Abstract
Despite the initial promise of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) in effectively combating tumor growth, the majority of patients with advanced non-small cell lung cancers (NSCLCs) inevitably develop resistance to these treatments. An infrequent genetic mutation known as BRAFV600E has been identified as a contributing factor to the emergence of acquired resistance to EGFR-TKIs. Genetic alterations in BRAF, particularly V600E, contribute to resistance to osimertinib. However, a combination therapy involving osimertinib, dabrafenib (a BRAF inhibitor), and trametinib has shown effectiveness in overcoming BRAF V600E-mediated resistance in advanced lung adenocarcinoma. This treatment regimen holds promise for similar cases. In our case report, the combination of osimertinib, dabrafenib, and trametinib effectively overcame osimertinib resistance and resulted in sustained partial remission.
Keywords: advanced lung adenocarcinoma, BRAFV600E mutation, EGFR-TKIs resistance, osimertinib, combination therapy
Introduction
Lung cancer stands as the leading cause of cancer-related deaths worldwide, with non-small cell lung cancer (NSCLC) accounting for approximately 85% of cases [1]. Substantial advancements have been made in targeting driver gene mutations in lung adenocarcinoma, the most common subtype of NSCLC. Notably, epidermal growth factor receptor (EGFR) mutations are present in 16% of advanced adenocarcinoma cases and are even more prevalent in Asian females, with a frequency of approximately 61.1% [2,3]. Consequently, EGFR-tyrosine kinase inhibitors (TKIs) have emerged as effective targeted therapies for lung adenocarcinoma [4]. Despite the initial efficacy of EGFR-TKIs, most advanced NSCLC cases inevitably develop acquired resistance (AR) to these treatments. One off-target downstream pathway contributing to AR in EGFR-mutated lung cancer is the V-Raf murine sarcoma viral oncogene homolog B1 (BRAF) V600E mutation [5–7]. Although the BRAFV600E mutation is considered uncommon, it plays a crucial role in AR for approximately 3% of patients undergoing second-line osimertinib treatment [8–11].
Dabrafenib and trametinib are FDA-approved BRAF and MEK inhibitors, respectively, utilized as first-line treatment for metastatic NSCLC patients with the BRAFV600E mutation [12–14]. In cases where osimertinib, an EGFR-TKI, proves ineffective for NSCLC patients exhibiting EGFR resistance with the BRAF V600E mutation, the combination of osimertinib with dabrafenib and trametinib has shown promise in overcoming resistance mediated by the BRAFV600E mutation in advanced lung adenocarcinoma [15–19].
Within this case report, we present a patient with lung adenocarcinoma who developed resistance to osimertinib and subsequently received the combination therapy of osimertinib, dabrafenib, and trametinib. The patient experienced partial regression of the tumor and maintained progression-free survival for over a year. Furthermore, continuous monitoring of the patient during treatment exhibited the manageable toxicity of the combination therapy.
It is worth noting that accurate identification of predictive genetic alterations is crucial for patient management, prognosis, and the understanding of AR mechanisms. Various sample types, detection methods, and genetic testing platforms can influence the accuracy of precision medicine and the selection of appropriate treatments [20]. Liquid biopsies, such as peripheral blood and pleural effusion analysis, have been proposed as alternatives to tissue biopsies for capturing tumor heterogeneity and clonal diversification [21–23]. Additionally, monitoring changes in serum carcinoembryonic antigen (CEA) levels has shown potential as a noninvasive option for monitoring and predicting outcomes in lung cancer patients [24].
Incorporating liquid biopsies and monitoring serum CEA levels can enhance the accuracy of genetic testing and contribute to patient management. Further research and clinical trials are necessary to validate the efficacy and safety of EGFR/BRAF/MEK co-inhibition in this patient population.
Results
Case report
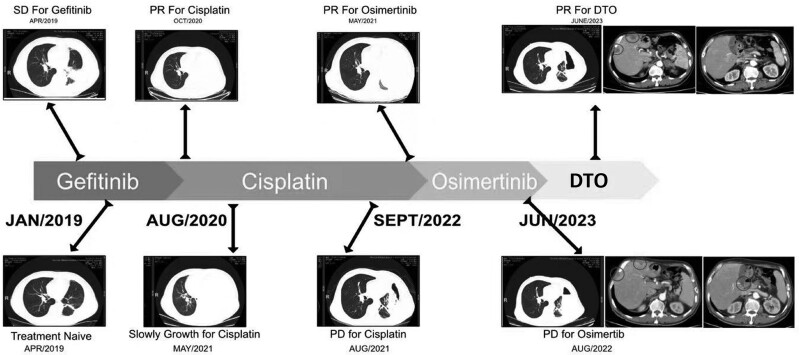
In January 2019, CT and blood tests were conducted on a 66-year-old male patient, revealing sporadic lung nodules, enlarged lymph nodes, pleural effusion, and elevated CEA levels. A biopsy confirmed the presence of lung adenocarcinoma. The initial diagnosis indicated stage IV left lung adenocarcinoma with malignant pleural effusion. Further examination through a lymph node biopsy revealed an EGFR exon 21 deletion, with an ECOG score of 2. Initially, the patient responded partially to EGFR-TKIs, but experienced abnormal liver function as a result. Subsequent reexamination of the patient after TKI treatment showed a significant increase in pleural effusion compared to pretreatment levels. After 2 cycles of cisplatin combined with pemetrexed, the patient’s disease remained uncontrolled.
Treatment with osimertinib was then initiated, resulting in a partial remission as the best response. However, with continued osimertinib treatment, the patient developed new liver and pancreatic metastases, leading to disease progression. To address this, a combined treatment involving osimertinib, dabrafenib, and trametinib was administered, resulting in a significant reduction in liver and pancreatic metastases and notable improvement in symptoms, with an ECOG score of 1. Adverse events were manageable. At the last follow-up in June 2023, the patient’s disease assessment still indicated a maintenance of partial remission. (Please refer to Figs. 1–3 for visual representations of the treatment course, tumor indicators, and genetic testing results.)
Fig. 1.
Radiologic images and time-line of patient’s clinical course. This figure displays radiologic images and a chronological time-line documenting the clinical course of patient 1. (The concept is adapted from Zeng et al’.s work in Cancer Drug Resistance 2021;4 : 1019-27.).
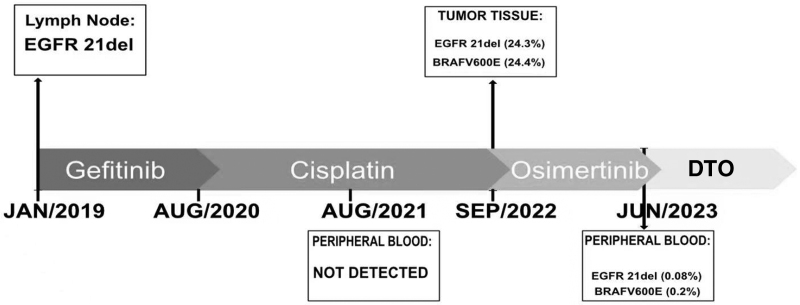
Fig. 3.
Mutations detected at different time-points from tissue or liquid biopsies in patient’s. This figure presents the mutations identified at various time-points through tissue or liquid biopsies in the patient. The concept is adapted from Zeng et al’.s study in Cancer Drug Resistance 2021;4 : 1019-27.
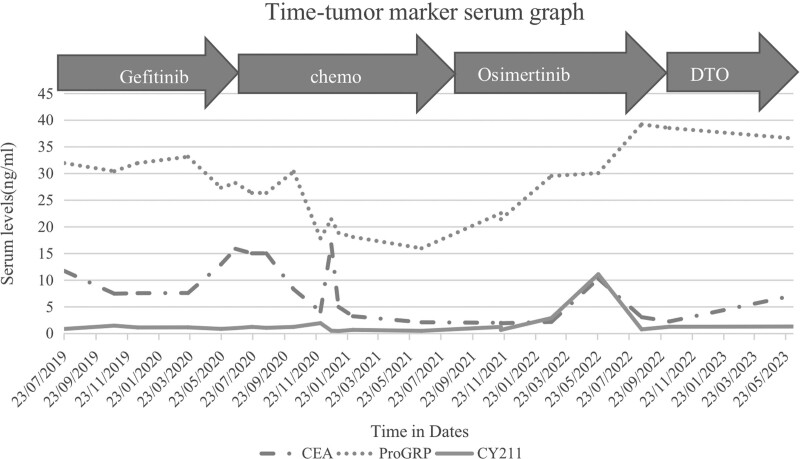
Fig. 2.
Changes in tumor serum levels during patient’s clinical course. In this figure, the fluctuations in tumor serum levels are illustrated, representing the changes observed throughout patient 1’s clinical course. (The concept is adapted from Zeng et al’.s research in Cancer Drug Resistance 2021;4 : 1019-27).
Efficacy and toxicity
In this 66-year-old male patient, the best response assessment for combination therapy after developing resistance to osimertinib was partial response, which has been maintained for over a year. During the course of combination treatment, the patient experienced recurring fever, with a maximum body temperature of 39 °C. Following a series of examinations, the fever was determined to be an adverse reaction associated with the drug therapy. After receiving symptomatic treatment, the patient’s body temperature returned to normal. Additionally, the patient developed mild rashes, fatigue, and nausea. However, after adjusting the dosage of the drug, the patient tolerated it well.
To the best of our knowledge, combined treatment targeting EGFR/BRAF/MEK has been investigated in eight cases, and we have compiled the clinical characteristics, treatment outcomes, and side effects in Table 1. Similarly, we have summarized the clinical characteristics, treatment outcomes, and side effects of the seven patients from our hospital in Table 2.
Table 1.
Overview of literature for osimertinib-induced BRAF V600 mutation with the reported efficacy and treatment toxicities
| Author | Cases | Baseline EGFR mutation | Previous treatment | Mutation profile at resistance to osimertinib | Treatment | Initial dose | Dose adjustment | Best overall response | Progression-free time | Adverse effect (grade) |
|---|---|---|---|---|---|---|---|---|---|---|
| Huang et al | 65 male | EGFR 19del | Gefitinib→Osimertinib | EGFR 19del/T790M BRAF V600E |
D + T + O | D:150 mg bid T:1 mg qd O:80 mg qd |
Not need | SD | >7.4months | Diarrhea(G1) Aronychia(G1) |
| Solassol et al | 68 female | EGFR 19del | Chemo→Afatinib→chemo→ICI→Osimertinib→chemo+anti-VEGF | EGFR 19del/T790M BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
Not need | SD | 6 months | NR |
| Ding et al | 63 male | EGFR 19del | Gefitinib→Osimertinib | EGFR 19del/T790M BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
Not need | SD | 9 month | Pyrexia(G1-2) Arontchia(G1-2) |
| Zhou et al | 69 male | EGFR L858R | Post-operative recurrence, gefitinib→chemo→osimertinib→chemo | EGFR L858R/T790M BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
Not need | PR | >2 months | Rash(G2) Decreased apptite(G2) |
| Meng et al | P1 : 56 female P2 : 66 male |
P1:EGFR 19del P2:EGFR 19del |
P1:Gefitinib→osimertinib P2:Afatinib→osimertinib |
P1:EGFR 19del BRAF V600E P2:EGFR 19del/T790M BRAF V600E |
P1: D + T + O P2: D + T + O |
P1/P2: D:150 mg bid T:2 mg qd O:80 mg qd |
P1:discontiuation P2:D:50 mg bid T:0.5 mg qd O:80 mg qd |
/ PR |
P1 : 6 weeks P2 : 13.4 months |
P1:Pneumonitis P2:Pyrexia(G2) Nausea,vomiting |
| Ribeiro et al | 50 female | EGFR 19del | Erlotinib→Osimertinib+SBRT→chemo+ICI→chemo | EGFR 19del/T790M BRAF V600E,PIK3CA mutation |
D + T + O | D:75 mg bid T:1 mg qd O:80 mg qd |
D:150 mg bid T:2 mg qd O:80 mg qd (Not succeed) |
PR | 8 months | Pyrexia,nausea dysgueusia(G1) Fatigue(G1→G2) |
| Mauclet et al | 60 female | EGFR 19del | Chemo+WBRT→ICI→erlotinib→osimertinib | EGFR 19del/T790M BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
D:75 mg bid T:1 mg qd O:40 mg qd |
PR | 7 months | Increased creatine kinase(G3) Pyrexia(G2) |
This table provides a summary of various literature sources that have investigated osimertinib-induced BRAF V600 mutation. It includes information on the effectiveness of the treatment (efficacy) as well as the adverse effects and side effects experienced by patients during the course of the treatment (treatment toxicities). The table serves as a comprehensive reference for understanding the outcomes and potential risks associated with osimertinib in relation to the specific BRAF V600 mutation.
Specific combination regimen, efficacy and toxicity of osimitinib-induced BRAF V600 mutations have been reported in the literature. (Concept adapted from Zeng et al. Cancer Drug Resist 2021;4 : 1019-27).
D, dabrafenib; O, osimertinib; T, trametinib.
Table 2.
Patients were followed up for the efficacy and toxicity of the combination therapy
| Cases | Baseline EGFR mutation | Previous treatment | Mutation profile at resistance to osimertinib | Treatment | Initial dose | Dose adjustment | Best overall response | Progression-free time | Adverse effect (grade) |
|---|---|---|---|---|---|---|---|---|---|
| 66 male | EGFR L858R | Gefitinib→chemo→Osimertinib | EGFR L858R BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
D:75 mg bid T:2 mg qd O:80 mg qod |
PR | >13months | Pyrexia(G2) Rash(G1) Fatigue(G1) Nausea(G1) |
| 56 female | EGFR 19del | Gefitinib→Osimertinib | EGFR T790M BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
D:100 mg bid T:1 mg qd O:80 mg qod |
SD | 6 months | Fatigue(G2) Pain(G2) |
| 79 female | EGFR T790M | Osimertinib→ Chemo+ICI |
EGFR L858R BRAF V600E |
D + T + O | D:75 mg bid T:2 mg qd O:80 mg qod |
Not need | - | 1 month | Fatigue(G2) Pain(G3) Nausea(G1) |
| 61 male | EGFR 19del | Osimertinib | EGFR L858R BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
Not need | PR | >5 months | Rash(G1) Fatugue(G1) |
| 77 female | EGFR 19del | Gefitinib→Osimertinib | EGFR L858R BRAF V600E |
D + T + O | D:100 mg bid T:2 mg qd O:80 mg qd |
D:75 mg bid T:2 mg qd O:80 mg qod |
PR | 18 months | Pyrexia(G2) decreased appetite(G2) Fatigue(G2) |
| 67 female | EGFR L858R |
Gefitinib→Osimertinib | EGFR L858R BRAF V600E |
D + T + O | D:100 mg bid T:2 mg qd O:80 mg qd |
Not need | PR | 14 months | Diarrhea(G1) Rash(G1) Fatigue(G1) |
| 68 female | EGFR L858R |
Osimertinib | EGFR L858R BRAF V600E |
D + T + O | D:150 mg bid T:2 mg qd O:80 mg qd |
D:75 mg bid T:2 mg qd O:80 mg qod |
PR | 20 months | Pyrexia(G2) decreased appetite(G1) Rash(G1) |
This table presents data related to patient follow-up during a combination therapy study. It includes information on the effectiveness of the combination therapy in terms of its efficacy (how well it achieved the desired treatment outcomes) and any observed toxicity (side effects or adverse reactions) experienced by the patients during the follow-up period. The table provides valuable insights into the performance and safety of the combination therapy in a clinical setting.
Efficacy and treatment toxicity of our hospital patients with osimertinib-induced BRAFV600 mutations.
D, dabrafenib; O, osimertinib; T, trametinib.
Discussion
The concurrent presence of BRAF V600E and EGFR-TKI resistance mutations poses a significant challenge in the treatment of advanced NSCLC. However, emerging evidence suggests that a promising therapeutic approach may lie in the combination of dabrafenib, trametinib, and osimertinib. This triple therapy has demonstrated remarkable efficacy in overcoming resistance and achieving meaningful clinical responses. Dabrafenib and trametinib, BRAF and MEK inhibitors respectively, effectively target the BRAF V600E mutation, leading to inhibition of the MAPK pathway and subsequent tumor regression [25].
A non-comparative, open-label international multicenter trial called BRF113928 demonstrated an overall response rate of 63% for the combination therapy of dabrafenib and trametinib, with response durations of 6 months or longer observed in 64% of responders [22]. These promising outcomes led to the approval of dabrafenib and trametinib by the Food and Drug Administration in 2017 for the first-line treatment of metastatic NSCLC patients carrying the BRAFV600E mutation. An updated phase 2 study (NCT01336634) revealed encouraging efficacy of dabrafenib plus trametinib as a second-line treatment, with a median progression-free survival of 10.2 months and a median overall survival of 18.2 months [24].
In our seven cases, the majority of patients responded well to the combination therapy, with 5 out of 7 patients achieving a progression-free survival (PFS) time of 6 months or longer. The objective response rate (ORR) reached 71.4%, and the median PFS reached 11 months. In addition to our own cases, we observed an ORR of 50%, with a PFS of 6 months or more in 75% of the eight patients in Table 1.
However, there have been no clinical trials conducted to verify the efficacy and safety of EGFR/BRAF/MEK co-inhibition in patients with EGFR exon 21 (E21) and BRAFV600E mutations after osimertinib treatment. Although this combination therapy demonstrates promising efficacy, it is essential to consider potential toxicities
In our seven cases, all 7 patients experienced adverse effects (AE), including fever (3/7), diarrhea (1/7), rash (4/7), fatigue (6/7), loss of appetite (2/7), nausea (2/7), and pain (2/7). Most adverse reactions were mild and were well tolerated after adjusting the drug dosage, and only one patient died within 1 month of the combination treatment. It is important to note that this patient’s death was not caused by the drug but rather by the development of her poor condition and overall physical state, as she had undergone multiple therapies prior to the combination treatment. In addition to our own cases, AE were experienced by eight patients (87.5%), including fever (4/8), nausea (2/8), nail inflammation (2/8), skin rash (1/8), fatigue (1/8), reduced appetite (2/8), vomiting (1/8), diarrhea (1/8), altered taste sensation (1/8), pneumonitis (1/8), and elevated creatine kinase levels (1/8). AE was mild.
Overall, whether reported in other studies or in our own case, the combined use of osimertinib, dabrafenib, and trametinib in patients with osimertinib resistance has shown significant benefits. By assessing patients’ physical status prior to combination therapy and closely monitoring them during treatment, adverse reactions can be managed with symptomatic treatment or adjustments to the dosage and frequency of drugs, effectively controlling the corresponding toxic side effects.
Furthermore, we found that fever occurred early in the treatment and was associated with a longer PFS in these cases. As depicted in Table 1, patients with fever (55.6%) tended to have a longer PFS (average PFS: 8.4 months, ranging from 4.7 to 13.4 months) compared to those without fever (average PFS: 4.2 months, ranging from 1.5 to 7.4 months). In our case, three out of the seven patients experienced varying degrees of fever, and febrile patients (42.9%) tended to have a longer PFS (average PFS: 17 months, ranging from 13 to 20 months) than those without fever (average PFS: 6.5 months, ranging from 1 to 14 months). Although the connection between fever and clinical outcomes remains unclear, there appears to be a tendency towards extended PFS in patients with fever in our case and in previously reported cases. Further prospective clinical trials with larger sample sizes are needed to explore the association between fever and clinical outcomes.
Accurate identification of genetic alterations is crucial for patient management, quality of life, prognosis, and understanding the mechanisms of different therapies. In this case, multiple genetic tests guided the long-term treatment management. However, when the patient developed resistance to gefitinib, no targeted driver mutation was found in the peripheral blood. Consequently, the treatment was switched to chemotherapy, and a liver biopsy was performed, revealing the presence of BRAF mutation. Subsequently, an EGFR 21 del mutation was identified through a lung biopsy, although no targeted driver mutation was found in the peripheral blood NGS test. This discrepancy may be attributed to the higher sensitivity of genetic tests derived from tumor tissues. However, despite the identification of these mutations, the patient experienced rapid disease progression and a short duration of response to treatment. This rapid progression with osimertinib in this case could be attributed to the presence of other undetected resistance mechanisms.
The primary manifestation of progressive disease in this patient was lymphangitis carcinoma of the lung, which posed challenges for tumor tissue biopsy. Liquid biopsy, such as peripheral blood and pleural effusion analysis, may be a better alternative as it can capture tumor heterogeneity and clonal diversification [26]. Monitoring the dramatic change of the driving mutation through circulating free DNA tests has been emphasized in several studies [27,28]. Therefore, liquid biopsy and tissue genetic analysis complement each other, and the selection of the appropriate sample type, method, and platform plays a vital role in the accuracy of precision medicine, particularly for patients experiencing drug resistance. Furthermore, a study suggested that serum CEA determinations are a feasible and noninvasive option for monitoring and prognosis [29]. Consistent with previous findings, the change in serum CEA, in this case, remained consistent with the anti-tumor response.
In conclusion, the combination therapy of osimertinib, dabrafenib, and trametinib shows promise as a targeted therapeutic option for patients with advanced NSCLC harboring both BRAF V600E and EGFR mutations. This treatment approach offers impressive efficacy while considering the importance of genetic testing, treatment monitoring, and the management of potential adverse events. Further prospective clinical trials with larger sample sizes are warranted to validate these findings and explore the long-term outcomes and potential applications of this therapeutic strategy.
Acknowledgements
We are sincerely grateful to all the members of The First Oncology Department at Jinzhou First Affiliated Hospital for their invaluable contributions through insightful discussions and unwavering support. We extend our heartfelt appreciation to Professor Xiaomei Liu for her innovative ideas and design concept. Furthermore, we express our deep gratitude to Li Xi for providing valuable statistical advice, which greatly enhanced the quality of our work.
This research was supported by Huilan Public Welfare Foundation under grant number HS2022-009 and the Exchange Training Program in the field of public health, under the grant number IIT-2022-010.
(1) Conception and design: Xiaomei Liu and Clint Taonaishe Chimbangu. (2) Administrative support: Xiaomei Liu. (3) Provision of study material: Zhou Ya, Li Xi, Zhao Jiayue, Meng Xiao, Wang Ying, Yu Xingxu, Xiaomei Liu, Clint Taonaishe Chimbangu. (4) Collection and assembly of data: Clint Taonaishe Chimbangu, Li Xi. (5) Data analysis and interpretation: Clint Taonaishe Chimbangu. (6) Manuscript writing: Clint Taonaishe Chimbangu. (7) Final approval of manuscript: All authors.
The data that supports the findings of this study are available upon reasonable request from the corresponding author. Please contact Clint Taonaishe Chimbangu at chimbangu@live.com for inquiries related to accessing the data.
Ethics statement: This article adheres to strict ethical guidelines, including informed consent from participants and maintaining anonymity and confidentiality throughout the research process. We affirm the originality of the content, avoidance of conflicts of interest, and compliance with all relevant regulations.
We confirm that the manuscript has been read and approved by all the named authors.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al.; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361:958–967. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai C-M, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 2018; 15:694–708. [DOI] [PubMed] [Google Scholar]
- 5.Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017; 17:637–658. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin Y-L, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A 2012; 109:E2127–E2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949–954. [DOI] [PubMed] [Google Scholar]
- 8.Yao Z, Torres NM, Tao A, Gao Y, Luo L, Li Q, et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 2015; 28:370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790Mpositive lung cancer and acquired resistance to osimertinib. JAMA Oncol 2018; 4:1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated nonsmall cell lung cancer. Br J Cancer 2019; 121:725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho CC, Liao WY, Lin CA, Shih JY, Yu CJ, Yang JC. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J Thorac Oncol 2017; 12:567–572. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Gan J, Guo K, Deng Y, Fang W. Acquired BRAF V600E mutation mediated resistance to osimertinib and responded to osimertinib, dabrafenib, and trametinib combination therapy. J Thorac Oncol 2019; 14:e236–e237. [DOI] [PubMed] [Google Scholar]
- 13.Mauclet C, Collard P, Ghaye B, Hoton D, Nana FA. Tumor response to EGFR/BRAF/MEK co-inhibition in a patient with EGFR mutated lung adenocarcinoma developing a BRAFV600 mutation as an acquired resistance mechanism. Lung Cancer 2021; 159:42–44. [DOI] [PubMed] [Google Scholar]
- 14.Solassol J, Vendrell JA, Senal R, Audran P, Leenhardt F, Quantin X. Challenging BRAF/EGFR co-inhibition in NSCLC using sequential liquid biopsies. Lung Cancer 2019; 133:45–47. [DOI] [PubMed] [Google Scholar]
- 15.Zhou F, Zhao W, Chen X, Zhang J, Zhou C. Response to the combination of dabrafenib, trametinib and osimertinib in a patient with EGFR-mutant NSCLC harboring an acquired BRAFV600E mutation. Lung Cancer 2020; 139:219–220. [DOI] [PubMed] [Google Scholar]
- 16.Meng P, Koopman B, Kok K, Ter Elst A, Schuuring E, van Kempen LC, et al. Combined osimertinib, dabrafenib and trametinib treatment for advanced non-small-cell lung cancer patients with an osimertinib-induced BRAF V600E mutation. Lung Cancer 2020; 146:358–361. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro MFSA, Knebel FH, Bettoni F, Saddi R, Sacardo KP, Canedo FSNA, et al. Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFRmutant/BRAF V600E lung adenocarcinoma patient. npj Precis Oncol 2021; 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding H, Zhuang Z, Xie J, Huang H, Tao Z, Liu Z. Durable clinical response of advanced lung adenocarcinoma harboring EGFR-19del/T790M/BRAFV600E mutations after treating with osimertinib and dabrafenib plus trametinib: a case report. Onco Targets Ther 2020; 13:7933–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. Common Terminology criteria for adverse events. Version 5.0. Published November 27, 2017. 2020. [Google Scholar]
- 20.Mu Y, Yang K, Hao X, Wang Y, Wang L, Liu Y, et al. Clinical characteristics and treatment outcomes of 65 patients with BRAF-mutated non-small cell lung cancer. Front Oncol 2020; 10:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planchard D, Besse B, Groen HJM, Souquet P-J, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016; 17:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jóri B, Schatz S, Kaller L, Kah B, Roeper J, Ramdani HO, et al. Comparison of resistance spectra after first and second line osimertinib treatment detected by liquid biopsy. Cancers (Basel) 2021; 13:2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schadendorf D, Robert C, Dummer R, Flaherty KT, Tawbi HA, Menzies AM, et al. Pyrexia in patients treated with dabrafenib plus trametinib across clinical trials in BRAFmutant cancers. Eur J Cancer 2021; 153:234–241. [DOI] [PubMed] [Google Scholar]
- 25.Subbiah V, Baik C, Kirkwood JM. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer 2020;6:797–810. [DOI] [PubMed] [Google Scholar]
- 26.Nichols D, Boyle TA, Noyes D, Latifi K, Feygelman V, Jackson I, et al. Evaluation of combined anti-PD-1 immunotherapy and radiation therapy in a preclinical mouse model of pneumonitis and fibrosis. J Thorac Dis 2018; 10:6254–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Miranda FS, Barauna VG, Dos Santos L, Costa G, Vassallo PF, Campos LCG. Properties and application of cell-free DNA as a clinical biomarker. Int J Mol Sci 2021; 22:9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arrieta O, Varela-Santoyo E, Cardona AF, Sánchez-Reyes R, Lara-Mejía L, Bassarmal SS, et al. Association of carcinoembryonic antigen reduction with progression-free and overall survival improvement in advanced non-small-cell lung cancer. Clin Lung Cancer 2021; 22:510–522. [DOI] [PubMed] [Google Scholar]
- 29.Zeng R, Luo L, Sun X, Bao Z, Du W, Dai R, et al. EGFR/BRAF/MEK co-inhibition for EGFR-mutated lung adenocarcinoma patients with an acquired BRAFV600E mutation: a case report and review of literature. Cancer Drug Resist 2021;4:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]