Abstract
Background
Many people receiving palliative care have reduced oral intake during their illness, and particularly at the end of their life. Management of this can include the provision of medically assisted hydration (MAH) with the aim of improving their quality of life (QoL), prolonging their life, or both. This is an updated version of the original Cochrane Review published in Issue 2, 2008, and updated in February 2011 and March 2014.
Objectives
To determine the effectiveness of MAH compared with placebo and standard care, in adults receiving palliative care on their QoL and survival, and to assess for potential adverse events.
Search methods
We searched for studies in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, CANCERLIT, CareSearch, Dissertation Abstracts, Science Citation Index and the reference lists of all eligible studies, key textbooks, and previous systematic reviews. The date of the latest search conducted on CENTRAL, MEDLINE, and Embase was 17 November 2022.
Selection criteria
We included all relevant randomised controlled trials (RCTs) of studies of MAH in adults receiving palliative care aged 18 and above. The criteria for inclusion was the comparison of MAH to placebo or standard care.
Data collection and analysis
Three review authors independently reviewed titles and abstracts for relevance, and two review authors extracted data and performed risk of bias assessment. The primary outcome was QoL using validated scales; secondary outcomes were survival and adverse events. For continuous outcomes, we measured the arithmetic mean and standard deviation (SD), and reported the mean difference (MD) with 95% confidence interval (CI) between groups. For dichotomous outcomes, we estimated and compared the risk ratio (RR) with 95% CIs between groups. For time‐to‐event data, we planned to calculate the survival time from the date of randomisation and to estimate and express the intervention effect as the hazard ratio (HR). We assessed the certainty of evidence using GRADE and created two summary of findings tables.
Main results
We identified one new study (200 participants), for a total of four studies included in this update (422 participants). All participants had a diagnosis of advanced cancer. With the exception of 29 participants who had a haematological malignancy, all others were solid organ cancers. Two studies each compared MAH to placebo and standard care. There were too few included studies to evaluate different subgroups, such as type of participant, intervention, timing of intervention, and study site. We considered one study to be at high risk of performance and detection bias due to lack of blinding; otherwise, risk of bias was assessed as low or unclear.
MAH compared with placebo
Quality of life
One study measured change in QoL at one week using Functional Assessment of Cancer Therapy ‐ General (FACT‐G) (scale from 0 to 108; higher score = better QoL). No data were available from the other study. We are uncertain whether MAH improves QoL (MD 4.10, 95% CI −1.63 to 9.83; 1 study, 93 participants, very low‐certainty evidence).
Survival
One study reported on survival from study enrolment to last date of follow‐up or death. We were unable to estimate HR. No data were available from the other study. We are uncertain whether MAH improves survival (1 study, 93 participants, very low‐certainty evidence).
Adverse events
One study reported on intensity of adverse events at two days using a numeric rating scale (scale from 0 to 10; lower score = less toxicity). No data were available from the other study. We are uncertain whether MAH leads to adverse events (injection site pain: MD 0.35, 95% CI −1.19 to 1.89; injection site swelling MD −0.59, 95% CI −1.40 to 0.22; 1 study, 49 participants, very low‐certainty evidence).
MAH compared with standard care
Quality of life
No data were available for QoL.
Survival
One study measured survival from randomisation to last date of follow‐up at 14 days or death. No data were available from the other study. We are uncertain whether MAH improves survival (HR 0.36, 95% CI 0.22 to 0.59; 1 study, 200 participants, very low‐certainty evidence).
Adverse events
Two studies measured adverse events at follow‐up (range 2 to 14 days). We are uncertain whether MAH leads to adverse events (RR 11.62, 95% CI 1.62 to 83.41; 2 studies, 242 participants, very low‐certainty evidence).
Authors' conclusions
Since the previous update of this review, we have found one new study. In adults receiving palliative care in the end stage of their illness, there remains insufficient evidence to determine whether MAH improves QoL or prolongs survival, compared with placebo or standard care. Given that all participants were inpatients with advanced cancer at end of life, our findings are not transferable to adults receiving palliative care in other settings, for non‐cancer, dementia or neurodegenerative diseases, or for those with an extended prognosis. Clinicians will need to make decisions based on the perceived benefits and harms of MAH for each individual's circumstances, without the benefit of high‐quality evidence to guide them.
Keywords: Adult, Humans, Neoplasms, Palliative Care
Plain language summary
Medically assisted hydration to assist people receiving palliative care
Review question
What are the benefits and risks of the medical administration of fluids for adults at the end stage of illness?
Key messages
‐ We are uncertain whether giving fluids through a drip either into a vein or under the skin, or via a tube into the stomach in the end stage of illness improves quality of life (well‐being) compared to either standard care (good mouth care to alleviate the sensation of thirst) or placebo. A placebo is a 'dummy', or sham treatment that looks the same but contains a non‐therapeutic amount of fluid.
‐ We are uncertain whether medical administration of fluids increases the length of time people live or if it leads to unwanted or harmful effects.
‐ We need more and better studies to investigate medical administration of fluids for people in the end stage of illness. Future studies should focus on better tests for determining if medical administration of fluids helps people, and on finding out when to start fluids and how much fluid will help, if any.
Why supplement fluid intake in the end stage of illness?
Palliative care is treatment, care, and support provided for people living with a life‐limiting illness. Adults receiving palliative care can experience a reduced desire for oral fluids as they approach the end of life. At this time they may develop symptoms related to the reduction in fluid intake that could impair their quality of life. A further concern is that without supplemental fluids their life span may be reduced as a potential of dying from complications secondary to a reduced oral intake rather than their underlying disease.
What did we want to find out?
We wanted to know whether medically administered fluids help adults in the end stage of illness.
We were interested in the effect of medically administered fluids on:
‐ the quality of life (well‐being) of people;
‐ how long they lived; and
‐ the development of any unwanted or harmful effects.
What did we do?
We searched for studies that investigated whether:
‐ medically assisted fluids compared to placebo with non‐therapeutic amounts of fluid; or
‐ medically assisted fluids compared to standard care including good mouth care to alleviate the sensation of thirst
was effective and whether it caused any unwanted effects in adults over 18 years of age who were receiving palliative care in the end stage of illness.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 4 studies that involved a total of 422 people. Two studies compared medically administered fluids to placebo, and two compared medically administered fluids to standard care. The largest study was in 200 people, and the smallest study was in 42 people; studies lasted between 2 and 14 days. The age of people in the studies ranged from 28 to 98 years, of whom 226 were women and 196 were men. The studies were conducted in countries around the world, including the UK, the USA, and Argentina. Two studies were funded by clinical research grants. We found no studies where people did not have an advanced cancer diagnosis.
Main results
In adults receiving medically administered fluids in the end stage of illness, compared to placebo:
‐ we are uncertain whether medically administered fluids improve quality of life;
‐ we are uncertain whether medically administered fluids increase how long people live, and whether they lead to the development of any unwanted or harmful effects.
In people receiving medically administered fluids in the end stage of illness, compared to standard care:
‐ we are uncertain whether medically administered fluids improve quality of life due to lack of information in the included studies;
‐ we are uncertain whether medically administered fluids increase how long people lived, and whether they lead to the development of any unwanted or harmful effects.
What are the limitations of the evidence?
We are not confident in the evidence, and the results of further research could differ from the results of this review. Three main factors reduced our confidence in the evidence. Firstly, the evidence does not cover all of the people we were interested in, as we only found studies that included people with advanced cancer. Secondly, studies were very small and there are not enough studies to be certain about the results of our outcomes. Finally, it is possible that people in the studies were aware of which treatment they were getting, which could have introduced bias.
How up‐to‐date is this evidence?
This review updates our previous review. The evidence is current to November 2022.
Summary of findings
Summary of findings 1. Medically assisted hydration compared with placebo for adults receiving palliative care.
| Medically assisted hydration compared with placebo for adults receiving palliative care | |||||
|
Patient or population: adults aged 18 years and older receiving palliative care Settings: inpatient Intervention: medically assisted hydration Comparator: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | MAH | ||||
|
Quality of life FACT‐G (scale from 0 to 108; higher score = better QoL) Follow‐up: 7 days |
The MD in QoL in the control group was 2.6. | The MD in QoL in the intervention group was 4.10 (from −1.63 lower to 9.83 higher). | ‐ | 93 (1 study) |
⨁◯◯◯ VERY LOW1 |
|
Survival Log‐rank analysis Study enrolment to last date of follow‐up or death |
We were unable to estimate HR. There was no clear evidence for an effect. | 93 (1 study) |
⨁◯◯◯ VERY LOW1 | ||
|
Adverse events NRS (scale from 0 to 10; lower score = less toxicity) Follow‐up: 2 days |
The mean injection site pain in the control group was 1.75. | The mean injection site pain in the intervention group was 0.35 (from −1.19 lower to 1.89 higher). | ‐ | 49 (1 study) |
⨁◯◯◯ VERY LOW1 |
| The mean injection site swelling in the control group was 1.41. | The mean injection site swelling in the intervention group was −0.59 (from −1.40 lower to 0.22 higher). | ‐ | |||
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FACT‐G: Functional Assessment of Cancer Therapy ‐ General; HR: hazard ratio; MAH: medically assisted hydration; MD: mean difference; NRS: numeric rating scale; QoL: quality of life | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for indirectness (studies only included participants with advanced cancer) and two levels for very serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
Summary of findings 2. Medically assisted hydration compared with standard care for adults receiving palliative care.
| Medically assisted hydration compared with standard care for adults receiving palliative care | ||||||
|
Patient or population: adults aged 18 years and older receiving palliative care Settings: inpatient Intervention: medically assisted hydration Comparator: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | MAH | |||||
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No data were available for this outcome. |
|
Survival Cox regression model Study enrolment to last date of follow‐up at 14 days or death |
There was no clear evidence for an effect (MAH group at 3 days: HR 0.36, 95% CI 0.22 to 0.59). | 200 (1 study) |
⨁◯◯◯ VERY LOW1 | |||
|
Adverse events Participant events Follow‐up: range 2 to 14 days |
Study population |
RR 11.62 (1.62 to 83.41) |
242 (2 studies) |
⨁◯◯◯ VERY LOW1 | ||
| 0/149 | 7/93 | |||||
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MAH: medically assisted hydration; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for indirectness (studies only included participants with advanced cancer), one level for serious study limitations (lack of blinding), and one level for serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 4, 2014) on 'Medically assisted hydration for adult palliative care patients' (Good 2014).
Description of the condition
The aim of palliative care is to improve the quality of life of people and their families who are living with a life‐limiting illness by treating physical, psychosocial, and spiritual symptoms (WHO 2021). Adults receiving palliative care have a variety of diagnoses including advanced cancer, neurodegenerative diseases, and dementia.
In people who are in the last few days to weeks of life, it is common to have a reduction in oral intake (Canihuante 2018; Lokker 2019). At the time that oral intake decreases, most people will have had a cessation of medical interventions and investigations. This is often associated with a deprescribing of medications that are not aimed at comfort, and a focus on maintaining quality of life (QoL).
There are several factors that can cause this reduction in oral intake in adults receiving palliative care including dysphagia, nausea, bowel obstruction, anorexia/cachexia syndrome, fluctuating consciousness, and loss of desire to drink. Concerns are often raised if patients' oral intake falls below a level where it is felt that they are able to maintain adequate hydration or where there are clinical signs of dehydration such as reduced skin turgor or poor urine output (Hui 2015; Lokker 2019). Whilst biochemical markers for dehydration can be used, routine blood tests have usually ceased at this time. Concerns have also been raised on the accuracy of biochemical markers and correlation with symptoms of poor hydration (Nwosu 2013).
Description of the intervention
This review focuses on the use of medically assisted hydration (MAH) in adults receiving palliative care in the last few days to weeks of life. MAH refers to fluids being given to patients via intravenous, subcutaneous, and enteral routes (Kingdon 2021; Ruegger 2015). There is no uniformity in prior literature as to how much fluid or what type of fluid should be given in this population (Forbat 2017). MAH is indicated when it is considered that a person's oral intake has fallen below threshold to maintain their hydration status, and where this could be detrimental to their QoL. The alternative to using MAH is standard care which is ensuring good oral care to prevent dryness in the mouth leading to symptoms of thirst (Druml 2016); this can be performed by both clinical staff or by carers and family members.
How the intervention might work
The aim of providing MAH is to improve QoL and to prolong survival in people receiving palliative care. QoL may be impaired by several mechanisms that can arise from or be caused by reduced oral intake including electrolyte imbalance, high urea and hypercalcaemia, accumulation of medications, such as opiates and anticholinergics, which can lead to drowsiness and myoclonus, as well as dehydration symptoms such as thirst and fatigue. Commencing a patient on MAH could help to reverse these processes and improve their QoL (Davies 2018; Krishna 2010).
When a person is no longer maintaining an adequate oral intake, there are concerns as to whether this will accelerate the dying phase or whether they will die from complications of their hydration status as opposed to the disease itself. It remains unclear whether MAH can enable people to live longer and improve their quality of life. However, these potential benefits need to be balanced against the adverse effects of any medical intervention used in this setting (Nakajima 2014b; Oehme 2018).
Why it is important to do this review
At present there is a lack of good‐quality evidence to support the use of MAH in adults receiving palliative care, with limited current guidelines to direct clinical management (Canihuante 2018; Hui 2015).
There remains variation in practice amongst clinicians, with no clear guidance over how MAH should be administered. Physicians' opinions around the role of MAH differ, with some believing it alleviates the symptoms of dehydration and has possible survival benefits, and others who are opposed to use of the therapy (Hui 2015; Oehme 2018; Raijmakers 2011). This second group worry that MAH may result in additional side effects for the patient, which may increase symptomatology and distress, without any perceived benefit. The most concerning of these is fluid overload, which may result in generalised oedema or complications, such as ascites and increased respiratory secretions (Hui 2015; Lokker 2019; Oehme 2018).
Previous studies have demonstrated wide variations in the number of people who receive MAH at the end of life, with prevalence estimates of the use of MAH ranging between 2% and 59% (Fritzson 2015; Krishna 2010; NICE 2017; O'Connor 2015).
The type of fluid used and quantity required also vary in practice (Canihuante 2018; Hui 2015; Nakajima 2014b). If MAH shows potential to improve QoL, then clarification regarding this will be beneficial.
Adults receiving palliative care can be cared for in inpatient, hospice, and community settings; however, few reviews have looked at whether MAH can be managed effectively in the community (Kingdon 2021).
Discussions around MAH can be emotive and cause distress to family, friends, and even patients, with concerns that not giving this will cause the patient to die due to complications of their hydration status and in discomfort. Having robust evidence to help guide such conversations would benefit both relatives and physicians in these discussions (Hui 2015; Poulose 2010).
As adults receiving palliative care progress closer to end of life, they often have a reduced level of consciousness and lose the ability to consent to treatment. Consequently, discussions about the benefits and risks of MAH are important to have prior to the patient losing the ability to consent or to ascertain who has been appointed to make decisions on their behalf (Druml 2016).
This review evaluated the existing literature to determine whether providing MAH for adults receiving palliative care leads to improved QoL. Secondary outcomes included evaluation of whether there is any increased harm attributable to this intervention, and whether there is any evidence that MAH improves survival.
There is a separate review considering medically assisted nutrition for adults receiving palliative care (Good 2014).
Objectives
To determine the effectiveness of medically assisted hydration (MAH) compared with placebo and standard care, in adults receiving palliative care on their quality of life (QoL) and survival, and to assess for potential adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that examined MAH compared with placebo or standard care. Randomised trials are the best design to minimise bias when evaluating the effectiveness of an intervention.
Types of participants
Adults aged 18 years and older receiving palliative care in any setting such as the home, hospice or hospital (WHO 2021), whose prognosis was limited and the focus of care was QoL (Doyle 2004). Diagnoses included (but were not limited to) incurable cancer, dementia, neurodegenerative diseases (e.g. motor neuron disease), HIV, chronic airways limitation, and chronic heart failure. We did not limit included participants to those in the terminal phase of their illness. We excluded participants who were having MAH as part of a perioperative, chemotherapy, or radiotherapy regimen, or because of chemotherapy or radiotherapy adverse effects.
Types of interventions
Medically assisted administration of fluids
Medically assisted administration of non‐nutritional fluids, administered via the subcutaneous tissue, venous system, or enterally (nasogastric tube, jejunostomy, gastrostomy).
Comparisons
Placebo, such as sham treatment with non‐therapeutic amounts of fluid.
Standard care, including regular oral care to prevent dryness in the mouth.
Types of outcome measures
We anticipated that studies on the effect of MAH in adults receiving palliative care may use a variety of outcome measures and included any study that reported any of the following outcome measures.
Primary outcomes
Quality of life, using validated scales, such as the Functional Assessment of Chronic Illness Therapy (FACIT), Functional Assessment of Cancer Therapy ‐ General (FACT‐G), or the European Organisation for Research and Treatment of Cancer (EORTC) Quality of life in people in palliative cancer care (QLQ‐C15‐PAL).
Secondary outcomes
Survival, measured from study enrolment to last date of follow‐up or death.
Adverse events. We reported any adverse events relating to MAH, including, but not limited to, pain and erythema at the treatment site, localised oedema, and generalised oedema due to fluid overload, such as ascites and increased respiratory secretions.
Search methods for identification of studies
Electronic searches
We searched the following databases without language restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL) searched up to 17 November 2022 (Appendix 1)
MEDLINE (Ovid) 1966 to 17 November 2022 (Appendix 2)
Embase (Ovid) 1980 to 17 November 2022 (Appendix 3)
Science Citation Index (ISI Web of Science) 1900 to 17 November 2022 (Appendix 4)
CINAHL (EBSCO) (Cumulative Index to Nursing and Allied Health Literature) 1982 to 17 November 2022 (Appendix 5)
CANCERLIT (up to November 2022)
CareSearch ‐ database listing conference proceedings and grey literature (up to November 2022)
Dissertation Abstracts (up to November 2022)
Searching other resources
For this update, we searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) for ongoing trials. In addition, we searched grey literature, key textbooks, checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We attempted to contact study authors for further details where only abstracts were published and for unpublished and ongoing trials. We contacted study authors for additional information where necessary. The search strategy was developed by the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS Review Group) Information Specialist and was independently peer reviewed. The PaPaS Information Specialist performed the searches.
Data collection and analysis
Selection of studies
Three review authors (PG, EB, WS) independently screened the title and abstract for each of the references identified in the literature search. Two review authors (PG, EB) read the full texts to assess eligibility based on the pre‐existing inclusion criteria. Any discrepancies between review authors were discussed and agreement reached by consensus. The selection process is recorded in a PRISMA flow diagram (Figure 1) (Moher 2009). We included studies in the review irrespective of whether measured outcome data were reported in a 'usable' way. Studies that were excluded from the review are recorded in the Characteristics of excluded studies table.
1.
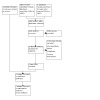
Study flow diagram.
Data extraction and management
Two review authors (PG, EB) independently extracted data using a standard, piloted form and checked for agreement before entry into Review Manager Web (RevMan Web 2022); the review authors also checked that the data had been entered correctly. In the event of disagreement, a third review author adjudicated (WS). We collated multiple reports of the same study so that each study, rather than each report, was the unit of interest. We collected characteristics of the included studies in sufficient detail to populate a Characteristics of included studies table.
We extracted the following information for each study.
Study methods (study design, allocation, blinding, setting, inclusion criteria)
Participants (sample size, exclusions/inclusions, number, disease, duration of study, prognosis, withdrawals and dropouts, site, e.g. hospital, hospice, home)
Intervention (type, route of delivery, control used)
Outcomes (QoL, survival, adverse events) including measures and time points
Numerical data for outcomes of interest
Adverse effects
Notes: study funding sources and study authors' declarations of interest
Assessment of risk of bias in included studies
Two review authors (PG, EB) independently assessed the trials based on the inclusion criteria. Any disagreements were discussed and resolved through consultation with a third review author (WS). We assessed all trials meeting the inclusion criteria using the Cochrane risk of bias assessment tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We completed a risk of bias table for each included study using the risk of bias tool. For each study, we assessed the risk of bias for the following domains.
Random sequence generation (checking for selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); or unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
Blinding of participants and personnel (checking for performance bias). We assessed the methods used to blind study participants and personnel from the knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how this was achieved). We assessed studies that were not double‐blinded as at high risk of bias.
Blinding of outcome assessment (checking for detection bias). We assessed the methods used to blind study participants and outcome assessors from the knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study had a clear statement that outcome assessors were unaware of treatment allocation, and ideally described how this was achieved); unclear risk of bias (study stated that outcome assessors were blind to treatment allocation but lacked a clear statement on how this was achieved). We assessed studies where outcome assessment was not blinded as at high risk of bias.
Incomplete outcome data (checking for attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or investigators used 'baseline observation carried forward' analysis, or both); unclear risk of bias (investigators used 'last observation carried forward' analysis); or high risk of bias (investigators used 'completer' analysis).
Selective reporting (checking for reporting bias). We assessed whether primary and secondary outcome measures were prespecified, and whether they were consistent with those reported. We assessed the methods as: low risk of bias (the study's prespecified outcomes were clear, and all expected outcomes of interest to the review were reported); high risk of bias (not all of the study's prespecified primary outcomes had been reported, or outcomes of interest were reported incompletely, or the primary and secondary outcome measures were not prespecified); unclear risk of bias (information insufficient to permit a judgement of low or high risk of bias).
Measures of treatment effect
For continuous outcomes, we measured the arithmetic mean and standard deviation (SD), and reported the mean difference (MD) with 95% confidence interval (CI) between groups. For dichotomous outcomes, we estimated and compared the risk ratio (RR) with 95% CIs between groups. For time‐to‐event (survival) data, we planned to calculate the survival time from the date of randomisation and to estimate and express the intervention effect as the hazard ratio (HR). In studies that performed multivariate survival analyses, using Cox proportional hazards regression models, and that reported HRs and CIs, we utilised these as summary data for describing trial findings.
Unit of analysis issues
We only included studies in which randomisation was by the individual participant. For trials containing multiple arms, we planned to include pair‐wise comparisons of each intervention arm to the control arm. For cluster‐randomised trials, we planned to seek direct estimates of the effect from an analysis that accounted for the cluster design. When the analysis in a cluster‐randomised trial did not account for the cluster design, we planned to use the approximately correct analysis approach, as presented in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a).
Dealing with missing data
Missing participant data were accounted for via the 'incomplete outcome data' domain of the risk of bias assessment, as described in Chapter 8 of the Cochrane Handbook (Higgins 2017). We contacted trial authors to request any missing numerical data.
Assessment of heterogeneity
We planned to assess heterogeneity using the I² statistic; however, there were insufficient data available to do so. We considered I² statistic to quantify heterogeneity, where values above 50% represented substantial heterogeneity, as per Chapter 10 of the Cochrane Handbook (Higgins 2021a). We planned to assess potential sources of heterogeneity through subgroup analyses; however, there were insufficient data available to do so.
Assessment of reporting biases
We planned to assess funnel plots if there were sufficient studies to support this (more than 10 studies for any outcome). However, given that we only identified four studies, we were unable to test for funnel plot asymmetry.
Data synthesis
We used Review Manager Web for data synthesis (RevMan Web 2022). For continuous outcomes, we planned to measure arithmetic mean and SD, and express effect sizes using the MD (where all studies utilised the same measurement scale), or the SMD (where studies used different scales), with 95% CIs. We planned to use a random‐effects model if there was significant clinical or statistical heterogeneity (or both). We considered I² values above 50% to represent substantial heterogeneity. For dichotomous outcomes, we synthesised outcomes by estimating and comparing the RR with 95% CIs. For time‐to‐event data, we planned to calculate the survival time from the date of randomisation and to estimate and express the intervention effect as the HR.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis based on: type of participant (cancer, non‐cancer, dementia, neurodegenerative diseases), intervention (intravenous, subcutaneous, enteral MAH), timing of intervention (in relation to death), and study site; however, there were insufficient data available to do so.
Sensitivity analysis
We planned to perform sensitivity analysis to determine the impact of including and excluding studies with a high risk of bias, and of using a fixed‐effect model.
Summary of findings and assessment of the certainty of the evidence
Two review authors (EB, AH) independently conducted the GRADE assessment using GRADEpro GDT and the guidelines provided in Chapter 14 of the Cochrane Handbook (GRADEpro GDT; Higgins 2021a). Any disagreements were discussed and resolved through consultation with a third review author (PG). We used GRADE to assess the certainty of the available evidence. The GRADE approach employs five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. We justified all decisions to downgrade the certainty of the evidence using footnotes. The GRADE system uses the following criteria to assign the level of certainty.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The GRADE system also uses the following criteria to assign a level of certainty to a body of evidence, per Chapter 14 of the Cochrane Handbook (Higgins 2021a).
High: randomised trials; or double‐upgraded observational studies.
Moderate: downgraded randomised trials; or upgraded observational studies.
Low: double‐downgraded randomised trials; or observational studies.
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series, case reports.
Factors that may decrease the level of certainty of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity, or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide CIs);
high probability of publication bias.
Factors that may increase the level of certainty of a body of evidence are:
large magnitude of effect;
all plausible confounding would reduce a demonstrated effect, or suggest a spurious effect when results show no effect;
dose‐response gradient.
We downgraded the level of certainty by one (−1) or two (−2), up to a maximum of −3, to very low, if we identified:
serious (−1) or very serious (−2) limitations to study quality;
important inconsistency (some (−1) or serious (−2));
some (−1) or major (−2) uncertainty about directness;
imprecise or sparse data (some (−1) or serious (−2));
high probability of reporting bias (−1).
Where meta‐analysis was not possible, we applied the GRADE domains (methodological limitations of studies, indirectness, imprecision, inconsistency, and likelihood of publication bias) guided by Murad 2017.
Summary of findings table
We included two summary of findings tables, one comparing MAH with placebo, and one comparing MAH with standard care, to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the certainty of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes QoL, survival, and adverse events.
Results
Description of studies
A description of included and excluded studies is provided in the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
For a full description of our screening process, see the study flow diagram (Figure 1). We used Covidence software to manage study screening and data collection and extraction (Covidence). The main database searches were conducted in January 2021 and updated in November 2022 (see Electronic searches) and retrieved 2928 records after de‐duplication, of which 2876 were excluded at the title and abstract screening stage. After full‐text screening of the remaining 52 records, we excluded 51 records of 51 studies (see Characteristics of excluded studies and Excluded studies for details). No studies were awaiting classification or ongoing. We included one new study, which together with the three studies included in the previous version of the review amounted to four records describing four unique studies.
The final review includes four unique published studies that randomised a total of 422 participants.
Included studies
We included four studies (one new at this update) with a total of 422 participants, of which 384 participants were available for analysis (Bruera 2005; Bruera 2013; Cerchietti 2000; Davies 2018). All of the included studies were published in English. A comprehensive description of these studies can be found in the Characteristics of included studies table.
Study design
Two studies compared MAH to placebo (Bruera 2005; Bruera 2013), and two studies compared MAH to standard care (Cerchietti 2000; Davies 2018). Three studies were multicentre studies either set in a hospital or hospice. One RCT was a feasibility study (Davies 2018).
Study population and setting
All participants in the included studies had advanced cancer, with no studies identified that included participants with non‐malignant conditions. The studies were conducted in countries around the world, including the UK, the USA, and Argentina. The participants were adults aged 28 to 98 years old. Two studies were open to people over the age of 16 years (Bruera 2005; Davies 2018), but no one under 18 years was recruited. Of the included participants, 226 were female and 196 were male. In three of the studies, participants were only included if it was thought that they were dehydrated (Bruera 2005; Bruera 2013; Cerchietti 2000). Cerchietti 2000 was the only single‐site study; the other three studies were all multisite and based in inpatient palliative care hospital units, oncology hospital units, and hospices (Bruera 2005; Bruera 2013; Davies 2018).
Intervention
The routes of administration were either intravenous or subcutaneous, and MAH was given as either boluses or continuous infusions. The amount of fluid given varied amongst the included studies. Davies 2018 based the amount of fluid given on participant weight with a scale of 1, 1.5, and 2 litres per day. All other studies administered at least 1000 mL of fluid per day in the treatment arm. For studies comparing MAH to placebo, the placebo consisted of 100 mL normal saline administered over four hours. For studies comparing MAH to standard care, standard care included ensuring good oral care to prevent dryness in the mouth.
Study size
The four included studies enrolled 422 participants, of whom 384 were included in the analyses. Trial size ranged from 42 to 200 participants.
Study duration
For the primary outcome, the study duration varied, ranging from two days, Bruera 2005; Cerchietti 2000, to one week, Bruera 2013, and 14 days, Davies 2018.
Outcome measures
Of the four included studies, one study measured QoL using the FACT‐G (Bruera 2013). Two studies reported survival (Bruera 2013; Davies 2018). One study comparing MAH with placebo reported univariate survival analysis, using log‐rank test‐based comparison of Kaplan‐Meier survival curves, from study enrolment to last date of follow‐up or death (Bruera 2013). One study comparing MAH with standard care reported multivariate survival analyses, using Cox proportional hazards regression models, from randomisation to follow‐up at 14 days (Davies 2018). Three studies reported on adverse events (Bruera 2005; Cerchietti 2000; Davies 2018). One study comparing MAH with placebo reported on intensity of adverse events using a numeric rating scale (NRS) from 0 to 10 (lower score = less toxicity) (Bruera 2005). Two studies comparing MAH with standard care reported on any adverse events relating to MAH, including pain and erythema at the treatment site, localised oedema, and generalised oedema due to fluid overload, such as increased respiratory secretions (Cerchietti 2000; Davies 2018).
Excluded studies
We excluded 51 full‐text articles from the review. The majority of these (46) were due to wrong study design. Three articles were duplicate articles, and a further two articles evaluated the wrong intervention. The reasons for exclusion are documented in the Characteristics of excluded studies table.
Risk of bias in included studies
We assessed the risk of bias of each study using the Cochrane risk of bias tool. We presented the overall findings in a risk of bias graph (Figure 2), which illustrates the review authors' judgements about each risk of bias domain as percentages across all included studies. Review authors' judgements about each risk of bias domain for each included study are shown in the risk of bias summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We assigned three studies to low risk of bias for random sequence generation (Bruera 2005; Bruera 2013; Davies 2018). Bruera 2005 used random numbers to determine the treatment allocation, with the code kept confidential until the study ended. Bruera 2013 used computer‐generated randomisation. Davies 2018 used computer‐generated randomisation, and the randomisation was co‐ordinated by the clinical trials unit. We assessed one study as at unclear risk of bias because they failed to provide sufficient details on the process (Cerchietti 2000).
Allocation concealment
We assessed two studies as at low risk of bias for allocation concealment (Bruera 2005; Bruera 2013). One study used sealed envelopes (Bruera 2005), and the other study had 1:1 randomisation performed by the study pharmacist (Bruera 2013). We assessed the two remaining studies as at unclear risk of bias because they failed to provide sufficient details on the process (Cerchietti 2000; Davies 2018).
Blinding
Blinding of participants and personnel (performance bias)
We assessed Bruera 2005 and Bruera 2013 as at low risk of bias, as in both studies there was blinding of participants and personnel. No information was given on blinding for Cerchietti 2000, which was therefore assessed as at unclear risk of bias. We assessed Davies 2018 as at high risk of bias as there was no blinding of participants and personnel.
Blinding of outcome assessment (detection bias)
We assessed two studies that reported blinding until the end of the study period as at low risk of bias (Bruera 2005; Bruera 2013). No blinding was described in Cerchietti 2000. We assessed the remaining study as at high risk of bias as there was no blinding of outcome assessment (Davies 2018).
Incomplete outcome data
We assessed three studies as being at low risk of attrition bias (Bruera 2005; Bruera 2013; Davies 2018), as either dropout rates were < 10% with reasons given for the dropouts, or dropouts were balanced across groups. We assessed the remaining study as at unclear risk of bias, as no data were given on participant dropout rate (Cerchietti 2000).
Selective reporting
There were no omissions in reporting identified in three of the included studies, and expected outcomes were consistent with those listed in the methods and were reported on for all participants (Bruera 2005; Bruera 2013; Davies 2018). We assessed one study as being at unclear risk of bias as the information presented was insufficient to permit a judgement of low or high risk (Cerchietti 2000).
Other potential sources of bias
We noted no other sources of significant bias in the included studies.
Effects of interventions
Comparison 1: MAH compared with placebo
See Table 1.
Two studies compared MAH with placebo, including data from Bruera 2005 (n = 49) and Bruera 2013 (n = 93).
1.1 Quality of life
One study measured change in QoL at one week using FACT‐G (scale from 0 to 108; higher score = better QoL) (Bruera 2013). There was no clear evidence for an effect (mean difference (MD) 4.10, 95% confidence interval (CI) −1.63 to 9.83; P = 0.16) (Table 3). No data were available for QoL for the other study (Bruera 2005). We are uncertain whether MAH improves QoL because the certainty of the evidence is very low (see Table 1), downgraded one level for indirectness (studies only included participants with advanced cancer) and two levels for very serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
1. Comparison 1: MAH versus placebo (quality of life from single‐study data).
| Outcome | MAH | Placebo | MD | Study ID | |||||
| Mean | SD | n | Mean | SD | n | MD (95% CI) | P value | ||
| Quality of life at 1 week | |||||||||
| FACT‐G (scale from 0 to 108; higher score = better QoL) | 6.7 | 11.2 | 44 | 2.6 | 16.7 | 49 | 4.10 (−1.63 to 9.83) | 0.16 | Bruera 2013 |
CI: confidence interval; FACT‐G: Functional Assessment of Cancer Therapy ‐ General; MAH: medically assisted hydration; MD: mean difference; QoL: quality of life; SD: standard deviation
1.2 Survival
One study measured survival from study enrolment to last date of follow‐up or death using Log‐rank analysis (Bruera 2013). We were unable to estimate hazard ratio (HR) from the data. There was no clear evidence for an effect. No data were available for survival for one study (Bruera 2005). We are uncertain whether MAH improves survival because the certainty of the evidence is very low (see Table 1), downgraded one level for indirectness (studies only included participants with advanced cancer) and two levels for very serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
1.3 Adverse events
One study measured adverse events at two days using NRS (scale from 0 to 10; lower score = less toxicity) (Bruera 2005). There was no clear evidence for pain (MD 0.35, 95% CI −1.19 to 1.89; P = 0.66) or swelling (MD −0.59, 95% CI −1.40 to 0.22; P = 0.16) at the injection site (Table 4). No data were available for adverse events for the other study (Bruera 2013). We are uncertain whether MAH leads to adverse events because the certainty of the evidence is very low (see Table 1), downgraded one level for indirectness (studies only included participants with advanced cancer) and two levels for very serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
2. Comparison 1: MAH versus placebo (adverse events from single‐study data).
| Outcome | MAH | Placebo | MD | Study ID | |||||
| Mean | SD | n | Mean | SD | n | MD (95% CI) | P value | ||
| Pain at the injection site at 2 days | |||||||||
| NRS (scale from 1 to 10; lower score = less toxicity) | 2.10 | 2.95 | 27 | 1.75 | 2.55 | 22 | 0.35 (−1.19 to 1.89) | 0.66 | Bruera 2005 |
| Swelling at the injection site at 2 days | |||||||||
| NRS (scale from 1 to 10; lower score = less toxicity) | 0.82 | 1.13 | 27 | 1.41 | 1.66 | 22 | −0.59 (−1.40 to 0.22) | 0.16 | Bruera 2005 |
CI: confidence interval; MAH: medically assisted hydration; MD: mean difference; NRS: numeric rating scale; SD: standard deviation
Comparison 2: MAH compared with standard care
See Table 2.
Two studies compared MAH with standard care, including data from Cerchietti 2000 (n = 42) and Davies 2018 (n = 200).
2.1 Quality of life
No data were available for this outcome for either study (see Table 2) (Cerchietti 2000; Davies 2018).
2.2 Survival
One study measured survival from randomisation to last date of follow‐up at 14 days or death using a Cox regression model (Davies 2018). The reported HR for survival at three days in the MAH group was 0.36 (95% CI 0.22 to 0.59). HR was only reported for survival at three days. No data were available for survival for the other study (Bruera 2013). We are uncertain whether MAH improves survival because the certainty of the evidence is very low (see Table 2), downgraded one level for indirectness (studies only included participants with advanced cancer), one level for serious study limitations (lack of blinding), and one level for serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
2.3 Adverse events
Two studies involving 242 participants measured adverse events at follow‐up (range 2 to 14 days) (Cerchietti 2000; Davies 2018). None of 149 participants receiving standard care, and 7 of 93 participants receiving MAH experienced adverse events (risk ratio 11.62, 95% CI 1.62 to 83.41; Analysis 1.1, Figure 4). We are uncertain whether MAH leads to adverse events because the certainty of the evidence is very low (see Table 2), downgraded one level for indirectness (studies only included participants with advanced cancer), one level for serious study limitations (lack of blinding), and one level for serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
1.1. Analysis.

Comparison 1: Comparison 2: MAH versus standard care, Outcome 1: Adverse events
4.

Forest plot: medically assisted hydration versus standard care: 1.1: adverse events.
Discussion
Summary of main results
We included four studies in the review with a total of 422 participants, of whom data were available for analysis from 384 participants, with the aim of determining if there was an effect of MAH on QoL, survival, and potential for adverse effects in adults receiving palliative care (Bruera 2005; Bruera 2013; Cerchietti 2000; Davies 2018). We identified one new study, Davies 2018, in addition to the three studies included in previous reviews (Bruera 2005; Bruera 2013; Cerchietti 2000). The included studies compared MAH with placebo (Bruera 2005; Bruera 2013), and MAH with standard care (Cerchietti 2000; Davies 2018). The following conclusions regarding the effectiveness of MAH compared with placebo and standard care on QoL, survival, and potential for adverse events in adults receiving palliative care should be interpreted taking into consideration the small number of eligible studies and the small numbers of participants in each treatment arm. We assessed the certainty of evidence as very low for studies comparing MAH with placebo due to indirectness and imprecision, and very low for studies comparing MAH with standard care due to indirectness, study limitations, and imprecision.
There were insufficient data to undertake meta‐analyses of the primary outcome of QoL or the secondary outcome of survival.
We are uncertain whether MAH improves QoL when compared to placebo because the certainty of the evidence is very low. No data were available for QoL for studies comparing MAH with standard care.
We are uncertain whether MAH improves survival when compared to placebo or standard care because the certainty of the evidence is very low.
We are uncertain whether MAH leads to adverse events when compared to placebo because the certainty of the evidence is very low. Whilst we were able to undertake a meta‐analysis for adverse events for the comparison MAH versus standard care, we are uncertain whether MAH leads to adverse events because the certainty of the evidence is very low.
There were insufficient data to evaluate different subgroups such as type of participant, intervention, timing of intervention, and study site.
Overall completeness and applicability of evidence
We identified only four studies for inclusion in the review, of which two compared MAH with placebo (Bruera 2005; Bruera 2013), and two compared MAH with standard care (Cerchietti 2000; Davies 2018); one of the RCTs was a feasibility study (Davies 2018). All participants were inpatients in a hospital or hospice setting with advanced cancer at end of life. There were no studies on adults receiving palliative care for non‐cancer, dementia, or neurodegenerative diseases, and no studies including participants in community settings. In terms of the intervention used, with the exception of two studies (Bruera 2005; Bruera 2013), there was no uniformity as to how much MAH was administered. This included types of fluid used, amount, and how quickly it was administered. The optimum route of administration was also not defined, with all studies using MAH via either the subcutaneous or intravenous routes. No studies used the enteral route for MAH.
We could not undertake meta‐analyses for the primary outcome of QoL or the secondary outcome of survival due to the paucity of data. Only one study reported QoL using validated scales (Bruera 2013). A variety of outcome measures were used in the studies to determine the effectiveness of MAH compared with placebo or standard care, including measurement of symptoms such as delirium (Bruera 2013; Cerchietti 2000; Davies 2018), hallucinations (Bruera 2005; Bruera 2013), myoclonus (Bruera 2005; Bruera 2013), fatigue (Bruera 2005; Bruera 2013), drowsiness (Bruera 2013), and well‐being (Bruera 2005; Bruera 2013). Only two studies determined survival (Bruera 2013; Davies 2018), using different methods, and only three studies reported adverse events (Bruera 2005; Cerchietti 2000; Davies 2018), with only local adverse reactions at the site of injection predefined at the start of the study in Bruera 2005. The variety in outcome measures and tools used in the included studies highlights the need for a standardised, validated measure for assessing the use of MAH in adults receiving palliative care. It is also important to highlight that alleviation of symptoms does not necessarily equate or have a linear relationship to improvement in QoL.
We were unable to undertake subgroup analysis based on the type of participant, intervention, timing of intervention in relation to death, and study site as there were too few included studies. As all participants were inpatients with advanced cancer at end of life, our findings are not transferable to adults receiving palliative care in other settings, for non‐cancer, dementia, or neurodegenerative diseases, or for those with an extended prognosis.
This review shows that there are few RCTs evaluating MAH for adults receiving palliative care for the outcomes QoL, survival, and adverse effects, and that the available RCTs are compounded by poor certainty of evidence.
Quality of the evidence
Only one study measured QoL using validated scales (Bruera 2013). We assessed the certainty of the evidence for QoL for the comparison MAH versus placebo to be very low, downgraded one level for indirectness (studies only included participants with advanced cancer) and two levels for very serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm). No studies comparing MAH with standard care measured QoL (Cerchietti 2000; Davies 2018).
We assessed the certainty of the evidence for the secondary outcomes of survival and adverse events for the comparison MAH versus placebo to be very low, downgraded one level for indirectness (studies only included participants with advanced cancer) and two levels for very serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm). We assessed the certainty of the evidence for survival and adverse events for the comparison MAH versus standard care to be very low, downgraded one level for indirectness (studies only included participants with advanced cancer), one level for serious study limitations (lack of blinding), and one level for serious imprecision (the analysis included small studies with wide CIs, including both appreciable benefit and harm).
It was therefore difficult to draw any conclusions given the current lack of robust evidence.
Potential biases in the review process
To minimise bias, three review authors independently assessed the search results, and two review authors performed data extraction and risk of bias assessment.
Agreements and disagreements with other studies or reviews
The existing literature surrounding the use of MAH for adults receiving palliative care is nonconclusive. It is largely consistent with our conclusion that there is insufficient evidence to either support or refute the use of MAH for adults receiving palliative care to improve QoL or survival. Most reviews recognise the poor certainty of evidence in the limited number of studies available and suggest further large‐scale, well‐designed studies.
Kingdon 2021 performed a systematic review of MAH in adults receiving palliative care but included prospective controlled studies and cohort studies as well as RCTs. Whilst including these studies, they reached the same conclusion that there is no current evidence to support the use of MAH in the palliative population for symptom control.
Canihuante 2018 identified systematic reviews on the use of MAH in adults receiving palliative care, finding four systematic reviews including three RCTs (Bruera 2005; Bruera 2013; Cerchietti 2000). The GRADE approach was also used, which agreed that the certainty of the evidence was very low for clinical dehydration improvement, thirst, delirium, local adverse effects, and low for survival and QoL. A potential difference in the grading may be due to the inclusion of non‐RCTs in the analysis; however, there was agreement in the finding that there is no clear evidence for use of MAH in this population.
Forbat 2017 reviewed the use of subcutaneous fluid in people with advanced illness. Of the 14 studies identified, eight were in the palliative population. The review was in agreement that there is a lack of documentation of the rate, volume, and frequency of fluid given in this population, which contributes to issues in developing guidelines.
Authors' conclusions
Implications for practice.
For adults receiving palliative care
There is insufficient evidence to either support or refute the use of medically assisted hydration (MAH) for adults receiving palliative care to improve quality of life (QoL) or survival.
For clinicians
We found insufficient evidence to either support or refute the use of MAH for adults receiving palliative care in terms of improving QoL and survival. There was also insufficient evidence on the risk of adverse events. Consequently, at present clinicians will need to make decisions based on the perceived benefits and harms of MAH for each individual's circumstances, without the benefit of high‐quality evidence to guide them.
For policymakers
There is insufficient evidence to support or refute the use of MAH in improving QoL or survival in adults receiving palliative care.
For funders of the intervention
There is insufficient evidence to support or refute the use of MAH in improving QoL or survival in adults receiving palliative care.
Implications for research.
Study design
High‐quality studies in the palliative care population remain difficult to perform successfully. The difficulty of research in a vulnerable population such as palliative care has been discussed in the literature. These difficulties start with consent, are followed by recruitment, elimination of confounders, and end with retention of participants throughout a study period (Rinck 1997). Ethical considerations such as consent and use of a placebo in trial design and conduct need to be carefully considered. There have been some innovative suggestions about how to overcome the issue of consent (Rees 2003), including the appointment of a 'personal consultee' such as a relation or friend, or a 'nominated consultee' such as a person independent to the study, to act in the participant's best interest (Davies 2018). Recruitment and retention have often been difficult to achieve in a population that can have rapid deterioration in their clinical condition, with most studies having small numbers of participants or falling short of their predetermined sample size (Bruera 2005; Oh 2015). There are also divergent views on the use of MAH in palliative care, which reflects the limited number of randomised controlled trials found. There remains a lack of standardisation of MAH used. All included studies used MAH either via the subcutaneous or intravenous routes. We found no studies using the enteral route for MAH. There was no standardisation in the amount of fluid or type of fluid that was given to the people receiving palliative care (Forbat 2017). In future studies it will be important to include some discussion about the fidelity of the interventions. Further studies are therefore needed to determine the optimum route and dosage.
Participant groups
The studies included in this review had narrowly defined patient populations. Palliative care is performed in hospitals, inpatient palliative care units, and in the community. Studies need to be performed in all these areas to allow external validity to different palliative care populations. It would also be helpful to define at what stage of their illness participants are given MAH. The reasons and aims of hydration in the last few days/weeks of life may be very different to those participants with a longer prognosis. An agreed‐upon diagnostic criteria for hydration status is essential for future trials, both to assess at entry and as an outcome. The prospective prediction of prognosis is difficult, and there is limited evidence to suggest that it may be better to stratify participants according to their performance status. In addition, all participants in the included studies were inpatients with advanced cancer, and it is important to examine MAH in adults receiving palliative care for non‐cancer, dementia, or neurodegenerative diseases, and to include studies in community settings.
Outcomes
It is important that clinically relevant outcomes are clearly defined and that they are the most clinically useful. The variety in outcome measures and tools used in the studies highlights the need for a standardised, validated measure for assessing the use of MAH in adults receiving palliative care. Despite much controversy about the effect MAH may have on survival, this was only included as an outcome in two included studies. Future studies should include survival of participants as an outcome. It is equally important that adverse events are well‐defined.
What's new
| Date | Event | Description |
|---|---|---|
| 19 June 2023 | New search has been performed | This review has been updated to include the results of a new search on 17 November 2022. |
| 19 June 2023 | New citation required but conclusions have not changed | One new study added (200 participants). |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 14 December 2020 | Amended | Updated text and sorting through of errors and warnings |
| 24 November 2020 | New citation required but conclusions have not changed | Updated with no changes |
| 8 May 2015 | Review declared as stable | This review will be assessed for further updating in 2019. |
| 16 April 2014 | New citation required and conclusions have changed | The search for this review was re‐run in April 2013 and March 2014. No new studies were found. Minor change to conclusions. We recommend that readers of the original review read this latest version. |
| 9 January 2014 | New search has been performed | We added a PRISMA flowchart to document the study selection process and added risk of bias tables. |
| 14 February 2011 | New search has been performed | The search for this review was re‐run in February 2011. No new studies were identified for inclusion in the review. |
| 6 October 2010 | Amended | Contact details updated. |
| 6 August 2008 | Amended | Converted to new review format |
Acknowledgements
We wish to thank the Cochrane Pain, Palliative and Supportive Care Review Group for their ongoing support in updating this review.
John Cavenagh, Mark Mather, and Peter Ravenscroft were authors on the original review but did not contribute to this update.
Cochrane Review Group funding acknowledgement: this project was funded by the National Institute for Health and Care Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Editorial and peer‐reviewer contribution
Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS) supported the authors in the development of this Cochrane Review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Neil O'Connell, PaPaS Co‐ordinating Editor, and Reader at Brunel University London
Managing Editor (conducted editorial checks and supported the editorial team): Anna Erskine, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
Assistant Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Kerry Harding, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
Contact Editor (provided editorial guidance throughout): Sarah Yardley
Information Specialist (developing and running search strategies): Joanne Abbott, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
Copy Editor (copy‐editing and proofreading): Lisa Winer, Cochrane Copy Edit Support
Peer reviewers (provided comments and recommended an editorial decision): Professor Emeritus Sam H Ahmedzai FRCP (clinical/content review), Bridget Candy, Honorary Senior Fellow, Marie Curie Research Department, UCL Division of Psychiatry, London (clinical/content review), Brian Duncan (consumer review), Nuala Livingstone (methods review), Adrian Tookman, Palliative Medicine Clinician (Retired) (clinical/content review)
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Palliative Care] explode all trees
#2 palliat*:ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Terminally Ill] this term only
#4 MeSH descriptor: [Terminal Care] explode all trees
#5 (terminal* near/6 care*):ti,ab,kw (Word variations have been searched)
#6 ((terminal* near/6 ill*) or terminal‐stage* or dying or (close near/6 death)):ti,ab,kw (Word variations have been searched)
#7 (terminal* near/6 disease*):ti,ab,kw (Word variations have been searched)
#8 (end near/6 life):ti,ab,kw (Word variations have been searched)
#9 hospice*:ti,ab,kw (Word variations have been searched)
#10 ("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage"):ti,ab,kw (Word variations have been searched)
#11 "advanced disease*":ti,ab,kw (Word variations have been searched)
#12 ("incurable illness*" or "incurable disease*"):ti,ab,kw (Word variations have been searched)
#13 ("advanced directive*" or "living will*" or "do‐not‐resuscitate order*"):ti,ab,kw (Word variations have been searched)
#14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 MeSH descriptor: [Fluid Therapy] this term only
#16 MeSH descriptor: [Dehydration] this term only
#17 (hydrat* or dehydrat* or rehydrat* or (fluid* near/6 therap*) or (fluid* near/6 balance*) or (fluid* near/6 manag*) or hypodermoclysis):ti,ab,kw (Word variations have been searched)
#18 #15 or #16 or #17
#19 #14 and #18
Appendix 2. MEDLINE search strategy
1 exp Palliative Care/
2 palliat*.tw.
3 Terminally Ill/
4 Terminal Care/
5 (terminal* adj6 care*).tw.
6 ((terminal* adj6 ill*) or terminal‐stage* or dying or (close adj6 death)).tw.
7 (terminal* adj6 disease*).tw.
8 (end adj6 life).tw.
9 hospice*.tw.
10 ("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage").tw.
11 "advanced disease*".tw.
12 ("incurable illness*" or "incurable disease*").tw.
13 ("advanced directive*" or "living will*" or "do‐not‐resuscitate order* ").tw.
14 or/1‐13
15 Fluid Therapy/
16 Dehydration/
17 (hydrat* or dehydrat* or rehydrat* or (fluid* adj6 therap*) or (fluid* adj6 balance*) or (fluid* adj6 manag*) or hypodermoclysis).tw.
18 15 or 16 or 17
19 14 and 18
Appendix 3. Embase search strategy
1 exp Palliative Care/
2 palliat*.tw.
3 Terminally Ill/
4 Terminal Care/
5 (terminal* adj6 care*).tw.
6 ((terminal* adj6 ill*) or terminal‐stage* or dying or (close adj6 death)).tw.
7 (terminal* adj6 disease*).tw.
8 (end adj6 life).tw.
9 hospice*.tw.
10 ("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage").tw.
11 "advanced disease*".tw.
12 ("incurable illness*" or "incurable disease*").tw.
13 ("advanced directive*" or "living will*" or "do‐not‐resuscitate order* ").tw.
14 or/1‐13
15 Fluid Therapy/
16 Dehydration/
17 (hydrat* or dehydrat* or rehydrat* or (fluid* adj6 therap*) or (fluid* adj6 balance*) or (fluid* adj6 manag*) or hypodermoclysis).tw.
18 15 or 16 or 17
19 14 and 18
Appendix 4. Science Citation Index (ISI Web of Science) search strategy
# 13 295 #12 AND #11
# 12 53,258 Topic=((hydrat* or dehydrat* or rehydrat* or (fluid* near/6 therap*) or (fluid* near/6 balance* ) or (fluid* near/6 manag* ) or hypodermoclysis))
# 11 37,433 #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
# 10 386 Topic=(("advanced directive*" or "living will*" or "do‐not‐resuscitate order*"))
# 9 412 Topic=(("incurable illness*" or "incurable disease*"))
# 8 3,316 Topic=("advanced disease*")
# 7 12,963 Topic=(("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage"))
# 6 2,135 Topic=(hospice*)
# 5 6,392 Topic=((end near/3 life))
# 4 1,176 Topic=((terminal* near/6 disease*))
# 3 1,527 Topic=((terminal* near/6 ill*))
# 2 906 Topic=((terminal* near/6 care*))
# 1 14,889 Topic=(palliat*)
Appendix 5. CINAHL search strategy
S23 S14 AND S22
S22 S15 OR S16 OR S17 OR S18 OR S19 OR S21
S21 (fluid* N6 manag* )
S20 (fluid* N6 balance* )
S19 (fluid* N6 therap*)
S18 hypodermoclysis
S17 (hydrat* or dehydrat* or rehydrat*)
S16 (MH "Dehydration")
S15 (MH "Fluid Therapy")
S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13 ("advanced directive*" or "living will*" or "do‐not‐resuscitate order*")
S12 ("incurable illness*" or "incurable disease*")
S11 "advanced disease*"
S10 ("end‐stage disease*" or "end stage disease* or end‐stage illness" or "end stage")
S9 hospice*
S8 (end n3 life)
S7 (terminal* N6 disease*)
S6 (terminal* N6 ill*)
S5 (terminal* N6 care*)
S4 (MH "Terminal Care+")
S3 (MH "Terminally Ill Patients+")
S2 palliat*
S1 (MH "Palliative Care")
Data and analyses
Comparison 1. Comparison 2: MAH versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adverse events | 2 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.62 [1.62, 83.41] |
Comparison 2. Analysis for Additional table 1. Comparison 1: MAH versus placebo (quality of life from single‐study data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Quality of life from single‐study data | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [‐1.63, 9.83] |
2.1. Analysis.

Comparison 2: Analysis for Additional table 1. Comparison 1: MAH versus placebo (quality of life from single‐study data), Outcome 1: Quality of life from single‐study data
Comparison 3. Analysis for Additional table 2. Comparison 1: MAH versus placebo (adverse events from single‐study data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Adverse events from single‐study data: pain at injection site | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐1.19, 1.89] |
| 3.2 Adverse events from single‐study data: swelling at injection site | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.40, 0.22] |
3.1. Analysis.

Comparison 3: Analysis for Additional table 2. Comparison 1: MAH versus placebo (adverse events from single‐study data), Outcome 1: Adverse events from single‐study data: pain at injection site
3.2. Analysis.

Comparison 3: Analysis for Additional table 2. Comparison 1: MAH versus placebo (adverse events from single‐study data), Outcome 2: Adverse events from single‐study data: swelling at injection site
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bruera 2005.
| Study characteristics | ||
| Methods | Randomised, controlled, double‐blind, multicentre trial Method of randomisation: truly random Study duration: 2 days | |
| Participants |
Sample size: 74 (13 were not eligible, 10 refused) 51 recruited |
|
| Interventions | 51 participants were randomised to 1 of 2 groups:
Route of administration: IV if IV access available (12 participants); subcutaneous if no IV access (37 participants) |
|
| Outcomes | Primary outcome:
Secondary method of analysis:
The target symptoms scored were also examined separately to look for individual improvement; these were sedation, fatigue, hallucinations, myoclonus, and a total aggregate score. MMSE Adverse effects |
|
| Identification | ||
| Notes | Some differences in performance status at randomisation, with intervention group having more participants in performance status 0, I, and II Study was underpowered, as recruitment was less than expected. Supported by the Brown Foundation, Houston, TX; and the Tobacco Settlement Foundation. No declarations of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random Numbers determining treatment allocation were calculated at M.D. Anderson Cancer Center ... and randomised code was kept confidential until the completion of the study" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes random numbers were delivered to the investigators in sealed enveloped" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind one investigator at each institution was unblinded and was responsible for preparing saline, droppers and pumps to maintain blinding" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigator at each institution responsible for assessing patient and the patient themselves were not aware of amount of fluid being administered" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient from each group did not complete the study due to family refusal in one case and...rapid deterioration in the other" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All initial outcomes reported. Comment: probably done |
Bruera 2013.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind, multicentre study Method of randomisation: computer‐generated simple randomisation scheme | |
| Participants | Advanced cancer (i.e. locally recurrent or metastatic disease) who were:
Sample size: 905 patients assessed for eligibility Excluded: 776 Included: 129 |
|
| Interventions | 129 participants were randomised to 1 of 2 groups.
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Identification | ||
| Notes | Underpowered ‐ powered for 150 participants, but recruitment stopped at 129 due to funding limitations Supported by University of Texas MD Anderson Cancer Center, Houston, TX 77030 Supported by Grant No: R01CA122292‐01 from National Cancer Institute National Institutes of Health grants National Cancer Institute grants No conflicts of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐generated simple randomization scheme" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "the study pharmacist randomly assigned patients in a 1:1 ratio to receive either 1,000 mL (hydration group) or 100 mL (placebo group)" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Blinding was achieved by having a separate infusion research nurse who was aware of the treatment assignment, set the infusion at the appropriate rate, covered and placed the infusion pump in a locked backpack, and started the infusion at the patient’s home each day. Blinding was further assured by the use of identical backpacks, counter weight (900 g) in the placebo backpack, and concealment of the rate of infusion on the infusion pump by a tap" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both the patient and research nurse conducting the study assessments were blinded to the study intervention and the randomization sequence" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient analysis presented in tabulated format with numbers given for patients who were not analysed in the final data; these were balanced across study arms. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All initial outcome measures reported. Comment: probably done |
Cerchietti 2000.
| Study characteristics | ||
| Methods | Randomised, comparative prospective, single‐centre trial Method of randomisation: unclear Blinding status: unclear Study duration: 48 hours | |
| Participants | Prognosis ‐ terminal‐stage advanced cancer patients ≥ 1 of the following symptoms: thirst; chronic nausea or delirium; dehydration diagnosed on physical examination, with or without renal failure; and inability to maintain an adequate water intake (< 50 mL/day fluid) Sample size: 50 Exclusions: 4 uncontrolled symptoms (pain in 2, severe dyspnoea in 2), 1 bowel obstruction syndrome requiring surgery, 3 severe constipation Participants were recruited from Argentina. |
|
| Interventions | 42 participants were randomised to 1 of 2 groups.
|
|
| Outcomes | Primary outcomes (VAS):
Secondary outcomes:
|
|
| Identification | ||
| Notes | No conflicts of interest declared. No grants or support reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", but method not adequately described. Comment: unclear if done |
| Allocation concealment (selection bias) | Unclear risk | Method used was not described. Comment: unclear if done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described. Comment: unclear if done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described. Comment: unclear if done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Method not described. Comment: unclear if done |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented. Comment: unclear if done |
Davies 2018.
| Study characteristics | ||
| Methods | Randomised control study Feasibility study Computer‐generated randomisation | |
| Participants | Inclusion criteria:
Exclusion criteria:
239 assessed and 39 excluded. 200 patients recruited to trial. A total of 12 sites used: 4 cancer care centres and 8 hospices all based in the UK Each site was allocated a specific intervention. Study ran from February 2015 to February 2016. |
|
| Interventions | Group A (127 participants)
Group B (73 participants)
|
|
| Outcomes | Primary outcome: feasibility study Secondary outcomes:
|
|
| Identification | Sponsorship source: University of Surrey Country: United Kingdom Setting: 4 cancer centres and 8 hospices Author's name: Andrew Davies Email: adavies12@nhs.net Address: Royal Surrey County Hospital, Egerton Road, Guildford, Surrey, GU2 7XX, UK |
|
| Notes | Funded by Research for Patient Benefit programme of the National Institute of Health Research in the UK No conflicts of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation process was co‐ordinated by the clinical trials unit, and the method utilised was computer generation using SAS Proc Plan (SAS Institute Inc., Cary, USA)" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Methodology unclear. Comment: unclear if done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was unblinded due to the nature of the two interventions" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The study was unblinded due to the nature of the two interventions" Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 200 patients were recruited to the study within 1 year ... and 199 (99.5%) participants completed the study. One patient was withdrawn due to an improvement in their condition" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All initial study outcomes were presented. Comment: probably not done |
FACIT‐F: Functional Assessment of Chronic Illness Therapy ‐ Fatigue; FACIT‐G: Functional Assessment of Chronic Illness Therapy ‐ General; IV: intravenous; MDAS: Memorial Delirium Assessment Scale; mEq: milliequivalents; MMSE: Mini Mental State Examination; QoL: quality of life; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arcand 2015 | Wrong study design |
| Bozzetti 2014 | Wrong study design |
| Bozzetti 2015 | Wrong study design |
| Brar 2018 | Wrong study design |
| Bruera 2010 | Duplicate |
| Bükki 2015 | Wrong study design |
| Cabañero‐Martínez 2019 | Wrong study design |
| Caccialanza 2018 | Wrong study design |
| Canihuante 2018 | Wrong study design |
| Chalany 2015 | Wrong study design |
| Chowdhury 2015 | Wrong study design |
| Dalal 2012 | Duplicate |
| Davies 2015 | Wrong study design |
| Druml 2016 | Wrong study design |
| Forbat 2017 | Wrong study design |
| Gent 2015 | Wrong study design |
| Good 2014 | Wrong study design |
| Grez 2017 | Wrong study design |
| Higashiguchi 2016 | Wrong study design |
| Ho 2015 | Wrong study design |
| Hui 2015 | Wrong study design |
| Ishiki 2013 | Wrong intervention |
| Ishiki 2015 | Wrong intervention |
| Lembeck 2016 | Wrong study design |
| Lokker 2019 | Wrong study design |
| Mayers 2019 | Wrong study design |
| Morita 2002 | Prospective controlled study examining fluid status of terminally ill cancer patients with intestinal obstruction. Excluded because there was no comparisons between groups with regards to hydration |
| Morita 2005 | Wrong study design |
| Morita 2006 | Multicentre, prospective, observational study examining artificial hydration therapy, laboratory findings, and fluid balance in terminally ill patients with abdominal malignancies. Excluded because there was no comparison of symptoms between the hydrated and non‐hydrated groups |
| Nakajima 2014a | Wrong study design |
| Nakajima 2014b | Wrong study design |
| Nakajima 2018 | Wrong study design |
| Nobuhisa 2018 | Wrong study design |
| Nwosu 2013 | Wrong study design |
| O'Connor 2015 | Wrong study design |
| Oehme 2018 | Wrong study design |
| Oh 2014 | Compared MAH to parenteral nutrition and not to the standard of care or placebo |
| Oh 2015 | Wrong study design |
| Otani 2013 | Wrong study design |
| Poulose 2010 | Wrong study design |
| Ruegger 2015 | Wrong study design |
| Smith 2017 | Wrong study design |
| Strand 2019 | Wrong study design |
| Torres‐Vigil 2011 | Wrong study design |
| Torres‐Vigil 2012 | Wrong study design |
| Viola 1997 | Wrong study design |
| Wade 2018 | Wrong study design |
| Waller 1994 | Wrong study design |
| XueQiTan 2018 | Wrong study design |
| Yamaguchi 2012 | Wrong study design |
| Yap 2016 | Wrong study design |
MAH: medically assisted hydration
Differences between protocol and review
During the final drafting of the review, the team uniformly agreed to change the title to 'Medically assisted hydration for adults receiving palliative care' to use the terms people/participants instead of patients. The review adopts the current Methodological Expectations of Cochrane Intervention Reviews (MECIR) methodological standards (Higgins 2021b). The GRADE approach for assessing the certainty of the evidence replaced the Oxford Quality Scale, Jadad 1996, and Rinck scale, Rinck 1997, with results presented in summary of findings tables. The Background incorporates the latest professional guidance on medically assisted hydration at end of life. We excluded prospective controlled studies included in previous reviews to minimise bias when evaluating the effectiveness of the intervention. We removed study size as an additional risk of bias domain in line with current Cochrane Pain, Palliative and Supportive Care Review Group guidance. We added three additional authors to the review team (Emma Buchan, Alison Haywood, and William Syrmis).
Contributions of authors
Emma Buchan: reviewed titles and abstracts, retrieved articles, assessed article quality, wrote the update, and will be responsible for future updates.
Alison Haywood: analysed and interpreted data, performed critical revision of review, and will be responsible for future updates.
William Syrmis: reviewed titles and abstracts, assessed article quality, performed critical revision of review.
Phillip Good: formulated question, wrote protocol, searched for studies, reviewed titles and abstracts, retrieved articles, assessed article quality, wrote the review, wrote the update, and will be responsible for future updates.
Contributors to previous updates:
Russell Richard: reviewed titles and abstracts, assessed article quality, performed critical revision of review.
William Syrmis: reviewed titles and abstracts, assessed article quality, performed critical revision of review.
Sue Jenkins‐Marsh: reviewed titles and abstracts, assessed article quality, performed critical revision of review.
Jane Stephens: reviewed titles and abstracts, assessed article quality, performed critical revision of review.
Sources of support
Internal sources
-
Mater Research – The University of Queensland, Australia
In‐kind and operational funds
-
St Vincent's Hospital Brisbane, Australia
In‐kind and operational funds
-
School of Pharmacy and Medical Sciences, Griffith University, Australia
In‐kind and operational funds
External sources
-
National Institute for Health and Care Research (NIHR), UK
Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS)
Declarations of interest
EB: none known; EB is a specialist palliative medicine physician trainee and manages adults receiving palliative care.
AH: none known.
WS: none known; WS is a specialist palliative medicine physician and manages adults receiving palliative care.
PG: none known; PG is a specialist palliative medicine physician and manages adults receiving palliative care.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bruera 2005 {published data only}
- Bruera E, Sala R, Rico MA, Moyano J, Centeno C, Willey J, et al. Effects of parenteral hydration in terminally ill cancer patients: a preliminary study. Journal of Clinical Oncology 2005;23:2366-71. [DOI] [PubMed] [Google Scholar]
Bruera 2013 {published data only}
- Bruera E, Hui D, Dalal S, Torres-Vigil I, Trumble J, Roosth J, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. Journal of Clinical Oncology 2013;21(1):111-8. [DOI: 10.1200/jco.2012.44.6518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerchietti 2000 {published data only}
- Cerchietti L, Navigante A, Sauri A, Palazzo F. Hypodermoclysis for control of dehydration in terminal-stage cancer. International Journal of Palliative Nursing 2000;6:370-4. [DOI] [PubMed] [Google Scholar]
Davies 2018 {published data only}
- Davies AN, Waghorn M, Webber K, Johnsen S, Mendis J, Boyle J. A cluster randomised feasibility trial of clinically assisted hydration in cancer patients in the last days of life. Palliative Medicine 2018;32(4):733‐43. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arcand 2015 {published data only}
- Arcand M. End-of-life issues in advanced dementia: Part 2: management of poor nutritional intake, dehydration, and pneumonia. Canadian Family Physician 2015;61(4):337-41. [PMC free article] [PubMed] [Google Scholar]
Bozzetti 2014 {published data only}
- Bozzetti F. Nutrition and/or hydration at the end of life. Nutritional Therapy and Metabolism 2014;32(4):206-7. [DOI: 10.5301/NTM.2014.12866] [DOI] [Google Scholar]
Bozzetti 2015 {published data only}
- Bozzetti F. Nutrition, hydration, and patient's preferences at the end of life. Supportive Care in Cancer 2015;23(6):1487-8. [DOI: 10.1007/s00520-014-2591-7] [DOI] [PubMed] [Google Scholar]
Brar 2018 {published data only}
- Brar K, Mekala HM, Lippmann S. Dehydration in terminal illness: Which path forward? The Journal of Family Practice 2018;67(10):E1-3. [PMID: ] [PubMed] [Google Scholar]
Bruera 2010 {published data only}
- Bruera E, Anderson KO, Palmer JL, Cohen MZ, Coldman B, Roberts LE, et al. A randomized, controlled trial of parenteral hydration in patients with advanced cancer. Journal of Clinical Oncology 2010;28(15 Suppl):TPS320. [Google Scholar]
Bükki 2015 {published data only}
- Bükki J. Author's response to: Bozzetti F, nutrition, hydration, and patient's preferences at the end of life. Supportive Care in Cancer 2015;23(6):1493. [DOI] [PubMed] [Google Scholar]
Cabañero‐Martínez 2019 {published data only}
- Cabañero‐Martínez MJ, Ramos‐Pichardo JD, Velasco‐Álvarez ML, García‐Sanjuán S, Lillo‐Crespo M, Cabrero‐García J. Availability and perceived usefulness of guidelines and protocols for subcutaneous hydration in palliative care settings. Journal of Clinical Nursing 2019;28(21-22):4012-20. [DOI: 10.1111/jocn.15036] [DOI] [PubMed] [Google Scholar]
Caccialanza 2018 {published data only}
- Caccialanza R, Constans T, Cotogni P, Zaloga GP, Pontes‐Arruda A. Subcutaneous infusion of fluids for hydration or nutrition: a review. Journal of Parenteral and Enteral Nutrition 2018;42(2):296-307. [DOI: 10.1177/0148607116676593] [DOI] [PubMed] [Google Scholar]
Canihuante 2018 {published data only}
- Canihuante J, Pérez P. Is parenteral hydration beneficial in terminally ill cancer patients? Medwave 2018;18(1):e7150. [DOI: ] [DOI] [PubMed] [Google Scholar]
Chalany 2015 {published data only}
- Chalany J. Subcutaneous rehydration in patients in terminal phase of dementia. Casopis Lekaru Ceskych 2015;154(1):14-8. [PubMed] [Google Scholar]
Chowdhury 2015 {published data only}
- Chowdhury S, Roy M, Freemantle S. The incidence of artificial feeding tube complications in non surgical upper GI cancer patients. Gut 2015;64(Suppl 1):A480-1. [DOI: ] [Google Scholar]
Dalal 2012 {published data only}
- Dalal S, Hui D, Torres-Vigil I, Palmer JL, Allo J, Frisbee-Hume S, et al. Parenteral hydration (PH) in advanced cancer patients: a multi-center, double-blind, placebo-controlled randomized trial. Journal of Clinical Oncology 2012;30(15 Suppl):9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2015 {published data only}
- Davies A, Waghorn M, Boyle J, Gallagher A, Johnsen S. Alternative forms of hydration in patients with cancer in the last days of life: study protocol for a randomised controlled trial. Trials 2015;16:464. [DOI: 10.1186/s13063-015-0988-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Druml 2016 {published data only}
- Druml C, Ballmer PE, Druml W, Oehmichen F, Shenkin A, Singer P et al. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clinical Nutrition 2016;35(3):545-56. [DOI: ] [DOI] [PubMed] [Google Scholar]
Forbat 2017 {published data only}
- Forbat L, Kunicki N, Chapman M, Lovell C. How and why are subcutaneous fluids administered in an advanced illness population: a systematic review. Journal of Clinical Nursing 2017;26(9-10):1204-16. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gent 2015 {published data only}
- Gent MJ, Fradsham S, Whyte GM, Mayland CR. What influences attitudes towards clinically assisted hydration in the care of dying patients? A review of the literature. BMJ Supportive and Palliative Care 2015;5(3):223-31. [DOI: ] [DOI] [PubMed] [Google Scholar]
Good 2014 {published data only}
- Good P, Richard R, Syrmis W, Jenkins‐Marsh S, Stephens J. Medically assisted hydration for adult palliative care patients. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD006273. [DOI: 10.1002/14651858.CD006273.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grez 2017 {published data only}
- Grez M, Perez-Cruz P, Rodriguez-Nunez A, Villouta F, Jaña C, Maldonado A, et al. Is it possible for caregivers to administer subcutaneous hydration to patients with advanced cancer at home? Feasibility and perceptions. Journal of Clinical Oncology 2017;35(31 Suppl 1):82. [DOI: ] [Google Scholar]
Higashiguchi 2016 {published data only}
- Higashiguchi T, Ikegaki J, Sobue K, Tamura Y, Nakajima N, Futamura A, et al. Guidelines for parenteral fluid management for terminal cancer patients. Japanese Journal of Clinical Oncology 2016;46(11):986-92. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ho 2015 {published data only}
- Ho S, Krishna LK. Artificial hydration at the end of life - treating the patient, family or physician. Annals of the Academy of Medicine 2015;44(12):558-60. [PubMed] [Google Scholar]
Hui 2015 {published data only}
- Hui D, Dev R, Bruera E. The last days of life: symptom burden and impact on nutrition and hydration in cancer patients. Current Opinion in Supportive and Palliative care 2015;9(4):346-54. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishiki 2013 {published data only}
- Ishiki H, Iwase S, Gyoda Y, Kanai Y, Ariyoshi K, Miyaji T, et al. Oral nutritional support can shorten the duration of parenteral hydration in end-of-life cancer patients: a randomized exploratory trial. European Journal of Cancer 2013;49:S274. [DOI: 10.1016/S0959-8049(13)70061-3] [DOI] [PubMed] [Google Scholar]
Ishiki 2015 {published data only}
- Ishiki H, Iwase S, Gyoda Y, Kanai Y, Ariyoshi K, Miyaji T, et al. Oral nutritional support can shorten the duration of parenteral hydration in end-of-life cancer patients: a randomized controlled trial. Nutrition and Cancer 2015;67(1):105‐11. [DOI: 10.1080/01635581.2015.976312] [DOI] [PubMed] [Google Scholar]
Lembeck 2016 {published data only}
- Lembeck ME, Pameijer CR, Westcott AM. The role of intravenous fluids and enteral or parenteral nutrition in patients with life-limiting Illness. The Medical Clinics of North America 2016;100(5):1131-41. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lokker 2019 {published data only}
- Lokker ME, Heide A, Oldenmenger WH, Rijt CC, Zuylen L. Hydration and symptoms in the last days of life. BMJ Supportive and Palliative Care 2021;11(3):335-43. [DOI: 10.1136/bmjspcare-2018-001729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayers 2019 {published data only}
- Mayers T, Kashiwagi S, Mathis BJ, Kawabe M, Gallagher J, Morales Aliaga ML, et al. International review of national‐level guidelines on end‐of‐life care with focus on the withholding and withdrawing of artificial nutrition and hydration. Geriatrics and Gerontology International 2019;19(9):847-53. [DOI: 10.1111/ggi.13741] [DOI] [PubMed] [Google Scholar]
Morita 2002 {published data only}
- Morita T, Tei Y, Inoue S, Suga A, Chihara S. Fluid status of terminally ill cancer patients with intestinal obstruction: an exploratory observational study. Supportive Care in Cancer 2002;10(6):474-9. [DOI] [PubMed] [Google Scholar]
Morita 2005 {published data only}
- Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, et al. Association between hydration volume and symptoms in terminally ill cancer patients with abdominal malignancies. Annals of Oncology 2005;16(4):640-7. [DOI] [PubMed] [Google Scholar]
Morita 2006 {published data only}
- Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, et al. Artificial hydration therapy, laboratory findings, and fluid balance in terminally ill patients with abdominal malignancies. Journal of Pain and Symptom Management 2006;31(2):130-9. [DOI] [PubMed] [Google Scholar]
Nakajima 2014a {published data only}
- Nakajima N, Takahashi Y, Ishitani K. The volume of hydration in terminally ill cancer patients with hydration-related symptoms: a prospective study. Journal of Palliative Medicine 2014;17(9):1037-41. [DOI: ] [DOI] [PubMed] [Google Scholar]
Nakajima 2014b {published data only}
- Nakajima N, Satake N, Nakaho T. Indications and practice of artificial hydration for terminally ill cancer patients. Current Opinion in Supportive and Palliative Care 2014;8(4):358-63. [DOI: ] [DOI] [PubMed] [Google Scholar]
Nakajima 2018 {published data only}
- Nakajima N. The effects of artificial hydration and nutrition therapy for terminally ill cancer patients based on the Japanese clinical guideline. Supportive Care in Cancer 2018;26(2 Suppl 1):S173. [DOI: ] [Google Scholar]
Nobuhisa 2018 {published data only}
- Nobuhisa N. How to distinguish starvation from refractory cachexia in terminal cancer patients and how to perform nutritional support? Palliative Medicine 2018;32(1 Suppl 1):138. [DOI: ] [Google Scholar]
Nwosu 2013 {published data only}
- Nwosu AC, Mayland CR, Mason SR, Khodabukus AF, Varro A, Ellershaw JE. Hydration in advanced cancer: can bioelectrical impedance analysis improve the evidence base? A systematic review of the literature. Journal of Pain and Symptom Management 2013;46(3):433-46. [DOI: ] [DOI] [PubMed] [Google Scholar]
O'Connor 2015 {published data only}
- O'Connor B, O'Neill C, Mc Donnell D, Ui Dhuibhir P, Lester L, Walsh D. Parenteral hydration: Review of prevalence and rationale in hospice inpatients. Supportive Care in Cancer 2015;23(1):S210. [DOI: ] [Google Scholar]
Oehme 2018 {published data only}
- Oehme J, Sheehan C. Use of artificial hydration at the end of life: A survey of Australian and New Zealand palliative medicine doctors. Journal of Palliative Medicine 2018;21(8):1145-51. [DOI: 10.1089/jpm.2018.0020] [DOI] [PubMed] [Google Scholar]
Oh 2014 {published data only}
- Oh SY, Jun HJ, Park SJ, Park IK, Lim GJ, Yu Y, et al. A randomized phase II study to assess the effectiveness of fluid therapy or intensive nutritional support on survival in patients with advanced cancer who cannot be nourished via enteral route. Journal of Palliative Medicine 2014;17(11):1266‐70. [DOI: 10.1089/jpm.2014.0082] [DOI] [PubMed] [Google Scholar]
Oh 2015 {published data only}
- Oh SY, Jun HJ, Song BK. Preference of cancer patients for parenteral nutrition in the end of life: in-depth interview from a pilot study. Annals of Oncology 2015;26:ix111. [DOI: ] [Google Scholar]
Otani 2013 {published data only}
- Otani S, Yoshimoto M, Tokuyasu N, Endo K, Takano S, Ikeguchi M, et al. The association between artificial hydration and symptoms in terminally ill cancer patients. Clinical Nutrition 2013;32:S155. [Google Scholar]
Poulose 2010 {published data only}
- Poulose JV, Krishna LR, Goh C. Artificial hydration at the end of life. Proceedings of Singapore Healthcare 2010;19(2 Suppl):S356. [DOI: ] [Google Scholar]
Ruegger 2015 {published data only}
- Ruegger J, Hodgkinson S, Field-Smith A, Ahmedzai SH. Care of adults in the last days of life: summary of NICE guidance. BMJ 2015;351:h6631-2. [DOI: 10.1136/bmj.h6631] [DOI] [PubMed] [Google Scholar]
Smith 2017 {published data only}
- Smith L, Ferguson R. Artificial nutrition and hydration in people with late-stage dementia. Home Healthcare Now 2017;35(6):321-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Strand 2019 {published data only}
- Strand AMR, Berg SF. Should dying patients be given hydration and nutrition? Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, Ny raekke 2019;139(1):15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Torres‐Vigil 2011 {published data only}
- Torres‐Vigil I, Cohen MZ, De La Rosa A, Burbach BE, Bruera E. Food or medicine: perceptions of advanced cancer patients and their caregivers regarding artificial hydration during the last weeks of life. Supportive Care in Cancer 2011;19(2 Suppl 1):S303‐4. [DOI: 10.1007/s00520-011-1184-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres‐Vigil 2012 {published data only}
- Torres-Vigil I, Cohen MZ, la Rosa A, Cárdenas-Turanzas M, Burbach BE, Tarleton KW, et al. Food or medicine: ethnic variations in perceptions of advanced cancer patients and their caregivers regarding artificial hydration during the last weeks of life. BMJ Supportive and Palliative Care 2012;2(3):276‐9. [DOI: 10.1136/bmjspcare-2012-000205] [DOI] [PMC free article] [PubMed] [Google Scholar]
Viola 1997 {published data only}
- Viola RA. Studying Fluid Status and the Dying: The Challenge of Clinical Research in Palliative Care [MSc thesis]. Canada: University of Ottawa, 1997. [Google Scholar]
Wade 2018 {published data only}
- Wade DT. Clinically assisted nutrition and hydration. BMJ 2018;362:k3869. [DOI: ] [DOI] [PubMed] [Google Scholar]
Waller 1994 {published data only}
- Wailer A, Hershkowitz M, Adunsky A. The effect of intravenous fluid infusion on blood and urine parameters of hydration and on state of consciousness in terminal cancer patients. American Journal of Hospice and Palliative Care 1994;11(6):22-7. [DOI] [PubMed] [Google Scholar]
XueQiTan 2018 {published data only}
- Xue Qi Tan E, Lai BM. Artificial nutrition and hydration can both prolong a person's life and dying process. Singapore Nursing Journal 2018;45(2):2-6. [Google Scholar]
Yamaguchi 2012 {published data only}
- Yamaguchi T, Morita T, Shinjo T, Inoue S, Takigawa C, Aruga E, et al. Effect of parenteral hydration therapy based on the Japanese national clinical guideline on quality of life, discomfort, and symptom intensity in patients with advanced cancer. Journal of Pain and Symptom Management 2012;43(6):1001‐12. [DOI: 10.1016/j.jpainsymman.2011.06.028] [DOI] [PubMed] [Google Scholar]
Yap 2016 {published data only}
- Yap SY, Siew BPT, Tay RY, Oei JAL. The use of artificial nutrition and hydration in an inpatient hospice in Singapore. Journal of Pain and Symptom Management 2016;52(6):e96. [DOI: ] [Google Scholar]
Additional references
Covidence [Computer program]
- Covidence. Version accessed 30 August 2021. Melbourne, Australia: Veritas Health Innovation, 2014. Available at covidence.org.
Doyle 2004
- Doyle D, Hanks G, Cherny NI, Calman K. Oxford Textbook of Palliative Medicine. 3rd edition. Oxford: Oxford University Press, 2004. [Google Scholar]
Fritzson 2015
- Fritzson A, Tavelin B, Axelsson B. Association between parenteral fluids and symptoms in hospital end-of-life care: an observational study of 280 patients. BMJ Supportive and Palliative Care 2015;5(2):160-8. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 30 August 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2017
- Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2021a
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological Expectations of Cochrane Intervention Reviews. Version February 2021. Cochrane: London.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Kingdon 2021
- Kingdon A, Spathis A, Brodrick R, Clarke G, Kuhn I, Barclay S. What is the impact of clinically assisted hydration in the last days of life? A systematic literature review and narrative synthesis. BMJ Supportive and Palliative Care 2021;11:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishna 2010
- Krishna LK, Poulose JV, Goh C. Artificial hydration at the end of life in an oncology ward in Singapore. Indian Journal of Palliative Care 2010;16(3):168-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murad 2017
- Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evidence Based Medicine 2017;22(3):85-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2017
- National Institute for Health and Care Excellence. Care of dying adults in the last days of life. Quality statement 4: Hydration. 2017. Available from www.nice.org.uk/guidance/qs144. [PubMed]
Raijmakers 2011
- Raijmakers NJ, Fradsham S, Zuylen L, Mayland C, Ellershaw JE, Heide A. Variation in attitudes towards artificial hydration at the end of life: a systematic literature review. Current Opinion in Supportive and Palliative Care 2011;5(3):265-72. [DOI] [PubMed] [Google Scholar]
Rees 2003
- Rees E, Hardy J. Novel consent process for research in dying patients unable to give consent. BMJ 2003;327(7408):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rinck 1997
- Rinck GC, den Bos GA, Kleijnen J, Haes HJ, Schade E, Veenhof CH. Methodologic issues in effectiveness research on palliative cancer care: a systematic review. Journal of Clinical Oncology 1997;15(4):1697-707. [DOI] [PubMed] [Google Scholar]
WHO 2021
- World Health Organization. WHO definition of palliative care. Available from www.who.int/cancer/palliative/definition/en/.
References to other published versions of this review
Good 2008
- Good P, Cavenagh J, Mather M, Ravenscroft P. Medically assisted hydration for adult palliative care patients. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD006273. [DOI: 10.1002/14651858.CD006273.pub2] [DOI] [PubMed] [Google Scholar]


