Abstract
Background
The aim of this prospective study was to evaluate the role of serum IL-6 as a potential predictive biomarker of postoperative complications (POC) in elective colorectal surgery.
Method
A total of 115 patients underwent colorectal surgery for malignancy. IL-6 was measured on the first and third postoperative days (POD1, POD3), and C-reactive protein (CRP) was measured on the POD3. POC was analysed in subgroups according to Clavien‒Dindo (CD), antibiotic (ATB) treatment, intensive care unit (ICU) and hospital length of stay. The predictive power of variables for evaluated endpoints was analysed using receiver-operating characteristic (ROC) analysis and described by area under the curve (AUC). ROC analysis was adopted for the identification of optimal cut-offs. Histological analysis was performed to verify IL-6 production by the tumour.
Results
Out of 115 patients who were analysed, 42% had POC. Patients with POC had significantly higher serum levels of IL-6 on POD1 (p < 0.001) and POD3 (p < 0.001). IL-6 early on POD1 as a predictor of antibiotic treatment, ICU stay and hospital stay (AUC 0.818; 0.811; 0.771) did not significantly differ from the AUC of CRP late on POD3 (0.879; 0.838, 0.752). A cut-off IL-6 value of 113 pg/ml on POD1 and 180.5 pg/ml on POD3 in severe complications (CD > 3a) resulted in 75% and 72% sensitivity, 78.6% and 99% specificity, negative predictive value 96.4% and 97% and positive predictive value 29% and 88.9%.
Conclusion
The serum level of interleukin-6 can predict severe (CD > 3a) POC early on POD1. On POD3, IL-6 is superior to CRP in terms of high positive predictive power of severe POC. Interestingly, the advantage of IL-6 on POD1 is early prediction of the need for antibiotic treatment, ICU stay and hospital stay, which is comparable to the CRP serum level late on the third POD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-03270-9.
Keywords: Interleukin-6, Postoperative complications, Colorectal surgery, Infection
Introduction
Elective colorectal surgery has relatively high postoperative mortality and morbidity due to its complexity. According to the literature, the mortality rate ranges from approximately 5 to 6%, and the morbidity rate ranges from approximately 20 to 40% [1]. Despite all improvements in surgical techniques, perioperative management and implementation of ERAS (enhanced recovery after surgery), avoidance of all postoperative complications (POC) is challenging, especially in old and fragile patients. Early detection and management of anastomotic leakage (AL) are essential to prevent severe complications and reduce mortality. These include stoma creation, prolonged length of hospital stay with negative economic aspects and peritonitis, which may lead to death. However, detection is challenging due to nonspecific signs early after operation. On the other hand, identification of patients with a low risk of POC allows early discharge with a low readmission rate. Interest in biomarkers for early prediction of AL is growing over time, and in the literature, many studies focus on different predictive systemic and peritoneal drain biomarkers. Instead of C-reactive protein (CRP), which predicts POC quite late, several studies focused on interleukin-6 (IL-6) serum levels from different points of view in colorectal surgery, but its role has not been clarified. In terms of postoperative complications, larger studies suggest that IL-6 might be helpful in predicting complications such as Clavien–Dindo (CD) > 3a, intra-abdominal infection or AL; however, conclusions differ, and some of them are rather heterogeneous. Therefore, the purpose of this study was to prospectively evaluate IL-6 in consecutive colorectal patients at our department as an appropriate biomarker to predict or detect complications early, which is an important task with multiple benefits in surgery and oncology.
There is strong evidence that many cell types produce IL-6. This cytokine is essential in the initial phase of the immune response; however, its biological impact is quite complex [2]. In the cell, IL-6 is recognized by a specific transmembrane receptor, IL-6R, which interacts with glycoprotein 130, acting as a signal transducer. IL-6 can also bind to a soluble form of the receptor (sIL-6R). This can trigger IL-6 signalling in cells not expressing IL-6R. This mechanism is usually called alternative or trans-signalling [3].
Many studies have shown the very important role of IL-6 signalling pathway activity in the biology of malignant tumours [4]. It is one of the principal mediators of the dialogue between malignant and other cells across the cancer ecosystem [5]. IL-6 serum levels are elevated in cancer patients, particularly in the advanced stages of malignancies, including colorectal cancer [6, 7]. IL-6 also participates in tumour spreading and metastasis formation in colorectal cancer [8, 9]. The elevated proinflammatory cytokines, namely, IL-1β and IL-6, can be considered markers of POC in colorectal cancer [10].
Surgical intervention inevitably elicits a proinflammatory response. However, it is also known that surgery and anaesthesia can result in a variety of metabolic and endocrine responses, which result in a generalized state of immunosuppression in the immediate postoperative period [11]. In complex surgery, it is essential to keep these two responses balanced [12]. To avoid the dramatic consequences of an overwhelming systemic inflammatory response syndrome, such as organ failure, an anti-inflammatory response (called “compensatory anti-inflammatory response syndrome”: CARS) is rapidly triggered by the host, including cortisol secretion by adrenal glands after afferent impulses from the site of injury [13, 14]. Mokart recently showed that IL-6 is a good independent early marker of postoperative sepsis, severe sepsis or septic shock after major oncological surgery [15]. The classic proinflammatory response is also activated in infectious complications, and increasing levels of inflammatory cytokines have also been reported in these complications [16].
We aimed to assess the predictive values and role of IL-6 and CRP in colorectal surgery. IL-6 serum levels might help predicting POC early and guide surgeons to provide more intensive care, prolong antibiotic (ATB) treatment and safe discharge of the patients.
Materials and methods
Patients
The prospective study included 122 consecutive patients operated on at University Hospital Brno Bohunice in the Czech Republic between May 2021 and September 2022. All patients were diagnosed with colorectal malignancy and underwent elective radical surgical resection with primary anastomosis (Table 1). Seven patients were excluded from a total of 122 patients due to resections indicated for inflammatory bowel disease (IBD). None of the patients was tested for Lynch syndrome. Since we started testing for microsatellite instability (MSI), 25 patients were tested. None of the patients had received immunosuppressive therapy in the last month before surgery or aspirin as a chemopreventive agent. All data were collected prospectively. This study was approved by the Medical Ethical Committee of the Faculty Hospital Brno (no. 10–270420/EK) in accordance with the ethical principles of the Declaration of Helsinki.
Table 1.
Patient characteristics, POC and subgroups of patients
| Patient characteristics (N = 115) | |
|---|---|
| Sex ratio (M:F) | 59 (51.3%):56 (48.7%) |
| Age (years) | 68 (39–89) |
| BMI (kg/m2) | 27 (17–43) |
| Physical status classification ASA | |
| 1/2/3/4 | 1 (0.9%)/33 (28.7%)/59 (51.3%)/22 (19.1%) |
| Comorbidities (overall) | 53 (46.1%) |
| Coronary artery disease | 24 (20.9%) |
| Diabetes mellitus | 30 (26.1%) |
| Thromboembolic disease | 27 (23.5%) |
| Rectal resections | 47 (40.9%) |
| TaTME | 16 (13.9%) |
| Laparoscopic/open TME | 14 (12.2%)/6 (5.2%) |
| Laparoscopic PME | 11 (9.6%) |
| Operating time (min) | 200 (90–380) |
| Adenocarcinoma | 38 (33.0%) |
| Adenoma with HG dysplasia | 4 (3.5%) |
| pCR after neoadjuvant CRT | 3 (2.6%) |
| No residual malignancy after LE | 2 (1.7%) |
| Upper/middle/distal rectum | 26 (55.3%)/11 (23.4%)/11 (23.4%) |
| Neoadjuvant therapy | 29 (61.7%) |
| Colonic resections | 68 (59.1%) |
| Laparoscopic/open left colectomy | 14 (12.2%)/6 (5.2%) |
| Laparoscopic/open right colectomy | 23 (20.0%)/23 (20.0%) |
| Transverse colon resection | 2 (1.7%) |
| Operating time (min) | 195 (75–380) |
| Adenocarcinoma | 55 (47.8%) |
| Adenoma with HG dysplasia | 10 (8.7%) |
| NET | 1 (0.9%) |
| Lymphoma | 2 (1.7%) |
| No complications/overall complications | 66 (57.4%)/49 (42.6%) |
| Mortality | 1 (0.9%) |
| Clavien‒Dindo | |
| 1/2 | 1 (0.9%)/34 (29.6%) |
| 3a/3b | 2 (1.7%)/3 (2.6%) |
| 4a/4b | 6 (5.2%)/2 (1.7%) |
| 5 | 1 (0.9%) |
| Reoperation | 10 (8.7%) |
| Anastomotic leak | 6 (5.2%) |
| Paralytic ileus | 15 (13%) |
| Urinary tract complications | 8 (7%) |
| Wound complications | 7 (6.1%) |
| Sepsis | 4 (3.5%) |
| Medical complications | 16 (13.9%) |
| Subgroups of patients | |
| CD > 3a | 12 (10.4%) |
| CD > 2 | 14 (12.2%) |
| ICU (days) | 4 (1; 60) |
| ICU > 5 days | 33 (28.7%) |
| Hospital stay (days) | 8 (6; 72) |
| Hospital stay > 10 days | 30 (26.1%) |
| Antibiotic treatment | 24 (20.9%) |
| Inflammatory complication | 29 (25.2%) |
M male, F female, TaTME transanal total mesorectal excision, TME total mesorectal excision, PME partial mesorectal excision, LC left colectomy, RC right colectomy, pCR pathologic complete response, CRT chemoradiotherapy, LE local excision, NET neuroendocrine tumour, HG high grade, BMI body mass index, ASA American Society of Anesthesiologists. Categorical variables are described by absolute (relative) frequencies. Continuous variables are described by median (minimum–maximum)
Oncological and surgical management
All patients with rectal tumours were assessed by a multidisciplinary team. Staging (according to the American Joint Committee on Cancer) was performed using whole-body positron emission tomography with magnetic resonance imaging (PET-MRI) [17]. Patients diagnosed with colon tumours were staged with abdominal and chest CT (computed tomography). Rigid rectoscopy and/or colonoscopy was performed to obtain a biopsy and exclude synchronous lesions. Neoadjuvant therapy, mostly chemoradiotherapy (CRT), was indicated by the oncologist according to recent guidelines [18, 19]. Adjuvant treatment after colon resections was decided by the oncologist, according to definitive histology.
Surgery management included the ERAS protocol, assessment of sphincters if a sphincter-saving procedure was possible (patients with middle and distal rectal tumours) and mechanical bowel preparation with oral antibiotics.
The procedure for rectal resection was described in detail previously [20]. Briefly, we operated mostly laparoscopically. Total mesorectal excision (TME) was performed for the middle (5–10 cm from the anal verge) and distal (0–5 cm) tumours, and partial mesorectal excision (PME) was performed for the upper tumours (10–15 cm). The transanal approach (using GelPOINT®) was used when TME could not be completed laparoscopically. The anastomosis was either hand sewn (in case of low anastomosis) or stapled. All patients were operated on between 10 and 12 weeks after neoadjuvant CRT, and all of them had created defunctioning loop ileostomy.
For right-sided colon tumours, handsewn anastomosis was usually performed, and complete mesocolic excision was indicated according to expert consensus [21]. In the case of descending colon/sigmoid tumours, left colectomy with side-to-end colorectal anastomosis was usually performed with a circular stapler. Tumours localized in the middle of the transverse colon were managed by transverse resection with side-to-side anastomosis. Indocyanine green (ICG) fluorescence angiography was used routinely to determine anastomotic perfusion.
Detection of IL-6 in resected tissue
The methodology of immunohistochemical analysis is available in Supplementary document no. 3.
Postoperative observations
We assessed postoperative morbidity and mortality in the first 90 days after the operation. Postoperative morbidity was evaluated using the CD classification. In CD classification, each POC is classified into one of five categories depending on its severity [22]. For a better assessment of POC, we also created subgroups of patients who had a postoperative inflammatory complication (PIC) and who needed ATB treatment. PIC included surgical site infections (SSI), parastomal abscesses, urinary tract infections, pneumonia, peritonitis and sepsis. To be more accurate, we also recorded the need for ATB treatment after surgery since not all PIC require antibiotics (COVID-19 infection and some SSI). Hospital and intensive care unit (ICU) stays were also recorded.
Study design
A total of 115 patients were included in the analysis. Patients were divided into six subgroups according to different characteristics, i.e. different types of complications. The subgroups consisted of the following: patients with CD > 2, CD > 3a, ICU hospital stay > 5 days and hospital stay > 10 days, patients who needed ATB treatment, and patients who suffered from PIC. Subgroups of patients with CD > 2 and CD > 3 are considered patients with major complications who require surgical, endoscopic or radiological intervention without general anaesthesia and intervention under general anaesthesia. These are the most serious, clinically relevant complications, which mainly influence the postoperative course. Patients assigned to the ATB treatment subgroup needed antibiotics in the postoperative period. The inflammatory complication subgroup consisted of patients who had incisional SSI, intra-abdominal infection (collection, abscess, peritonitis), sepsis, respiratory and urinary or intestinal infection with identification of the organism(s) by culture or non-culture-based microbiologic testing methods. We believe the aspect of ATB treatment and PIC can add additional and more precise information about postoperative course, and if predicted, it might serve as a guide for early ATB treatment. Prolonged ICU and hospital stays are connected with adverse postoperative course and were defined as ICU stay > 5 days and hospital stay > 10 days. All patients were analysed for serum levels of IL-6 on postoperative day (POD) 1 and POD3 and CRP on POD3. We hypothesized that high serum levels of IL-6 could serve as a predictive factor for POC, hospital and ICU stays and the need for ATB. The results were also analysed to set up the cut-off values of predictive factors.
Blood sample analysis
All blood samples were drawn using routine blood tests in the morning from the peripheral or central venous system. A blood sample was routinely analysed in the hospital biochemical laboratory, where Li heparin plasma was used to determine the CRP (mg/l) and IL-6 (pg/ml) values. Automatic analyses were carried out in the clinical chemistry module c702 of Cobas 8000 (F. Hoffman-La Roche Ltd.; hereinafter, Roche) via immunoturbidimetric CRP test (Ref. 07876424 190) and in the immunochemical module e801 of Cobas 8000 via electrochemiluminescence noncompetitive IL-6 immunoassay (Ref. 07027532190).
Statistics
Standard descriptive statistics were applied in the analysis — absolute and relative frequencies for categorical variables and median supplemented by minimum–maximum range for continuous variables. The predictive power of variables for evaluated endpoints was analysed using receiver-operating characteristic (ROC) analysis and described by area under the curve (AUC), its 95% confidence interval and statistical significance. ROC analysis was adopted to identify optimal cut-offs for continuous variables at the point of the maximum sum of sensitivity (SN) and specificity (SP). Cut-off values were obtained using the Youden index. The area under the receiver operating characteristic curve (AUROC) is a widely recognized metric in medical diagnostics. It quantifies a diagnostic test’s ability to distinguish between different conditions or groups of patients. An AUROC value of 1.0 signifies a perfect test, while 0.5 suggests that the test is no better than chance, represented as a diagonal line on the graph. An AUROC exceeding 0.9 indicates a highly effective test with strong discriminatory power [23]. To compare ROC curves, the p-value was computed using the method by Hanley and McNeil. Analysis was computed using SPSS 28.0.1.1 (IBM Corporation 2021), and p = 0.05 was adopted as the level of statistical significance in all analyses. No correction for multiple testing was applied.
Results
Characteristics of patients
Of the 115 analysed patients, 40.9% underwent rectal resection, mostly in the third clinical stage (61.7%), and 59.1% of patients underwent colonic resection, mostly in the second pathological stage (35.3%). Most procedures were performed laparoscopically (67.8%), and the most common pathological finding was adenocarcinoma (80.9%). Neoadjuvant therapy was administered in 29 (25.2%) patients only for rectal tumours. Six patients had MSI (5 colon, 1 rectum), 19 patients were microsatellite stable (MSS) (11 colon, 8 rectum) and 90 patients were not tested. Because of a small sample, we did not perform analysis further analysis. Detailed characteristics are given in Table 1.
Detection of IL-6 and IL-6R in cancer samples
To verify the tumour as the source of IL-6, colorectal carcinoma from the patients was subjected to histological analysis. The detailed description and histological section specimen picture are available in Supplementary document no. 3.
Postoperative complications
A total of 49 (42%) patients had complications, and one patient with many medical comorbidities died from septic shock after a right colectomy due to progressive small bowel ischemia, which required reoperations. The most common complication according to CD classification was grade 2, which mostly included paralytic ileus, urinary tract infection and SSI. Reoperation was indicated for all six cases of AL, and in five cases, ileostomy was created. AL occurred in four cases after right colectomy and only in two cases after rectal resections, due to higher percentage (55.3%) of upper rectal tumours (see Table 1).
IL-6 and CRP as predictors of postoperative complications
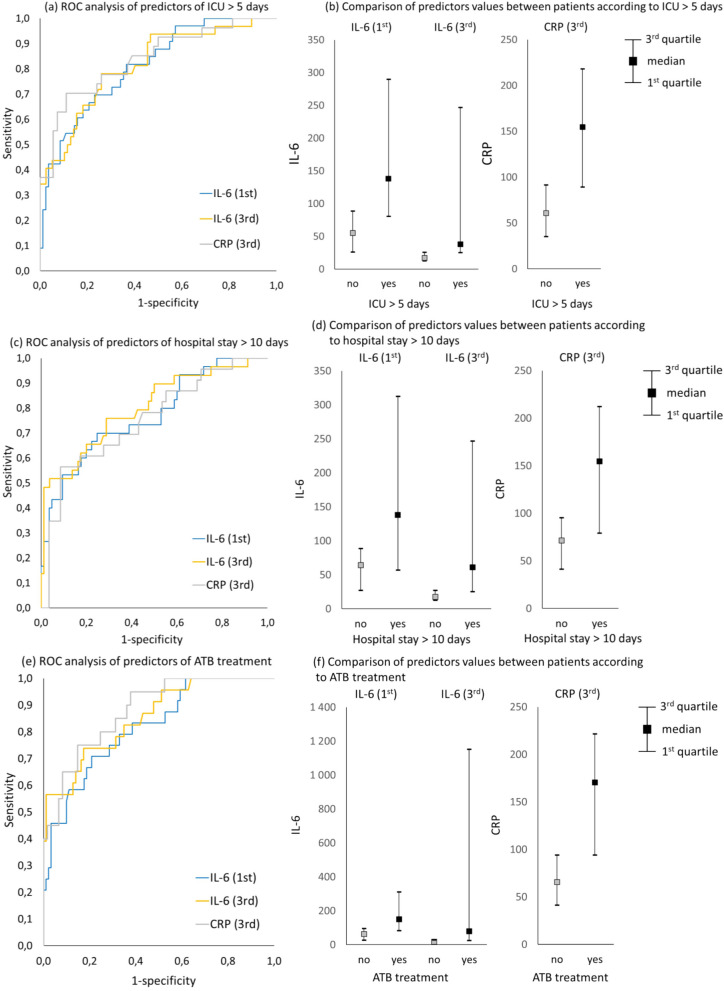
Biomarkers IL-6 and CRP as predictive factors are presented in Table 2. IL-6 and CRP values were significantly higher in particular subgroups of patients with POC (p < 0.001). The best marker in terms of greatest AUC (0.914; CI 0.817–1.000) was the IL-6 value on the third day after the operation, except for the prediction of ICU stay more than 5 days (AUC 0.814; CI 0.724–0.904) and inflammatory complications (AUC 0.839; 0.758–0.920), where CRP proved to be superior (AUC 0.838 and 0.904). Moreover, IL-6 on POD1 as a predictor of POC was statistically significant (p < 0.001) and, therefore, could predict complications, most accurately the need for antibiotic treatment (AUC 0.818; CI 0.725–0.912). No statistically significant differences were found in IL-6 levels depending on the tumour height in the rectum (Fig. 1, Supplementary documents nos. 1 and 2).
Table 2.
Strength of predictors between patient subgroups
| Predictor (POD) | Patient subgroups | AUC (95% CI) | p-value | |
|---|---|---|---|---|
| Clavien‒Dindo > 3a | Clavien‒Dindo < 3a | |||
| IL-6 (1st) | N = 12; 189 (46; 2 746) | N = 103; 66 (9; 936) | 0.804 (0.673; 0.934) | < 0.001 |
| IL-6 (3rd) | N = 11; 1 153 (19; 50 000) | N = 98; 19 (3; 473) | 0.914 (0.817; 1.000) | < 0.001 |
| CRP (3rd) | N = 10; 192 (63; 420) | N = 71; 76 (14; 342) | 0.869 (0.755; 0.983) | < 0.001 |
| Clavien‒Dindo > 2 | Clavien‒Dindo < 2 | |||
| IL-6 (1st) | N = 14; 145 (31; 2 746) | N = 101; 66 (9; 936) | 0.751 (0.612; 0.890) | 0.002 |
| IL-6 (3rd) | N = 13; 263 (18; 50 000) | N = 96; 19 (3; 473) | 0.865 (0.755; 0.976) | < 0.001 |
| CRP (3rd) | N = 10; 192 (63; 420) | N = 71; 76 (14; 342) | 0.869 (0.755; 0.983) | < 0.001 |
| ICU > 5 days | ICU < 5 days | |||
| IL-6 (1st) | N = 33; 138 (32; 2 746) | N = 82; 56 (9; 419) | 0.811 (0.727; 0.895) | < 0.001 |
| IL-6 (3rd) | N = 32; 43 (9; 50 000) | N = 77; 18 (3; 83) | 0.814 (0.724; 0.904) | < 0.001 |
| CRP (3rd) | N = 27; 155 (32; 420) | N = 54; 62 (14; 185) | 0.838 (0.742; 0.934) | < 0.001 |
| Hospital stay > 10 days | Hospital stay < 10 days | |||
| IL-6 (1st) | N = 30; 138 (26; 2 746) | N = 85; 65 (9; 352) | 0.771 (0.669; 0.872) | < 0.001 |
| IL-6 (3rd) | N = 29; 61 (8; 50 000) | N = 80; 18 (3; 1 387) | 0.800 (0.700; 0.899) | < 0.001 |
| CRP (3rd) | N = 23; 155 (32; 292) | N = 58; 73 (14; 420) | 0.752 (0.631; 0.873) | < 0.001 |
| ATB treatment | No ATB treatment | |||
| IL-6 (1st) | N = 24; 152 (43; 2 746) | N = 91; 64 (9; 352) | 0.818 (0.725; 0.912) | < 0.001 |
| IL-6 (3rd) | N = 23; 79 (15; 50 000) | N = 86; 18 (3; 114) | 0.854 (0.766; 0.942) | < 0.001 |
| CRP (3rd) | N = 20; 171 (63; 420) | N = 61; 66 (14; 201) | 0.879 (0.798; 0.959) | < 0.001 |
| Inflammatory complication | No inflammatory complication | |||
| IL-6 (1st) | N = 29; 138 (31; 2 746) | N = 86; 61 (9; 352) | 0.798 (0.705; 0.890) | < 0.001 |
| IL-6 (3rd) | N = 28; 52 (15; 50 000) | N = 81; 18 (3; 114) | 0.839 (0.758; 0.920) | < 0.001 |
| CRP (3rd) | N = 22; 178 (63; 420) | N = 59; 64 (14; 178) | 0.904 (0.833; 0.974) | < 0.001 |
POD postoperative day, AUC area under the curve, CI confidence interval, ICU intensive care unit, ATB antibiotics. Continuous variables are described by median (minimum–maximum). N is the number of patients with or without specific complication. IL-6 serum levels are measured in pg/ml and CRP in mg/l. Parentheses include minimum and maximum values
Fig. 1.
ROC curves and box plot graphs of IL-6 on POD1 and POD3 and CRP on POD3 as predictors of ICU (a, b), hospital length of stay (c, d) and ATB treatment (e, f). Axis y represents true-positive rate (sensitivity), and axis x represents false-positive rate (1—specificity). The statistical analysis and significance of the data are shown in Table 3
We also analysed our results to set the cut-off values of each marker (Table 3). We observed that IL-6 on POD3 with a cut-off ≥ 180.5 was the strongest predictive factor in terms of POC CD > 3a, with the best combination of sensitivity (72.7%), specificity (99%), negative predictive value (NPV) (97.0%) and positive predictive value (PPV) (88.9%). Although the highest sensitivity (90%) was observed in patients with a CRP cut-off higher than 113.8 on POD3 in terms of CD > 3 and CD > 2 complications, it mostly had a negative predictive value (98.2%). The best statistically significant (p < 0.001) positive predictive value (88.9%) was seen in IL-6 ≥ 180.5 on POD3 in CD > 3a complications; therefore, it might be the best predictor. Interestingly, we showed that IL-6 POD1 predicts ICU and hospital length of stay and ATB treatment (AUC 0.733, 0.726, 0.750) comparable to CRP on POD3 (AUC 0.796, 0.740, 0.801); there were no statistically significant differences between ROC curves (p = 0.333, p = 0.678, and p = 0.813). See supplementary document no. 2.
Table 3.
Cut-off values, NPV, PPV, sensitivity and specificity of predictors
| Positive class for ROC analysis | Predictor cutoff (POD) | AUC (95% CI) | p-value | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|---|---|---|
| Clavien‒Dindo > 3a | IL-6 (1st) ≥ 113.0 | 0.768 (0.619; 0.918) | 0.002 | 75.0 | 78.6 | 96.4 | 29.0 |
| IL-6 (3rd) ≥ 180.5 | 0.859 (0.699; 1.000) | < 0.001 | 72.7 | 99.0 | 97.0 | 88.9 | |
| CRP (3rd) ≥ 113.8 | 0.844 (0.721; 0.968) | < 0.001 | 90.0 | 78.9 | 98.2 | 37.5 | |
| Clavien‒Dindo > 2 | IL-6 (1st) ≥ 113.0 | 0.713 (0.558; 0.867) | 0.010 | 64.3 | 78.2 | 94.0 | 29.0 |
| IL-6 (3rd) ≥ 34 | 0.808 (0.684; 0.933) | < 0.001 | 84.6 | 77.1 | 97.4 | 33.3 | |
| CRP (3rd) ≥ 113.8 | 0.844 (0.721; 0.968) | < 0.001 | 90.0 | 78.9 | 98.2 | 37.5 | |
| ICU > 5 days | IL-6 (1st) ≥ 89.3 | 0.733 (0.627; 0.838) | < 0.001 | 69.7 | 76.8 | 86.3 | 54.8 |
| IL-6 (3rd) ≥ 25.0 | 0.761 (0.660; 0.862) | < 0.001 | 78.1 | 74.0 | 89.1 | 55.6 | |
| CRP (3rd) ≥ 109.0 | 0.796 (0.682; 0.910) | < 0.001 | 70.4 | 88.9 | 85.7 | 76.0 | |
| Hospital stay > 10 days | IL-6 (1st) ≥ 89.3 | 0.726 (0.617; 0.836) | < 0.001 | 70.0 | 75.3 | 87.7 | 50.0 |
| IL-6 (3rd) ≥ 60.9 | 0.740 (0.619; 0.861) | < 0.001 | 51.7 | 96.3 | 84.6 | 83.3 | |
| CRP (3rd) ≥ 146.0 | 0.740 (0.605; 0.874) | < 0.001 | 56.5 | 91.4 | 84.1 | 72.2 | |
| ATB treatment | IL-6 (1st) ≥ 104.0 | 0.750 (0.633; 0.866) | < 0.001 | 70.8 | 79.1 | 91.1 | 47.2 |
| IL-6 (3rd) ≥ 34.2 | 0.782 (0.668; 0.897) | < 0.001 | 73.9 | 82.6 | 92.2 | 53.1 | |
| CRP (3rd) ≥ 113.8 | 0.801 (0.679; 0.924) | < 0.001 | 75.0 | 85.2 | 91.2 | 62.5 | |
| Inflammatory complication | IL-6 (1st) ≥ 136.5 | 0.729 (0.610; 0.848) | < 0.001 | 55.2 | 90.7 | 85.7 | 66.7 |
| IL-6 (3rd) ≥ 34.0 | 0.753 (0.640; 0.865) | < 0.001 | 67.9 | 82.7 | 88.2 | 57.6 | |
| CRP (3rd) ≥ 113.8 | 0.827 (0.714; 0.940) | < 0.001 | 77.3 | 88.1 | 91.2 | 70.8 |
POD postoperative day, AUC area under the curve, CI confidence interval, NPV negative predictive value, PPV positive predictive value, ICU intensive care unit, ATB antibiotics. Continuous variables are described by median (minimum–maximum). Cut-off values for IL-6 serum levels are measured in pg/ml and CRP in mg/l. Parentheses include minimum and maximum values
Discussion
The main purpose of ERAS in colorectal surgery is to reduce postoperative stress and to allow faster return of physiological functions and shorter hospital stay. To achieve these statements, postoperative complications must be detected early to prevent morbidity and mortality, and this remains a problem. In the literature, many studies focus on different predictive systemic and peritoneal drain biomarkers (inflammatory, microbiological, markers of ischemia) and frequently mention CRP, procalcitonin (PCT), neutrophil-to-lymphocyte ratio, white blood cell count and IL-6. Additionally, changes in serum albumin, nutritional parameters and sarcopenia have been studied as predictive preoperative biomarkers to detect high-risk patients before surgery [24, 25].
One of the most investigated biomarkers, which has also changed management in colorectal surgery, is CRP, displaying high sensitivity for infectious POC with a high NPV. According to a multicentric PREDICT study, a change in the CRP level exceeding 50 mg/l between any two PODs can accurately rule out AL (NPV 99%). Moreover, CRP monitoring in patients after TME can predict safe discharge on POD5 [26–28]. Nevertheless, a specific cut-off value to definitely rule out AL is not yet clear, and values range from 94 to 190 ml/l at different time points from POD3 to POD5. Therefore, some studies suggest monitoring the trajectory of biomarker changes, as it might be more accurate [29]. In addition, corticosteroids and statins may decrease CRP [30]. The drawback of CRP is that it predicts POC quite late (from POD3, improving later after operation) and usually has only a strong NPV. Our values of CRP on POD3 were consistent with those of known studies. In our subgroups, CRP allowed us to rule out infectious complications, the need for ATB treatment (AUC 0.904, 0.879) and serious POC CD > 3a, which included all cases of reoperation for AL (AUC 0.869). Cut-off values ranged from 109 to 146 mg/l between the observed categories of complications. It is worth to mention that Holmgren et al. described elevation of preoperative CRP in patients with AL after colonic resection but not rectal resection. They also found that patients with AL after rectal resections had significantly elevated preoperative serum levels of inflammation-related proteins CXCL6 (C–X–C motif chemokine ligand 6) and CCL11 (C–C motif chemokine ligand 11) [31].
Serum cytokine IL-6 has been investigated as a predictor of early detection of postoperative sepsis [15]. However, homogenous data on serum IL-6 and POC and early discharge after colorectal surgery seem to be limited. The promising advantage of IL-6 is its ability to detect POC early after surgery. Rettig et al. studied 137 patients after major abdominal surgery and found that POC is associated with high IL-6 serum levels on POD1. Only 67 patients (48.9%) underwent colorectal surgery [16]. We confirmed this conclusion in our study on POD1 as well as POD3 (p < 0.001). Serum IL-6 as a predictor of AL leak in colorectal surgery was investigated in several studies [32–35]. In these four studies, the main limitations are small samples (range 22–84 patients), different sampling times and different analytical methods. This could also be a problem in our study; therefore, we did not analyse AL alone. In detail, Reisinger et al. showed no correlation between IL-6 and POC but did not demonstrate any data on IL-6. Ellebæk et al. showed an increased median IL-6 level in patients with and without AL (a total of 26 patients, 4 had AL) on POD1 compared to the preoperative level but without any statistical analysis. Similar results were seen by Slotwińsky et al. in 22 patients with no statistical significance of IL-6 between the groups without and with postoperative infectious complications. Conversely, Alonso et al. studied the relationship between intra-abdominal infection and tumour recurrence and showed higher serum IL-6 in patients with AL or intra-abdominal abscess on POD2 and POD4 (p = 0.014, 0.009) and in patients with recurrence (p < 0.05). These significant outcomes are probably due to a better study design (30 patients with complications vs. 30 patients without). Our results support a correlation between IL-6 and not only infectious complications but also CD > 3a and CD > 2 on POD1 and POD3. Interestingly, we found that patients with the need of ATB treatment and longer ICU and hospital length of stay had significantly higher IL-6 serum levels (p = 0.001).
The following studies improved the statistical analysis and focused on IL-6 as a potential early predictor of complications. Boersema et al. in 2018 found that in 47 patients after colorectal surgery, the serum IL-6 ratio (preoperative/POD) cannot predict postoperative ileus but can predict infectious complications on POD1 and POD 3 with a larger AUC than CRP (0.825 and 0.801 vs. 0.732 and 0.731) [36]. We cannot properly compare these results with those in our study since a ratio was used. Interesting data presented by Zawadzki et al. focused on 32 patients with rectal tumours, and they found that IL-6 on POD3 can predict AL (p < 0.001, AUC 0.82, cut-off > 65.9 pg/ml, SN 100% and SP 76%, PPV 31, NPV 100), but preoperative IL-6 cannot (p = 0.286). Moreover, changes in IL-6 were not affected by the type of surgical approach (robotic or open) or the length or extent of surgery; however, only five patients had AL [37]. Consistent with this study, we also found that IL-6 on POD3 was very strong, particularly in the detection of POC CD > 3a (p < 0.001, AUC 0.85, cut-off > 180.5, SN 72% and SP 99, PPV 88, NPV 97). Surprising is the fact that on POD3, IL-6 has a high PPV (88%) of serious complications (CD > 3a) and is thus superior to CRP, which has dominantly NPV. In affected patients, this might provide a clinical implication in terms of more intensive care, control abdominal CT scan, or escalation/prolonged ATB treatment. However, these implications need to be further studied before introduction into clinical practice.
Two studies combined patients with colorectal cancer and benign disease (IBD, diverticulosis and others), and the results were inconclusive. Zielińska-Borkowska et al. are the only study that reported no predictive value of IL-6 for AL on POD1 in uni- and multivariate analyses (p > 0.05, AUC 0.61). This was a prospective study in a total of 157 patients; however, 36% of patients had a benign disease, and no information about anti-inflammatory drugs was given [38]. In the second study by Sammour et al., of a sample of 206 patients, 35% had benign disease, and IL-6 on POD1 was significant in detecting AL (p = 0.048, AUC 0.65); however, the use of anti-inflammatory drugs was not recorded [39]. A study that focused on IL-6 as a predictor of intra-abdominal septic complications (AL, abscess, fistula) in 118 patients with only Crohn’s disease found IL-6 as a significant predictor of POC on POD1, POD3 and POD5 (p < 0.001 for all, AUC 0.71, 0.86, 0.82), and 45% of patients had anti-inflammatory treatment [40]. There is an evident discrepancy between studies when detecting POC on POD1. It seems that IL-6 as a predictive factor for benign disease is affected by medication and might not be as accurate as for patients with colorectal tumours. Our study adds evidence that IL-6 on POD1 is capable of predicting POC in colorectal patients with high NPV.
Finally, some large studies also exist. In a Danish study, [40] authors analysed 401 patients divided by age (210 old and 191 young, threshold 70 years), where preoperative high levels of IL-6 but not CRP in the old were associated with major complications (CD > 3a). On POD1, a twofold increase in IL-6 predicted major complications only in patients < 70 years, and on POD3, a twofold increase in IL-6 from preoperative levels predicted major complications in both age groups (OR (odds ratio) = 1.75, 1.24–2.46, p = 0.002) [41]. A possible explanation for the age difference could be increased IL-6 levels in older and malnourished patients [8, 42–45]. In a multicentric prospective New Zealand study, Su’a et al. analysed 283 patients who underwent only colonic surgery (no rectum) and showed [46] a statistically significant difference between AL and no AL on POD 1 (AUC 0.68, p = 0.03, a cut-off value of 10.8 pg/mL gave an NPV of 99.1%, sensitivity 0.85, specificity 0.83) and POD 2 (AUC 0.69, p = 0.02). The explanation for low AUC and cut-off values compared to our study is probably the fact that these authors combined benign and malignant diagnoses as discussed before, and the ratio between those is unavailable. Nevertheless, the conclusions of these two studies support our results that measuring IL-6 postoperatively has a potential benefit in predicting POC.
There are some limitations of this study. We selected complications in six different subgroups as mentioned in methodology due to a small sample size, and therefore, we did not analyse anastomotic leakage alone. Also, there were some missing data (samples), which might have biased the results. Our study also lacks external validation.
Conclusion
In this study, we found that the serum level of interleukin-6 can predict severe (CD > 3a) POC early on POD1 with high NPV. On POD3, IL-6 is superior to CRP in terms of high positive predictive power of severe POC. To our knowledge, no study has investigated the advantage of IL-6 on POD1 as early predictor of the need for antibiotic treatment, ICU stay and hospital stay, which is comparable to the CRP serum level late on the third POD. It allows early prediction and could help decide which patients will not potentially benefit from prolonged ATB treatment, and it can guide ICU and hospital discharge. Surgeons should be aware of the need to initiate more intensive care when detecting high IL-6 values, as it could improve the severe course of early postoperative recovery.
Supplementary Information
Additional file 1: Supplementary document 1: Figure 1a ROC analysis of predictors of Dindo-Clavien > 3a. Figure 1b ROC analysis of predictors of Dindo-Clavien > 2. Figure 1c ROC analysis of predictors of ICU > 5 days. Figure 1d ROC analysis of predictors of hospital stay > 10 days. Figure 1e ROC analysis of predictors of ATB treatment. Figure 1f ROC analysis of predictors of inflammatory complication. Figure 2a Comparison of predictors values between patients according to Dindo-Clavien > 3a. Figure 2b Comparison of predictors values between patients according to Dindo-Clavien > 2. Figure 2c Comparison of predictors values between patients according to ICU > 5 days. Figure 2d Comparison of predictors values between patients according to hospital stay > 10 days. Figure 2e Comparison of predictors values between patients according to ATB treatment. Figure 2f Comparison of predictors values between patients according to inflammatory complication.
Additional file 2: Supplementary document 2: Table 1. Comparation of characteristics of patients according to presence of endpoints and their prediction power. Table 2. Comparison of binarized characteristics of patients (cut-off derived employing ROC analysis) according to presence of endpoints and their prediction power. Table 3 IL-6 levels depending on height of rectal tumour.
Additional file 3: Supplementary document 3: Fig. 1. Section of adenocarcinoma of the rectum from a male patient (T3 N2 M0, grade 3). Negative control shows the specificity of the reaction (A). Positivity for αSMA (B), IL-6 (C) and IL-6R (D) is also demonstrated. IL-6-positive leukocytes are present in the vessel (E). The tumour is infiltrated by IL-6-positive leukocytes (F) that are also on the surface of the tumour tissue (G). These cells also exhibited IL-6R (H). The bar is 300 µm.
Acknowledgements
The authors are grateful to Helena Stýblová for technical assistance and Marie Kasparek Kvicala, MSc, for language assistance.
Abbreviations
- POC
Postoperative complications
- IL-6
Interleukin 6
- POD
Postoperative day
- CRP
C-reactive protein
- CD
Clavien-Dindo
- ATB
Antibiotics
- ICU
Intensive care unit
- ROC
Receiver-operating characteristic
- AUC
Area under the curve
- ERAS
Enhanced recovery after surgery
- AL
Anastomotic leakage
- CARS
Compensatory anti-inflammatory response syndrome
- IBD
Inflammatory bowel disease
- MSI
Microsatellite instability
- PET-MRI
Positron emission tomography with magnetic resonance imaging
- CT
Computed tomography
- TME
Total mesorectal excision
- PME
Partial mesorectal excision
- CRT
Chemoradiotherapy
- ICG
Indocyanine green
- PIC
Postoperative inflammatory complications
- SSI
Surgical site infections
- AUROC
Area under the receiver operating characteristic curve
- NPV
Negative predictive value
- PPV
Positive predictive value
- PCT
Procalcitonin
- CXCL6
C–X–C motif chemokine ligand 6
- CCL11
C–C motif chemokine ligand 11
- SN
Sensitivity
- SP
Specificity
- OR
Odds ratio
Authors’ contributions
VP, conducted surgery and patients care and manuscript writing; LL, conducted histochemistry and imaging; KSm, manuscript preparation (cancer cell biology review) and data interpretation; MS, manuscript preparation, surgery and patients care; KSk, study design and patients care; MB, conducted biochemical analysis and IL-6 serology; JJ, conducted statistical analysis and graphical design; LK, conducted pathologic tissue analyses; and ZK, conducted surgical strategy and data review, clinical data interpretation and manuscript writing. All authors reviewed the final version of the manuscript.
Funding
This work was funded by Operational Programme Research, Development, and Education within the project National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102) — funded by the European Union — Next-Generation EU and by Charles University project Cooperatio ONCO and supported by the Ministry of Health, Czech Republic — conceptual development of research organization (FNBr, 65269705).
Availability of data and materials
All the source data are stored and can be accessed upon request by corresponding authors.
Declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethical Committee of the Faculty Hospital Brno (no. 10–270420/EK) in accordance with the ethical principles of the Declaration of Helsinki. It was reviewed by the Medical Ethical Committee of the Faculty Hospital Brno (no. 10–270420/EK), and the approved text was signed by every involved participant. The authors obtained consent from all participants for their participation in this research project, and the authors maintain a record of their consent.
Consent to publication
The authors publish no identifiable data. The authors obtained consent (approved as above) from all participants for publication of their anonymized data and results of this research project, and the authors maintain a record of their consent.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alves A. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140(3):278. doi: 10.1001/archsurg.140.3.278. [DOI] [PubMed] [Google Scholar]
- 2.Lacina L, Brábek J, Král V, Kodet O, Smetana K. Interleukin-6: a molecule with complex biological impact in cancer. Histol Histopathol. 2019;34(2):125–136. doi: 10.14670/HH-18-033. [DOI] [PubMed] [Google Scholar]
- 3.Španko M, Strnadová K, Pavlíček AJ, et al. IL-6 in the ecosystem of head and neck cancer: possible therapeutic perspectives. Int J Mol Sci. 2021;22(20):11027. doi: 10.3390/ijms222011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rašková M, Lacina L, Kejík Z, et al. The role of IL-6 in cancer cell invasiveness and metastasis—overview and therapeutic opportunities. Cells. 2022;11(22):3698. doi: 10.3390/cells11223698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vokurka M, Lacina L, Brábek J, Kolář M, Ng YZ, Smetana K. Cancer-associated fibroblasts influence the biological properties of malignant tumours via paracrine secretion and exosome production. Int J Mol Sci. 2022;23(2):964. doi: 10.3390/ijms23020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kučera J, Strnadová K, Dvořánková B, et al. Serum proteomic analysis of melanoma patients with immunohistochemical profiling of primary melanomas and cultured cells: pilot study. Oncol Rep. Published online September 17, 2019. 10.3892/or.2019.7319 [DOI] [PMC free article] [PubMed]
- 7.Łukaszewicz-Zając M, Mroczko B. Circulating biomarkers of colorectal cancer (CRC)—their utility in diagnosis and prognosis. J Clin Med. 2021;10(11):2391. doi: 10.3390/jcm10112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brábek J, Jakubek M, Vellieux F, et al. Interleukin-6: molecule in the intersection of cancer, ageing and COVID-19. Int J Mol Sci. 2020;21(21):7937. doi: 10.3390/ijms21217937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodla L, Xue X. The role of inflammatory mediators in colorectal cancer hepatic metastasis. Cells. 2022;11(15):2313. doi: 10.3390/cells11152313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi XY, Liu MX, Xu K, et al. Peritoneal cytokines as early biomarkers of colorectal anastomotic leakage following surgery for colorectal cancer: a meta-analysis. Front Oncol. 2022;11:791462. doi: 10.3389/fonc.2021.791462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon. 2011;9(1):38–43. doi: 10.1016/j.surge.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Castellheim A, Brekke OL, Espevik T, Harboe M, Mollnes TE. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol. 2009;69(6):479–491. doi: 10.1111/j.1365-3083.2009.02255.x. [DOI] [PubMed] [Google Scholar]
- 13.Meisel C, Meisel A. Suppressing immunosuppression after stroke. N Engl J Med. 2011;365(22):2134–2136. doi: 10.1056/NEJMcibr1112454. [DOI] [PubMed] [Google Scholar]
- 14.Bouras M, Roquilly A, Mahé PJ, et al. Cortisol total/CRP ratio for the prediction of hospital-acquired pneumonia and initiation of corticosteroid therapy in traumatic brain-injured patients. Crit Care. 2019;23(1):394. doi: 10.1186/s13054-019-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokart D, Capo C, Blache JL, et al. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br J Surg. 2002;89(11):1450–1456. doi: 10.1046/j.1365-2168.2002.02218.x. [DOI] [PubMed] [Google Scholar]
- 16.Rettig TCD, Verwijmeren L, Dijkstra IM, Boerma D, van de Garde EMW, Noordzij PG. Postoperative interleukin-6 level and early detection of complications after elective major abdominal surgery. Ann Surg. 2016;263(6):1207–1212. doi: 10.1097/SLA.0000000000001342. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. 10.1245/s10434-010-0985-4 [DOI] [PubMed]
- 18.Pox C, Aretz S, Bischoff S, et al. S3-Leitlinie Kolorektales Karzinom Version 1.0 - Juni 2013 AWMF-Registernummer: 021/007OL. Z Für Gastroenterol. 2013;51(08):753–854. doi: 10.1055/s-0033-1350264. [DOI] [PubMed] [Google Scholar]
- 19.Rectal Cancer NCCN Guidelines. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx#rectal
- 20.Svoboda M, Procházka V, Grolich T, Pavlík T, Mazalová M, Kala Z. Does pathological complete response after neoadjuvant therapy influence postoperative morbidity in rectal cancer after transanal total mesorectal excision? J Gastrointest Cancer. Published online May 7, 2022. 10.1007/s12029-022-00826-y [DOI] [PubMed]
- 21.Tejedor P, Francis N, Jayne D, et al. Consensus statements on complete mesocolic excision for right-sided colon cancer—technical steps and training implications. Surg Endosc. 2022;36(8):5595–5601. doi: 10.1007/s00464-021-08395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM. 2006;8(01):19–20. doi: 10.1017/S1481803500013336. [DOI] [PubMed] [Google Scholar]
- 24.Joliat GR, Schoor A, Schäfer M, Demartines N, Hübner M, Labgaa I. Postoperative decrease of albumin (ΔAlb) as early predictor of complications after gastrointestinal surgery: a systematic review. Perioper Med. 2022;11(1):7. doi: 10.1186/s13741-022-00238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Yang R, Xu J, Fang K, Abdelrahim M, Chang L. Sarcopenia as a predictor of postoperative risk of complications, mortality and length of stay following gastrointestinal oncological surgery. Ann R Coll Surg Engl. 2021;103(9):630–637. doi: 10.1308/rcsann.2021.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cazelles A, Giacca M, Monsinjon M, Hain E, Frontali A, Panis Y. Monitoring of C-reactive protein decreases length of stay after laparoscopic total mesorectal excision for cancer: a prospective case-matched study in 236 patients. Colorectal Dis. 2021;23(5):1158–1166. doi: 10.1111/codi.15573. [DOI] [PubMed] [Google Scholar]
- 27.Stephensen BD, Reid F, Shaikh S, Carroll R, Smith SR, Pockney P. C-reactive protein trajectory to PREDICT colorectal anastomotic leak: PREDICT study. Br J Surg. 2020;107(13):1832–1837. doi: 10.1002/bjs.11812. [DOI] [PubMed] [Google Scholar]
- 28.Warschkow R, Beutner U, Steffen T, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg. 2012;256(2):245–250. doi: 10.1097/SLA.0b013e31825b60f0. [DOI] [PubMed] [Google Scholar]
- 29.El Zaher HA, Ghareeb WM, Fouad AM, et al. Role of the triad of procalcitonin, C-reactive protein, and white blood cell count in the prediction of anastomotic leak following colorectal resections. World J Surg Oncol. 2022;20(1):33. doi: 10.1186/s12957-022-02506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su’a BU, Mikaere HL, Rahiri JL, Bissett IB, Hill AG. Systematic review of the role of biomarkers in diagnosing anastomotic leakage following colorectal surgery. Br J Surg. 2017;104(5):503–512. doi: 10.1002/bjs.10487. [DOI] [PubMed] [Google Scholar]
- 31.Holmgren K, Jonsson P, Lundin C, et al. Preoperative biomarkers related to inflammation may identify high-risk anastomoses in colorectal cancer surgery: explorative study. BJS Open. 2022;6(3):zrac072. doi: 10.1093/bjsopen/zrac072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellebæk MB, Baatrup G, Gjedsted J, Fristrup C, Qvist N. Cytokine response in peripheral blood indicates different pathophysiological mechanisms behind anastomotic leakage after low anterior resection: a pilot study. Tech Coloproctology. 2014;18(11):1067–1074. doi: 10.1007/s10151-014-1204-2. [DOI] [PubMed] [Google Scholar]
- 33.Alonso S, Pascual M, Salvans S, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol EJSO. 2015;41(2):208–214. doi: 10.1016/j.ejso.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 34.Slotwiński R, Olszewski WL, Chaber A, Slodkowski M, Zaleska M, Krasnodebski IW. The soluble tumor necrosis factor receptor I is an early predictor of local infective complications after colorectal surgery. J Clin Immunol. 2002;22(5):289–296. doi: 10.1023/a:1020022006043. [DOI] [PubMed] [Google Scholar]
- 35.Reisinger KW, Poeze M, Hulsewé KWE, et al. Accurate prediction of anastomotic leakage after colorectal surgery using plasma markers for intestinal damage and inflammation. J Am Coll Surg. 2014;219(4):744–751. doi: 10.1016/j.jamcollsurg.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Boersema GSA, Wu Z, Menon AG, Kleinrensink GJ, Jeekel J, Lange JF. Systemic inflammatory cytokines predict the infectious complications but not prolonged postoperative ileus after colorectal surgery. Mediators Inflamm. 2018;2018:1–9. doi: 10.1155/2018/7141342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zawadzki M, Krzystek-Korpacka M, Gamian A, Witkiewicz W. Serum cytokines in early prediction of anastomotic leakage following low anterior resection. Videosurgery Miniinvasive Tech. 2018;13(1):33–43. doi: 10.5114/wiitm.2018.72785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zielińska-Borkowska U, Dib N, Tarnowski W, Skirecki T. Monitoring of procalcitonin but not interleukin-6 is useful for the early prediction of anastomotic leakage after colorectal surgery. Clin Chem Lab Med CCLM. 2017;55(7). 10.1515/cclm-2016-0736 [DOI] [PubMed]
- 39.Sammour T, Singh PP, Zargar-Shoshtari K, Su’a B, Hill AG. Peritoneal cytokine levels can predict anastomotic leak on the first postoperative day. Dis Colon Rectum. 2016;59(6):551–556. doi: 10.1097/DCR.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 40.Xie T, Zhao C, Ding C, et al. Postoperative interleukin-6 predicts intra-abdominal septic complications at an early stage after elective intestinal operation for Crohn’s disease patients. Inflamm Bowel Dis. 2018;24(9):1992–2000. doi: 10.1093/ibd/izy090. [DOI] [PubMed] [Google Scholar]
- 41.Dolin T, Christensen I, Johansen A, et al. Pre- and perioperative inflammatory biomarkers in older patients resected for localized colorectal cancer: associations with complications and prognosis. Cancers. 2021;14(1):161. doi: 10.3390/cancers14010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagoe T, Tsuji T, Sawai T, et al. Increased serum levels of interleukin-6 in malnourished patients with colorectal cancer. Cancer Lett. 2003;202(1):109–115. doi: 10.1016/j.canlet.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Murdaca G, Paladin F, Casciaro M, Vicario CM, Gangemi S, Martino G. Neuro-inflammaging and psychopathological distress. Biomedicines. 2022;10(9):2133. doi: 10.3390/biomedicines10092133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonçalves RS dos SA, Maciel ÁCC, Rolland Y, Vellas B, De Souto Barreto P. Frailty biomarkers under the perspective of GeroScience: a narrative review. Ageing Res Rev. 2022;81:101737. doi: 10.1016/j.arr.2022.101737. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Qiu L, Liu Q, et al. Senescent fibroblasts generate a CAF phenotype through the Stat3 pathway. Genes. 2022;13(9):1579. doi: 10.3390/genes13091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su’a B, Milne T, Jaung R, et al. Detection of anastomotic leakage following elective colonic surgery: results of the prospective biomarkers and anastomotic leakage (BALL) study. J Surg Res. 2022;273:85–92. doi: 10.1016/j.jss.2021.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary document 1: Figure 1a ROC analysis of predictors of Dindo-Clavien > 3a. Figure 1b ROC analysis of predictors of Dindo-Clavien > 2. Figure 1c ROC analysis of predictors of ICU > 5 days. Figure 1d ROC analysis of predictors of hospital stay > 10 days. Figure 1e ROC analysis of predictors of ATB treatment. Figure 1f ROC analysis of predictors of inflammatory complication. Figure 2a Comparison of predictors values between patients according to Dindo-Clavien > 3a. Figure 2b Comparison of predictors values between patients according to Dindo-Clavien > 2. Figure 2c Comparison of predictors values between patients according to ICU > 5 days. Figure 2d Comparison of predictors values between patients according to hospital stay > 10 days. Figure 2e Comparison of predictors values between patients according to ATB treatment. Figure 2f Comparison of predictors values between patients according to inflammatory complication.
Additional file 2: Supplementary document 2: Table 1. Comparation of characteristics of patients according to presence of endpoints and their prediction power. Table 2. Comparison of binarized characteristics of patients (cut-off derived employing ROC analysis) according to presence of endpoints and their prediction power. Table 3 IL-6 levels depending on height of rectal tumour.
Additional file 3: Supplementary document 3: Fig. 1. Section of adenocarcinoma of the rectum from a male patient (T3 N2 M0, grade 3). Negative control shows the specificity of the reaction (A). Positivity for αSMA (B), IL-6 (C) and IL-6R (D) is also demonstrated. IL-6-positive leukocytes are present in the vessel (E). The tumour is infiltrated by IL-6-positive leukocytes (F) that are also on the surface of the tumour tissue (G). These cells also exhibited IL-6R (H). The bar is 300 µm.
Data Availability Statement
All the source data are stored and can be accessed upon request by corresponding authors.