Abstract
Nitrogen dioxide (NO2) is a regulated pollutant that is associated with numerous health impacts. Recent advances in epidemiology indicate high confidence linking NO2 exposure with increased mortality, an association that recent studies suggest persists even at concentrations below regulatory thresholds. While large disparities in NO2 exposure among population subgroups have been reported, U.S. NO2-attributable mortality rates and their disparities remain unquantified. Here we provide the first estimate of NO2-attributable all-cause mortality across the contiguous U.S. (CONUS) at the census tract-level. We leverage fine-scale, satellite-informed, land use regression model NO2 concentrations and census tract-level baseline mortality data to characterize the associated disparities among different racial/ethnic subgroups. Across CONUS, we estimate that the NO2-attributable all-cause mortality is ∼170,850 (95% confidence interval: 43,970, 251,330) premature deaths yr–1 with large variability across census tracts and within individual cities. Additionally, we find that higher NO2 concentrations and underlying susceptibilities for predominately Black communities lead to NO2-attributable mortality rates that are ∼47% higher compared to CONUS-wide average rates. Our results highlight the substantial U.S. NO2 mortality burden, particularly in marginalized communities, and motivate adoption of more stringent standards to protect public health.
Keywords: Air pollution, NO2 mortality, environmental justice, inequity, health impacts
Introduction
Nitrogen dioxide (NO2) is a health-harming gaseous pollutant that can lead to premature death.1 In the U.S., vehicle emissions are the largest single contributor to ambient NO2 concentrations.2 Indeed, NO2 concentrations are often used as a surrogate for the complex vehicle tailpipe emission mixture (e.g., HEI, 20221). NO2 is also a precursor of other health-damaging pollutants such as ozone (O3) and contributes to fine particulate matter (PM2.5).3 Through successful air pollution regulations, NO2 concentrations have declined,4,5 but adverse health outcomes associated with NO2 exposure persist for all air pollution levels, even those below regulatory limits6−8 and typically impact minoritized populations the most.9,10
Assessing mortality risks attributable to NO2 is an active and rapidly evolving field of research. Findings from the 2016 Integrated Science Assessment,2 the scientific foundation for the current U.S. EPA National Ambient Air Quality Standards (NAAQS), found suggestive evidence linking long-term NO2 exposure to all-cause mortality. New epidemiological evidence11 confirms these impacts with moderate confidence which consequently led to the updated and more stringent World Health Organization NO2 Air Quality Guidelines (AQGs).12 Subsequently, higher confidence in these associations have been reported by the Health Effects Institute.1 However, the causality of these impacts is still uncertain. Given that NO2 and other traffic-related air pollutants are often highly correlated in both space and time, it remains unclear whether NO2 is a causal agent or simply an indicator of the complex traffic-related air pollution mixture.1 Given its short atmospheric lifetime (∼hours), NO2 can exhibit tight spatial gradients with high concentrations close to emission sources such as heavily trafficked roads but gradually declining concentrations that fall to background levels at distances of ∼700 m.13 With more than 45 million people living within 300 feet of major roadways, of which a vast majority are people of color (POC),14 NO2 exposure does not impact all population subgroups equally. Indeed, despite nation-wide decreases in NO2 concentrations in the past decade,4 disparities in NO2 exposure persist, with racial/ethnic population subgroups still carrying a disproportionate burden.9,15
Several studies have estimated the U.S. mortality burden associated with PM2.5 and O3; however, the U.S.-wide NO2-attributable mortality burden has not yet been assessed. Given recent epidemiological findings of increased confidence in the links between NO2 and mortality risks, there is a need to understand these impacts on a national scale. In a global study, Song et al.16 recently estimated the U.S. NO2-attributable mortality burden but only for highly urbanized sites. Previous works have shown large disparities in NO2 exposure,4,9,10,15 but none have looked at the associated disparities in NO2 mortality burdens. Disparities in NO2-attributable pediatric asthma burdens have been quantified; however, such studies are limited by the resolution of available baseline asthma rates which are typically only available at coarser-than-tract resolutions.4 In this work, we fill these research gaps by estimating contiguous U.S. (CONUS)-wide NO2-attributable health impacts at the census tract-level, enabling us to determine neighborhood-scale equity implications. To provide this health-critical estimate, we leverage high-resolution modeled NO2 concentrations (∼1 km), the latest evidence linking long-term NO2 exposure to mortality and tract-level mortality rates.
Methods and Materials
NO2 Concentrations
Surface NO2 concentrations at ∼1 km horizontal resolution are derived from global data sets that incorporate a land use regression model (LURM) and satellite data.5 The underlying LURM represents mean 2010–2012 NO2 concentrations (Larkin et al.17), which were scaled to subsequent years using NO2 column densities from satellite data sets.5 The annual normalized mean bias for this data set, against in situ observations in 2015, was 0.14 ppbv with a correlation coefficient of 0.77 (additional comparisons in Kerr et al.4). We use concentration data from 2015, averaged to underlying tracts and consistent with available all-cause mortality baseline mortality rates. Tract boundaries are obtained from the U.S. Census Bureau and correspond to the 2010 decadal census.18 This highly spatially resolved NO2 data set is appropriate given the heterogeneity of NO2, particularly in urban areas, i.e., areas that are often burdened by high NO2 concentrations, and allows us to determine the NO2-related mortality burden and associated disparities at equity-relevant neighborhood scales which would otherwise be underestimated at coarser resolutions.19
Population and Demographic Data
We use census tract age-stratified population data and demographic information from the American Community Survey (ACS).20 Specifically, we obtain 5-year estimates from 2015 to 2019 which incorporate a larger sample size, thus reducing the margin of error compared to data sets covering shorter time periods. For each tract, the population data represent each 5-yr age group ranging from 30 to 85+ years. Using the racial/ethnic characterizations from the ACS demographic data, population subgroups include non-Hispanic: White, Black, American Indian or Alaska Native, Asian, Native Hawaiian/Other Pacific Islander, people identifying with another race or with two or more races, and the Hispanic/Latino subgroup. We conduct our analysis at the census tract-level for CONUS but also present estimates for a selection of the top 15 most populous Metropolitan Statistical Areas (MSAs) using the U.S. TIGER/Line spatial boundaries from the U.S. Census Bureau.21
Health and Equity Impacts
To estimate the NO2-attributable mortality for each census tract within the U.S. we use an epidemiologically derived log–linear concentration–response function consistent with previous literature and described in eq 1(5)
| 1 |
where MortCT represents the estimated attributable mortality at the census tract level expressed as a function of the tract baseline all-cause mortality rate (BMRCT) and the population (POPCT) for each 5-year age group together with the bracketed term representing the Attributable Fraction (AF); the fraction of the underlying all-cause mortality attributable to NO2 with β representing the concentration–response coefficient and xCT the NO2 concentrations.
Census tract baseline mortality incidence rates for each 5-year age group were obtained from Industrial Economic (IEc 2010–2015) and derived from USALEEP abridged life tables with modifications for broader use in national health benefits analyses.22 We derived β from a relative risk (RR) of 1.04 (95% confidence interval (CI) 1.01–1.06)1 per 10 μg m–3 from the recent Health Effect Institute (HEI) systematic review and meta-analysis1 converted to ppb equivalent (1 ppb NO2 = 1.88 μg m–3) in line with previous studies.23 Evidence for NO2-related health impact thresholds is limited; therefore, here we use the full NO2 concentration range. Presented health impacts (Figure 1) hold for ages 30+ with uncertainties reflecting the RR 95% CI.
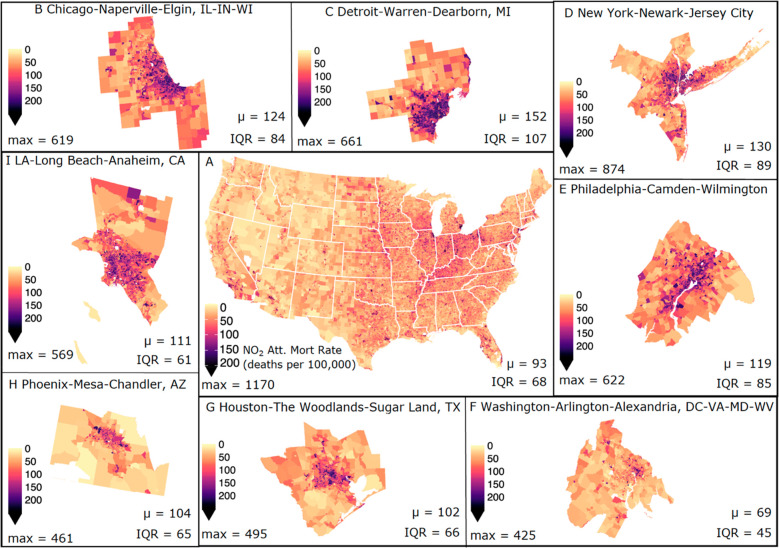
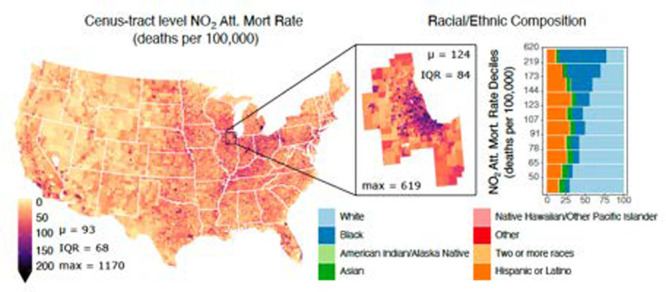
Figure 1.
NO2-attributable mortality rates. Spatial distribution of the estimated NO2-attributable mortality rates expressed in deaths per 100,000 per year across (A) the Contiguous U.S. (CONUS), and a selection of the top 15 most populous MSAs: (B) Chicago-Naperville-Elgin, IL-IN-WI, (C) Detroit-Warren-Dearborn, MI, (D) New York-Newark-Jersey City, NY-NJ-PA, (E) Philadelphia-Camden-Wilmington, PA-NJ-DE-MD, (F) Washington-Arlington-Alexandria, DC-VA-MD-WV, (G) Houston-The Woodlands-Sugar Land, TX, (H) Phoenix-Mesa-Chandler, AZ, and (I) Los Angeles-Long Beach-Anaheim, CA, with MSA-averaged NO2-attributable mortality rates (μ; deaths per 100,000 per year), IQRs, and maximum NO2-attributable mortality rates (max; deaths per 100,000 per year), annotated at the bottom of each panel.
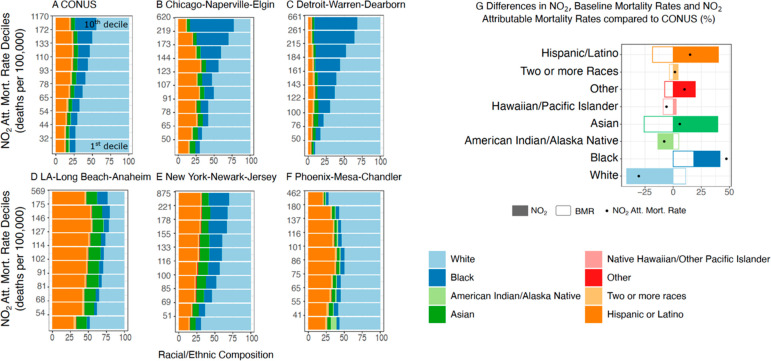
To assess the tract-level NO2-attributable mortality disparities across the U.S., we analyze the racial/ethnic composition within each NO2-attributable mortality decile (Figure 2A–F) and compare BMRs, NO2 concentrations, and NO2-attributable mortality rates for the 10th decile (>90th percentile) of each racial/ethnic population subgroup to CONUS-level average rates (Figure 2G).
Figure 2.
Racial and Ethnic Disparities. The racial and ethnic composition (in %) of the population for deciles of NO2-attributable mortality rates for (A) the contiguous U.S. and (B–F) across selected MSAs (in deaths per 100,000 per year). (G) Comparison of NO2 concentrations, Baseline Mortality Rates (BMRs), and estimated NO2-attributable mortality rates for the upper 10th percentile of each population subgroup relative to CONUS-wide averages (in %). All race groups, except Hispanic or Latino, include only those identifying as non-Hispanic.
Results and Discussion
Health Impacts
Across CONUS we estimate that NO2 concentrations are linked to ∼170,850 (CI: 43,970, 251,330) premature deaths yr–1 or ∼90 deaths per 100,000 people yr–1 (henceforth “deaths”; Figure 1A). Reflective of large spatial heterogeneity in NO2 concentrations across CONUS, we note higher NO2-attributable mortality rates for the Midwest and Northeast with a maximum burden of 1170 deaths found within Monroe County, NY (Rochester, NY MSA). Averaging NO2-attributable mortality rates across each of the 380 MSAs within CONUS, we find the highest rate of 152 deaths in the Detroit-Warren-Dearborn MSA (Figure 1C) and lowest (28 deaths) in the Hinesville, GA MSA. For the Detroit MSA, averaged NO2-attributable mortality rates are 1.6 times the CONUS average, largely driven by NO2 concentrations 1.4 times the CONUS levels. A similar pattern is found in the Chicago MSA where rates are 1.3 times the CONUS mean due to elevated NO2. The Youngstown-Warren-Boardman, PA MSA also has higher than average mortality rates (1.6 times); however, it has NO2 concentrations similar to the CONUS average, but with an average BMR 1.4 times CONUS levels. To isolate the singular impact of NO2 exposure on estimated mortality burdens, we estimate the AF (eq 1) and find that across CONUS ∼7% of the underlying baseline mortality is attributable to NO2, with a maximum of ∼21% in New York City. We note that NO2-attributable mortality rates within urban cores are even higher than MSA averaged rates, as NO2 concentrations increase within heavily trafficked urban areas. For example, the NO2 mortality rate for the City of Chicago is 1.7 times the CONUS MSA mean level as NO2 concentrations within the city are almost double.
Within MSAs, we note large tract-level variability in the NO2-related mortality rates. For example, rates for tracts within the Detroit and New York-Newark-Jersey City MSAs reach a maximum of ∼660 and ∼870 deaths with an interquartile range (IQR) of 107 and 89 deaths, respectively. The large variability in estimated NO2-related mortality rates among tracts within an MSA reflects the wide range in both NO2 concentrations (1.8–22.8 ppb) and BMRs (180–5560 deaths per 100,000), highlighting the importance of spatially resolved air pollution and health data and the potential for estimates conducted at coarser resolutions to underestimate the true health impact.
Disparities in NO2-Attributable Mortality
Across CONUS, NO2-related mortality burdens disproportionately impact communities of color. Specifically, tracts with the largest estimated NO2-attributable mortality rates (10th decile) are 29% Black, 5% Asian, 18% Hispanic or Latino, and 45% White, while the racial/ethnic makeup of the total population is 12% Black, 5% Asian, 18% Hispanic or Latino, and 61% White (Figure 2A).
For individual MSAs, the burden on communities of color is amplified. For example, in the Chicago and Detroit MSAs ∼60% of the population in tracts with the highest NO2-related mortality rates is Black (Figure 2B,C), a subgroup that comprises less than ∼21% of the total population for these MSAs. Similarly, in the New York MSA, the Black and Hispanic/Latino subgroups represent 16% and 24% of the total MSA population; however, each represents ∼30% of the population where high NO2-attributable mortality rates occur (Figure 2E). Reflecting a higher portion of Hispanic/Latino communities in the Los Angeles-Long Beach-Anaheim, MSA (45%), this subgroup constitutes the majority of NO2-attributable mortality deciles (>41%), except the first decile, which is 48% White. In the Phoenix-Mesa-Chandler MSA, the White population comprises the majority of all NO2-attributable mortality deciles (>50%, Figure 2F), reflecting a predominately White MSA (77%).
Estimated NO2 health impacts do not depend solely on pollutant concentrations but also on underlying susceptibilities of exposed populations.24 In Figure 2G we assess the two driving factors that determine NO2-attributable mortality rates for the top 10% of each population subgroup relative to CONUS-wide averages: (1) NO2 concentrations and (2) BMRs. NO2-attributable mortality rates in predominately White or American Indian/Alaska Native tracts are lower than CONUS average rates (−31% and −8%; Figure 2G), largely driven by lower NO2 concentrations. Native Hawaiian/Other Pacific Islanders also experience lower NO2 mortality burdens compared to CONUS average rates (−6%); however, lower rates are driven by lower BMRs. Conversely, the NO2 mortality burden is 6% and 15% higher than average in communities that are predominately Asian or Hispanic/Latino stemming from higher NO2 exposure and lower BMRs. Tracts within which populations are predominately Black have both higher NO2 exposures as well as higher underlying susceptibilities, leading to disproportionately large NO2-attributable mortality rates (+47%) compared to other subgroups.
Discussion
In this work, we present a tract-level CONUS-wide estimate of NO2-attributable mortality and identify large variability in NO2 mortality burdens across tracts. Additionally, we show disparities in NO2-attributable mortality rates across CONUS and highlight the disproportionate burden on communities of color, driven by higher-than-average NO2 exposure and higher underlying susceptibilities.
Our CONUS-wide estimate of approximately 171,000 annual premature deaths from NO2 exposure for ages 30+ is broadly consistent with a recent estimate by Song et al.16 who estimate premature NO2 mortality in 13,169 urban areas globally. For U.S. urban centers, Song et al. estimate ∼38,000 premature deaths per year. However, our estimate is CONUS-wide and uses a different RR with no concentration threshold together with tract-level all-cause mortality rates, as opposed to national-level;25 as such, a direct comparison is not possible. Evidence suggesting an increase in mortality risks associated with long-term exposure to NO2, even at low concentration levels, is continuously emerging;6−8 thus, here we opt to quantify the NO2-related premature mortality impacts at all exposure levels. We note that the NO2 exposures used to determine the RR used here1 are generally well-below the EPA’s annual NAAQS of 53 ppb, suggesting large public health risks persist, even at levels below regulatory limits. While all CONUS tracts exhibit NO2 concentrations below the NAAQS, ∼77% exceed the more stringent World Health Organization (WHO) AQG of 10 μg m–3. We estimate that if NO2 concentrations are reduced to the WHO AQG in all tracts exceeding it, ∼42% of NO2-attributable CONUS deaths would be prevented (∼71,980 avoided deaths) but disparities within individual MSAs would persist (Figure S1).
The pollution-attributable morality burden for the U.S. is more commonly estimated for PM2.5,4,26−29 as links between long-term exposure to PM2.5 and adverse health outcomes are long-established. Estimates for U.S.-wide PM2.5-related mortality burdens range from ∼50,000 to ∼131,000 premature deaths4,26−29 depending on the year and methodology of the study. Our NO2 mortality estimate is higher compared to existing PM2.5-related literature; however, a direct comparison is nontrivial. Discrepancies in estimates can be linked to use of coarser health data which can underestimate health impacts by around 15%,30 choice of RR, and the use of thresholds below which no health impacts are incurred. Transportation is one of the largest contributors to NO2, which leads to peak concentrations along highways and major road networks with levels exceeding the WHO AQGs within cities and urban areas.5 A large portion of people living close to these hotspots are POC, who also generally experience higher-than-average susceptibilities. We therefore hypothesize that steeper NO2 concentration gradients within highly susceptible urban populations is also a likely driver of our higher NO2 mortality estimate.4 Additionally, the relationship between NO2 and all-cause mortality is unadjusted for confounding pollutants such as PM2.5, and therefore our estimated NO2 mortality burden is not independent of PM2.5 influences. LURM-derived NO2 concentrations at ∼1 km scales also have uncertainties; e.g., NO2 estimates are based on empirical relationships rather than physics-backed models and rely on roadway information with no data on transportation sources (e.g., vehicle types or traffic counts17) which can influence NO2 spatial variability.30
Our finding highlighting the unjust NO2 mortality burden on POC complements previous exposure-focused studies suggesting higher NO2 and PM2.5 concentrations for marginalized communities.9,15,31−33 Studies looking at the associated health disparities are scarce, especially for NO2. A recent U.S. study found that in the 2010s, disparities in NO2-attributable pediatric asthma increased despite decreases in overall pollutant concentrations.4 The disparities in NO2-related mortality presented in this work are estimated using the same RR for all population subgroups and do not account for varying RR values across different races. For PM2.5, Spiller et al.29 show that not using race-specific RR could underestimate PM2.5 health impacts especially among older Black Americans and therefore lead to lower estimated disparities in health outcomes. For NO2 mortality, race-specific RRs have been quantified;34 however, these were restricted to ages 65+. Despite the lack of 30+ race-specific RRs, our conclusions using tract-level baseline mortality data are similar to those of Spiller et al.29 for PM2.5; i.e., we find higher attributable mortality rates for minoritized populations, especially Black populations. Nonetheless, it is unclear how the NO2 race-specific RRs for ages 30+ would influence our results.
While several studies have confirmed the unjust burden of NO2 exposure on marginalized communities,4,9,10,15 none have quantified the CONUS all-cause NO2-attributable mortality burden and associated disparities. Our use of census tract pollutant and health data enables us to capture wider and more representative variability in underlying population subgroup susceptibilities and associated disparities in NO2 mortality burdens, a result which would otherwise be underrepresented if using county-, state-, or national-level data.4 Our results indicate that policies aimed at reducing NOx emissions such as those pertaining to the transportation sector35,36 could reduce long-standing environmental injustices and motivate more stringent air quality standards.
Acknowledgments
We thank William Raich, Henry Roman, and Melanie Jackson from Industrial Economic and Neal Fann, Elizabeth Chan and Ali Kamal from the U.S. EPA for deriving and providing the census tract-level all-cause mortality rates used in this study. The NO2 concentrations used in this study can be found here:https://disc.gsfc.nasa.gov/datasets/SFC_NITROGEN_DIOXIDE_CONC_1/summary. D.E.H. acknowledges support from National Science Foundation CAREER Award CAS-Climate-2239834. S.C.A. and G.H.K. acknowledge support from NASA (Grant No. 80NSSC21K0511).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.3c00500.
Disparities in NO2-attributable mortality rates following reductions in NO2 concentrations to the WHO Air Quality Guidelines (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Health Effects Institute . Systematic Review and Meta-analysis of Selected Health Effects of Long-Term Exposure to Traffic-Related Air Pollution HEI Special Report 23 ;Health Effects Institute, 2022.
- U.S. EPA . Integrated Science Assessment for Oxides of Nitrogen-Health Criteria. EPA/600/R-15/068. 2016; www.epa.gov/isa, accessed 2023-10-18.
- Jacob D. J.Introduction to Atmospheric Chemistry ;Princeton University Press, 1999. [Google Scholar]
- Kerr G. H.; Martin R. V.; Donkelaar A. V.; Brauer M.; Bukart K.; Wozniak S.; Goldberg D. L.; Anenberg S. C.. Increasing disparities in air pollution health burdens in the United States. In ESS Open Archive 2022. 10.1002/essoar.10512159.1 [DOI]
- Anenberg S. C., et al. Long-term trends in urban NO2 concentrations and associated paediatric asthma incidence: estimates from global datasets. Articles Lancet Planet Health 2022, 6 10.1016/S2542-5196(21)00255-2. [DOI] [PubMed] [Google Scholar]
- Yazdi M. D.; et al. Long-term effect of exposure to lower concentrations of air pollution on mortality among US Medicare participants and vulnerable subgroups: a doubly-robust approach. Lancet Planet Health 2021, 5, e689–e697. 10.1016/S2542-5196(21)00204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.; et al. Low-Concentration Air Pollution and Mortality in American Older Adults: A National Cohort Analysis (2001–2017). Environ. Sci. Technol. 2022, 56, 7194–7202. 10.1021/acs.est.1c03653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.; et al. Long-Term Exposure to Low-Level NO2 and Mortality among the Elderly Population in the Southeastern United States. Environ. Health Perspect. 2021129 10.1289/EHP9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L. P.; Harris M. H.; Apte J. S.; Marshall J. D. National and Intraurban Air Pollution Exposure Disparity Estimates in the United States: Impact of Data-Aggregation Spatial Scale. Environ. Sci. Technol. Lett. 2022, 9, 786. 10.1021/acs.estlett.2c00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss S. E.; et al. Local- and regional-scale racial and ethnic disparities in air pollution determined by long-term mobile monitoring. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 10.1073/pnas.2109249118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu P.; Atkinson R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2020, 144, 105998. 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO global air quality guidelines ;World Health Organization, 2021. [Google Scholar]
- Levy I.; et al. Elucidating multipollutant exposure across a complex metropolitan area by systematic deployment of a mobile laboratory. Atmos Chem. Phys. 2014, 14, 7173–7193. 10.5194/acp-14-7173-2014. [DOI] [Google Scholar]
- EPA. Research on Near Roadway and Other Near Source Air Pollution. https://www.epa.gov/air-research/research-near-roadway-and-other-near-source-air-pollution, accessed 2023-10-18. (2020).
- Wang Y.; et al. Disparities in ambient nitrogen dioxide pollution in the United States. Proc. Natl. Acad. Sci. U. S. A. 120, 2023 10.1073/pnas.2208450120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; et al. Premature mortality attributable to NO2 exposure in cities and the role of built environment: A global analysis. Sci. Total Environ. 2023, 866, 161395. 10.1016/j.scitotenv.2023.161395. [DOI] [PubMed] [Google Scholar]
- Larkin A.; et al. Global Land Use Regression Model for Nitrogen Dioxide Air Pollution. Environ. Sci. Technol. 2017, 51, 6957–6964. 10.1021/acs.est.7b01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S., Census Bureau. 2019TIGER/Line Shapefiles. https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html, accessed 2023-10-18. [Google Scholar]
- Mohegh A.; Goldberg D.; Achakulwisut P.; Anenberg S. C. Sensitivity of estimated NO2-attributable pediatric asthma incidence to grid resolution and urbanicity. Environmental Research Letters 2021, 16, 014019. 10.1088/1748-9326/abce25. [DOI] [Google Scholar]
- Manson S.; Schroeder J.; Van Riper D.; Kugler T.; Ruggles S.. IPUMS National Historical Geographic Information System: Version 17.0 [5-Year Data [2015–2019, Block Groups & Larger Areas]]; IPUMS: Minneapolis, MN, 2022; 10.18128/D050.V17.0. [DOI]
- U.S. Census Bureau . TIGER/Line Shapefile, 2019, nation, U.S., Current Metropolitan Statistical Area/Micropolitan Statistical Area (CBSA) National. https://catalog.data.gov/dataset/tiger-line-shapefile-2019-nation-u-s-current-metropolitan-statistical-area-micropolitan-statist (2019).
- Raich W.; Fant C.; Jackson M.; Roman H.. Memorandum Supporting Near-Source Health Benefits Analyses Using Fine-Scale Incidence Rates ;Industrial Economics, Inc., 2020.
- Khreis H.; et al. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Yang P.; Zhang Y.; Wang K.; Doraiswamy P.; Cho S. H. Health impacts and cost-benefit analyses of surface O3 and PM2.5 over the U.S. under future climate and emission scenarios. Environ. Res. 2019, 178, 108687. 10.1016/j.envres.2019.108687. [DOI] [PubMed] [Google Scholar]
- Stieb D. M.; et al. Systematic review and meta-analysis of cohort studies of long term outdoor nitrogen dioxide exposure and mortality. PLoS One 2021, 16, e0246451 10.1371/journal.pone.0246451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind A. L.; Tessum C. W.; Coggins J. S.; Hill J. D.; Marshall J. D. Fine-scale damage estimates of particulate matter air pollution reveal opportunities for location-specific mitigation of emissions. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 8775–8780. 10.1073/pnas.1816102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessum C. W.; et al. Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 6001–6006. 10.1073/pnas.1818859116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann N.; Coffman E.; Timin B.; Kelly J. T. The estimated change in the level and distribution of PM2.5-attributable health impacts in the United States: 2005–2014. Environ. Res. 2018, 167, 506–514. 10.1016/j.envres.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller E., Proville J., Roy A.; Muller N. Z.. Mortality Risk from PM2.5: A Comparison of Modeling Approaches to Identify Disparities across Racial/Ethnic Groups in Policy Outcomes. Environ. Health Perspect., 129, 2021 10.1289/EHP9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southerland V. A.; et al. Assessing the distribution of air pollution health risks within cities: A neighborhood-scale analysis leveraging high-resolution data sets in the bay area, california. Environ. Health Perspect, 129, 2021 10.1289/EHP7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M. D.; et al. Estimating Intra-Urban Inequities in PM2.5-Attributable Health Impacts: A Case Study for Washington, DC. Geohealth, 5, 2021 10.1029/2021GH000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer J.; Hardman I.; Shimshack J.; Voorheis J. Disparities in PM 2.5 air pollution in the United States. Science 2020, 369, 575–578. 10.1126/science.aaz9353. [DOI] [PubMed] [Google Scholar]
- Jbaily A.; et al. Air pollution exposure disparities across US population and income groups. Nature 2022, 601, 228–233. 10.1038/s41586-021-04190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum K. Do.; et al. Long-term nitrogen dioxide exposure and cause-specific mortality in the U.S. Medicare population. Environ. Res. 2022, 207, 112154. 10.1016/j.envres.2021.112154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visa M. A.; et al. Neighborhood-scale air quality, public health, and equity implications of multi-modal vehicle electrification. Environmental Research: Infrastructure and Sustainability 2023, 3, 035007. 10.1088/2634-4505/acf60d. [DOI] [Google Scholar]
- Camilleri S. F.; et al. Air quality, health and equity implications of electrifying heavy-duty vehicles. Nat. Sustain 2023, 10.1038/s41893-023-01219-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.