Abstract
Introduction:
Previous analyses of tracheostomy in paediatric burns was hindered by a lack of multi-institution or nationwide analysis. This study aims to explore the effects of tracheostomy in paediatric burn patients in such an analysis. De-identified data was obtained from the TriNetX Research Network database.
Methods:
Two cohorts were identified using ICD and CPT codes: paediatric burn patients with tracheostomy (cohort 1) and paediatric burn patients without tracheostomy (cohort 2). Cohorts were matched according to age at diagnosis and pulmonary condition, specifically influenza and pneumonia, respiratory failure, acute upper respiratory infection, and pulmonary collapse. Cohorts were also matched for age at burn diagnosis and surface area burned. Several parameters including infection following a procedure, sepsis, volume depletion, respiratory disorders, laryngeal disorders, pneumonia, and other metrics were also compared.
Results:
A total of 152 patients were matched according to age and pulmonary condition. Cohort 1 and cohort 2 had a mean age of 4.45 ± 4.06 and 4.39 ± 3.99 years, respectively. Matched patients with tracheostomy had a higher risk for pneumonia, respiratory failure, other respiratory disorders, diseases of the vocal cord and larynx, sepsis, volume depletion, pulmonary edema, and respiratory arrest. The risk ratios for these outcomes were 2.96, 3.5, 3.13, 3.9, 2.5, 2.5, 3.3, and not applicable. Analysis of longitudinal outcomes of paediatric burn patients with tracheostomy vs. those without demonstrated the tracheostomy cohort suffered much worse morbidity and experienced higher health burden across several metrics.
Conclusion:
The potential benefits of tracheostomy in paediatric burn patients should be weighed against these outcomes.
Keywords: Paediatrics, Burn, Airway, Ventilation, Outcomes
1. Introduction
Non-intentional injury is the leading cause of death in children with burns accounting for a large portion of these deaths. Advancements in burn care including better treatment of infections, appropriate fluids, and nutritional management, among others leading to reduced rates of sepsis and respiratory failure. In addition to percentage of total body surface area (TBSA) burned and age, sepsis and respiratory failure are two major risk factors for death in paediatric burn patients [1,2]. The prevalence and risks of respiratory failure led surgeons to place tracheostomies in many of these patients. The primary indications for endotracheal intubation in paediatric burn patients include deteriorating respiratory status, pneumonia, and suspected prolonged ventilation [3,4]. Cuffed endotracheal intubation is now preferred in young paediatric burn patients due to lower rates of air leak and airway manipulation [5]. Previous analysis showed that increased TBSA was not linked to increased incidence of tracheostomy [6]. Rather, patients are converted to tracheostomy due to suspected prolonged ventilation, full-thickness burns to the face with anticipated wound closure difficulties and difficult nasotracheal access, airway access failure following endotracheal intubation, complications secondary to endotracheal intubation, pulmonary sepsis, and pulmonary failure [7,8]. Conversion to tracheostomy is not common, with one study reporting only 2/26 massively burned paediatric burn patients undergoing tracheostomy [7]. No consensus is present on the timing of tracheostomy in paediatric burn patients, with 32% of centers performing tracheostomy within 2 weeks and 36% waiting 4 weeks or longer before performing tracheostomy [8].
About 15–19% of paediatric patients that undergo tracheostomy procedure are at risk for numerous short- and long-term complications [9]. Early complications include air leaks, hemorrhage, injury to surrounding structures, pulmonary oedema, respiratory arrest, and death. Delayed complications include mucus plugging, stomal problems, tracheal lesions or granulation, hemorrhage, life-long difficulty swallowing, laryngotracheal scarring, and stenosis, with up to 15% of patients suffering from delayed consequences alone [4,9,10]. Furthermore, growing paediatric body size and respiratory tract further exacerbate the complications. Tracheostomy can also prove a significant financial burden for institutions and patients. One study found that in two years, the total cost of tracheostomy in 502 patients was $53.3 million with hospital costs accounting for 64.4% of the total cost [9]. Tracheostomy complications in burn patients such as pneumonia, respiratory failure, pulmonary edema, diseases of the vocal cords and larynx, other respiratory disorders, and sepsis are outcomes associated with varying morbidity, mortality, and financial burdens. For example, paediatric sepsis is a major cause of morbidity and mortality in the paediatric population. In children, sepsis alone is associated with hospital mortality of 10.3%, a mean length of hospital stay of 31 days, and an increase in treatment cost of $41,000 (in 1995 dollars) [11]. When compounded by severe burns, these outcomes can become even more severe. Furthermore, outcomes such as diseases of the vocal cords and larynx may involve reconstructive operations, further increasing the burden placed on patients and centers and adds additional risks.
For decades, with lack of consensus among medical institutional practices, controversy remains regarding the placement of tracheostomy in paediatric burn patients [12,13]. Almost all previously published literature on the efficacy of tracheostomy in paediatric burn patients are reported by single institutions with a limited number of patients. Furthermore, most analyses describe the prevalence of outcomes in the paediatric burn population who receive tracheostomy alone, rather than comparing these rates to counterparts who did not receive tracheostomy. For nearly two decades no updated literature has emerged aiming at multi-institutional or a large population encompassing nationwide analysis within the paediatric burn tracheostomy cohort. To fill this gap, the purpose of this study is to explore the risk of adverse events in paediatric burn patients that receive tracheostomy on a multi-institutional national scale.
2. Materials and methods
De-identified patient data was obtained from the TriNetX Research Network database. Two cohorts were identified: paediatric burn patients with tracheostomy (cohort 1) and paediatric burn patients without tracheostomy (cohort 2, the control). TriNetX (www.trinetx.com) is a global federated health research network providing de-identified access to retrospective electronic medical records (diagnoses, procedures, medications, laboratory values, genomic information) from approximately 72 million patients in 51 large Healthcare Organizations (HCOs). The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No Protected Health Information (PHI) or personal data is available to the users of the platform, as such this project was exempt from IRB review.
Burn patients ≤ 18 years old were identified using the ICD and CPT codes T20-T25 (burns and corrosions of external body surface, specified by site) and T30-T32 (burns and corrosions of multiple and unspecified body regions). Tracheostomy was identified using the ICD and CPT codes 0B11 (respiratory system/bypass/trachea), 1005887 (tracheostomy, emergency procedure), 1014613 (tracheostomy, planned [separate procedure]), 31600 (tracheostomy, planned [separate procedure]), 31601 (tracheostomy, planned [separate procedure]; younger than 2 years), 31603 (tracheostomy, emergency procedure; transtracheal), 31605 (tracheostomy, emergency procedure; cricothyroid membrane), 31610 (tracheostomy, fenestration procedure with skin flaps), Z43.0 (encounter for attention to tracheostomy), and Z93.0 (tracheostomy status). Cohorts 1a and 2a were matched by age at burn diagnosis and pulmonary condition, defined as J09-J18 (influenza and pneumonia), J96 (respiratory failure, not elsewhere classified), J00-J06 (acute upper respiratory infection), and J98.1 (pulmonary collapse). Cohorts 1b and 2b were matched according to age at burn diagnosis and T31.0-T31.9 (TBSA).
Based on previous studies and consulting institutional paediatric burn surgeons, the outcomes compared between cohorts were death, T81.4 (infection following a procedure), L00-L08 (infection of the skin and subcutaneous tissue), A40-A41 (sepsis), E86 (volume depletion), R57.1 (hypovolemic shock), J81 (pulmonary edema), R09.2 (respiratory arrest), J96 (respiratory failure, not elsewhere classified), J98 (other respiratory disorders), J38 (diseases of vocal cords and larynx), and J12-J18 (pneumonia). To preserve patient anonymity, TriNetX does not report follow-up times of patients or cohorts. The final TriNetX data analysis was performed on 6/14/2021 and all outcomes are reported as risk ratios with 95% confidence intervals, as tabulated by the TriNetX analytic feature. The flow diagram of the retrospective analysis between the cohorts is shown in Fig. 1A and B.
Fig. 1 –
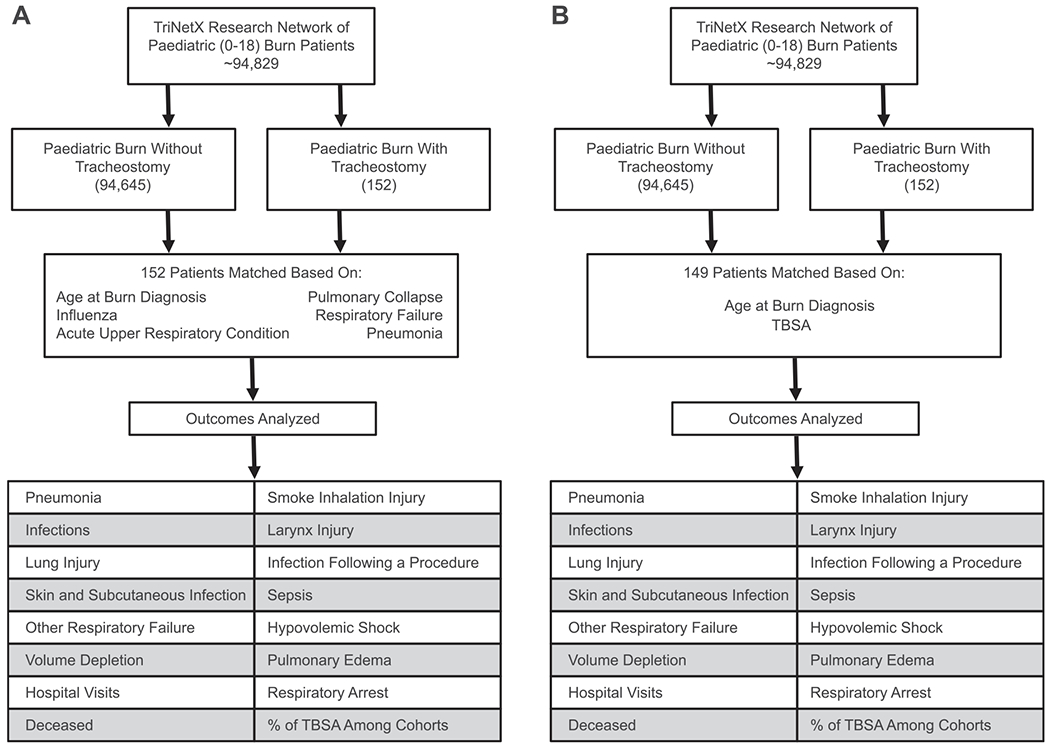
(A) Flow diagram of the outcomes analyzed in paediatric burn patients with and without tracheostomy when matching for age and pulmonary condition. (B) Flow diagram of the outcomes analyzed in paediatric burn patients with and without tracheostomy when matching for age and TBSA.
3. Results
A total of 152 patients in 25 HCOs were identified in cohort 1 with a mean age of 4.45 ± 4.06, while a total of 94,645 patients in 48 HCOs were identified in cohort 2 with a mean age of 4.17 ± 4.25. After matching by age and pulmonary condition, both cohorts consisted of 152 patients, designated as cohort 1a and cohort 2a which had a mean age of 4.45 ± 4.06 and 4.39 ± 3.99, respectively. After matching by age and pulmonary condition as described above, cohorts 1a and 2a both had 70 patients (46%) with influenza or pneumonia, 93 patients (61.2%) with respiratory not elsewhere classified, 50 patients (32.9%) with acute upper respiratory infection, and 65 patients (42.8%) with pulmonary collapse. Another level of matching was performed by age and TBSA, comprising of 149 patients, where cohort 1b had a mean age of 4.46 ± 4.08, and cohort 2b had a mean age of 4.46 ± 4.13. The cohort demographics after matching can be found in Table 1 and the distribution of TBSA in matched cohorts 1a,2a, 1b, and 2b can be found in Fig. 2.
Table 1 –
Cohort demographics after matching.
| Cohort 1a | Cohort 2a | Cohort 1b | Cohort 2b | |
|---|---|---|---|---|
| Mean Age (S.D.) | 4.45 (4.06) | 4.39 (3.99) | 4.46 (4.08) | 4.46 (4.13) |
| Male | 53.9% | 59.2% | 53% | 58.4% |
| Female | 46.1% | 40.8% | 47% | 41.6% |
| White | 50% | 51.3% | 49.7% | 45% |
| Black or African American | 28.9% | 21% | 28.9% | 23.5% |
| Not Hispanic or Latino | 61.1% | 62.5% | 61.1% | 49% |
| Hispanic or Latino | 18.4% | 13.8% | 18.1% | 12.1% |
| Unknown Ethnicity | 20.4% | 23.7% | 20.8% | 38.9% |
Fig. 2 –
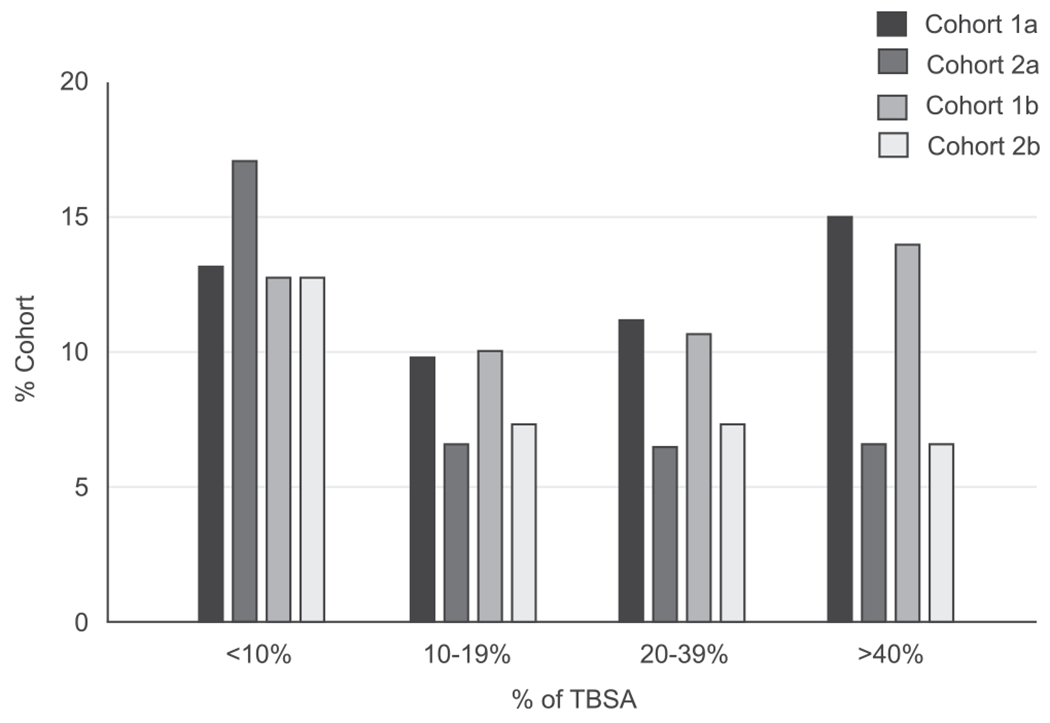
% TBSA in paediatric burn patients with and without tracheostomy when matching for age/pulmonary condition (cohorts 1a and 2a) and age/TBSA (cohorts 1b and 2b).
In the unmatched cohort 1, tracheostomy was performed within 2 weeks of burn in 106 patients, 2–4 weeks in 8 patients, and after 1 month in 38 patients.
Cohort 1a was found to have statistically significant increases in pneumonia, respiratory failure, and other respiratory disorders as compared to cohort 2a. In cohort 1a, 71 patients developed pneumonia, 77 patients developed respiratory failure, and 97 patients developed other respiratory disorders, as compared to 24, 22, and 31 patients in cohort 2a, respectively (p < 0.05). When matching according to age and pulmonary condition, the risk ratio for pneumonia, respiratory failure, and other respiratory disorders are 2.96 (95% CI = 1.97,4.43), 3.5 (95% CI = 2.31,5.31), and 3.13 (95% CI = 2.24,4.38), respectively (Table 2). Cohort 1a had significantly higher number of repeated hospital visits (53) compared to cohort 2a (21) (risk ratio = 2.52, 95% CI = 1.6, 3.97) (Table 2). Cohort 1b also had a significantly higher number of repeated hospital visits (52) compared to cohort 2b (17) (risk ratio= 3.06, 95% CI = 1.86, 5.04) (Table 3).
Table 2 –
Comparison of comorbidities and complications associated with tracheostomy [Cohort 1a] and without tracheostomy [Cohort 2a] when matching for age and pulmonary condition.
| Outcome | Cohort 1a n (%) | Cohort 2a n (%) | Risk Ratio | 95% Confidence Interval | P |
|---|---|---|---|---|---|
| Pneumonia | 71 (46.7) | 24 (15.8) | 2.96 | (1.97, 4.43) | < 0.0001 |
| Respiratory Failure | 77 (50.7) | 2 (14.5) | 3.5 | (2.31, 5.31) | < 0.0001 |
| Other Respiratory Disorders | 97 (63.8) | 31 (20.4) | 3.13 | (2.24, 4.38) | < 0.0001 |
| Hospital Visits | 53 (34.9) | 21 (13.8) | 2.52 | (1.6, 3.97) | < 0.0001 |
| Diseases of the Vocal Cords and Larynx | 39 (25.6) | 10 (6.58) | 3.9 | (2.02, 7.53) | < 0.0001 |
| Sepsis | 25 (16.4) | 10 (6.58) | 2.5 | (1.24, 5.02) | 0.007 |
| Volume Depletion | 25 (16.4) | 10 (6.58) | 2.5 | (1.24, 5.02) | 0.007 |
| Pulmonary Edema | 33 (21.7) | 10 (6.58) | 3.3 | (1.69, 6.45) | 0.0002 |
| Respiratory Arrest | 10 (6.58) | 0 (0) | N/A | N/A | 0.0013 |
| Death | 16 (10.5) | 10 (6.58) | 1.6 | (0.75, 3.41) | 0.218 |
| Infection of the Skin and Subcutaneous Tissue | 29 (19.1) | 19 (12.5) | 1.53 | (0.896, 2.60) | 0.1157 |
| Infection Following a Procedure | 10 (6.58) | 10 (6.58) | 1 | (0.429, 2.33) | 1 |
| Hypovolemic Shock | 10 (6.58) | 10 (6.58) | 1 | (0.429, 2.33) | 1 |
Table 3 –
Comparison of comorbidities and complications associated with tracheostomy [Cohort 1b] and without tracheostomy [Cohort 2b] in the paediatric burn population when matching for age and TBSA.
| Outcome | Cohort 1b n (%) | Cohort 2b n (%) | Risk Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|---|
| Hospital Visits | 52 (34.9) | 17 (11.4) | 3.06 | (1.86, 5.04) | < 0.0001 |
| Diseases of the Vocal Cords and Larynx | 38 (25.5) | 10 (6.71) | 3.8 | (1.97, 7.34) | < 0.0001 |
| Pneumonia | 70 (47) | 10 (6.71) | 7 | (3.76, 13) | < 0.0001 |
| Infections of the Skin and Subcutaneous Tissue | 28 (18.8) | 10 (6.71) | 2.8 | (1.41, 5.56) | 0.0018 |
| Sepsis | 25 (16.8) | 10 (6.71) | 2.5 | (1.24, 5.02) | 0.007 |
| Volume Depletion | 25 (16.8) | 10 (6.71) | 2.5 | (1.24, 5.02) | 0.007 |
| Pulmonary Edema | 32 (21.5) | 10 (6.71) | 3.2 | (1.63, 6.27) | 0.0002 |
| Respiratory Failure | 76 (51) | 10 (6.71) | 7.6 | (4.09, 14.1) | < 0.0001 |
| Other Respiratory Disorders | 94 (63.1) | 10 (6.71) | 9.4 | (5.1, 17.3) | < 0.0001 |
| Hypovolemic Shock | 10 (6.71) | 0 (0) | N/A | N/A | 0.0013 |
| Respiratory Arrest | 10 (6.71) | 0 (0) | N/A | N/A | 0.0013 |
| Death | 16 (10.7) | 10 (6.71) | 1.6 | (0.751, 3.41) | 0.2181 |
| Infection Following a Procedure | 10 (6.71) | 10 (6.71) | 1 | (0.429, 2.33) | 1 |
To maximize the protection of patient anonymity, cohort sizes from n = 1–10 patients are reported as n = 10 by TriNetX. Cohort sizes n > 10 are reported as an exact number. Cohort sizes of n = 0 are reported as such by TriNetX. Several outcomes were identified in which one of the cohorts had n ≤ 10 patients (n = 10 is reported by TriNetX in this instance) but still had statistically significant differences between cohorts.
Cohort 1a was found to have statistically significant increases in the risk of diseases of the vocal cords and larynx, sepsis, volume depletion, pulmonary edema, and respiratory arrest as compared to cohort 2a. In cohort 1a, 39 patients developed diseases of the vocal cords and larynx, 25 patients developed sepsis, 25 patients developed volume depletion, and 33 patients developed pulmonary edema, compared to ≤ 10 patients in cohort 2a for these outcomes. In cohort 1a, ≤ 10 patients developed respiratory arrest compared to 0 patients in cohort 2a. When matching for age and pulmonary condition, the risk ratio for diseases of the vocal cords and larynx, sepsis, volume depletion, pulmonary edema, and respiratory arrest are 3.9 (95% CI = 2.02, 7.53), 2.5 (95% CI = 1.24, 5.02), 2.5 (95% CI = 1.24, 5.02), 3.3 (95% CI = 1.69, 6.45), and N/A, respectively (Table 2).
Cohort 1b was found to have statistically significant increases in the risk of diseases of the vocal cords and larynx, pneumonia, infections of the skin and subcutaneous tissue, sepsis, volume depletion, hypovolemic shock, pulmonary edema, respiratory arrest, respiratory failure, and other respiratory disorders as compared to cohort 2b. In cohort 1b, 38 patients developed diseases of the vocal cords and larynx, 70 patients developed pneumonia, 28 patients developed infections of the skin and subcutaneous tissue, 25 patients developed sepsis, 25 patients developed volume depletion, 32 patients developed pulmonary edema, 76 patients developed respiratory failure, and 94 patients developed with other respiratory disorders, compared to ≤ 10 patients in cohort 2b for these outcomes. Additionally, in cohort 1b, ≤ 10 patients developed hypovolemic shock and respiratory arrest compared to 0 patients in cohort 2b. When matching for age and TBSA, the risk ratio for diseases of the vocal cords and larynx, pneumonia, infections of the skin and subcutaneous tissue, sepsis, volume depletion, pulmonary edema, respiratory failure, other respiratory disorders, hypovolemic shock, and respiratory arrest are 3.8 (95% CI = 1.97, 7.34), 7 (95% CI = 3.76, 13), 2.8 (95% CI = 1.41, 5.56), 2.5 (95% CI = 1.24, 5.02), 2.5 (95% CI = 1.24, 5.02), 3.2 (95% CI = 1.63, 6.27), 7.6 (95% CI = 4.09, 14.1), 9.4 (95% CI = 5.1, 17.3), N/A, and N/A, respectively (Table 3).
When matching for age and pulmonary condition, we found no statistical difference in death, infections of the skin and subcutaneous tissue, infection following a procedure, or hypovolemic shock (Table 2). When matching for age and TBSA, we found no statistical difference in death or infection following a procedure (Table 3). Inhalation injury was present in n ≤ 10 patients in all cohorts.
4. Discussion
The trend of tracheostomy placement in the paediatric burn patient population has waxed and waned for decades, largely due to disagreements over the complications vs. the benefits of tracheostomy [12,13]. Much of the literature reports the adverse events associated with tracheostomy as it stands alone, rather than comparing this cohort to burn patients that did not undergo a tracheostomy. The conclusions of these studies highlight a range of complications from no risk of complication [3,14] to persistent tracheocutaneous fistula, hemorrhage, pneumothorax, tracheomalacia, granulomas, and tracheal stenosis [15,16]. Our study’s findings aim to clarify the risks of various complications that paediatric burn patients face when undergoing tracheostomy. Although matching cohorts according to age and the patient’s deteriorating pulmonary condition is most appropriate in this population, as it is the likely indication for tracheostomy, our study has also matched the cohorts according to age and TBSA, and reported this as a proxy for illness severity. Our study’s findings demonstrate that paediatric burn patients with tracheostomy are at an increased risk for pneumonia, respiratory failure, other respiratory disorders, diseases of the vocal cords and larynx, sepsis, volume depletion, pulmonary edema, and respiratory arrest. This is the largest analysis of tracheostomy in the paediatric burn population, with previous studies reaching up to 88 patients [10].
Patients are often converted from endotracheal intubation to tracheostomy due to suspected prolonged ventilation, fullthickness burns to the face with anticipated wound closure difficulties and no nasotracheal access, airway access failure following endotracheal intubation, complications secondary to endotracheal intubation, pulmonary sepsis, and pulmonary failure [17,18]. These indications directed the matching criteria of age at burn diagnosis, influenza and pneumonia, respiratory failure, acute upper respiratory infection, and pulmonary collapse. Burn age at diagnosis was an additional matching criterion as infants are more likely to suffer worse prognoses than children and adolescents [19]. One recent study of 65 paediatric burn patients who underwent tracheostomy in China found that upper airway obstruction and respiratory insufficiency with mechanical ventilator support were the two major driving indications for tracheostomy [15]. Most tracheostomies placed in this institution were prophylactic tracheostomies without prior intubation. Inhalation injury is unlikely to be a confounding variable in our study or be the major cause of respiratory complications in either cohort as n ≤ 10 patients in all cohorts suffered inhalation injury; as such inhalation injury was not a matching criterion.
The results of this study highlight an increased risk of tracheostomy in the paediatric burn population for pneumonia, respiratory failure, other respiratory disorders, diseases of the vocal cords and larynx, sepsis, volume depletion, pulmonary edema, and respiratory arrest as compared to their non-tracheostomy counterparts. The risk ratios of diseases of the vocal cords and larynx, sepsis, volume depletion, pulmonary edema, and respiratory arrest are reported at their minimum value. The risk ratios are calculated using the TriNetX analysis function with the underlying assumption that 10 patients in cohort 2 suffered from each of the outcomes. The calculations were performed in this manner due to the way TriNetX reports n ≤ 10 as n = 10. If the number of patients is less than 10 for these outcomes, the risk ratio and statistical significance of these findings will be greater.
Clinicians should consider the increased risk of adverse outcomes associated with tracheostomy and pursue preventative therapies for said outcomes. Patients with tracheostomy are inherently at increased risk for complications, such as diseases of the vocal cords and larynx, however, the risk of other complications may be reduced with changes in medical management. More careful hemodynamic and volume management in this cohort is indicated due to the increased risk for volume depletion and pulmonary edema. Additionally, we found that paediatric burn patients with tracheostomy are at an increased risk of infections, such as pneumonia and sepsis, as compared to their non-tracheostomy counterparts. Increased infectious risk should be recognized by clinicians and interventions such as more frequent infection screening is indicated. While our study reports the largest analysis of tracheostomies in the paediatric burn population, the findings of this study are retrospective and should be used to guide future prospective, randomized studies to make more concrete recommendations. Updated practice guidelines need to be developed which stratify risks associated with a tracheostomy, indications for tracheostomy, and placement timing of tracheostomy [6].
The risks associated with tracheostomy proliferate as each of the potential adverse outcomes has its own accompanying risks of morbidity and mortality. Some tracheostomy complications even require surgical repair, compounding the risks to the patient. One adverse outcome associated with tracheostomy with particular risk to paediatric patients is sepsis. Septic children have a mortality rate of over 10% even without severe burns or tracheostomy. The potential implications of tracheostomy procedures on patient morbidity, patient mortality, and burden to healthcare due to complications and alternatives to tracheostomy must be considered in certain situations. Given the morbidity and increased financial burdens associated with tracheostomy, the significant practice variation of tracheostomy timing and placement rates between centers should be further analyzed [8].
Several limitations should be considered for this study. One of the inherent limitations of using the TriNetX database is that TriNetX does not report patients in an exact whole number if n ≤ 10, but rather as a cohort of 10. This is done to protect the privacy of the patients and the institution where the data was extracted. This means if cohort 1 and 2 are reported to both have 10 patients in their respective cohorts, both cohorts can have as low as 1 or a maximum of 10 patients. As a result, it limits the number of patients in each cohort especially in rare populations like paediatric burn population with tracheostomy and thus affecting the downstream statistical analysis. Follow up time on patients could not be determined as this is also sensitive information that TriNetX does not share for privacy purposes.Additionally, individual institution data is not provided by TriNetX to further protect patient privacy. Finally, due to the variation of coding protocols at different institutions, some patients may have received a diagnosis code that describes burn locations, but not TBSA burned. This limited the ability to match patients according to TBSA or determine true cohort TBSA demographics.
5. Conclusions
Paediatric burn patients that undergo tracheostomy are at an increased risk for pneumonia, respiratory failure, other respiratory disorders, diseases of the vocal cords and larynx, sepsis, volume depletion, and pulmonary edema. The potential benefits of tracheostomy placement in paediatric burn patients should be weighed against these outcomes.
Funding
Funding was provided by the ‘Remembering the 15’ Burn Research Endowment, and the Joseph D and Lee Hage Jamail Distinguished Professor Endowment for Burn Research and Education, at the University of Texas Medical Branch - Galveston.
Abbreviations:
- TBSA
total body surface area
- HCOs
Healthcare Organizations
Footnotes
Presented at the 53rd American Burn Association Annual Meeting on April 7–9, 2021.
CRediT authorship contribution statement
Nicholas J. Iglesias, Anesh Prasai, George Golovko, Deepak K. Ozhathil, and Steven E. Wolf worked on the conception and design of the study, or acquisition of data, or analysis and interpretation of data. Nicholas J. Iglesias, Anesh Prasai, George Golovko, Deepak K. Ozhathil, and Steven E. Wolf were responsible for drafting the article or revising it critically for important intellectual content. All authors have approved the final version of this manuscript.
Declaration of Interest
Authors declare no conflicts of interest other than SEW is the Editor of this journal.
REFERENCES
- [1].Barrow RE, Spies M, Barrow LN, Herndon DN. Influence of demographics and inhalation injury on burn mortality in children. Burns 2004;30:72–7. 10.1016/j.burns.2003.07.003 [DOI] [PubMed] [Google Scholar]
- [2].Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care 2009;13:R183. 10.1186/cc8170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Coin CE, Purdue GF, Hunt JL. Tracheostomy in the young pediatric burn patient. Arch Surg 1998;133(537–9):9–40. 10.1001/archsurg.133.5.537 [DOI] [PubMed] [Google Scholar]
- [4].Rocourt DV, Hall M, Kenney BD, Fabia R, Groner JI, Besner GE. Respiratory failure after pediatric scald injury. J Pedia Surg 2011;46:1753–8. 10.1016/j.jpedsurg.2011.04.018 [DOI] [PubMed] [Google Scholar]
- [5].Dorsey DP, Bowman SM, Klein MB, Archer D, Sharar SR. Perioperative use of cuffed endotracheal tubes is advantageous in young pediatric burn patients. Burns 2010;36:856–60. 10.1016/j.burns.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Calhoun KH, Deskin RW, Garza C, McCracken MM, Nichols RJ Jr, Hokanson JA, et al. Long-term airway sequelae in a pediatric burn population. Laryngoscope 1988;98:721–5. 10.1288/00005537-198807000-00006 [DOI] [PubMed] [Google Scholar]
- [7].Sheridan RL, Schnitzer JJ. Management of the high-risk pediatric burn patient. J Pedia Surg 2001;36:1308–12. 10.1053/jpsu.2001.25805 [DOI] [PubMed] [Google Scholar]
- [8].Silver GM, Freiburg C, Halerz M, Tojong J, Supple K, Gamelli RL. A survey of airway and ventilator management strategies in North American pediatric burn units. J Burn Care Rehabil 2004;25:435–40. 10.1097/01.bcr.0000138294.39313.6b [DOI] [PubMed] [Google Scholar]
- [9].Watters KF. Tracheostomy in infants and children. Respir Care 2017;62:799–825. 10.4187/respcare.05366 [DOI] [PubMed] [Google Scholar]
- [10].Clemens J, Fischer C, Willging JP, McCall J. 7: Complications after tracheotomy in pediatric burn patients. Otolaryngol-Head Neck Surg 1996;115:P163. 10.1016/s0194-5998(96)80869-2 [DOI] [Google Scholar]
- [11].Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003;167:695–701. 10.1164/rccm.200207-682OC [DOI] [PubMed] [Google Scholar]
- [12].Barret JP, Desai MH, Herndon DN. Effects of tracheostomies on infection and airway complications in pediatric burn patients. Burns 2000;26:190–3. 10.1016/s0305-4179(99)00113-8 [DOI] [PubMed] [Google Scholar]
- [13].Palmieri TL, Jackson W, Greenhalgh DG. Benefits of early tracheostomy in severely burned children. Crit Care Med 2002;30:922–4. 10.1097/00003246-200204000-00036 [DOI] [PubMed] [Google Scholar]
- [14].Jones JE, Rosenberg D. Management of laryngotracheal thermal trauma in children. Laryngoscope 1995;105:540–2. 10.1288/00005537-199505000-00019 [DOI] [PubMed] [Google Scholar]
- [15].Chen B, Kuang F, Zhang Z, Chen L, Liu Z, Zhang X, et al. Early tracheostomy in severely burned pediatric patients: 16-year experience at a tertiary burn center in China. Res Sq: Res Sq 2020. [Google Scholar]
- [16].Sen S, Heather J, Palmieri T, Greenhalgh D. Tracheostomy in pediatric burn patients. Burns 2015;41:248–51. 10.1016/j.burns.2014.10.005 [DOI] [PubMed] [Google Scholar]
- [17].Aggarwal S, Smailes S, Dziewulski P. Tracheostomy in burns patients revisited. Burns 2009;35:962–6. 10.1016/j.burns.2009.03.005 [DOI] [PubMed] [Google Scholar]
- [18].Hunt JL, Purdue GF, Gunning T. Is tracheostomy warranted in the burn patient? Indications and complications. J Burn Care Rehabil 1986;7:492–5. 10.1097/00004630-198611000-00009 [DOI] [PubMed] [Google Scholar]
- [19].Sharma RK, Parashar A. Special considerations in paediatric burn patients. Indian J Plast Surg 2010;43:S43–50. 10.4103/0970-0358.70719 [DOI] [PMC free article] [PubMed] [Google Scholar]


