Abstract
Aims
Timing and surgical strategies in acute infective endocarditis are still questionable. We sought to investigate clinical outcomes of patients undergoing mitral valve repair (MVR) compared with mitral valve replacement [mitral valve prosthesis (MVP)] for acute infective endocarditis.
Methods
From 2004 to 2019, 109 consecutive patients with acute mitral valve infective endocarditis were retrospectively investigated. Patients were divided into two groups according to surgical strategy: MVR 53/109 (48.6%) versus MVP 56/109 (51.4%). Primary end points were in-hospital mortality and overall survival at 10 years. Secondary end point was the freedom from infective endocarditis relapse.
Results
Our institutional surgical approach for infective endocarditis allowed us to achieve MVR in 48.6% of patients. Hospital mortality was comparable between the two groups [MVR: 1/53 (1.9%) versus MVP: 2/56 (3.6%), P = 1.000]. Overall 10-year survival was 80.0 ± 14.1 and 77.2 ± 13.5% for MVR and MVP, respectively (P = 0.648). MVR showed a lower incidence of infective endocarditis relapse compared with MVP (MVR: 93.6 ± 7.1 versus MVP: 80.9 ± 10.8%, P = 0.041). At Cox regression, infective endocarditis relapse was an independent risk factor for death (hazard ratio 4.03; 95% confidence interval 1.41–11.52; P = 0.009).
Conclusion
The tendency to postpone surgery in stable patients with mitral infective endocarditis allowed achievement of MVR in almost 50% of patients. Although repair remains the approach of choice in our institution, no differences between MVR and MVP were reported in terms of early/late survival. However, MVP had a higher incidence of infective endocarditis relapse that represents an independent risk of mortality.
Keywords: infective endocarditis, mitral valve repair, mitral valve replacement, valve surgery
Introduction
Benefits of reparative strategies have been largely established for the surgical treatment of degenerative mitral valve diseases.1,2 However, clinical advantages of repair over replacement strategies seem to be less pronounced when new onset of mitral regurgitation is the consequence of an acute infective endocarditis.
Despite several studies reporting a superior overall survival of mitral valve repair (MVR) techniques over replacement,3–6 this approach might be limited or not feasible when a large amount of mitral tissue needs to be resected or, even worse, is lacking, thus limiting the reparability or increasing the complexity of the repair. In this scenario, concerns have been raised about durability of the repair, especially in technically challenging cases. Moreover, complex repair may require prolonged cardiopulmonary bypass time that might be cumbersome in this subset of patients. Additionally, complex repair techniques are related to a higher need for reinterventions at mid to long term.7
Conversely, replacement by means of a prosthetic valve [mitral valve prosthesis (MVP)] is nowadays limited to poorer surgical candidates thus significantly influencing perioperative and short-term results. Of note, this approach should be discouraged in patients with infective endocarditis secondary to intravenous drug abuse to limit the amount of prosthetic material and the incidence of recurrences.8
In this retrospective single-center study, we sought to critically investigate clinical outcomes derived from our institutional approach to patients with severe mitral valve regurgitation secondary to active infective endocarditis. We focused our analysis on the effects of repair or replacement at long-term follow-up.
Methods
Patients’ selection
We included all patients who underwent mitral valve surgery, either repair or replacement, because of active infective endocarditis on native mitral valve between January 2004 and December 2019. The Institutional Review Board approved this study (PN 1815). According to the ESC criteria, active infective endocarditis was defined as surgery performed within 6 weeks of antibiotic treatment onset, in the presence of positive culture of the remnants of tissue retrieved during surgery as well as macroscopic evidence of endocarditis. We therefore included patients with age older than 18 years with diagnosis of active infective endocarditis primarily affecting the mitral valve. Patients with previous mitral valve surgery were excluded from the current analysis.9
Finally, our study population consisted of 109 patients undergoing surgery for acute infective endocarditis on the native mitral valve. Of those, 53 patients (48.6%) underwent MVR whereas 56 patients (51.4%) had MVP.
Preoperative, intraoperative and postoperative data were retrieved from the institutional electronic medical records, while clinical follow-up consisted of routine postoperative outpatient visits as well as data collection by means of telephone interviews with patients and/or referral cardiologists. No patients were lost to follow-up.
Preoperative management
Empiric antibiotic therapy was promptly initiated once diagnosis of infective endocarditis was confirmed according to the modified Duke criteria. Therapy was, therefore, tailored once the causative pathogen had been identified. Surgical intervention was considered and discussed case by case following the Heart Team decision involving anesthesiologists, cardiac surgeons, cardiologists and infectivologists. Furthermore, according to the ESC guidelines,9 surgery was deemed urgent in cases of uncontrolled infections, high risk of major embolism and in case of hemodynamic impairment because of infective endocarditis-related structural complications conditioning cardiac heart failure. Conversely, when urgency/emergency criteria were not met, surgery was postponed after completion of at least 4 weeks of antibiotic therapy. The goal of this approach was to raise the probability of blood as well as tissue sterilization, minimizing the risk of acute re-infection.
All operations were performed by experienced surgeons with extensive expertise in mitral valve repair and replacement.
According to Carpentier's general principle, the aims of the MVR were to restore a proper leaflet motion and coaptation and stabilize to annulus whenever possible.10 Thus, the first-line strategy in any surgical intervention was to assess the mitral valve reparability and proceed to MVR. After extensive removal of pathological or infected tissue, replacement was performed whenever the above general principles were deemed not achievable.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and categorical variables as numbers (percentages). Continuous variables were compared by using Student's t-test or the Mann–Whitney–Wilcoxon test, as appropriate, whereas categorical variables were compared by using Pearson's χ2 test or Fisher's exact test, as appropriate. P-values of 0.05 or less were considered statistically significant.
Overall survival as defined above was estimated using the Kaplan–Meier method and survival curves were compared using the Mantel–Cox log-rank test. A Cox regression model was applied in order to identify predictors for mortality. The significant level α = 0.2 in the univariate analysis was used to select variables for the multivariate model (using backward stepwise elimination).
Primary end points were perioperative mortality and overall survival at 10 years of follow-up. Secondary end point was the event-free survival intended as survival free from endocarditis.
Results
Preoperative
Patient baseline characteristics are depicted in Table 1. No differences have been reported among the two groups and the Operative Risk Evaluation (EuroSCORE II). LVEF values were comparable despite the tendency in the MVR group to have a higher incidence of male patients and intravenous drug abusers. However, this did not reach statistical relevance. A total of 22 (20.2%) patients had had previous cardiac surgery: 9 had had previous aortic valve replacement whereas 13 had had previous coronary artery bypass grafting (CABG).
Table 1.
Preoperative patient characteristics
| Variable | Overall population (n = 109) | Mitral valve repair (MVR) (n = 53) | Mitral valve replacement (MVP) (n = 56) | P-value |
| Age (years) (mean ± SD) | 58.9 ± 15.5 | 57.7 ± 15.7 | 60 ± 15.2 | 0.437 |
| Male [n (%)] | 74 (67.9%) | 40 (75.5%) | 34 (60.7%) | 0.099 |
| Hypertension [n (%)] | 62 (56.9%) | 30 (56.6%) | 32 (57.1%) | 0.955 |
| S-creatinine (mg/dl) (mean ± SD) | 1.45 ± 1.35 | 1.29 ± 1.09 | 1.72 ± 2.05 | 0.205 |
| Atrial fibrillation [n (%)] | 24 (22%) | 10 (18.9%) | 14 (25%) | 0.440 |
| BSA (m2) (mean ± SD) | 1.77 ± 0.11 | 1.77 ± 0.11 | 1.78 ± 0.10 | 0.907 |
| BMI (kg/m2) (mean ± SD) | 23.78 ± 2.6 | 23.8 ± 2.7 | 23.7 ± 2.6 | 0.847 |
| Smoker [n (%)] | 32 (29.4%) | 18 (34%) | 14 (25%) | 0.304 |
| LVEF (%) (mean ± SD) | 56.74 ± 9.1 | 56.79 ± 8.3 | 56.7 ± 9.83 | 0.956 |
| NYHA 4 [n (%)] | 18 (17.6%) | 10 (19.2%) | 8 (16%) | 0.669 |
| EuroSCORE II (mean ± SD) | 7.27 ± 9.35 | 6.7 ± 8.31 | 8.15 ± 10.88 | 0.542 |
| Severe mitral regurgitation [n (%)] | 97 (89%) | 47 (88.7%) | 50 (89.3%) | 0.920 |
| Dialysis [n (%)] | 3 (2.7%) | 1 (1.9%) | 2 (3.6%) | 1 |
| Diabetes with insulin (%) | 13 (11.9%) | 6 (11.3%) | 7 (12.5%) | 0.849 |
| Previous stroke [n (%)] | 24 (22%) | 13 (24.5%) | 11 (19.6%) | 0.538 |
| Hypercholesterolemia | 39 (35.8%) | 23 (43.4%) | 16 (28.6%) | 0.107 |
| Previous cardiac surgery [n (%)] | 22 (20%) | 11 (20.8%) | 11 (19.6%) | 0.885 |
BSA, body surface area; LVEF, left ventricular ejection fraction; MVP, mitral valve prosthesis; MVR, mitral valve repair; NYHA, New York Heart Association; S-creatinine, serum creatinine; SD, standard deviation.
Causative microorganism
Causative microorganisms are summarized in Table 2. The most frequently isolated pathogens were Staphylococcus aureus (17,4%), Streptococcus viridans (12.8%) and Enterococcus (10.1%) whereas in 34 cases (31.1%), blood cultures resulted as negative.
Table 2.
Causative microorganisms
| Pathogen | Total (n = 109) | Percentage |
| Staphylococcus spp. | 27 | 24.8% |
| Staphylococcus aureus | 19 | 17.4% |
| MSSA | 11 | 10.1% |
| MRSA | 8 | 7.3% |
| CNS (coagulase-negative Staphylococcus) | 8 | 7.3% |
| Staphylococcus epidermidis | 4 | 3.7% |
| Staphylococcus haemoliticus | 1 | 0.9% |
| Staphylococcus lugdudensis | 2 | 1.8% |
| Staphylococcus capitis | 1 | 0.9% |
| Streptococcus spp. | 28 | 25.7% |
| Streptococcus viridans | 14 | 12.8% |
| Streptococcus sanguinis | 8 | 7.3% |
| Streptococcus mitis | 4 | 3.7% |
| Streptococcus anginosus | 1 | 0.9% |
| Streptococcus defectivus | 1 | 0.9% |
| Streptococcus gallolyticus | 9 | 8.3% |
| Former nutritional variants | 2 | 1.8% |
| Abiotrofia defective | 1 | 0.9% |
| Granulicatella adiacens | 1 | 0.9% |
| Other Streptococcaceae | 3 | 2.8% |
| Enterococcus faecalis | 11 | 10.1% |
| Actinomyces odontolyiticus | 2 | 1.8% |
| HACEK group | 2 | 1.8% |
| Gram negative other than HACEK | 3 | 2.8% |
| Candida spp. | 2 | 1.8% |
| Negative blood culture | 34 | 31.1% |
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Surgical technique
Surgical details are listed in Table 3. In 14 cases (12.8%), surgery was performed under urgency/emergency conditions because of hemodynamic instability (MVR 5 patients, 9.4%; MVP 9 patients, 16.1%, P = 0.301). Twenty-five patients (22.9%) underwent concomitant aortic valve replacement and five patients had concomitant CABG (4.6%). Valve replacement in the MVP group was performed via median sternotomy in all cases: 71.4% (40/56) of patients received a mechanical valve. Of note, posterior subvalvular apparatus was preserved unless largely involved in the infective endocarditis process. Conversely, median sternotomy was performed in 77.4% of patients of the MVR group whereas others underwent MVR via right anterior mini-thoracotomy (12/53, 22.6% patients). In particular, the MVR group required valvuloplasty associated with annuloplasty in 86.8% of cases (46/53), whereas a repair without annular stabilization was performed in 13.2% of patients (7/53). Technical details of MVR are reported in Table 4.
Table 3.
Operative outcomes
| Variables | Total (n = 109) | Mitral valve repair (MVR) (n = 53) | Mitral valve replacement (MVP) (n = 56) | P-value |
| Urgent/emergency surgery [n (%)] | 14 (12.8%) | 5 (9.4%) | 9 (16.1%) | 0.301 |
| Concomitant aortic valve replacement [n (%)] | 25 (22.9%) | 14 (26.4%) | 11 (19.6%) | 0.401 |
| Concomitant CABG [n (%)] | 5 (4.6%) | 4 (7.6%) | 1 (1.8%) | 0.198 |
| CPB time (min), median (25th–75th IQR) | 113 (95–13) | 125 (104–143) | 83 (80–96) | 0.006 |
| Aortic cross-clamp time (min), median (25th–75th IQR) | 186 (80–102) | 90 (75–106) | 83 (80–96) | 0.238 |
CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; IQR, interquartile range; MVP, mitral valve prosthesis; MVR, mitral valve repair.
Table 4.
Surgical techniquesa used for mitral valve repair
| Surgical techniquea for MVR | Frequency |
| Edge to edge | 10 (18.9%) |
| Plicature | 2 (3.8%) |
| Commissural closure | 2 (3.8%) |
| Leaflet extension | 6 (11.3%) |
| P2 resection | 25 (47.2%) |
| Other segments resection | 3 (5.7%) |
| Gore-Tex chordal replacement | 7 (13.2%) |
| Prosthetic ring | 46 (86.8%) |
| Repair without ring | 7 (13.2%) |
MVR, mitral valve repair.
Techniques described are used alone or combined.
Perioperative
In-hospital mortality was 2.8% (3/109 patients). Of these, one patient in the MVR group died from cardiogenic shock whereas in the MVP group, two patients died from multiorgan failure and invasive fungal infection, respectively. Postoperative course and complications are reported in Table 5. Of note, no patients experienced cerebrovascular accidents during hospitalization. Mean hospital stay was 10.5 ± 9.10 days (MVR: 11.04 ± 10.4 days versus MVP: 10.07 ± 8.09 days, P = 0.580). During this time frame, no patients experienced early infective endocarditis relapse.
Table 5.
Postoperative outcomes
| Variables | Overall population (n = 109) | Mitral valve repair (MVR) (n = 53) | Mitral valve replacement (MVP) (n = 56) | P-value |
| In-hospital mortality [n (%)] | 3 (2.8%) | 1 (1.9%) | 2 (3.6) | 1 |
| Length of stay (days) (mean ± SD) | 10.5 ± 9.10 | 11.04 ± 10.4 | 10.07 ± 8.09 | 0.580 |
| ICU stay (h) (median, IQR) | 22 (18–54) | 24 (18–66) | 21 (18–42) | 0.449 |
| Prolonged ventilation (MAV >48 h) | 5 (4.6%) | 4 (7.5%) | 1 (1.8%) | 0.190 |
| IABP [n (%)] | 5 (4.6%) | 3 (5.7%) | 2 (3.6%) | 0.602 |
| Bleeding requiring surgical revision [n (%)] | 6 (5.5%) | 2 (3.8%) | 4 (7.1%) | 0.679 |
| Blood transfusion [n (%)] | 62 (56.9%) | 36 (67.9%) | 26 (46.4%) | 0.024 |
| Acute renal injury (KDIGO ≥2) [n (%)] | 6 (5.5%) | 4 (7.5%) | 2 (3.6%) | 0.429 |
| Postoperative LVEF (mean ± SD) | 55.06 ± 8.38 | 55.91 ± 8.05 | 54.16 ± 8.76 | 0.399 |
IABP, intra-aortic balloon pump; IQR, interquartile range; LVEF, left ventricular ejection fraction; MAV, mechanical assisted ventilation; MVP, mitral valve prosthesis; MVR, mitral valve repair; SD, standard deviation.
Follow-up
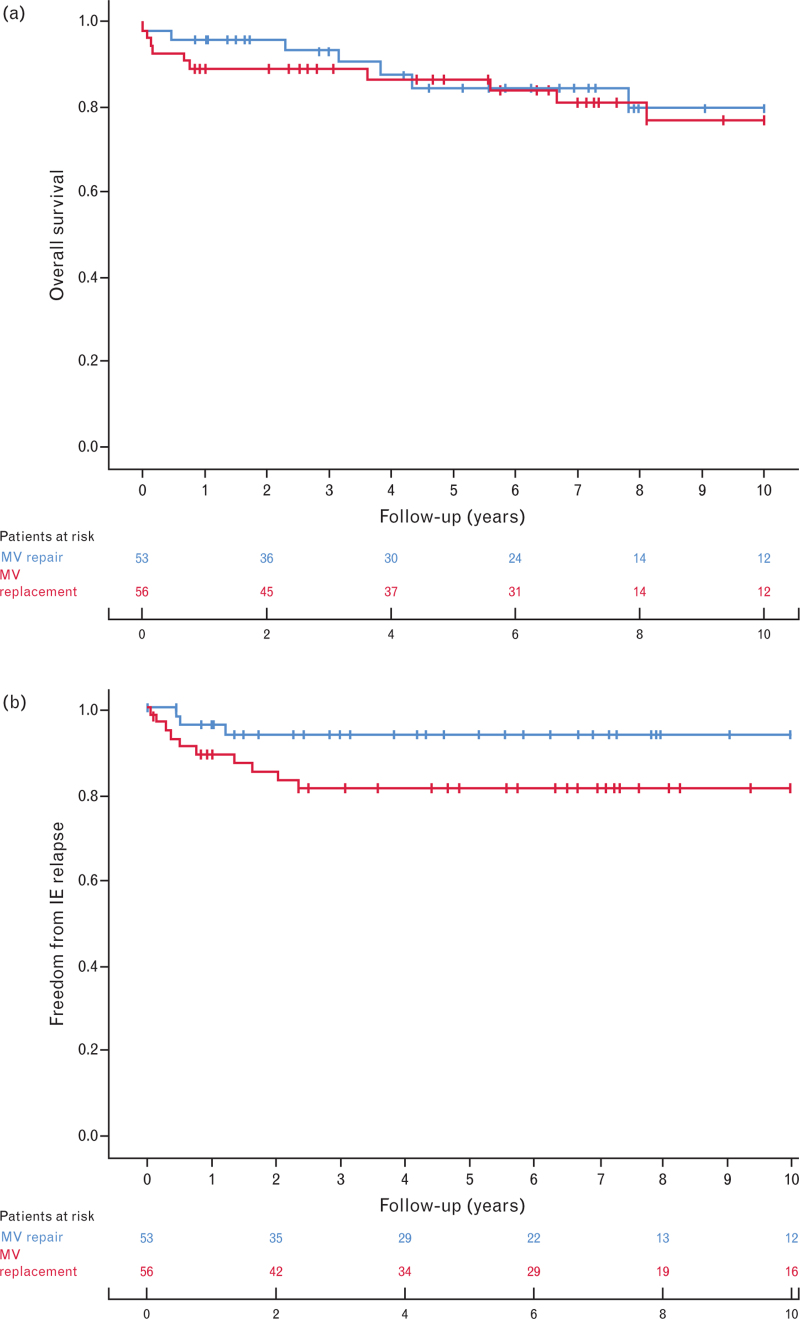
Mean follow-up was 74 months [95% confidence interval (CI) 23–126] and the follow-up time was comparable between the two groups (MVP: 69 ± 52 months versus MVP: 78 ± 52 months, P = 0.294). All-cause mortality rate was 15.6% (17/109) for the entire population and no differences were found between the two groups (MVR 7/53, 13.2% versus MVP 10/56, 17.9%, P = 0.503). Survival rate at 5 years of follow-up was 84.7 ± 5.1% for MVR and 86.9 ± 9.0% for MVP whereas at 10-year follow-up, survival was 80.0 ± 14.1 and 77.2 ± 13.5% for MVR and MVP, respectively (P = 0.648) (Fig. 1a). Cardiac-related death occurred in three MVR and five MVP patients (P = 0.717).
Fig. 1.
(a) Survival analysis with Kaplan–Meier curve for overall survival. (b) Kaplan–Meier curve for survival freedom from endocarditis relapse.
Infective endocarditis relapse was confirmed in 13 patients [MVR = 3/53 (5.7%) versus MVP = 10/56 (16.1%), P = 0.049]; in particular, 3 of these required reintervention (1 in the MVR group and 2 in the MVP group). Kaplan–Meier event-free survival showed a significantly higher incidence of endocarditis relapse in the MVP group (10-year: MVR = 93.6 ± 7.1; MVP = 80.9 ± 10.8%, P = 0.041; Fig. 1b). Multivariate Cox regression analysis depicted endocarditis relapse as an independent predictor of mortality (hazard ratio = 4.03; 95% CI 1.41–11.52; P = 0.009). Cox regression analysis is reported in detail in Table 6.
Table 6.
Univariable and multivariable Cox regression analysis to identify independent predictors of all-cause mortality at follow-up
| Univariable | Multivariable | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| MRSA/MSSA | 1.11 | 0.60–2.04 | 0.737 | |||
| REDO | 0.56 | 0.14–2.23 | 0.417 | |||
| Drug abuser | 0.85 | 0.41–1.75 | 0.668 | |||
| MVR versus MVP | 0.63 | 0.39–1.01 | 0.058 | 0.99 | 0.37–2.68 | 0.986 |
| Early surgery | 0.81 | 0.38–1.76 | 0.597 | |||
| IE recurrence | 4.03 | 1.41–11.52 | 0.009 | 2.98 | 1.37–6.47 | 0.006 |
HR, hazard ratio; IE, infective endocarditis; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; MVP, mitral valve prosthesis; MVR, mitral valve repair.
Eight redo operations were performed on mitral valve/mitral prosthesis during the follow-up: three for re-endocarditis as outlined above (one in the MVR group and two in the MVP group), three for worsening of mitral regurgitation after a previous MVR and two for prosthesis degeneration.
The incidence of cardiac-related need for rehospitalization was 29.4% (32/109) at long-term follow-up [MVR = 28.3% (15/53); MVP = 30.4% (17/56), P = 0.814].
Discussion
This study sought to examine the clinical outcomes of patients undergoing MVR compared with MVP as treatment for acute infective endocarditis. One hundred and nine patients were enrolled from January 2014 to December 2019 where 48.6% underwent MVR and 51.4% of patients had MVP. We found no advantages in terms of overall survival reported at long-term follow-up when MVR was performed over MVP. However, MVP was associated with a higher incidence of infective endocarditis relapse, which was found to be an independent predictor of mortality.
The principle of endocarditis surgery is to completely eradicate infection and restore functional anatomy of the involved valve. Since its first report in the 1990s, MVR in infective endocarditis has gained popularity and it has been largely proposed as the treatment of choice.11 Nowadays, leading centers in mitral valve surgery reach 80% of repairs in infective endocarditis on mitral valve.12 However, in the ‘real world’ the median of MVR performed in infective endocarditis is around 15% with a wide variability, ranging from 10 to 80%.12,13
In this study, MVR was retained as feasible in 50% of patients with acute infective endocarditis when the mitral valve was involved in an infectious process. Unless the indications for urgent/emergent surgery were met according to the ESC guidelines, surgery was postponed after 4 weeks of antibiotic therapy.9 This approach aimed to achieve a full blood sterilization, maximizing medical therapy before surgery, lowering the incidence of acute infective endocarditis relapse and reducing complications in the postoperative course for either the MVR or the MVP group. Defauw et al.14 described a different timing for surgical intervention and showed early results akin to those in our study. In their study, surgery was performed within a few days of optimal antibiotics therapy to minimize progression of the disease, which resulted in no early postoperative reinfection. Similarly, de Kerchove et al.12 described an early surgical approach (within a median delay of 9 days – unless urgent) with mitral valve surgery being performed. They reported only one case of early relapse of infective endocarditis despite 4.6% of cases requiring early reintervention for suture dehiscence (four in the MVP group and one in the MVR group) thus suggesting that early surgery performed on active inflammatory tissue may jeopardize the effectiveness of the surgical procedure. However, both centers reported an intraoperative and perioperative mortality of around 15%, respectively. This was five-fold higher than what we found in our population (2.8%; 3/109) following a maximization of medical therapy reducing the risk of late bacteremia. These differences in early results keep topic controversies between pros and cons of an early versus a delayed surgical approach. Moreover, de Kerchove et al.,12 Cuerpo et al.15 and Ruttmann et al.16 refuted the general idea of ‘urgent surgery reduces itself the repair probability on the mitral valve’ being able to proceed to MVR in more than 60 and 80% of cases.
We speculate a watchful waiting approach in nonurgent/emergency clinical scenarios that allows the infective endocarditis process to expand and reduce healthy tissue available for a stable and durable repair. The appropriate timing for the treatment of infective endocarditis involving ‘left-side valves’ is still a matter of debate and results in literature showed to be inconclusive in demonstrating a clear superiority of a ‘prompt’ rather than a ‘delayed’ approach.
Our population was balanced in terms of preoperative baseline characteristics. We did not outline any survival benefit of having the mitral valve repaired rather than replaced (MVR: 80.0 ± 14.1% versus MVP: 77.2 ± 13.5%, P = 0.648). This was similar to results retrieved from the Spanish nationwide registry in which the tendency to have a better survival in the MVR group did not reach statistical significance.15 Other studies showed similar findings with no differences in terms of long-term survival in patients receiving MVR when compared with MVP.17 In contrast, a large US registry involving more than 1900 patients reported a significantly higher perioperative mortality at long-term follow-up in patients with infective endocarditis who underwent MVP when compared with MVR.18 Other groups reported similar findings where MVR showed survival benefits, especially for composite end points, including infective endocarditis relapse, mortality and need for redo surgery.16
Regarding the recurrences of infective endocarditis, we found a significantly higher incidence of relapse at 10-year follow-up in the MVP group compared with the MVR group (MVR: 3/53, 5.7% versus MVP: 10/56, 16.1%, P = 0.049). Most of these events occurred within the first 3 years of follow-up. These outcomes were confirmed in a recent meta-analysis by He et al.,13 where MVR was associated with a lower incidence of recurrences, thus suggesting that the prosthetic valve itself represents a plausible risk factor for endocarditis relapse.18,19 Opposite results were reported by Cuerpo et al.,15 along with Ruttmann et al.16 and Defauw et al.,14 where no differences were found in terms of infective endocarditis relapse. Despite controversies, in this study, we highlighted infective endocarditis as an independent predictor of mortality regardless of the surgical strategy (hazard ratio 4.03; 95% CI 1.41–11.52; P = 0.009).
Limitations
The main limitation is the retrospective form of this current analysis. Despite the fact that we only included patients with evidence of mitral valve endocarditis involvement receiving MVR or MVP with/without associated procedures, the presence of patients who underwent urgent surgery for hemodynamic instability could have influenced early results in terms of morbidity and mortality. Although the preoperative variables were not statistically different, confounding factors may persist given the different surgical strategies chosen (MVR/MVP) and indicating the different mitral valve involvement between the two groups. Finally, the small sample size of this single-center report and lack of randomization limit the generalization of our findings.
Conclusion
In conclusion, controversial data have been reported for early and late mortality and infective endocarditis relapse in patients undergoing MVR or MVP for infective endocarditis affecting the mitral valve. In this regard, a clear recommendation of a repair rather than replacement approach seems to be inappropriate. We do believe the therapeutic approach should be based on the individual circumstances as well as on the single-center results and experience. Further investigations are warranted, although powered randomized analyses comparing MVR versus MVP are difficult to perform in the context of infective endocarditis and they might be unethical.
Acknowledgements
Data availability statement: The data underlying this article will be shared on reasonable request to the corresponding author.
IRB Approval PN 1815.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Revised 8 July, 2023
References
- 1.Jung JC, Jang MJ, Hwang HY. Meta-analysis comparing mitral valve repair versus replacement for degenerative mitral regurgitation across all ages. Am J Cardiol 2019; 123:446–453. [DOI] [PubMed] [Google Scholar]
- 2.Gillinov AM, Blackstone EH, Nowicki ER, et al. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg 2008; 135:885–893. 893.e1–893.e2. [DOI] [PubMed] [Google Scholar]
- 3.Okada Y, Nakai T, Muro T, Ito H, Shomura Y. Mitral valve repair for infective endocarditis: Kobe experience. Asian Cardiovasc Thorac Ann 2020; 28:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tepsuwan T, Rimsukcharoenchai C, Tantraworasin A, et al. Comparison between mitral valve repair and replacement in active infective endocarditis. Gen Thorac Cardiovasc Surg 2019; 67:1030–1037. [DOI] [PubMed] [Google Scholar]
- 5.Lee HA, Cheng YT, Wu VC, et al. Nationwide cohort study of mitral valve repair versus replacement for infective endocarditis. J Thorac Cardiovasc Surg 2018; 156:1473–1483. [DOI] [PubMed] [Google Scholar]
- 6.Harky A, Hof A, Garner M, Froghi S, Bashir M. Mitral valve repair or replacement in native valve endocarditis? Systematic review and meta-analysis. J Card Surg 2018; 33:364–371. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Miao Q, Liu X, Zhang C, Ma G, Liu J. Repair versus replacement for active endocarditis of the mitral valve: 9 years of experience. J Card Surg 2022; 37:3713–3719. [DOI] [PubMed] [Google Scholar]
- 8.Cecchi E, Corcione S, Lupia T, et al. Infective endocarditis in people who inject drugs: report from the Italian Registry of Infective Endocarditis. J Clin Med 2022; 11:4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier A, Adams DH, Filsoufi F. Carpentier's reconstructive valve surgery: from valve analysis to valve reconstruction. Maryland Heights, Missouri: Saunders/Elsevier; 2010. [Google Scholar]
- 11.Dreyfus G, Serraf A, Jebara VA, et al. Valve repair in acute endocarditis. Ann Thorac Surg 1990; 49:706–711. [DOI] [PubMed] [Google Scholar]
- 12.de Kerchove L, Price J, Tamer S, et al. Extending the scope of mitral valve repair in active endocarditis. J Thorac Cardiovasc Surg 2012; 143: (4 Suppl): S91–S95. [DOI] [PubMed] [Google Scholar]
- 13.He K, Song J, Luo H, et al. Valve replacement or repair in native mitral valve infective endocarditis-which is better? A meta-analysis and systematic review. J Card Surg 2022; 37:1004–1015. [DOI] [PubMed] [Google Scholar]
- 14.Defauw RJ, Tomšič A, van Brakel TJ, Marsan NA, Klautz RJM, Palmen M. A structured approach to native mitral valve infective endocarditis: is repair better than replacement? Eur J Cardiothorac Surg 2020; 58:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuerpo GP, Valerio M, Pedraz A, et al. Mitral valve repair in infective endocarditis is not inferior to valve replacement: results from a Spanish nationwide prospective registry. Gen Thorac Cardiovasc Surg 2019; 67:585–593. [DOI] [PubMed] [Google Scholar]
- 16.Ruttmann E, Legit C, Poelzl G, et al. Mitral valve repair provides improved outcome over replacement in active infective endocarditis. J Thorac Cardiovasc Surg 2005; 130:765–771. [DOI] [PubMed] [Google Scholar]
- 17.Perrotta S, Fröjd V, Lepore V, Schersten H, Jeppsson A, Svensson G. Surgical treatment for isolated mitral valve endocarditis: a 16-year single-centre experience. Eur J Cardio-thorac Surg 2018; 53:576–581. [DOI] [PubMed] [Google Scholar]
- 18.Toyoda N, Itagaki S, Egorova NN, et al. Real-world outcomes of surgery for native mitral valve endocarditis. J Thorac Cardiovasc Surg 2017; 154:1906.e9–1912.e9. [DOI] [PubMed] [Google Scholar]
- 19.Oliver L, Leauthier M, Jamme M, et al. Mitral valve repair is better than mitral valve replacement in native mitral valve endocarditis: results from a prospective matched cohort. Arch Cardiovasc Dis 2022; 115:160–168. [DOI] [PubMed] [Google Scholar]