Abstract
Background
To examine whether general and abdominal adiposity was a risk factor for the new‐onset of inflammatory bowel disease (IBD) and the potential mediating effect of metabolic and inflammation status.
Methods
A total of 492,998 individuals free of IBD recruited from 2006 to 2010 in the UK Biobank were included in our study, with ongoing follow‐up linking to the health‐related outcome. Multivariable Cox regression models were used to evaluate the associations between general adiposity (body mass index) and abdominal adiposity (waist circumference) and the subsequent risk of IBD and its subtype. We also investigated the potential mediating effects of metabolic and inflammation status by carrying out exploratory mediation analyses.
Results
During a median follow‐up of 12.5 years, we documented 2954 incident IBD cases (915 Crohn's disease [CD] and 2039 ulcerative colitis). After adjustment for important confounders, body mass index (hazard ratio [HR] highest quintile [Q5] vs. lowest quintile [Q1] = 1.18, 95% confidence interval [CI] 1.04–1.32; P‐trend = 0.006) and waist circumference (HR Q5 vs. Q1 = 1.30, 95% CI 1.14–1.49; P‐trend <0.001) showed a positive association with the risk of IBD. The associations were partially mediated by metabolic status (24%; 15%), C‐reactive protein (36%; 19%) and inflammation score (82%; 46%).
Conclusions
Adiposity bore a risk factor for incident IBD, whereas unhealthy metabolism, especially inflammation, seemed to be an important intermediate condition between the association. Our findings provide evidence for possible mechanisms relating adiposity to IBD from an epidemiological perspective, and experimental studies are needed for further demonstration.
Keywords: adiposity, BMI, Crohn's disease, IBD, inflammation, inflammatory bowel disease, mediation analysis, metabolic status, ulcerative colitis, waist circumference
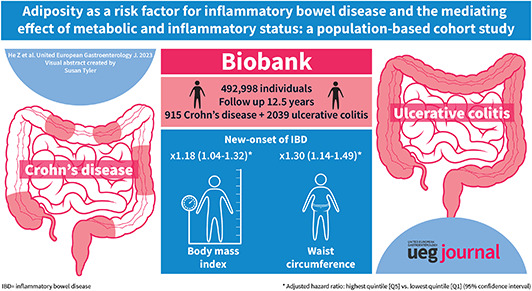
Key summary.
Summarize the established knowledge on this subject
Prior evidence for associations between adiposity and the risk of inflammatory bowel disease (IBD) was inconsistent.
Possible mechanisms by which adiposity relates to IBD risk are unclear.
What are the significant and/or new findings of this study?
Our study found a positive association between adiposity and the risk of incident IBD, and the positive associations could be partially mediated by unhealthy metabolism and inflammation.
Screening and prevention of IBD are necessary for obese populations, especially those accompanied by unhealthy metabolism and inflammation.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic relapsing inflammatory disease mainly involving the gastrointestinal tract, characterized by mucosal inflammation, including the two major subtypes of Crohn's disease (CD) and ulcerative colitis (UC). 1 The prevalence and incidence of IBD over the past half century have increased remarkably in countries and regions that have adapted a “Westernized” lifestyle, 1 manifested by decreased physical activity and tremendous changes in eating habits, which have also been demonstrated to be risk factors for obesity. 2 Notably, the prevalence of obesity has also increased worldwide in the past few decades 3 and the incidence and prevalence of IBD were observed to be growing in parallel to the obesity pandemic. 4 Previous literature put forward that 15%–40% are obese and an additional 20%–40% are overweight among IBD adults. 5 Therefore, a better understanding of whether and how obesity relates to IBD is deserved.
Several studies have been conducted to explore whether adiposity and fat distribution had effects on the development of IBD but have provided inconsistent and conflicting evidence. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Although most prospective cohort studies put forward the idea that general adiposity represented by body mass index increased the risk of CD and decreased the risk of UC, 8 , 11 , 12 , 13 a few studies showed the inverse or null finding. 8 , 10 , 12 , 13 Similarly, the meta‐analyses based on cohort studies also showed inconsistent results. 6 , 9 As for abdominal adiposity measured by waist circumference, limited evidence was provided for its positive association with risk of CD. 7
Additionally, the pathomechanism by which body adiposity might affect the development of IBD remains unclear. 14 It has been well established that inflammation plays a role in the pathophysiology of IBD 15 and the coexistence of obesity, unhealthy metabolism and systematic inflammation. 16 , 17 Obesity has been reported to relate to multiple chronic diseases through metabolic homeostasis and inflammation, 18 , 19 but has not been explored regarding IBD.
Therefore, this study aimed to clarify the associations of general adiposity and abdominal adiposity with the risk of IBD, as well as the potential effects of metabolic status and inflammation status on the association.
MATERIALS AND METHODS
Between 2006 and 2010, over 500,000 people consented to participate in the UK Biobank study and were asked to complete self‐administrated questionnaires, verbal interviews, physical measurements, biological sample collection at recruitment, as well as ongoing follow‐up for the health‐related outcome, as described earlier. 20 The UK Biobank has received ethical approval from the North West Multicenter Research Ethics Committee (REC reference: 21/NW/0157) and participants were asked to sign the consent when recruited. We leveraged 492,998 participants for analyses when excluded participants who were without adiposity data (n = 3210), pregnant or unsure (n = 338), with a prior diagnosis of IBD when recruited (n = 5905) or unclear IBD diagnosis at the follow‐up visit (n = 9) among the 502,460 individuals (Figure 1). Information on the current study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline.
FIGURE 1.
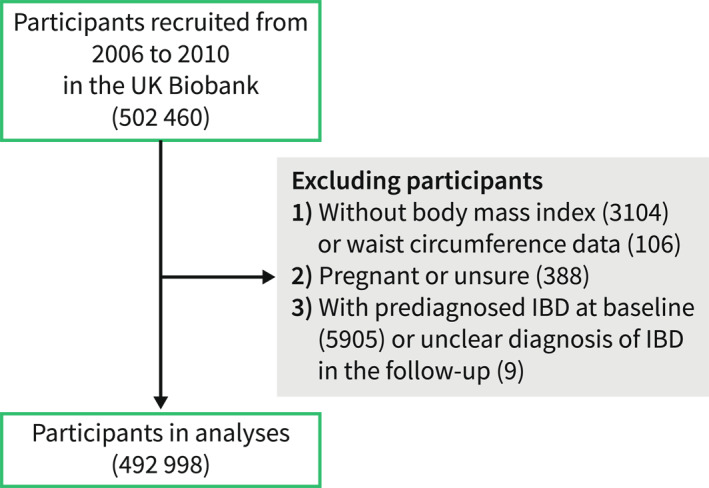
Flowchart of the study.
MEASUREMENT OF ADIPOSITY
Exposures were body mass index (constructed as weight in kilograms divided by height in meters squared) and waist circumference, which were used to measure the general and abdominal adiposity in the present study. Waist circumference showed a high correlation (Spearman correlation coefficient = 0.806) with visceral adipose tissue mass measured by dual‐energy X‐ray absorptiometry. Information on height (cm), weight (kg), and waist circumference (cm) of each participant was collected during the initial assessment center visit. Other adiposity measures including body fat percentage (%), whole body fat mass (kg), whole body fat‐free mass (kg), and hip circumference documented in assessment, and constructed adiposity index including the ratio of waist circumference and height, the ratio of waist circumference and hip circumference, body roundness index (BRI), 21 a body shape index (ABSI), 22 were also included in our study. Body roundness index and ABSI are newly developed anthropometric indices, calculated as 364.2–365.5 × {1 − ((waist circumference/2π)/(0.5 × height)) 2 }0.5 and waist circumference/(body mass index2/3 × height1/2), suggested as alternatives to traditional visceral adiposity tissue and body fat percentage. 22
ASSESSMENT OF COVARIATES
Confounders might cause effects on the associations of adiposity with IBD risk, including age, sex (female, male), race (white, others), Townsend deprivation index (TDI), education, smoking status (current, previous, never), alcohol drinking (current, previous, never), physical activities, dietary quality index, total energy intake and comorbidities represented by Charlson comorbidity index (CCI). 23 TDI was calculated prior to participants joining the UK Biobank and measured the material deprivation among a population. The diet index was constructed by seven food groups (vegetables, fruits, whole grains, refined grains, processed meats, and unprocessed meats, fish) collected by the food frequency questionnaire: higher the index, healthier the diet. 24
We considered metabolic status and inflammation represented by C‐reactive protein levels and a low‐grade inflammation (INFLA) score as potential mediators. Participants who satisfied less than two of the following four criteria were considered healthy metabolism 25 : (1) abnormal triglycerides (≥1.7 mmol/L) or lipid‐lowering medications, (2) increased diastolic blood pressure (≥85 mmHg) or systolic blood pressure (≥130 mmHg) or anti‐hypertensive drugs, (3) elevated fasting glucose (≥5.6 mmol/L) or medications for diabetes (insulin and oral anti‐diabetic) and (4) low high‐density lipid (HDL)‐cholesterol (1.29 mmol/L for women and 1.04 mmol/L for men). C‐reactive protein (plasma levels in mg/L), white blood cell count (10^9 cells/Liter), platelet count (10^9 cells/Liter), and neutrophil‐to‐lymphocyte ratio (the ratio of neutrophil count and lymphocyte count) were assayed in blood samples and used to evaluate the INFLA score. After assigning scores by dividing the four biological markers into deciles, the total INFLA score ranged from −16 to +16, with a higher score indicating higher inflammation levels. 26 Detailed descriptions and missing information of each covariate are presented in Supplementary Table 1‐2.
ASCERTAINMENT OF OUTCOME
Participants were followed up on an ongoing basis for health data by linking to the national electronic health databases. The newly developed IBD events were obtained by the International Statistical Classification of Diseases and Related Health Problems (ICD) codes from the Hospital Episode Statistics (the 10th Revision of ICD [ICD 10] and the Ninth Revision of ICD [ICD 9]) and the death registry (ICD 10), and read v2 and read v3 codes (mapped to ICD codes) from the primary care data. The ICD 9 codes and ICD 10 codes for IBD were 555 (CD) and 556 (UC) and K50 (CD) and K51 (UC), respectively. ICD codes have been identified with high diagnostic accuracy in Great Britain. 27 Multiple‐sourced health data allowed us to capture diagnostic information on IBD to the maximum extent, with a significant advantage over studies relying only on single‐sourced data. As described in a previous study conducted based on the UK Biobank database, combining sources for disease diagnosis showed high positive predictive values (91%). 28
STATISTICAL ANALYSIS
Participants' characteristics were described according to IBD status and subtype. Variables in continuous and categorical were expressed as mean (standard deviation [SD]) and proportions, and missing variables were imputed as and mean (for continuous variables) and mode (for categorical variables), and created the ‘NA’ category for physical activities and metabolic status because of the large missing rate.
Cox proportional hazard regression models were used to evaluate the hazard ratio (HR) and 95% confidence interval (CI) for the association between adiposity and risk of IBD, CD and UC, and all models satisfied the proportional hazard assumption. Multivariable models were adjusted for age, sex and ethnicity in the minimally adjusted model and TDI, smoking status, drinking status, education, physical activities and diet index were further added in the fully adjusted model. Hazard ratio was calculated for fifths of body mass index and waist circumference relative to the lowest group, as well as for specific unit increase (5 kg/m2 or 10 cm). To flexibly describe the shape of the associations, the non‐linear relationship was depicted by the restricted cubic spline and tested. We additionally explored whether body adiposity represented by other measures correlated with the risk of IBD.
We explored to what extent metabolic status and inflammation played a role in the explored associations between adiposity and IBD risk. First, we examined whether adiposity had different effects on IBD depending on the existence of metabolic status (healthy, unhealthy), levels of C‐reactive protein (low, high according to the median values), and INFLA score (low, high according to the median values) by subgroup analyses. Second, we examined the mediating effects of unhealthy metabolism and inflammation on the associations using structural equation models. Specifically, the individual regression path was modeled in the mediation model for the following associations: (1) the total effect: between exposure (adiposity) and outcome (IBD); (2) a path: between exposure and mediators (metabolic status, C reactive protein, INFLA score); (3) b path: between the mediators and the outcome adjusting for exposure. The indirect effect was calculated as the product of a and b and the proportion mediated was developed by dividing the indirect effect of the total effect (Figure 3a).
FIGURE 3.
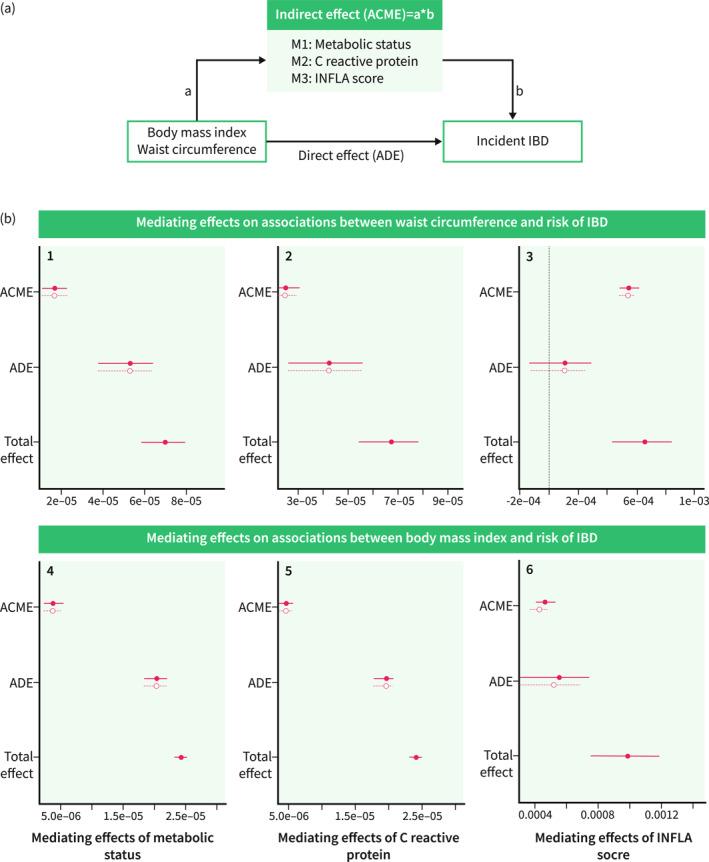
The schematic diagram (a) and mediation effects (b) of metabolic status, C‐reactive protein and INFLA score on the association of adiposity with risk of incident inflammatory bowel disease (IBD). The x‐axis represents the mediation effect estimates and the y‐axis represents the three different effects (mediating effect, direct effect, total effect). B1, B2, B3 showed the mediation effects of metabolic status, C‐reactive protein and INFLA score on the associations between body mass index and risk of IBD, and B4, B5, B6 showed the mediation effects of metabolic status, C‐reactive protein and INFLA score on the associations between waist circumference and risk of IBD. ACME, average causal mediation effects; ADE, average direct effects. The mediating effects of the three mediators (M1: metabolic status; M2: C reactive protein; M3: INFLA score) were separately examined in the individual structural equation model.
Furthermore, we reinvestigated the association between adiposity and risk of IBD in a series of sensitivity analyses: (1) changed the classification methods for body mass index (<25, 25–30, ≥30 kg/m2 [general obesity]) 29 and waist circumference (<88 cm for female or <102 cm for male, ≥88 cm for female or ≥102 cm for male [abdominal obesity]) 30 according to specific values; (2) changed the body mass index calculated from height and weight to the body mass index measured by impedance measurement; (3) further adjusted for total energy intake; (4) further adjusted for comorbidities; (5) excluded participants with missing data on physical activities; (6) lag one‐year analysis by excluding participants with new onset of IBD in the first year during follow‐up.
All analyses were conducted by R 4.2.1 with ‘survival’ and ‘mediation’ packages, and two‐sided P < 0.05 suggested statistical significance.
RESULTS
Overall, the mean (SD) age was 56.53 (8.09) years in the included 492,998 participants, and 268,372 (54.4%) of them were female. The mean (SD) of body mass index and waist circumference was 27.44 (4.80) kg/m2 and 90.30 (13.48) cm, respectively (Table 1). During a median (inter‐quartile range) follow‐up of 12.5 (1.5) years, there were 2954 incident IBDs, with 915 CD and 2039 UCs. After adjustment of potential confounders in the multivariable‐adjusted model, body mass index (HR per unit = 1.06, 95% CI 1.02–1.10; HR highest quintile [Q5] vs. lowest quintile [Q1] = 1.18, 95% CI 1.04–1.32; P‐trend = 0.006) and waist circumference (HR per unit = 1.06, 95% CI 1.03–1.10; HR Q5 vs. Q1 = 1.30, 95% CI 1.14–1.49; P‐trend <0.001) per unit increase and higher quintiles showed positive associations with the risk of IBD, consistent associations were also observed in the risk of CD. However, adiposity, especially measured by body mass index did not relate to the risk of UC, although associations were found in the minimally adjusted models (Table 2). The above associations were flexibly plotted in the restricted cubic spline, and a non‐linear relationship of body mass index with risk of CD was detected (Figure 2). Furthermore, adiposity measured using whole body fat mass (HR per SD = 1.04, 95% CI 1.01–1.08), body fat percentage (HR per SD = 1.05, 95% CI 1.00–1.11), the ratio of waist circumference and hip circumference (HR per SD = 1.12, 95% CI 1.06–1.17), the ratio of waist circumference and height (HR per SD = 1.09, 95% CI 1.05–1.13), ABSI (HR per SD = 1.10, 95% CI 1.05–1.15), BRI (HR per SD = 1.08, 95% CI 1.05–1.12) were positively associated with the risk of IBD (Supplementary Table 3).
TABLE 1.
Characteristics of participants according to inflammatory bowel disease (IBD) status and subtype.
| Overall | Non‐IBD | IBD | CD | UC | |
|---|---|---|---|---|---|
| n | 492,998 | 490,044 | 2954 | 915 | 2039 |
| Age (years) | 56.53 (8.09) | 56.52 (8.09) | 57.29 (8.04) | 57.16 (8.18) | 57.35 (7.98) |
| Sex | |||||
| Female | 268,372 (54.4) | 266,896 (54.5) | 1476 (50.0) | 516 (56.4) | 960 (47.1) |
| Male | 224,626 (45.6) | 223,148 (45.5) | 1478 (50.0) | 399 (43.6) | 1079 (52.9) |
| TDI | |||||
| Low deprivation | 164,336 (33.3) | 163,480 (33.4) | 856 (29.0) | 257 (28.1) | 599 (29.4) |
| Moderate deprivation | 164,332 (33.3) | 163,387 (33.3) | 945 (32.0) | 293 (32.0) | 652 (32.0) |
| High deprivation | 164,330 (33.3) | 163,177 (33.3) | 1153 (39.0) | 365 (39.9) | 788 (38.6) |
| Ethnicity | |||||
| White | 466,786 (94.7) | 464,004 (94.7) | 2782 (94.2) | 865 (94.5) | 1917 (94.0) |
| Others | 26,212 (5.3) | 26,040 (5.3) | 172 (5.8) | 50 (5.5) | 122 (6.0) |
| Smoking status | |||||
| Current | 51,898 (10.5) | 51,465 (10.5) | 433 (14.7) | 160 (17.5) | 273 (13.4) |
| Never | 271,516 (55.1) | 270,237 (55.1) | 1279 (43.3) | 417 (45.6) | 862 (42.3) |
| Previous | 169,584 (34.4) | 168,342 (34.4) | 1242 (42.0) | 338 (36.9) | 904 (44.3) |
| Drinking | |||||
| Current | 453,732 (92.0) | 451,101 (92.1) | 2631 (89.1) | 805 (88.0) | 1826 (89.6) |
| Never | 21,727 (4.4) | 21,555 (4.4) | 172 (5.8) | 60 (6.6) | 112 (5.5) |
| Previous | 17,539 (3.6) | 17,388 (3.5) | 151 (5.1) | 50 (5.5) | 101 (5.0) |
| Education | |||||
| College and above | 158,513 (32.2) | 157,781 (32.2) | 732 (24.8) | 239 (26.1) | 493 (24.2) |
| High school and below | 334,485 (67.8) | 332,263 (67.8) | 2222 (75.2) | 676 (73.9) | 1546 (75.8) |
| Physical activity | |||||
| Low | 74,360 (15.1) | 73,877 (15.1) | 483 (16.4) | 154 (16.8) | 329 (16.1) |
| Middle | 161,349 (32.7) | 160,438 (32.7) | 911 (30.8) | 293 (32.0) | 618 (30.3) |
| High | 159,664 (32.4) | 158,761 (32.4) | 903 (30.6) | 275 (30.1) | 628 (30.8) |
| NA | 97,625 (19.8) | 96,968 (19.8) | 657 (22.2) | 193 (21.1) | 464 (22.8) |
| Metabolic status | |||||
| Healthy | 224,236 (45.5) | 223,121 (45.5) | 1115 (37.7) | 339 (37.0) | 776 (38.1) |
| Unhealthy | 197,887 (40.1) | 196,515 (40.1) | 1372 (46.4) | 420 (45.9) | 952 (46.7) |
| NA | 70,875 (14.4) | 70,408 (14.4) | 467 (15.8) | 156 (17.0) | 311 (15.3) |
| C reactive protein (mg/L) | 2.58 (4.18) | 2.57 (4.17) | 3.70 (5.65) | 4.71 (7.08) | 3.24 (4.80) |
| INFLA | −0.09 (6.05) | −0.10 (6.05) | 2.01 (6.36) | 2.78 (6.64) | 1.67 (6.20) |
| Diet score | 3.66 (1.36) | 3.66 (1.36) | 3.51 (1.39) | 3.50 (1.40) | 3.51 (1.38) |
| CCI Index | 0.27 (0.90) | 0.27 (0.90) | 0.40 (1.03) | 0.38 (0.96) | 0.41 (1.06) |
| Body mass index (kg/m2) | 27.44 (4.80) | 27.43 (4.80) | 27.95 (5.02) | 28.13 (5.30) | 27.87 (4.88) |
| Waist circumference (cm) | 90.30 (13.48) | 90.29 (13.47) | 92.44 (13.63) | 92.40 (13.80) | 92.46 (13.56) |
| Body fat percentage (%) | 31.45 (8.48) | 31.45 (8.48) | 31.58 (8.65) | 32.57 (8.82) | 31.14 (8.54) |
| Whole body fat mass (kg) | 24.86 (9.49) | 24.86 (9.49) | 25.47 (9.89) | 26.20 (10.46) | 25.14 (9.61) |
| Whole body fat‐free mass (kg) | 53.22 (11.42) | 53.22 (11.42) | 54.12 (11.58) | 52.97 (11.48) | 54.64 (11.58) |
| The ratio of waist circumference and hip circumference | 0.87 (0.09) | 0.87 (0.09) | 0.89 (0.09) | 0.88 (0.09) | 0.89 (0.09) |
| Height (cm) | 168.45 (9.28) | 168.45 (9.28) | 168.60 (9.39) | 167.69 (9.37) | 169.00 (9.38) |
| The ratio of waist circumference and height | 0.54 (0.08) | 0.54 (0.08) | 0.55 (0.08) | 0.55 (0.08) | 0.55 (0.08) |
| A body shape index (ABSI) | 0.77 (0.05) | 0.77 (0.05) | 0.77 (0.05) | 0.77 (0.05) | 0.78 (0.05) |
| Body roundness index (BRI) | 4.17 (1.59) | 4.17 (1.59) | 4.43 (1.67) | 4.50 (1.73) | 4.40 (1.64) |
Note: The continuous and categorical variables were described as mean (SD) and proportion (%).
Abbreviations: CCI, Charlson comorbidity index; CD, Crohn'’s diseases; IBD, inflammatory bowel diseases; SD, standard deviation; TDI, Townsend deprivation index; UC, ulcerative colitis.
TABLE 2.
Hazard Ratios (HRs) and 95% CIs per fifth and specified unit increase in adiposity measures for the risk of inflammatory bowel disease (IBD).
| Cases | Person years | Minimally adjusted model HR (95% CI) | P | Fully adjusted model HR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Inflammatory bowel disease | ||||||
| Body mass index | ||||||
| Per 5 kg/m2 | 1.11 (1.07, 1.15) | <0.001 | 1.06 (1.02, 1.10) | 0.004 | ||
| Q1 | 501 | 1,200,655 | Ref | Ref | ||
| Q2 | 558 | 1,201,876 | 1.06 (0.94, 1.20) | 0.317 | 1.05 (0.93, 1.19) | 0.409 |
| Q3 | 573 | 1,197,337 | 1.07 (0.95, 1.21) | 0.253 | 1.03 (0.92, 1.17) | 0.580 |
| Q4 | 629 | 1,193,695 | 1.17 (1.04, 1.32) | 0.008 | 1.09 (0.97, 1.23) | 0.152 |
| Q5 | 693 | 1,181,936 | 1.34 (1.19, 1.50) | <0.001 | 1.18 (1.04, 1.32) | 0.007 |
| P trend | <0.001 | 0.006 | ||||
| Waist circumference | ||||||
| Per 10 cm | 1.11 (1.08, 1.14) | <0.001 | 1.06 (1.03, 1.10) | <0.001 | ||
| Q1 | 406 | 1,115,916 | Ref | Ref | ||
| Q2 | 541 | 1,171,117 | 1.23 (1.08, 1.40) | 0.002 | 1.19 (1.04, 1.35) | 0.010 |
| Q3 | 579 | 1,200,035 | 1.25 (1.10, 1.43) | 0.001 | 1.18 (1.03, 1.34) | 0.017 |
| Q4 | 666 | 1,229,713 | 1.38 (1.21, 1.58) | <0.001 | 1.25 (1.09, 1.43) | 0.001 |
| Q5 | 762 | 1,258,719 | 1.54 (1.35, 1.76) | <0.001 | 1.30 (1.14, 1.49) | <0.001 |
| P trend | <0.001 | <0.001 | ||||
| Crohn's disease | ||||||
| Body mass index | ||||||
| Per 5 kg/m2 | 1.16 (1.09, 1.23) | <0.001 | 1.11 (1.04, 1.19) | 0.001 | ||
| Q1 | 169 | 1,197,764 | Ref | Ref | ||
| Q2 | 160 | 1,198,842 | 0.94 (0.76, 1.17) | 0.587 | 0.94 (0.76, 1.17) | 0.597 |
| Q3 | 167 | 1,194,974 | 0.99 (0.79, 1.22) | 0.895 | 0.97 (0.78, 1.20) | 0.781 |
| Q4 | 183 | 1,190,988 | 1.08 (0.88, 1.34) | 0.461 | 1.03 (0.83, 1.28) | 0.761 |
| Q5 | 236 | 1,179,593 | 1.41 (1.15, 1.72) | 0.001 | 1.28 (1.04, 1.57) | 0.018 |
| P trend | <0.001 | 0.008 | ||||
| Waist circumference | ||||||
| Per 10 cm | 1.17 (1.11, 1.23) | <0.001 | 1.12 (1.07, 1.18) | <0.001 | ||
| Q1 | 129 | 1,114,099 | Ref | Ref | ||
| Q2 | 171 | 1,168,671 | 1.30 (1.04, 1.64) | 0.024 | 1.26 (1.00, 1.59) | 0.046 |
| Q3 | 171 | 1,197,406 | 1.34 (1.06, 1.70) | 0.015 | 1.27 (1.00, 1.61) | 0.049 |
| Q4 | 211 | 1,226,699 | 1.67 (1.32, 2.11) | <0.001 | 1.53 (1.21, 1.94) | <0.001 |
| Q5 | 233 | 1,255,285 | 1.82 (1.44, 2.31) | <0.001 | 1.58 (1.25, 2.01) | <0.001 |
| P trend | 1.15 (1.09, 1.21) | <0.001 | 1.11 (1.06, 1.17) | <0.001 | ||
| Ulcerative colitis | ||||||
| Body mass index | ||||||
| Per 5 kg/m2 | 1.09 (1.04, 1.14) | <0.001 | 1.03 (0.99, 1.08) | 0.186 | ||
| Q1 | 332 | 1,199,615 | Ref | Ref | ||
| Q2 | 398 | 1,200,530 | 1.12 (0.97, 1.30) | 0.117 | 1.11 (0.95, 1.28) | 0.180 |
| Q3 | 406 | 1,195,714 | 1.12 (0.96, 1.29) | 0.140 | 1.07 (0.92, 1.24) | 0.395 |
| Q4 | 446 | 1,192,607 | 1.22 (1.06, 1.41) | 0.007 | 1.12 (0.97, 1.29) | 0.131 |
| Q5 | 457 | 1,181,077 | 1.30 (1.13, 1.50) | <0.001 | 1.13 (0.98, 1.31) | 0.101 |
| P trend | 1.06 (1.03, 1.10) | <0.001 | 1.02 (0.99, 1.06) | 0.136 | ||
| Waist circumference | ||||||
| Per 10 cm | 1.09 (1.05, 1.13) | <0.001 | 1.04 (1.00, 1.08) | 0.049 | ||
| Q1 | 277 | 1,115,083 | Ref | Ref | ||
| Q2 | 370 | 1,170,019 | 1.19 (1.02, 1.39) | 0.030 | 1.15 (0.98, 1.34) | 0.087 |
| Q3 | 408 | 1,198,972 | 1.21 (1.03, 1.42) | 0.022 | 1.13 (0.96, 1.32) | 0.144 |
| Q4 | 455 | 1,228,312 | 1.26 (1.07, 1.49) | 0.005 | 1.14 (0.96, 1.34) | 0.127 |
| Q5 | 529 | 1,257,155 | 1.42 (1.21, 1.66) | <0.001 | 1.19 (1.01, 1.40) | 0.041 |
| P trend | 1.08 (1.04, 1.12) | <0.001 | 1.03 (0.99, 1.07) | 0.107 | ||
Note: Minimally adjusted model: Cox model adjusted for age, sex and ethnicity; fully adjusted model: further adjusted for TDI, smoking status, drinking status, education, physical activities and diet index. P values with bold indicate statistically significant (P < 0.05).
Abbreviations: CI, confidence interval; IBD, inflammatory bowel disease; HR, hazard ratio; SD, standard deviation.
FIGURE 2.
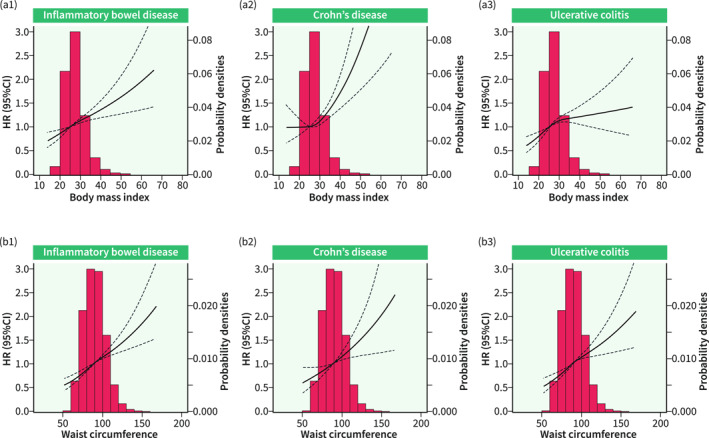
Restricted cubic spline for the association between adiposity and inflammatory bowel disease (IBD) risk. P for non‐linear relationship of plots A1, A2, A3, B1, B2, and B3 was 0.147, 0.024, 0.070, 0.358, 0.946 and 0.309, respectively.
In the exploratory analyses of the effects of metabolic status and inflammation, the associations of body mass index and waist circumference with the risk of IBD became insignificant and with lower estimates in the subgroup analyses stratified by metabolic status, C‐reactive protein levels and INFLA score although no interactions were observed (Supplementary Table 4). In the mediation analyses, we found that the partial association of body mass index and waist circumference with IBD can be explained by the potential mediators investigated. To be specific, for body mass and waist circumferences, the mediating effect estimates (proportion of mediating effect) were 1.68E‐05 (24%) and 3.78E‐06 (15%) of metabolic status, 2.50E‐05 (36%) and 4.58E‐06 (19%) for the level of C‐reactive protein, and 5.45E‐04 (82%) and 4.48E‐04 (46%) for INFLA score. Especially, the association between body mass index and the risk of IBD could be mostly explained (82%) by the INFLA score, with a mediating effect estimate (95% CI) of 5.45E‐04 (4.84E‐04, 6.08E‐04) (Table 3, Figure 3b).
TABLE 3.
Mediation effects of potential factors on the adiposity and risk of inflammatory bowel disease (IBD).
| Mediation effects | The proportion of mediation effect | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Proportion | 95% CI | P | |
| Body mass index | ||||||
| Metabolic status | 1.68E‐05 | 1.08E‐05, 2.25E‐05 | <0.001 | 24% | 15%, 38% | <0.001 |
| C reactive protein | 2.50E‐05 | 2.08E‐05, 3.03E‐05 | <0.001 | 36% | 27%, 53% | <0.001 |
| INFLA | 5.45E‐04 | 4.84E‐04, 6.08E‐04 | <0.001 | 82% | 66%, 129% | <0.001 |
| Waist circumference | ||||||
| Metabolic status | 3.78E‐06 | 2.24E‐06, 5.44E‐06 | <0.001 | 15% | 9%, 22% | <0.001 |
| C reactive protein | 4.58E‐06 | 3.68E‐06, 5.73E‐06 | <0.001 | 19% | 15%, 24% | <0.001 |
| INFLA | 4.48E‐04 | 3.96E‐04, 5.02E‐04 | <0.001 | 46% | 38%, 61% | <0.001 |
Note: P values with bold indicate statistically significant (P < 0.05).
Abbreviation: CI, confidence interval.
Additional sensitivity analyses were conducted to test the robustness of the primary findings. When categorizing body mass index and waist circumference by specific values, participants with general obesity (≥30) and abdominal obesity (≥88 cm for females, ≥102 cm for males) were also at higher risk of IBD and CD (Supplementary Table 5). Re‐analyzing the data using another measure of body mass index (Supplementary Table 6) and further adjusting for total energy intake or comorbidities did not change main findings (Supplementary Table 7). Similarly, the results were consistent when we excluded participants with missing values of physical activities or with new onset of IBD in the first year during follow‐up (Supplementary Table 8).
DISCUSSION
In this large prospective cohort study of UK adults, there were associations of general adiposity (body mass index) and abdominal adiposity (waist circumference), as well as other adiposity measures, with higher risk of IBD after multiple adjustments of potential confounders. The associations of body mass index and waist circumference with IBD could be partially mediated by unhealthy metabolism and inflammation, especially INFLA score.
Studies have explored whether general adiposity measured by body mass index is positively associated with IBD risk and the findings were conflicting. A pooled analysis of five prospective cohorts involving 601,009 individuals, 13 as well as two other relatively small‐sized cohort studies observed that obesity was related to a higher risk of CD instead of UC, 8 , 12 consistent with the findings of a meta‐analysis based on over a million participants. 9 However, inverse associations between body mass index and risk of UC 6 , 11 and null associations of body mass index with neither CD nor UC were also raised in some studies. 10 For the waist circumference, only a recent cohort study conducted based on the Korean population found that Individuals with abdominal obesity bore an increased risk of developing CD proportional to waist circumference but not UC with 10 million individuals data, providing limited evidence. 7 Overall, the evidence for adiposity and IBD was insufficient, and prior studies exploring the effects of adiposity on IBD had differences in the extent of population's age and ethnicity, sample size, classification and measurement of obesity. The present study leveraged data from a large‐scale cohort study using obesity data constructed and directly measured using impedance, and multiple adiposity measurements and classification, providing strong evidence for the associations between adiposity and risk of IBD from the perspective of general and abdominal obesity. In addition to dividing the population into quintiles according to body mass index and waist circumference, we classify the population according to specific cut‐off values given its clinically relevant guidance. Furthermore, considering the potential effects of diets and baseline comorbidities, we included overall diet quality score in the fully adjusted model and additionally adjusted for total energy intake and overall comorbidities represented by CCI, which was less taken into account in prior studies. Noteworthy, recent studies investigating the risk of new‐onset IBD after bariatric surgery presented inconsistent results, and whether the effects result from weight loss or the operation were unclear, 31 , 32 , 33 which deserved to be further explored.
The biological mechanisms underlying the positive association between adiposity and the risk of IBD are not fully understood. 14 Considering the common existence and possible co‐pathogenesis of obesity, metabolic abnormalities and inflammation, 16 , 17 and the metabolic and inflammatory nature of IBD, 34 we explored the potential effects of metabolic and inflammatory status in the associations by testing modification and mediation effects. The decreased even disappearing associations in the subgroup analyses indicated potential confounding roles. In the further mediation analyses, we provided evidence for the mediating roles of metabolic status, level of C‐reactive protein and INFLA score. The INFLA score contained four well‐established markers of systemic inflammation and aggregated measured low‐grade inflammation. Our results raised 79% and 43% mediating effects of INFLA on the associations of body mass index and waist circumference with IBD, suggesting the critical roles of low‐grade inflammation in the process, similar to the finding that systematic inflammation‐mediated diseases, 19 such as cognitive control, 35 blood pressure. 36 Interestingly, metabolic inflammation in obesity and related disorders partly proved to originate from the dysbiosis of gut microbiota, 37 , 38 one of the core links in the occurrence of IBD. Therefore, the roles and complex interaction of metabolism, inflammation and gut microbiota in the associations of adiposity with the development of IBD deserved to be further proven and explored.
The strength of our study was that we had access to utilize a well‐characterized and well‐administrated cohort with a large sample size. The cohort study prospectively followed‐up for more than 10 years with low loss to follow‐up. 39 Also, multiple‐source‐derived data collection and calculation methods for adiposity allowed us to examine the associations between adiposity and IBD risk from multiple perspectives. The current study also has several limitations. First, metabolic and inflammatory indicators would have been repeatedly measured in ideal analysis instead of the baseline time point as used in our analysis, to identify long‐term levels of metabolic status and systemic inflammation more effectively. Second, although the models used adjusted for a wide range of important confounders, and the prospective study design of the UK Biobank avoided the potential selection biases of other observational studies, biases such as confounder and reverse causation bias cannot be completely avoided no matter in correlation or mediating analyses. Intervention studies such as randomized controlled trials should be conducted to prove our findings. Finally, our cohort might not be representative of the overall population because of the disparity in age and ethnicity. However, as previously reported, the valid assessment of exposure‐disease relationships might be generalized widely although the study participants are not representative enough. 40
CONCLUSIONS
This large‐scale cohort study involving approximately 0.5 million UK adults demonstrated the positive associations of general adiposity (body mass index) and abdominal adiposity (waist circumference) with IBD and was partially mediated by unhealthy metabolism and inflammation. Interventional and experimental studies examining the specific mechanism by which adiposity may influence the development of IBD are warranted in the future.
AUTHOR CONTRIBUTIONS
Zixuan He: Methodology (lead); formal analysis (equal); writing‐original draft (lead). Tian Fu: Methodology (equal); formal analysis (lead); writing‐original draft (equal). Shiyuan Lu: Methodology (supporting); formal analysis (lead); writing‐original draft (equal); writing‐review and editing (equal). Yuhao Sun: Methodology (supporting); formal analysis (supporting); writing‐original draft (supporting). Yao Zhang: Formal analysis (supporting); writing‐review and editing (supporting). Wenming Shi: Formal analysis (supporting); writing‐original draft (supporting); writing‐review and editing (supporting). Zhaoshen Li: Conceptualization (equal); methodology (supporting); formal analysis (supporting); writing‐original draft (supporting); writing‐review and editing (supporting). Guarantor of the article: Dr Jie Chen and Professor Minzi Deng. All authors approved the final version of the manuscript. Minzi Deng: Conceptualization (equal); methodology (lead); formal analysis (supporting); writing‐original draft (supporting); writing‐review and editing (equal). Jie Chen: Conceptualization (lead); methodology (lead); formal analysis (supporting); writing‐original draft (supporting); writing‐review and editing (equal). Yu Bai: Conceptualization (lead); methodology (equal); formal analysis (supporting); writing‐original draft (supporting); writing‐review and editing (equal).
CONFLICT OF INTEREST STATEMENT
No financial conflicts of interest among the authors.
ETHICS APPROVAL
The UK Biobank has received ethical approval from the North West Multicenter Research Ethics Committee (REC reference: 21/NW/0157) and participants were asked to sign the consent when recruited.
Supporting information
Supplementary Information S1
ACKNOWLEDGMENTS
This research was carried out using the UK Biobank study under application number 73595. We are grateful to the UK Biobank participants and the team for their participation and assistance. Yu Bai was supported by the National Natural Science Foundation of China (Grant No. 82170567), the National Key R&D Program of China (Grant No. 2018YFC1313103) and 234 Discipline Climbing Plan of Changhai Hospital, Second Military Medical University/Naval Medical University (Grant No. 2019YXK004); Minzi Deng was supported by the Natural Science Foundation of Hunan Province (Grant No. 2021JJ30999); Zixuan He is supported by the Shanghai Sailing Program (Grant No. 23YF1458700), and the Youth Start‐up Fund of Naval Medical University (Grant No. 2022QN065).
He Z, Fu T, Lu S, Sun Y, Zhang Y, Shi W, et al. Adiposity as a risk factor for inflammatory bowel disease and the mediating effect of metabolic and inflammatory status: a population‐based cohort study. United European Gastroenterol J. 2023;11(10):973–984. 10.1002/ueg2.12468
Zixuan He, Tian Fu and Shiyuan Lu contributed equally.
Contributor Information
Minzi Deng, Email: dengmz@csu.edu.cn.
Jie Chen, Email: med_chenjie@zju.edu.cn.
Yu Bai, Email: md.baiyu@foxmail.com.
DATA AVAILABILITY STATEMENT
Data can be obtained from the UK Biobank (www.ukbiobank.ac.uk/).
REFERENCES
- 1. Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. 10.1038/s41575-020-00360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–1170. 10.1161/circulationaha.111.039453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 4. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. e42; quiz e30. 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 5. Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14(2):110–121. 10.1038/nrgastro.2016.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milajerdi A, Abbasi F, Esmaillzadeh A. A systematic review and meta‐analysis of prospective studies on obesity and risk of inflammatory bowel disease. Nutr Rev. 2022;80(3):479–487. 10.1093/nutrit/nuab028 [DOI] [PubMed] [Google Scholar]
- 7. Je Y, Han K, Chun J, Kim Y, Kim JH, Hoon Youn Y, et al. Association of waist circumference with the risk of inflammatory bowel disease: a nationwide cohort study of 10 million individuals in Korea. J Crohn's Colitis. 2022;17(5):681–692. 10.1093/ecco-jcc/jjac193 [DOI] [PubMed] [Google Scholar]
- 8. Harpsøe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843–855. 10.1093/ije/dyu045 [DOI] [PubMed] [Google Scholar]
- 9. Rahmani J, Kord‐Varkaneh H, Hekmatdoost A, Thompson J, Clark C, Salehisahlabadi A, et al. Body mass index and risk of inflammatory bowel disease: a systematic review and dose‐response meta‐analysis of cohort studies of over a million participants. Obes Rev. 2019;20(9):1312–1320. 10.1111/obr.12875 [DOI] [PubMed] [Google Scholar]
- 10. Chan SS, Luben R, Olsen A, Tjonneland A, Kaaks R, Teucher B, et al. Body mass index and the risk for Crohn's disease and ulcerative colitis: data from a European Prospective Cohort Study (The IBD in EPIC Study). Am J Gastroenterol. 2013;108(4):575–582. 10.1038/ajg.2012.453 [DOI] [PubMed] [Google Scholar]
- 11. Jensen CB, Ängquist LH, Mendall MA, Sørensen TIA, Baker JL, Jess T. Childhood body mass index and risk of inflammatory bowel disease in adulthood: a population‐based cohort study. Am J Gastroenterol. 2018;113(5):694–701. 10.1038/s41395-018-0031-x [DOI] [PubMed] [Google Scholar]
- 12. Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, et al. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21(2):361–368. 10.1097/mib.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan SSM, Chen Y, Casey K, Olen O, Ludvigsson JF, Carbonnel F, et al. Obesity is associated with increased risk of Crohn's disease, but not ulcerative colitis: a pooled analysis of five prospective cohort studies. Clin Gastroenterol Hepatol. 2022;20(5):1048–1058. 10.1016/j.cgh.2021.06.049 [DOI] [PubMed] [Google Scholar]
- 14. Bilski J, Mazur‐Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9(12):780. 10.3390/biom9120780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. 10.1038/nature06005 [DOI] [PubMed] [Google Scholar]
- 16. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375–c391. 10.1152/ajpcell.00379.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esser N, Legrand‐Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. 10.1016/j.diabres.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 18. Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3). 10.1210/endrev/bnaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–723. 10.1111/obr.12551 [DOI] [PubMed] [Google Scholar]
- 20. Sun Y, Yuan S, Chen X, Sun J, Kalla R, Yu L, et al. The contribution of genetic risk and lifestyle factors in the development of adult‐onset inflammatory bowel disease: a prospective cohort study. Am J Gastroenterol. 2023;118(3):511–522. 10.14309/ajg.0000000000002180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas DM, Bredlau C, Bosy‐Westphal A, Müller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–2271. 10.1096/fasebj.27.1_supplement.360.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7):e39504. 10.1371/journal.pone.0039504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130–1139. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 24. Fu T, Ye S, Sun Y, Dan L, Wang X, Chen J. Greater adherence to cardioprotective diet can reduce inflammatory bowel disease risk: a longitudinal cohort study. Nutrients. 2022;14(19):4058. 10.3390/nu14194058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hinnouho G.‐M, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. 2015;36(9):551–559. 10.1093/eurheartj/ehu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, et al. A score of low‐grade inflammation and risk of mortality: prospective findings from the Moli‐sani study. Haematologica. 2016;101(11):1434–1441. 10.3324/haematol.2016.144055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burns EM, Rigby E, Mamidanna R, Bottle A, Aylin P, Ziprin P, et al. Systematic review of discharge coding accuracy. J Public Health. 2012;34(1):138–148. 10.1093/pubmed/fdr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_pdp.pdf
- 29. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486–2497. 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 31. Braga Neto MB, Gregory M, Ramos GP, Loftus EV, Ciorba MA, Bruining DH, et al. De‐novo inflammatory bowel disease after bariatric surgery: a large case series. J Crohn's Colitis. 2018;12(4):452–457. 10.1093/ecco-jcc/jjx177 [DOI] [PubMed] [Google Scholar]
- 32. Kermansaravi M, Valizadeh R, Farazmand B, Mousavimaleki A, Taherzadeh M, Wiggins T, et al. De novo inflammatory bowel disease following bariatric surgery: a systematic review and meta‐analysis. Obes Surg. 2022;32(10):3426–3434. 10.1007/s11695-022-06226-2 [DOI] [PubMed] [Google Scholar]
- 33. Allin KH, Jacobsen RK, Ungaro RC, Colombel JF, Egeberg A, Villumsen M, et al. Bariatric surgery and risk of new‐onset inflammatory bowel disease: a nationwide cohort study. J Crohn's Colitis. 2021;15(9):1474–1480. 10.1093/ecco-jcc/jjab037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adolph TE, Meyer M, Schwärzler J, Mayr L, Grabherr F, Tilg H. The metabolic nature of inflammatory bowel diseases. Nat Rev Gastroenterol Hepatol. 2022;19(12):753–767. 10.1038/s41575-022-00658-y [DOI] [PubMed] [Google Scholar]
- 35. Cannavale CN, Bailey M, Edwards CG, Thompson SV, Walk AM, Burd NA, et al. Systemic inflammation mediates the negative relationship between visceral adiposity and cognitive control. Int J Psychophysiol. 2021;165:68–75. 10.1016/j.ijpsycho.2021.03.010 [DOI] [PubMed] [Google Scholar]
- 36. Yang YD, Zheng CJ, Dong YH, Zou Z, Lv Y, Wang Z, et al. Sex difference in the mediation roles of an inflammatory factor (hsCRP) and adipokines on the relationship between adiposity and blood pressure. Hypertens Res. 2019;42(6):903–911. 10.1038/s41440-019-0222-x [DOI] [PubMed] [Google Scholar]
- 37. Richard ML, Sokol H. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol. 2019;16(6):331–345. 10.1038/s41575-019-0121-2 [DOI] [PubMed] [Google Scholar]
- 38. Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20(1):40–54. 10.1038/s41577-019-0198-4 [DOI] [PubMed] [Google Scholar]
- 39. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health‐related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information S1
Data Availability Statement
Data can be obtained from the UK Biobank (www.ukbiobank.ac.uk/).


