Abstract
Menopause is associated with a decline in overall health in women. One health aspect impacted is glucose metabolism. As women experience menopause, their metabolism declines dramatically. The current study addressed the influence of ovarian somatic cells on the improvement of metabolic health through transplantations of young, germ cell-depleted ovaries. The purpose of this study is to expand the understanding of female reproductive health on metabolism. Control mice were grouped by age and treatment mice were age-matched. Treatment mice were placed into one of three groups: 1) mice received germ cell-depleted ovaries, 2) mice received germ cell-containing ovaries, and 3) mice received ovarian somatic cells via injection directly to their original ovary. All mice were subject to a glucose tolerance test, during which a bolus of dextrose was administered, and blood glucose levels were collected and recorded. Mice were euthanized between 680 and 700 days. Metabolic results showed an improvement of glucose metabolism in both germ cell-depleted and germ cell-containing groups compared to controls. No significance difference was noted between the germ cell-containing and germ cell-depleted groups. Somatic cell injection groups also showed improved glucose metabolism compared to controls. This experiment has shown that post-reproductive health is positively influenced by reproductive status. Additionally, somatic cells play an important role in the restoration of health to post-reproductive mice.
Keywords: Menopause, Glucose metabolism, Ovary, Aging
1. Introduction
Menopause is defined as the period in women when menstrual cycles cease and is characterized by reproductive decline. Menopause is additionally associated with a sharp decline in many aspects of health. Before menopause, females hold a significant health advantage over their male counterparts; however, post-menopausal females experience an increased risk of disease compared to males of the same age (Hodis and Mack, 2009). Menopause is associated with increased cardiovascular disease, increased risk of osteoarthritis, reduced muscular strength, and reduced cognitive ability (Mason et al., 2011). Similarly, women who undergo an ovariectomy or hysterectomy experience a decline in health mimicking that of a true menopause. In mice, glucose metabolism reflected what is seen in post-menopausal models (Tawfik et al., 2015).
Menopause is associated with a decreased metabolism and an increased risk for metabolic syndrome in a majority of women. Metabolic syndrome is characterized by increased weight gain and lipid deposition, insulin resistance, and low glucose metabolism. Existing treatments for menopause-related symptoms focus on hormone replacement therapy. Hormone replacement therapy, while effective at treating some symptoms of menopause, has not proven successful in the treatment of metabolic disease or in the treatment of other menopause-related health related changes (Lobo, 2008).
Ovarian failure is associated with the failure and depletion of ovarian germ cells. Germ cells are the reproductive cells of the body, and experience follicular cycles within the ovary. Germ cells are most commonly associated with the term ‘egg’ in females. Ovarian somatic cells additionally play a central role in health. Somatic cells are not reproductive cells and are characterized by all other cells within the ovary. Previous studies showed that germ cell-depletion significantly improves skeletal muscle endurance (Habermehl and Mason, 2019). This experiment aims to evaluate the extent to which germ cells and somatic cells influence the metabolic decline associated with menopause. In current experiments, we chemically depleted ovarian germ cells in pre-pubertal ovaries and evaluated glucose metabolism in young and post-reproductive mice with and without ovarian germ cell-depletion. To further evaluate somatic cell influence on post-reproductive health, we conducted somatic cell injections into the ovary and evaluated glucose metabolism.
2. Materials and methods
Female mice of the CBA/J strain were used in this experiment because they prematurely lose their ovarian follicles, leading them to become reproductively senescent at approximately 300 days of age (Thung et al., 1956; Jones and Krohn, 1961; Faddy et al., 1987). A reduction in the number of ovarian follicles in humans is associated with the onset of menopause, and thus was an ideal model in the study of age and menopause associate disease.
Female CBA/J mice aged 75 days, 200 days, 450 days, and 500 days were obtained from Jackson Laboratory (Bar Harbor, ME, USA). The mice were housed in individual, ventilated cages (Green Line IVC Sealsafe Plus, Tecniplast, West Chester, PA, USA). The laboratory environment included fresh, filtered air (15 changes/h), temperature (21 ± 2 °C, humidity of 50 ± 20%), a light-dark cycle (12:12 h), and a specific, pathogen-free colony. Corn cob bedding (7097 Corncob, Harlan Tecklad, Bartonville, IL, USA) was changed weekly. Cages also contained deionized water and added enrichments. Mice were supplied with a certified laboratory diet ad libitum (2018 Teclad Glodal 18% Protein Rodent Diet, Harlan Teklad, Bartonville, IL, USA). Mice were maintained an American Association for Accreditation of Laboratory Animal Care (AALAC)-approved facility in accordance with the National Institutes of Health animal-use guidelines. Animal care and use protocols were developed under National Research Council guidelines found in the Guide for the Care and Use of Laboratory Animals. This project was approved by the Utah State University Institutional Animal and Care and Use Committee (IACUC-2277).
Anesthetics were used for both donor and recipient mice that underwent surgery. Analgesia was provided to recipient mice for 48 h post operation, or longer if deemed necessary. Donor mice were euthanized via cervical dislocation. A thoracotomy was performed immediately after cervical dislocation, followed by rapid exsanguination via cardiocentesis. Mice with acute weight loss were treated with moistened food and subcutaneous fluids. Mice with acute urine straining or rectal/vaginal prolapse were manually cleaned and treated with Desitin®. Mice were monitored at least twice a day and body weight was recorded monthly, more frequently if concerns arose. Moribund, aged mice that exerted overt clinical signs (catatonia) were euthanized. Criteria for euthanasia specific for aged mice were determined in coordination with the attending veterinarian and included, but were not limited to, mice found in poor condition with or without crusting around the perineum and diarrhea, urine staining, persistent vaginal prolapse, chronic vulva/ rectal swelling, kyphosis, respiratory distress, anorexia, poor coat condition and lack of grooming, moribund mentation, hind-limb weakness/paresis, wounds not healing, limited mobility, neoplastic growth, and unusual weight loss (or gain). From peak weight to death, aged, female CBA/J mice have an average weight loss of approximately 12% per month (Mason et al., 2010). An increased rate of weight loss, but not total weight loss, was the most critical factor for determining a moribund state. Unexpected deaths were uncommon, but included neoplastic growths (most commonly mammary), decubitus ulcers (extremely old animals) and uncontrolled cataleptic seizures (normally 11–13 months of age). Mice that received germ cell-containing ovarian transplants lived on average 770 days. Mice that received germ cell-depleted ovarian transplants lived an average of 898 (Mason et al., 2011). In this study, control mice were euthanized at age 200, 300, 700, and 900 days. Treatment mice were euthanized at 700 days.
3. Experimental design
400-day-old mice were randomly selected as control, germ cell-containing transplant recipients, and germ cell-depleted transplant recipients.
3.1. Controls
Control groups were created using the following criteria:
100-day-old mice that were reproductively cycling (n = 19)
200-day-old mice that were both acyclic and cyclic during the study (n = 10)
400-day-old mice that were acyclic (n = 5)
600-day old mice that were acyclic and from a previous study (n = 3)
3.2. Ovarian transplant groups
The ovarian transplant recipient groups were created using the following criteria:
400-day-old mice that were acyclic at the time of surgery and received 60-day-old ovaries from donor mice that were cycling (germ cell-containing) (n = 9) (Fig. 1).
400-day-old mice that were acyclic at time of surgery and received 60-day-old germ cell-depleted (GD) ovaries from donor mice that were cycling (n = 11) (Fig. 1).
Fig. 1.
28-day-old mice with young ovaries (YO) were treated with 4-vinylcyclohexene diepoxide (VCD) or Oil for 15 day. At 60 days of age, germ cell-depleted (GD) ovaries and germ cell-containing (GC) ovaries were removed and transplanted into 400-day mice. Unaltered ovaries from 28-day-old mice (YO) were digested and injected into the ovary and ovarian bursa of 60-day-old ovarian somatic cell recipient mice (OSC). Mice metabolism was evaluated via glucose tolerance test starting at 460 days. Mice were collected when treatment groups were 700 ± 10 days.
3.3. Somatic cell injection groups
Ovarian somatic cells were transplanted into the ovary using the following criteria:
400-day-old mice that were acyclic at time of surgery and received somatic cell injections to both individual ovaries. Somatic cells were obtained from 60-day-old mice that were cycling (n = 5) (Fig. 1).
3.4. Age at manipulation
Normally, CBA/J female mice become sexually competent at 45–60 days of age. Manipulation was conducted at 28 days of age to avoid major up-regulation of the reproductive system at the onset of puberty and to eliminate influences the female gonad might have in addition to direct effects of gonadal hormones. Manipulation was conducted by injecting a placebo of sesame oil or treatment with 4-vinylcyclohexene diepoxide (VCD) to deplete germ cells from young ovaries (n = 20). Germ cell-depleted donor mice received injections intraperitoneal of 160 mg/kg VCD once daily for 15 days. A previous 15-day VCD dosing protocol was created to stop reproductive cycling and deplete ovarian primordial and primary follicles (Mason et al., 2009).
While rodents do not undergo menopause, they do have an estropause-like decrease in reproductive function. This decline in reproductive function begins at around 240 days of age and decreases gradually until reproductive incompetence at 330 days of age (Mason et al., 2018).
4. Surgical procedures
4.1. Ovarian transplants
Female mice of the CBA/J strain become reproductively active between 45 and 60 days old. At 60 days of age, cycling was confirmed in donor mice, and donor mice were anesthetized and had their ovaries removed. Cycles were confirmed using vaginal lavage. Donor mice were euthanized via cervical dislocation following removal of ovaries.
Recipient mice underwent ovarian transplant surgery and received donor ovaries as previously described (Mason et al., 2009). Briefly, mice were anesthetized, and a vertical incision was made in the lateral aspect of the abdomen. The ovarian fat pad was externalized, and a small opening was made in the ovarian bursa. The ovary was extracted and the donor ovary was placed within the empty ovarian bursa. The ovarian bursa was replaced within the body and the abdomen was sutured. The skin was stapled closed. This procedure was performed on both the left and right ovaries. All mice were allowed to recover for one month before metabolism was measured.
4.2. Injections
At 60 days of age, cycling was confirmed in donor mice, and donor mice were anesthetized and had their entire reproductive tract removed. Cycles were confirmed using vaginal lavage. Donor mice were euthanized via cervical dislocation following removal of ovaries.
Ovaries were isolated from the ovarian bursa from donor mice and placed in a glass convex dish. 50 μl of digestion buffer (1% collagenase V, 100 U DNase, 1% dispase with 290 μl PBS + ) was added, and ovaries were manually torn with 19 gauge needs for 5 min. Ovary solution was then placed into a 1.5 mL Eppendorf tube. lOOul buffer was used to wash the glass dish and added to Eppendorf tube. Another 345 μL digestion buffer was added to the ovary solution, total fluid within the tube at 500 μL. Ovary solution was incubated at 37 °C for 5 min with frequent pipetting, and 5 min with mild pipetting. 500 μL of serum-containing media was added to the solution and allowed to sit for 5 min. The solution was spun down at 500 g for 5 min. The supernatant was again removed, and pellet was resuspended with 1.0 mL PBS-. The ovary solution was filtered through a 40 μm filter into a 50 mL tube and spun down at 500 g for 5 min. The supernatant was again removed, and remaining pellet was resuspended in 500 μL of germ cell culture media containing a ZVF and AAV-GFP marker. Cells were plated in the first well of a four-well-plate and cultured overnight at 38°, 6% CO2. The next day, media was removed and deposited into the third well. The original well was rinsed twice with PBS/BSA, after which 100 μL of 0.25% trypsin/EDTA was added and allowed to sit for up to 10 min. After cells have rounded, 400 μL of germ ell media was added to the well and pipetted to a single cell suspension. 500 μL was then added and suspension was incubated for 6 h. Pure somatic cells were created in the first well in this way and were used in somatic cell injections.
Female mice were injected with ovarian somatic cells as previously done. Briefly, surgical procedures to externalize the ovary were performed using the same operating procedure as ovarian transplants. Instead of removing the ovary, somatic cells were transported via a microinjection needle into the ovary and ovarian bursa. The ovary was then returned to the abdomen wall. This procedure was done for both ovaries. The abdomen was sutured closed and the skin was closed with skin staples. Mice were allowed to recover for one month before metabolism was evaluated.
5. Exclusion criteria
Exclusion from analysis was based on gonadal input, defined as cyclic changes on vaginal cytology, presumably due to the cyclic influence of ovarian hormones. Absence of gonadal input was assumed to be indication of the lack of cyclic influence of ovarian hormones. Cycling was determined by vaginal lavage. Control mice that displayed cytological evidence of gonadal input 13 months of age or prior to surgery at 17 months of age were excluded from analysis. Ovarian transplant recipients that failed to display evidence of gonadal input postoperatively were also excluded from analysis.
6. Metabolic function-glucose test
An intraperitoneal (IP) glucose tolerance test (GTT) was performed in mice that were feed-deprived for 4–5 h previous. Fasting time was selected based on metabolic differences between mice and humans and due to aged female's intolerance to overnight fasting (Ayala et al., 2010). Blood glucose levels were measured using FreeStyle Freedom Lite Blood Glucose Monitoring System (Abbott Diabetes Care Inc. Almeda, CA, USA). Blood samples were obtained from a small nick at the tip of the tail, 2 h prior to testing and again immediately prior to glucose administration (t0), and at 15, 30, 60, 90, 120, and 180 min after injection. Glucose injection consisted of 20% d-glucose (2.8 g/kg lean body mass). Calibration of the FreeStyle Freedom Lite Blood Glucose Monitoring System was performed using control test solutions provided by the manufacturer.
Two drops of blood were collected, the first of which was discarded. The second drop was placed on the FreeStyle Freedom Lite test strip. Blood glucose reading was expressed as mg/dL. Glucose measurements at each point were then graphed and results expressed as Area Under the Curve (AUC).
7. Statistical analysis
Statistical analysis was performed with GraphPad Prism 7.04 (GraphPad Software, Inc., La Jolla, CA, USA). D'Agostino-Person Omnibus test was performed to determine normality. Data were analyzed with a two-factor ANOVA and a Tukey-Kramer post-hoc test was used to determine differences between the groups. Student's two-tailed t-test was performed on individual treatments assuming unequal distribution of variance. Test results were considered significant for p values p < 0.05.
8. Results
Blood glucose levels for each treatment and control mouse was mapped in mg/dL against time. Averages were also charted, as seen in Fig. 3. At t = 15 min, all mice groups experienced peak blood glucose levels. As seen in Fig. 3, Old Control mice on average peaked higher than other treatment and control groups after 15 min post-glucose injection. Additionally, the curve remains high as the glucose is metabolized, finally falling to similar levels to other groups after 90 min. The three other groups, Oil Control, Age Match Control, and GD Treatment groups follow similar curves. GD and GC both exhibited lower levels of blood glucose than the Age Match controls.
Fig. 3.
Blood glucose levels in mg/dL across time.
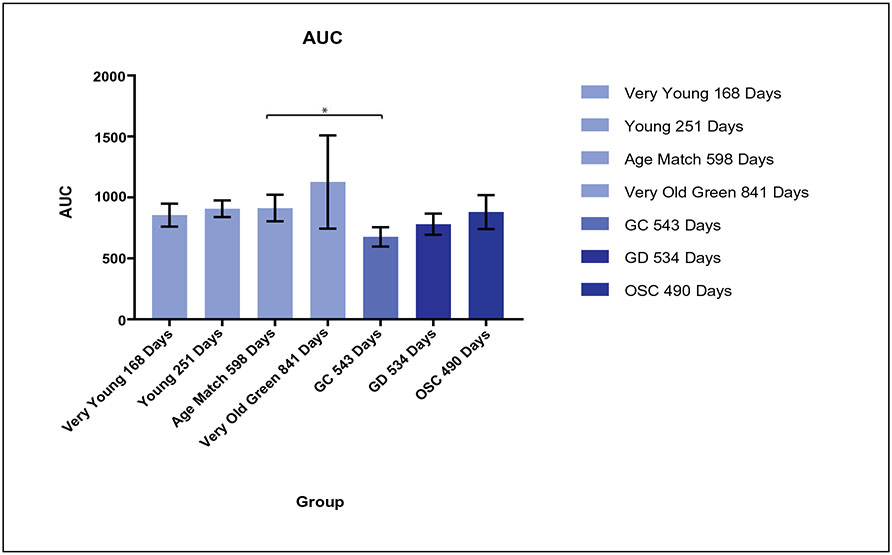
Young mice glucose metabolism exhibited a low area under the curve (AUC), as can be seen in Fig. 2. As age of control mice increased, the area under the curve also increased. Very old mice (age 841 days) exhibited the highest area under the curve. Germ Cell-Containing (GC) Treatment group exhibited significantly lower AUC than the Age Match Control group (p < 0.012). The AUC of the GD and GC was only slightly elevated over Young Control mice. GD mice, despite having an AUC below that of the Age Match controls, were not significantly different (p = 0.3). There was no significant difference between the GD and GC treatment groups (p = 0.06). While comparable levels, no significant conclusions can be drawn between these two groups.
Fig. 2.
Area Under the Curve (AUC) for young, old, age match, and very old control groups, and for germ cell containing (GC), germ cell-depleted (GD), and ovarian somatic cell injection (OSC) groups. Asterisk denotes a statistically significant relationship.
Ovarian Somatic Cell Injection (OSC) Groups also exhibited lower blood glucose level peaks compared to Old Controls, Age Match Controls, and Young Controls. OSC did not have an AUC significantly lower than Age Match Controls (p = 0.657). Additionally, OSC did not have a significant AUC compared to GD Treatment (p = 0.343), nor did OSC have a significant AUC compared to GC (p = 0.087).
9. Discussion
As females age, glucose intolerance increases. This intolerance can be characterized by a dramatic increase in blood glucose levels above fasting blood glucose levels after food consumption, as well as by an increased recovery time after food consumption for blood glucose levels to return to resting levels. A decrease in metabolic efficacy leads to other health problems, including metabolic syndrome and diabetes. These conditions can cause a decline in quality of life as well as overall lifespan. Loss of metabolic function has been previously associated with the loss of ovarian function in post-menopausal mice (Romero-Aleshire et al., 2009).
Our control groups follow an expected trend following that of a metabolic curve in post-reproductive women. As the age of mice increased, so also did circulating blood glucose level and subsequent Area Under the Curve (AUC). Old mice exhibited a less efficient metabolic system, as can be seen both in Figs. 2 and 3. In both these Figures, the Area Under the Curve for old mice (age 841 days) is elevated beyond that of Age-Match Control (age 598 days) and Young Mice Control (age 168 days) groups. Elevated circulating blood glucose levels is a risk factor for disease and often leads to insulin resistance, leading to metabolic syndrome, and is seen in the old mice comparable to a naturally occurring menopause in women.
GD and GC groups both had reduced circulating blood glucose levels compared to their age match counterparts, suggesting that germ cells and somatic cells play a role in overall post-menopausal health. While conclusions are not statistically supported for GD vs. Age Match controls, the general trend may lead to an understanding about somatic cells in post-menopausal health. The Somatic Cell Injection (OSC) group similarly had reduced levels. These results suggest there is a potential treatment option for menopause-related health decline directed at somatic cell therapy.
Currently, control of menopause-related symptoms has been addressed using Hormone Replacement Therapy (HRT). Few studies have been conducted on the effect of HRT on metabolism of post-menopausal women, but existing studies show mixed results and no proven treatment for metabolic syndrome or resistance (Lovre et al., 2017). HRT typically targets the hormone estrogen, which is decreased during menopause. Germ cells produce aromatase, which converts androgens to estrogens. Our research suggests that while germ cells may contribute to menopause hormone changes, these changes may not be exclusive in affecting overall metabolic health. Because both germ cell-depleted and somatic cell injection groups experienced improved metabolic health over old and age-match control groups, we propose that somatic cells also play a critical role in metabolic health. As a result, HRT may be less effective in correcting the decline in metabolic health due to the fact that somatic cells largely lack production of the hormones augmented by this therapy. We propose that ovarian somatic cell-based treatment may be more effective at treating metabolic decline than HRT. While this study lacked the direct evaluation of hormone, further studies will be conducted to test this idea.
Overall, young ovarian transplantation has a positive influence on glucose metabolism. GC groups confirm reduced blood glucose levels in post-reproductive mice. No statistical supported conclusions can be concluded for OSC and GC groups. In addition to this finding, somatic cell injections also had a positive influence on glucose metabolism. All treatment groups (OSC, GD, GC) showed reduced blood glucose levels.
Glucose metabolism is only one part of the complex metabolic system influenced by menopause. Continued studies in other aspects of metabolic health of post-reproductive mice have the potential to increase understanding of the roles somatic and germ cells. Future studies in this lab involve evaluating circulating triglyceride levels of GC, GD, and OSC mice to understand if germ cell-depletion influence triglyceride levels.
10. Conclusions
While women are living longer, the age of onset of menopause has stayed relatively constant. The relationship between somatic cells and germ cells in overall health is complex and still not well understood. Women are living longer with menopause and with the health decline associated with menopause. A health decline is associated with menopause that includes the decline in glucose metabolism. The decline in the efficacy of glucose metabolism may be improved with young ovaries that have been germ cell-depleted.
Additionally, somatic cell injections proved to be successful in restoring glucose metabolism. This understanding has the potential to develop a somatic cell treatment for post-menopausal metabolism conditions. In developing existing understanding of somatic and germ cell communication, the hope is to develop a treatment to restore the health of post-menopausal women.
Acknowledgements
The authors thank Dr. Aaron Olsen, Mrs. Lisa DeSoi, and Kate Parkinson for help with the mice. Additionally, the authors thank Utah State University, School of veterinary Medicine, Department of Animal, Dairy, and Veterinary Sciences.
Funding
Research reported in this publication was supported by the Utah Agricultural Experiment Station, Utah State University, and by the School of Veterinary Medicine, Department of Animal, Dairy and Veterinary Sciences, Utah State University.
References
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. , 2010. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech 3 (9–10), 525–534. 10.1242/dmm.006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy MJ, Telfer E, Gosden RG, 1987. The kinetics of pre-antral follicle development in ovaries of CBA/Ca mice during the first 14 weeks of life. Cell Tissue Kinet. 20 (6) (551–540). [DOI] [PubMed] [Google Scholar]
- Habermehl TL, Mason JB, 2019. Decreased sarcopenia in aged females with young ovary transplants was preserved in mice that received germ cell-depleted young ovaries. J. Clin. Med 8 (1), 40. 10.3390/jcm8010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis HN, Mack J, 2009. Coronary heart disease and hormone replacement therapy after menopause. J. Clim 12, 71–75. 10.1080/13697130903095178. [DOI] [PubMed] [Google Scholar]
- Jones EC, Krohn PL, 1961. The relationships between age, numbers of oocytes and fertility in virgin and multiparous mice. J. Endocrinol 21, 436–495. [DOI] [PubMed] [Google Scholar]
- Lobo RA, 2008. Metabolic syndrome after menopause and the role of hormones. Maturitis 60 (1), 10–18. 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Lovre D, Lindsey SH, Mauvais-Jarvis F, 2017. Effect of menopausal hormone therapy on components of metabolic syndrome. Ther. Adv. Cardiovasc. Dis 11 (1), 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR, 2009. Transplantation of young ovaries to old mice increased lifespan in transplant recipients. J. Gerontol. A 64A, 1207–1211. 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR, 2010. Ovarian status influenced the rate of body-weight change but not the total amount of body-weight gained or lost in female CBA/J mice. Exp. Gerontol 45 (6), 435–441. 10.1016/j.exger.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR, 2011. Transplantation of young ovaries restored cardioprotective influence in post-reproductive-aged mice: ovarian transplantation and cardiomyopathy. Aging Cell 10, 448–456. 10.1111/j.1474-9726.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Parkinson KC, Habermehl TL, 2018. Orthotopic ovarian transplantation procedures to investigate the life- and health-span influence of ovarian senescence in female mice. J. Vis. Exp (132), e54438. 10.3791/56638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Aleshire MJ, Diamon-Stanic MK, Hasty AH, Hoyer PB, Brooks HL, 2009. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am. J. Physiol 297 (3). 10.1152/ajpregu.90762.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik SH, Mahmoud BF, Saad MI, Shehata M, Kamel MA, Helmy MH, 2015. Similar and additive effect of ovariectomy and diabetes on insulin resistance and lipid metabolism. Biochem. Res. Int 2015. 10.1155/2015/567945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thung PJ, Boot LM, Muhlbock O, 1956. Senile changes in the oestrous cycle and in ovarian structure in some inbread strains of mice. Acta Endocrinol. 23, 8–32. [DOI] [PubMed] [Google Scholar]