Abstract
A gene coding for a putative α-glucosidase has been identified in the open reading frame yvdL (now termed malL), which was sequenced as part of the Bacillus subtilis genome project. The enzyme was overproduced in Escherichia coli and purified. Further analyses indicate that MalL is a specific oligo-1,4-1,6-α-glucosidase (sucrase-maltase-isomaltase). MalL expression in B. subtilis requires maltose induction and is subject to carbon catabolite repression by glucose and fructose. Insertional mutagenesis of malL resulted in a complete inactivation of the maltose-inducible α-glucosidase activity in crude protein extracts and a Mal− phenotype.
Disaccharides such as maltose, sucrose, and trehalose can serve as sole carbon and energy sources for Bacillus subtilis (12, 17, 33). In most cases, the accumulation of sugars is coupled with phosphate bond energy (20). Sucrose and trehalose utilization is dependent on the uptake of the sugars by their specific permeases, which are phosphoenolpyruvate-dependent phosphotransferase systems (PTS) correlated with the phosphorylation of the sugar (9, 12, 26). The phosphorylated sugars are further hydrolyzed in the cytoplasm by a specific phosphosucrase (17, 31) or phospho-α-1,1-glucosidase (10, 12). The latter reaction results in glucose-6-phosphate and glucose in a ratio of 1:1 from 1 mol of trehalose-6-phosphate (10). In a further reaction, the resulting glucose can serve as a substrate for an ATP-dependent glucose kinase (29). Internal glucose presumably also is produced by hydrolysis of other disaccharides such as maltose. Previous studies indicated that maltose is taken up by a non-PTS system, because uncouplers negatively affected maltose transport. This finding led to the conclusion that B. subtilis does not possess an enzyme II for maltose and that maltose uptake is a proton motive-driven process (33).
Amino acid sequence analysis.
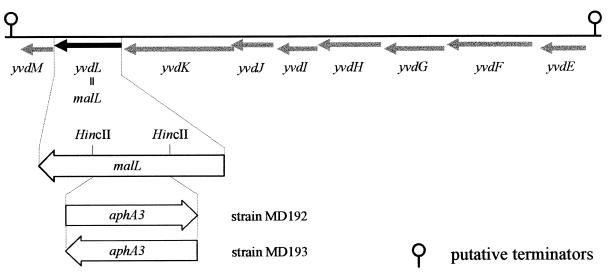
Newly available sequence data from the B. subtilis genome sequencing project make possible the identification of several genes coding for potential α-glucosidases (15, 32). The predicted gene products deduced from the DNA sequence of one gene cluster (yvdE to yvdM) from 3,545.7 to 3,558.0 kb on the B. subtilis genomic map (Fig. 1) (32) showed amino acid similarities to proteins involved in maltose/maltodextrin utilization systems, which presumably belong to the ABC transporter family. Therefore, we have chosen this region for further investigation with regard to maltose utilization. The derived amino acid sequence from yvdL exhibits high similarities to several α-glucosidases (32), indicating that the protein belongs to the glucosidase protein family. Therefore, and because of the results presented in this work, we designated the gene, predicted to encode a protein composed of 561 amino acids with a calculated molecular mass of 66 kDa and a pI of 4.98, malL.
FIG. 1.
The yvdE to yvdM region from 3,545 to 3,558 kb of B. subtilis. The yvdL gene (black arrow, now named malL) encoding the α-glucosidase is depicted below the DNA. Restriction sites used for malL inactivation by the aphA3 cassette are listed. Potential transcription terminators as proposed in the SubtiList data bank (15, 32) are denoted at the ends of the DNA.
Expression of α-glucosidase in B. subtilis.
If an α-glucosidase involved in maltose utilization exists in B. subtilis, one would expect it to be maltose inducible. Therefore, we investigated the dependence of para-nitrophenyl-α-d-glucopyranoside (PNPG) hydrolysis (PNPG is a synthetic substrate analogous for many α-glucosidases) on the presence or absence of maltose by MalL in crude cell extracts. MalL activity was determined as previously described for the phospho-α-1,1-glucosidase TreA (10, 12) with C minimal medium containing all the required components (18) and sugars as mentioned; cells were harvested at an optical density at 600 nm (OD600) as indicated (Fig. 2). As expected, high α-glucosidase activity was detected only in cultures grown in C minimal medium containing 10 mM maltose, whereas only residual PNPG-hydrolyzing activity was present in cells grown without maltose (grown on 0.4% K-glutamate). The latter activity is about 15-fold lower than the maltose-induced level. These data lead to the conclusion that the α-glucosidase expression (MalL; see below) is maltose inducible.
FIG. 2.
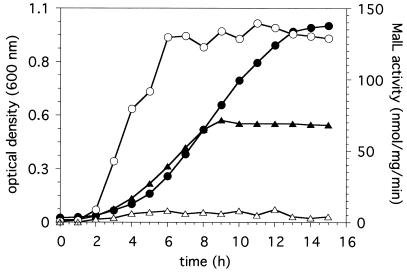
Growth phase-dependent MalL activity. B. subtilis strains were grown in minimal media containing 10 mM maltose. Aliquots were harvested at the indicated times, and MalL activity was determined with PNPG as a substrate and expressed in nanomoles of product formed minute−1 milligram of crude protein extract−1 for the wild-type (○) and the MalL− strain MD193 (▵). The corresponding OD600s of the cultures are indicated for the wild-type (•) and the MalL− strain MD193 (▴). The growth and MalL activities of strains MD192 and MD193 were identical.
In carbohydrate utilization, the presence of different rapidly metabolized carbohydrates leads to a sequential expression of different sugar utilization systems. Glucose and fructose are sugars which are preferentially metabolized. This preferential metabolism leads to the repression of other sugar-metabolizing systems. In bacilli, this regulatory mechanism, designated carbon catabolite repression (CCR), contains the central component CcpA, which is essential for the mediation of CCR after interaction with HPr phosphorylated at Ser46 (6, 7). The phosphorylation of HPr Ser46 is catalyzed by an ATP-dependent HPr kinase (22). Introducing the ptsH1 mutation, changing serine 46 to alanine, or the inactivation of CcpA results in the loss of CCR in many CCR systems (6, 7, 13). However, additional mechanisms of CCR have been proposed, including specific regulators, e.g., the contribution of the xylose or trehalose repressors and glucose-6-phosphate acting as an anti-inducer (4, 5), inducer exclusion (3, 27), the contribution of the glucose kinase (29, 30, 35), and potentially the regulation of enzymatic functions (10).
No α-glucosidase activity is detectable when wild-type cells are grown in 10 mM glucose or in a combination of 10 mM maltose and glucose (data not shown). The same results were observed under the same growth conditions when glucose was substituted for 10 mM fructose (data not shown), suggesting that, besides being inducible by maltose, α-glucosidase is subject to glucose- and fructose-promoted CCR. Previous studies reported that a maltose-inducible α-glucosidase is under CCR (7). However, in strains harboring a ccpA and/or a ptsH1 mutation, glucose-promoted CCR of the maltose-inducible α-glucosidase persisted (7). Therefore, additional mechanisms mediating the CCR of the maltose-inducible α-glucosidase which are independent of CcpA must be postulated.
We have also found that the expression of the maltose-inducible α-glucosidase is dependent on the growth phase in C minimal media containing 10 mM maltose. The highest MalL activity (139 nmol of PNPG hydrolyzed min−1 mg of crude protein extract−1) occurred about 6 h after dilution of the cultures, corresponding to the mid-log phase of growth (Fig. 2). Upon reaching its maximal value, the specific activity of maltose-inducible PNPG hydrolysis remained constant, even when the culture entered stationary phase.
Cloning of the α-glucosidase-encoding gene.
The malL gene was cloned by amplification via PCR (19) with a set of appropriate primers. B. subtilis chromosomal DNA was used as a template with the oligonucleotides 5′-CGATGTGAAAGGAGAAGGATCCATGAGTG and 5′-GATATTCTGCAGTATCTGTTATCACTCCG, introducing a BamHI site 5′ and a PstI site 3′ to malL. The resulting 1,731-bp DNA fragment was ligated to the appropriate cloning sites of plasmid pQE-9 (21) after digestion with BamHI and PstI. In the resulting plasmid, pMalL, malL transcription is under an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter and the gene for the α-glucosidase is fused N-terminally in frame to the His-tag coding region of the plasmid. The encoded protein has a 12-residue N-terminal extension including the affinity tag (underlined): Met-Arg-Gly-Ser-His-His-His-His-His-His-Gly-Ser-Met …. Plasmid pMalL was transformed into Escherichia coli RB791 (2) and selected on Luria broth (25) supplemented with ampicillin (100 μg/ml). The addition of 2 mM IPTG to liquid cultures yielded an intense protein band in crude cell extracts migrating at the expected molecular mass of 66 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gels (16) which were stained with Coomassie blue R250. Thus, the construct pMalL results in the overexpression of MalL in E. coli.
Inactivation of MalL.
To show that MalL is indeed involved in maltose utilization in B. subtilis and that malL encodes the observed maltose-inducible α-glucosidase, we have constructed two malL mutations by insertional mutagenesis (Fig. 1 and Table 1) of the cloned gene in plasmid pMalL. In the first step, malL was inactivated on the plasmid, leading to plasmids pMalLK1 and pMalLK2, which were obtained by replacing an internal 549-bp HincII fragment of pMalL by a 1,494-bp SmaI/StuI DNA fragment of plasmid pDG792 (11) carrying the aphA3 gene (Fig. 1). Recombinants were selected in E. coli on Luria broth plates supplemented with ampicillin (100 μg/ml) and kanamycin (30 μg/ml). The orientation of the aphA3 cassette was determined by digestion with AvaI, whose cleavage site is asymmetrically located in aphA3. The resulting plasmid, pMalK1, carries aphA3 in the same orientation as malL, whereas in pMalLK2 aphA3 is oriented in the opposite direction. Strains MD192 and MD193 were constructed by transformation (14) of a 2,676-bp BamHI/PstI DNA fragment obtained from plasmids pMalLK1 and pMalLK2, respectively, in B. subtilis, followed by selection on kanamycin (30 μg/ml) for recombinants, which had arisen by a double crossover. The correct insertion in the chromosome of the integrants has been verified by PCR (see above) (19) with chromosomal DNA of B. subtilis wild type and the resulting strains MD192 and MD193 as templates. Both B. subtilis mutants were also analyzed for maltose-inducible α-glucosidase activity. No significant enzymatic activity could be observed even in the maltose-induced state (Fig. 2).
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant genotype or phenotype | Reference or sourcea |
|---|---|---|
| Strains | ||
| Bacillus subtilis | ||
| 168 | trpC2 (wild type) | BGSC, 1A1 |
| MD192 | trpC2 malL::aphA3 | pMalLK2 tf> 168 |
| MD193 | trpC2 malL::aphA3 | pMalLK1 tf> 168 |
| Escherichia coli RB791 | F′ (lacIq L8) hsdR+hsdM | 2 |
| Plasmids | ||
| pDG792 | pMTL23 derivative containing the aphA3 antibiotic cassette | 11 |
| pMalL | pQE-9 derivative containing malL fused in frame to the His-tag coding region under tac promoter control | This work |
| pMalLK1 | pMalL derivative carrying malL::aphA3 | This work |
| pMalLK2 | pMalL derivative carrying malL::aphA3 | This work |
| pQE-9 | Expression vector for His-tag fusions under T5 promoter control | 21 |
tf> indicates transformation of DNA mentioned. BGSC, Bacillus Genetic Stock Center, Ohio State University, Columbus.
We also analyzed the phenotype of the B. subtilis α-glucosidase mutants. On solid C minimal maltose medium, malL inactivation leads to a Mal− phenotype (data not shown), although some residual growth is still observable. This finding agrees with the behavior of cultures in liquid C minimal maltose medium (shown in Fig. 2 for B. subtilis wild type and mutant MD193). Wild-type cells showed a typical growth curve reaching an OD600 of more than 1.0. In contrast, the cultures of the malL mutations start with identical doubling times, but growth stopped when the culture reached an OD600 of 0.5. However, malL mutant strains and wild-type strains exhibited no difference in growth when glucose (10 mM) was substituted for maltose (data not shown). One explanation for the incomplete Mal− phenotype of the malL strains could be the impurity of the maltose (possibly containing contaminating glucose) we used in this assay. Another interpretation for this phenotype may be the existence of an additional maltose utilization system. Nevertheless, the results presented clearly identify MalL as the only maltose-inducible α-glucosidase which is also subject to CCR.
Purification of the α-glucosidase.
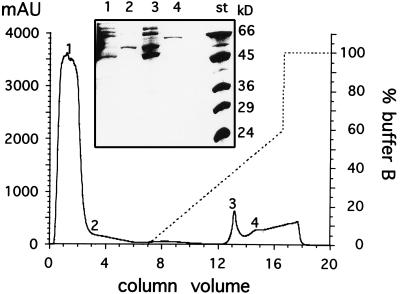
E. coli RB791/pMalL was grown in 100 ml of Luria broth at 37°C. Expression of malL was induced by the addition of 2 mM IPTG when the culture reached an OD600 of 0.5. Growth was allowed to proceed for another 5 h, and the cells were harvested by centrifugation at 5,000 × g. The resulting cell pellet was washed once in lysis buffer (25 mM imidazole [pH 7.0], 10 mM potassium chloride, 1 mM magnesium chloride, 1 mM calcium chloride, 2 mM 1,4-dithiothreitol), resuspended in the same buffer, and frozen at −70°C. Frozen cell pellets were thawed on ice and sonicated six times for 30 s each at 40 W with 30-s intervals with a Labsonic U sonicator (B. Braun, Melsungen, Germany). After centrifugation for 30 min at 40,000 × g in a Sorvall SS34 rotor, overproduced soluble MalL was present in the supernatant. The crude extract was passed over a 1-ml Ni2+-loaded HiTrap chelating column (Pharmacia, Freiburg, Germany) which had been equilibrated with lysis buffer with a Pharmacia Äkta purifier apparatus. The column was washed with 5 column volumes of lysis buffer until the absorption at 280 nm showed a stable baseline, and protein was eluted with a 10-ml linear gradient between lysis buffer and lysis buffer containing 500 mM imidazole at a flow rate of 1 ml/min (Fig. 3). Fractions (2 ml for column washing and 0.5 ml for gradient elution) were collected. The fusion protein typically eluted at about 250 mM imidazole. During protein purification, we analyzed the total and specific MalL activities from each purification step and the enrichment of MalL protein by SDS-PAGE (Fig. 3). The enrichment of MalL activity was calculated to be 45-fold. A 100-ml culture yielded about 2.4 mg of pure protein. Purified MalL was reasonably stable when stored in elution buffer at 4°C for at least 4 weeks.
FIG. 3.
Elution profile in Ni2+ HiTrap chelating column and purification of MalL. Protein absorption at 280 nm (solid line) and the imidazole gradient as the percentage of buffer B (dashed line) are presented as a function of the column volume (in milliliters). Analysis of selected fractions (indicated by numbers on the elution profile) on SDS-PAGE is shown in the insert. Pure MalL is in fraction 4. Molecular mass standards with the indicated sizes in kilodaltons (kD) are shown in lane st. mAU, milli-absorption units.
Physical properties of MalL activity.
To compare the stability of MalL with that of other α-glucosidases, we examined its activity under the influence of different parameters. A standard MalL activity test was used (containing 3 μg of purified protein per ml at 25°C) to determine the optimum pH. The pH of the reaction buffer was varied with either HCl or NaOH or supplemented with different concentrations of NaCl and KCl. With PNPG as the substrate, the pH profile was essentially symmetrical with optimal activity at pH 7.0. At pHs 5.75 and 8.0, the activity was reduced to 50%. MalL activity was inhibited by salt and exhibited 50% inhibition at 500 mM NaCl and 40% inhibition at 1 M KCl. These findings differ from the previously described effects of salt on the homologous phospho-α-1,1-glucosidase TreA, whose enzymatic activity is stimulated up to 10-fold at the appropriate salt concentrations (10).
Substrate specificity and kinetic parameters of MalL.
The phenotype of the malL mutants and the analysis of the amino acid sequence of MalL suggested that the gene encodes an α-glucosidase. However, the enzyme specificity cannot be determined definitively from the sequence analysis. Therefore, the specific activity of purified MalL was monitored by determining the release of free glucose after disaccharide hydrolysis by coupling the enzymatic activity with hexokinase and glucose-6-phosphate dehydrogenase following the method described by Seno and Charter (28). For this purpose, we used the glucose determination kit HK10 from Sigma (Deisenhofen, Germany) with modifications as described by the manufacturer. The reaction mixture contained 50 mM Tris-HCl, (pH 7.0), 25 mM MgCl2, 0.5 mM NAD, 1 mM ATP, and 0.8 U of glucose-6-phosphate dehydrogenase and was incubated at 25°C. Glucose-6-phosphate dehydrogenase activity was assayed with NAD as the cofactor by monitoring the change in OD340. Kinetic parameters were determined with 3 μg of purified MalL in a 1-ml reaction volume. Using substrates at 8 mM allowed us to conclude initially that in addition to PNPG, MalL hydrolyzes sucrose, maltose, and isomaltose, with specific activities of 4.47, 1.7, and 2.02 μmol of glucose per min per mg of protein, respectively. Under these assay conditions, no hydrolysis of trehalose, ortho-nitrophenyl galactopyranoside (ONPG), or melibiose and only weak lactose hydrolysis (0.04 μmol/min/mg) were observed. Analysis of the Km and Vmax of MalL for different substrates showed that MalL has 22- to 76-fold-higher affinities for isomaltose and maltose than for sucrose (Table 2). However, the Vmax for sucrose is faster than that for cleavage of isomaltose and maltose. The Km for PNPG is similar to that for maltose, but the Vmax for cleavage of PNPG is up to 100-fold lower than that observed for various disaccharides. From these data, it can be concluded that MalL efficiently hydrolyzes α-glycosidic 1,4- and 1,6-disaccharides but not α-1,1- or β-glycosidic bonds. The enzyme must also discriminate between galactosides and glucosides, because no hydrolysis of melibiose (α-1,6-galactopyranosyl-α-d-glucose) was detected. Therefore, we categorize this enzyme as an oligo-1,4-1,6-α-glucosidase. By virtue of its ability to hydrolyze sucrose and PNPG, this enzyme may be assigned the general designation oligo-α-glucosidase.
TABLE 2.
MalL substrate specificity and kinetic parameters
| Carbohydrate | Systematic name of carbohydrate | Vmax (μmol min−1 mg of protein−1) | Km (mM) |
|---|---|---|---|
| Sucrose | 1-O-α-d-glucopyranosyl-β-d-fructofuranoside | 357 | 10.2 |
| Isomaltose | 6-O-α-d-glucopyranosyl-d-glucose | 162 | 0.455 |
| Maltose | 4-O-α-d-glucopyranosyl-d-glucose | 65 | 0.135 |
| PNPG | p-Nitrophenyl-α-d-glucopyranoside | 3.3 | 0.210 |
The substrate specificity of MalL, its ability to hydrolyze PNPG, and its release of glucose as an end product of disaccharide cleavage clearly distinguish MalL from the amylomaltase, maltodextrin phosphorylase, and maltodextrin glucosidase enzymes involved in maltosaccharide catabolism in E. coli and Streptococcus pneumoniae and the phospho-α-glucosidases described for B. subtilis, E. coli, and Fusarium mortiferum (12, 23, 34). MalL of B. subtilis bears a greater resemblance to the α-glucosidase or maltase MalA of Staphylococcus xylosus (8) or to those found in yeast; this is also reflected in the considerable sequence similarity between these proteins (data not shown). However, S. xylosus MalA is a specific α-1,4-glucosidase which does not cleave isomaltose (8).
For utilization of sucrose at concentrations of 1 mM or less, B. subtilis possesses a sucrose-specific permease (SacP) which belongs to the phosphoenolpyruvate-dependent PTS as well as a phosphosucrase (1, 9). At higher sucrose concentrations, sucrose is cleaved extracellularly (31) and the resulting monosaccharides are taken up by the glucose- and fructose-specific PTS-dependent permeases (24). Therefore, a functional role for MalL in sucrose metabolism seems doubtful, at least when externally hydrolyzed sucrose is used as a carbon source. However, MalL might play a role in the breakdown of internal storage polysaccharides containing α-1,4 and α-1,6 bonds.
It appears most likely that MalL has a direct function in maltose and/or isomaltose metabolism. The location of malL in a cluster of genes with high homologies to maltose utilization systems suggests that these genes encode an ABC transporter for maltose. As previously suggested, maltose uptake in B. subtilis is an energy-dependent mechanism. The most plausible explanation for the negative effect of uncouplers on maltose transport was the role of the proton motive force in this process (33). Tangney and coworkers also reported the contribution of a putative maltose phosphorylase (33). However, it is possible that two maltose utilization systems exist in B. subtilis; this would explain the partial Mal− phenotype in the malL mutants as discussed above. In future work we will focus on the putative maltose and/or isomaltose transport and utilization system encoded by the yvdE to yvdM region and the molecular mechanisms of the regulation of these genes via induction and glucose repression.
Acknowledgments
We thank U. Ehmann for her interest in this work and many helpful suggestions and K. Oliva for editing the manuscript. This work was carried out in the laboratories of W. Hillen, whose support is greatly appreciated.
Financial support was obtained from the Deutsche Forschungsgemeinschaft (Da248/2-2, Da248/5-2), SFB473, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Arnaud M, Vary P, Zagorec M, Klier A, Débarbouillé M, Postma P, Rapoport G. Regulation of the sacPA operon of Bacillus subtilis: identification of phosphotransferase system components involved in SacT activity. J Bacteriol. 1992;174:3161–3170. doi: 10.1128/jb.174.10.3161-3170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brent R, Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci USA. 1981;78:4202–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahl M K. Enzyme IIGlc contributes to trehalose metabolism in Bacillus subtilis. FEMS Microbiol Lett. 1997;148:233–238. [Google Scholar]
- 4.Dahl M K, Hillen W. Contributions of XylR, CcpA and HPr to catabolite repression of the xyl operon in Bacillus subtilis. FEMS Microbiol Lett. 1995;132:79–83. [Google Scholar]
- 5.Dahl M K, Schmiedel D, Hillen W. Glucose and glucose-6-phosphate interaction with Xyl repressor proteins from Bacillus spp. may contribute to regulation of xylose utilization. J Bacteriol. 1995;177:5467–5472. doi: 10.1128/jb.177.19.5467-5472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 7.Deutscher J, Reizer J, Fischer C, Galinier A, Saier M H, Jr, Steinmetz M. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J Bacteriol. 1994;176:3336–3344. doi: 10.1128/jb.176.11.3336-3344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeter O, Brückner R. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol Microbiol. 1996;21:739–749. doi: 10.1046/j.1365-2958.1996.301398.x. [DOI] [PubMed] [Google Scholar]
- 9.Fouet A, Arnaud M, Klier A, Rapoport G. Bacillus subtilis sucrose-specific enzyme II of the phosphotransferase system: expression in Escherichia coli and homology to enzymes II from enteric bacteria. Proc Natl Acad Sci USA. 1987;84:8773–8777. doi: 10.1073/pnas.84.24.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotsche S, Dahl M K. Purification and characterization of the phospho-α-1,1-glucosidase (TreA) of Bacillus subtilis. J Bacteriol. 1995;177:2721–2726. doi: 10.1128/jb.177.10.2721-2726.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guérout-Fleury A-M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 12.Helfert C, Gotsche S, Dahl M K. Cleavage of trehalose-phosphate in Bacillus subtilis is catalyzed by a phospho-α-(1,1)-glucosidase encoded by the treA gene. Mol Microbiol. 1995;16:111–120. doi: 10.1111/j.1365-2958.1995.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 13.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the Gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 14.Kunst F, Msadek T, Rapoport G. Signal transduction network controlling degradative enzyme synthesis and competence in Bacillus subtilis. In: Piggot P J, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C: American Society for Microbiology; 1994. pp. 1–20. [Google Scholar]
- 15.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature (London) 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lepesant J-A, Kunst F, Pascal M, Kejzlarová-Lepesant J, Steinmetz M, Dedonder R. Specific and pleiotropic regulatory mechanisms in the sucrose system of Bacillus subtilis 168. In: Schlessinger D, editor. Microbiology—1976. Washington, D.C: American Society for Microbiology; 1976. pp. 58–69. [Google Scholar]
- 18.Msadek T, Kunst F, Henner D, Klier A, Rapoport G, Dedonder R. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol. 1990;172:824–834. doi: 10.1128/jb.172.2.824-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 20.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiagen. The QIAexpressionist. 3rd. ed. Hilden, Germany: Qiagen Inc.; 1997. [Google Scholar]
- 22.Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stülke J, Karamata D, Saier M H, Jr, Hillen W. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol Microbiol. 1998;27:1157–1170. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 23.Rimmele M, Boos W. Trehalose-6-phosphate hydrolase of Escherichia coli. J Bacteriol. 1994;176:5654–5664. doi: 10.1128/jb.176.18.5654-5664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saier M H, Jr, Reizer J. The bacterial phosphotransferase system: new frontiers 30 years later. Mol Microbiol. 1994;13:755–764. doi: 10.1111/j.1365-2958.1994.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Schöck F, Dahl M K. Analysis of DNA flanking the treA gene of Bacillus subtilis reveals genes encoding a putative specific enzyme IITre and a potential regulator of the trehalose operon. Gene. 1996;175:59–63. doi: 10.1016/0378-1119(96)00120-5. [DOI] [PubMed] [Google Scholar]
- 27.Schöck F, Dahl M K. Expression of the tre operon of Bacillus subtilis 168 is regulated by the repressor TreR. J Bacteriol. 1996;178:4576–4581. doi: 10.1128/jb.178.15.4576-4581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seno E T, Charter K F. Glycerol catabolic enzymes and their regulation in wild type and mutant strains of Streptomyces coelicolor A3(2) J Gen Microbiol. 1983;129:1403–1413. doi: 10.1099/00221287-129-5-1403. [DOI] [PubMed] [Google Scholar]
- 29.Skarlatos, P., and M. K. Dahl. The glucose kinase of Bacillus subtilis. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 30.Späth C, Kraus A, Hillen W. Contribution of glucose kinase to glucose repression of xylose utilization in Bacillus megaterium. J Bacteriol. 1997;179:7603–7605. doi: 10.1128/jb.179.23.7603-7605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinmetz M, Aymerich S. The Bacillus subtilis sac-deg constellation: how and why? In: Zukowski M M, Ganesan A T, Hoch J A, editors. Genetics and biotechnology of Bacilli. Vol. 3. San Diego, Calif: Academic Press; 1990. pp. 303–311. [Google Scholar]
- 32.SubtiList. Data release R14.2. In: Mozer I, Danchin A, editors. http://www.pasteur.fr./Bio/Subtilist.html. Paris, France: Institut Pasteur; 1997. [Google Scholar]
- 33.Tangney M, Buchanan C J, Priest F G, Mitchell W J. Maltose uptake and its regulation in Bacillus subtilis. FEMS Microbiol Lett. 1992;97:191–196. doi: 10.1016/0378-1097(92)90385-2. [DOI] [PubMed] [Google Scholar]
- 34.Thompson J, Gentry-Weeks C R, Nguyen N Y, Folk J E, Robish S A. Purification from Fusarium mortiferum ATCC 25557 of a 6-phosphoryl-O-α-d-glucopyranosyl:6-phosphoglucohydrolase that hydrolyzes maltose 6-phosphate and related phospho-α-d-glucosides. J Bacteriol. 1995;177:2505–2512. doi: 10.1128/jb.177.9.2505-2512.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner E, Marcandier S, Egeter O, Deutscher J, Götz F, Brückner R. Glucose kinase-dependent catabolite repression in Staphylococcus xylosus. J Bacteriol. 1995;177:6144–6152. doi: 10.1128/jb.177.21.6144-6152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]