Abstract
Background:
High weight gain in pregnancy is associated with greater postpartum weight retention, yet long-term implications remain unknown. We aimed to assess whether gestational weight change was associated with mortality over 50 years later.
Methods:
The Collaborative Perinatal Project (CPP) was a prospective U.S. pregnancy cohort (1959–1965). The CPP Mortality Linkage Study linked CPP participants to the National Death Index and Social Security Death Master File for vital status through 2016. Adjusted hazard ratios (HRs) with 95% confidence intervals (CI) estimated associations between gestational weight gain/loss according to the 2009 recommendations and mortality by pre-pregnancy body mass index (BMI). The primary endpoint was all-cause mortality. Secondary endpoints included cardiovascular and diabetes underlying causes of mortality.
Findings:
Among 46,042 participants, 45·3% (n=20839) self-identified as Black and 46·2% (n=21287) as White. Median follow-up time was 52 years (interquartile range 45–54) and 38·9% died. For those with pre-pregnancy underweight weight (n=3809; 9·4%), change above recommendations was associated with increased cardiovascular (HR=1·84 [1·08–3·12]) mortality and increased all-cause (HR=1·14 [95% CI 0·86–1·51]) mortality, but this estimate was less precise and statistically insignificant. For those with pre-pregnancy normal weight (n=27921; 68·6%), weight change below recommendations was associated with lower diabetes mortality (HR=0·62 [0·48–0·79]) and weight change above recommendations was associated with increased all-cause (HR=1·09 [1·01–1·18]) and cardiovascular (HR=1·20 [1·04–1·37]) mortality. For those with pre-pregnancy overweight (n=6251; 15·4%), weight change above recommendations was associated with elevated all-cause (HR=1·12 [1·01–1·24]) and diabetes mortality, as well as elevated cardiovascular (HR=1·12 [0·94–1·33]) mortality, but this estimate was less precise and statistically insignificant. For those with pre-pregnancy obesity (n=2708; 6·7%), all associations between gestational weight change and mortality were highly imprecise with no meaningful associations.
Interpretation:
This study’s novel findings support the importance of achieving healthy gestational weight gain within recommendations, adding that the implications may extend beyond the pregnancy window to long-term health, including cardiovascular and diabetes mortality.
Funding:
National Institutes of Health.
INTRODUCTION
Routine weight measurement during pregnancy is widely adopted in US prenatal care.1 In 2009, the National Academy of Medicine (NAM) published gestational weight gain recommendations, balancing available evidence to optimize the health of birthing people and their offspring.2 An important outcome considered in these recommendations was postpartum weight retention. A systematic review of nine studies found that exceeding recommendations was associated with postpartum weight retention of 3·06 kg (95% CI 1·50–4·63) at three years and 4·72 kg (2·94–6·50) at 15 years postpartum. Higher gestational weight gain is also associated with higher fat mass and greater waist circumference postpartum.3 Moreover, visceral adipose tissue is increased postpartum, even without retained weight.3 Given the strong associations between mid-life excess weight, particularly excess visceral adipose tissue, and the development of cardiovascular disease and diabetes, there is plausible evidence that high gestational weight gain causes cardiometabolic diseases and associated mortality risk.4,5 Yet, some studies have reported positive associations between excessive or higher gestational weight gain with diabetes and cardiovascular health, and others have no association with diabetes.6–8
Under this hypothesis that high gestational weight gain may increase obesity-related cardiometabolic diseases and subsequent mortality, the corollary is that low gestational weight gain may lower these risks, yet we lack critical relevant data. While gestational weight gain below recommendations has been consistently associated with lower postpartum weight retention, 9 one study has found that in individuals with pre-pregnancy underweight or normal weight, weight loss in pregnancy was associated with an increased cardiovascular disease risk 16 years post-pregnancy.7
No studies to date have investigated associations between gestational weight change and mortality risk. Chronic disease development occurs over many years, and diagnosis typically does not occur until later in life, highlighting the important need for long-term follow-up. The longest follow-up on chronic diseases related to gestational weight gain is 21 years.8 This study examined associations between gestational weight change and all-cause and cardiovascular and diabetes mortality in a racially diverse cohort up to 50 years post-pregnancy. Given strong associations between pre-pregnancy body mass index (BMI) and gestational weight change and current pre-pregnancy BMI-specific recommendations, all analyses were stratified by pre-pregnancy BMI.2,10
MATERIALS & METHODS
Study Population
The Collaborative Perinatal Project (CPP) is an observational cohort study of 48,197 pregnant people (58,760 pregnancies) enrolled at their first prenatal visit at 12 U.S. clinical centers between 1959–1966.11 Participants’ vital status was ascertained as of 2016 for the CPP Mortality Linkage Study.12 For this analysis, the index pregnancy was the last CPP pregnancy for the participants with multiple study pregnancies (n=8,772; 18·2%). Exclusions were made for participants with missing identifiable linkage information (n=1637), pregnancy-related deaths (n=9), and non-singleton births (n=524), resulting in a final sample of 46,042 (Figure S1).
There were no institutional review boards or rules for using human subjects at the inception of CPP during the late 1950s, but general informed consent for participation was obtained. Institutional review board approval was obtained for the CPP Mortality Linkage Study by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Emmes Corporation, which performed the abstraction of identifying information from historic CPP records and facilitated the linkages.
Exposure Assessment
Participants self-reported their pre-pregnancy weight and height at their first prenatal visit. Participants’ weight at each prenatal visit and delivery admission was recorded. Total gestational weight change (gain/loss) was calculated as measured weight at delivery minus pre-pregnancy weight. Weight data were cleaned in two ways to identify potentially implausible observations. First, total weight change <0·1 (−10·4 kg; n=41) and >99·9 (29·0 kg; n=40) percentiles were considered implausible. Second, implausible total weight change was assessed based on the entire weight trajectory extracted from prenatal records using conditional percentiles;13 implausible values were defined as those inconsistent (>±3 standard deviation; SD) with expected weight based on participants’ longitudinal prenatal trajectory (n=536; 1·2%). Implausible total weight change was set to missing and imputed.
Pre-pregnancy BMI (kg/m2) was classified as underweight (<18·5), normal weight (18·5–24·9), overweight (25·0–29·9), or obesity (≥30·0). There were no universal gestational weight gain guidelines in the 1950’s and 1960’s; therefore, for applicability to current public health guidelines, total weight change was classified as below, within, or above the 2009 NAM pre-pregnancy BMI specific recommendations.2: We also used quintiles defined within each BMI category as a data-driven approach. Figure S2 provides a comparison of the recommendations and quintiles. A total weight change for gestational age z-score was also calculated. Internal week-specific mean and SD references were estimated using serial weight gain from 6 to 42 weeks’ gestation among participants without preexisting diabetes or hypertension, gestational diabetes, impaired glucose tolerance in pregnancy, gestational hypertension, or preeclampsia/eclampsia, who delivered between 37 weeks 0 days and 41 weeks 6 days and had more than three repeated weight gain measurements (414,726 weight measurements; n=24,845).14 Using estimated reference means and SDs, total weight change for gestational age at delivery z-scores were estimated for the entire cohort and classified into quintiles for analysis.
Outcome Assessment
As of December 31, 2016, participants’ vital status was ascertained using linkages with the National Death Index (NDI)15 and Social Security Death Master File (SSDMF). The NDI contains records of all U.S. deaths from 1979 onward and identifies matches based on probabilistic linkage. The SSDMF covers the entire follow-up period from 1958 onward. All-cause mortality was defined by a recorded death in the NDI augmented by the SSDMF if there was no match with the NDI; 89% of deaths were sourced from the NDI, which is considered more accurate than the SSDMF.12,16 Follow-up time was estimated as the difference between index pregnancy date of delivery and date of death or December 31, 2016.
In the NDI, underlying cause of death was defined by the World Health Organization & International Conference for the Ninth Revision of the International Classification of Diseases recommendations as “(a) disease or injury which initiated the train of events leading directly to death, or (b) circumstances of the accident or violence which produced the fatal injury”.15,17 These coding decisions are automated based on international coding rules. Underlying causes of death were grouped according to ICD-10 codes and comparable ICD-9 codes (Table S1).18
The primary endpoint was all-cause mortality. Secondary endpoints included cardiovascular and diabetes underlying causes of mortality based on our hypotheses that excess gestational weight gain may lead to weight retention or differential adipose stores leading to chronic diseases. Other major causes of death outside of the main hypotheses are included in the supplement.
Statistical Analysis
Mean (SD) total gestational weight change was reported across characteristics by pre-pregnancy BMI. Cox proportional hazards regression estimated all-cause and cause-specific mortality hazard ratios (HRs) with each exposure. Cause-specific hazards accounted for competing risks of deaths due to different causes. The primary exposure was total gestational weight change according to the 2009 NAM recommendations. Analyses adjusted for index pregnancy covariates, including age, race/ethnicity, pre-pregnancy BMI, parity, marital status, income, education, smoking, gestational age at delivery, pre-pregnancy conditions, including diabetes, cardiovascular, respiratory, renal, neurological, and cancer/tumors, site, and pregnancy year (Table S2). To avoid over-adjustment bias, we did not adjust for factors on the causal pathway, including birthweight, gestational diabetes, hypertensive disorders of pregnancy, or mode of delivery.19 We do not have information on postpartum weight retention; however, it also is on the causal pathway and should not be included in the models. Models were adjusted for gestational length to address the inherent correlation between gestational length and gestational weight change.20,21 Secondary analyses classified gestational weight change as quintiles, with quintile 3 as the reference. Sensitivity analyses utilized total weight change for gestational age z-scores in quintiles as an alternative approach to account for the correlation between gestational weight gain and gestational length.21
Misclassification in death status may arise from linkages with the NDI and SSDMF. We previously assessed sensitivity and specificity specific to our data to address this concern.20 By comparing the vital status classification to vital status determined by an expert genealogist in a random sample of 1250 participants, we estimated the sensitivity to be 0.87 and the specificity to be 0.84. These estimates help account for potential misclassification in death status and provide a more accurate understanding of the data’s reliability. Using these estimates, we conducted a quantitative bias analysis to examine the influence of outcome misclassification on our findings.22 Additionally, while delivery weight was obtained from the medical records, reducing the possibility for errors, there may be misclassification in total weight gain due to underreporting of pre-pregnancy weight. Prior studies have found that the correlation between self-reported pre-pregnancy weight and measured weight was high (r=0.90–0.99), with errors ranging from 0.34 to 2.94 kg.23 We performed a quantitative bias analysis to assess the influence of exposure misclassification on our findings. We employed sensitivity and specificity estimates of 0.9 while also considering additional variation at a level of 0.8. This analysis allowed us to thoroughly examine the potential effects of misclassification on our findings.
Missing data on exposure, covariates, and cause of death were multiply imputed using chained equations under the missing at random assumption (see Table S3 for missing data details); the model included the exposure, outcome, and covariates. Regression models were estimated for each of the 10 imputed data sets, and results combined using Rubin’s rule.24 Statistical significance was defined as P<0·05. All tests were two-sided and used SAS version 9·4 (SAS Institute, Cary, NC) or R (Vienna, Austria).
Role of the Funding Source
The study sponsors played no role in the study design, collection, analysis, data interpretation, writing of the report, or decision to submit the paper for publication. The corresponding author had full access to the study data and had final responsibility for the decision to submit the paper for publication.
RESULTS
Most participants (68·6%; n=27921) had a normal weight pre-pregnancy BMI, and 45·3% (n=20839) self-identified as Black and 46·2% (n=21287) as White (Table S4). Mean [SD] weight gain was highest among participants with underweight (10·4 [4·0] kg) and lowest among participants with obesity (6·5 [7·2] kg) (Table 1). Over a median (interquartile range) follow-up of 52 years (45–54), 38·9% (n=17901/46042) of participants died. The mortality rate (95% confidence interval [CI]) per 1000 person-years increased with increasing pre-pregnancy BMI (<18·5: 6·6 [6·3–7·0]; 18·5–24·9: 7·3 [7·1–7·4]; 25·0–29·9: 10·5 [10·2–10·9]; ≥30·0: 14·4 [13·7–15·0]). Cardiovascular disease accounted for 30·0% (n=5369/17901) of deaths; ischemic heart disease (13·2%; n=2368/17901), cerebrovascular (5·5%; n=984/17901), other cardiovascular diseases (4·8%; n=852/17901), hypertensive diseases (2·9%; n=521/17901), arrhythmia (1·9%; n=340/17901), and heart failure (1·7%; n=304/17901). Diabetes accounted for 4·9% (n=882/17901) of deaths.
TABLE 1.
Demographic and pregnancy characteristics of study participants and gestational weight change across characteristics by pre-pregnancy BMI status, n=46042.
| Index Pregnancy Characteristicsa | Pre-pregnancy BMI Status, kg/m2 | |||
|---|---|---|---|---|
| < 18·5 | 18·5 – 24·9 | 25·0 – 29·9 | ≥ 30·0 | |
| Gestational Weight Change, Mean (SD) | ||||
| Overall | 10·4 (4·0) | 9·7 (4·5) | 8·7 (5·8) | 6·5 (7·2) |
| Age, years | ||||
| < 20 | 10·5 (4·1) | 10·0 (4·8) | 9·4 (6·3) | 8·0 (8·4) |
| 20–24 | 10·4 (4·0) | 9·8 (4·4) | 8·9 (5·8) | 6·9 (7·1) |
| 25–29 | 10·3 (4·1) | 9·6 (4·4) | 8·7 (5·6) | 6·3 (7·1) |
| 30–34 | 10·3 (3·7) | 9·4 (4·6) | 8·7 (5·5) | 6·4 (7·2) |
| ≥ 35 | 9·7 (4) | 9·2 (4·5) | 7·9 (5·8) | 5·6 (7·1) |
| Race/ethnicity | ||||
| White | 10·6 (3·9) | 9·8 (4·3) | 8·8 (5·5) | 5·7 (7·1) |
| Black | 10·1 (4·0) | 9·7 (4·7) | 8·7 (6·0) | 7·0 (7·3) |
| Puerto Rican | 10·8 (4·4) | 9·1 (4·8) | 8·2 (5·5) | 5·8 (7·1) |
| Other | 10·3 (4·1) | 9·9 (4·6) | 9·4 (6·3) | 6·2 (7·5) |
| Parity | ||||
| 0 | 10·6 (4·0) | 9·9 (4·6) | 9·3 (6·1) | 6·5 (7·7) |
| 1 | 10·1 (3·9) | 9·6 (4·4) | 8·8 (5·9) | 6·5 (7·1) |
| 2 | 10·3 (4·0) | 9·6 (4·4) | 8·7 (5·6) | 6·3 (7·4) |
| 3 | 10·2 (4·1) | 9·6 (4·4) | 8·5 (5·8) | 6·0 (6·6) |
| ≥ 4 | 10·3 (4·1) | 9·8 (4·7) | 8·5 (5·7) | 6·6 (7·3) |
| Marital Status | ||||
| Single | 10·0 (4·2) | 9·7 (4·8) | 9·2 (6·2) | 7·9 (7·6) |
| Married/common law | 10·4 (4·0) | 9·7 (4·4) | 8·6 (5·7) | 6·3 (7·1) |
| Other | 11·5 (4·1) | 10·1 (5·1) | 9·0 (6·1) | 6·4 (7·5) |
| Smokingb, cigarettes per day | ||||
| Non-smoking | 10·6 (4·1) | 9·8 (4·5) | 8·9 (5·8) | 6·7 (7·0) |
| 1–4 | 10·4 (4·1) | 10 (4·6) | 9·0 (6·0) | 7·2 (7·7) |
| 5–9 | 10·4 (4·1) | 9·9 (4·6) | 9·0 (6·0) | 6·4 (7·7) |
| 10–19 | 10·1 (4·0) | 9·5 (4·4) | 8·5 (5·4) | 5·8 (7·1) |
| ≥20 | 10·2 (3·8) | 9·4 (4·5) | 8·0 (5·5) | 5·1 (7·7) |
| Income, annual USD | ||||
| ≤1999 | 10·6 (4·2) | 9·8 (4·8) | 8·9 (6·0) | 7·0 (7·6) |
| 2000–3999 | 10·3 (4·1) | 9·7 (4·6) | 8·8 (5·9) | 6·6 (7·2) |
| 4000–5999 | 10·6 (3·9) | 9·8 (4·3) | 8·4 (5·5) | 6·1 (7·1) |
| 6000–7999 | 10·2 (3·6) | 9·6 (4·3) | 9·1 (5·7) | 6·0 (7·2) |
| 8000–9999 | 9·9 (3·8) | 9·5 (4·1) | 7·9 (5·2) | 4·9 (6·2) |
| ≥10000 | 10·0 (3·8) | 9·8 (4·3) | 8·0 (5·5) | 5·6 (7·0) |
| Education | ||||
| <High School | 10·5 (4·3) | 9·7 (4·9) | 8·0 (5·7) | 6·3 (7·2) |
| Some high school | 10·7 (4·0) | 9·9 (4·6) | 9·1 (6) | 6·7 (7·4) |
| High School Graduate | 10·2 (3·9) | 9·8 (4·4) | 8·8 (5·7) | 6·3 (7·1) |
| Some College | 9·9 (3·5) | 9·3 (4·0) | 8·8 (5·3) | 6·2 (6·7) |
| Prior Pregnancy Loss | ||||
| No | 10·3 (3·9) | 9·6 (4·4) | 8·6 (5·7) | 6·3 (7·1) |
| Yes | 10·5 (4·1) | 9·8 (4·6) | 8·8 (5·9) | 6·6 (7·4) |
| Preexisting diabetesc | ||||
| No | 10·4 (4·0) | 9·7 (4·5) | 8·7 (5·8) | 6·4 (7·2) |
| Yes | 9·3 (3·0) | 8·2 (4·5) | 7·8 (5·2) | 7·3 (7·6) |
| Preexisting cardiovascular conditionsd | ||||
| No | 10·4 (4·0) | 9·7 (4·5) | 8·7 (5·7) | 6·5 (7·2) |
| Yes | 10·1 (4·0) | 9·7 (4·7) | 8·7 (6·2) | 6·3 (7·3) |
| Preexisting respiratory conditionse | ||||
| No | 10·4 (4·0) | 9·7 (4·5) | 8·7 (5·8) | 6·4 (7·2) |
| Yes | 10·0 (4·1) | 9·4 (4·7) | 8·5 (5·4) | 6·7 (7·2) |
| Preexisting renal conditions | ||||
| No | 10·4 (4·0) | 9·7 (4·5) | 8·7 (5·8) | 6·5 (7·2) |
| Yesf | 10·4 (4·1) | 9·7 (4·6) | 8·7 (5·8) | 5·9 (7·3) |
| Preexisting neurological conditionsg | ||||
| No | 10·3 (4·0) | 9·7 (4·5) | 8·8 (5·8) | 6·6 (7·2) |
| Yes | 10·7 (4·3) | 9·8 (4·7) | 8·3 (5·7) | 5·6 (7·3) |
| Preexisting cancer/tumors | ||||
| No | 10·4 (4·0) | 9·7 (4·5) | 8·7 (5·8) | 6·5 (7·3) |
| Yesh | 10·6 (4·0) | 9·5 (4·4) | 8·3 (5·6) | 5·6 (7·0) |
Abbreviations: BMI, body mass index; USD, United States dollars.
Missing data: total weight change n=6284; age, n=2; pre-pregnancy BMI, n=5353; parity n=1645; marital status, n=5; smoking, n=1612; income, n=4773; education, n=2415; prior pregnancy loss, n=1380; Preexisting diabetes, n=661; Preexisting cardiovascular conditions, n=717; Preexisting respiratory conditions, n=717; Preexisting renal conditions, n=717; Preexisting neurological conditions, n=717; Preexisting cancer/tumors, n=717.
Categories determined according to quartiles among smokers.
Type unknown.
Cardiovascular conditions included hypertension, rheumatic fever, and any other cardiovascular diseases.
Respiratory conditions included tuberculosis, asthma, other chronic pulmonary diseases, and other conditions requiring thoracic surgery.
Renal conditions included pyelitis, glomerulonephritis and other conditions requiring kidney, urinary, or bladder surgery.
Neurological conditions included neuromuscular diseases, convulsive disorders, psychosis, alcohol or drug addiction, or other neurological diseases.
Cancer and tumors included any history of cancer, and gastrointestinal, kidney, urinary, bladder, or gynecological tumors.
Pre-pregnancy BMI <18·5 kg/m2
Mortality was lowest among those with weight change within recommendations (1·2 per 1000 person-years [95% CI 1·0–1·5]) and highest among those with weight change both below (1·8 [1·6–2·0]) and above (1·9 [1·2–2·9]) recommendations (Figure 1; Table S5). Weight change below recommendations was associated with increased all-cause (HR=1·11 [95% CI 0·97–1·26]) and cardiovascular (HR=1·18 [0·89–1·58]) mortality and decreased diabetes (HR=0·68 [0·25–1·89]) mortality, but the estimates were imprecise and not statistically significant (Figure 1; Table 2; Table S5). Weight change above recommendations was associated with increased cardiovascular (HR=1·84 [1·08–3·12]) mortality and all-cause (HR=1·14 [0·86–1·51]) mortality, but the estimate was less precise and not statistically significant. Compared to quintile 3, quintiles 1 (HR=1·14 [0·95– 1·37]) and 2 (HR=1·13 [0·96–1·34]) were associated with elevated all-cause mortality, but with imprecise and not statistically significant estimates (Figure 2; Table S6). Quintile estimates for cardiovascular and diabetes mortality were highly imprecise and not statistically significant (Table 3).
Figure 1. Total gestational weight change classified according to the 2009 National Academy of Medicine recommendations and the association with all-cause mortality (n=46,042).
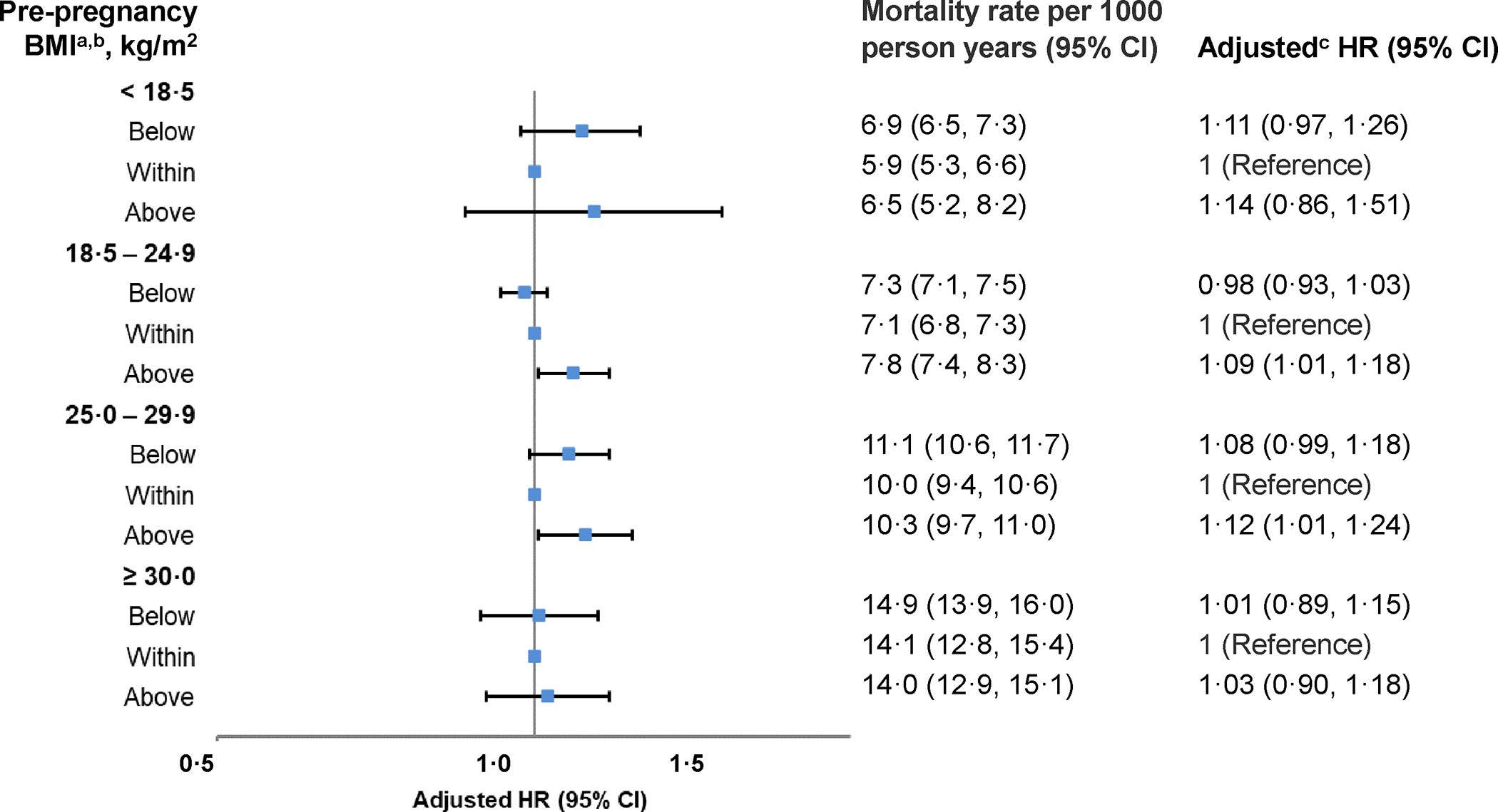
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; National Academy of Medicine, NAM.
a Recommendations for total gestational weight gain (2009): Pre-pregnancy BMI <18·5 kg/m2 (12·5–18·0 kg); 18·5–24·9 kg/m2 (11·5–16·0 kg); 25·0–29·9 kg/m2 (7·0–11·5 kg); ≥ 30·0 kg/m2 (5·0–9·0 kg). A total of 3809 (9·4%) participants had a pre-pregnancy BMI that was <18·5 kg/m2 and among this group, 68·2%, 26·6%, and 5·3% had a gestational weight change that was considered below, within, or above recommendations, respectively.
c Analyses adjusted for the following index pregnancy variables: age, smoking, race/ethnicity, parity, marital status, income, education, preexisting diabetes, preexisting cardiovascular conditions, preexisting cancer/tumors, preexisting neurological conditions, preexisting renal conditions, preexisting respiratory conditions, prior pregnancy loss, site, study year, pre-pregnancy body mass index, gestational week at delivery.
Table 2.
Total gestational weight change classified according to the 2009 National Academy of Medicine recommendations and the association with cardiovascular and diabetes mortality (n=46,042).
| Weight Change by 2009 NAM Recommendation | |||
|---|---|---|---|
| Below | Within | Above | |
| Pre-pregnancy BMI Status | Cardiovascular Disease Mortality | ||
| < 18·5 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 1·8 (1·6, 2·0) | 1·2 (1·0, 1·5) | 1·9 (1·2, 2·9) |
| Unadjusted HR (95% CI) | 1·34 (1·00, 1·79) | 1 (Reference) | 1·73 (1·01, 2·97) |
| Adjusted HR (95% CI)c | 1·18 (0·89, 1·58) | 1 (Reference) | 1·84 (1·08, 3·12) |
| 18·5 – 24·9 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 2·0 (1·9, 2·1) | 2·1 (1·9, 2·2) | 2·5 (2·2, 2·8) |
| Unadjusted HR (95% CI) | 1·01 (0·92, 1·11) | 1 (Reference) | 1·22 (1·06, 1·41) |
| Adjusted HR (95% CI)c | 0·93 (0·84, 1·02) | 1 (Reference) | 1·20 (1·04, 1·37) |
| 25·0 – 29·9 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 3·8 (3·5, 4·2) | 3·4 (3·1, 3·7) | 3·4 (3·1, 3·8) |
| Unadjusted HR (95% CI) | 1·16 (1·01, 1·34) | 1 (Reference) | 1·03 (0·88, 1·22) |
| Adjusted HR (95% CI)c | 1·08 (0·94, 1·26) | 1 (Reference) | 1·12 (0·94, 1·33) |
| ≥ 30·0 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 5·9 (5·2, 6·5) | 5·2 (4·4, 6·0) | 5·2 (4·6, 6·0) |
| Unadjusted HR (95% CI) | 1·23 (0·99, 1·52) | 1 (Reference) | 1·05 (0·85, 1·30) |
| Adjusted HR (95% CI)c | 1·12 (0·90, 1·40) | 1 (Reference) | 1·08 (0·86, 1·34) |
| Pre-pregnancy BMI Status | Diabetes Mortality | ||
| < 18·5 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 0·2 (0·1, 0·2) | 0·3 (0·2, 0·4) | 0·2 (0·0, 0·7) |
| Unadjusted HR (95% CI) | 0·87 (0·36, 2·08) | 1 (Reference) | 0·73 (0·10, 5·12) |
| Adjusted HR (95% CI)c | 0·68 (0·25, 1·89) | 1 (Reference) | 0·90 (0·13, 6·35) |
| 18·5 – 24·9 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 0·3 (0·2, 0·3) | 0·3 (0·3, 0·4) | 0·4 (0·3, 0·5) |
| Unadjusted HR (95% CI) | 0·74 (0·58, 0·93) | 1 (Reference) | 0·99 (0·63, 1·56) |
| Adjusted HR (95% CI)c | 0·62 (0·48, 0·79) | 1 (Reference) | 0·95 (0·61, 1·47) |
| 25·0 – 29·9 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 0·6 (0·5, 0·7) | 0·5 (0·4, 0·6) | 0·9 (0·7, 1·1) |
| Unadjusted HR (95% CI) | 1·07 (0·72, 1·59) | 1 (Reference) | 1·69 (1·19, 2·40) |
| Adjusted HR (95% CI)c | 0·97 (0·64, 1·48) | 1 (Reference) | 1·77 (1·23, 2·54) |
| ≥ 30·0 kg/m2 | |||
| Mortality rate per 1000 person years (95% CI)b | 1·4 (1·1, 1·7) | 1·5 (1·1, 2·0) | 1·4 (1·1, 1·8) |
| Unadjusted HR (95% CI) | 0·86 (0·59, 1·25) | 1 (Reference) | 0·86 (0·58, 1·27) |
| Adjusted HR (95% CI)c | 0·76 (0·51, 1·13) | 1 (Reference) | 0·83 (0·55, 1·27) |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; National Academy of Medicine, NAM.
Recommendations for total gestational weight gain (2009): Pre-pregnancy BMI <18·5 kg/m2 (12·5–18·0 kg); 18·5–24·9 kg/m2 (11·5–16·0 kg); 25·0–29·9 kg/m2 (7·0–11·5 kg); ≥ 30·0 kg/m2 (5·0–9·0 kg).
Mortality rate estimated obtained from a single imputed dataset.
Analyses adjusted for the following index pregnancy variables: age, smoking, race/ethnicity, parity, marital status, income, education, preexisting diabetes, preexisting cardiovascular conditions, preexisting cancer/tumors, preexisting neurological conditions, preexisting renal conditions, preexisting respiratory conditions, prior pregnancy loss, site, study year, pre-pregnancy body mass index, gestational week at delivery.
Figure 2. Total gestational weight change in pre-pregnancy body mass index specific quintiles and the adjusted association with all-cause mortality stratified by pre-pregnancy body mass index (n=46,042).
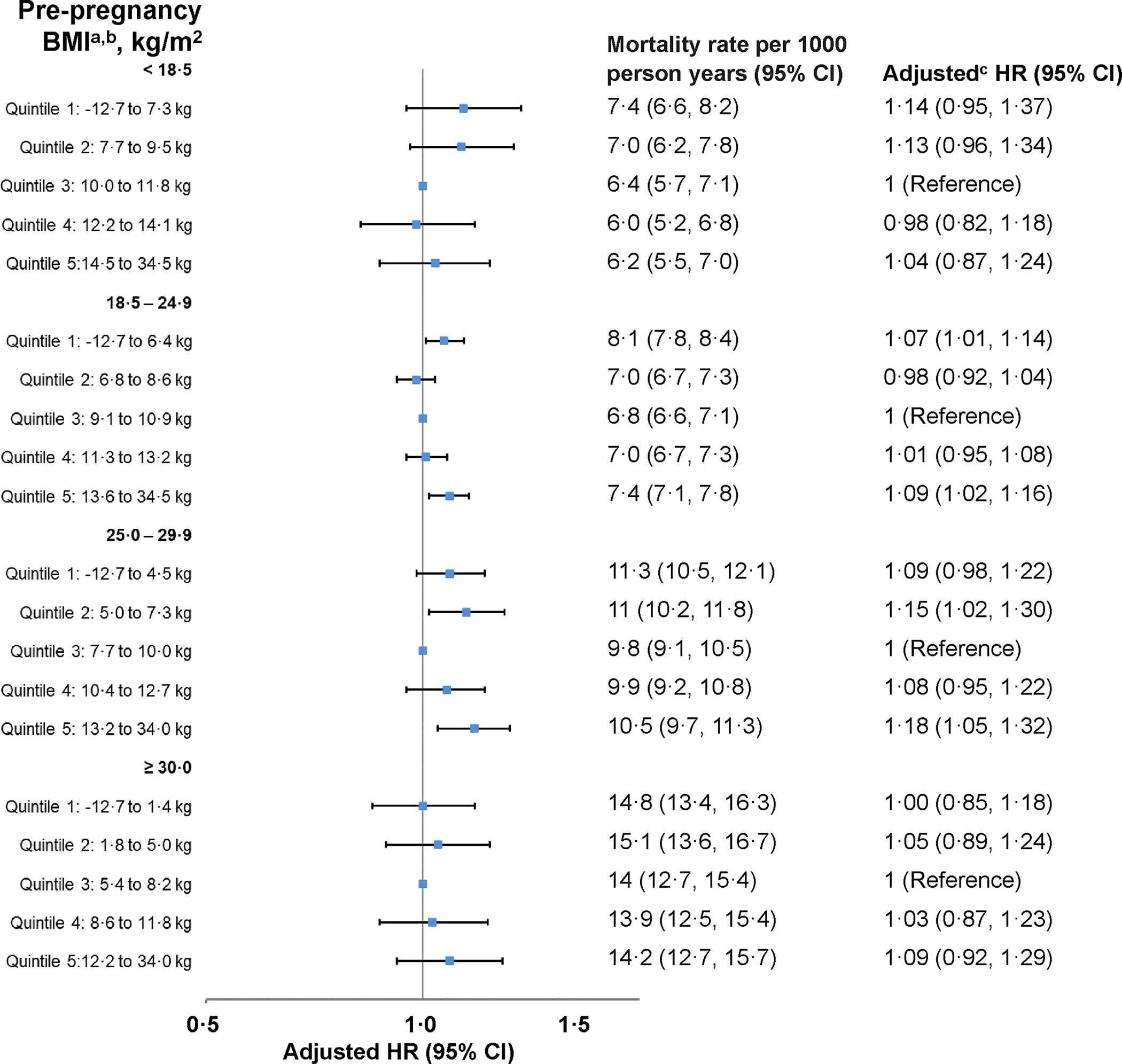
Abbreviations: BMI, body mass index; CI, confidence interval; IQR, interquartile range; HR, hazard ratio.
a Quintiles of gestational weight change were defined within each pre-pregnancy BMI category. Overall, the minimum weight change was −12·7 kg; however, the 5th percentile was −4·5 kg. The maximum weight change was 34·0 kg; however, the 95th percentile was 18·1 kg.
b Mortality rate estimated obtained from a single imputed dataset.
c Analyses adjusted for the following index pregnancy variables: age, smoking, race/ethnicity, parity, marital status, income, education, preexisting diabetes, preexisting cardiovascular conditions, preexisting cancer/tumors, preexisting neurological conditions, preexisting renal conditions, preexisting respiratory conditions, prior pregnancy loss, site, study year, pre-pregnancy body mass index, gestational week at delivery.
Table 3.
Total gestational weight change in pre-pregnancy body mass index specific quintiles and the association with cardiovascular and diabetes cause specific mortality, stratified by pre-pregnancy body mass index (n=46,042).
| Total Gestational Weight Changea | |||||
|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Pre-pregnancy BMI Status (kg/m 2 ) | Cardiovascular Disease Mortality | ||||
| < 18·5 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 1·8 (1·4, 2·2) | 1·7 (1·4, 2·2) | 1·7 (1·4, 2·1) | 1·5 (1·1, 1·9) | 1·4 (1·0, 1·8) |
| Unadjusted HR (95% CI) | 1·09 (0·78, 1·53) | 1·09 (0·78, 1·52) | 1 (Reference) | 0·89 (0·61, 1·30) | 0·97 (0·65, 1·43) |
| Adjusted HR (95% CI)c | 1·01 (0·70, 1·44) | 1·08 (0·78, 1·51) | 1 (Reference) | 0·93 (0·63, 1·37) | 1·09 (0·74, 1·61) |
| 18·5 – 24·9 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 2·4 (2·2, 2·5) | 1·9 (1·7, 2·0) | 1·9 (1·7, 2) | 2·1 (1·9, 2·3) | 2·2 (2·0, 2·4) |
| Unadjusted HR (95% CI) | 1·24 (1·11, 1·39) | 0·96 (0·85, 1·09) | 1 (Reference) | 1·07 (0·94, 1·22) | 1·14 (1·01, 1·30) |
| Adjusted HR (95% CI)c | 1·07 (0·95, 1·20) | 0·91 (0·81, 1·03) | 1 (Reference) | 1·08 (0·95, 1·22) | 1·15 (1·01, 1·30) |
| 25·0 – 29·9 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 4·0 (3·6, 4·5) | 3·6 (3·2, 4·1) | 3·3 (2·9, 3·7) | 3·2 (2·8, 3·7) | 3·7 (3·2, 4·1) |
| Unadjusted HR (95% CI) | 1·19 (0·99, 1·43) | 1·12 (0·91, 1·38) | 1 (Reference) | 0·89 (0·71, 1·13) | 1·12 (0·92, 1·36) |
| Adjusted HR (95% CI)c | 1·10 (0·91, 1·33) | 1·13 (0·91, 1·39) | 1 (Reference) | 0·97 (0·77, 1·21) | 1·24 (1·01, 1·52) |
| ≥ 30·0 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 5·8 (4·9, 6·7) | 5·9 (5·1, 7) | 4·9 (4·2, 5·8) | 5·6 (4·8, 6·6) | 5·1 (4·2, 6·0) |
| Unadjusted HR (95% CI) | 1·21 (0·95, 1·55) | 1·27 (1·00, 1·63) | 1 (Reference) | 1·08 (0·83, 1·40) | 1·05 (0·82, 1·36) |
| Adjusted HR (95% CI)c | 1·10 (0·85, 1·43) | 1·18 (0·91, 1·52) | 1 (Reference) | 1·11 (0·85, 1·45) | 1·08 (0·83, 1·41) |
| Pre-pregnancy BMI Status | Diabetes Mortality | ||||
| < 18·5 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 0·1 (0·1, 0·3) | 0·2 (0·1, 0·4) | 0·1 (0·1, 0·3) | 0·2 (0·1, 0·4) | 0·3 (0·2, 0·5) |
| Unadjusted HR (95% CI) | 1·45 (0·44, 4·75) | 1·89 (0·66, 5·44) | 1 (Reference) | 0·89 (0·20, 3·99) | 1·83 (0·64, 5·19) |
| Adjusted HR (95% CI)c | 1·23 (0·33, 4·57) | 2·36 (0·70, 7·96) | 1 (Reference) | 1·19 (0·24, 5·96) | 2·54 (0·76, 8·43) |
| 18·5 – 24·9 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 0·3 (0·2, 0·4) | 0·2 (0·2, 0·3) | 0·3 (0·2, 0·3) | 0·3 (0·3, 0·4) | 0·3 (0·3, 0·4) |
| Unadjusted HR (95% CI) | 1·27 (0·86, 1·87) | 1·02 (0·68, 1·52) | 1 (Reference) | 1·40 (0·97, 2·02) | 1·56 (1·10, 2·23) |
| Adjusted HR (95% CI)c | 0·95 (0·62, 1·44) | 0·91 (0·62, 1·34) | 1 (Reference) | 1·46 (0·98, 2·16) | 1·63 (1·13, 2·35) |
| 25·0 – 29·9 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 0·4 (0·3, 0·6) | 0·8 (0·6, 1·1) | 0·4 (0·3, 0·6) | 0·6 (0·4, 0·8) | 1·0 (0·7, 1·2) |
| Unadjusted HR (95% CI) | 1·01 (0·61, 1·67) | 1·65 (0·98, 2·77) | 1 (Reference) | 1·33 (0·81, 2·18) | 2·09 (1·33, 3·28) |
| Adjusted HR (95% CI)c | 0·87 (0·52, 1·47) | 1·68 (1·00, 2·81) | 1 (Reference) | 1·44 (0·87, 2·38) | 2·24 (1·41, 3·55) |
| ≥ 30·0 kg/m 2 | |||||
| Mortality rate per 1000 person years (95% CI)b | 1·5 (1·1, 2·1) | 1·3 (0·9, 1·8) | 1·4 (1·0, 1·9) | 1·3 (0·9, 1·8) | 1·7 (1·3, 2·3) |
| Unadjusted HR (95% CI) | 1·02 (0·65, 1·60) | 0·86 (0·53, 1·41) | 1 (Reference) | 0·93 (0·56, 1·56) | 1·11 (0·70, 1·75) |
| Adjusted HR (95% CI)c | 0·90 (0·57, 1·44) | 0·78 (0·47, 1·30) | 1 (Reference) | 0·93 (0·55, 1·58) | 1·10 (0·68, 1·79) |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Quintiles of gestational weight change were defined within each pre-pregnancy BMI category.
Mortality rate estimated obtained from a single imputed dataset.
Analyses adjusted for the following index pregnancy variables: age, smoking, race/ethnicity, parity, marital status, income, education, preexisting diabetes, preexisting cardiovascular conditions, preexisting cancer/tumors, preexisting neurological conditions, preexisting renal conditions, preexisting respiratory conditions, prior pregnancy loss, site, study year, pre-pregnancy body mass index, gestational week at delivery.
Pre-pregnancy BMI 18·5–24·9 kg/m2
Mortality was lowest among those with weight change within recommendations (7·1 per 1000 person-years [6·8–7·3]) and highest among those with weight change above (7·8 [7·4–8·3]) recommendations (Figure 1; Table 2). Weight change below recommendations was associated with lower diabetes mortality (HR=0·62 [0·48–0·79]) (Table 2). Weight change above recommendations was associated with increased all-cause (HR=1·09 [1·01–1·18]) and cardiovascular (HR=1·20 1·04–1·37]) mortality. Both quintile 1 (HR=1·07 [1·01–1·14]) and quintile 5 (HR=1·09 [1·02–1·16]) were associated with increased all-cause mortality. Quintile 5 was associated with increased cardiovascular (HR=1·15 [1·01–1·30]) and diabetes (HR=1·63 [1·13–2·35]) mortality (Figure 2; Table 3; Table S6).
Pre-pregnancy BMI 25·0–29·9 kg/m2
Mortality was lowest among those with weight change within recommendations (10·0 per 1000 person-years 9·4–10·6]) and highest among those with weight change below (11·1 [10·6–11·7]) recommendations (Figure 1; Table 2). Weight change below recommendations was associated with increased all-cause (HR=1·08 [0·99–1·18]) and cardiovascular (HR=1·08 [0·94–1·26]) mortality but with imprecise and not statistically significant estimates (Table S5). Weight change above recommendations was associated with elevated all-cause (HR=1·12 [1·01–1·24]) and diabetes (HR=1·77 [1·23–2·54]) mortality, as well as cardiovascular (HR=1·12 [0·94–1·33]) mortality, but these estimates were imprecise and not statistically significant. Compared to quintile 3, quintile 1 (HR=1·09 [0·98–1·22]), which lack precision and statistical significance, and quintile 2 (HR=1·15 [1·02–1·30]) were associated with elevated all-cause mortality (Figure 2; Table S6); similar estimates in magnitude were observed for cardiovascular mortality, but they were not statistically significant (Table 3). Quintile 5 was associated with elevated all-cause (HR=1·18 [1·05–1·32]), cardiovascular (HR=1·24 [1·01–1·52]), and diabetes (HR=2·24 [1·41–3·55]) mortality.
Pre-pregnancy BMI ≥30·0 kg/m2
All associations between gestational weight change and mortality were imprecise and not statistically significant (Figure 1–2, Tables 2–3, Tables S5–S6).
Exploratory analyses with other major causes of death
Additional major causes of death were cancer (32·4%; n=5800/17901), respiratory disease (7·3%; n=1308/17901), dementia (3·5%; n=627/17901), infectious disease (3·5%; n=628/17901), kidney disease (2·1%; n=379/17901), and liver disease (2·0%; n=355/17901). Associations between gestational weight change and these causes of death are shown in Table S7.
Sensitivity analyses using gestational weight change z-scores
Sensitivity analyses based on gestational weight gain z-scores yielded similar findings, although estimates with the lowest weight gain were slightly attenuated for those with pre-pregnancy overweight (Table S8).
Sensitivity analyses for exposure and outcome misclassification
Our analysis (Table S9) indicates that exposure misclassification is likely to have a negligible and inconsequential impact on the observed associations. Similarly, most estimates were not altered when considering outcome misclassification, although a few observed associations were slightly underestimated (Table S10). Under the most extreme scenarios of potential misclassification, the observed estimates for the above recommendations may be underreported. In contrast, the estimates below recommendations were generally similar, except for those with pre-pregnancy underweight, where they may be underreported.
DISCUSSION
This study provided the first assessment of long-term mortality associations with gestational weight change based on a diverse cohort of ~46,000 U.S. pregnant people who were pregnant between 1959 and 1966 and followed for more than 50 years post-pregnancy. We found that excessive gestational weight gain (i.e., above 2009 NAM recommendations), was associated with a 9% to 12% increased all-cause mortality risk among individuals with normal weight or overweight, respectively. The risk was also elevated by 14% among those with underweight, but estimates were imprecise and lacked statistical significance. Moreover, excessive gain was associated with an 84% and 20% increased risk of mortality due to cardiovascular disease among those with pre-pregnancy underweight or normal weight, respectively, and a 77% increased risk of mortality due to diabetes among those with overweight. Additionally, weight gain below recommendations was associated with a lower mortality risk due to diabetes among with normal weight. While existing literature has demonstrated that healthy gestational weight gain is crucial for optimizing pregnancy outcomes such as birthweight,2 this study demonstrates that for individuals without pre-pregnancy obesity, gestational weight gain may have a lasting impact on health beyond pregnancy, increasing mortality risk from cardiometabolic diseases. No prior studies have examined gestational weight change and its association with mortality. Existing research focuses on cardiovascular disease and type 2 diabetes post-pregnancy.6,8,9 A 21-year follow-up study found a 42% increase in type 2 diabetes for those exceeding recommendations,8 but this was not replicated in a study with only 10–17 years of follow-up, which may have been insufficient for diabetes development.6 A 16-year follow-up study revealed an increased hypertension risk for all BMI classes and cardiovascular disease risk for underweight/normal-weight individuals with high gestational weight gain.7 This current study demonstrates that high gestational weight change on mortality up to 50 years post-pregnancy through cardiovascular and diabetes mortality links.
The findings regarding excessive weight gain during pregnancy support the hypothesis that gaining too much weight contributes to the accumulation of visceral adiposity. This, in turn, increases the risk of chronic diseases and subsequent mortality. It is noteworthy that even if individuals return to their pre-pregnancy weight after childbirth, they still retain higher levels of visceral fat six years later.25 Despite considering various important factors, we cannot establish a causal relationship, and excessive gestational weight gain may be a predictive factor rather than a direct cause of later health problems and mortality. However, it remains crucial to prioritize primary prevention of excessive weight gain during pregnancy due to its significant impact on various maternal and offspring outcomes.2 It is important to note that a considerable proportion (20–64%) of pregnant U.S. individuals exceed the recommended weight gain guidelines.26 Additionally, future studies should investigate whether interventions targeting weight retention and, specifically, reducing visceral fat after childbirth can help mitigate long-term risks.
While we hypothesized low weight gain prevents postpartum weight retention and subsequent obesity-related cardiometabolic mortality, we found low weight gain or loss was associated with increased mortality in individuals without pre-pregnancy obesity, although some of the estimates were not statistically significant and all require replication. This finding aligns with a prior study showing an elevated cardiovascular disease risk 16 years later for individuals without pre-pregnancy overweight or obesity who lost weight during pregnancy.7 The biological basis for this association, however, remains unclear, necessitating further research.
Gestational weight change was not associated with long-term mortality among individuals with obesity before pregnancy. Mortality rates were approximately double in individuals with obesity compared to normal weight. This high baseline mortality risk does not appear to be altered by gestational weight gain.
Gestational weight gain recommendations were last updated in 2009, when no evidence was available concerning long-term mortality.2 Our findings generally suggest that for pregnant people with pre-pregnancy underweight and normal weight a lower weight gain than currently recommended may be desirable in the context of long-term mortality. For those with underweight, generally, no variation in mortality was observed across quintiles 3 and 4, noting that most estimates were imprecise and not statistically significant, corresponding to a favorable range of 10 to 14 kg. For those with normal weight, the lowest mortality was generally observed with quintiles 2 to 3, corresponding to a favorable range of 6·8 to 10·9 kg. Alternatively, for those with overweight a lower upper limit may be ideal for long-term maternal health. The lowest mortality was observed with quintile 3, corresponding to 7·7 to 10·0 kg. Nevertheless, these findings require replication and must be considered in the context of short-term maternal and offspring health outcomes.
This study comprised pregnant individuals in the 1950’s-1960s. Utilizing a historic cohort is imperative to study the long-term health implications of risk factors, including gestational weight change. While participant characteristics in this historic cohort may differ from contemporary obstetric populations, we utilized current weight gain recommendations and stratified findings by pre-pregnancy BMI to strengthen relevance to current populations who are more likely to be overweight or obese and experience higher than recommended weight gain in pregnancy.27 This research underscores the importance of excessive weight gain prevention. In 2021, the U.S. Preventive Services Task Force found ‘adequate evidence’ that behavioral interventions lower the likelihood of excessive weight gain and that healthcare providers should offer pregnant people behavioral counseling to increase healthy weight gain.28 There is an urgent need to identify and implement novel interventions to help pregnant people achieve healthy weight gain.
In addition to the long-term follow-up of over 50 years, this study has unique strengths. The study’s racial diversity (almost 50% Black) was an important strength. Most research on pregnancy exposures and long-term outcomes has been limited to primarily White individuals.29 Additionally, the findings were robust to adjustment for many important covariates and multiple sensitivity analyses. We utilized covariate adjustment and weight gain for gestational age z-scores to account for the inherent correlation between gestational length and weight gain.21 Under reasonable assumptions, quantitative bias analyses conducted for both exposure and outcome misclassification demonstrated the overall robustness of our results, and the extent of misclassification bias is expected to be minimal. Nevertheless, there may also be misclassification in pre-pregnancy BMI, but this is likely minimal.23 Additional limitations include a lack of follow-up after delivery limiting the ability to understand the process for developing chronic diseases, and assess how an individual’s risk may be altered with postpartum interventions. We cannot assess how an individual’s risk may be altered with postpartum interventions. Note that our findings are limited to associations observed with the participants’ last study pregnancy. Unfortunately, we were unable to examine gestational weight changes across all pregnancies. However, focusing on the participants’ last study pregnancy allowed us to provide insights specific to that period. Further research is needed to investigate gestational weight changes across multiple pregnancies. Moreover, some participants may have had additional pregnancies after the study period, and we could not examine gestational weight change across all lifetime pregnancies. Lastly, these findings represent underlying causes of death and not contributing causes. This is an important distinction; underlying causes of death are mutually exclusive mortality categories that do not represent overall disease prevalence. More research into contributing causes of death is warranted.
In this first study relating weight change during pregnancy to long-term mortality, the data suggest that excessive weight gain may put many birthing people at an increased risk for earlier mortality, particularly due to cardiometabolic diseases. Effective efforts are urgently needed to help pregnant people achieve healthy weight gain during pregnancy.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Gestational weight gain is associated with adverse pregnancy outcomes, with the greatest extent of the evidence related to offspring birthweight. However, very little is known about the implications for the long-term health of the birthing person. There are a few studies on cardiometabolic health post-pregnancy in relation to gestational weight change. To understand the current evidence base for the association between gestational weight change and long-term mortality, we performed a literature review using PubMed in October 2022 using the search terms “pregnancy weight gain” or “gestational weight gain” and “mortality” not “infant mortality”. No studies were identified investigating the association of gestational weight gain with long-term mortality.
The added value of this study
Our study adds critical knowledge to gestational weight gain research by examining the associations with all-cause mortality and cause-specific mortality over 50 years post-pregnancy. Based on the current 2009 National Academy of Medicine recommendations, we found that excessive gestational weight gain was associated with a 9% to 12% increased all-cause mortality risk among pregnant people with a pre-pregnancy BMI in the normal or overweight range, respectively. Moreover, we found that excessive weight gain was associated with an 84% and 20% increased risk of mortality due to cardiovascular disease among those with a pre-pregnancy BMI in the underweight and normal range, respectively, and a 77% increased risk of mortality due to diabetes among those in the overweight range.
Implications of all the available evidence
In this first study relating weight gain during pregnancy to long-term mortality, the data suggest that excessive weight gain may put many birthing people at an increased risk for earlier mortality, particularly due to cardiometabolic diseases. Effective efforts are urgently needed to help pregnant people achieve healthy weight gain during pregnancy.
ACKNOWLDEGEMENT:
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contract # HHSN275200800002I/27500013).
Footnotes
CONFLICT OF INTEREST: The authors have no conflicts of interest to declare.
DATA SHARING:
Data from the Collaborative Perinatal Project is publicly available at https://www.archives.gov/research/electronic-records/nih.html (National Archives Identifier: 606622). Researchers interested in the linked mortality data should contact the NICHD for details on a data-sharing agreement and a confidentiality agreement with the National Death Index.
REFERENCES
- 1.American College of Obstetricians Gynecologists Weight gain during pregnancy. Committee opinion no. 548. Obstet Gynecol 2013; 121(1): 210–2. [DOI] [PubMed] [Google Scholar]
- 2.Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: Institute of Medicine and National Research Council, 2009. [PubMed] [Google Scholar]
- 3.Janumala I, Toro-Ramos T, Widen E, et al. Increased Visceral Adipose Tissue Without Weight Retention at 59 Weeks Postpartum. Obesity 2020; 28(3): 552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray GA, Jablonski KA, Fujimoto WY, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. The American journal of clinical nutrition 2008; 87(5): 1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Després J-P. Body Fat Distribution and Risk of Cardiovascular Disease. Circulation 2012; 126(10): 1301–13. [DOI] [PubMed] [Google Scholar]
- 6.Moll U, Olsson H, Landin-Olsson M. Impact of pregestational weight and weight gain during pregnancy on long-term risk for diseases. PLoS One 2017; 12(1): e0168543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkegaard H, Bliddal M, Støvring H, et al. Maternal weight change from prepregnancy to 18 months postpartum and subsequent risk of hypertension and cardiovascular disease in Danish women: A cohort study. PLOS Medicine 2021; 18(4): e1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Mamun A, Mannan M, O’Callaghan MJ, Williams GM, Najman JM, Callaway LK. Association between gestational weight gain and postpartum diabetes: evidence from a community based large cohort study. PloS One 2013; 8(12): e75679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. The American Journal of Clinical Nutrition 2011; 94(5): 1225–31. [DOI] [PubMed] [Google Scholar]
- 10.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evidence Report/Technology Assessment 2008; (168): 1–223. [PMC free article] [PubMed] [Google Scholar]
- 11.Niswander KR. The collaborative perinatal study of the National Institute of Neurological Diseases and Stroke. The Woman and Their Pregnancies 1972. [Google Scholar]
- 12.Pollack AZ, Hinkle SN, Liu D, et al. Vital Status Ascertainment for a Historic Diverse Cohort of US Women. Epidemiology 2020; 31(2): 310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Hutcheon JA. Identifying outliers and implausible values in growth trajectory data. Annals of Epidemiology 2016; 26(1): 77–80.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. The American Journal of Clinical Nutrition 2013; 97(5): 1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Death Index user’s guide. In: National Center for Health Statistics, editor. Hyattsville, MD; 2013. [Google Scholar]
- 16.Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology 2001; 12(2): 259–61. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Manual of the international statistical classification of diseases, injuries, and causes of death: based on the recommendations of the ninth revision conference, 1975, and adopted by the Twenty-ninth World Health Assembly: World Health Organization,; 1977. [Google Scholar]
- 18.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep 2001; 49(2): 1–32. [PubMed] [Google Scholar]
- 19.Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. American journal of obstetrics and gynecology 2017; 217(2): 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinkle SN, Mitchell EM, Grantz KL, Ye A, Schisterman EF. Maternal Weight Gain During Pregnancy: Comparing Methods to Address Bias Due to Length of Gestation in Epidemiological Studies. Paediatr Perinat Epidemiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutcheon JA, Bodnar LM. Good Practices for Observational Studies of Maternal Weight and Weight Gain in Pregnancy. Paediatric and Perinatal Epidemiology 2018; 32(2): 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MP MR, Lash TL. Applying Quantitative Bias Analysis to Epidemiologic Data (Statistics for Biology and Health) 2nd ed. Edition ed: Springer; 2021 [Google Scholar]
- 23.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obesity Reviews 2017; 18(3): 350–69. [DOI] [PubMed] [Google Scholar]
- 24.Little R, Rubin D. Statistical inference with missing data. New York: Wiley; 2002. [Google Scholar]
- 25.Janumala I, Toro-Ramos T, Widen E, et al. Increased visceral adipose tissue without weight retention at 59 weeks postpartum. Obesity 2020; 28(3): 552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstetrics & Gynecology 2015; 125(4): 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laughon SK, Branch DW, Beaver J, Zhang J. Changes in labor patterns over 50 years. Am J Obstet Gynecol 2012; 206(5): 419.e1–.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson KW, Barry MJ, Mangione CM, et al. Behavioral Counseling Interventions for Healthy Weight and Weight Gain in Pregnancy. JAMA 2021; 325(20): 2087. [DOI] [PubMed] [Google Scholar]
- 29.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation 2021; 143(18). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Collaborative Perinatal Project is publicly available at https://www.archives.gov/research/electronic-records/nih.html (National Archives Identifier: 606622). Researchers interested in the linked mortality data should contact the NICHD for details on a data-sharing agreement and a confidentiality agreement with the National Death Index.


