Abstract
Background
Antibody-drug conjugates are cancer therapeutics that combine specificity and toxicity. A highly cytotoxic drug is covalently attached to an antibody that directs it to cancer cells. The conjugation of the drug-linker to the antibody is a key point in research and development as well as in industrial production. The consensus is to conjugate the drug to a surface-exposed part of the antibody to ensure maximum conjugation efficiency. However, the hydrophobic nature of the majority of drugs used in antibody-drug conjugates leads to an increased hydrophobicity of the generated antibody-drug conjugates, resulting in higher liver clearance and decreased stability.
Methods
In contrast, we describe a non-conventional approach in which the drug is conjugated in a buried part of the antibody. To achieve this, a ready-to-click antibody design was created in which an azido-based non-canonical amino acid is introduced within the Fab cavity during antibody synthesis using nonsense suppression technology. The Fab cavity was preferred over the Fc cavity to circumvent issues related to cleavage of the IgG1 lower hinge region in the tumor microenvironment.
Results
This antibody design significantly increased the hydrophilicity of the generated antibody-drug conjugates compared to the current best-in-class designs based on non-canonical amino acids, while conjugation efficiency and functionality were maintained. The robustness of this native shielding effect and the versatility of this approach were also investigated.
Conclusions
This pioneer design may become a starting point for the improvement of antibody-drug conjugates and an option to consider for protecting drugs and linkers from unspecific interactions.
Keywords: antibody-drug conjugates, native shielding, non-canonical amino acids, fab cavity, hydrophilicity, hydrophobicity
Statement of Significance: This work describes for the first time to our knowledge a ready-to-click antibody design based on non-canonical amino acids enabling the generation of highly hydrophilic antibody-drug conjugates and highlights the importance of the conjugation site selection as well as the drug-linker design for the generation of less hydrophobic antibody-drug conjugates.
INTRODUCTION
To date, a total of thirteen antibody-drug conjugates (ADCs) have been approved for cancer therapy by various regulatory organizations, such as the Food and Drug Administration (USA), the European Medicines Agency (EU), the Pharmaceuticals and Medical Devices Agency (Japan), and the National Medical Products Administration (China). The concept of ADCs can be seen as conferring greater cytotoxicity to the antibody, or higher specificity to the drug. The antibody part of the ADC recognizes the tumor-associated antigen on the tumor cell surface. Once the ADC is internalized, the drug, covalently attached to the antibody via a linker, is released and kills the tumor cell. Among the thirteen approved ADCs, eleven are based on IgG1, while two of them are based on IgG4. The use of these antibody formats allows a long half-life and stability of the ADCs. Since the mammalian cell machinery allows excellent quality control of proline isomerization, disulfide-bridge formation, N-glycosylation, and other post-translational modifications required for the antibody quality, antibodies are industrially produced in chinese hamster ovary (CHO) cells for eleven of the currently approved ADCs, or in NS0 and Sp2/0 cells for the two remaining. The majority of drugs used for ADCs currently approved or having reached clinical trials target DNA (anthracyclines, benzodiazepines, calicheamicins, duocarmycins), RNA polymerase II (amatoxins), topoisomerase I (camptothecins), or microtubules (auristatins, maytansinoids, tubulysins). A linker, which may be cleavable or non-cleavable, covalently links the drug to the antibody. These drug-linkers are generally produced chemically. Once the drug-linker and the antibody are produced, they are conjugated by chemical, enzymatic, or a combination of enzymatic and chemical methods. Conjugation efficiency is a key issue in research and development since homogeneous products are required to draw meaningful conclusions about functional and biochemical properties. For production, reduced conjugation efficiency has a significant time and cost impact. To ensure maximum conjugation efficiency, surface-exposed conjugation sites are conventionally chosen. However, the drugs used for ADCs are often highly hydrophobic. This leads to an increased hydrophobicity of the generated ADCs, resulting in higher liver clearances by the Kupffer cells and sinusoidal endothelial cells [1, 2], which then reduces the therapeutic efficacy. Aside from the therapeutic aspect, this hydrophobicity also decreases ADC stability due to a higher propensity to aggregate. In contrast, we opted for a non-conventional approach, which can be seen as analogous to a Trojan Horse. We decided to conjugate the drug in a buried part of the antibody and specifically chose the cavity in the Fab region over the cavity in the Fc region (Figure 1a). Indeed, one issue of the IgG1-based antibodies is the cleavage in their lower hinge region in the tumor microenvironment by metalloproteinases [3]. While the IgG1 remains full-length upon single chain cleavage, due to strong non-covalent interactions between both CH3 domains, the full cleavage separates the Fc part from the (Fab)2 part. Hence, if the drug is located on the Fc region and full cleavage occurs, the Fab region, capable of targeting the tumor cell, no longer has a cytotoxic effect.
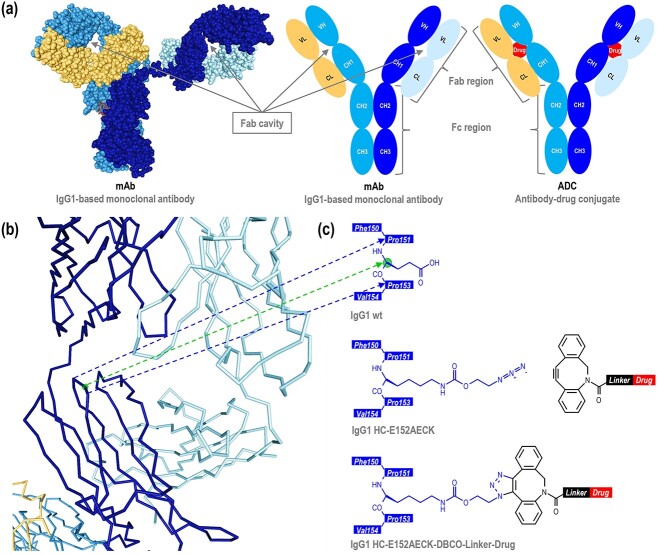
Figure 1.
Antibody design. (a) 3D (PDB: 1HZH) and schematic representations of a monoclonal antibody, and schematic representation of an antibody-drug conjugate with the drug within the Fab cavity. (b) 3D representation (PDB: 1HZH) of one of the two Fab regions. Each point represents the α-carbon of the amino acid. The amino groups, the carboxy groups, and the side chains are not represented to simplify the visualization. The sphere represents the α-carbon of the glutamic acid HC-152. (c) Schematic representations of the native antibody sequence with the sphere representing the α-carbon of the glutamic acid HC-152, the antibody sequence with AECK introduced at the position HC-152, and the antibody sequence with AECK at the position HC-152 conjugated to a DBCO-based drug-linker. Only one of the two triazole regioisomers [1, 4] is represented. Amino acids are numbered according to the IgG1-Eu described by Edelman et al. [19].
Enzymatic and combination of enzymatic and chemical methods enable addressing the N-terminus, C-terminus, or limited positions within the antibody, implying very limited design possibilities. On the other hand, chemical methods based on engineered cysteines and non-canonical amino acids allow for addressing more positions and generating tailor-made ADCs. The non-canonical amino acids, due to their unique structures and reactive groups, enable new designs and conjugation strategies. Therefore, this technology was used to create a new design. Although the orthogonal system also targets endogenous nonsense codons, Roy et al. [4] have shown that amber (uag) suppression does not impact the growth, viability, yields, or quality of the produced antibody. Reduced cell viability was only observed with some non-canonical amino acids, due to the non-canonical amino acid itself, independently of the orthogonal system or amber suppression. They also described its suitability for industrial application, outperforming with 3 g/L the yields previously reported [5–7]. Hence, the amber suppression technology was chosen to create a monoclonal antibody (mAb) design in which the non-canonical amino acid is located in a buried position of the Fab. This mAb design was assessed in respect of synthesis, conjugation, as well as of biochemical and functional properties of the generated ADCs.
RESULTS AND DISCUSSION
Antibody design
Based on 3D structures of human IgG1 [8], murine IgG1 [9], humanized IgG1-based Fab [10–14], and humanized IgG2-based Fab [15], we aimed to identify positions whose amino acid residue 1) has its α-carbon/β-carbon bond or β-carbon/γ-carbon bond oriented towards the inside of the Fab cavity, 2) while being sufficiently far from the center of the cavity, to provide enough space for the drug-linker and to ensure the maximum shielding effect. For the selected positions, the possible rotamers of the lysine and tyrosine residues were then evaluated. Among the canonical amino acids, lysine is structurally the closest to azido-ethoxy-carbonyl-lysine (AECK), a non-canonical amino acid used for the generation of ADCs [4, 7]. Similarly, tyrosine is structurally the closest canonical amino acid to p-azido-phenylalanine (AzF), another non-canonical amino acid used for the generation of ADCs [16–18]. Positions were numbered according to the IgG1-Eu described by Edelman et al [19]. We determined the position HC-152 in combination with AECK as the best-suited design (Figure 1b). For AzF however, rotamers at this position that are suitable for conjugation may not guarantee an inward orientation of the drug. Therefore, AzF was not further investigated. Position HC-152 has the advantage of being located C-terminal of the proline residue HC-151, a checkpoint for IgG folding. Isomerization of this proline to cis-conformation is essential for the folding of the CH1 domain and its assembly with the CL domain [20]. Furthermore, it is located N-terminal of the proline residue HC-153, also one of the three cis prolines of the CH1 domain. As a result, the side chain orientation of the amino acid HC-152 is more likely to be energetically maintained. In combination with AECK containing an azido group, dibenzocyclooctyne (DBCO) containing the alkyne counterpart was chosen to conjugate the drug-linker to the antibody by strain-promoted azide-alkyne cycloaddition (SPAAC) (Figure 1c). Unlike bicyclononyne (BCN) which forms a single triazole regioisomer upon SPAAC, or simple alkyne which forms a single triazole regioisomer upon copper-catalyzed azide-alkyne cycloaddition (CuAAC), DBCO enables the generation of two possible regioisomers, thus maximizing the chances of addressing this difficult-to-access position.
Antibody synthesis
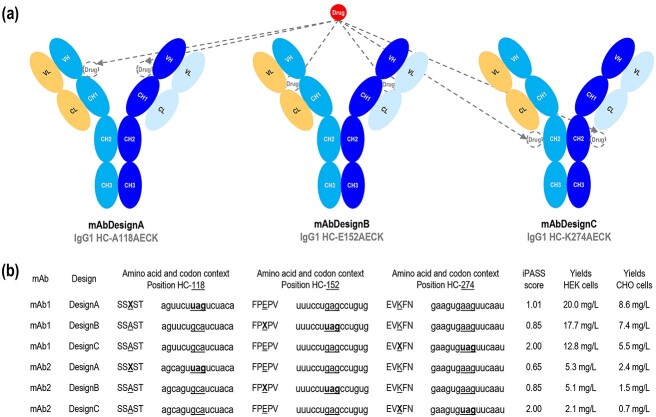
To determine whether the production of this antibody design would be suitable for industrial application, two additional antibody designs based on different positions for the incorporation of AECK were tested as references. The first reference design is based on position HC-118 (IgG1-Eu numbering). This position was described for the incorporation of the non-canonical amino acids acetyl-phenylalanine (AcF) [5, 6, 21–23], AzF [16–18], and cyclopropene-lysine (CypK) [24, 25] to generate ADCs, allowing an enhanced ADC stability over the position HC-119 [6]. Indeed, this is the position used in ARX517 and ARX788 (A118AcF), respectively anti-PSMA and anti-HER2 ADCs currently investigated in clinical phases I and II. More widely, this is also the position used in the THIOMAB™ technology based on engineered cysteines (A118C) [26, 27]. The second reference design is based on position HC-274. This position was described for the incorporation of AECK [4, 7] and cyclopentadienyl-ethoxy-carbonyl-lysine (CpHK) [28] to generate ADCs, showing the highest ADC hydrophilicity over several positions [7]. Both positions HC-118 and HC-274 were reported as suitable positions for the industrial production of antibodies containing non-canonical amino acids using amber suppression [4, 6, 7]. In this article, mAb design A refers to the antibody design in which the non-canonical amino acid AECK is introduced at position HC-118, mAb design B at position HC-152, and mAb design C at position HC-274 (Figure 2a). Since human embryonic kidney (HEK) cells are more commonly used in research for antibody synthesis, due to their ease of transfection with polyethylenimine (PEI), and CHO cells are mainly used for industrial production, the feasibility assessment of the designs was performed in both systems. A Methanosarcina mazei orthogonal system in combination with amber suppression was chosen to introduce the non-canonical amino acid AECK, and two IgG1-based monoclonal antibodies (mAb1 and mAb2) were used as models. As a proof-of-concept, antibody synthesis was performed by transient transfections. Four plasmids, encoding the antibody heavy chain, the antibody light chain, the orthogonal M. mazei AECK-transfer ribonucleic acid (AECK-tRNA), and the orthogonal M. mazei AECK-tRNA synthetase (AECK-RS), were co-transfected in equimolar ratios. Yields were quantified by affinity chromatography using the MabSelect™ SuRe™ column. The protein A of this column is modified to only recognize the heavy chain at its CH2-CH3 interface, in contrast to the native protein A which additionally recognizes the variable domain of the heavy chain for VH3-type antibodies. As a result, the suppression product can be selectively purified and quantified in a single step. Analysis of the synthesis showed that yields obtained from design B were between those of designs A and C, for both mAb1 and mAb2, in both HEK and CHO systems (Figure 2b). Interestingly, iPass (identification of permissive amber sites for suppression) scores, calculated based on amber codon context and supposed to predict suppression efficiency in HEK cells with the orthogonal M. mazei pyrrolysine-tRNA/pyrrolysine-RS pair [29], did not correspond at all with the obtained yields (Figure 2b), emphasizing the fact that although codon context is important for suppression efficiency, other factors are much more determining.
Figure 2.
Antibody synthesis. (a) Schematic representations of the mAb designs A, B, and C, with their intended drug location. (b) Amino acid context, codon context, theoretical iPASS score, and antibody yields in CHO and HEK systems for designs A, B, and C with mAb1 and mAb2. The reference amino acid or reference codon is indicated as underlined. X represents the non-canonical amino acid. iPASS scores were calculated based on the amber codon context by using the website shiny.bio.lmu.de:3838/iPASSv2. An iPASS score of ≥1 should indicate above-average relative non-canonical amino acid incorporation efficiency in HEK cells with the orthogonal M. mazei pyrrolysine-tRNA/pyrrolysine-RS pair. Antibody yields were quantified from the culture supernatants by affinity chromatography using the MabSelect™ SuRe™ column in an ÄKTA pure 25 L system. Transient transfections were independently performed three times. Amino acids are numbered according to IgG1-Eu described by Edelman et al. [19].
ADC hydrophobicity
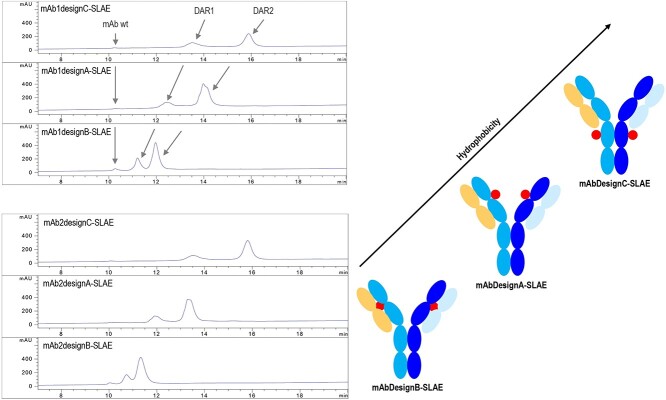
To evaluate ADC hydrophobicity, hydrophobic interaction chromatography (HIC) was utilized. This mild condition-based method enables the analysis of ADCs in a non-denatured state. DBCO-C4-PEG3-VC-PABC-AE (named SLAE, for standard linker auristatin E) was conjugated to purified antibodies. Auristatin E (AE) used in SLAE is widely known as monomethyl auristatin E, the main reference for ADC technologies. After conjugation, the generated ADCs were then purified, and HIC-ultraviolet (HIC-UV) analysis showed that ADCs based on design B were the most hydrophilic among the three investigated designs (Figure 3). The drug-to-antibody ratio = 2 (DAR2) species of design B were even more hydrophilic than the DAR1 species of design A. Interestingly, design C, which was reported to lead to the less hydrophobic ADCs among several designs [7], here led to the less hydrophilic ADCs.
Figure 3.
ADC hydrophobicity . Left: HIC chromatograms (214 nm) of ADCs based on mAb1 or mAb2, with designs C, A, and B (from top to bottom), and containing SLAE (DBCO-C4-PEG3-VC-PABC-AE). The arrows indicate the corresponding DAR species in each sample. For the chromatograms presented here, 125 ng of mAb1 wild-type (wt) or mAb2 wt were added to the sample (5 μg) as internal standard before injection. Right: schematic representations of ADCs based on designs C, A, and B (from top to bottom). The arrow indicates the increasing hydrophobicity. SLAE stands for standard linker auristatin E and is schematically represented in Figure 4a.
Shielding effect
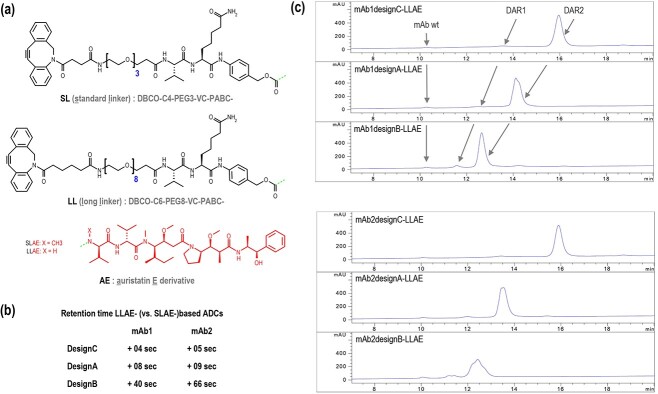
To determine the limit of this shielding effect, antibodies were conjugated with a drug via a longer linker (Figure 4a), to place the drug further outside the cavity. For this purpose, DBCO-C6-PEG8-VC-PABC-AE, named LLAE (for long linker auristatin E) was used to generate new ADCs. Compared to ADCs based on SLAE, ADCs based on LLAE showed an increase in retention time of 4 seconds with mAb1 and 5 seconds with mAb2 for the ADCs based on design C. For ADCs based on design A, an increase in retention time of 8 seconds with mAb1 and 9 seconds with mAb2 was observed. For ADCs based on design B, an increase in retention time of 40 seconds with mAb1 and 66 seconds with mAb2 was observed (Figure 4b). This strong hydrophobic shift for design B indicates a drastically reduced shielding effect. However, ADCs based on design B still remained much more hydrophilic than the ADCs based on designs A and C (Figure 4c), showing that even with this unusually long linker, the shielding effect is still present.
Figure 4.
Shielding effect . (a) Chemical structures of the standard linker (SL), long linker (LL), and auristatin E derivatives (AE). SLAE is the combination of SL and AE, in which X is a methyl substituent. LLAE is the combination of LL and AE, in which X is a hydrogen substituent. The dashes at the C-terminus of the linker and at the N-terminus of the drug represent the bond between the linker and the drug. (b) Differences in retention time (RT) between ADCs based on LLAE and ADCs based on SLAE were calculated as follows: RT difference = RTLLAE-based ADC—RTSLAE-based ADC. (c) HIC chromatograms (214 nm) of ADCs based on mAb1 or mAb2, with designs C, A, and B (from top to bottom), containing LLAE (DBCO-C6-PEG8-VC-PABC-AE). The arrows indicate the corresponding DAR species in each sample. For the chromatograms presented here, 125 ng of mAb1 wt or mAb2 wt were added to the sample (5 μg) as internal standard before injection.
Conjugation efficiency
To determine conjugation efficiency, areas under the curve obtained from the HIC-UV chromatograms were analyzed. Even though the non-canonical amino acid AECK is more difficult to access in design B, the conjugation efficiencies were in fact similar between the three different designs (Table 1). Conjugation with LLAE was more efficient than with SLAE (Table 1). This may be explained by the fact that the conjugation conditions, in particular the dimethyl sulfoxide (DMSO) concentration, were not optimal for SLAE (which is less polar than LLAE). Even though LLAE resulted in more hydrophobic ADCs, due to the drug being more exposed on the surface of the antibody, it showed that 100% conjugation efficiency could be achieved (Table 1), which corroborates the fidelity of this M. mazei orthogonal system [4] and the excellent conjugability of this non-canonical amino acid [7].
Table 1.
DAR, conjugation efficiency, DAR0, DAR1, and DAR2 species of the generated ADCs. Samples (5 μg) were analyzed by HIC (214 nm) without addition of the reference mAb wt.
| ADC | DAR | Conjugation efficiency |
DAR0 species |
DAR1 species |
DAR2 species |
|---|---|---|---|---|---|
| mAb1DesignA-SLAE | 1.83 | 92% | 0% | 17% | 83% |
| mAb1DesignB-SLAE | 1.69 | 85% | 2% | 26% | 72% |
| mAb1DesignC-SLAE | 1.67 | 84% | 0% | 33% | 67% |
| mAb2DesignA-SLAE | 1.79 | 90% | 0% | 21% | 79% |
| mAb2DesignB-SLAE | 1.77 | 88% | 1% | 21% | 78% |
| mAb2DesignC-SLAE | 1.69 | 85% | 0% | 31% | 69% |
| mAb1DesignA-LLAE | 2.00 | 100% | 0% | 0% | 100% |
| mAb1DesignB-LLAE | 1.96 | 98% | 0% | 4% | 96% |
| mAb1DesignC-LLAE | 1.95 | 97% | 0% | 5% | 95% |
| mAb2DesignA-LLAE | 1.98 | 99% | 0% | 2% | 98% |
| mAb2DesignB-LLAE | 1.90 | 95% | 1% | 8% | 91% |
| mAb2DesignC-LLAE | 1.96 | 98% | 0% | 4% | 96% |
In vitro efficacy
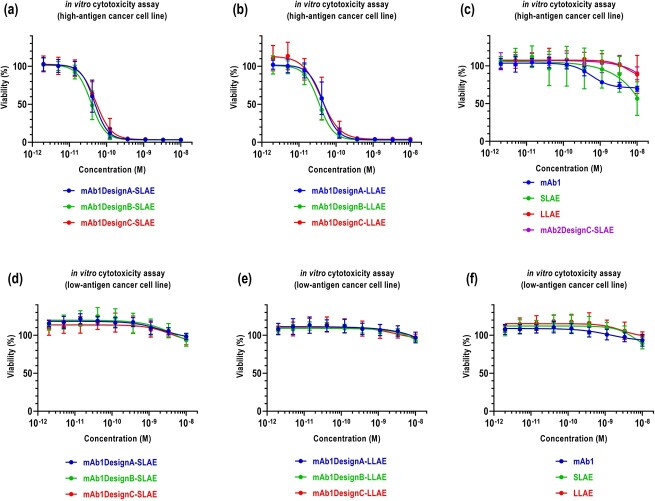
To determine whether shielding the drug in the Fab cavity reduces the ADC efficacy at the cellular level, in vitro cytotoxic assays were performed. As the cleavable linker is less accessible for design B and therefore more strongly dependent on lysosomal degradation compared to designs A and C, it was expected that ADCs based on the latter two designs show better efficacy. However, analysis of cytotoxic activity on high-antigen expressing cancer cell lines showed that ADCs based on design B perform as well as ADCs based on designs A and C (Figure 5a,b). Additionally, ADCs based on design B did not show cytotoxicity on low-antigen expressing cancer cell lines, similar to ADCs based on designs A and C (Figure 5a,b). The antibody wild-type (wt) alone, the drug-linkers alone, and the isotype ADC showed low cytotoxicity (Figure 5c).
Figure 5.
In vitro efficacy. Cytotoxic activity of (a) SLAE-based ADCs, (b) LLAE-based ADCs, (c) mAb wt, drug-linkers, and isotype ADC on high antigen-expressing cancer cell lines. Cytotoxic activity of (d) SLAE-based ADCs, (e) LLAE-based ADCs, (f) mAb wt and drug-linkers on low antigen-expressing cancer cell lines. The means and the standard deviations are the results of 3 independent experiments performed in triplicates.
Versatility
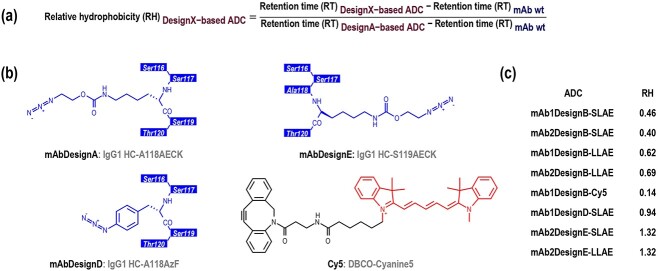
The proof-of-concept of this approach has been completed with auristatin E derivatives as ADC payload. To determine if this is not only applicable to auristatin E derivatives, this approach was repeated with a chemical molecule having a very different structure. DBCO-Cyanine5 (Figure 6b), a highly hydrophobic fluorophore, rarely used compared to its more hydrophilic sulfonated equivalent (DBCO-sulfo-Cyanine5), was conjugated to mAb1 designs A and B (Figure 3a) (Figure 4c). Due to its higher hydrophobicity, design C was not investigated anymore. As similarity in respect of hydrophobicity and conjugation efficiency was observed between both antibodies (Figure 3, 4, table 1), further investigations were done with either mAb1 or mAb2. To quantify and compare the hydrophobicity of ADC designs, the relative hydrophobicity (RH) was calculated based on the retention times (RT) from HIC-UV chromatograms, using the equivalent ADCs based on design A as reference (Figure 6a). For this, 125 ng of the corresponding antibody wt were added to the sample (2 μg) as internal standard before injection into the HIC-UV system. It was observed that the shielding effect is also present when conjugating DBCO-Cyanine5 (Cy5) (RH = 0.14) (Figure 6c). It seems that the shorter the linker, the stronger the shielding effect (RH = 0.62, 0.46, and 0.14 with mAb1DesignB conjugated with LLAE, SLAE, and Cy5 respectively) (Figure 6c). This suggests that the potential of this approach with our auristatin E-based drug-linkers can further be exploited.
Figure 6.
Relative hydrophobicity . (a) Formula of the relative hydrophobicity (RH). For the determination of the relative hydrophobicity, 125 ng of mAb wt were added to the sample (2 μg) as internal standard before injection into the HIC-UV system. Relative hydrophobicity was calculated with design A-based ADC sharing the same antibody and the same drug or fluorophore as reference. A low RH value indicates a low hydrophobicity. (b) Schematic representations of the antibody sequence with AECK introduced at position HC-118 (mAbDesignA), the antibody sequence with AzF introduced at position HC-118 (mAbDesignD), the antibody sequence with AECK introduced at position HC-119 (mAbDesignE), and the chemical structure of Cy5 (DBCO-Cyanine5). Amino acids are numbered according to IgG1-Eu described by Edelman et al. [19]. (c) Relative hydrophobicity (RH) of the generated ADCs. RH of design A-based ADCs, which are equal to 1 by definition, are shown in Table S1.
Additional reference design
Since design A (AECK introduced at position HC-118) was used as a reference but unlike design C has not yet been described in the literature, an additional antibody design, here referred to as design D, was generated. In this design, the non-canonical amino acid p-azido-phenylalanine (AzF) is introduced at position HC-118 (Figure 6b) using an Escherichia coli-based orthogonal system in mammalian cells, as described by other groups [16–18]. Designs A and D differ only by the nature of the non-canonical amino acid. Although AzF has a very different structure than AECK, both designs displayed a similar hydrophobicity (RH = 0.94) (Figure 6c), indicating that design B (RH = 0.46) is also superior to this reference design [16–18].
Relevance of the conjugation site
Another antibody design, here referred to as design E, was also generated to investigate the introduction of AECK at position HC-119 compared to position HC-118 (Figure 6b). ADCs based on design E were significantly more hydrophobic than those based on design A (RH = 1.32 and 1.32 with mAb2DesignE conjugated with SLAE and LLAE, respectively) (Figure 6c), possibly due to the drug-linker oriented outward from the antibody and being more exposed. This may explain the increased unspecific premature cleavage of the valine-citrulline linker in both mouse and rat plasma matrices observed by Tian et al. [6] for the position HC-119 compared to HC-118 using AcF, and highlights, even more, the importance of the careful selection of the conjugation site.
CONCLUSION
Similar to a Trojan Horse design, we conjugated the drug into the Fab cavity of the antibody, via the non-canonical amino acid AECK introduced at position HC-152 using nonsense suppression technology. This approach resulted in a significant increase in the hydrophilicity of the ADCs, far surpassing the current best-in-class designs. The yields of this antibody design (mAb design B) in CHO and HEK systems turned out to be even higher than those of the reference antibody design (mAb design C), a design already described as suitable for industrial application, suggesting that yields of 3 g/L reported by Roy et al. [4] may even be improved. Moreover, the conjugation efficiency and cytotoxic activity on tumor cells were also maintained, while low-antigen cells remained unaffected. Although a shielding effect was observed for our design with all drug-linkers, it was observed that the shorter the linker, the better the shielding effect. This suggests that the design of the auristatin E-based drug-linkers used in this approach may still be optimized to fully exploit the potential of this approach. This includes optimizing or redesigning the linker to allow the drug to be more deeply embedded in the Fab cavity. Also of interest, the investigation of bidentate linkers [30–32] may be an option to consider for the generation of ADCs with a higher DAR. Although this work focused on the generation and early evaluation of ADC designs, it will remain interesting to measure the impact of the high hydrophilicity of design B on the long-term stability, pharmacokinetic properties, and in vivo antitumor activity, according to the ADC candidates and indications.
During the course of this project, Coumans et al. [33] described a similar approach using engineered cysteines. In their approach, amino acid residues of the Fab region were replaced by cysteines, and drugs such as duocarmycin, auristatin E, and auristatin F were successfully conjugated. These designs, with the drug shielded in the Fab cavity, resulted in an outstanding hydrophilicity. An in vivo study [33] was performed with mice lacking the carboxylesterase 1c (CES1c), to ensure that differences in ADC efficacy were not due to unspecific premature cleavage of the valine-citrulline linker for designs in which more exposed positions are chosen. It was demonstrated that ADCs based on the design HC-P41C almost led to the complete elimination of the tumor, while ADCs based on a standard design did not show a significant reduction of the tumor volume at the same dose. Analysis of the pharmacokinetics showed a correlation with the antitumor activity.
Although unspecific interactions of drugs with endogenous enzymes, transporters, receptors, or anti-drug antibodies have not been extensively reported in the literature yet, the approaches described in this article and by Coumans et al. [33] may be considered to address such issues. Unlike current linker-based approaches to reduce hydrophobicity, these antibody-based approaches allow for shielding the drug by surrounding it, while removal of this native shield is simply achieved by lysosomal degradation of the antibody in the targeted cell. Due to the high frequency of optimal rotamers of the residue HC-152 combined with the long linear side chain of AECK, the antibody design described in this article increases the propensity of the drug-linker to be conjugated from the inside of the Fab cavity. Aside from this structural aspect, this design presents the rare advantage of enabling both direct and reliable drug conjugation. By rendering obsolete the processes of functionalization, buffer exchange, or enzymatic conversion, usually associated with extensive handling and characterization, this clickable design promotes reliable assessment and parallelization of candidates for research applications and represents an attractive way to avoid the introduction of additional batch-to-batch variations for industrial production.
MATERIAL AND METHODS
3D structures
PDB structures 1HZH [8], 1IGY [9], 6OGE [10], 4HKZ [11], 6B9Z [12], 6MH2 [13], and 1N8Z [14] 5SX4 [15] were used as models. Rcsb.org with viewer = NGL (WebGL) was used for the initial visualization of the backbone and side chains. Rcsb.org, with viewer = Jsmol (JavaScript), was used for visualization of hydrophobic regions, patches, and amino acids. WinCoot (0.9.4.1) was used for single amino acid mutation and rotamers identification.
Antibodies
Amino acids were numbered according to the IgG1-Eu described by Edelman et al. [19]. The constant domains of the heavy chain are of m17/−1/−2 allotype, where HC-214 is a lysine (G1m17), HC-356 is a glutamic acid (G1m-1), HC-358 is a methionine (G1m-1), and HC-431 is an alanine (G1m-2). The constant domains of the light chain are of Km3 allotype, where LC-153 is an alanine and LC-191 is a valine. mAb1 heavy chain variable domain is of VH3 humanized type, mAb2 heavy chain variable domain is of VH1 humanized type. mAb1 and mAb2 light chain variable domains are of VKI humanized types. Signal peptides and signal peptide types remain undisclosed. Shiny.bio.lmu.de:3838/iPASSv2/ [29] was used to calculate theoretical iPass scores according to the user guide.
Orthogonal synthetases and tRNAs
Orthogonal M. mazei AECK-RS is also known as M. mazei pyrrolysine-RS and was not modified. Orthogonal E. coli AzFRS is a modified version of the E. coli tyrosine-RS (Y37T-D182S-F183A-D265R, sequential numbering) as described by Chin et al. [34]. Orthogonal M. mazei AECK-tRNA is a modified version of the M. mazei pyrrolysine-tRNA (a10g-u14g-u16g-u20c-u25c-a52c, sequential numbering) as described by Serfling et al. [35]. Orthogonal E. coli AzF-tRNA is a modified version of the E. coli tyrosine-tRNA(−gua) (g35c, sequential numbering) as described previously [36, 37]. The introduction of a nuclear export signal (NES) to the N-terminus of the orthogonal M. mazei AECK-RS, supposed to increase its cytosolic localization and suppression in HEK cells [35, 38], did not significantly increase the yields in our HEK system and strongly reduced the overall yields in our CHO system, independently of the suppression efficiency. Therefore, antibody synthesis was performed in all systems without this NES.
Plasmids and DNA sequences
pcDNA3.4 was used as a final vector for HC and LC and was initially ordered at ProteoGenix S.A.S. pcDNA3.1_Zeo(+) was used as a final vector for orthogonal aa-RSs and was initially ordered at BioCat GmbH. pcDNA3.1_Hygro(+) was used as a final vector for orthogonal tRNAs and was initially ordered at Life Technologies GmbH. DNA sequences for heavy chain constant domains, i.e. from HC-A118 to HC-K447, and DNA sequences for light chain constant domains, i.e. from LC-R108 to LC-C214, were optimized for CHO systems using the GeneOptimizer Algorithm (Geneart AG) and were initially ordered in pcDNA3.1(+) vectors at BioCat GmbH. DNA sequences for heavy chain variable domains and light chain variable domains as well as signal peptides for mAb1 and mAb2 (optimization undisclosed) were initially ordered at BioCat GmbH in pUC57–1.8 k vectors. Orthogonal M. mazei AECK-RS and orthogonal E. coli AzF-tRNA synthetase (AzF-RS) were not DNA-optimized and were initially ordered in a pcDNA3.1(+) vector at BioCat GmbH. The DNA sequence for the NES was optimized as described by Serfling et al. [35]. For M. mazei AECK-tRNA and orthogonal E. coli AzFtRNA, the U6 promoter, as in BLOCK-iT™ U6 RNAi Entry Vector (Invitrogen, reference K494500), was used as tRNA promoter, following the CMV promoter, and followed by the orthogonal tRNA, a cca-tail, and a tttttt-terminator, in 8 copies, each separated by a HindIII site. The sequences were ordered at Life Technologies GmbH. For all constructs, no tag was introduced. For all constructs intended to be translated, the aug codon of the Kozak sequence present in the vector was used as a start codon.
Cloning
Gibson assembly technology was mainly used to modify vectors, signal peptides, transcription, and translation elements, as well as to generate amber mutants and final plasmids. For Gibson assembly, polymerase chain reaction (PCR) primers (melting temperature (Tm) annealing part = 60–64 °C; Tm non-annealing part = 48–52 °C) were ordered at Integrated DNA Technologies, BV., and used to generate DNA fragments using a Q5® Hot Start High-Fidelity DNA Polymerase (New England Biolabs GmbH, reference M0493L). The generated DNA fragments were extracted and purified from agarose gel electrophoresis using the Zymoclean™ Gel DNA Recovery Kit (Zymo Research Europe GmbH, reference D4008) according to the manufacturer’s protocol. Purified DNA fragments were then mixed between 1:1 and 1:10 molar ratio for Gibson assembly using NEBuilder® HiFi DNA Assembly, Master Mix (New England Biolabs GmbH, reference M5520AA) at 50 °C for 30 min. After purification using the DNA Clean & Concentrator™-5 (Zymo Research Europe GmbH, reference D4014) according to the manufacturer’s protocol, 20–40 ng of the generated product was used to transform E. coli JM109 electrocompetent cells (prepared in-house) using a GenePulser Cell™ electroporation system (Bio-Rad Laboratories GmbH). Clones were selected from Luria-Bertani-agar plates containing 100 μg/ml ampicillin, and grown in 10 mL Luria-Bertani Broth (Scharlau, reference 02–406-500) medium containing 100 μg/ml ampicillin for 16 hours. Plasmids were extracted and purified using the QIAprep® Spin Miniprep Kit (Qiagen GmbH, reference 27,106) according to the manufacturer’s protocol, analyzed by agarose gel electrophoresis, and sequenced by LGC Genomics Berlin.
Plasmid preparation
Transformed E. coli JM109 cells were grown, respectively for midi- or maxi-preparation, in 100 or 250 mL Luria-Bertani Broth (Scharlau, reference 02–406-500) medium containing 100 μg/ml ampicillin for 16 hours. Plasmid was extracted and purified using the PureLink™ HiPure Plasmid Midiprep Kit (Invitrogen/Thermo Fisher Scientific, K210005) or the PureLink™ HiPure Plasmid Maxiprep Kit (Invitrogen/Thermo Fisher Scientific, K210007) according to the manufacturer‘s protocol. Eluate was incubated with isopropanol (0.7 volumes per 1 volume eluate) at 25 °C for 2 min, before centrifugation (15,000 x g, 4 °C, 60 min). The supernatant was discarded, before new centrifugation (15,000 x g, 4 °C, 5 min). The supernatant was discarded, and 70% cold ethanol was gently introduced, before new centrifugation (10,000 x g, 4 °C, 5 min). The supernatant was discarded, before new centrifugation (15,000 x g, 4 °C, 5 min). The supernatant was carefully removed and discarded, and the pellet was dried (25 °C, 5–10 min). For midiprep or maxiprep, respectively 50 μL or 150 μL ultrapure water were then introduced directly on the pellet, and incubated at 37 °C for 5 min. The resuspended pellet was shaken on a Vibrax® VXR basic (IKA) at 1500 revolutions per minute, 25 °C for 30 min. After the final centrifugation (15,000 x g, 4 °C, 30 min), the supernatant was collected.
Antibody synthesis by transient transfection
Although not as optimal as the Expi293™ and ExpiCHO™ systems, HEK and CHO systems with established in-house protocols for further investigation by cell-free protein synthesis were used for antibody synthesis. For FreeStyle™ 293-F cells (Gibco/Thermo Fisher Scientific, reference R79007), HEK TF medium (Xell AG, reference 861–0001) supplemented with 6 mM UltraGlutamine™ (Lonza, reference BE17-605E/U1) was used. Cells were maintained between 0.5x106 and 3x106 cells/ml at 37 °C, 5% CO2, 90 rpm (50 mm shaking diameters) in non-baffled flasks without vent cap (cap not tightly closed). For CHO-K1 cells (Deutsche Sammlung von Mikroorganismen und Zellkulturen, reference ACC 110), adapted in-house for serum-free and suspension culture, ProCHO™5 medium (Lonza, reference BELN12-766Q) supplemented with 6 mM UltraGlutamine™ (Lonza, reference BE17-605E/U1) was used. Cells were maintained between 0.5x106 and 4x106 cells/ml at 37 °C, 5% CO2, 90 rpm (50 mm shaking diameters) in baffled flasks without vent cap (cap not tightly closed). For transfection, CHO and HEK cells were centrifuged (300 x g, 25 °C, 5 min), resuspended in fresh pre-equilibrated medium into a concentration of 4x106 cells/ml, and incubated at 37 °C for 2 hours. For 106 cells, 625 ng of plasmids were introduced in a 150 mM sodium chloride solution (50 ng/μl final). For 106 cells, 2500 ng of polyethylenimine (PEI) linear molecular weight 25,000 (Polysciences, Inc, reference 23,966–2) (1 mg/ml) were introduced in a 150 mM sodium chloride solution (200 ng/μl final). The PEI-based mix was introduced into the plasmid mix, and incubated at 25 °C for 10 min. After complexation, the mix was introduced drop by drop on the cells while gently swirling. Cells were incubated for 5 hours at 37 °C, 5% CO2, 55 rpm (50 mm shaking diameters) for HEK cells, and 50 rpm (50 mm shaking diameters) for CHO cells. An equivalent volume [1] of fresh pre-equilibrated medium containing 4 mM of azido-ethoxy-carbonyl-lysine (AECK) (Iris Biotech GmbH, reference HAA2080) or p-azido-phenylalanine (AzF) (Bachem, reference 4020250.0001) and 2.5 mM valproic acid (VPA) (Sigma, reference P4543-10G) [39] was introduced on the cells, followed by an incubation of 9 days at 31 °C [39, 40], 70 rpm (50 mm shaking diameters) for HEK cells and 60 rpm (50 mm shaking diameters) for CHO cells. For both HEK and CHO cells, 20 mL of 300 g/l glucose and 30 mL of 200 mM UltraGlutamine™ (Lonza, reference BE17-605E/U1) per liter of medium were supplemented 4 days after transfection. All samples and solutions were filtered or centrifuged (16,000 x g, 4 °C, 60 min) with supernatant collection.
Antibody purification
After centrifugation (10,000 x g, 4 °C, 30 min), the pH of the cell culture supernatant was controlled and if necessary adjusted (pH 6.0–7.0) for optimal binding to protein A. Before introduction in the FPLC system, the sample was filtered at 0.2 μm (Sarstedt, reference 92.3940.001, reference 83.1826.102, or reference 83.3940.001 according to the sample volume) with filtrate collection, or was centrifuged (16,000 x g, 4 °C, 30 min) with supernatant collection. The sample was introduced at 2.5 mL/min in a HiTrap MabSelect SuRe™ 5 mL column (Cytiva, reference 11,003,494), pre-equilibrated with 5 column volumes (CV) of 20 mM sodium phosphate 150 mM sodium chloride pH 6.9 solution in an ÄKTA pure™ 25 L (Cytiva) at 4 °C. After a 20 CV-wash at 5 mL/min, the antibody was eluted with a linear gradient of 50 mM sodium citrate pH 3.0 solution at 2.5 mL/min over 8 CV, and eluates were immediately neutralized with 15% of 1 M Tris pH 9.0 solution (13% final). In the case of isocratic elution, eluates were neutralized with 25% of 1 M Tris pH 9.0 solution (20% final). After neutralization, eluates were formulated in the conjugation buffer, i.e. phosphate-buffered saline (PBS) containing 137 mM sodium chloride, 2.7 mM potassium chloride, and 10 mM sodium phosphate at pH 7.4 (VWR International GmbH, reference E404-200TABS) at 4–6 mg/ml (Nanodrop quantification based on absorbance at 280 nm) using Amicon® Ultra-4 10 kDa (Millipore, reference UFC8010), followed by sample centrifugation (16,000 x g, 4 °C, 60 min) with supernatant collection. For quantification purposes, the elution fractions were directly discarded. All solutions were filtered (Sarstedt, reference 92.3940.001, reference 83.1826.102, or reference 83.3940.001 according to the solution volume) with filtrate collection.
Drug- and fluorophore-linkers
DBCO-C4-PEG3-VC-PABC-AE (MedChemTronica, reference HY-111012) and DBCO-C6-PEG8-PABC-AE (Iris Biotech GmbH) were used as drug-linker models. DBCO-Cyanine5 (BroadPharm, reference BP-23775) was used as a fluorophore-linker model.
Antibody conjugation
The final volume of the conjugation reaction was chosen according to the nature and the concentration of the drug-linker or fluorophore-linker while keeping the final antibody concentration higher than 0.5 mg/ml. The antibody (initially in PBS) was incubated (25 °C, 15 min) in 10%, 15%, or 18% of dimethyl sulfoxide (DMSO) (Sigma, reference D8418-100ML), and the drug-linker or fluorophore-linker (initially in 100% DMSO) was incubated (25 °C, 15 min) in 60%, 50%, or 40% of DMSO before conjugation. Conjugation occurred at 10–20% final DMSO (80–90% final PBS), with 2–4 molar equivalents of drug-linker or fluorophore-linker per site of conjugation, at 25 °C for 16 hours. Conjugates were purified and formulated again in PBS pH 7.4 at 0.5–1.0 mg/ml (Nanodrop quantification based on absorbance at 280 nm) using Amicon® Ultra-0.5 30 kDa (Millipore, reference UFC5030), followed by sample centrifugation (16,000 x g, 4 °C, 60 min) with supernatant collection or were introduced at 0.5 mL/min in a HiTrap™ rProtein A FastFlow 1 mL column (Cytiva, reference 17,507,902), pre-equilibrated with 5 CV of 20 mM sodium phosphate 150 mM sodium chloride pH 6.9 solution in an ÄKTA pure™ 25 L (Cytiva) at 4 °C. After a 10 CV-wash at 1 mL/min, the antibody was isocratically eluted with 3 CV of 50 mM sodium citrate pH 3.0 solution at 0.5 mL/min and immediately neutralized with 25% of 1 M Tris pH 9.0 solution (20% final). After neutralization, eluates were formulated in the conjugation buffer (PBS pH 7.4) at 0.5–1.0 mg/ml (quantification based on absorbance at 280 nm) using Amicon® Ultra-0.5 10 kDa (Millipore, reference UFC5010), followed by sample centrifugation (16,000 x g, 4 °C, 60 min) with supernatant collection. All samples and solutions were filtered (Sarstedt, reference 92.3940.001, reference 83.1826.102, or reference 83.3940.001 according to the sample or solution volume) with filtrate collection or centrifuged (16,000 x g, 4 °C, 60 min) with supernatant collection.
Hydrophobic interaction chromatography
Hydrophobic interaction chromatography was performed on a TSKgel Butyl-NPR, 2.5 μm particle size, 4.6 mm ID × 3.5 cm column (Tosoh, reference 0014947) using an Agilent 1260 Infinity HPLC-DAD system (Agilent Technologies Deutschland GmbH). Mobile phase A consisted of 25 mM sodium phosphate, 1.5 M ammonium sulfate, pH 6.9 and mobile phase B consisted of 80% (v/v) 25 mM sodium phosphate, pH 6.9, and 20% (v/v) isopropanol. The column was pre-equilibrated with 5 CV of mobile phase A and 2 or 5 μg of the sample (0.25 mg/ml in PBS) were subjected to a gradient of 0% to 100% mobile phase B over 20 min at a flow of 0.4 mL/min. Detection was performed at 214 nm, and peaks and area under the curve (AUC) were automatically detected and calculated by the OpenLab CDS 2.14.29 software. For the determination of the relative hydrophobicity, 125 ng of mAb1 wt or mAb2 wt were added to the sample (2 or 5 μg) as internal standard before injection.
In vitro cytotoxicity assays
Samples (1.5x10−12 M to 1x10−8 M) were introduced in wells (Corning, reference Costar 3917) containing 1000 adherent cancer cells (100 μL total volume), seeded 24 hours before, and usually maintained between 15.6x103 and 62.5x103 cells/cm2 for the high-antigen cancer cell line (undisclosed) and between 28.5x103 and 228.5x103 cells/cm2 for the low-antigen cancer cell line (undisclosed) in Dulbecco’s Modified Eagle’s Medium—High glucose with 25 mM HEPES (Sigma, reference D6171-500ML), 10% FBS Supreme (PAN, reference P30–3031), 4 mM Stable Glutamine 200 mM (100X) (PAN, reference P04–82100), 1 mM sodium pyruvate 100 mM (Biowest, reference L0642–100) at 37 °C, 5% CO2 and 100% humidity. After 120 hours, supernatants (100 μL) were discarded, and 50 μL of a solution composed of 20% (v/v) CellTiter-Glo® 2.0 Cell Viability Assay (Promega GmbH, reference G9243) and 80% (v/v) PBS were introduced in the well, and chilled at 25 °C for at least 20 min protected from light. Luminescence was measured with a FLUOstar Omega plate reader (BMG Labtech).
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Dorothea C. Hallier (Fraunhofer-Gesellschaft) for the support with the ÄKTA pure 25 L; Yanan Sun, Jakob Steff, and Felix Bredendiek (Freie Universität Berlin) for the support with the 1260 Infinity; Anika Andersson (Fraunhofer-Gesellschaft) for the support with the culture of adherent cancer cell lines; Anne Zemella (Fraunhofer-Gesellschaft) for the training on orthogonal translation systems; and Sophie Marinoff (Glycotope GmbH) for the factual inputs in respect of ADC design.
Contributor Information
Nathanaël Rakotoarinoro, Institute for Cell Therapy and Immunology branch Bioanalytics and Bioprocesses, Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V., 14476 Potsdam-Golm, Germany; Institute of Pharmacy, Freie Universität Berlin, 14195 Berlin, Germany.
Yan F K Dyck, Institute of Pharmacy, Freie Universität Berlin, 14195 Berlin, Germany.
Simon K Krebs, Institute for Cell Therapy and Immunology branch Bioanalytics and Bioprocesses, Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V., 14476 Potsdam-Golm, Germany; Institute of Biotechnology, Technische Universität Berlin, 13355 Berlin, Germany.
Miriam-Kousso Assi, Institute for Cell Therapy and Immunology branch Bioanalytics and Bioprocesses, Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V., 14476 Potsdam-Golm, Germany; Department of Biotechnology, Hamburg University of Applied Sciences, 21033 Hamburg, Germany.
Maria K Parr, Institute of Pharmacy, Freie Universität Berlin, 14195 Berlin, Germany.
Marlitt Stech, Institute for Cell Therapy and Immunology branch Bioanalytics and Bioprocesses, Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V., 14476 Potsdam-Golm, Germany.
AUTHORS‘ CONTRIBUTIONS
NR originated the concept, outlined the project, designed mAbs, synthetases, tRNAs, and plasmids, established the platforms for cell-based orthogonal translation systems, cell-based mAb synthesis, purification, and conjugation, prepared the draft manuscript and the figures. NR, YFKD, and MKA generated the data presented in the manuscript. NR and YFKD evaluated project feasibility. YFKD, NR, and MKP set up the methods for mAb/ADC biochemical characterization. MKA, NR, and MS set up the methods for mAb/ADC functional characterization. SKK established the platform for Gibson assembly and related cloning techniques. MKP and MS approved, supported, and supervised the project. YFKD, SKK, MKA, MKP, and MS revised and edited the manuscript. All authors agreed to the final version of the manuscript.
FUNDING
The authors acknowledge the Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V., the Freie Universität Berlin, the Bundesministerium für Bildung und Forschung (BMBF), the Ministerium für Wissenschaft, Forschung und Kultur (MWFK), and the Investitionsbank des Landes Brandenburg (ILB) via the Europäischer Fonds für regionale Entwicklung (EFRE) for enabling the work featured in this article.
CONFLICT OF INTEREST STATEMENT
NR, SKK, MKA, and MS are employees of the Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V.. YFKD and MKP have nothing to disclose.
ANIMAL RESEARCH STATEMENT
Not applicable.
ETHICS AND CONSENT STATEMENT
Not applicable.
DATA AVAILABILITY STATEMENT
All data are incorporated into the article and its online supplementary material.
References
- 1. Lyon, RP, Bovee, TD, Doronina, SOet al. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat Biotechnol 2015; 33: 733–5. [DOI] [PubMed] [Google Scholar]
- 2. Meyer, D, Bou, L, Shum, Set al. An in vitro assay using cultured Kupffer cells can predict the impact of drug conjugation on in vivo antibody pharmacokinetics. Mol Pharm 2020; 17: 802–9. [DOI] [PubMed] [Google Scholar]
- 3. Zhang, N, Deng, H, Fan, Xet al. Dysfunctional antibodies in the tumor microenvironment associate with impaired anticancer immunity. Clin Cancer Res 2015; 21: 5380–90. [DOI] [PubMed] [Google Scholar]
- 4. Roy, G, Reier, J, Garcia, Aet al. Development of a high yielding expression platform for the introduction of non-natural amino acids in protein sequences. MAbs 2020; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Axup, JY, Bajjuri, KM, Ritland, Met al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A 2012; 109: 16101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tian, F, Lu, Y, Manibusan, Aet al. A general approach to site-specific antibody drug conjugates. Proc Natl Acad Sci U S A 2014; 111: 1766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. VanBrunt, MP, Shanebeck, K, Caldwell, Zet al. Genetically encoded Azide containing amino acid in mammalian cells enables site-specific antibody-drug conjugates using click cycloaddition chemistry. Bioconjug Chem 2015; 26: 2249–60. [DOI] [PubMed] [Google Scholar]
- 8. Saphire, EO, Parren, PW, Pantophlet, Ret al. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 2001; 293: 1155–9. [DOI] [PubMed] [Google Scholar]
- 9. Harris, LJ, Skaletsky, E, McPherson, A. Crystallographic structure of an intact IgG1 monoclonal antibody. J Mol Biol 1998; 275: 861–72. [DOI] [PubMed] [Google Scholar]
- 10. Hao, Y, Yu, X, Bai, Yet al. Cryo-EM structure of HER2-trastuzumab-pertuzumab complex. PloS One 2019; 14: e0216095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donaldson, JM, Zer, C, Avery, KNet al. Identification and grafting of a unique peptide-binding site in the fab framework of monoclonal antibodies. Proc Natl Acad Sci U S A 2013; 110: 17456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King, JD, Ma, Y, Kuo, YCet al. Template-catalyzed, Disulfide conjugation of monoclonal antibodies using a natural amino acid tag. Bioconjug Chem 2018; 29: 2074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luthra, A, Langley, DB, Schofield, Pet al. Human antibody Bispecifics through phage display selection. Biochemistry 2019; 58: 1701–4. [DOI] [PubMed] [Google Scholar]
- 14. Cho, HS, Mason, K, Ramyar, KXet al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin fab. Nature 2003; 421: 756–60. [DOI] [PubMed] [Google Scholar]
- 15. Sickmier, EA, Kurzeja, RJ, Michelsen, Ket al. The Panitumumab EGFR complex reveals a binding mechanism that overcomes Cetuximab induced resistance. PloS One 2016; 11: e0163366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kern, JC, Cancilla, M, Dooney, Det al. Discovery of pyrophosphate Diesters as Tunable, soluble, and bioorthogonal linkers for site-specific antibody–drug conjugates. J Am Chem Soc 2016; 138: 1430–45. [DOI] [PubMed] [Google Scholar]
- 17. Kern, JC, Dooney, D, Zhang, Ret al. Novel phosphate modified Cathepsin B linkers: improving aqueous solubility and enhancing payload scope of ADCs. Bioconjug Chem 2016; 27: 2081–8. [DOI] [PubMed] [Google Scholar]
- 18. Brandish, PE, Palmieri, A, Antonenko, Set al. Development of anti-CD74 antibody–drug conjugates to target glucocorticoids to immune cells. Bioconjug Chem 2018; 29: 2357–69. [DOI] [PubMed] [Google Scholar]
- 19. Edelman, GM, Cunningham, BA, Gall, WEet al. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A 69: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feige, MJ, Groscurth, S, Marcinowski, Met al. An unfolded CH1 domain controls the assembly and secretion of IgG antibodies. Mol Cell 2009; 34: 569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kularatne, SA, Deshmukh, V, Ma, Jet al. A CXCR4-targeted site-specific antibody-drug conjugate. Angew Chem Int Ed Engl 2014; 53: 11863–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim, RK, Yu, S, Cheng, Bet al. Targeted delivery of LXR agonist using a site-specific antibody-drug conjugate. Bioconjug Chem 2015; 26: 2216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu, S, Pearson, AD, Lim, RKet al. Targeted delivery of an anti-inflammatory PDE4 inhibitor to immune cells via an antibody-drug conjugate. Mol Ther 2016; 24: 2078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oller-Salvia, B, Kym, G, Chin, JW. Rapid and efficient generation of stable antibody-drug conjugates via an encoded Cyclopropene and an inverse-electron-demand Diels-Alder reaction. Angew Chem Int Ed Engl 2018; 57: 2831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oller-Salvia, B. Genetic encoding of a non-canonical amino acid for the generation of antibody-drug conjugates through a fast bioorthogonal reaction. J Vis Exp 2018; 139: 58066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Junutula, JR, Raab, H, Clark, Set al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 2008; 26: 925–32. [DOI] [PubMed] [Google Scholar]
- 27. Shen, BQ, Xu, K, Liu, Let al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol 2012; 30: 184–9. [DOI] [PubMed] [Google Scholar]
- 28. St Amant, AH, Huang, F, Lin, Jet al. A diene-containing noncanonical amino acid enables dual functionality in proteins: rapid Diels-Alder reaction with Maleimide or proximity-based dimerization. Angew Chem Int Ed Engl 2019; 58: 8489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartoschek, MD, Ugur, E, Nguyen, TAet al. Identification of permissive amber suppression sites for efficient non-canonical amino acid incorporation in mammalian cells. Nucleic Acids Res 2021; 49: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verkade, JMM, Wijdeven, MA, Van Geel, Ret al. A polar Sulfamide spacer significantly enhances the manufacturability, stability, and therapeutic index of antibody-drug conjugates. Antibodies (Basel) 2018; 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruins, JJ, Damen, JAM, Wijdeven, MAet al. Non-genetic generation of antibody conjugates based on Chemoenzymatic tyrosine click chemistry. Bioconjug Chem 2021; 32: 2167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wijdeven, MA, vanGeel, R, Hoogenboom, JHet al. Enzymatic glycan Remodeling–metal free click (GlycoConnect) technology provides homogenous antibody-drug conjugates with improved stability and therapeutic index without antibody sequence engineering. MAbs 2022; 14: 207846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coumans, R, Ariaans, G, Spijker, HJet al. A platform for the generation of site-specific antibody–drug conjugates that allows for selective reduction of engineered Cysteines. Bioconjug Chem 2020; 31: 2136–46. [DOI] [PubMed] [Google Scholar]
- 34. Chin, JW, Cropp, TA, Anderson, JCet al. An expanded eukaryotic genetic code. Science 2003; 301: 964–7. [DOI] [PubMed] [Google Scholar]
- 35. Serfling, R, Lorenz, C, Etzel, Met al. Designer tRNAs for efficient incorporation of non-canonical amino acids by the pyrrolysine system in mammalian cells. Nucleic Acids Res 2018; 46: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stech, M, Rakotoarinoro, N, Teichmann, Tet al. Synthesis of fluorescently Labeled antibodies using non-canonical amino acids in eukaryotic cell-free systems. Methods Mol Biol 2021; 2305: 175–90. [DOI] [PubMed] [Google Scholar]
- 37. Krebs, SK, Rakotoarinoro, N, Stech, Met al. A CHO-based cell-free dual fluorescence reporter system for the straightforward assessment of amber suppression and scFv functionality. Front Bioeng Biotechnol 2022; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nikić, I, Estrada Girona, G, Kang, JHet al. Debugging eukaryotic genetic code expansion for site-specific click-PAINT super-resolution microscopy. Angew Chem Int Ed Engl 2016; 55: 16172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wulhfard, S, Baldi, L, Hacker, DLet al. Valproic acid enhances recombinant mRNA and protein levels in transiently transfected Chinese hamster ovary cells. J Biotechnol 2010; 148: 128–32. [DOI] [PubMed] [Google Scholar]
- 40. Wulhfard, S, Tissot, S, Bouchet, Set al. Mild hypothermia improves transient gene expression yields several fold in Chinese hamster ovary cells. Biotechnol Prog 2008; 24: 458–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.