Abstract
Pathogens face a tradeoff with respect to virulence; while more virulent strains often have higher per-contact transmission rates, they are also more likely to kill their hosts earlier. Because virulence is a heritable trait, there is concern that a disease-modifying vaccine, which reduces the disease severity of an infected vaccinee without changing the underlying pathogen genotype, may result in the evolution of higher pathogen virulence. We explored the potential for such virulence evolution with a disease-modifying HIV-1 vaccine in an agent-based stochastic epidemic model of HIV in United States men who have sex with men (MSM). In the model, vaccinated agents received no protection against infection, but experienced lower viral loads and slower disease progression. We compared the genotypic set point viral load (SPVL), a measure of HIV virulence, in populations given vaccines that varied in the degree of SPVL reduction they induce. Sensitivity analyses were conducted under varying vaccine coverage scenarios. With continual vaccination rollout under ideal circumstances of 90% coverage over thirty years, the genotypic SPVL of vaccinated individuals evolved to become greater than the genotypic SPVL of unvaccinated individuals. This virulence evolution in turn diminished the public health benefit of the vaccine, and in some scenarios resulted in an accelerated epidemic. These findings demonstrate the complexity of viral evolution and have important implications for the design and development of HIV vaccines.
Keywords: HIV, vaccine, viral evolution, virulence, modeling
Introduction
Human immunodeficiency virus (HIV) remains one of the leading causes of mortality and morbidity worldwide, particularly in low- and middle-income countries [1]. While antiretroviral therapy (ART) and pre-exposure prophylaxis (PrEP) have substantially reduced HIV transmission, continuing problems with accessibility, acceptability, and affordability have limited the uptake of these interventions in many communities [2]. HIV vaccines have the potential to address these gaps in HIV prevention. Because the development of an infection-blocking vaccine for HIV has proven elusive, alternative vaccine mechanisms are under consideration, including non-sterilizing disease-modifying vaccines [3]. These have the potential to improve individual outcomes by reducing disease severity in infected vaccinees and potentially reduce population-level HIV burden by lowering viral load and subsequent per-act transmission risks [4,5]. No approved vaccine in humans is exclusively disease-modifying, but many have disease-modifying effects in addition to protecting against infection, including SARS-CoV-2 vaccines [6–10], seasonal flu vaccines [11], and the bacillus Calmette-Guérin vaccine for tuberculosis [12], all of which can reduce disease severity among vaccinees. Several promising experiments with HIV vaccines in animal models have demonstrated a disease-modifying effect, with experimental vaccines that lower initial viremia or set point viral load (SPVL) of vaccinated subjects following challenge with SIV [13–17]. These vaccine candidates primarily induced T-cell responses.
A key determinant of the public health burden of HIV is pathogen virulence, a trait which is positively associated with severity of illness and with transmission [18]. Since SPVL, the plasma viral load at the beginning of the chronic stage, strongly affects progression to AIDS, evolutionary biologists have used this as a proxy for virulence. HIV SPVL, which is partially heritable from infector to infectee [19], is thought to exhibit a classic virulence-transmission trade-off, whereby an intermediate level of pathogen virulence maximizes the cumulative chance of transmission by producing enough viral copies to have sufficient per-exposure likelihood of transmission without prematurely killing the host and cutting future transmission opportunities short [18]. Given the evolutionary trade-off between transmission and host mortality, a disease-modifying vaccine may create an environment conducive to the evolution of higher HIV virulence by reducing the fitness cost of high SPVL and providing a longer span over which high virulence viruses may be transmitted. Figure 1 provides a conceptual schematic of the optimal virulence in the balance of transmission and host survival.
Figure 1. Optimal virulence as a product of host survival and transmission probability.
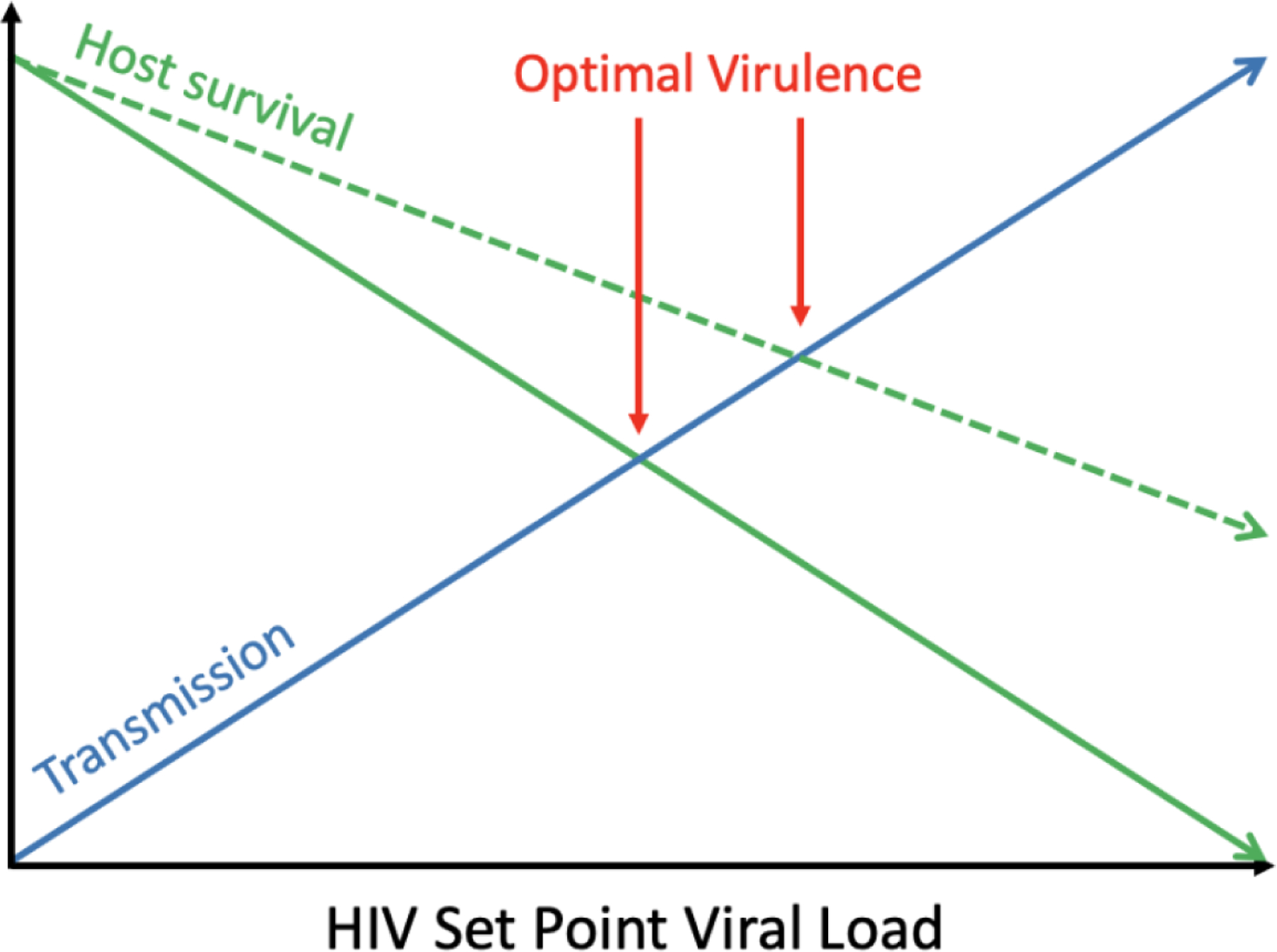
While transmission probability increases with higher SPVL (blue line), host survival decreases (green line). In this conceptual figure, fitness is optimized when these lines intersect. We hypothesize that a disease modifying vaccine that extends host survival (dotted green line) could select for viruses with higher SPVLs.
Mathematical modeling provides the means to evaluate potential evolutionary outcomes of novel therapeutics prior to their introduction in human populations. Recently, a compartmental evo-epidemiological model was used to examine the bounds of vaccine-driven virulence evolution for SARS-CoV-2, demonstrating that virulence evolution was a theoretical possibility, but only when vaccine effect on disease severity was not robust or long-lasting [20]. In the field of HIV prevention, the application of modeling is particularly important given the diversity and lability of the HIV-1 genome, and the evidence for viral evolution in response to innate immunity and human interventions [21–25].
We have previously used a dynamic agent-based network model, Evonet, to explore the evolution of resistance to a vaccine similar to RV-144, a preventative HIV-1 vaccine that demonstrated partial efficacy in a phase III trial [26]. In that study, selection for vaccine resistant strains diminished the efficacy of the vaccine over time. In the present study, we extend this model to study the evolution of HIV-1 virulence in response to a potential disease-modifying vaccine. To our knowledge, this is the first study to evaluate the potential evolution of virulence under a disease-modifying vaccine for HIV-1.
Methods
Base Model
We added a disease-modifying vaccine intervention component to a previously developed agent-based dynamic network model [26–29]. The model, Evonet, is a publicly available modifiable evolutionary framework built upon the dynamic network simulation package, EpiModel [30]. Additional details and information about Evonet can be accessed at https://github.com/EvoNetHIV. The baseline model simulates HIV-1 transmission in a network that approximates epidemic characteristics among men who have sex with men (MSM) in the United States, and is parameterized using validated behavioral data from this population. HIV-1 transmission is dependent on sexual network structure, the timing and frequency of sexual acts within those networks, and the use of condoms, ART and/or PrEP. To approximate ART use in US MSM, we modeled treatment scenarios wherein 70% of HIV-positive agents initiated ART an average of one year after infection, resulting in 65% of HIV-positive agents being virologically suppressed, a level achieved at year zero and maintained throughout the remaining simulations. To assess viral evolution separately from any possible interaction from ART, we also ran all scenarios with no ART (see Supplement).
Newly infected agents were assigned a SPVL that was partly based on the SPVL of the HIV-infected sexual contact. The genetic heritability of SPVL was modeled by assuming the average proportion of the variation in SPVL determined by donor SPVL was 36% [19,31]. Consistent with clinical findings, individual peak and daily viral loads were determined by the agent’s SPVL [33], as was the daily probability of moving through the four CD4+ count categories towards death due to AIDS [34]. Higher SPVLs resulted in higher daily viral loads, which increased per-act probabilities of transmission following contacts between sero-discordant agents.
Determinants of SPVL
Each agent’s phenotypic SPVL (the SPVL that one would measure in a patient) was the sum of: that agent’s underlying genotypic SPVL, which was a heritable property of the virus that infected the agent; a random, host-specific effect with mean zero that was determined at the time of infection; and the effect of vaccination on phenotypic SPVL. Upon infection, the recipient’s genotypic SPVL was set to the donor’s genotypic SPVL plus a random within-host evolution term with mean zero that allowed for the introduction of viruses with different genotypic SPVLs. To facilitate discussions about SPVLs in vaccinated agents, we use the term intrinsic SPVL to signify what the phenotypic SPVL would be in the absence of vaccination. For ease of computation, however, our analysis assessing change in the underlying SPVL used genotypic SPVL as a proxy for intrinsic SPVL, which should be approximately equal since we focus on population averages. This is because the host-specific and within-host evolution terms are both normal random numbers with a mean of zero, so the expected (though not necessarily the observed) values for the phenotypic SPVL in the absence of vaccination and the genotypic SPVL in the presence or absence of vaccination equal the donor’s genotypic SPVL.
Vaccine module
When a vaccinated agent became infected, the vaccine temporarily reduced that agent’s phenotypic SPVL by either 0.5, 1.0, 1.5, or 2.0 log10 copies/ml, depending on the vaccine scenario. Vaccine efficacy was assumed to last 3 years, during which the infected vaccinated agent’s disease progression and transmission rates were determined by this temporary phenotypic SPVL. After the end of vaccine efficacy, the viral load and rates of CD4 decline reverted to what they would be in the absence of a vaccine (i.e., they were determined by that agent’s intrinsic SPVL). Since we focused on disease modifying vaccines, vaccination did not decrease the risk of seroconversion for the vaccinated individual. Table 1 contains the key vaccine-related parameters used in the analyses.
Table 1.
Key vaccine parameters
| Parameter | Description | Value |
|---|---|---|
| Prevalence | Prevalence of HIV-1 at baseline | 10% |
| Coverage | Percentage of total population who are vaccinated | 10%, 30%, 50%, 70%, or 90% |
| Vaccine decrement | Difference between genotypic and temporary phenotypic SPVL due to vaccine | 0.5, 1.0, 1.5, 2.0 log10 copies/ml |
| Efficacy duration | Length of time before vaccinated agents lose vaccine effect | 3 years |
| Vaccination campaign duration | Length of time for which vaccination coverage is maintained | 30 years |
| Heritability | Proportion of new infectee’s SPVL attributable to the SPVL of the donor, vs random chance | 36% |
Evolution in the model
Among agents infected at baseline, genotypic SPVLs were assigned randomly using a normal distribution with a mean of 4.5 log10 copies/ml [35]. When the model began and agents started transmitting to susceptible agents, the donor’s genotypic SPVL contributed, on average, 36% to the infectee’s SPVL, with the random random/environmental/mutational factors contributing the remaining 64%. Because vaccination temporarily reduced viral load, infected vaccinees experienced lower disease severity and transmitted to others at a lower rate. Because we relied on a transmission function in which transmission rates increased exponentially with log SPVL, only those vaccinees with high SPVLs were likely to transmit the virus to others [36]. For example, in a model with a vaccine reducing phenotypic SPVL by 2.0 log10 copies/ml, an infected agent with an intrinsic SPVL of 5.0 log10 copies/ml would have initial disease progression rates and risks of transmitting to others consistent with a SPVL of 3.0 log10 copies/ml, whereas a similar person with an intrinsic SPVL of 4.0 log10 copies/ml would have progression and transmission rates consistent with a SPVL of 2.0 log10 copies/ml. When either of these individuals came into contact with an uninfected agent, transmission was more likely to occur by the individual with the higher intrinsic SPVL.
Modeling strategy
We compared the population SPVL between the base model without vaccination and the four vaccines that caused either a 0.5 log10, 1.0 log10, 1.5 log10, or 2.0 log10 copies/ml reduction in the phenotypic SPVL of infected vaccinees. We began the model with a 10-year burn-in period with no vaccination. Vaccination began at model year 0 and reached the designated coverage level within five years, after which coverage was maintained for 25 years, followed by 20 years of no vaccine to explore the duration of any lingering vaccine-driven changes in SPVL. Our primary analysis focused on a vaccination coverage of 90% of the total population. In sensitivity analyses, we examined vaccine coverage levels between 10% and 90% at 20% increments. Vaccine efficacy was assumed to last 3 years. Any uninfected agent who was never vaccinated or whose previous vaccine was no longer in effect was eligible for vaccination. All simulations began with a population of 10,000 agents, 10% of whom were infected with HIV-1 to approximate the prevalence of HIV-1 in United States MSM [37]. Each scenario was run with 50 replicates, with results presented as a mean of values for all replicates. We included a scenario with a vaccine effect of 1log10 SPVL reduction at 60% coverage to compare with a 2log10 effect vaccine at 30% coverage, creating the same mean vaccine effect over the whole population with differing individual vaccine effects to explore whether degree of virulence evolution is comparable (supplement). All experiments were run with Evonet in R version 4.2.0. The total number of simulations was 4 vaccine scenarios * 5 vaccine coverage levels * 50 replicates, with one no-vaccine control, and a preventive vaccine comparison.
Analysis
We described the occurrence of evolution by comparing the genotypic SPVL of new infections over time between vaccine and no-vaccine scenarios. Selection for viruses with higher SPVLs can influence overall transmission dynamics, and we explored these by qualitatively comparing epidemic characteristics (incidence and prevalence of HIV-1) between vaccine and no-vaccine scenarios. We also calculated mean infections averted in each vaccination scenario using the cumulative incidence of the vaccine runs compared to the no-vaccine scenario. We compared mean survival time among unvaccinated agents infected after 30 years of the vaccine campaign to agents infected at the same time in the no-vaccine control using a Chi-squared test. We provide 95% confidence intervals based on a normal distribution for these estimates.
Results
We simulated four vaccination scenarios that produce a 0.5 log10, 1.0 log10, 1.5 log10, or 2.0 log10 copies/ml reduction in SPVL for infected vaccinees, all at 90% coverage; and with a no-vaccination comparison scenario (Figure 2).
Figure 2. Mean phenotypic SPVL of infected agents over time under four vaccine scenarios.
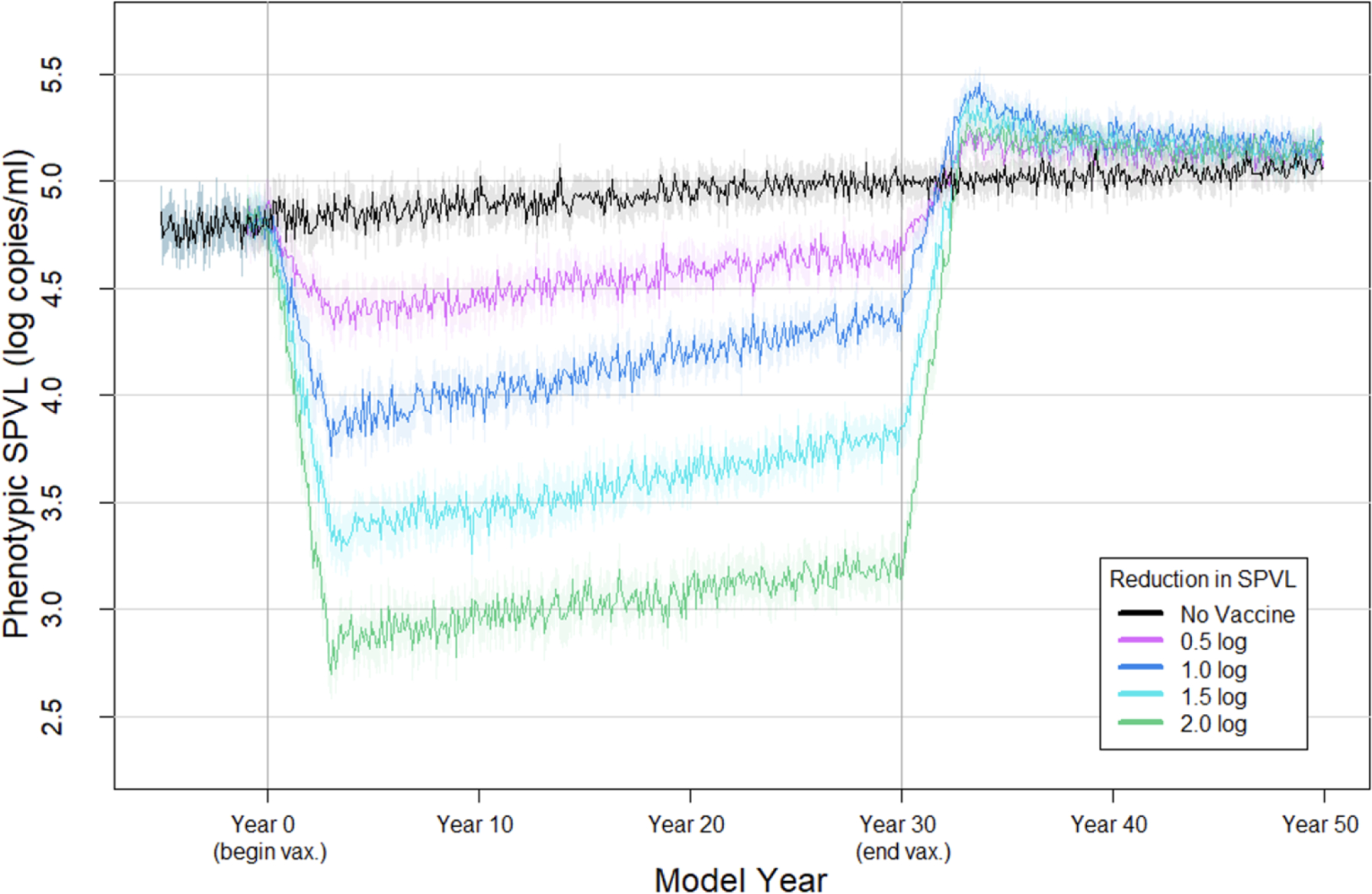
Each line represents the mean phenotypic SPVL of newly infected agents at each time point (every 30 days) in a no vaccine scenario or with 90% coverage of a vaccine that reduces SPVL by 0.5, 1.0, 1.5, or 2.0 log10 copies/ml, over 50 replicates. Shaded area reflects the 95% confidence interval.
Changes in SPVL by vaccine effect and coverage level
Figure 3 shows the mean and standard deviation of the incident genotypic SPVL from 50 replicate simulations for each of these scenarios. By 30 years after rollout, changes in genotypic SPVL were observed for all four vaccines. Across the four vaccine scenarios, genotypic SPVL after vaccine roll-out followed a non-monotonic pattern, with the highest mean genotypic SPVL observed in the scenario with an intermediate vaccine effect (1.0 log10 vaccine). At year 30 of vaccine rollout, the mean genotypic SPVLs of newly infected agents in the no vaccine scenario, the 0.5 log10 vaccine, the 1.0 log10 vaccine, the 1.5 log10 vaccine, and the 2 log10 vaccine scenarios were 4.99 log10 copies/ml (95%CI: 4.91, 5.08), 5.19 log10 copies/ml (95%CI: 5.11, 5.28), 5.40 log10 copies/ml (95%CI: 5.31, 5.49), 5.29 log10 copies/ml (95%CI: 5.21, 5.38), and 5.22 log10 copies/ml (95%CI: 5.12, 5.30), respectively (see Figure 3 inset).
Figure 3. Mean genotypic SPVL of new infections over time under 90% coverage of a disease modifying vaccine with varying effects on phenotypic SPVL.
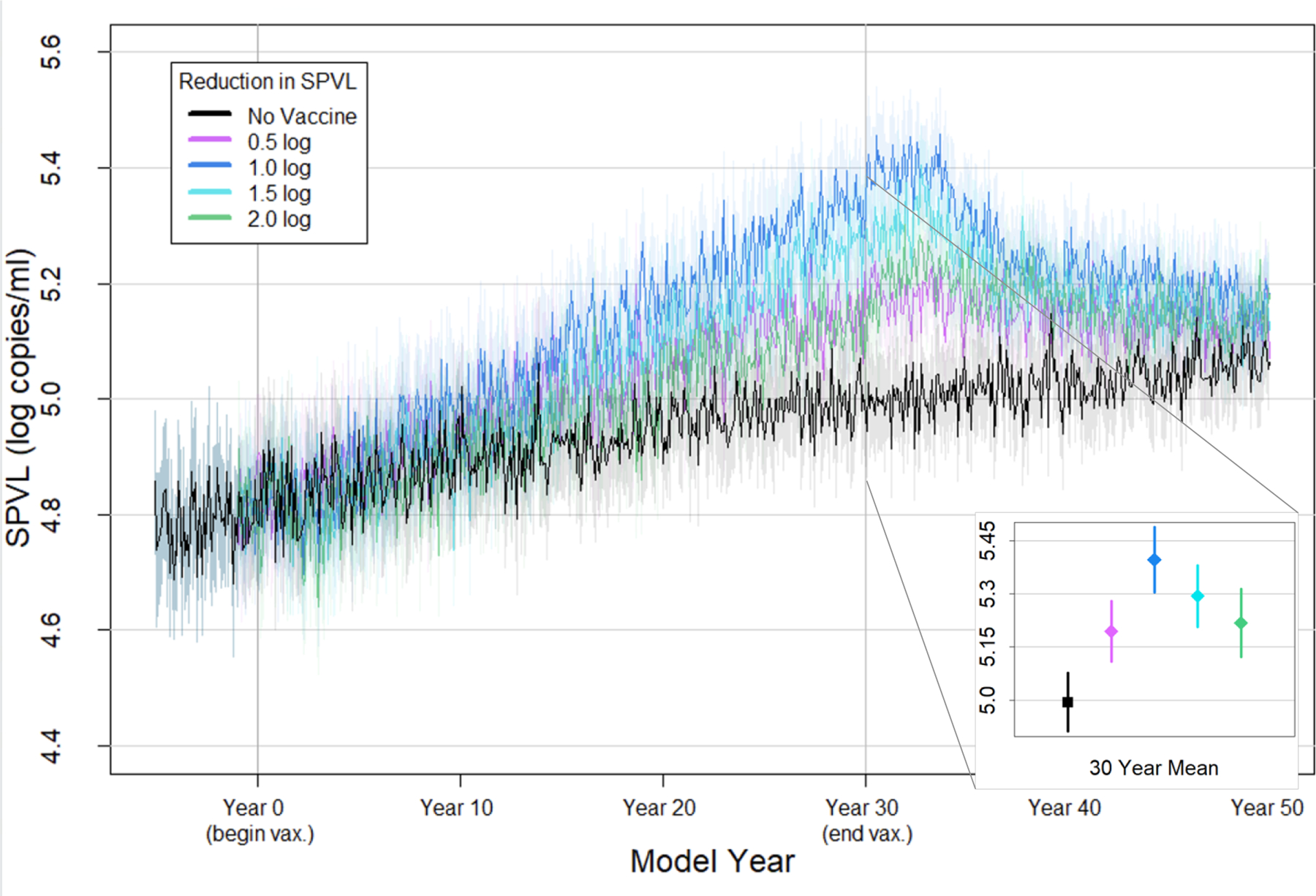
Mean genotypic SPVL of all new infections at each time point (every 30 days) over 50 replicates. Shaded area reflects the 95% confidence interval.
Inset: focus on mean genotypic SPVL of new infections during year 30 after vaccine rollout, with bars indicating the 95% confidence interval
Sensitivity analyses - varying vaccine coverage
We next examine the impact of vaccination with a disease-modifying vaccine under different coverage levels (10–90%). Figure 4 presents these results for the scenario with the greatest degree of vaccine-driven evolution (phenotypic SPVL reduction = 1.0 log10 copies/ml); results for the other vaccine scenarios are presented in the supplement. As expected, higher coverage resulted in greater divergence in genotypic SPVL between vaccine and no-vaccine scenarios, though coverage of vaccine at 20% still resulted in elevated genotypic SPVL by year 30.
Figure 4. Average genotypic SPVL under a 1log10 vaccine campaign with varying coverage levels.
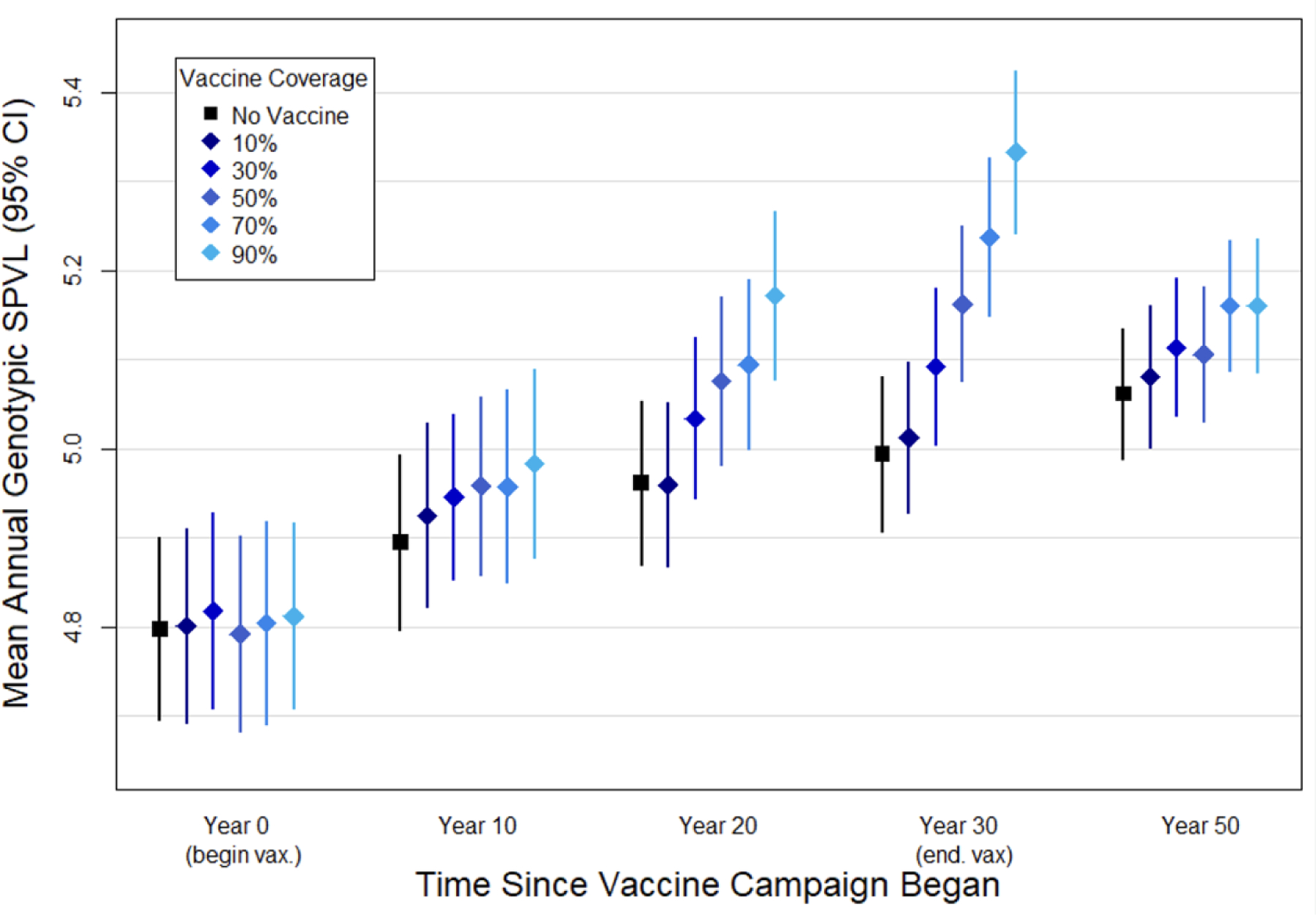
Points represent the mean genotypic SPVL at the time point indicated, showing a no-vaccine scenario and varying coverage levels of a vaccine that reduces phenotypic SPVL by 1.0 log10 copies/ml, over fifty replicates. Bars represent the 95% confidence interval.
Public health implications - incidence
Figure 5 depicts HIV-1 incidence over time in the no-vaccine scenario compared with 10–90% coverage of a 1.0 log10 vaccine. In the no-vaccine scenario, HIV-1 incidence initially rises steadily and begins to level out at one case per 100 person-years by year 20. In coverage scenarios of 50% and greater, vaccination results in higher incidence than in the no-vaccine scenario 30 years after vaccine rollout. With 90% vaccine coverage, incidence rose as high as 1.4 cases per 100 person-years 33 years after vaccine rollout. For the other three vaccines, the increase in incidence due to vaccine-driven evolution was less marked (figures shown in supplement). The disease modifying vaccine does not directly prevent infection in those taking it but is still expected to have indirect effects on incidence by reducing the risk of onward transmission by an infected vaccinee because of the decreased phenotypic SPVL. HIV-1 incidence does decrease following vaccination, but this beneficial effect was eroded by approximately 20 years after the start of a vaccine campaign at all vaccine coverage levels, due to increased transmission caused by selection for higher intrinsic SPVLs.
Figure 5. HIV-1 incidence per 100 person-years with a 1.0 log10 vaccine at varying coverage levels.
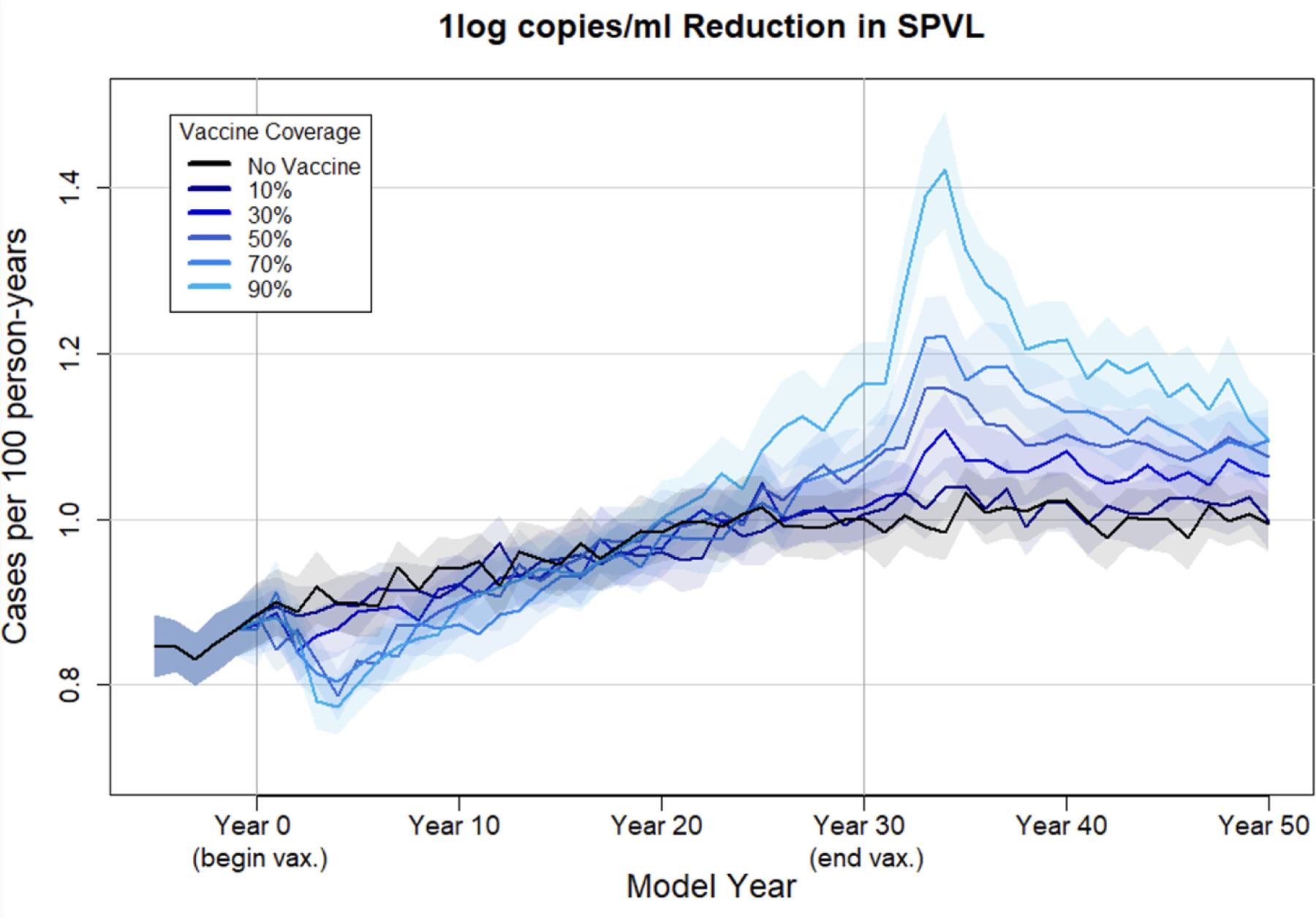
Mean incidence (per 100 person-years) at each time point (every 30 days) over 50 replicates under varying coverage levels of a vaccine that reduces phenotypic SPVL by 1.0 log10 copies/ml. Shaded area reflects the 95% confidence interval.
Public health implications - prevalence
We also examined HIV-1 prevalence to demonstrate the combined effect of changes in HIV influence as well as the increased longevity among agents due to the disease-modifying vaccine effects. Figure 6 depicts HIV-1 prevalence over time in the no-vaccine scenario compared with 10–90% coverage of a 1.0 log10 vaccine. Vaccination briefly decreased HIV-1 prevalence for a 1.0 log10 vaccine at most coverage levels due to the decreased chances of infection with lower phenotypic SPVLs. By 20 years after the start of the vaccine campaign, however, prevalence in the vaccine scenarios began to rise above that in the no-vaccine scenarios. The difference in prevalence in the high coverage vaccine scenarios was fairly small, and was most marked for the 90% coverage level. In the no-vaccine scenario, prevalence reaches equilibrium at approximately 13%. In the higher coverage vaccine scenarios, prevalence begins to increase 15 years after vaccination begins, exceeding 14% in the 90% coverage scenario. For the other three vaccines, the increase in prevalence due to vaccine-driven evolution was less evident (figures shown in supplement).
Figure 6. HIV-1 prevalence with a 1.0 log10 vaccine at varying coverage levels.
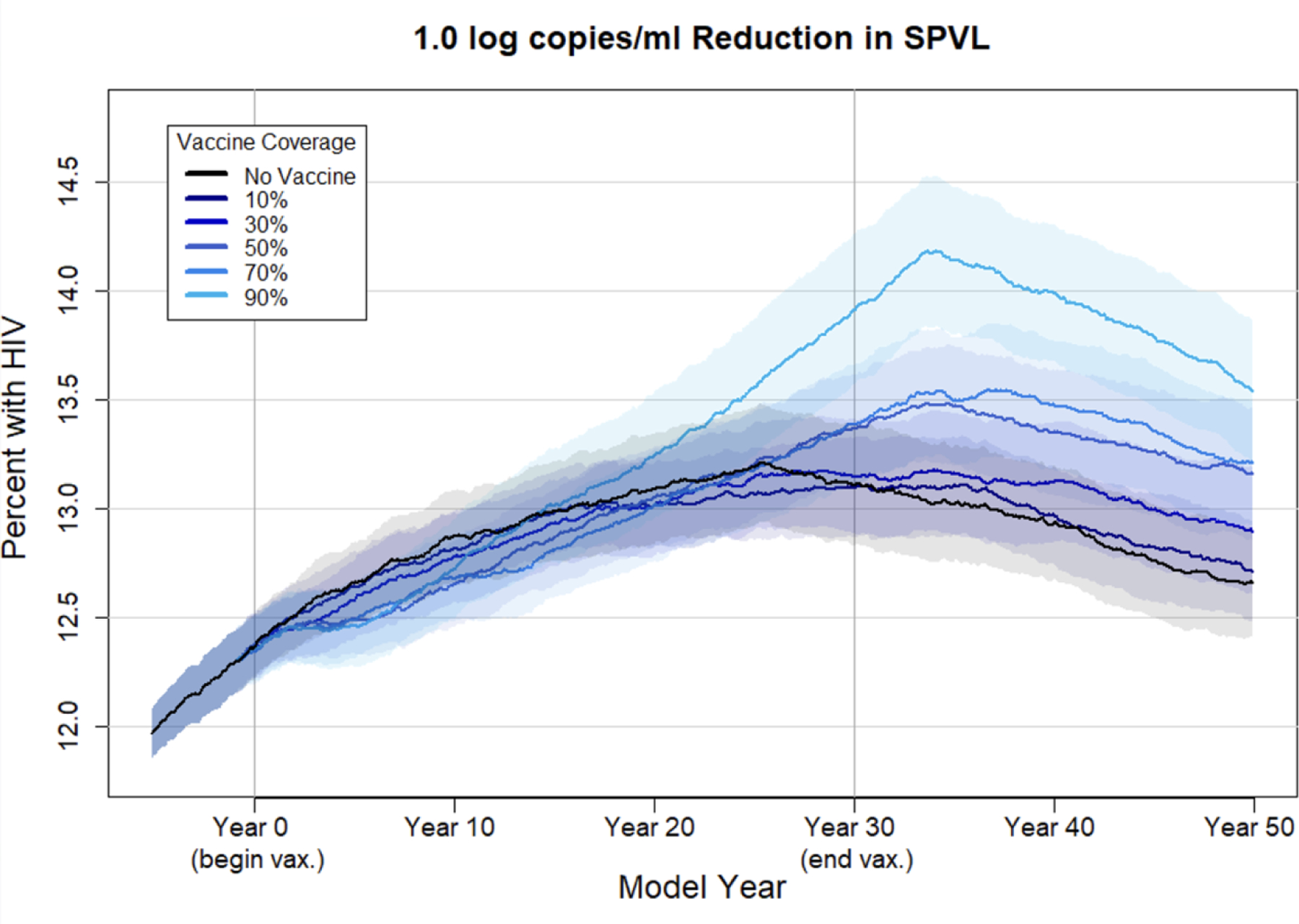
Mean HIV-1 prevalence at each time point (every 30 days) over 50 replicates under varying coverage levels of a vaccine that reduces phenotypic SPVL by 1.0 log10 copies/ml. Shaded area reflects the 95% confidence interval.
Public health implications - infections averted
The percent of cumulative infections averted by the vaccine relative to the no-vaccine scenario decreased over time as evolution of higher intrinsic SPVLs began to outweigh the initial benefits of the 1.0 log10 vaccine. This trend was the most drastic for the 90% coverage scenario: by 10, 20, and 30 years after the start of the vaccine campaign, the percent of infections averted was 7.41% (95%CI: 4.9 – 9.9%), 3.91% (95%CI: 1.4 – 6.5%), and −0.95% (95%CI: −3.7 – 1.81%), respectively, compared to the no-vaccine scenario, and by 40 years after rollout (i.e., 10 years after the end of the vaccine campaign) it was significantly negative at −7.83% (95%CI: −10.7 - −5.0%). Figure 7 depicts the proportion of infections averted in the vaccine scenarios for a 1.0 log10 difference vaccine at coverage levels between 10 and 90% of the population in the years post-vaccination. Proportion of deaths averted follows a similar pattern (results not shown).
Figure 7. Proportion of infections averted due to a 1.0 log10 vaccine at varying coverage levels.
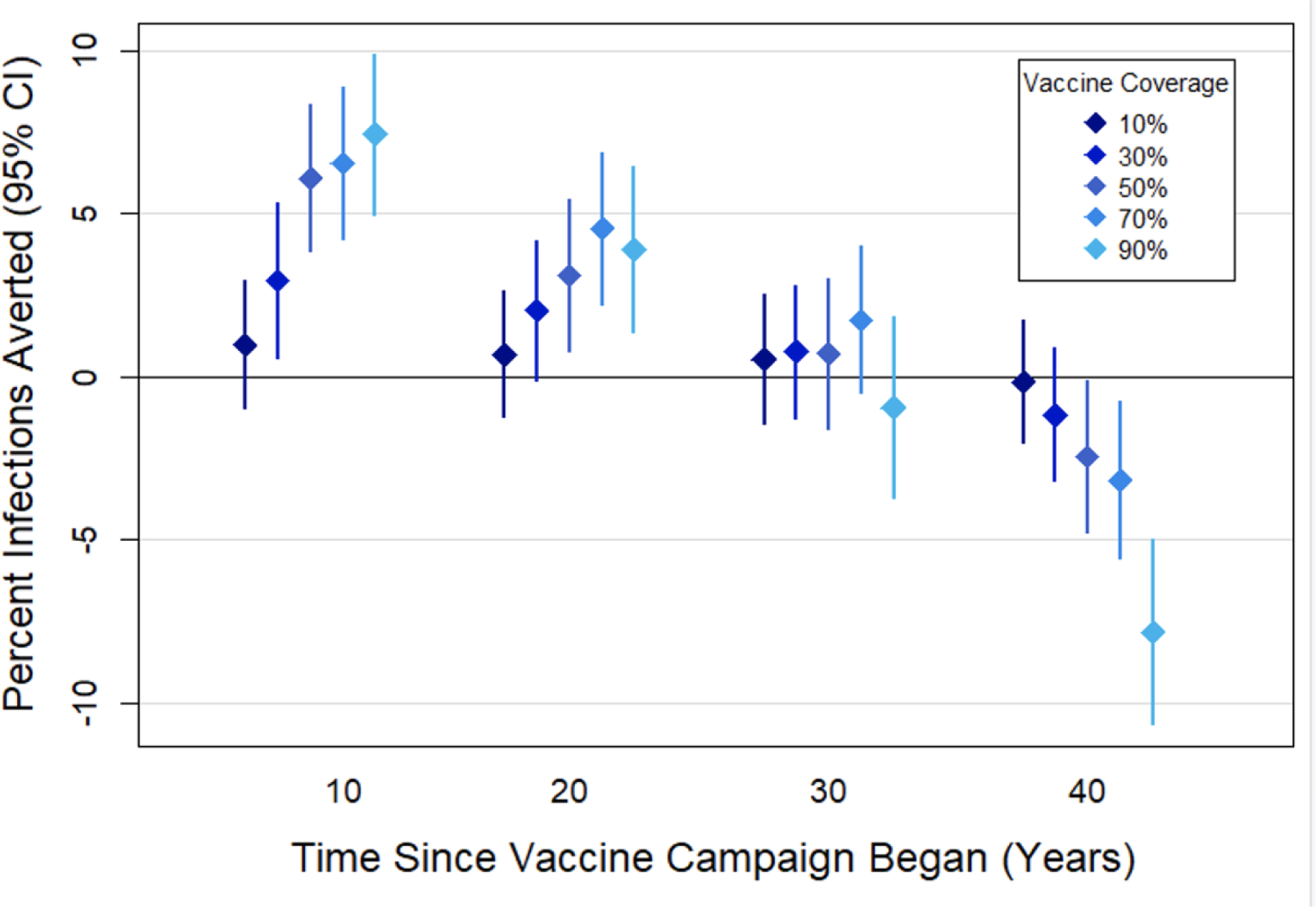
The mean proportion of cumulative infections averted by the vaccine relative to the no-vaccine scenario at varying coverage levels of a vaccine that reduced phenotypic SPVL by 1.0 log10 copies/ml. Bars represent 95% confidence intervals.
Public health implications - survival among unvaccinated
Vaccine-driven virulence evolution has the potential to impact HIV-1 mortality and survival among people who were not vaccinated at the time of infection. As the mean genotypic SPVL increases over time, agents who are unvaccinated at infection will experience more rapid decline associated with higher SPVLs while vaccinated agents are protected from severe disease. Too few agents are unvaccinated at infection in the 90% coverage scenarios to reliably ascertain survival differences; therefore, we used the 70% coverage scenarios for this comparison (Figure 8). In the first ten years of a vaccine campaign with 70% coverage of a vaccine that reduces phenotypic SPVL by 1.0 log10 copies/ml, the median survival time of an unvaccinated person who became infected (9.98 years) is not statistically different from the survival time of an infected person in the no-vaccine scenario: 10.2 years (Chi squared= 0.3, 1 df, p= 0.6). By the last ten years of the vaccine campaign, however, median survival time of an unvaccinated infectee is 7.39 years (95%CI: 7.19, 7.62), which differs significantly from the no-vaccine survival time of 9.31 years (95%CI: 8.09, 10.8) (Chi squared= 8.3, 1 df, p= 0.004).
Figure 8. Kaplan-Meier survival curves for agents unvaccinated at infection in the first ten years of the vaccine campaign compared to the final 10 years.
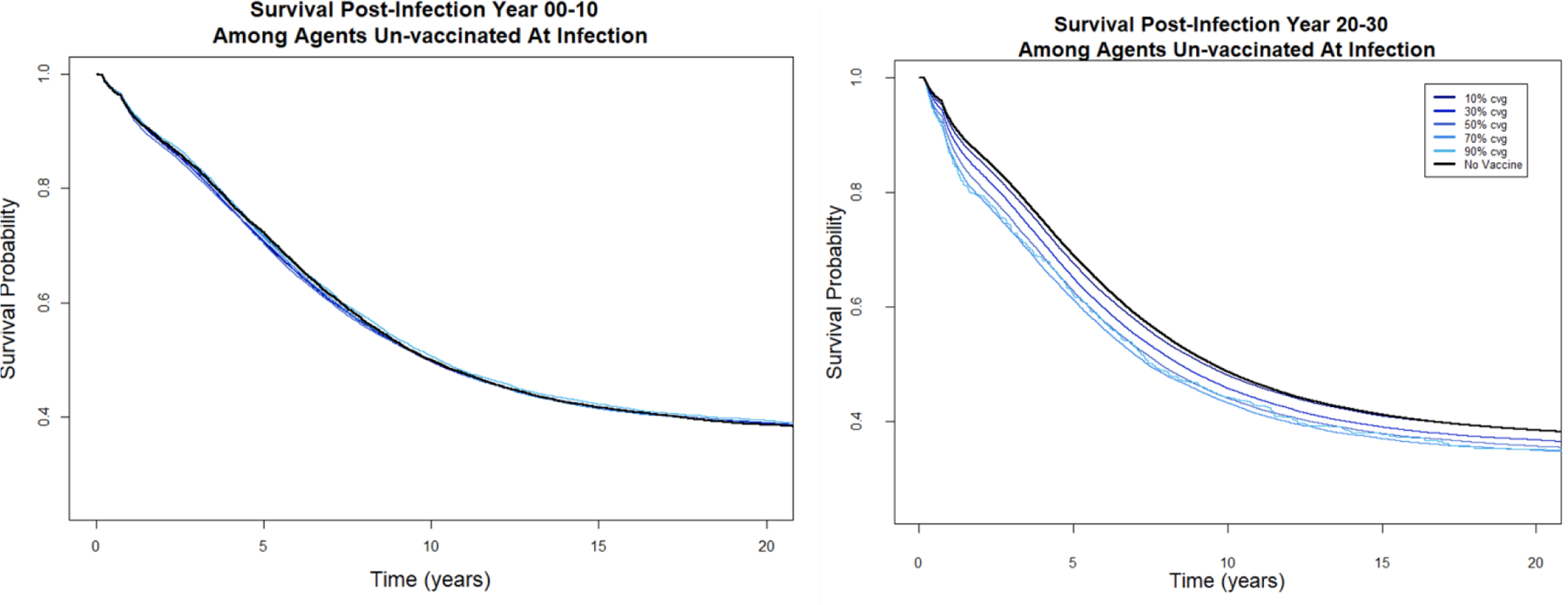
First plot: survival among agents who became infected between year 0 and 10, with each line (overlapping and not distinguishable) representing survival under different coverage levels of a 1.0 log10 disease modifying vaccine. Second plot: survival among agents who became infected between year 20 and 30, with each line representing survival under different coverage levels of a 1.0 log10 disease modifying vaccine.
Discussion
While we expect that a vaccine that prevents HIV-1 infection should be broadly beneficial to public health, we hypothesized that a disease-modifying vaccine may have contradictory effects: initial reductions in incidence and mortality due to viral load reduction, followed by steady increases in mortality and viral transmission as the vaccine selects for more virulent, high-SPVL viruses. This selection occurs because vaccination lowers the transmissibility of low-SPVL infections while extending the lifespan of agents infected with high-SPVL viruses. We simulated the evolutionary impacts of disease-modifying vaccines with varied effects in an epidemic scenario based on the United States MSM HIV-1 epidemic using different vaccine coverage levels. In all scenarios, the average incident genotypic SPVL of agents infected in the vaccine scenarios rose over time following vaccine rollout, while the same measure in the no-vaccine scenario increased much more gradually as expected for an exponential transmission function. The increased intrinsic SPVL observed was non-monotonic, and was greatest for vaccines that reduced the phenotypic or experienced SPVL of infected agents by intermediate amounts, and in scenarios with higher vaccine coverage.
Our findings are consistent with previous modeling studies of virulence evolution from disease-modifying vaccines for theoretical scenarios or for specific pathogens besides HIV. In a statistical model of a theoretical disease-modifying (or “anti-growth”) vaccine, Gandon and coauthors demonstrated that under certain conditions virulence can stabilize at higher levels following vaccine rollout [38]. Using simulations of a disease-modifying malaria vaccine, this group later confirmed that virulence can evolve under vaccination in an endemic scenario, and that this can result in higher mortality [39]. Another malaria vaccine paper [40] described a statistical model for a disease-modifying malaria vaccine, and noted that secondary cases may also increase as a result of prolonged infectious period. Our model differs substantially from these studies of disease-modifying vaccines not only in pathogen considered, but in methodology. While theirs were statistical or compartmental, ours was agent-based and stochastic, suggesting that the shared finding of long-term increases in virulence may be robust to differing mathematical approaches.
Several studies have previously examined the possible public health impacts of a future disease-modifying HIV vaccine. None of these included the potential for virulence evolution, likely because most were published prior to much of the current understanding of the genetic heritability of SPVL. Two studies examined the impacts of disease-modifying vaccines on the epidemiology of HIV-1 in South Africa [41] or Brazil [42], and found that population-level benefits may be achieved if the vaccines reduce transmissibility indirectly by lowering viral load. Two studies modeled the public health impacts of disease modifying vaccines, but also incorporated the potential for risk compensation where vaccinated individuals engage in riskier sexual behavior because of their perceived immunity [43,44]. One of these [44] also incorporated the chance that the virus could mutate to escape the vaccine effect. Without consideration of virulence evolution, previous studies of disease modifying vaccines have likely consistently predicted overall positive public health benefits from vaccination. While our study did not include risk compensation, which should further reduce the overall public-health benefit of the vaccine, we still found conditions under which a disease-modifying HIV-1 vaccine could be detrimental to public health over the long term.
In our evaluation, higher virulence evolution was observed under specific conditions that are not likely to occur in a pragmatic vaccine rollout scenario, unless high levels of vaccine coverage are achieved. For instance, at vaccine coverage levels comparable to existing HIV prevention methods in North America, such as PrEP (around 30% of the eligible population) [45], HIV virulence was not statistically significantly greater than the no-vaccine control by year 30. Indeed, the additional preventive coverage afforded by people who were adherent on PrEP, many of whom would also be vaccinated, would further reduce chances for evolution to occur by effectively reducing the pool of susceptible individuals.
We focused on vaccination in a North American MSM population, but other populations may have important considerations that warrant further exploration. In some Sub-Saharan African countries, for instance, the HIV epidemic is characterized by primarily heterosexual transmission, as well as a much more heterogenous population with key risk groups and different network dynamics [46]. Because the per-act HIV transmission risk associated with vaginal intercourse is lower than with anal intercourse [47], the chances a vaccinated infected individual with a lowered phenotypic SPVL transmits may be so low-level that virulence evolution is limited in a heterosexual network. However, in higher-risk sub-groups with more frequent exposure and higher HIV prevalence such a commercial sex workers [48], it may be possible that transmission is frequent enough for virulence evolution to occur. Future work should explore these potential dynamics to add to our understanding of the population health implications of a disease modifying vaccine with potential for virulence evolution in other populations.
Our study is subject to several caveats and limitations. To maintain our focus on key evolutionary tradeoffs, we ignored a large number of immunological and virological factors (e.g., the emergence of X4 variants, immunological exhaustion, and microbial translocation) that affect HIV-1 progression in ways that do not depend directly on viral load. Our model, likewise, ignores the possibility that viruses evolve to escape vaccine-inducing immune responses in addition to evolving to have different SPVLs. While we could in principle have incorporated these and other details in this model, we felt that attempting to add too many of details at this early stage (i.e., at a time when there are not yet any DMVs in use) would have interfered with our ability to communicate the broader concepts explored in this paper. Once a specific disease modifying vaccine with known properties starts to roll out, it may become more important to incorporate these kinds of details into our model.
A more specific caveat is that our study relies on a transmission rate function [36], in which the probability of transmission increases 2.89-fold for each 10-fold increase in viral load over the full range of viral loads. While this transmission rate function is based on detailed statistical analyses of curated transmission rate data, their underlying dataset included only a few individuals with viral loads greater than 6.0. Thus, it is hard to be fully confident that an agent with a SPVL of 6.5, for example, will be 2.89-fold more infectious than a similar agent with a SPVL of 5.5, as one would infer from their transmission rate function. Ethical reasons make it difficult to collect more data of the sort analyzed by Hughes et al. (i.e., data on transmission rates in untreated, sero-discordant couples) now that ART is widely available. Thus, we are left to make inferences based on what is arguably the best available transmission rate function, while acknowledging that there is limited data about transmission rates in people with very high SPVLs. However, we note that a recent study of highly virulent “VB” strains circulating in Europe [49] supports the idea that very high SPVL viruses have substantially higher per-act transmission rates. We reran the main experiments using a different proposed transmission function [31], and observed that vaccination did result in evolution of higher intrinsic SPVLs but retained an overall health benefit (see supplement).
Finally, there are several epidemiological details that could affect our quantitative conclusions. For example, we have assumed that vaccine effects last for three years and that the vaccine is gradually rolled out over a five-year period, after which time coverage is maintained at a constant level. This may not capture the heterogeneity and inconsistency of a vaccine roll out in an actual population, but we do not anticipate that this would substantially affect the evolutionary environment we studied. We also used a public health definition of vaccine coverage, measured as a percentage of the total population. As a result, our higher coverage scenarios had lower than targeted coverage because high HIV-1 prevalence limited the number of eligible individuals. Lastly, we did not examine risk compensation, whereby vaccinated individuals accept more behavioral risks due to a perceived protection or invulnerability from vaccination, but this risk compensation may serve to further lessen the overall benefits of our hypothetical vaccine [50].
Conclusions
Viral evolution represents a substantial challenge to the development of vaccines for the prevention and management of HIV-1. We have demonstrated that HIV-1 can evolve to become more virulent in the case of a disease-modifying vaccine. Particularly at intermediate levels of reduction in disease severity, vaccine-driven virulence evolution accelerated the HIV-1 epidemic over time despite temporary reductions in disease severity. To our knowledge this is the first modeling study of HIV-1 viral evolution in response to a disease-modifying vaccine. Virulence evolution should be an important consideration in the process of designing future vaccines.
Supplementary Material
Acknowledgements
This work was supported by the US National Institutes of Health [grant numbers R01-AI108490, R01-GM125440]. The funders had no role in the study design; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the article for publication. Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, P2C HD042828, to the Center for Studies in Demography & Ecology at the University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Vermund SH. Global HIV Epidemiology: A Guide for Strategies in Prevention and Care. Curr HIV/AIDS Rep 2014;11:93–8. 10.1007/s11904-014-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Corey L, Gray GE, Buchbinder SP. The aspirational necessity of HIV prevention. J Int AIDS Soc 2019;22:e25289. 10.1002/jia2.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Engl J Med 2007;356:2073–81. 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- [4].Van Braeckel E, Leroux-Roels G. HIV vaccines: can CD4+ T cells be of help? Hum Vaccines Immunother 2012;8:1795–8. 10.4161/hv.21760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gupta SB, Jacobson LP, Margolick JB, Rinaldo CR, Phair JP, Jamieson BD, et al. Estimating the benefit of an HIV-1 vaccine that reduces viral load set point. J Infect Dis 2007;195:546–50. 10.1086/510909. [DOI] [PubMed] [Google Scholar]
- [6].Lipsitch M, Dean NE. Understanding COVID-19 vaccine efficacy. Science 2020. 10.1126/science.abe5938. [DOI] [PubMed]
- [7].Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, et al. Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19-Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance - Nine States, June-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1291–3. 10.15585/mmwr.mm7037e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet Lond Engl 2021;397:1819–29. 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Giudice GD, et al. Influenza vaccine immunology. Immunol Rev 2011;239:167–77. 10.1111/j.1600-065X.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- [12].Colditz GA. Efficacy of BCG Vaccine in the Prevention of Tuberculosis: Meta-analysis of the Published Literature. JAMA 1994;271:698. 10.1001/jama.1994.03510330076038. [DOI] [PubMed] [Google Scholar]
- [13].Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol 2006;80:5875–85. 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 2006;312:1530–3. 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Polacino PS, Stallard V, Klaniecki JE, Pennathur S, Montefiori DC, Langlois AJ, et al. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in Macaques. J Virol 1999;73:8201–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine 2002;20:1949–55. 10.1016/s0264-410x(02)00076-2. [DOI] [PubMed] [Google Scholar]
- [17].Mattapallil JJ, Douek DC, Buckler-White A, Montefiori D, Letvin NL, Nabel GJ, et al. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J Exp Med 2006;203:1533–41. 10.1084/jem.20060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shirreff G, Pellis L, Laeyendecker O, Fraser C. Transmission Selects for HIV-1 Strains of Intermediate Virulence: A Modelling Approach. PLOS Comput Biol 2011;7:e1002185. 10.1371/journal.pcbi.1002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Müller V, Fraser C, Herbeck JT. A Strong Case for Viral Genetic Factors in HIV Virulence. Viruses 2011;3:204–16. 10.3390/v3030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miller IF, Metcalf CJE. Assessing the risk of vaccine-driven virulence evolution in SARS-CoV-2. R Soc Open Sci n.d.;9:211021. 10.1098/rsos.211021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Edlefsen PT, Rolland M, Hertz T, Tovanabutra S, Gartland AJ, deCamp AC, et al. Comprehensive Sieve Analysis of Breakthrough HIV-1 Sequences in the RV144 Vaccine Efficacy Trial. PLOS Comput Biol 2015;11:e1003973. 10.1371/journal.pcbi.1003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012;490:417–20. 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Boutwell CL, Rolland MM, Herbeck JT, Mullins JI, Allen TM. Viral Evolution and Escape during Acute HIV-1 Infection. J Infect Dis 2010;202:S309–14. 10.1086/655653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Allen TM, Altfeld M, Geer SC, Kalife ET, Moore C, O’Sullivan KM, et al. Selective Escape from CD8+ T-Cell Responses Represents a Major Driving Force of Human Immunodeficiency Virus Type 1 (HIV-1) Sequence Diversity and Reveals Constraints on HIV-1 Evolution. J Virol 2005;79:13239–49. 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu Y, McNevin JP, Holte S, McElrath MJ, Mullins JI. Dynamics of Viral Evolution and CTL Responses in HIV-1 Infection. PLOS ONE 2011;6:e15639. 10.1371/journal.pone.0015639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Herbeck JT, Peebles K, Edlefsen PT, Rolland M, Murphy JT, Gottlieb GS, et al. HIV population-level adaptation can rapidly diminish the impact of a partially effective vaccine. Vaccine 2018;36:514–20. 10.1016/j.vaccine.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Herbeck JT, Mittler JE, Gottlieb GS, Goodreau SM, Murphy JT, Cori A, et al. Evolution of HIV virulence in response to widespread scale up of antiretroviral therapy: a modeling study. Virus Evol 2016;2. 10.1093/ve/vew028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stansfield SE, Mittler JE, Gottlieb GS, Murphy JT, Hamilton DT, Detels R, et al. Sexual role and HIV-1 set point viral load among men who have sex with men. Epidemics 2018. 10.1016/j.epidem.2018.08.006. [DOI] [PMC free article] [PubMed]
- [29].Goodreau SM, Stansfield SE, Murphy JT, Peebles KC, Gottlieb GS, Abernethy NF, et al. Relational concurrency, stages of infection, and the evolution of HIV set point viral load. Virus Evol 2018;4. 10.1093/ve/vey032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jenness SM, Goodreau SM, Morris M. EpiModel: An R Package for Mathematical Modeling of Infectious Disease over Networks. J Stat Softw 2018;84:8. 10.18637/jss.v084.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci U S A 2007;104:17441–6. 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stansfield SE, Herbeck JT, Gottlieb GS, Abernethy NF, Murphy JT, Mittler JE, et al. Test-and-treat coverage and HIV virulence evolution among men who have sex with men. Virus Evol 2021;7:veab011. 10.1093/ve/veab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Robb ML, Eller LA, Kibuuka H, Rono K, Maganga L, Nitayaphan S, et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med 2016;374:2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cori A, Pickles M, van Sighem A, Gras L, Bezemer D, Reiss P, et al. CD4+ cell dynamics in untreated HIV-1 infection: overall rates, and effects of age, viral load, sex and calendar time. AIDS Lond Engl 2015;29:2435–46. 10.1097/QAD.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Herbeck JT, Gottlieb GS, Li X, Hu Z, Detels R, Phair J, et al. Lack of Evidence for Changing Virulence of HIV-1 in North America. PLoS ONE 2008;3:e1525. 10.1371/journal.pone.0001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012;205:358–65. 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rosenberg ES, Grey JA, Sanchez TH, Sullivan PS. Rates of Prevalent HIV Infection, Prevalent Diagnoses, and New Diagnoses Among Men Who Have Sex With Men in US States, Metropolitan Statistical Areas, and Counties, 2012–2013. JMIR Public Health Surveill 2016;2. 10.2196/publichealth.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gandon S, Mackinnon M, Nee S, Read A. Imperfect vaccination: some epidemiological and evolutionary consequences. Proc R Soc Lond B Biol Sci 2003;270:1129–36. 10.1098/rspb.2003.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature 2001;414:751. 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- [40].Smith RJ. Could Low-Efficacy Malaria Vaccines Increase Secondary Infections in Endemic Areas? In: Deutsch A, Parra RB de la, Boer RJ de, Diekmann O, Jagers P, Kisdi E, et al. , editors. Math. Model. Biol. Syst. Vol. II Epidemiol. Evol. Ecol. Neural Syst. Brain Innov. Math. Methods, Boston, MA: Birkhäuser; 2008, p. 3–9. 10.1007/978-0-8176-4556-4_1. [DOI] [Google Scholar]
- [41].Blower SM, Bodine EN, Grovit-Ferbas K. Predicting the potential public health impact of disease-modifying HIV vaccines in South Africa: the problem of subtypes. Curr Drug Targets Infect Disord 2005;5:179–92. 10.2174/1568005054201616. [DOI] [PubMed] [Google Scholar]
- [42].Fonseca MGP, Forsythe S, Menezes A, Vuthoori S, Possas C, Veloso V, et al. Modeling HIV Vaccines in Brazil: Assessing the Impact of a Future HIV Vaccine on Reducing New Infections, Mortality and Number of People Receiving ARV. PLoS ONE 2010;5. 10.1371/journal.pone.0011736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Smith RJ, Blower SM. Could disease-modifying HIV vaccines cause population-level perversity? Lancet Infect Dis 2004;4:636–9. 10.1016/S1473-3099(04)01148-X. [DOI] [PubMed] [Google Scholar]
- [44].Davenport MP, Ribeiro RM, Chao DL, Perelson AS. Predicting the Impact of a Nonsterilizing Vaccine against Human Immunodeficiency Virus. J Virol 2004;78:11340–51. 10.1128/JVI.78.20.11340-11351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mann LM, Le Guillou A, Goodreau SM, Marcus JL, Sanchez T, Weiss KM, et al. Correlations between community-level HIV preexposure prophylaxis coverage and individual-level sexual behaviors among United States MSM. AIDS 2022;36:2015. 10.1097/QAD.0000000000003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Green D, Tordoff DM, Kharono B, Akullian A, Bershteyn A, Morrison M, et al. Evidence of sociodemographic heterogeneity across the HIV treatment cascade and progress towards 90-90-90 in sub-Saharan Africa – a systematic review and meta-analysis. J Int AIDS Soc 2020;23:e25470. 10.1002/jia2.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS Lond Engl 2014;28:1509–19. 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kharsany ABM, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J 2016;10:34–48. 10.2174/1874613601610010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wymant C, Bezemer D, Blanquart F, Ferretti L, Gall A, Hall M, et al. A highly virulent variant of HIV-1 circulating in the Netherlands. Science 2022;375:540–5. 10.1126/science.abk1688. [DOI] [PubMed] [Google Scholar]
- [50].Peebles K, Mittler JE, Goodreau SM, Murphy JT, Reid MC, Abernethy N, et al. Risk compensation after HIV-1 vaccination may accelerate viral adaptation and reduce cost-effectiveness: a modeling study. Sci Rep 2021;11:6798. 10.1038/s41598-021-85487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


