Abstract
Like its homologs throughout the biological world, the l-isoaspartyl protein repair methyltransferase of Escherichia coli, encoded by the pcm gene, can convert abnormal l-isoaspartyl residues in proteins (which form spontaneously from asparaginyl or aspartyl residues) to normal aspartyl residues. Mutations in pcm were reported to greatly reduce survival in stationary phase and when cells were subjected to heat or osmotic stresses (C. Li and S. Clarke, Proc. Natl. Acad. Sci. USA 89:9885–9889, 1992). However, we subsequently demonstrated that those strains had a secondary mutation in rpoS, which encodes a stationary-phase-specific sigma factor (J. E. Visick and S. Clarke, J. Bacteriol. 179:4158–4163, 1997). We now show that the rpoS mutation, resulting in a 90% decrease in HPII catalase activity, can account for the previously observed phenotypes. We further demonstrate that a new pcm mutant lacks these phenotypes. Interestingly, the newly constructed pcm mutant, when maintained in stationary phase for extended periods, is susceptible to environmental stresses, including exposure to methanol, oxygen radical generation by paraquat, high salt concentrations, and repeated heating to 42°C. The pcm mutation also results in a competitive disadvantage in stationary-phase cells. All of these phenotypes can be complemented by a functional pcm gene integrated elsewhere in the chromosome. These data suggest that protein denaturation and isoaspartyl formation may act synergistically to the detriment of aging E. coli and that the repair methyltransferase can play a role in limiting the accumulation of the potentially disruptive isoaspartyl residues in vivo.
Spontaneous chemical reactions which occur in the cytoplasm of a cell under physiological conditions often result in damage to the cell’s critical macromolecules, a process which may be enhanced by exposure to environmental changes, such as increased temperature, reactive oxygen species, altered osmotic conditions, and various chemicals (32). While damage to DNA and mechanisms for its repair have been extensively studied, many chemical changes also occur in proteins (30). The significance of such protein damage may be greatest for cells which are not dividing or those in which protein turnover is curtailed by nutrient limitation or environmental stresses.
Organisms ranging from bacteria to mammals and plants have developed strategies for coping with one product of spontaneous protein damage, the l-isoaspartyl residues which arise from aspartyl and asparaginyl residues in proteins (5). These abnormal residues can affect protein structure and enzyme activity (reviewed in reference 30) but are recognized and methylated by l-isoaspartyl protein carboxyl methyltransferases (EC 2.1.1.77), such as the product of the Escherichia coli pcm gene (8, 12). Methylation stimulates the reformation of an unstable succinimide intermediate which can yield a normal l-aspartyl residue upon hydrolysis, thus ultimately catalyzing net repair of the damaged site (17). In plants, methyltransferase activity is induced during seed production and in response to dehydration and other stresses (19), while the enzyme activity has been strongly correlated with aging in mammals by the recent finding that mice lacking the gene accumulate damaged proteins and die at an early age (11).
The construction of an E. coli strain carrying a pcm deletion has been described previously (12). This mutant strain showed reduced ability to survive for prolonged periods in stationary phase and increased sensitivity to heat and osmotic shock (12, 13). Similar, though more severe, phenotypes have been associated with mutations in rpoS, a gene located about 1.5 kb downstream of pcm and encoding ςS, an alternative RNA polymerase sigma subunit crucial to the cellular response to starvation and other stresses (reviewed in reference 14). We recently determined that a nonsense mutation located near the 5′ end of rpoS and present in many common laboratory strains (but not MC1000, the parent strain for these mutants) had been inadvertently introduced into the pcm mutant (31). The amber mutation reduced RpoS activity (as measured by the activity of HPII catalase, induced at stationary phase under the control of RpoS) to about 10% of normal. This unexpected complication made it necessary to reevaluate the pcm mutant to determine whether some or all of the phenotypes previously observed (12, 13) might actually be due to the secondary mutation in rpoS.
The experiments reported here demonstrate that the phenotypes originally attributed to the pcm mutation could be complemented by a functional copy of rpoS integrated elsewhere on the E. coli chromosome but not by a functional pcm gene. A new pcm mutant strain was constructed which lacked the rpoS nonsense mutation; this strain survived as well as its parent for 10 days in stationary phase or when subjected to the other stresses which reduced the viability of the original mutant. The new pcm mutant, however, did display specific phenotypes when cells maintained in stationary phase were subjected continuously or repeatedly to environmental stresses with the potential to unfold proteins. These phenotypes were complemented by a wild-type pcm gene and demonstrate the inability of aging cells to cope with additional stresses in the absence of the isoaspartyl repair enzyme.
MATERIALS AND METHODS
Strains and plasmids.
E. coli strains and plasmids used in the phenotypic assays are described in Table 1. Strain JV1026 was constructed by first inserting a 3.8-kb BamHI-BclI fragment from pCL1 (12), carrying the surE-pcm operon and its promoter, into pLDR10 (7) adjacent to the lambda recombination sequence attP. A NotI restriction fragment of this plasmid lacking the origin of replication was then self-ligated and introduced into E. coli WM2269, harboring pLDR8, a plasmid with a temperature-sensitive replication origin carrying the lambda int gene (7). Colonies expressing the ampicillin resistance marker from pLDR10 when grown at 42°C (the nonpermissive temperature for pLDR8) were screened to confirm the loss of the helper plasmid. The construction of HC1011 was done similarly, except that pLDR11 (7) was used as the integration vector and the integrated DNA was a 4.8-kb EcoRI fragment carrying rpoS+ from a derivative of pMMkatF2 (20). The resulting attB::pcm+ or attB::rpoS+ insertion was transduced into strain CL1010 by using bacteriophage P1 (25) and confirmed by PCR amplification with attB- and gene-specific primers and by Southern hybridization.
TABLE 1.
Genotypes of E. coli strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| MC1000a | λ− e14−araD139 Δ(araA-leu)7697 galE15 galK16 Δ(codB-lac)3 rpsL150 mcrB1 relA1 spoT1 | CGSCb, 2 |
| CL1010 | MC1000 Δpcm::KmrrpoS396 | 12 |
| HC1011 | MC1000 Δpcm::KmrrpoS396 attB::rpoS+ | This paper |
| JV1012 | MC1000 rpoS13::Tn10 | 31 |
| JV1026 | MC1000 Δpcm::KmrrpoS396 attB::pcm+ | This paper |
| JV1068 | MC1000 Δpcm::Cmr | This paper |
| JV1083 | MC1000 Δpcm::CmrattB::pcm+ | This paper |
| JV1090 | MC1000 malE52::Tn10 | This paper |
| JV1093 | MC1000 malE52::Tn10 Δpcm::Cmr | This paper |
| JV1094 | MC1000 malE57::Tn5 | This paper |
| JV1098 | MC1000 malE52::Tn10 Δpcm::CmrattB::pcm+ | This paper |
Used as wild-type parent strain for all experiments.
E. coli Genetic Stock Center, Yale University, New Haven, Conn.
Strain JV1068 was constructed with pKAS46, which is dependent on the pir gene product for its maintenance and carries a kanamycin resistance (Kmr) marker and a wild-type (Strs) rpsL gene (26). A chloramphenicol resistance (Cmr) marker was used to replace nearly all of the pcm coding sequence (from MluI to ClaI) in pCL1 and was oriented in the same direction as pcm, paralleling the construction of the original mutant strain CL1010 (12). An XbaI fragment carrying the marker and flanking DNA was then inserted into pKAS46, and the resulting plasmids were maintained in CC118λ (pir+). For chromosomal gene replacement, these plasmids were electroporated (by standard methods with a Bio-Rad Gene Pulser apparatus) into strain MC1000 (which lacks the pir gene and is Strr), and Kmr single recombinants were screened for Cmr. These colonies were then streaked on plates containing streptomycin (1 mg/ml) to select for loss of the vector sequences, including rpsL. Strr colonies were restreaked on the same medium and then screened for the Kms Cmr phenotype, which would indicate that gene replacement had occurred. The identities of the recombinant strains were confirmed by transduction and linkage analysis, PCR amplification with primers complementary to pcm, and sequencing of pcm and rpoS.
The complementing strain JV1083 was constructed by transducing the pcm+ gene and the linked Apr marker from strain JV1023 (MC1000 attB::pcm+) into JV1068. Strains TST1 (malE52::Tn10) and TSM7 (malE57::Tn5) were obtained from the E. coli Genetic Stock Center and used in the construction of reciprocally marked strains for the growth advantage in stationary phase (GASP) assay. Selection for Tcr after infection of MC1000 and JV1068 with bacteriophage P1 grown on TST1 permitted the isolation of strains JV1090 and JV1093; strain JV1094 was constructed similarly by transduction of MC1000 with phage grown on TSM7. Strain JV1093 was then transduced with phage grown on strain JV1023 and selected for Apr to generate the complementing strain JV1098.
PCR and sequencing.
Oligonucleotide primers were synthesized with an Oligoassembler apparatus (Pharmacia Biotech, Piscataway, N.J.), and PCR amplifications were performed with an MJ Research (Watertown, Mass.) thermal cycler and Taq DNA polymerase (Promega Corp., Madison, Wis.). For longer PCR products, Taq Extender (Stratagene Cloning Systems, La Jolla, Calif.) was used according to the manufacturer’s instructions. For sequencing, chromosomal DNA from colonies or overnight cultures was amplified directly (9). Sequencing was carried out with an Applied Biosystems 373A or 377 automated sequencing apparatus.
Enzyme assays.
Cytosolic extracts were prepared by gentle sonication of E. coli grown to stationary phase (18 to 20 h in 5 ml of Luria-Bertani [LB] broth) and resuspended in a 0.5-ml solution of 5 mM potassium phosphate, 5 mM disodium EDTA, 10% glycerol, and 25 μM phenylmethylsulfonyl fluoride (pH 7.0) as described previously (31). The protein concentration was estimated by the method of Lowry et al. (16) after trichloroacetic acid precipitation. A vapor phase assay to measure base-labile methyl esters produced by transfer of the methyl group from S-adenosyl-l-[methyl-14C]methionine to a specific isoaspartyl-containing peptide substrate (Lys-Ala-Ser-Ala-isoAsp-Leu-Ala-Lys-Tyr) by methyltransferase in the extract was performed essentially as described previously (12). Methylation was carried out for 20 min at 37°C, 1.5 M Na2CO3 was used to release labeled methanol from the methyl esters, and peptide-specific counts were determined by subtraction of a control sample assayed in the absence of peptide substrate. HPII catalase activity was determined by means of a spectrophotometric assay for H2O2 breakdown in extracts that had been heated to inactivate HPI catalase (31).
Stationary-phase and stress survival tests.
Resistance to lethal heat shock was measured by using cultures grown for approximately 20 h in LB broth. Cells from these cultures were spun down and resuspended to 1/10 their original density in 0.85% NaCl, and 50-μl aliquots were then heated to 55°C for 10 min in a thermal cycler, using a thin-walled polycarbonate 96-well plate. Viable counts were performed by making serial dilutions in 0.85% NaCl and plating them on LB agar (incubated for 16 to 20 h at 37°C) to determine the number of bacteria that had survived; these results were then compared to counts of the original cultures. Oxidative stress testing was carried out similarly, except that H2O2 was added to the diluted cells to a final concentration of 15 or 30 mM and the cells were incubated for 1 h at 37°C. For osmotic stress, the culture aliquots were resuspended in 2.5 M NaCl and maintained at 37°C for 4 h.
Long-term stationary-phase survival was measured by growing cultures for 20 to 24 h at 37°C in 1.5 ml of M9 minimal medium or LB broth, using 15-ml plastic screw-cap tubes and a roller drum for aeration. At this point, aliquots were removed for “day 0” viable counts. The cultures were then maintained in the original culture medium for an additional 10 days on the roller drum at 37°C, and aliquots were removed for viable counts every 1 to 2 days. To determine the effect of methanol (0.5 or 1%), paraquat (0.1 or 0.25 mg/ml), or salt (0.5 or 1 M) on the stationary-phase cells, long-term cultures were grown and maintained in LB broth as described above. The desired agent was added to the indicated concentration on day 0, following the removal of aliquots for counting. The effect of pulsed heating was determined by transferring cultures maintained as above to a 42°C incubator for 1 or 2 h every 24 h; in this case, aliquots were removed for counting prior to heating.
GASP assay.
The ability of aged cells to outcompete fresh overnight cultures (termed the GASP phenotype) was determined essentially as described previously (33, 34). Parental and mutant strains to be tested were marked with chromosomal antibiotic resistance genes, grown overnight in LB broth, and maintained for an additional 10 days in the same medium, with aeration. After 9 days, viable counts were performed to determine the numbers of living cells remaining; on day 10, the aged cultures were diluted 1:1,000, based on the number of live cells, into fresh overnight cultures of a parental strain marked with a different antibiotic resistance. The mixture was maintained for an additional 10 days, and aliquots were removed every 1 to 2 days, diluted, and plated on appropriate antibiotic-containing media in order to determine the number of viable cells of each strain.
RESULTS AND DISCUSSION
The original pcm mutant strain shows rpoS-like phenotypes that can be complemented by rpoS but not pcm.
Previously, a strain with pcm deleted was shown to be defective in stationary-phase survival and in resistance to heat and to increased osmotic strength (12, 13). Similar, though more severe, phenotypes are characteristic of mutations in rpoS (encoding the sigma factor ςS, a key positive regulator of the cellular response to stationary phase), which is located only 1.5 kb downstream of pcm. As additional phenotypes, including resistance to heat and osmotic stress, were investigated, a more significant overlap was observed (Table 2) between the phenotypes of the original pcm mutant (CL1010) and those of an rpoS mutant (JV1012). The relatively small difference in oxidative stress resistance between CL1010 and the Pcm+ parent strain, MC1000, noted previously (12) became much more pronounced upon increasing the incubation time to 60 min and the peroxide concentration to 30 mM (Table 2). Lowered resistance to peroxide is one of the characteristic phenotypes of an rpoS mutation, because ςS directs the transcription of the stationary-phase catalase, HPII (15). We also observed little difference between the pcm and rpoS mutants in survival of lethal heat shock (55°C) or treatment with 2.5 M NaCl (Table 2).
TABLE 2.
Enzyme activities and short-term stress survival of original and new pcm mutants and complemented strains
| Strain | Relevant genotype | Enzyme activitya
|
% Survival of stationary-phase cellsb
|
||||
|---|---|---|---|---|---|---|---|
| Pcmc | HPII catalased | Heate | Saltf | 15 mM H2O2g | 30 mM H2O2g | ||
| MC1000 | Parent strain | 3.3 ± 0.61 | 36.0 ± 2.5 | 7.2 | 59 | 80 | 9.0 |
| CL1010 | Δpcm::KmrrpoS396 | 0.08 ± 0.04 | 3.1 ± 0.40 | <1.0 × 10−5 | 3.0 | 34 | <1.0 × 10−5 |
| JV1012 | rpoS13::Tn10 | 3.6 ± 0.25 | 0.63 ± 0.22 | <2.2 × 10−5 | 2.4 | 1.0 | <1.0 × 10−5 |
| JV1026 | Δpcm::KmrrpoS396 attB::pcm+ | 1.9 ± 0.25 | 3.9 ± 0.97 | <2.5 × 10−5 | 2.1 | 39 | 5.0 × 10−5 |
| HC1011 | Δpcm::KmrrpoS396 attB::rpoS+ | 0.05 ± 0.03 | 30.2 ± 2.4 | 4.0 | ND | 80 | 4.0 |
| JV1068 | Δpcm::Cmr | 0.0 ± 0.004 | 37.0 ± 1.3 | 5.2 | 54 | 82 | 10 |
Averages of at least four trials; error range represents 1 standard deviation.
Values are averages of at least two trials in duplicate; ND, not determined.
Pcm activity in picomoles of methyl groups transferred to an isoaspartyl-containing peptide substrate per minute per milligram of total protein.
HPII activity in micromoles of hydrogen peroxide decomposed per minute per milligram of total protein.
Diluted 1:10 in 0.85% NaCl and heated to 55°C for 10 min.
Diluted 1:10 in 2.5 M NaCl and incubated at 37°C for 4 h.
Diluted 1:10 in H2O2 at indicated concentration; incubated at 37°C for 1 h.
We have recently determined that this previously characterized pcm mutant in fact carries an amber mutation in rpoS derived from the JC7623 strain used in its construction and inadvertently cotransduced into the MC1000 background (31). This allele, now designated rpoS396, reduces RpoS activity, as measured by ςS-dependent HPII catalase activity at stationary phase, to about 10% of that in MC1000 (Table 2). Complementation studies were performed to determine whether the phenotypes observed for CL1010 were due to the pcm mutation, the rpoS mutation, or the combination. Previously, efforts at plasmid complementation had yielded ambiguous results which might be attributed to the difficulty of ensuring plasmid maintenance in long-term stationary-phase cultures, so we inserted a wild-type copy of pcm or rpoS into the bacteriophage lambda attachment site (attB) on the E. coli chromosome (strains JV1026 and HC1011, respectively) for these experiments. The complementing copy of pcm restored isoaspartyl methyltransferase activity to at least 50% of normal without affecting HPII catalase activity; similarly, the complementing rpoS restored HPII to wild-type levels but did not change the Pcm activity (Table 2).
When cultures were grown to stationary phase in minimal medium and monitored by plate counts of viable cells for 10 days (Fig. 1), the pcm+ complementing strain (JV1026) did not show any significant improvement in survival over the pcm mutant (CL1010). However, the introduction of a functional rpoS gene restored stationary-phase survival to wild-type levels (HC1011). Similarly, the reduced survival of heat, osmotic, and oxidative stresses observed for the pcm mutant strain was not relieved by a functional copy of pcm (Table 2, compare CL1010 to JV1026) but the phenotypes were complemented by the rpoS+ construct (Table 2, HC1011). Complementation with rpoS alone thus appears to be sufficient to overcome the survival defects of CL1010. These results suggest that the amber mutation in rpoS is responsible for the phenotypes previously reported for pcm mutants by Li and Clarke (12).
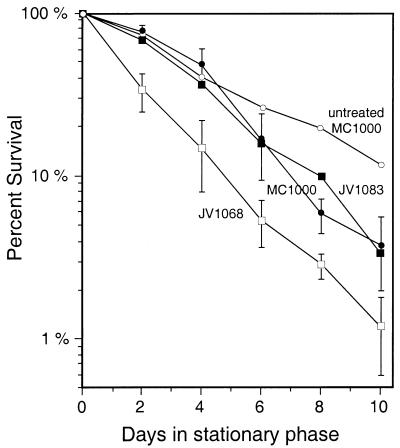
FIG. 1.
Stationary-phase survival of pcm mutants and complemented strains. Cultures of the indicated strains were grown in M9 medium for 24 h (day 0), and the number of viable cells remaining was determined at intervals for the next 10 days. The number of CFU is shown as a percentage of the maximum number of CFU; the cultures did not always reach their maximum levels within the first 24 h. Survival of the original pcm deletion mutant strain, CL1010 (––□––) is shown compared to that of its parent, MC1000 (○), to those of strains complemented with a chromosomal wild-type pcm (JV1026) (▪) or rpoS (HC1011) (▴) gene, and to that of a newly constructed pcm deletion mutant strain, JV1068 (—□—).
A newly constructed pcm mutant survives normally in stationary phase.
In order to determine whether the pcm defect made any contribution to the observed phenotypes, a new pcm mutant was constructed which was free from the secondary rpoS mutation. This was accomplished by using a suicide vector to integrate a near-complete deletion of the pcm gene directly into the chromosome of MC1000 (see Materials and Methods), a strain in which the chromosomal rpoS had been sequenced and shown to be wild type (31). The resulting strain (JV1068) had no detectable Pcm activity (Table 2).
As shown in Fig. 1, the new pcm mutant survived long-term maintenance in minimal medium as successfully as the parental strain; this was also the case when the cells were grown in LB broth or when the experiment was extended to 40 days (data not shown). When tested for viability after 55°C heat shock, treatment with 2.5 M NaCl for 4 h, or treatment with 15 or 30 mM H2O2 for 1 h, the mutants again showed no survival defects (Table 2). These results demonstrate that the original phenotypes attributed to the pcm mutation were indeed due solely to the secondary rpoS mutation. Furthermore, despite the physical proximity of pcm to rpoS, its disruption had no effect on ςS activity as judged by measurement of HPII catalase (Table 2) or by means of rpoS-lacZ translational fusions (data not shown).
Although we would expect isoaspartyl damage to reach significant levels during this long incubation in stationary phase, inactivation of the only known repair mechanism results in no detectable loss of viability under these conditions. Thus, we must conclude that unless this damage is being repaired by some other means, the formation of isoaspartyl residues is not in itself terribly detrimental to E. coli under otherwise stable conditions. We therefore became interested in determining whether the significance of the isoaspartyl residues might increase when the bacteria encounter changing environmental conditions.
Susceptibility of pcm mutants to environmental stresses in extended stationary-phase cultures.
When subjected to short-term environmental stresses (Table 2) or to conditions which reduce culture viability very quickly (e.g., desiccation, maintenance at 42°C, or exposure to chloramphenicol [data not shown]), there was no observable difference between the survival of the parental strain and that of the pcm mutant JV1068. We therefore concentrated on subjecting stationary-phase cultures to conditions which reduced the viability of the cultures more slowly, allowing time for isoaspartyl formation. Initially, we found two stresses which differentially affected the survival of the parental and pcm mutant strains: low concentrations of either methanol or paraquat.
To test the effect of methanol, strains were grown to stationary phase in LB broth and then methanol was added to a final concentration of 0.5% (vol/vol; 156 mM). Under these conditions, the parental strain, MC1000, survived nearly as well as it did when no methanol was added (Fig. 2). The pcm mutant (JV1068), however, showed a distinct and reproducible drop in viability: within 2 days, viable cells in the mutant culture were reduced to about 40% of the wild-type level. Although both strains declined gradually over time, survival of the mutant cells remained at about 20 to 40% of the level of wild-type cells for several days. The difference between the two strains was smaller toward the end of the 10-day period, presumably because the concentration of methanol (or some toxic product) was gradually reduced by evaporation and/or metabolic activity.
FIG. 2.
Effect of methanol on stationary-phase survival of the pcm mutant strain JV1068. Methanol was added to 0.5% (vol/vol) to cultures grown overnight in LB broth, and the number of viable cells remaining was monitored for 10 days thereafter. The points are shown as the percentage of the day 0 CFU remaining viable and are averages of five trials: the error bars represent 1 standard deviation. The strains used were as follows: the untreated parental strain (untreated MC1000) (○), a methanol-treated parental strain (MC1000) (•), a pcm mutant (JV1068) (□), and a pcm mutant complemented with attB::pcm+ (JV1083) (▪).
This survival defect could be readily complemented either by a wild-type pcm gene integrated into the chromosome at attB (Fig. 2, JV1083) or by a plasmid-encoded copy (data not shown). Both of these complementing strains survived as well as MC1000. Increasing the methanol concentration to 1% did not greatly affect the outcome of the experiment, while 5% methanol was sufficient to cause a rapid loss of viability in both wild-type and mutant strains, to the extent that no viable cells were recoverable after about 5 days (data not shown). Neither n-butanol nor isopropanol had a differential effect on the pcm mutant at the same concentrations (0.5 or 1%) as used for methanol (data not shown).
The longevity of the pcm mutant was also significantly compromised when we added paraquat to the long-term stationary-phase cultures. Paraquat, a redox-cycling drug, results in the production of reactive oxygen species, including hydrogen peroxide and superoxide and hydroxyl radicals (10). The advantage of this agent over the peroxide treatment we used in our short-term experiments is that paraquat can be repeatedly reduced and reoxidized and thus produces reactive oxygen species continuously, whereas oxidative stress produced by direct application of peroxide lasts only until the peroxide is inactivated by catalase.
When maintained in the presence of 0.1 mg of paraquat/ml (Fig. 3), the pcm mutant (JV1068) typically survived nearly as well as the parental strain (MC1000) for about 4 days, but then its viability dropped dramatically to below the limit of detection. The parental strain was also affected by the addition of paraquat but could invariably be recovered from the cultures after 10 days, as could the pcm mutant when complemented with the attB::pcm+ construct (JV1083). Increasing the paraquat concentration to 0.25 or 0.5 mg/ml resulted in faster reduction in viability for both strains, but the mutant cultures again dropped rapidly to undetectable levels while the parental and complemented strains remained recoverable for 2 to 4 additional days (data not shown). A concentration of 1 mg/ml was sufficient to completely kill both cultures within 2 days (data not shown). Paraquat had no effect on either strain when cultures were grown in tightly closed tubes filled to the cap with growth medium to limit oxygen (data not shown), supporting the idea that paraquat’s effect is indeed related to oxygen radical formation. These results suggest that the l-isoaspartyl methyltransferase is required for survival when cells are under continual oxidative stress.
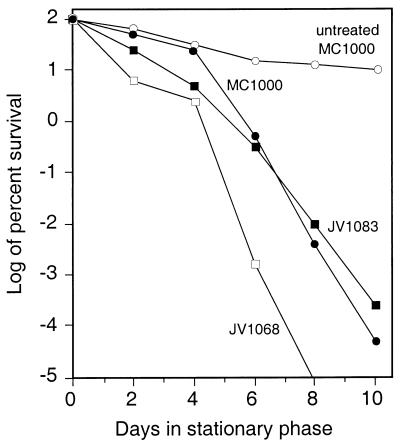
FIG. 3.
Effect of paraquat on stationary-phase survival of the pcm mutant strain JV1068. Paraquat was added to a concentration of 0.1 mg/ml to cultures grown overnight in LB broth, and the number of viable cells remaining was monitored for 10 days thereafter. The points are shown as logs of the percentage of the day 0 CFU remaining viable (averages of at least five trials) for the untreated parental strain (untreated MC1000) (○) and for the paraquat-treated parental strain (MC1000) (•), a pcm mutant (JV1068) (□), and a pcm mutant complemented with attB::pcm+ (JV1083) (▪). Viable JV1068 cells remaining on days 8 and 10 were below the limit of detection, which was approximately 50 cells/ml.
Interestingly, neither methanol (up to 1%) nor paraquat (up to 0.5 mg/ml) appeared to reduce the viability of the mutant when grown in minimal medium rather than in LB broth (data not shown). This may be attributable to the development of a generally higher level of stress resistance when grown in minimal medium (24), or the phenotypes may be dependent on the more dynamic nature of LB cultures or the higher metabolic rates of stationary-phase cells in LB broth (33).
One explanation for the effect of methanol would be toxicity resulting from the formation of formaldehyde, one immediate oxidation product of methanol; however, we found no differential effect of formaldehyde (0.005 or 0.015%) on pcm mutants (data not shown). Another possibility would be that methanol increases protein denaturation. Like ethanol (28), whose known effects include protein unfolding (4, 21), methanol can induce the transcription of heat shock genes in E. coli (29). This is an attractive idea because denaturation is also one of the effects of oxidative stress on proteins (6), providing a link between the two phenotypes described above. If this hypothesis were correct, we would expect other denaturing stresses, such as ethanol, heat, or osmotic stress (1, 21, 27), to have a stronger effect on pcm than on the parental strain.
The pcm mutant did not show impaired survival when ethanol was added to stationary-phase cultures at a concentration of 1 or 5%—indeed, ethanol-treated cultures of either MC1000 or JV1068 actually survived better than untreated cultures (data not shown). Presumably, this occurs because alcohol dehydrogenase, expressed at low levels under aerobic conditions (3), permits the nutrient-limited bacteria to obtain some carbon and energy by metabolizing the ethanol. However, stationary-phase cultures treated with 0.5 M NaCl (Fig. 4A) showed a phenotype similar to that seen with methanol. Viability of the pcm mutant (JV1068) decreased by a small amount relative to that of the parent strain; the decrease was repeatable and could be complemented by a wild-type pcm gene (JV1083). Increasing the salt concentration to 1 M gave similar results (data not shown). Although incubating the cultures continuously at 42°C resulted in rapid loss of viability for both strains, shifting them to 42°C for 2 h every day again produced a repeatable difference between the two strains (Fig. 4B), which could be complemented by introducing a functional copy of pcm.
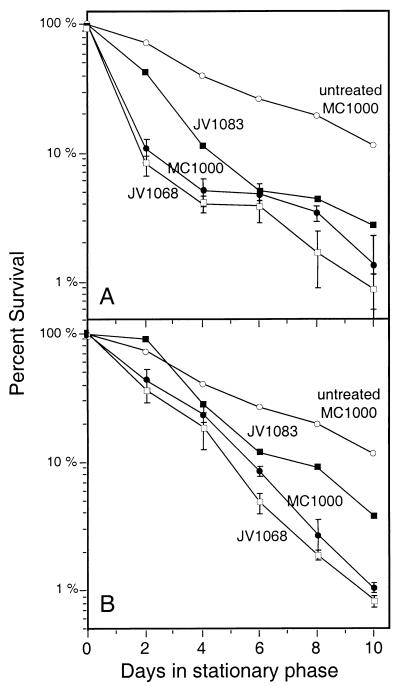
FIG. 4.
Effects of osmotic and heat stresses on stationary-phase survival of the pcm mutant strain JV1068. (A) Survival curve showing long-term maintenance in LB broth of the untreated parental strain (untreated MC1000) (○) and the effect of adding NaCl to 0.5 M on the parental strain (MC1000) (•), the pcm mutant (JV1068) (□), and the pcm mutant complemented with attB::pcm+ (JV1083) (▪). The points shown are averages of six trials; the error bars represent 1 standard deviation. (B) Survival curve for the untreated parental strain (untreated MC1000) (○) and the effect of heating the parental strain (MC1000) (•), the pcm mutant (JV1068) (□), and the complemented pcm mutant (JV1083) (▪) to 42°C for 2 h per day. The points shown are averages of at least three trials; the error bars represent 1 standard deviation.
When an isoaspartyl residue forms, it introduces a kink into the polypeptide backbone, due to the routing of the backbone through what was formerly the side chain of an aspartyl or asparaginyl residue and the consequent placement of an extra methylene group between two adjacent residues (see reference 30 for a review). This might alter the protein’s conformation sufficiently to increase its susceptibility to subsequent denaturation resulting from heat, oxidative attack, or other stresses, or the isoaspartyl damage might inhibit refolding once the protein becomes denatured. Alternatively, the presence of isoaspartyl residues might expose new sites to oxidative or other damage, or conversely, partial unfolding by environmental agents might make aspartyl or asparaginyl residues which were previously buried accessible to the solvent, increasing the amount of isoaspartyl damage. Further experiments will be needed to evaluate the ability of these hypotheses to account for the synergistic effect of stress and pcm mutations.
Deletion of pcm affects the GASP phenotype.
E. coli cultures aged for 10 days in LB broth acquire mutations which appear to enhance their ability to maintain metabolic activity under starvation conditions. When mixed with fresh overnight cultures of the same strain, the aged cells outcompete the younger ones and take over the culture within a few days, a phenomenon termed the GASP phenotype (33, 34). We asked whether this competitive situation might provide a means of identifying subtle defects in long-term survival resulting from the pcm mutation.
The MC1000 parental strain, the pcm mutant JV1068, and the attB::pcm complementing strain JV1083 were marked by transducing the antibiotic resistance marker from strains carrying either a Tn10 (Tcr) or Tn5 (Kmr) insertion in the malE locus (see Materials and Methods) (Table 1). The resulting strains were grown to stationary phase in LB broth and then aged for 10 days. Aliquots of the aged cultures were added at a 1:1,000 ratio to fresh overnight cultures of either the Tcr or Kmr derivative of MC1000 so that each resulting culture contained one Tcr strain and one Kmr strain. Plate counts were done to monitor the number of viable cells of each type.
As shown in Fig. 5A, aged parental cells exhibited the GASP phenotype and became dominant in the cultures within 10 days, as did the pcm mutants with the complementing attB::pcm+ construction (Fig. 5B). Although there was some variability in the time required for this shift to occur, the numbers of the two strains reached equality in 3 to 6 days, and the aged cells were invariably in the majority after 10 days. In a total of seven trials for MC1000 and six trials for the complementing strain, no exceptions were seen. When the aged competitor was the pcm mutant, however, the results were much more variable; three representative outcomes from eight trials are shown in Fig. 5C to E. In two of the eight cultures, the aged pcm mutants demonstrated GASP phenotypes similar to that seen for the wild type (Fig. 5C). However, in four cases, the mutants were delayed in reaching equality with the fresh MC1000 (Fig. 5D), and in two experiments, they failed to become the dominant strain at all (Fig. 5E).
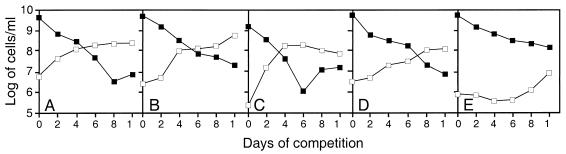
FIG. 5.
Deletion of pcm affects the development of the GASP phenotype. A culture of the competitor strain of interest (open symbols) which had been maintained in stationary phase for 10 days was mixed in a 1:1,000 ratio with a fresh overnight culture of JV1094 (Kmr parental strain; closed symbols). The competitor strains were as follows: (A) aged JV1090 (Tcr parental strain); (B) aged JV1098 (Tcr pcm mutant complemented by attB::pcm+); (C to E) aged JV1093 (Tcr pcm mutant). Each panel represents the result of a single experiment, representative of at least six experiments (A and B) or typical of the three different types of outcomes observed for the pcm mutants in eight experiments (C to E). Results similar to those shown in panels C and E were observed twice each, while results similar to those shown in panel D were observed four times.
The reduced ability of pcm mutants to display the GASP phenotype suggests that although we were unable to detect a direct effect of the Pcm repair mechanism on the viability of unstressed cells during stationary phase, it may nonetheless provide an important competitive advantage. The need for strains lacking the methyltransferase to spend more metabolic energy in degrading and replacing damaged proteins in order to maintain key cellular functions may affect their competitive ability. Another possibility is that wild-type strains may be able to respond more rapidly to any nutrients which become available, damage to their key enzymes having already been repaired. The variability noted in this experiment is not unexpected, since the particular mutations which occur or are selected for during the aging period would affect the outcome of the experiment, as would the particular proteins which become damaged by the formation of isoaspartyl residues. In view of this inherent randomness, the fact that aged pcm mutants did not reliably take over the culture while aged MC1000 never failed to do so seems to suggest a clear reduction in competitive ability which could certainly provide a significant selective advantage for Pcm+ bacteria.
Summary.
Since a rapidly dividing E. coli cell can produce enough material for an entirely new cell in as little as 20 min, there might seem to be little point in repairing any polypeptide species that become damaged. However, protein synthesis represents a significant expenditure of energy for E. coli and other bacteria, and they apparently make a considerable investment in protein repair systems as well. The best-known examples of such protein repair are the refolding and disaggregation functions performed by chaperones (see reference 22 for a review) and the enzymatic reduction of methionine sulfoxides to their original form by the peptide methionine sulfoxide reductase (18). The necessity for such mechanisms becomes more obvious when one considers the oligotrophic environments in which E. coli spends most of its time when not fortunate enough to encounter a vertebrate host. When protein synthesis is constrained by limited nutrient availability, suboptimal temperatures, or other environmental conditions, existing proteins become much more valuable. Thus, repair of any protein damage which can be recognized and corrected enzymatically, as is the case for isoaspartyl formation, may represent a more frugal use of scarce resources than the synthesis of new proteins.
The phenotypes which we have demonstrated here, using newly constructed pcm mutants, are subtle, but they nevertheless point to a role for the isoaspartyl methyltransferase in the stationary-phase longevity of E. coli. The pcm mutants showed reduced viability in stationary phase, but only when exposed to an additional environmental stress, such as methanol or paraquat. In this sense, the Pcm repair system might be somewhat analogous to the yeast chaperone Hsp104, whose greatest effect on survival is observed upon exposure to both heat and ethanol rather than in response to the individual stresses (23).
Our observations are consistent with the idea that isoaspartyl damage indeed contributes to the loss of viability in the pcm mutants. They also suggest that such damage may be relatively innocuous in a fully folded protein under normal conditions but become detrimental to the cell under potentially denaturing conditions. Future experiments will examine the possible link between protein unfolding and isoaspartyl damage and investigate whether there are particular substrates for the Pcm methyltransferase whose repair is most crucial.
ACKNOWLEDGMENTS
We thank P. C. Loewen (pMMkatF2), W. Messer (pLDR plasmids), K. A. Skorupski (pKAS46), and M. Berlyn of the E. coli Genetic Stock Center (MC1000, TST1, TSM7) for making strains available to us. We are grateful to Jeffrey Ichikawa and other members of the Clarke laboratory for reading the manuscript and for helpful discussions.
This work was supported by grant GM26020 from the National Institutes of Health (S.C. and H.C.) and NIH Postdoctoral Fellowship AG05684 (J.E.V.).
REFERENCES
- 1.Beckmann R P, Lovett M, Welch W J. Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol. 1992;117:1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 3.Clark D, Cronan J E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980;141:177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark D P, Beard J P. Altered phospholipid composition in mutants of Escherichia coli sensitive or resistant to organic solvents. J Gen Microbiol. 1979;113:267–274. doi: 10.1099/00221287-113-2-267. [DOI] [PubMed] [Google Scholar]
- 5.Clarke S. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res. 1987;30:808–821. doi: 10.1111/j.1399-3011.1987.tb03390.x. [DOI] [PubMed] [Google Scholar]
- 6.Davies K J A, Lin S W, Pacific R E. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured proteins. J Biol Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- 7.Diederich L, Rasmussen L J, Messer W. New cloning vectors for integration into the λ attachment site attB of the Escherichia coli chromosome. Plasmid. 1992;28:14–24. doi: 10.1016/0147-619x(92)90032-6. [DOI] [PubMed] [Google Scholar]
- 8.Fu J C, Ding L, Clarke S. Purification, gene cloning, and sequence analysis of an l-isoaspartyl protein carboxyl methyltransferase from Escherichia coli. J Biol Chem. 1991;266:14562–14572. [PubMed] [Google Scholar]
- 9.Gussow D, Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989;17:4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan H M, Fridovich I. Paraquat and Escherichia coli. J Biol Chem. 1979;254:10846–10852. [PubMed] [Google Scholar]
- 11.Kim E, Lowenson J D, MacLaren D C, Clarke S, Young S G. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc Natl Acad Sci USA. 1997;94:6132–6137. doi: 10.1073/pnas.94.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Clarke S. A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance. Proc Natl Acad Sci USA. 1992;89:9885–9889. doi: 10.1073/pnas.89.20.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Ichikawa J K, Ravetto J J, Kuo H-C, Fu J C, Clarke S. A new gene involved in stationary-phase survival located at 59 minutes on the Escherichia coli chromosome. J Bacteriol. 1994;176:6015–6022. doi: 10.1128/jb.176.19.6015-6022.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 15.Loewen P C, Triggs B L. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984;160:668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.McFadden P N, Clarke S. Conversion of isoaspartyl peptides to normal peptides by coupled enzymatic/nonenzymatic reactions: implications for the cellular repair of damaged proteins. Proc Natl Acad Sci USA. 1987;84:2595–2599. doi: 10.1073/pnas.84.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moskovitz J, Rahman M A, Strassman J, Yancey S O, Kushner S R, Brot N, Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mudgett M B, Clarke S. Hormonal and environmental responsiveness of a developmentally regulated protein repair l-isoaspartyl methyltransferase in wheat. J Biol Chem. 1994;269:25605–25612. [PubMed] [Google Scholar]
- 20.Mulvey M R, Sorby P A, Triggs-Raine B L, Loewen P C. Cloning and physical characterization of katE and katF required for catalase HPII expression in Escherichia coli. Gene. 1988;73:337–345. doi: 10.1016/0378-1119(88)90498-2. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen V T, Morange M, Bensaude O. Protein denaturation during heat shock and related stress: Escherichia coli β-galactosidase and Photinus pyralis luciferase inactivation in mouse cells. J Biol Chem. 1989;264:10487–10492. [PubMed] [Google Scholar]
- 22.Parsell D A, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez Y, Taulien J, Borkovich K A, Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992;11:2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegele D A, Almirón M, Kolter R. Approaches to the study of survival and death in stationary-phase Escherichia coli. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 151–169. [Google Scholar]
- 25.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 26.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 27.Tanford C. Protein degradation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- 28.VanBogelen R A, Kelley P M, Neidhardt F C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987;169:26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Dyk T K, Smulski D R, Reed T R, Belkin S, Vollmer A C, LaRossa R A. Responses to toxicants of an Escherichia coli strain carrying a uspA′::lux genetic fusion and an E. coli strain carrying a grpE′::lux fusion are similar. Appl Environ Microbiol. 1995;61:4124–4127. doi: 10.1128/aem.61.11.4124-4127.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visick J E, Clarke S. Repair, refold, recycle: how bacteria can deal with spontaneous and environmental damage to proteins. Mol Microbiol. 1995;16:835–845. doi: 10.1111/j.1365-2958.1995.tb02311.x. [DOI] [PubMed] [Google Scholar]
- 31.Visick J E, Clarke S. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J Bacteriol. 1997;179:4158–4163. doi: 10.1128/jb.179.13.4158-4163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson K. Microbial stress proteins. Adv Microb Physiol. 1990;31:183–223. doi: 10.1016/s0065-2911(08)60122-8. [DOI] [PubMed] [Google Scholar]
- 33.Zambrano M M, Kolter R. GASPing for life in stationary phase. Cell. 1996;86:181–184. doi: 10.1016/s0092-8674(00)80089-6. [DOI] [PubMed] [Google Scholar]
- 34.Zambrano M M, Siegele D A, Almirón M, Tormo A, Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993;259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]