Abstract
Airway management is required during general anaesthesia and is essential for life-threatening conditions such as cardiopulmonary resuscitation. Evidence from recent trials indicates a high incidence of critical events during airway management, especially in neonates or infants. It is important to define the optimal techniques and strategies for airway management in these groups. In this joint European Society of Anaesthesiology and Intensive Care (ESAIC) and British Journal of Anaesthesia (BJA) guideline on airway management in neonates and infants, we present aggregated and evidence-based recommendations to assist clinicians in providing safe and effective medical care. We identified seven main areas of interest for airway management: i) preoperative assessment and preparation; ii) medications; iii) techniques and algorithms; iv) identification and treatment of difficult airways; v) confirmation of tracheal intubation; vi) tracheal extubation, and vii) human factors. Based on these areas, Population, Intervention, Comparison, Outcomes (PICO) questions were derived that guided a structured literature search. GRADE (Grading of Recommendations, Assessment, Development and Evaluation) methodology was used to formulate the recommendations based on those studies included with consideration of their methodological quality (strong ‘1’ or weak ‘2’ recommendation with high ‘A’, medium ‘B’ or low ‘C’ quality of evidence). In summary, we recommend: 1. Use medical history and physical examination to predict difficult airway management (1С). 2. Ensure adequate level of sedation or general anaesthesia during airway management (1B). 3. Administer neuromuscular blocker before tracheal intubation when spontaneous breathing is not necessary (1С). 4. Use a videolaryngoscope with an age-adapted standard blade as first choice for tracheal intubation (1B). 5. Apply apnoeic oxygenation during tracheal intubation in neonates (1B). 6. Consider a supraglottic airway for rescue oxygenation and ventilation when tracheal intubation fails (1B). 7. Limit the number of tracheal intubation attempts (1C). 8. Use a stylet to reinforce and preshape tracheal tubes when hyperangulated videolaryngoscope blades are used and when the larynx is anatomically anterior (1C). 9. Verify intubation is successful with clinical assessment and end-tidal CO2 waveform (1C). 10. Apply high-flow nasal oxygenation, continuous positive airway pressure or nasal intermittent positive pressure ventilation for postextubation respiratory support, when appropriate (1B).
Keywords: airway management, difficult airway, neonate, paediatric anaesthesia, practice guidelines
Airway management is required for anaesthetised patients undergoing surgical or diagnostic procedures and is essential for life-threatening conditions such as cardiopulmonary resuscitation and critical care. Several guidelines have been published to standardise airway management and tracheal intubation procedures for routine and emergency situations in patients with normal, known, or anticipated difficult airway.1–4 However, there are no specific guidelines for neonates and infants.
Children have a unique anatomy and physiology that can present clinicians with significant challenges.5 Younger children, term neonates, and pre-term neonates are at the highest risk for respiratory and traumatic complications from airway management. Most devices available for airway management are not specifically designed or tested for use in children.3,6–8
This practice guideline aims to provide an evidence-based approach to airway management in neonates and infants. It was developed by a core group of experts in paediatric airway management, with the intention to serve anaesthetists working in a variety of paediatric settings, from highly specialised to district centres. As expertise and resources differ across centres, these practice guidelines are not a standard of care, however, they should serve as a basis for developing local institutionally approved operating procedures and best practice guidelines.
Methods
In 2021, 23 internationally recognised experts in airway management formed a task force aimed at writing updated practice guidelines for airway management in neonates and infants. The proposal was submitted to the European Society of Anaesthesiology and Intensive Care (ESAIC) and to the Editorial Board of the British Journal of Anaesthesia (BJA) for logistic support and endorsement. A joint endeavour between ESAIC and BJA was approved.
Clinical queries were developed in the form of seven Population, Intervention, Comparison, Outcome (PICO) groups and further developed into five elements for the search strategy. The complete list of PICO groups was then revised and approved by the task force, and generated the following research questions:
-
1.
Is physical assessment the best way to predict difficult airway management? Can physical assessment be improved by further measurements? Which normal values should be used?
-
2.
What type of preparation and planning should be mandatory before starting airway management in neonates and infants? Is neuromuscular block recommended for tracheal intubation if maintaining spontaneous breathing is not necessary?
-
3.
Is direct laryngoscopy the first-choice technique for tracheal intubation in neonates and infants? Should direct laryngoscopy be replaced by other techniques? What is the definition of a difficult intubation? Is a tracheal intubation algorithm needed?
-
4.
What should be the gold standard for anticipated difficult airway management, and who should be involved? Where should difficult airway management be performed?
-
5.
Which technique should be used for detecting the correct position of the tracheal tube in neonates and infants?
-
6.
What is the best strategy for safe tracheal extubation in neonates and infants after difficult intubation, either anticipated or unanticipated? Should extubation or removal of an airway device be performed under deep anaesthesia or when the patient is awake?
-
7.
What is the impact of human factors and the need for developing a specific paediatric airway curriculum?
Criteria for considering studies for data analysis
Types of studies: Data analysis was based on all randomised, parallel, quasi-randomised studies (including crossover design) and observational studies that addressed the above queries. Systematic reviews and meta-analyses were considered on a case-by-case basis when meeting inclusion criteria. Data from quasi-randomised, observational and large retrospective studies were included as very few, if any, randomised controlled trials (RCTs) were anticipated.
Types of participants: The qualitative and quantitative analysis of the literature was confined to children up to 1 yr of age, with or without specified comorbidities. Studies including a mix of paediatric and adult populations were reviewed only if they included a relevant number of infants. In case there was a lack of data for neonates and infants undergoing airway management in the operating room, and if considered relevant, data were extrapolated from non-operating room settings (i.e. neonatal and paediatric intensive care unit and emergency department).
Types of interventions: We included the following interventions: i) physical assessment for detecting or predicting a potentially difficult airway; ii) child and staff preparation for airway management, including pharmacological treatment; iii) direct laryngoscopy for tracheal intubation; iv) specific competency and techniques for expected difficult intubation; v) chest auscultation confirmation of correct tracheal tube position; vi) strategies for tracheal extubation in children in whom tracheal intubation was difficult, including use of an airway management debriefing; vii) influence of human factors and competencies on successful airway management.
Types of comparators: Any technique or strategy for preparation, or both, initial and intraoperative management, and extubation different from the above-mentioned ‘interventions’ were considered as comparators, both in routine care and difficult airway management, either expected or unexpected.
Types of outcomes: First-pass tracheal intubation success, number of attempts until successful intubation was achieved, and any complication during and after airway management were considered as outcomes.
Search methods for identification of studies
An information specialist (AC) developed the literature search strategy in close collaboration with ND and the ESAIC group methodologists (AA, PK, and CSR). The literature search was conducted in PubMed, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL). A similar search strategy was used for all databases. The electronic database search was run on November 17, 2021 by AC, and included articles published since 2011 to increase clinical relevance (Appendix 1). Panel members were also encouraged to add any missing papers of interest that they were aware of, and to conduct related searches themselves. The resulting titles were screened by two independent authors. A third author was assigned to resolve conflicts for inclusion or exclusion.
Search results
After removing duplicates, the authors screened titles with abstracts in a two-stage procedure. Relevant papers were retrieved for full text assessment and data extraction by task force subgroups who compiled and wrote the literature review for their respective PICO groups. The methodologist was responsible for choosing topics for possible meta-analyses based on the quality of the available data, reliability of the search (sensitivity), and the predefined inclusion and exclusion criteria. For this guideline, we found no data suitable for meta-analysis.
For simplicity of the search, screening, inclusion and exclusion of studies, all retrieved titles and articles were allocated into five folders: A) preparation of airway management, B) tracheal intubation, C) difficult airway, D) tracheal extubation, and E) human factors. The retrieved articles were:
-
A)
From 972 publications on preparation, after removal of duplicates and date restrictions, the remaining 466 titles were screened, resulting in 60 abstracts. For the next step, 22 full articles were included.
-
B)
From 3991 publications on tracheal intubation, after removal of duplicates and date restrictions, the remaining 2486 titles were screened, resulting in 532 abstracts. For the next step, 94 full articles were included.
-
C)
From 570 publications on difficult airway, after removal of duplicates and date restrictions, the remaining 278 titles were screened, resulting in 191 abstracts. For the next step, 86 full articles were included.
-
D)
From 6507 titles on tracheal extubation, after removal of duplicates and date restrictions, the remaining 4637 titles were screened, resulting in 613 abstracts. For the next step, 180 full articles were included.
-
E)
From the 994 titles on human factors, 992 were screened, 64 abstracts were selected, and 37 full articles were used for a short scoping review.
A detailed description of the search strategy and PICO are shown in Appendix 1.
Data collection and analysis
Selection of studies: all publications meeting inclusion criteria were included. At least two authors in each PICO group assessed the relevant full text articles independently by using Covidence software. Disagreements were resolved by a member not involved in the screening.
Data extraction and management: Each task force group extracted data from relevant studies in a similar fashion with guidance from the methodologist using similar spreadsheets, including information on study design, population characteristics, interventions, and outcome measures. Task force group authors reached a consensus regarding extracted data through discussion, initially within the group, and secondly within the entire task force.
Assessment of risk of bias in included studies: Risk of bias was assessed for each PICO group in accordance with the Cochrane Handbook for Systematic Reviews of Interventions source for RCTs,9 and was assessed for the following domains:
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of outcome assessors (performance and detection bias)
Incomplete outcome data, intention-to-treat (attrition bias)
Selective reporting
Trials were assessed as having a low risk of bias if all of the domains were considered adequate, medium risk if one domain was inadequate, and high risk if more than one domain was considered inadequate or unclear. Disagreement regarding assessment of the risk of bias was settled in a discussion with the methodologist (AA). For non-RCTs, checklists from SIGN (Scottish Intercollegiate Guidelines Network;https://www.sign.ac.uk/what-we-do/methodology/checklists/) were applied.
Assessment of the quality of the evidence: In accordance with the ESAIC guidelines policy, the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology was used to assess methodological quality and to formulate recommendations.
Decisions to downgrade the level of evidence for a recommendation were based on the quality and type of the included literature, observed inconsistencies, indirectness or directness of the evidence, overall impression, and the presence of publication bias as proposed by GRADE. Decisions to upgrade the level of evidence for recommendations were based on study quality and magnitude of the effect ratio, dose-response gradient, and plausible confounding. The GRADE definitions are summarised in Table 1. A more detailed account of GRADE is available at https://www.uptodate.com/home/grading-guide.
Table 1.
GRADE definitions. GRADE, Grading of Recommendations, Assessment, Development and Evaluation.
| Grade of recommendation | Clarity of risk/benefit | Quality of supporting evidence |
| 1A Strong recommendation, high-quality evidence |
Benefits clearly outweigh risk and burdens, or vice versa | Consistent evidence from well performed randomised, controlled trials or overwhelming evidence of some other form; further research is unlikely to change our confidence in the estimate of benefit and risk |
| 1B Strong recommendation, moderate quality evidence |
Benefits clearly outweigh risk and burdens, or vice versa | Evidence from randomised, controlled trials with important limitations (inconsistent results, methodological flaws, indirect or imprecise), or very strong evidence of some other research design; further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate |
| 1C Strong recommendation, low-quality evidence |
Benefits appear to outweigh risk and burdens, or vice versa | Evidence from observational studies, unsystematic clinical experience, or from randomised, controlled trials with serious flaws; any estimate of effect is uncertain |
| 2A Weak recommendation=suggestion, high-quality evidence |
Benefits closely balanced with risks and burdens | Consistent evidence from well-performed randomised, controlled trials or overwhelming evidence of some other form; further research is unlikely to change our confidence in the estimate of benefit and risk |
| 2B Weak recommendation=suggestion, moderate-quality evidence |
Benefits closely balanced with risks and burdens, some uncertainty in the estimates of benefits, risks, and burdens | Evidence from randomised, controlled trials with important limitations (inconsistent results, methodological flaws, indirect or imprecise), or very strong evidence of some other research design; further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate |
| 2C Weak recommendation=suggestion, low-quality evidence |
Uncertainty in the estimates of benefits, risks, and burdens; benefits may be closely balanced with risks and burdens | Evidence from observational studies, unsystematic clinical experience, or from randomised, controlled trials with serious flaws; any estimate of effect is uncertain |
Development of recommendations: After the above procedures, each group developed recommendations relevant to their PICO group and clinical questions. These were then discussed and rediscussed, as required, with the entire expert panel in light of the data synthesis (when available), the risk of bias, and the quality of the evidence.
One general limitation across all PICO groups was heterogeneity of the case mix, such that many studies included children >1 yr old. Consequently, the task force assessed external validity and generalisability of the findings of the included studies for each recommendation. When considered reasonable, evidence from older patients was used for drafting recommendations, suggestions, or clinical practice statements.
A two-step Delphi process using an online survey was used to produce expert recommendations and degree of agreement. A third Delphi round was performed by teleconference to discuss methodological quality of the supporting literature and when rephrasing recommendations was mandated. The same voting and consensus processes were applied to each recommendation, suggestion, or clinical practice statement.
Summary of recommendations, suggestions and clinical practice statements.
PICO 1. Preoperative airway assessment to predict difficulty
Is physical examination the best way to predict difficult airway management? Can physical examination be implemented by further measures? Which normal values should be used?
Recommendation: We recommend use of medical history and physical examination to predict difficult airway management in neonates and infants (1C).
Evidence summary: No evidence is available supporting or refuting the use of physical assessment to predict difficult airway management in neonates and infants. However, clinician experience supported by several retrospective studies identified some physical risk factors for a difficult airway in neonates and infants: micro-, retro-, or prognathia, limited mouth opening, facial asymmetry, fixed cervical spine, labio-palatine cleft, and oral or neck mass.10–12 These physical features commonly exist in neonates and infants with syndromes associated with anticipated difficult airway management such as the Pierre Robin, Crouzon, and Treacher-Collins syndromes.11,13 However, there are no studies prospectively evaluating the role of physical features in predicting difficult airway management (mask ventilation or tracheal intubation) in neonates and infants with an apparently normal airway. While several adult based studies have suggested simple summations or weighted risk scores for predicting ‘unanticipated’ difficult tracheal intubation based on physical measures (e.g. symptoms of a pathological airway; inter-incisor gap; mandible luxation; thyromental distance; head and neck movement; and Mallampati score), none of these have been validated in children nor is it always possible to assess these parameters.14–16 The Mallampati score is not feasible in neo-nates and infants as it requires a cooperative patient who can follow verbal commands. Other measures (e.g. measuring thyromental distance) are not always possible and are subject to dramatic changes as neonates and infants grow rapidly in the first months of life. Therefore, no normal values are available for neonates and infants.
A single centre case series of eight infants aged 3 weeks to 1 yr has shown the potential benefit of using volumetric computer tomography (CT) imaging to delineate abnormalities of the lower trachea and lungs (Supplementary Table S1).17 However, the case series did not provide data for upper airway abnormalities that can be associated with difficult airway management. No other measures were found to help with prediction of difficult airway in neonates and infants.
PICO 2. Preparation for airway management and pharmacological treatment (outside resuscitation)
What preparation and planning should be mandatory before airway management in neonates and infants? Is neuromuscular block mandatory if spontaneous breathing is not necessary (pharmacology)?
Recommendation: We recommend use of an adequate level of sedation or general anaesthesia in neonates and infants during airway management to ensure patient comfort and safety (1B).
Recommendation: We recommend use of neuromuscular block before tracheal intubation when maintaining spontaneous breathing is not necessary (1C). The risks and benefits of neuromuscular blocking agent administration should be balanced for the individual patient and team skills.
Evidence summary: Except in cases of resuscitation (e.g. in the delivery room), tracheal intubation in neonates and infants with minimal or no anaesthesia is a largely abandoned clinical practice. The reasons for promoting awake intubation in the past were varied, including fear of adverse effects such as pulmonary aspiration, poor tolerance of infants to hypo-xaemia, lack of knowledge of pharmacology in the youngest children, and the significant medicolegal implications of dealing with this population.
Seven RCTs,18–24 13 (five retrospective, eight prospective) observational studies,25–37 and one systematic review38 were identified that examined different sedation or anaesthesia regimens (Supplementary Tables S2 and S3). The age of patients included in the RCTs ranged from 26 weeks to 3 yr, and included a total of 486 patients. The age of patients in the observational studies was from 24 weeks to 7 yr, and studied 10 759 intubation attempts. One observational study was interrupted prema-turely,28 thus five retrospective studies27,31,35–37 and seven observational studies25,26,29,30,32–34 remained for analysis. Very heterogenous drug regimens were used in neonates and infants. Some studies assessed the effects of opioids during intubation, the differences between inhalation and i.v. anaes-thesia,24,33,37 the optimal dose for propofol,29,38 the use of neuromuscular blocking agents,21–23,31,34–36 and a variety of drugs used for premedication.25–27,30,32 All studies assessed orotracheal intubation except one RCT and two observational studies that recruited patients for nasotracheal intubation.26,36 Data on emergent intubation were only available in two observational studies.25,26 Finally, only one observational study compared orotracheal intubation in a group that maintained spontaneous ventilation and in a group with controlled venti-lation.31 To determine the best preparation for neonates and infants for undergoing orotracheal intubation, we extracted data on first attempt success, number of attempts, and adverse events reported in each group. Many studies did not report adverse events (e.g. hypotension, hypertension, hypercapnia, hypoxaemia, upper airway trauma, myoclonus, hypothermia, or laryngospasm), nor the occurrence of severe adverse events (e.g. death, tonic-clonic seizures, pneumothorax, sepsis, digestive tract perforation, pulmonary haemorrhage, cardiac arrest, supraventricular tachycardia, pulmonary hypertension, aspiration syndrome, or hyponatraemia). We did not find evidence of an increase in the total number of adverse events related to use of sedative or general anaesthetic drugs.
Several studies compared a group without sedation or anaesthesia with an anaesthetised group. Compared with no sedation or anaesthesia, anaesthesia increased the success rate of intubation on the first attempt, and reduced the number of attempts and incidence of complications. Some studies compared several anaesthesia regimens, in some cases up to 13 different regimens of sedative drugs30 or the combination of more than two drugs in the same group. Anticholinergic drugs were used in the majority of studies before induction of anaesthesia, with atropine the most frequently used agent. Use of an anticholinergic drug was not associated with a lower incidence of bradycardia. Based on the available evidence, neonates and infants receiving opioids will have lower noci-ception as measured with the premature infant pain profile than those who did not receive opioids.20 Use of a neuro-muscular blocking agent was found to improve the quality of intubation conditions and to decrease the median number of orotracheal intubation attempts. Two RCTs that used rocuro-nium reported a higher rate of successful first attempt tracheal intubation, even at doses as low as 0.2 mg kg−1 in one RCT.22,23 Atracurium was used in one underpowered RCT that failed to detect a clinically relevant difference.21 Suxamethonium was addressed in two observational studies.27,32 In summary, use of a neuromuscular blocking agent increases the success of tracheal intubation and reduces the incidence of complications such as laryngospasm.
PICO 3. Tracheal intubation
Is direct laryngoscopy or videolaryngoscopy the first-choice technique for tracheal intubation in neonates and infants?
Recommendation: We recommend the use of a video-laryngoscope with an age-adapted standard blade (Macintosh or Miller) as first choice for tracheal intubation of neonates and infants (1B), including for tracheal intubation in the lateral position (1C).
Clinical practice statement: a videolaryngoscope should also be used for teaching purposes using a dual approach: direct laryngoscopy for the trainee and videolaryngoscopy for the tutor. The screen can serve as guide for feedback during the intubation manoeuvre performed by the trainee.
Clinical practice statement: Training is a mandatory and essential prerequisite for correct use of a videolaryngos-cope. The use of a videolaryngoscope is warranted in anaesthesia suites, intensive care units, and emergency departments.
Evidence summary: Five RCTs comparing videolaryngo-scopy (VL) with direct laryngoscopy (DL) were assessed (Supplementary Table S4).8,39–41 The first is an RCT that enrolled 564 infants and has indicated that videolaryngoscopy with a standard Miller blade was superior to direct laryngos-copy. Infants <6.5 kg in weight had greater first-attempt success rate for orotracheal intubation when videolaryngoscopy was used compared with direct laryngoscopy (92% vs. 81%).39 Another RCT among neonatology residents compared video-laryngoscopy with direct laryngoscopy for intubation of premature neonates.40 Overall intubation success rate was greater in the videolaryngoscopy group (57% vs. 33%). First-year residents and all residents intubating their first patient had higher intubation success rates with videolaryngoscopy than with direct laryngoscopy (58% vs. 23% and 50% vs. 17%, respectively). Among residents with <6 months’ tertiary neonatal experience, when the instructor was able to view the video-laryngoscope screen success rate was 66% (69 of 104) compared with 41% (42 of 102) when the screen was covered (odds ratio [OR] 2.81, 95% confidence interval [CI] 1.54–5.17).41 When pre-medication was used, the success rate in the intervention group (videolaryngoscopy) was 72% (56 of 78) compared with 44% (35 of 79) in the control group (direct laryngoscopy) (OR 3.2, 95% CI 1.6–6.6). A multicentre RCT compared video-laryngoscopy with direct laryngoscopy in neonates and infants with apnoeic oxygenation added in both groups. First attempt tracheal intubation success rate with no desaturation was greater with videolaryngoscopy (89% [108 of 121]; 95% CI 83.7–94.8%) compared with direct laryngoscopy (79% [97 of 123]; 95% CI 71.6–86.1%), with an adjusted absolute risk difference of 9.5% (CI O.8–18.1%).6
Two RCTs compared videolaryngoscopy with direct laryn-goscopy for tracheal intubation with the patient in the lateral position.42,43 The videolaryngoscopy group had a higher first-attempt success rate and provided a more favourable glottic view compared with conventional direct laryngoscopy with a Miller blade. These studies used the C-MAC (Karl Storz, Tut-tlingen, Germany) videolaryngoscope in comparison to direct laryngoscope for tracheal intubation. One single-centre RCT (Supplementary Table S5) enrolled 120 children, 1–24 months of age, undergoing elective surgery, and showed that there was no difference between direct laryngoscopy with a Miller or Macintosh blade for laryngoscopic views and intubation conditions.44
Should apnoeic oxygenation become standard of care during tracheal intubation?
Recommendation: We recommend the use of apnoeic oxygenation during tracheal intubation in neonates (1B).
Clinical practice statement: In infants, the of apnoeic oxygenation (low or high flow) during tracheal intubation should be based on the risk of hypoxaemia in the patient and the experience of the provider.
Evidence summary: Five RCTs have shown that supplemental oxygen increases the safe apnoea time in neonates and infants and reduces the incidence of hypoxaemia during tracheal intubation (Supplementary Table S6).45–49 Supplemental oxygen can be delivered directly via the airway instrumentation device AirTraq (Prodol Meditec S.A., Vizcaya, Spain), Truview (Truphatek International Ltd, Netanya, Israel), Oxiport (Truphatek International®, Israel), nasopharyngeal airway or via nasal cannula.
One RCT which studied use of high-flow nasal oxygenation (HFNO) before orotracheal intubation in 251 patients (median age 28 weeks)46 showed a success rate for orotracheal intubation on the first attempt of 50% (62 of 124 patients) when HFNO was used and 32% (40 of 127 patients) when standard care (morphine and midazolam, without HFNO) was used. An observational study found that the incidence of desaturation during attempts at tracheal intubation in paediatric patients was significantly lower when 5 L min−1 oxygen was provided via a nasal cannula.50 One RCT comparing the efficacy of vid-eolaryngoscopy and direct laryngoscopy in neonates and infants showed that the incidence of desaturation during intubation was low in both groups.6 Even though the study was designed to compare direct laryngoscopy vs. video-laryngoscopy, the authors stated that the low incidence of adverse events could be partially attributed to supplementary oxygen administration prolonging the safe apnoea time.6,50 The optimal oxygen flow remains to be determined; it should be reported in L kg−1 min−1 to assess the effectiveness and permit comparisons.6,50
There are sparse data for anticipated difficult airways. We found only a single case report in a preterm neonate with multiple airway abnormalities and repeated attempts at tracheal intubation.51
Cuffed or uncuffed tracheal tube as standard of care?
Recommendation: Cuffed and uncuffed tubes can both be safely used (cuffed tubes in children >3 kg) (1C).
Clinical practice statement: For the safe use of cuffed tubes, we recommend adherence to the manufacturer's instructions, including size and cuff inflation pressure (minimal cuff pressure to avoid air leak, not exceeding 20–30 cm H2O), to reduce the risk of postextubation stridor. Anatomical variation, clinical conditions, and degree of prematurity might warrant the use of an uncuffed tube.
Evidence summary: Cuffed tracheal tubes are increasingly used in neonates, infants, and preschool children, with RCTs showing fewer tube exchanges for selecting the best size without affecting postextubation complications (Supplementary Table S7).52–54 The effect of cuffed tubes on the incidence of postintubation stridor or complications in neo-nates remains unclear because of a lack of sufficient data.54–56
Retrospective studies and case series indicate the need to be vigilant regarding cuff inflation pressure limits.57–59 There are insufficient data to support the routine use of a cuffed tracheal tube in children <3 kg with external tube diameter being the limiting factor.58
What is unanticipated difficult intubation in neonates and infants?
Suggestion: We suggest defining unanticipated difficult intubation as: ‘two failed tracheal intubation attempts’ to facilitate (i) comparison between studies and (ii) assessment of the effectiveness of interventions (2C).
Evidence summary: A standardised definition of difficult intubation in neonates and infants is required, especially when difficult intubation is unanticipated by medical history and physical assessment. Four large prospective observational databases were considered, all of which define difficult tracheal intubation as a minimum of two failed attempts (Supplementary Table S8).3,4,60,61 This definition will allow standardised reporting of future studies.
Nasal or oral route for tracheal intubation in neonates and infants?
Clinical practice statement: the nasal route is often preferred for success rate in neonates, and the oral route for infants, but limited data are available to recommend the nasal or oral route. When the nasal route is chosen, the risk of bleeding and nose preparation with topical vasoconstrictors should be considered.
Evidence summary: Limited data are available to recommend the preferred intubation route (Supplementary Table S9). Nasal intubation is preferred in some institutions for ease in securing the tracheal tube, especially when prolonged intubation is expected.62
Can a supraglottic airway device be an alternative to a tracheal tube in difficult airway or emergency situations?
Recommendation: We recommend use of a supraglottic airway device for rescue oxygenation and ventilation when tracheal intubation has failed or if face mask ventilation is inadequate (1B).
Evidence summary: All studies are single-centre RCTs (Supplementary Table S10).63–66 A supraglottis airway device permits oxygenation and ventilation in neonates and infants either during elective surgery or resuscitation, and can be used as an alternative to either a tracheal tube or face mask ventilation. The incidence of major adverse respiratory events during elective surgery was significantly higher for use of a tracheal tube than for use of a supraglottic airway device, with a risk ratio of 5.3 (95% CI 1.6–17.4).63 Two RCTs in neonates aged ≥34 weeks reported that supraglottic airway devices were more effective than face mask for oxygenation during cardiopulmonary resuscitation, potentially avoiding the need for tracheal intubation.64,65 One multicentre RCT reported fewer attempts and faster placement of a supraglottic airway device compared with tracheal intubation (32 vs. 66 s).66
Is a neonatal and infant tracheal intubation algorithm (cognitive aid) necessary?
Recommendation: We recommend development of a multi-disciplinary consensus based tracheal intubation cognitive aid for neonates and infants to harmonise clinical practices and potentially reduce tracheal intubation related morbidity and mortality and to enable assessment of long term outcomes (1C).
Evidence summary: Two prospective observational multi-centre studies (NECTARINE and NEAR4NEOS) reported a high incidence of difficult tracheal intubation and adverse tracheal intubation-associated events in neonates and infants (Supplementary Table S11).3,67 The high variability of clinical approaches could have contributed to the high incidence of adverse events. The NECTARINE trial reported frequent use of neuromuscular blocking agents (72.6%) and limited use of videolaryngoscopy.3 Practices independently associated with reduced adverse events in the NEAR4NEOS registry included videolaryngoscopy (OR 0.46, 95% CI 0.28–0.73) and neuro-muscular block (OR 0.38, 95% CI 0.25–0.57).67 A prospective multicentre trial to investigate the benefits of neuromuscular blocking agents was abandoned early because of a lack of funding causing it to be underpowered.21
PICO 4. Difficult airway management
What should be the gold standard for anticipated difficult airway management and who should be involved?
Clinical practice statement: For anticipated difficult airway management, at least one type of videolaryngoscope, flexible intubating scope, or rigid or semirigid scope should be available, including appropriate sizes for the patient's age, in addition to routinely used equipment such as supraglottic airway devices and face masks.
Evidence summary: There is no ‘one solution fits all’ for difficult airway management for neonates and infants. In any medical care area where infants might need respiratory support, established equipment for bag-mask ventilation, for opening of the upper airway (such as oral or nasal airways), supraglottic airway devices, suction tubes, laryngoscopes, and tracheal tubes in appropriate sizes should be readily available. Every instrument for instrumentation of the difficult airway has limitations in certain situations. As the source of airway difficulties varies individually, individuals’ airway restrictions inevitably demand one of the various types of airway devices.
Recommendation: We recommend limiting the number of tracheal intubation attempts by reassessing the clinical condition and by considering a change to a different technique, different provider, or both after each attempt (1C).
Clinical practice statement: after four attempts, clinicians should consider aborting intubation attempts and waking the patient if feasible.
Evidence summary: Multiple attempts at tracheal intubation with the same technique result in a higher probability of complications and can cause airway oedema or bleeding. This reduces the chance of success of subsequent attempts with the same or other techniques (Supplementary Table S12). A single centre retrospective analysis of 1341 intubations in healthy infants for routine operative procedures found an increased risk for hypoxaemia (OR 1.78, 95% CI 1.30–2.43) when multiple intubation attempts occurred.68 In a prospective observational single centre trial including 171 intubations on neonatal intensive care wards, occurrence of more than two intubations was identified as the only independent risk factor (OR 6.7, 95% CI 1.3–33.6) for adverse outcomes (brady-cardia, hypotension, or hypertension).69 Comparable results have been reported in a retrospective analysis of an international multicentre database for neonatal intubations because of respiratory failure.70 Two attempts had an OR of 1.6 and three or more attempts an OR of 1.8 for severe adverse events. Thus, after an initial failed attempt, assistance by additional personnel with special airway expertise and supportive devices must be consulted immediately.
Clinical practice statement: There is insufficient evidence to recommend which patients should be intubated with hyperangulated blades. However, in cases where standard blades fail and the airway is difficult (anterior larynx, suspected cervical spine injury, or limited movement), the next step should be an alternative advanced technique including use of hyperangulated blades with a stylet, flexible or rigid bronchoscopy alone or in combination with video-laryngoscopy, or flexible bronchoscopy via a supraglottic airway device.
Evidence summary: When conventional direct laryngos-copy fails to provide a sufficient view of the glottis for tracheal intubation, videolaryngoscopy is commonly used as the first advanced technique.
Hyperangulated (non-standard) videolaryngoscopes are often superior in visualising the glottis when conventionally shaped (standard) blades fail.71–73
There is a difference in intubation success rate in infants and neonates when different types of videolaryngoscopes are used. The success rate for intubation using hyperangulated videolaryngoscopes is lower than with Miller blade video-laryngoscopes. In conventional direct laryngoscopy, a good view of the glottis is usually associated with easy intubation. This might not be the case when performing indirect video-laryngoscopy, particularly when using a hyperangulated blade.74–77 Because videolaryngoscopy provides a full view of the glottis without aligning the oral and the tracheal axis, passage of a tracheal tube can be more difficult. This is particularly true if a hyperangulated blade is used, resulting in greater misalignment of the oral and tracheal axes and a longer and more angulated route for the tracheal tube to pass into the trachea. This makes use of a stylet mandatory when a hyperangulated videolaryngoscope is used. This can result in more time needed for tracheal intubation and lower overall success rates, especially in small children.71,72,78–84 Modification of the shape of a stylet by adding an angle of 15 degrees has been described when using a hyperangulated blade.85,86
Based on this evidence, other alternative techniques that have been studied in this population for tracheal intubation when direct laryngoscopy is difficult include flexible bron-choscopy, intubation through a supraglottic airway device, or combined use of videolaryngoscopy with flexible bronchoscopy. Data from the Pediatric Difficult Intubation Registry suggest that these advanced techniques have similar success rates, and it is not possible to recommend any single technique. The choice of intubation technique will depend on the equipment available, the experience of the clinician managing the patient, and the airway anatomy of the patient. It is important that practitioners not persist with failing techniques, and that an alternative be used early in the process, as severe complications associated with airway management occur with repeated attempts at laryngoscopy and intubation.
Recommendation: We recommend use of a stylet to reinforce and preshape tracheal tubes when a hyperangulated laryngoscope blade is used or when the larynx is anatomically anterior (1C).
Clinical practice statement: Routine use of a stylet to improve the success rate of tracheal intubation by novice practitioners and trainees cannot be recommended when performing laryngoscopy with standard blades. Bougies of appropriate size can be used to facilitate a difficult intubation or to guide tube insertion.
Evidence summary: Using a stylet during laryngoscopy with standard blades to reinforce or preshape the tracheal tube does not appear to increase the success rates of tracheal intubation in neonates and infants and might be traumatic to neonatal and infant airways (Supplementary Table S13). A retrospective analysis of >5000 neonatal intubations, one RCT, and two meta-analyses (with low quality of evidence) did not identify benefits for first time success rate or complications if a stylet was used or not.55,58,87,88 Nevertheless, a stylet or bougie might be essential if reinforcement or stronger angulation of the tracheal tube is needed or when the larynx is anatomically anterior and angulation of the tracheal tube is essential to facilitate intubation.85,89
Recommendation: We recommend flexible bronchoscopy by the nasal route in case of restricted mouth opening (1C).
Clinical practice statement: Flexible fibreoptic tracheal intubation can be performed through a supraglottic airway device, a specially designed face mask or via one nostril while a nasopharyngeal tube is in place in the other nostril for oxygenation. Intubation through a special face mask can be easier, especially for trainees or novices, and when performed via the nasal route. Another provider can assist with mask ventilation during the intubation. If not using a supraglottic airway device, trainees and novices might choose the nasal route for fibreoptic intubation, unless contraindicated, under supervision of an expert physician.
Evidence summary: In most situations of difficult airway management, use of a flexible bronchoscope is a suitable solution for tracheal intubation (Supplementary Table S14). There are no alternatives if a fully visualised transnasal tracheal intubation is required or if mouth opening is severely restricted. The nasal passage is easier for less experienced trainees because of more straightforward guidance of the scope compared with the oral route.90
Suggestion: We suggest use of a rigid bronchoscope as an advanced technique when the laryngeal inlet is obstructed by swelling and in cases of upper airway stenosis or compression or in congenital or postsurgical tracheal constriction or tortuosity (2C). If necessary, a multidisciplinary team (including an otolaryngologist) should be involved.
Evidence summary: Flexible intubating fibreoptic scopes are not the single best solution for all problems with the pae-diatric airway because of its fundamental limitations: a limited field of view, interference from bleeding and secretions, and flexibility of the device as only the tip of the bron-choscope can be steered. If it is necessary to advance a bronchoscope through a narrow space, such as laryngeal stenosis or tracheal obstruction, use of a rigid endoscope can be advantageous. If preloaded with a tracheal tube, the rigid scope can be used to guide the tracheal tube beyond the stenosis. In cases of long tracheal stenosis (for example extra-luminal compression by a mediastinal mass), placement of a rigid bronchoscope might be required. This enables navigation through the narrow region while splinting the airway open, and at the same time allowing ventilation via a side port.78,91–95 The main limitation of rigid and semirigid scopes is possible difficulty in aligning the oral and tracheal axes, for example in cases of severely restricted mouth opening or severely restricted retroflexion of the head.96–98 A trial including 26 children (12 of them infants) with difficult airway reported quicker intubation with a semirigid scope than with a fibreoptic scope (52 vs. 83 s) (Supplementary Table S15).97
Clinical practice statement: After induction of general anaesthesia, when tracheal intubation fails, oxygenation and ventilation via a supraglottic airway device or face mask are severely impaired or impossible, and spontaneous breathing cannot be restored, a surgical tracheotomy should be performed. Of several techniques described, evidence is lacking for superiority of one technique over another.
Surgical cricothyroidotomy and percutaneous needle crico-thyroidotomy are not suitable options in neonates and infants; for the former because the small size of the cricothyroid membrane will likely render insertion of a tracheal tube impossible, and for the latter because of the unfavourable anatomy.
Clinical practice statement: When the expertise and equipment are available, extracorporeal membrane oxygenation (ECMO) can be considered as a rescue intervention for a difficult airway when waking the patient is not an option. However, there is lack of evidence supporting such a recommendation, and the decision and timing to proceed with ECMO is left to local guidelines.
Evidence summary: Current literature does not provide clear guidance on how and when to perform emergency front-of-neck access to the trachea as a life saving intervention in neonates and small children. The small size of the cricothy-roid membrane is not compatible with surgical or percutaneous cricothyrotomy in neonates and infants and the compressibility of the trachea makes a percutaneous approach relatively impractical. Therefore, a tracheostomy is preferable.99 Even with maximal extension of the head and neck, the combination of the high location of the larynx in the neck and the relationship between the mandible and the trachea forces percutaneous access to the airway to be approached at a very steep angle, resulting in tracheal compression and the risk of posterior wall perforation with subsequent problems leading to overall failure. A single pae-diatric case report described the need to rescue the airway with surgical access after unsuccessful percutaneous crico-thyroidotomy.100 Based on little evidence, we propose that a surgical tracheostomy represents the preferred emergency access to the trachea in neonates and infants.101,102 Access to the trachea is provided with a tracheostomy tube or a standard cuffed tracheal tube of appropriate size for age. This procedure should be carried out by the most competent physician available. A coordinated multidisciplinary team approach should be considered. Local airway leaders should prepare a standard operating procedure based on their specific expertise and availability of other involved disciplines in their institutions.
Clinical practice statement: No evidence is available supporting or refuting management of difficult airways in a particular location (operating room or intensive care unit) if adequate expertise, equipment, and medications for managing anticipated and unanticipated difficult airways are available.
Evidence summary: We found no evidence to support a particular location for difficult airway management, either during tracheal intubation or extubation. However, as expertise, equipment, and medications are all mandatory to ensure safe management, the operating room has all of these immediately available.
PICO 5. Confirmation of tracheal intubation
Which technique should be used for confirmation of successful tracheal intubation and correct positioning of the tube in neonates and infants?
Recommendation: We recommend immediate verification of successful intubation with both clinical assessment (bilateral and symmetrical breath sounds) and end-tidal carbon dioxide (EtCO2) with sustained EtCO2 waveforms. In cases of difficult intubation or complex patients, use of videolaryngoscopy for a second look, in combination with EtCO2 waveforms and ul-trasonography, should be considered to confirm successful tracheal intubation (1C).
Evidence summary: A large, multicentre, retrospective, observational study that included all paediatric ages (<18 yr) compared the efficacy of waveform capnography (quantitative EtCO2 measurement with a waveform) and colorimetric CO2 detection in confirming tracheal intubation, but did not separate infants for analysis.103 This study reported no significant difference between the two techniques for the primary endpoints of oesophageal intubation with delayed recognition, cardiac arrest, or oxygen desaturation, but was considered underpowered.
Two observational studies analysed ultrasonography in children including infants.104,105 Both studies used ultrasound for rapid confirmation and assessed the anterior neck at the level of the cricothyroid membrane to detect either tracheal intubation or inadvertent oesophageal intubation. One study added an additional two points of assessment on the anterior chest wall at the level of each nipple along the midclavicular line to check for lung sliding.104 The reference comparator was capnography for successful intubation and chest radiography for correct tracheal tube placement. Both the three-point and single-point ultrasonography assessments had 100% sensitivity and specificity for successful intubation. In a study using a single-point assessment, capnography failed to detect proper placement in two patients (a 17-yr-old and a 3-month-old) and oesophageal intubation (a 2-month-old), but these were correctly detected by ultrasonography.105 The three point study also assessed endobronchial intubation vs.trachealintubation, with sensitivity and specificity for ultrasonography of 86% (95% CI 56.7–96.0%) and 98% (95% CI 94.8–99.5%), respectively.104 Interobserver agreement with ultrasonography for the three point study was high. Median ultrasonography operation time was 11 s (inter-quartile range: 10–17 s) vs. 12 min (inter-quartile range: 9–16 min) for chest radiography in the three-point study, and was 17 s (95% CI: 12.9–21.2 s) and 14 min (95% CI 12.3–15.8 min) in the single point study.
One RCT and one observational study in neonates compared use of a respiratory function monitor to measure gas flows (i.e. flow waves) vs. waveform capnography and colorimetric EtCO2 detection, respectively.106,107 Both studies showed that flow waves were accurate and faster in assessing correct tracheal tube placement, whereas EtCO2 failed to detect CO2 in 16–31% of successful intubations resulting in unnecessary reintubations. Flow waves require attachment to a modern ventilator and expertise to analyse appropriately. Failures of EtCO2 can be exacerbated in micro-premature ne-onates because of the low volume of CO2 expired and diluted in the gas flow of the CO2 sampling line.
Finally, guidelines on unrecognised oesophageal intubation suggest the routine use of videolaryngoscopy to allow team communication and verbalisation of correct tube positioning. Even though these guidelines are for intubation of adults, to some extent they can be applied to neonates and infants, where correct tracheal intubation should be confirmed quickly to avoid hypoxaemia.108 The articles included are summarised in Supplementary Table S16.
Suggestion: We suggest verifying optimal tracheal tube position in the tracheobronchial tree in case of complex patients or those with continued clinical instability with chest radiography, visualisation of the carina with a flexible fibre-scope, chest ultrasonography, or all (2C).
Evidence summary: Malposition of the tracheal tube in infants can lead to life-threatening complications including hypo-xaemia, pneumothorax, and unintentional extubation.109,110 A method for estimating appropriate insertion depth of a tracheal tube in neonates has been extensively studied (Supplementary Table S17). Despite antero-posterior chest radiography considered as the gold standard for determining correct tracheal tube tip position,106,111,112 there are limitations to this method, including lack of immediacy, no definite interpretation guidelines, and radiation exposure. The commonly used ‘7-8-9 rule’ (7 cm at the lips for 1 kg, 8 cm for 2 kg, 9 cm for 3 kg), also known as the ‘weight+6’ rule in term infants,113 can overestimate tracheal tube insertion depth in preterm infants.111,114 Gesta-tional age and nasotragal length formulas have been proposed to estimate tracheal tube tip depth in infants, particularly premature and low birth weight neonates, with mixed results.115–120 As ultrasonography has become more popular, it has been studied to determine its predictive value compared with chest radiography.112 Two observational studies in neo-nates which assessed the feasibility of using ultrasonography to confirm location of the tracheal tube in the tracheobronchial tree used chest radiography performed before ultrasonography as the comparator.111,112 Although both studies had low numbers, they determined that ultrasonography was feasible to assess tracheal tube position in neonates without anatomical pathology of the neck or chest. Although chest radiography does not provide immediate confirmation, it remains the current gold standard.
PICO 6. Strategies for tracheal extubation
What is the best strategy for safe extubation in neonates and infants after difficult intubation, either anticipated or unanticipated?
Suggestion: We suggest assessing clinical signs like conjugate gaze, facial grimace, eye-opening and purposeful movements to predict successful awake extubation. If measurable, a tidal volume >5 ml kg−1 can support readiness to extubate (2C).
Clinical practice statement: Equipment for reintubation should be immediately available at every tracheal extubation, especially in cases of difficult tracheal intubation or previous failed extubation. The specific airway pathology, anatomical reason for difficult intubation (i.e. airway swelling), and number of intubation attempts should be considered.
Evidence summary: Whereas the literature is scarce regarding tracheal extubation in infants with difficult airway, studies have identified predictors for successful extubation in infants without difficult airways after general anaesthesia. One prospective cohort study highlighted a multifactorial approach including factors such as conjugate gaze, facial grimace, eye-opening, purposeful movements, and tidal volume >5 ml kg−1 to predict successful tracheal extubation in paediatric surgical patients with no airway abnormality and after volatile anaesthesia (Supplementary Table S18).121 Insufficient evidence exists to recommend using tube exchangers in neonates and infants (during extubation) when a clinician or physician expects a difficult reintubation.
Suggestion: We suggest use of intraoperative corticoste-roids, nebulised epinephrine, or both to prevent and treat postextubation stridor when significant airway manipulation has occurred (1C).
Evidence summary: One RCT that examined prevention and treatment of postextubation airway obstruction in chil-dren122 showed a beneficial role for nebulised L-epinephrine, with no dose-response effect evident.122 A systematic review and meta-analysis that analysed the effectiveness of cortico-steroids in preventing postextubation stridor and extubation failure supported corticosteroid use intraoperatively (Supplementary Table S19).123
Recommendation: We recommend use of HFNO, continuous positive airway pressure (CPAP), or nasal intermittent positive pressure ventilation (NIPPV) for postextubation respiratory support when clinically appropriate (1B).
Evidence summary: After tracheal extubation, there is strong evidence for application of NIPPV to reduce the incidence of extubation failure and reintubation within 48 h.124–127 HFNO has gained popularity as postextubation respiratory support for premature neonates. Whereas some studies failed to show benefit over NIPPV to prevent reintubation, there is increasing evidence for comparable beneficial effects of HFNO and nasal CPAP as postextubation respiratory support with less risk of nasal trauma.128–133 A summary of the articles included is in Supplementary Table S20.
Should tracheal extubation or removal of the airway device be performed under deep anaesthesia or when the patient is awake?
Clinical practice statement: The decision to remove a supra-glottic airway device or tracheal tube under deep anaesthesia in neonates and infants to minimise overall airway complications should depend on the following factors:
-
a)
experience of the anaesthesiologist,
-
b)
airway complications (e.g. bronchospasm or laryngospasm) at induction of anaesthesia or difficult or traumatic intubation or mask ventilation,
-
c)
overall risk of airway complications and reactivity (e.g. presence of upper or lower airway infection, asthma, airway anomalies, masses, reduced pulmonary oxygen reserve, thoracoabdominal asynchrony, ventilatory frequency, conjugate gaze, pharyngeal tone, high oxygen requirement),
-
d)
other comorbidities and risk factors (e.g. cardiac, metabolic, sepsis, actual or former prematurity),
-
e)
type of surgery (e.g. maxillofacial or craniofacial, ear-–nose–throat, neurosurgery or plastic surgery) and type of anaesthesia (total i.v. or inhalation anaesthesia),134
-
f)
high risk of pulmonary aspiration of gastric content (emergency surgery),
-
g)
availability of resources and backup for ensuring overall safety of the child,
-
h)
complete recovery from sedatives and neuromuscular blocking agents.
Evidence summary: Early removal of supraglottic airway devices in children under general anaesthesia is often advocated to reduce the risk of laryngospasm, coughing, airway obstruction/activation, desaturation, or haemodynamic instability, and to increase operating room turnover and efficiency. However, the literature often fails to distinguish between deep and awake extubation (Supplementary Table S21). A Cochrane systematic review assessed 15 RCTs of which 10 were exclusively in children and one included adults and children.135 All trials had a mixed population of children ranging from infants to 18 yr old and used the LMA Classic® for elective general anaesthesia. There was insufficient evidence in favour of or against early or late removal of a supraglottic airway device to reduce the risk of laryngospasm and desaturation. However, early removal was associated with a higher risk of airway obstruction (Relative Risk [RR] 2.82, 95% CI 1.28–6.22). Subgroup analyses based on age remained underpowered despite the larger proportion of paediatric trials. A small RCT failed to detect a difference between awake and deep supraglottic airway device removal in children (average age 33 months), but was underpowered.136
In a systematic review, the feasibility of awake tracheal extubation or with deep anaesthesia was assessed in 1881 children across 17 RCTs, with six trials assessing tracheal extubation and 11 RCTs assessing removal of supraglottic airway devices.137 Most of the studies had a mixed-age population and only one included infants. There was reduced risk of overall airway complications (OR 0.56, 95% CI 0.33–0.96), cough (OR 0.30, 95% CI 0.12–0.72), and desaturation (OR 0.49, 95% CI 0.25–0.95) with deep extubation. There was increased risk of airway obstruction in children with deep extubation (OR 3.38, 95% CI 1.69–6.73), but no significant difference for laryngospasm, desaturation, or breath-holding regardless of the airway device, while deep extubation appeared to reduce the risk of coughing with a tracheal tube but not for a supra-glottic airway device.
Wake Up Safe, a multi-institutional paediatric anaesthesia patient safety organisation and quality improvement registry, published the largest series of adverse events related to removal of airway devices under deep anaesthesia for children aged 7 weeks to 19 yr.138 Of 3652 events, 66 met inclusion criteria with the most prevalent being laryngospasm (55%), airway obstruction (11%), emesis (8%), apnoea (6%), and bronchospasm (6%). There were two events that led to cardiac arrest, one of which led to death. Overall 19 respiratory cases resulted in cardiac arrest, 15 of which were considered preventable. Table 2 summarises the situations and actions to consider if a difficult extubation is expected.
Table 2.
Situations and actions to be undertaken in case of anticipated difficult extubation.
| MANAGEMENT OF ANTICIPATED DIFFICULT EXTUBATION | |
| SITUATION | ACTION |
| PREPARATION | |
| Where | Extubation in the most appropriate location (operating room, intensive care). When in doubt transfer to the operating room |
| When | Daytime, when possible |
| Who | Ensure presence of competent and experienced physicians in paediatric difficult airway management |
| If high risk of failed extubation or history of advanced airway management, request ENT specialist or a competent physician able to perform eFONA or surgical access to trachea | |
| What | Have appropriate airway equipment available and ready to use for reintubation |
| Have adequate respiratory support systems present (i.e. CPAP, oxygen nasal cannula including high-flow systems) in case of risk of airway obstruction or desaturation | |
| Optimise any reversible confounder that may complicate extubation (i.e. high FO2 requirement, hypercapnia, acidosis, volume overload, haemodynamic instability, hypovolaemia, anaemia, neuromuscular block, hypothermia) | |
| EXTUBATION | |
| Wean and reverse | From anaesthesia, sedatives and neuromuscular agents |
| Time-out | Inform the team about the plan |
| Discharge | Have a clear strategy for the post-anaesthesia recovery in case of risk of secondary airway failure and obstruction |
Extubation is always an elective manoeuvre, and the plan should be developed and discussed beforehand. Based on the extubation strategy published by Weatherall and colleagues (Pediatr Anesth 2022; 32: 592−9). CPAP, continuous positive airway pressure; eFONA, emergency front of neck access; ENT, ear nose and throat; FiO2, fraction of inspired oxygen.
Human factors and paediatric airway competencies
What is the impact of human factors?
Clinical practice statement: Addressing human factors-related errors and improving communication and teamwork might reduce patient harm.
Similar to other complex systems with frequent humanhuman and human-machine interactions, adverse events in anaesthesia usually occur as a result of unpredictable combinations of human and organisational failures.139 Several human factor components are critical for anaesthesia safety including teamwork, communication, situational awareness, human error, cultural factors, and hierarchy.140–143 Cognitive processes in decision making and cognitive factors such as overconfidence, fixation errors, and loss aversion play a crucial role in decision-making in the fast-moving and complex anaesthesia environment.144 For example, fixation errors such as focussing on tracheal intubation rather than oxygenation can contribute to anaesthesia-related morbidity and mortal-ity.145 Clinicians may fear a loss of reputation and perseverate on a failing technique instead of calling for help (loss aversion). Human factors played a role in every incident reported to the 4th National Audit Project (NAP4) of the Royal College of Anaesthetists and Difficult Airway Society.146
No studies have specifically examined the impact of human factors on airway management in neonates and infants. Nevertheless, a retrospective review found that human factors played a role in 42.5% of operating theatre incidents in pae-diatric anaesthesia, of which 52.2% were airway or respiratory incidents.147 The most common were errors in judgement (43%), failure to check (17.8%), technical failures of skill (9.2%), inexperience (7.7%), inattention or distraction (5.6%), and communication issues (5.6%).147 Rule-based mistakes (28.0%) and latent (system) errors (24.9%) were the most common mechanisms involved.147 Other studies found similar numbers, with human factors contributing to more than half of all critical incidents in paediatric anaesthesia (58.5%) and more than half of the critical incidents were respiratory or airway events.148 One prospective observational study found that human factors such as communication and coordination failures played a role in many (non-airway-related) adverse events in paediatric cardiac anaesthesia, although cognitive compensation processes mitigated many errors.149 In the neonatal intensive care setting, team stress was associated with adverse events during tracheal intubation,150 and an increased number of observers increased trainee stress during simulated neonatal intubation.151 In general, issues at an organisational or system level might be easier to target than failures or unsafe acts of individuals.139 For clinical teams, simulation based team training has been shown to improve emergency team performance in paediatric emergencies.152 Assessment tools for team technical and behavioural skills have been developed, however it remains unclear whether they can help improve outcomes. Knowledge of human factors errors does not reduce their incidence, and systems changes remain the best way to mitigate the vast majority of individual mistakes.
What is the need for developing a specific paediatric airway curriculum?
Clinical practice statement: paediatric airway management requires a specific set of skills and structured training. Implementing and maintaining skills using neonatal intubation simulation training is advised when exposure during neonatal practice is not frequent enough.
Compared with adults, children have increased perioperative risk with a higher incidence of adverse events associated with airway management in neonates and infants.3,67,153 The APRICOT study, a prospective, observational multicentre cohort study with a total of 30 874 children, including 361 (1.2%) neonates and 2912 (9.4%) infants, found an incidence of severe perioperative critical events in 5.2% of children, with higher incidence in younger children and with a beneficial effect of the experience and seniority of the team.153 Of these critical events, a high percentage were respiratory critical events and five of nine children who suffered a cardiac arrest <1 yr old.153 An inverse relationship between the incidence of complications and the number of paediatric anaesthesia cases per year performed by the anaesthetist has also been reported.154
With regards to airway management analysis of the NEAR4NEOS registry, an association between training level and outcomes of neonatal intubation with first-attempt success rate of 23% for residents, 53% for fellows, and 60% for consultants was found.155 Similar data with higher success rates and fewer adverse events with higher training levels were reported for paediatric intensive care units. Data from the NEAR4KIDS registry also indicated a significant improvement in first-attempt tracheal intubation success rates for ICU fellows during their fellowship along with a reduction of tracheal intubation-related adverse events. Interestingly, there was no significant difference in tracheal intubation-related adverse events in the first quarter compared with the rest of the academic year.156 Finally, specific techniques have been investigated: oral fibrescope intubation in children <2 yr of age was significantly faster when performed by experts compared with residents.90 It is well known that complications increase with increasing airway manipulations and intubation attempts.153 These data show improved outcomes with higher levels of physician training.
No studies have directly assessed whether airway management skills are transferable from adult patients to older children or infants and neonates. However, a manikin study comparing intubation performance by 52 paediatricians and 52 anaesthetists indicated better neonatal and paediatric intubation skills for anaesthetists,157 suggesting a degree of transferability of intubation skills. This is highlighted by studies involving paediatricians who report their last intubation occurring more than 1 year ago.158 Simulation-based training for neonatal tracheal intubation is not comparable to adult simulation training. Neonatal mannequins have low fidelity and often do not adequately replicate the neonatal and infant airway. Because of often limited reproduction of anatomical conditions, differences in consistency of artificial tissues, and lack of natural secretions and slipperiness, training with neonatal mannequins does not fully prepare trainees for neonatal intubation. This expert opinion was supported by a questionnaire by participants of mannequin based neonatal tracheal intubation training.159 At least familiarity with the devices and algorithms and team performance can be improved by simulation training.
For training of neonatal and infant intubation in the real world, videolaryngoscopes with standard blades (Macintosh or Miller) should be used to provide a team view of the procedure and enable continuous supervision and teaching.160–162 The equipment necessary for safe airway management should be used regularly by those who manage difficult neonatal airways.
Final remarks and discussion
These practice guidelines were developed by an international task force on behalf of the ESAIC and BJA with the aim of providing evidence-based recommendations. The task force decided to limit the guidelines to the neonatal and infant population (up to 1 yr of age) because these patients have been identified as the most vulnerable during airway management. They experience a higher risk of complications such as hypoxaemia, cardiac arrest, airway trauma, and neurologic injury during airway management than older children.
The task force recommends a careful physical examination to identify patients at risk of difficult airway management. Medical history and physical assessment represent the best ways to predict a difficult airway and plan airway management. Use of videolaryngoscopy in this patient population is strongly recommended. Most recent evidence on neonatal and infant airway management demonstrates the benefit of vid-eolaryngoscopy in reducing the number of attempts at tracheal intubation and increasing first-pass tracheal intubation success compared with direct laryngoscopy. However, direct laryngoscopy and videolaryngoscopy are not mutually exclusive techniques. With standard blade videolaryngoscopes, it is possible to perform both direct laryngoscopy and indirect videolaryngoscopy simultaneously. One of the difficulties in interpreting the evidence before the task force was the lack of clarity over the role of indirect laryngoscopy (where only the video screen is used) and video-assisted direct laryngoscopy where a video-enabled laryngoscope is used to perform direct laryngoscopy. The use of indirect videolaryngoscopy and video-assisted direct laryngoscopy facilitates teaching, improves team communication, and ensures that personnel are involved in all steps of airway management, including appropriate placement of the tracheal tube. However, the task force emphasises the importance of teaching the correct use and potential pitfalls of videolaryngoscopy in order to minimise harm to children with the transition to use of video-laryngoscopy, which will probably replace traditional direct laryngoscopes in the near future. Although the role of videoassisted direct laryngoscopy remains unclear, it is a technique that nicely straddles the two techniques and should be an important focus during paediatric airway training.
The guidelines highlight the importance of oxygenation during the tracheal intubation process. Oxygen administration during airway instrumentation can increase the safe apnoea time and improve the chance of successful tracheal intubation. The task force is not able to recommend one technique of oxygenation over another. Regardless of the technique used to provide supplementary oxygen, care must be taken to ensure that oxygen administration is limited by a pressure-limiting valve and continued provision of anaesthesia to avoid accidental awareness.163 The guidelines emphasise the importance of limiting the number of attempts at tracheal intubation as they are associated with increased incidence of adverse events. The task force agreed to limit the number of tracheal intubation attempts to four (one by a physician in training and three by a senior anaesthesiologist) and to consider waking the patient if the airway cannot be secured. The task force recommends an adequate depth of anaesthesia and the use of neuromuscular blocking agents before tracheal intubation attempts. The practice of awake neonatal intubation should be limited to specific clinical conditions. Neuro-muscular blocking agents facilitate tracheal intubation and should be considered before laryngoscopy and tracheal intubation.
Although airway management guidelines traditionally focus on tracheal intubation, the task force recognises that tracheal extubation in children <1yrofagecanbeascriticalastracheal intubation, and therefore recommends careful planning for tracheal extubation.164 Clinicians should plan safe tracheal extubation and, if necessary, be prepared to secure the airway again after extubation in the most appropriate location with sufficient staff and equipment readily available. Use of tube exchangers introduced and left in the tracheal tube before extubation when intubation was difficult or the patient had complex airway management is anecdotal and cannot be recommended, but the task force emphasises that a reintubation plan should always be considered in these situations.165,166
Although there is little published evidence on the impact of human factors on airway management in this population, the task force recognises its importance in airway management outcomes, and recommends optimising communication, debriefing, and education about the pitfalls of innate human behaviour in patient safety.
Implementation of neonatal and infant airway management guidelines into daily clinical practice is critical to improving patient care and outcomes. Considering the different settings across the globe, it is important that the guidelines are critically interpreted and adapted and approved for local practice. For this reason, the task force recommends the following: (1) the full text be sent by ESAIC to national and international anaesthesiology societies for endorsement; (2) implementation by local institutions; (3) creation of E-learning tools to interpret and adapt the guidelines to clinical practice; and (4) creation of a certification process for successful skill acquisition by clinicians.
Limitations and further research
These guidelines have certain limitations that should be taken into consideration for future improvements.
-
(1)
These guidelines are largely based on studies from anaesthesia practice that are aimed at addressing practice in the operating room. The guidelines need to be carefully applied to other contexts such as intensive care units, delivery suites, emergency departments, and out-of-hospital settings.
-
(2)
The task force of paediatric anaesthesia experts recommends development of a universal algorithm that can be applied to non-anaesthesiologists as well. It would be beneficial for patients if experts from different paediatric specialties collaborate on a universal algorithm.
-
(3)
The number of studies supporting these guidelines are few and of varying quality with few RCTs in this population. Areas in need of further study include, but are not limited to oxygen flow rates and optimal techniques for oxygenation during intubation, outcomes when using video-laryngoscopy with hyperangulated blades for difficult airway management, and deep or awake extubation of the trachea.
-
(4)
Airway management of infants is a large topic and the task force had to make hard decisions on which PICOs and clinical questions to select. This might result in disagreements with other opinion makers and colleagues. For example, regarding a topic such as the use of surfactant in neonates, we acknowledge the importance of this intervention, but believe it is beyond the scope of these guidelines, and it is extensively covered by two recent systematic reviews and new guidelines on management of respiratory distress in infants and neonates.167,168
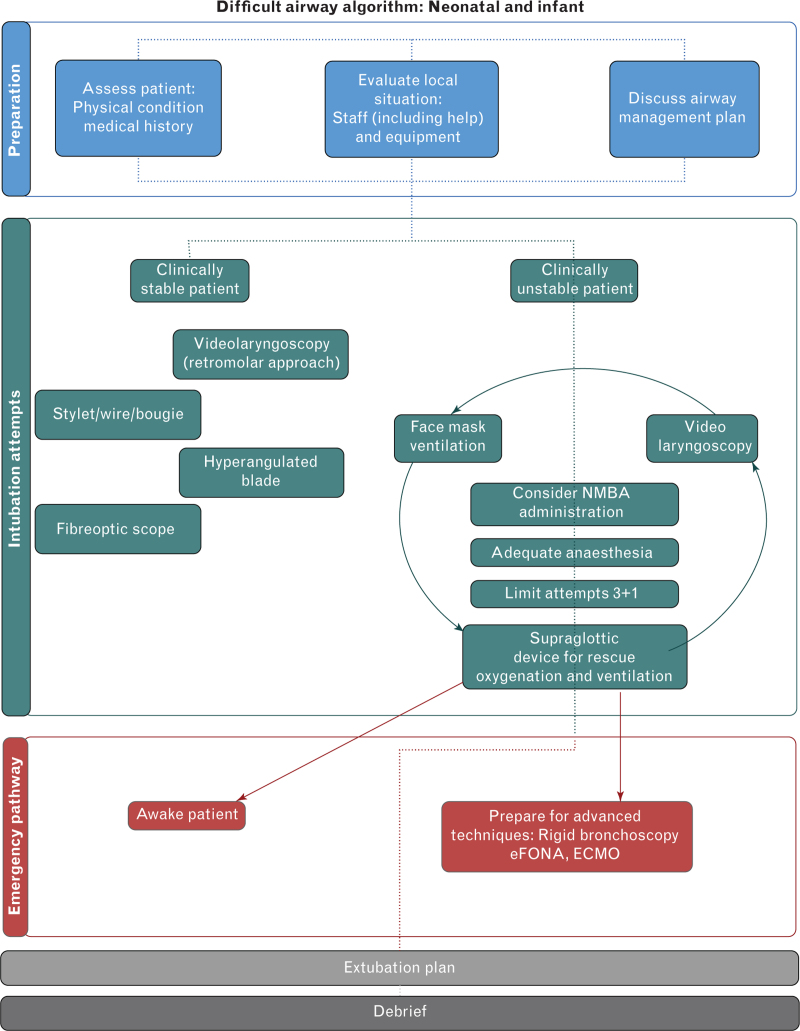
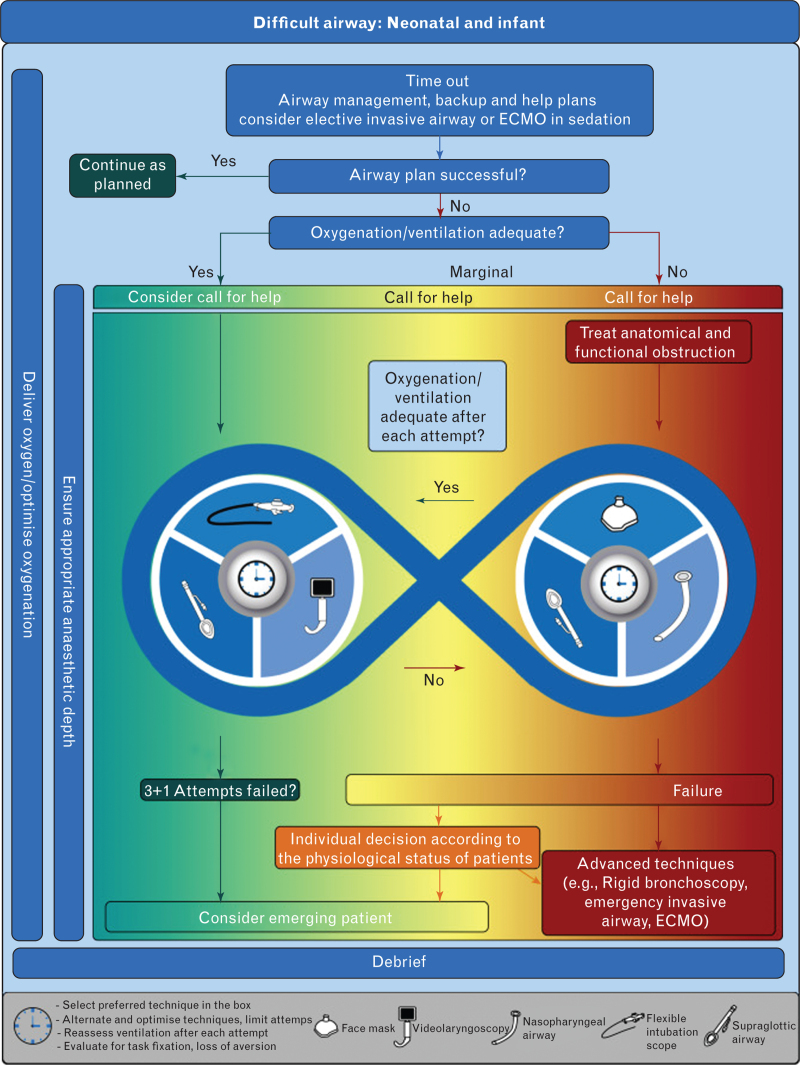
In conclusion, an international task force reviewed the literature and reached consensus on practice guidelines for airway management in children <1 yr of age. Algorithms and cognitive aids for neonatal and infant difficult airway management are reproduced in Figures 1 and 2. These guidelines represent a summation of the literature and expert consensus, and identify gaps in the literature that warrant future research.
Fig. 1.
Difficult airway algorithm for neonates and infants.
The algorithm is divided into three sections: preparations, intubation attempts, and emergency pathway. During preparation the local situation and the expertise should be evaluated and the plan discussed with the team. Once intubation attempts are started, the clinical conditions of the patient should be evaluated after every attempt. The emergency pathway should be entered in case of an unstable patient. Every difficult case should be reviewed and discussed to learn from errors. ECMO, extracorporeal membrane oxygenation; eFONA, emergency front of neck access; NMBA, neuromuscular blocking agents.
Fig. 2.
Difficult airway cognitive aid for neonates and infants.
Start with a time-out to identify and discuss the airway management plan with the team. During intubation attempts, the clinical condition of the patient, evaluated at every attempt, should guide the decision process. The colours represent the ability to oxygenate, from green (adequate oxygenation) to yellow (marginal oxygenation) to red (inadequate oxygenation). Limit the number of attempts to four (one initial attempt, plus three). Ensure adequate sedation or anaesthesia and administer oxygen at all times. Consider human factors (task fixation, loss of aversion). Debrief at the end of the case to learn from errors. Based on Figure 4 from Apfelbaum and colleagues1 (Anesthesiology 2022; 136: 31–81). ECMO, extracorporeal membrane oxygenation.
Supplementary Material
Acknowledgements relating to this article
The authors thank Pierre Harlet, Sophie Debouche, and Saman Sepehr at the ESAIC Guidelines office for secretarial and logistic assistance, and Deena Campbell, Ilene Manacher, and Tom Abbott from the British Journal of Anaesthesia for their professional graphic assistance.
Declaration of interest: ND is member of the ESAIC research committee and Chair of the task force; TA is an editor and TE is a member of the associate editorial board of the British Journal of Anaesthesia; AA is senior methodologist of the ESAIC; CRS is methodologist and chair of the mentorship program of the ESAIC; MKB is a member of the ESAIC forum patients/paediatric; PGK is a medical advisor to Verathon, Inc. (20001 North Creek Parkway Bothell, WA 98011 USA).
Funding: Equally funded by European Society of Anaesthesiology and Intensive Care and the British Journal of Anaesthesia.
Author's contributions: All Authors were members of the task force and equally contributed in developing this guidelines.
Appendix A. Supplementary data: Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2023.08.040.
Footnotes
Supplemental digital content is available for this article.
References
- 1.Apfelbaum JL, Hagberg CA, Connis RT, et al. 2022 American society of anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology 2022; 136:31–81. [DOI] [PubMed] [Google Scholar]
- 2.Matava CT, Kovatsis PG, Lee JK, et al. Pediatric airway management in COVID-19 patients: consensus guidelines from the society for pediatric anesthesia's pediatric difficult intubation collaborative and the Canadian pe-diatric anesthesia society. Anesth Analg 2020; 131:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disma N, Virag K, Riva T, et al. Difficult tracheal intubation in neonates and infants. NEonate and Children audiT of Anaesthesia pRactice IN Europe (NECTARINE): a prospective European multicentre observational study. Br J Anaesth 2021; 126:1173–1181. [DOI] [PubMed] [Google Scholar]
- 4.Fiadjoe JE, Nishisaki A, Jagannathan N, et al. Airway management complications in children with difficult tracheal intubation from the Pediatric Difficult Intubation (PeDI) registry: a prospective cohort analysis. Lancet Respir Med 2016; 4:37–48. [DOI] [PubMed] [Google Scholar]
- 5.Jagannathan N, Asai T. Difficult airway management: children are different from adults, and neonates are different from children. Br J Anaesth 2021; 126:1086–1088. [DOI] [PubMed] [Google Scholar]
- 6.Riva T, Engelhardt T, Basciani R, et al. Direct versus video laryngoscopy with standard blades for neonatal and infant tracheal intubation with supplemental oxygen: a multicentre, non-inferiority, randomised controlled trial. Lancet Child Adolesc Health 2023; 7:101–111. [DOI] [PubMed] [Google Scholar]
- 7.Stein ML, Park RS, Kiss EE, et al. Efficacy of a hybrid technique of simultaneous videolaryngoscopy with flexible bronchoscopy in children with difficult direct laryngoscopy in the Pediatric Difficult Intubation Registry. Anaesthesia 2023; 78:1093–1101. [DOI] [PubMed] [Google Scholar]
- 8.Burjek NE, Nishisaki A, Fiadjoe JE, et al. Video-laryngoscopy versus fiber-optic intubation through a supraglottic airway in children with a difficult airway: an analysis from the Multicenter Pediatric Difficult Intubation Registry. Anesthesiology 2017; 127:432–440. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane; 2022. Available from: www.training.cochrane.org/handbook. [Accessed 24 September 2023]. [Google Scholar]
- 10.Venkat Raman V, Beer D. Perioperative airway complications in infants and children with Crouzon and Pfeiffer syndromes: a single-center experience. Pediatr Anesth 2021; 31:1316–1324. [DOI] [PubMed] [Google Scholar]
- 11.Nargozian C. The airway in patients with craniofacial abnormalities. Pediatr Anesth 2004; 14:53–59. [DOI] [PubMed] [Google Scholar]
- 12.Thomas K, Hughes C, Johnson D, Das S. Anesthesia for surgery related to craniosynostosis: a review. Part 1. Pediatr Anesth 2012; 22:1033–1041. [DOI] [PubMed] [Google Scholar]
- 13.Glover CD, Fernandez AM, Huang H, et al. Perioperative outcomes and management in midface advancement surgery: a multicenter observational descriptive study from the Pediatric Craniofacial Collaborative Group. Pediatr Anesth 2018; 28:710–718. [DOI] [PubMed] [Google Scholar]
- 14.Senol D, Ozbag D, Dedeoglu N, et al. Comparison of anthropometric and conic beam computed tomography measurements of patients with and without difficult intubation risk according to modified mallampati score: new markers for difficult intubation. Niger J Clin Pract 2021; 24:1609. [DOI] [PubMed] [Google Scholar]
- 15.Shahhosseini S, Montasery M, Saadati M, Shafa A. Comparative evaluation of difficult intubation predictors in children under two years of ages. Anesth Pain Med 2021; 11:e118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadepalli C, Stepien KM, Sharma R, Jovanovic A, Tol G, Bentley A. Airway abnormalities in adult mucopoly-saccharidosis and development of salford mucopoly-saccharidosis airway score. J Clin Med 2021; 10:3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan JZ-Y, Crossett M, Ditchfield M. Dynamic volumetric computed tomographic assessment of the young paedi-atric airway: initial experience of rapid, non-invasive, four-dimensional technique. J Med Imaging Radiat Oncol 2013; 57:141–148. [DOI] [PubMed] [Google Scholar]
- 18.Avino D, Zhang W-H, de Villé A, Johansson A-B. Remi-fentanil versus morphine- midazolam premedication on the quality of endotracheal intubation in neonates: a noninferiority randomized trial. J Pediatr 2014; 164:1032–1037. [DOI] [PubMed] [Google Scholar]
- 19.Norman E, Wikström S, Rosén I, Fellman V, Hellström-Westas L. Premedication for intubation with morphine causes prolonged depression of electrocortical background activity in preterm infants. Pediatr Res 2013; 73:87–94. [DOI] [PubMed] [Google Scholar]
- 20.Badiee Z, Vakiliamini M, Mohammadizadeh M. Remi-fentanil for endotracheal intubation in premature infants: a randomized controlled trial. J Res Pharm Pract 2013; 2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durrmeyer X, Breinig S, Claris O, et al. Effect of atropine with propofol vs atropine with atracurium and sufenta-nil on oxygen desaturation in neonates requiring nonemergency intubation. JAMA 2018; 319:1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feltman DM, Weiss MG, Nicoski P, Sinacore J. Rocuro-nium for nonemergent intubation of term and preterm infants. J Perinatol 2011; 31:38–43. [DOI] [PubMed] [Google Scholar]
- 23.Gelberg J, Kongstad L, Werner O. Intubation conditions in young infants after propofol and remifentanil induction with and without low-dose rocuronium. Acta Anaesthesiol Scand 2014; 58:820–825. [DOI] [PubMed] [Google Scholar]
- 24.Rajan S, Gotluru P, Andews S, Paul J. Evaluation of endotracheal intubating conditions without the use of muscle relaxants following induction with propofol and sevoflurane in pediatric cleft lip and palate surgeries. J Anaesthesiol Clin Pharmacol 2014; 30:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caldwell CD, Watterberg KL. Effect of premedication regimen on infant pain and stress response to endotra-cheal intubation. J Perinatol 2015; 35:415–418. [DOI] [PubMed] [Google Scholar]
- 26.Carbajal R, Lode N, Ayachi A, et al. Premedication practices for tracheal intubation in neonates transported by French medical transport teams: a prospective observational study. BMJ Open 2019; 9:e034052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean PN, Hoehn EF, Geis GL, et al. Identification of the physiologically difficult airway in the pediatric emergency department. Acad Emerg Med 2020; 27:1241–1248. [DOI] [PubMed] [Google Scholar]
- 28.de Kort EHM, Prins SA, Reiss IKM, et al. Propofol for endotracheal intubation in neonates: a dose-finding trial. Arch Dis Child Fetal Neonatal Ed 2020; 105:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Kort EHM, Andriessen P, Reiss IKH, van Dijk M, Simons SHP. Evaluation of an intubation readiness score to assess neonatal sedation before intubation. Neona-tology 2019; 115:43–48. [DOI] [PubMed] [Google Scholar]
- 30.Durrmeyer X, Daoud P, Decobert F, et al. Premedication for neonatal endotracheal intubation: results from the epidemiology of procedural pain in neonates study. Pediatr Crit Care Med 2013; 14:e169–e175. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Marcinkiewicz AG, Adams HD, Gurnaney H, et al. A retrospective analysis of neuromuscular blocking drug use and ventilation technique on complications in the Pediatric Difficult Intubation Registry using propensity score matching. Anesth Analg 2020; 131:469–479. [DOI] [PubMed] [Google Scholar]
- 32.Howell J, Tarquinio K, Montgomery V, et al. Association of vagolytic and ketamine use with tracheal intubation associated adverse events. Crit Care Med 2013; 41:A219–A220. [Google Scholar]
- 33.Kim E-H, Lee J-H, Song I-K, Kim J-T, Kim B-R, Kim H-S. Effect of head position on laryngeal visualisation with the McGrath MAC videolaryngoscope in paediatric patients. Eur J Anaesthesiol 2016; 33:528–534. [DOI] [PubMed] [Google Scholar]
- 34.Krick J, Gray M, Umoren R, Lee G, Sawyer T. Premed-ication with paralysis improves intubation success and decreases adverse events in very low birth weight infants: a prospective cohort study. J Perinatol 2018; 38:681–686. [DOI] [PubMed] [Google Scholar]
- 35.Le CN, Garey DM, Leone TA, Goodmar JK, Rich W, Finer NN. Impact of premedication on neonatal intubations by pediatric and neonatal trainees. J Perinatol 2014; 34:458–460. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa Y, Ades A, Foglia EE, et al. Premedication with neuromuscular blockade and sedation during neonatal intubation is associated with fewer adverse events. J Perinatol 2019; 39:848–856. [DOI] [PubMed] [Google Scholar]
- 37.Sgrò S, Morini F, Bozza P, Piersigilli F, Bagolan P, Picardo S. Intravenous propofol allows fast intubation in neonates and young infants undergoing major surgery. Front Pediatr 2019; 7:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Kort EHM, Halbmeijer NM, Reiss IKM, Simons SHP. Assessment of sedation level prior to neonatal intubation: a systematic review. Pediatr Anesth 2018; 28:28–36. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Marcinkiewicz AG, Kovatsis PG, Hunyady AI, et al. First-attempt success rate of video laryngoscopy in small infants (VISI): a multicentre, randomised controlled trial. Lancet 2020; 396:1905–1913. [DOI] [PubMed] [Google Scholar]
- 40.Volz S, Stevens TP, Dadiz R. A randomized controlled trial: does coaching using video during direct laryngos-copy improve residents’ success in neonatal intubations? J Perinatol 2018; 38:1074–1080. [DOI] [PubMed] [Google Scholar]
- 41.O'Shea JE, Thio M, Kamlin CO, et al. Videolaryngoscopy to teach neonatal intubation: a randomized trial. Pediatrics 2015; 136:912–919. [DOI] [PubMed] [Google Scholar]
- 42.Salama ER, el Amrousy D. GlideScope® cobalt video laryngoscope versus direct Miller laryngoscope for lateral position-tracheal intubation in neonates with myelodysplasia: a prospective randomized study. Saudi J Anaesth 2018; 13:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain D, Mehta S, Gandhi K, Arora S, Parikh B, Abas M. Comparison of intubation conditions with CMAC Miller videolaryngoscope and conventional Miller laryngoscope in lateral position in infants: a prospective randomized trial. Pediatr Anesth 2018; 28:226–230. [DOI] [PubMed] [Google Scholar]
- 44.Varghese E, Kundu R. Does the Miller blade truly provide a better laryngoscopic view and intubating conditions than the Macintosh blade in small children? Pediatr Anesth 2014; 24:825–829. [DOI] [PubMed] [Google Scholar]
- 45.Dias R, Dave N, Chhabria R, Shah H, Garasia M. A randomised comparative study of Miller laryngoscope blade versus Oxiport ® Miller laryngoscope blade for neonatal and infant intubations. Indian J Anaesth 2017; 61:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodgson KA, Owen LS, Kamlin COF, et al. Nasal high-flow therapy during neonatal endotracheal intubation. N Engl J Med 2022; 386:1627–1637. [DOI] [PubMed] [Google Scholar]
- 47.Windpassinger M, Plattner O, Gemeiner J, et al. Pharyn-geal oxygen insufflation during AirTraq laryngoscopy slows arterial desaturation in infants and small children. Anesth Analg 2016; 122:1153–1157. [DOI] [PubMed] [Google Scholar]
- 48.Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br JAnaesth 2017; 118:232–238. [DOI] [PubMed] [Google Scholar]
- 49.Steiner JW, Sessler DI, Makarova N, et al. Use of deep laryngeal oxygen insufflation during laryngoscopy in children: a randomized clinical trial. Br J Anaesth 2016; 117:350–357. [DOI] [PubMed] [Google Scholar]
- 50.Soneru CN, Hurt HF, Petersen TR, Davis DD, Braude DA, Falcon RJ. Apneic nasal oxygenation and safe apnea time during pediatric intubations by learners. Pediatr Anesth 2019; 29:628–634. [DOI] [PubMed] [Google Scholar]
- 51.Staudt GE, Reddy SK, Moore AD. Apneic oxygenation via nasal cannula for intubation of a premature neonate with multiple airway anomalies. BMJ Case Rep 2019; 12:e227494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss M, Dullenkopf A, Fischer JE, Keller C, Gerber AC. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth 2009; 103:867–873. [DOI] [PubMed] [Google Scholar]
- 53.Peyton J, Foglia E, Lee GS. Pediatric airway anatomy and tracheal tubes: it is not all about the cuff. Anesth Analg 2021; 133:891–893. [DOI] [PubMed] [Google Scholar]
- 54.Williams ZC, Kim SS, Naguib A, Shafy SZ, Tobias JD. Use of cuffed endotracheal tubes in infants less than 5 kilograms: a retrospective cohort study. J Pediatr Surg 2022; 57:375–381. [DOI] [PubMed] [Google Scholar]
- 55.de Orange FA, Andrade RG, Lemos A, Borges PS, Figueiroa JN, Kovatsis PG. Cuffed versus uncuffed endo- tracheal tubes for general anaesthesia in children aged eight years and under. Cochrane Database Syst Rev 2017; 2017: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dariya V, Moresco L, Bruschettini M, Brion LP. Cuffed versus uncuffed endotracheal tubes for neonates. Cochrane Database Syst Rev 2022; 1:CD013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambers NA, Ramgolam A, Sommerfield D, et al. Cuffed vs. uncuffed tracheal tubes in children: a randomised controlled trial comparing leak, tidal volume and complications. Anaesthesia 2018; 73:160–168. [DOI] [PubMed] [Google Scholar]
- 58.Kamlin COF, O’Connell LAF, Morley CJ, et al. A randomized trial of stylets for intubating newborn infants. Pediatrics 2013; 131:e198–e205. [DOI] [PubMed] [Google Scholar]
- 59.Thomas RE, Erickson S, Hullett B, et al. Comparison of the efficacy and safety of cuffed versus uncuffed endo-tracheal tubes for infants in the intensive care setting: a pilot, unblinded RCT. Arch Dis Child Fetal Neonatal Ed 2021; 106:614–620. [DOI] [PubMed] [Google Scholar]
- 60.Engelhardt T, Virag K, Veyckemans F, Habre W. Airway management in paediatric anaesthesia in Europe–insights from APRICOT (Anaesthesia Practice in ChildrenObservational Trial): a prospective multicentre observational study in 261 hospitals in Europe. Br J Anaesth 2018; 121:66–75. [DOI] [PubMed] [Google Scholar]
- 61.Sawyer T, Foglia EE, Ades A, et al. Incidence, impact and indicators of difficult intubations in the neonatal intensive care unit: a report from the National Emergency Airway Registry for Neonates. Arch Dis Child Fetal Neonatal 2019; 104:F461–F466. [DOI] [PubMed] [Google Scholar]
- 62.Tippmann S, Haan M, Winter J, et al. Adverse events and unsuccessful intubation attempts are frequent during neonatal nasotracheal intubations. Front Pediatr 2021; 9:675238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drake-Brockman TFE, Ramgolam A, Zhang G, Hall GL, von Ungern-Sternberg BS. The effect of endotracheal tubes versus laryngeal mask airways on perioperative respiratory adverse events in infants: a randomised controlled trial. Lancet 2017; 389:701–708. [DOI] [PubMed] [Google Scholar]
- 64.Yang C, Zhu X, Lin W, et al. Randomized, controlled trial comparing laryngeal mask versus endotracheal intubation during neonatal resuscitation–a secondary publication. BMC Pediatr 2016; 16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trevisanuto D, Cavallin F, Nguyen LN, et al. Supreme laryngeal mask airway versus face mask during neonatal resuscitation: a randomized controlled trial. J Pediatr 2015; 167:286–291. e1. [DOI] [PubMed] [Google Scholar]
- 66.Wanous AA, Wey A, Rudser KD, Roberts KD. Feasibility of laryngeal mask airway device placement in neonates. Neonatology 2017; 111:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foglia EE, Ades A, Sawyer T, et al. Neonatal intubation practice and outcomes: an international registry study. Pediatrics 2019; 143:e20180902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gálvez JA, Acquah S, Ahumada L, et al. Hypoxemia, bradycardia, and multiple laryngoscopy attempts during anesthetic induction in infants. Anesthesiology 2019; 131:830–839. [DOI] [PubMed] [Google Scholar]
- 69.Saisamorn F, Sriwiset C, Sirisomboon R, Paes B, Kitsommart R. Indications and outcomes of neonatal intubation: a single-center, prospective study in a middle-income country. Pediatr Neonatol 2022; 63:125–130. [DOI] [PubMed] [Google Scholar]
- 70.Lee JH, Turner DA, Kamat P, et al. The number of tracheal intubation attempts matters! A prospective multi-institutional pediatric observational study. BMC Pediatr 2016; 16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peyton J, Park R, Staffa SJ, et al. A comparison of video-laryngoscopy using standard blades or non-standard blades in children in the Paediatric Difficult Intubation Registry. Br J Anaesth 2021; 126:331–339. [DOI] [PubMed] [Google Scholar]
- 72.Abdelgadir IS, Phillips RS, Singh D, Moncreiff MP, Lumsden JL. Videolaryngoscopy versus direct laryngos-copy for tracheal intubation in children (excluding neo-nates). Cochrane Database Syst Rev 2017; 5:CD011413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sola C, Saour A-C, Macq C, Bringuier S, Raux O, Dadure C. Children with challenging airways: what about Glide-Scope® video-laryngoscopy? Anaesth Crit Care Pain Med 2017; 36:267–271. [DOI] [PubMed] [Google Scholar]
- 74.Er A, Çağlar A, Çitlenbik H, et al. Which device is favorable for intubation attempts of pediatric residents on four different pediatric airway simulations? Pediatr Emerg Care 2022; 38:e272–e277. [DOI] [PubMed] [Google Scholar]
- 75.Moritz A, Holzhauser L, Fuchte T, Kremer S, Schmidt J, Irouschek A. Comparison of Glidescope Core, C-MAC Miller and conventional Miller laryngoscope for difficult airway management by anesthetists with limited and extensive experience in a simulated Pierre Robin sequence: a randomized crossover manikin study. PLoS One 2021; 16:e0250369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abid ES, McNamara J, Hall P, et al. The impact of video-laryngoscopy on endotracheal intubation success by a pediatric/neonatal critical care transport team. Prehosp Emerg Care 2021; 25:325–332. [DOI] [PubMed] [Google Scholar]
- 77.Zhang B, Gurnaney HG, Stricker PA, Galvez JA, Isserman RS, Fiadjoe JE. A Prospective observational study of technical difficulty with GlideScope-guided tracheal intubation in children. Anesth Analg 2018; 127:467–471. [DOI] [PubMed] [Google Scholar]
- 78.Kaufmann J, Laschat M, Hellmich M, Wappler F. A randomized controlled comparison of the Bonfils fiberscope and the GlideScope Cobalt AVL video laryngoscope for visualization of the larynx and intubation of the trachea in infants and small children with normal airways. Pediatr Anesth 2013; 23:913–919. [DOI] [PubMed] [Google Scholar]
- 79.Kim J-T, Na H-S, Bae J-Y, et al. GlideScope® video laryngoscope: a randomized clinical trial in 203 paedi-atric patients. Br J Anaesth 2008; 101:531–534. [DOI] [PubMed] [Google Scholar]
- 80.Vlatten A, Aucoin S, Litz S, Macmanus B, Soder C. A comparison of the STORZ video laryngoscope and standard direct laryngoscopy for intubation in the Pedi-atric airway - a randomized clinical trial. Pediatr Anesth 2009; 19:1102–1107. [DOI] [PubMed] [Google Scholar]
- 81.Fiadjoe JE, Gurnaney H, Dalesio N, et al. A prospective randomized equivalence trial of the GlideScope Cobalt® video laryngoscope to traditional direct laryngoscopy in neonates and infants. Anesthesiology 2012; 116:622–628. [DOI] [PubMed] [Google Scholar]
- 82.Macnair D, Baraclough D, Wilson G, Bloch M, Engelhardt T. Pediatric airway management: comparing the Berci-Kaplan Video Laryngoscope with direct laryn-goscopy. Pediatr Anesth 2009; 19:577–580. [DOI] [PubMed] [Google Scholar]
- 83.Gupta N, Girdhar KK, Singh P, Gupta A. Comparison of CMAC Miller blade and McGrath for intubation in neo-nates and infants undergoing surgery. Trends Anaesth Crit Care 2020; 30:e77. [Google Scholar]
- 84.Mutlak H, Rolle U, Rosskopf W, et al. Comparison of the TruView infant EVO2 PCD™ and C-MAC video laryngoscopes with direct Macintosh laryngoscopy for routine tracheal intubation in infants with normal airways. Clinics 2014; 69:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Min JJ, Oh EJ, Shin YH, Kwon E, Jeong JS. The usefulness of endotracheal tube twisting in facilitating tube delivery to glottis opening during GlideScope intubation in infants: randomized trial. Sci Rep 2020; 10:4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee J, Cho S, Choe H, et al. Effects of tip-manipulated stylet angle on intubation using the GlideScope® vid-eolaryngoscope in children: a prospective randomized controlled trial. Pediatr Anesth 2021; 31:802–808. [DOI] [PubMed] [Google Scholar]
- 87.Gray MM, Rumpel JA, Brei BK, et al. Associations of stylet use during neonatal intubation with intubation success, adverse events, and severe desaturation: a report from NEAR4NEOS. Neonatology 2021; 118:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O'Shea JE, O’Gorman J, Gupta A, et al. Orotracheal intubation in infants performed with a stylet versus without a stylet. Cochrane Database Syst Rev 2017; 6:CD011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strutt JR, Thompson NR, Stotesbery JL, Horvath B. Emergency endotracheal intubation with a rigid stylet of an infant with severe subglottic stenosis. J Emerg Med 2020; 58:e157–e160. [DOI] [PubMed] [Google Scholar]
- 90.Jagannathan N, Sequera-Ramos L, Sohn L, et al. Randomized comparison of experts and trainees with nasal and oral fibreoptic intubation in children less than 2 yr of age. Br J Anaesth 2015; 114:290–296. [DOI] [PubMed] [Google Scholar]
- 91.Tan A, Nolan JA. Anesthesia for children with anterior mediastinal masses. Pediatr Anesth 2022; 32:4–9. [DOI] [PubMed] [Google Scholar]
- 92.Pearson JK, Tan GM. Pediatric anterior mediastinal mass. Semin Cardiothorac Vasc Anesth 2015; 19:248–254. [DOI] [PubMed] [Google Scholar]
- 93.Latham GJ, Greenberg RS. Anesthetic considerations for the pediatric oncology patient - part 2: systems-based approach to anesthesia. Pediatr Anesth 2010; 20:396–420. [DOI] [PubMed] [Google Scholar]
- 94.Kriege M, Alflen C, Strauß H, Ott T, Noppens RR. Usage of a semi-rigid intubation endoscope is not superior to a video laryngoscope. A prospective, randomised, controlled trial comparing the SensaScope vs. the McGrath Series 5 in surgical patients. Trends Anaesth Crit Care 2018; 18:23–28. [Google Scholar]
- 95.Dharmarajan H, Liu Y-CC, Hippard HK, Chandy B. Difficult airway intubation simulation using Bonfils fiberscope and rigid fiberscope for surgical training. Int J Pediatr Otorhinolaryngol 2018; 105:171–175. [DOI] [PubMed] [Google Scholar]
- 96.Nowakowski M, Williams S, Gallant J, Ruel M, Robitaille A. Predictors of difficult intubation with the bonfils rigid fiberscope. Anesth Analg 2016; 122:1901–1906. [DOI] [PubMed] [Google Scholar]
- 97.Kaufmann J, Laschat M, Engelhardt T, Hellmich M, Wappler F. Tracheal intubation with the Bonfils fiberscope in the difficult pediatric airway: a comparison with fiberoptic intubation. Pediatr Anesth 2015; 25:372–378. [DOI] [PubMed] [Google Scholar]
- 98.Rudolph C, Henn-Beilharz A, Gottschall R, Wallenborn J, Schaffranietz L. The unanticipated difficult intubation: rigid or flexible endoscope? Minerva Anestesiol 2007; 73:567–574. [PubMed] [Google Scholar]
- 99.Black AE, Flynn PER, Smith HL, Thomas ML, Wilkinson KA. Development of a guideline for the management of the unanticipated difficult airway in pediat-ric practice. Pediatr Anesth 2015; 25:346–362. [DOI] [PubMed] [Google Scholar]
- 100.Okada Y, Ishii W, Sato N, Kotani H, Iiduka R. Management of pediatric ‘cannot intubate, cannot oxygenate’. Acute Med Surg 2017; 4:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Both CP, Diem B, Alonso E, et al. Rabbit training model for establishing an emergency front of neck airway in children. Br J Anaesth 2021; 126:896–902. [DOI] [PubMed] [Google Scholar]
- 102.Ulmer F, Lennertz J, Greif R, Butikofer L, Theiler L, Riva T. Emergency front of neck access in children: a new learning approach in a rabbit model. Br J Anaesth 2020; 125:e61–e68. [DOI] [PubMed] [Google Scholar]
- 103.Langhan ML, Emerson BL, Nett S, et al. End-tidal carbon dioxide use for tracheal intubation. Pediatr Crit Care Med 2018; 19:98–105. [DOI] [PubMed] [Google Scholar]
- 104.Mori T, Nomura O, Hagiwara Y, Inoue N. Diagnostic accuracy of a 3-point ultrasound protocol to detect esoph-ageal or endobronchial mainstem intubation in a pediatric emergency department. J Ultrasound Med 2019; 38:2945–2954. [DOI] [PubMed] [Google Scholar]
- 105.Galicinao J, Bush AJ, Godambe SA. Use of bedside ultra-sonography for endotracheal tube placement in pediatric patients: a feasibility study. Pediatrics 2007; 120:1297–1303. [DOI] [PubMed] [Google Scholar]
- 106.van Os S, Cheung P-Y, Kushniruk K, O’Reilly M, Aziz K, Schmölzer GM. Assessment of endotracheal tube placement in newborn infants: a randomized controlled trial. J Perinatol 2016; 36:370–375. [DOI] [PubMed] [Google Scholar]
- 107.Schmölzer GM, Poulton DA, Dawson JA, Kamlin COF, Morley CJ, Davis PG. Assessment of flow waves and colori-metric CO2 detector for endotracheal tube placement during neonatal resuscitation. Resuscitation 2011; 82:307–312. [DOI] [PubMed] [Google Scholar]
- 108.Chrimes N, Higgs A, Hagberg CA, et al. Preventing unrecognised oesophageal intubation: a consensus guideline from the Project for Universal Management of Airways and international airway societies. Anaesthesia 2022; 77:1395–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pereira SS, Raja E-A, Satas S. Weight-based guide overestimates endotracheal tube tip position in extremely preterm infants. Am J Perinatol 2019; 36:1498–1503. [DOI] [PubMed] [Google Scholar]
- 110.Bartle RM, Miller AG, Diez AJ, Smith PB, Gentile MA, Puia-Dumitrescu M. Evaluating endotracheal tube depth in infants weighing less than 1 kilogram. Respir Care 2019; 64:243–247. [DOI] [PubMed] [Google Scholar]
- 111.Dennington D, Vali P, Finer NN, Kim JH. Ultrasound confirmation of endotracheal tube position in neonates. Neonatology 2012; 102:185–189. [DOI] [PubMed] [Google Scholar]
- 112.Descamps C, Beissel A, Vo-van P, et al. Role of ultraso-nography in the assessment of correct endotracheal tube placement in neonates. Acta Paediatr 2020; 109:1057–1059. [DOI] [PubMed] [Google Scholar]
- 113.Tochen ML. Orotracheal intubation in the newborn infant: a method for determining depth of tube insertion. J Pediatr 1979; 95:1050–1051. [DOI] [PubMed] [Google Scholar]
- 114.Peterson J, Johnson N, Deakins K, Wilson-Costello D, Jelovsek JE, Chatburn R. Accuracy of the 7-8-9 rule for endotracheal tube placement in the neonate. J Perinatol 2006; 26:333–336. [DOI] [PubMed] [Google Scholar]
- 115.Uygur Ö, Öncel MY, Şimşek GK, et al. Is nasal septum–tragus length measurement appropriate for endotracheal tube intubation depth in neonates? a randomized controlled study. Am JPerinatol 2021; 38:728–733. [DOI] [PubMed] [Google Scholar]
- 116.Puttawong D, Manopunya S, Visrutaratna P, Kosarat S, Khuwuthyakorn V, Tantiprabha W. Accuracy of various recent recommendations to estimate the optimal depth of orotracheal tube in Thai neonates. J Matern Fetal Neonatal Med 2022; 35:3343–3347. [DOI] [PubMed] [Google Scholar]
- 117.Priyadarshi M, Thukral A, Sankar MJ, et al. ‘Lip-to-Tip’ study: comparison of three methods to determine optimal insertion length of endotracheal tube in neo-nates. Eur J Pediatr 2021; 180:1459–1466. [DOI] [PubMed] [Google Scholar]
- 118.Takeuchi S, Arai J, Nagafuji M, et al. Ideal endotracheal tube insertion depth in neonates with a birthweight less than 750 g. Pediatr Int 2020; 62:932–936. [DOI] [PubMed] [Google Scholar]
- 119.Flinn AM, Travers CP, Laffan EE, O’Donnell CPF. Estimating the endotracheal tube insertion depth in new-borns using weight or gestation: a randomised trial. Neonatology 2015; 107:167–172. [DOI] [PubMed] [Google Scholar]
- 120.Leung C. Optimal insertion depth for endotracheal tubes in extremely low-birth-weight infants. Pediatr Crit Care Med 2018; 19:328–331. [DOI] [PubMed] [Google Scholar]
- 121.Templeton TW, Goenaga-Díaz EJ, Downard MG, et al. Assessment of common criteria for awake extubation in infants and young children. Anesthesiology 2019; 131:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.da Silva PSL, Fonseca MCM, Iglesias SBO, Junior EL, de Aguiar VE, de Carvalho WB. Nebulized 0.5, 2.5 and 5 ml L-epinephrine for post-extubation stridor in children: a prospective, randomized, double-blind clinical trial. Intensive Care Med 2012; 38:286–293. [DOI] [PubMed] [Google Scholar]
- 123.Kimura S, Ahn JB, Takahashi M, Kwon S, Papatheodorou S. Effectiveness of corticosteroids for post-extubation stridor and extubation failure in pediatric patients: a systematic review and meta-analysis. Ann Intensive Care 2020; 10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lemyre B, Davis PG, de Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev 2017; 2:CD003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia Y, Liu C, Xiao M. A prospective randomized controlled trial of nasal intermittent positive pressure ventilation for prevention of extubation failure in very low birth weight infants. Chinese Pediatr Emerg Med 2014; 12:215–219. [Google Scholar]
- 126.Ramaswamy VV, Bandyopadhyay T, Nanda D, et al. Efficacy of noninvasive respiratory support modes as postextubation respiratory support in preterm neonates: a systematic review and network meta-analysis. Pediatr Pulmonol 2020; 55:2924–2939. [DOI] [PubMed] [Google Scholar]
- 127.Komatsu DFR, Diniz EM, de A, Ferraro AA, Ceccon MEJR, Vaz FAC. Randomized controlled trial comparing nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure in premature infants after tracheal extubation. Rev Assoc Med Bras 2016; 62:568–574. [DOI] [PubMed] [Google Scholar]
- 128.Manley BJ, Owen LS, Doyle LW, et al. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med 2013; 369:1425–1433. [DOI] [PubMed] [Google Scholar]
- 129.Daish H, Badurdeen S. Question 2: humidified heated high flow nasal cannula versus nasal continuous positive airway pressure for providing respiratory support following extubation in preterm newborns. Arch Dis Child 2014; 99:880–882. [DOI] [PubMed] [Google Scholar]
- 130.Colleti Junior J, Azevedo Rde, Araujo O, Carvalho WB de. High-flow nasal cannula as a post-extubation respiratory support strategy in preterm infants: a systematic review and meta-analysis. J Pediatr (Rio J) 2020; 96:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Charki S, Patil P, Hadalgi L, et al. Heated humidified high-flow nasal cannula versus nasal continuous positive airways pressure for respiratory support in preterm ne-onates – a noninferiority trial at a tertiary care center. J Clin Neonatol 2020; 9:168. [Google Scholar]
- 132.Yengkhom R, Suryawanshi P, Gupta B, Deshpande S. Heated humidified high-flow nasal cannula vs. nasal continuous positive airway pressure for post-extubation respiratory support in preterm infants: a randomized controlled trial. J Trop Pediatr 2021; 67:fmaa082. [DOI] [PubMed] [Google Scholar]
- 133.Collins CL, Holberton JR, Barfield C, Davis PG. A randomized controlled trial to compare heated humidified high-flow nasal cannulae with nasal continuous positive airway pressure postextubation in premature infants. J Pediatr 2013; 162:949–954. e1. [DOI] [PubMed] [Google Scholar]
- 134.Karam C, Zeeni C, Yazbeck-Karam V, et al. Respiratory adverse events after LMA® mask removal in children: a randomized trial comparing propofol to sevoflurane. Anesth Analg 2023; 136:25–33. [DOI] [PubMed] [Google Scholar]
- 135.Mathew PJ, Mathew JL. Early versus late removal of the laryngeal mask airway (LMA) for general anaesthesia. Cochrane Database Syst Rev 2015; 2015:CD007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abbasi S, Siddiqui KM, Qamar-ul-Hoda M. Adverse respiratory events after removal of laryngeal mask airway in deep anesthesia versus awake state in children: a randomized trial. Cureus 2022; 14:e24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koo C-H, Lee S, Chung S, Ryu J-H. Deep vs. awake extu-bation and LMA removal in terms of airway complications in pediatric patients undergoing anesthesia: a systemic review and meta-analysis. J Clin Med 2018; 7:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vitale L, Rodriguez B, Baetzel A, Christensen R, Haydar B. Complications associated with removal of airway devices under deep anesthesia in children: an analysis of the Wake up Safe database. BMC Anesthesiol 2022; 22:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reason J. Safety in the operating theatre - Part 2: human error and organisational failure. Qual Saf Health Care 2005; 14:56–60. [PMC free article] [PubMed] [Google Scholar]
- 140.Jones CPL, Fawker-Corbett J, Groom P, Morton B, Lister C, Mercer SJ. Human factors in preventing complications in anaesthesia: a systematic review. Anaesthesia 2018; 73:12–24. [DOI] [PubMed] [Google Scholar]
- 141.Flin R, Patey R, Glavin R, Maran N. Anaesthetists’ nontechnical skills. Br J Anaesth 2010; 105:38–44. [DOI] [PubMed] [Google Scholar]
- 142.Greenberg CC, Regenbogen SE, Studdert DM, et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg 2007; 204:533–540. [DOI] [PubMed] [Google Scholar]
- 143.Rosenstein AH, O’Daniel M. Impact and implications of disruptive behavior in the perioperative arena. J Am Coll Surg 2006; 203:96–105. [DOI] [PubMed] [Google Scholar]
- 144.Stiegler MP, Tung A. Cognitive processes in anesthesiology decision making. Anesthesiology 2014; 120:204–217. [DOI] [PubMed] [Google Scholar]
- 145.Fioratou E, Flin R, Glavin R. No simple fix for fixation errors: cognitive processes and their clinical applications. Anaesthesia 2010; 65:61–69. [DOI] [PubMed] [Google Scholar]
- 146.Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the fourth national Audit Project of the royal College of anaesthetists and the difficult airway society. Part 1: anaesthesia. Br J Anaesth 2011; 106:617–631. [DOI] [PubMed] [Google Scholar]
- 147.Marcus R. Human factors in pediatric anesthesia incidents. Pediatr Anesth 2006; 16:242–250. [DOI] [PubMed] [Google Scholar]
- 148.Lee J-H, Kim E-K, Song I-K, et al. Critical incidents, including cardiac arrest, associated with pediatric anesthesia at a tertiary teaching children's hospital. Pediatr Anesth 2016; 26:409–417. [DOI] [PubMed] [Google Scholar]
- 149.Barach P, Johnson JK, Ahmad A, et al. A prospective observational study of human factors, adverse events, and patient outcomes in surgery for pediatric cardiac disease. J Thorac Cardiovasc Surg 2008; 136:1422–1428. [DOI] [PubMed] [Google Scholar]
- 150.Umoren RA, Sawyer TL, Ades A, et al. Team stress and adverse events during neonatal tracheal intubations: a report from NEAR4NEOS. Am J Perinatol 2020; 37:1417–1424. [DOI] [PubMed] [Google Scholar]
- 151.Bensouda B, Mandel R, Mejri A, Lachapelle J, St-Hilaire M, Ali N. Effect of an audience on trainee stress and performance during simulated neonatal intubation: a randomized crossover trial. BMC Med Educ 2018; 18:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thim S, Henriksen TB, Laursen H, Schram AL, Paltved C, Lindhard MS. Simulation-based emergency team training in pediatrics: a systematic review. Pediatrics 2022; 149:e2021054305. [DOI] [PubMed] [Google Scholar]
- 153.Habre W, Disma N, Virag K, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med 2017; 5:412–425. [DOI] [PubMed] [Google Scholar]
- 154.Auroy Y, Ecoffey C, Messiah A, Rouvier B. Relationship between complications of pediatric anesthesia and volume of pediatric anesthetics. Anesth Analg 1997; 84:234–235. [DOI] [PubMed] [Google Scholar]
- 155.Johnston L, Sawyer T, Ades A, et al. Impact of physician training level on neonatal tracheal intubation success rates and adverse events: a report from National Emergency Airway Registry for Neonates (NEAR4NEOS). Neonatology 2021; 118:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Branca A, Tellez D, Berkenbosch J, et al. The new trainee effect in tracheal intubation procedural safety across PICUs in North America: a report from National Emergency Airway Registry for Children. Pediatr Crit Care Med 2020; 21:1042–1050. [DOI] [PubMed] [Google Scholar]
- 157.van Sambeeck SJ, van Kuijk SMJ, Kramer BW, Vermeulen PM, Vos GD. Endotracheal intubation skills of pediatricians versus anesthetists in neonates and children. Eur J Pediatr 2019; 178:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Donoghue AJ, Ades AM, Nishisaki A, Deutsch ES. Video-laryngoscopy versus direct laryngoscopy in simulated pediatric intubation. Ann Emerg Med 2013; 61:271–277. [DOI] [PubMed] [Google Scholar]
- 159.Soghier LM, Walsh HA, Goldman EF, Fratantoni KR. Simulation for neonatal endotracheal intubation training: how different is it from clinical practice? Simul Healthc 2022; 17:e83–e90. [DOI] [PubMed] [Google Scholar]
- 160.O'Shea JE, Kirolos S, Thio M, Kamlin COF, Davis PG. Neonatal videolaryngoscopy as a teaching aid: the trainees’ perspective. Arch Dis Child Fetal Neonatal Ed 2021; 106:168–171. [DOI] [PubMed] [Google Scholar]
- 161.Moussa A, Luangxay Y, Tremblay S, et al. Video-laryngoscope for teaching neonatal endotracheal intubation: a randomized controlled trial. Pediatrics 2016; 137:e20152156. [DOI] [PubMed] [Google Scholar]
- 162.Saran A, Dave N, Karnik P. Efficacy and safety of videolaryngoscopy-guided verbal feedback to teach neonatal and infant intubation. A prospective randomised cross over study. Indian J Anaesth 2019; 63:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Disma N, Riva T, Hansen TG, Engelhardt T. Changing landscape of tracheal intubation in the very young. Eur J Anaesthesiol 2023; 40:307–309. [DOI] [PubMed] [Google Scholar]
- 164.Eisler LD, Hua M, Li G, Sun LS, Kim M. A multivariable model predictive of unplanned postoperative intubation in infant surgical patients. Anesth Analg 2019; 129:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wise-Faberowski L, Nargozian C. Utility of airway exchange catheters in pediatric patients with a known difficult airway. Pediatr Crit Care Med 2005; 6:454–456. [DOI] [PubMed] [Google Scholar]
- 166.Cheon EC, Palac HL, Paik KH, et al. Unplanned, postoperative intubation in pediatric surgical patients. Anes-thesiology 2016; 125:914–928. [DOI] [PubMed] [Google Scholar]
- 167.Ramaswamy VV, Abiramalatha T, Bandyopadhyay T, Boyle E, Roehr CC. Surfactant therapy in late preterm and term neonates with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2022; 107:393–397. [DOI] [PubMed] [Google Scholar]
- 168.Sweet DG, Carnielli VP, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology 2023; 120:3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.