss6904a1] [PMID https://www.ncbi.nlm.nih.gov/pubmed/32214087] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarewicz D., Ziemińska E., Łazarewicz J. W. (2003). Dantrolene inhibits NMDA-induced 45Ca uptake in cultured cerebellar granule neurons. Neurochemistry International, 43(4–5), 273–278. [DOI: 10.1016/S0197-0186(03)00012-3] [PMID https://www.ncbi.nlm.nih.gov/pubmed/12742069] [DOI] [PubMed] [Google Scholar]
- Manaenko A., Chen H., Kammer J., Zhang J. H., Tang J. (2011). Comparison evans blue injection routes: intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. Journal of Neuroscience Methods, 195(2), 206–210. [DOI: 10.1016/j.jneumeth.2010.12.013] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21168441] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3026886] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza R., Sharma B. (2018). Selective modulator of peroxisome proliferator-activated receptor-α protects propionic acid induced autism-like phenotypes in rats. Life Sciences, 214, 106–117. [DOI: 10.1016/j.lfs.2018.10.045] [PMID https://www.ncbi.nlm.nih.gov/pubmed/30366038] [DOI] [PubMed] [Google Scholar]
- Mody I. (1995). NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends in Pharmacological Sciences, 16(10), 356–359. [DOI: 10.1016/S0165-6147(00)89070-7] [PMID https://www.ncbi.nlm.nih.gov/pubmed/7491714] [DOI] [PubMed] [Google Scholar]
- Morakotsriwan N., Wattanathorn J., Kirisattayakul W., Chaisiwamongkol K. (2016). Autistic-like behaviors, oxidative stress status, and histopathological changes in cerebellum of valproic acid rat model of autism are improved by the combined extract of purple rice and silkworm pupae. Oxidative Medicine and Cellular Longevity, 2016, 3206561. [DOI: 10.1155/2016/3206561] [PMID https://www.ncbi.nlm.nih.gov/pubmed/27034733] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4806649] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A., Ahmad S. F., Attia S. M., Bakheet S. A., Al-Harbi N. O., Al-Ayadhi L. Y. (2018). Activation of IL-17 receptor leads to increased oxidative inflammation in peripheral monocytes of autistic children. Brain, Behavior, and Immunity, 67, 335–344. [DOI: 10.1016/j.bbi.2017.09.010] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28935156] [DOI] [PubMed] [Google Scholar]
- Norden D. M., Fenn A. M., Dugan A., Godbout J. P. (2014). TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia, 62(6), 881–895. [DOI: 10.1002/glia.22647] [PMID https://www.ncbi.nlm.nih.gov/pubmed/24616125] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4061706] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K. F., Deshpande L. S., DeLorenzo R. J. (2013). Hypothermia reduces calcium entry via the N-methyl-D-aspartate and ryanodine receptors in cultured hippocampal neurons. European Journal of Pharmacology, 698(1–3), 186–192. [DOI: 10.1016/j.ejphar.2012.10.010] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23085028] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3536497] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole D. P., Amadesi S., Veldhuis N. A., Abogadie F. C., Lieu T., Darby W., et al. (2013). Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. The Journal of Biological Chemistry, 288(8), 5790–5802. [DOI: 10.1074/jbc.M112.438184] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23288842] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3581372] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakkar K., Bayraktutan U. (2016). Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-alpha and apoptosis. Biochimica et Biophysica Acta, 1862(1), 56–71. [DOI: 10.1016/j.bbadis.2015.10.016] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26527181] [DOI] [PubMed] [Google Scholar]
- Ross H. E., Guo Y., Coleman K., Ousley O., Miller A. H. (2013). Association of IL-12p70 and IL-6:IL-10 ratio with autism-related behaviors in 22q11.2 deletion syndrome: A preliminary report. Brain, Behavior, and Immunity, 31, 76–81. [DOI: 10.1016/j.bbi.2012.12.021] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23353117] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3669236] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol D. A., Frye R. E. (2014). Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Frontiers in Physiology, 5, 150. [DOI: 10.3389/fphys.2014.00150] [PMID https://www.ncbi.nlm.nih.gov/pubmed/24795645] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4001006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T., Eder C. (2009). Importance of the non-selective cation channel TRPV1 for microglial reactive oxygen species generation. Journal of Neuroimmunology, 216(1–2), 118–121. [DOI: 10.1016/j.jneuroim.2009.07.008] [PMID https://www.ncbi.nlm.nih.gov/pubmed/19683814] [DOI] [PubMed] [Google Scholar]
- Singh P., Sharma B. (2016). Reversal in cognition impairments, cholinergic dysfunction, and cerebral oxidative stress through the modulation of ryanodine receptors (RyRs) and cysteinyl leukotriene-1 (CysLT1) receptors. Current Neurovascular Research, 13(1), 10–21. [DOI: 10.2174/1567202612666151026105610] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26500103] [DOI] [PubMed] [Google Scholar]
- Smith S. E., Li J., Garbett K., Mirnics K., Patterson P. H. (2007). Maternal immune activation alters fetal brain development through interleukin-6. The Journal of Neuroscience, 27(40), 10695–10702. [DOI: 10.1523/JNEUROSCI.2178-07.2007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/17913903] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2387067] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorby-Adams A. J., Marcoionni A. M., Dempsey E. R., Woenig J. A., Turner R. J. (2017). The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. International Journal of Molecular Sciences, 18(8), 1788.[DOI: 10.3390/ijms18081788] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28817088] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5578176] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Nagai Y., Wada K., Koike T. (2012). Calcium leak through ryanodine receptor is involved in neuronal death induced by mutant huntingtin. Biochemical and Biophysical Research Communications, 429(1–2), 18–23. [DOI: 10.1016/j.bbrc.2012.10.107] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23131566] [DOI] [PubMed] [Google Scholar]
- Taheri S., Gasparovic C., Huisa B. N., Adair J. C., Edmonds E., Prestopnik J., et al. (2011). Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke, 42(8), 2158–2163. [DOI: 10.1161/STROKEAHA.110.611731] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21719768] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3584170] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye C., Bolton P. (2013). Neural connectivity abnormalities in autism: insights from the Tuberous Sclerosis model. BMC Medicine, 11(1), 55. [DOI: 10.1186/1741-7015-11-55] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23445933] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3751657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uutela M., Lindholm J., Louhivuori V., Wei H., Louhivuori L. M., Pertovaara A., et al. (2012). Reduction of BDNF expression in Fmr1 knockout mice worsens cognitive deficits but improves hyperactivity and sensorimotor deficits. Genes, Brain, and Behavior, 11(5), 513–523. [DOI: 10.1111/j.1601-183X.2012.00784.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22435671] [DOI] [PubMed] [Google Scholar]
- Wang B., Zhao J., Yu M., Meng X., Cui X., Zhao Y., et al. (2014). Disturbance of intracellular calcium homeostasis and CaMKII/CREB signaling is associated with learning and memory impairments induced by chronic aluminum exposure. Neurotoxicity Research, 26(1), 52–63. [DOI: 10.1007/s12640-013-9451-y] [PMID https://www.ncbi.nlm.nih.gov/pubmed/24366850] [DOI] [PubMed] [Google Scholar]
- Wei H., Chadman K. K., McCloskey D. P., Sheikh A. M., Malik M., Brown, et al. (2012). Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochimica et Biophysica Acta, 1822(6), 831–842. [DOI: 10.1016/j.bbadis.2012.01.011] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22326556] [DOI] [PubMed] [Google Scholar]
- Wei H., Zou H., Sheikh A. M., Malik M., Dobkin C., Brown W. T., et al. (2011). IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. Journal of Neuroinflammation, 8, 52. [DOI: 10.1186/1742-2094-8-52] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21595886] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3114764] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wang X., Gao J., Liang S., Hao Y., Sun C., et al. (2017). Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuro-inflammation in the rat model of autism. Life Sciences, 173, 43–54. [DOI: 10.1016/j.lfs.2017.01.012] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28161158] [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sun Y., Wang F., Wang Z., Peng Y., Li R. (2012). Downregulating the canonical Wnt/β-catenin signaling pathway attenuates the susceptibility to autism-like phenotypes by decreasing oxidative stress. Neurochemical Research, 37(7), 1409–1419. [DOI: 10.1007/s11064-012-0724-2] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22374471] [DOI] [PubMed] [Google Scholar]
- Zou C., Shi Y., Ohli J., Schüller U., Dorostkar M. M., Herms J. (2016). Neuroinflammation impairs adaptive structural plasticity of dendritic spines in a preclinical model of Alzheimer's disease. Acta Neuropathologica, 131(2), 235–246. [DOI: 10.1007/s00401-015-1527-8] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26724934] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4713725] [DOI] [PMC free article] [PubMed] [Google Scholar]