Abstract
Background
Gintonin is a new material of ginseng that acts through the ginseng-derived lysophosphatidic acid (LPA) receptor ligand. The gintonin-enriched fraction (GEF) inhibits amyloid plaque accumulation in the cortex and hippocampus, improves cognitive dysfunction by increasing acetylcholine levels, and promoted hippocampal neurogenesis in an animal model of Alzheimer's disease. We evaluated the effect of the GEF on the cognitive performance of subjects with subjective memory impairment (SMI).
Methods
In this eight-week, randomized, assessor- and participant-blinded, placebo-controlled study, participants with SMI were assigned to three groups receiving placebo, GEF 300 mg/day or GEF 600 mg/day. The Korean versions of the Alzheimer's Disease Assessment Scale (K-ADAS), Mini-Mental State Examination (K-MMSE), and Stroop color-word test (K-SCWT) were also evaluated along with the safety profiles.
Results
One hundred thirty-six participants completed the study. After eight weeks, we analyzed intergroup differences in primary or secondary outcome score changes. When we compared the GEF group with the placebo group, we observed significant improvements in the K-ADAS and K-SCWT scores. The GEF group did not show a significant improvement in K-MMSE and BDI scores compared to the placebo group. No adverse events were observed in the gintonin and placebo groups for eight weeks.
Conclusion
The GEF is safe and effective in improving subjective cognitive impairment related to both the K-ADAS and K-SCWT in this study. However, further large-scale and randomized controlled studies are warranted to secure other cognitive function tests besides the K-ADAS and K-SCWT, and to confirm the findings of the current study.
Keywords: Ginseng, Gintonin, Subjective memory impairment, K-ADAS, K-SCWT
Graphical abstract
1. Introduction
Panax ginseng Meyer is an herbal medicine known as a traditional tonic, which improves memory function after long-term intake in humans. Ginseng contains various bioactive compounds. Recent studies on gintonin, a non-saponin ginseng component, have shown several characteristics that other ginseng components, such as ginseng saponins, do not have. Gintonin contains a high concentration of exogenous lysophosphatidic acids (LPAs), which can enhance cognitive function through various mechanisms involving G protein-coupled LPA receptors [1]. The brain is rich in LPAs, which perform vital pluripotent functions ranging from brain development to cognitive processes. Previous in vitro and in vivo investigations have shown that gintonin might be responsible for improvements in cognitive function in preclinical studies [2,3]. For example, gintonin-mediated activation of G protein-coupled LPA receptors in neurons attenuates the production and accumulations of β-amyloid (Aβ), a key cause to Alzheimer's disease development, that derives from the amyloid precursor protein (APP) via amyloidogenic pathways. In addition, gintonin attenuates Aβ-induced neurotoxicity to protect neurons and rather activates non-amyloidogenic pathways [4]. Thus, gintonin stimulates the formation of the soluble amyloid precursor protein α (sAPPα), which has neuroprotective properties and anti-apoptotic effects of neurons [4,5]. Gintonin-mediated G-protein-coupled LPA receptors activation is also coupled to glutamate receptors, through which gintonin stimulates synaptic transmission, resulting in long-term potentiation (LTP) induction in hippocampus [6,7]. Furthermore, gintonin enhances choline acetyltransferase activity, an acetylcholine synthase enzyme, leading to increased acetylcholine production in the hippocampus and cortex [3,4]. In addition, gintonin stimulated hippocampal neurogenesis in an animal model of Alzheimer's disease (AD) and improved cognitive performance [3,4]. Thus, the underlying mechanisms of gintonin on anti-AD efficacy is clear in its preclinical studies.
On the other hand, AD including dementia, is growing concerns in aging populations worldwide, presenting a significant clinical and socio-economic burdens to both families and caregivers [8]. Recent studies have indicated that the pathophysiological, functional, and structural changes in the brain associated with AD can occur slowly and progressively for up to 20 years before clinical symptoms become evident [9]. Therefore, identifying and modifying the early stages of AD is crucial for preventing or improving the clinical progress of dementia [10,11]. Subjective memory impairment (SMI) in the elderly is characterized by a perceived decline in memory function relative to the subject's prior abilities, without any accompanying abnormalities in other cognitive functions. SMI or mild cognitive impairment (MCI) is very frequent in aging societies of over 65 years and substantially affects daily functions in severe conditions [12]. Although the presence of SMI is strongly linked to an increased risk of progression to AD, taking preventive medications for AD at the SMI stage is not recommended because of their limited clinical benefits over many kinds of adverse events (AEs), which commonly include nausea, vomiting, diarrhea, or sleep disturbance [[12], [13], [14], [15], [16]]. Therefore, the role of dietary supplements and traditional herbal medicines with neuroprotective effects has been emphasized, although most of these have insufficient scientific evidence to improve cognitive function [17]. Based on previous preclinical studies related to anti-AD drugs, gintonin may be a candidate for the amelioration of memory impairment in SMI humans.
This study aims to evaluate the cognitive enhancing effects and safety of the GEF in individuals with SMI using a randomized, placebo-controlled clinical trial design, with both the assessors and participants blinded to the treatment assignment.
2. Materials and methods
2.1. Study subjects
Subjects between the ages of 50 and 85 years who exhibited subjective memory impairment were recruited from the Neurology Department of Seoul National University Hospital, as previously detailed in a report [18]. In brief, subjective memory impairment was defined as a subject's self-reported feeling of reduced memory function, without any objective cognitive impairment, and was evaluated through the Korean Mini-Mental State Examination (K-MMSE), with a minimum score of 23 [18]. We excluded subjects who met any of the following describe criteria (Table 1): Subjects who (1) were taking ginseng and/or health functional foods containing ginseng components; (2) were allergic to or hypersensitive to ginseng; (3) had a diagnosis of liver disease or had serum alanine transaminase, aspartate aminotransferase, or bilirubin levels ≥ three times the upper limit of the normal range; (4) had chronic kidney disease with a serum creatinine level ˃ 2.0 mg/dl or on dialysis; (5) had a medical status that interfered with food absorption or oral administration of the drug; (6) were being treated for major mental illnesses such as mood disorders or schizophrenia; (7) had been diagnosed with dementia; (8) were taking medications for dementia, drugs or other dietary supplements that could affect memory and cognitive function, or other drugs on clinical trials for cognitive improvement within 4 weeks from the time of inclusion; (9) were addicted to alcohol or other drugs; (10) were pregnant, planning a pregnancy, or breastfeeding; or (11) had participated in another clinical trial within one month of visit.
Table 1.
List of Study Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Age (year old) | 50 ≤ 85 | < 50 or 85 < |
| K-MMSE score | ≥ 23 | < 23 |
| Liver function test (AST, ALT, or bilirubin levels) | < three times the upper limit of the normal range | ≥ three times the upper limit of the normal range |
| Renal function (serum creatinine level) | ≤ 2.0 mg/dl | ˃ 2.0 mg/dl |
| Cognitive function | Subjective memory complain | Dementia |
| Participation another clinical trial | > one month | ≤ one month |
| Taking medications, food affecting cognition | > 4 weeks | ≤ 4 weeks |
| Allergic to ginseng | None | Yes |
| Other medical conditions | GI absorption dysfunction Major mental illness Addiction Pregnancy (planning breast feeding) |
AST (aspartate aminotransferase); ALT (alanine transaminase); GI (Gastrointestinal).
The clinical trial was registered with the Korea Center for Disease Control and Prevention ‘s Clinical Research Information Service, under the registration number KCT0004636 on October 01, 2019. Additionally, the study protocol and supporting documentation received approval from the Institutional Review Board (IRB) of SNUH (registration numbers: 1908-103-1056 and 1804-122-940). The study was conducted in accordance with the regulations set forth by the SNUH IRB as well as the guidelines established by the International Conference on Harmonization for Good Clinical Practice. All participants who were enrolled provided written informed consent.
2.2. Study design and procedures
The study was designed as a randomized, placebo-controlled trial, with both the assessors and participants being blinded to the treatment assignments. Prior to the intervention, the participants underwent a thorough review of their medical histories, physical examinations, and laboratory tests at baseline as described in a previous report [18,19]. Participants who met the inclusion criteria were assigned to either the test or control group in a 1:1 ratio using randomization. The sample size for the study was determined based on the results of a prior study involving 80 dementia patients, which showed a difference of 1.3 points in K-MMSE score changes between the test and placebo groups, with a standard deviation of 0.4 points for the score changes in each group [19]. A total of 154 subjects were recruited from the 65, 25, and 64 in the test (GEF 300 mg/day and GEF 600 mg/day) and control (placebo) groups, with a 5% dropout rate, significance level of 5%, and power of 80%. The allocation concealment and randomization processes were conducted by a third-party organization, Biofood Contract Research Organization (CRO), which was not involved in the research process. The subjects were enrolled in the study and assigned a randomization number. Biofood CRO then used a computer-generated random list to assign the participants to either the test or control group based on their randomization number. Block randomization was used to allocate the participants to the test and control groups. All group assignment data for each participant were kept confidential and sealed by Biofood CRO.
One tablet is 400 mg containing GEF 150 mg and 250 mg excipient compounds. The GEF group received either two or four tablets of GEF 150 mg, along with 250 mg excipient compounds such as dextrin, crystalline cellulose, caramel pigment, gardenia yellow pigment, magnesium stearate, and silicon dioxide, once daily for eight weeks. Meanwhile, the control group received either two or four tablets of crystalline cellulose 374 mg, along with 26 mg of other excipient compounds such as caramel pigment, gardenia yellow pigment, magnesium stearate, and silicon dioxide to make the same 400 mg tablet, once daily for the same duration. The GEF and placebo tablets used in the study were manufactured by Gintonin KU Biotech Co., Ltd. in a good manufacturing practice facility and stored at room temperature. The daily dosage of GEF administered to the participants was determined based on a mouse in vivo experiments that observed cognitive improvement after 25-100 mg/kg/day of GEF and on conversion of dosage in human adults with a body weight of 60 kg [6,20]. The tablets used in this study (placebo and GEF intake groups) appeared to be identical. Randomization was carried out at the SNUH Clinical Research Unit using a computer–generated list of random numbers. Follow-up examinations were conducted four and eight weeks after treatment, during which the participants underwent physical and laboratory examinations and were monitored for any AEs.
2.3. Measurements
At baseline, four weeks, and eight weeks following gintonin administration, the K-MMSE [21], Korean version of the Alzheimer's Disease Assessment Scale (ADAS-K) [22], and Korean version of the Stroop Color-Word Test (K-SCWT) were used to evaluate cognitive functions as described in a previous report [23]. Two neurology specialists, who were blinded to patient allocation and clinical information, conducted the outcome assessments. Treatment-emergent AEs were identified and recorded in accordance with the Medical Dictionary for Regulatory Activities (MedDRA) [24].
2.4. Primary and the secondary end points
The primary endpoint was designated as changes in MMSE total scores at four and eight weeks from the baseline, and the secondary endpoints were specified as changes in K-ADAS total scores, ADAS scores in the cognitive and non-cognitive domains (ADAS-Cog and ADAS-noncog), number of correct answers in word- and color-reading domains of the K-SCWT test, and CDR scores at four and eight weeks from the baseline.
2.5. Statistical analysis
SPSS® (version 28.0; IBM, Armonk, New York, USA) was utilized for all statistical analyses. Efficacy analyses were conducted based on the per-protocol set, while safety analyses were performed based on the safety set (SAF). SAF was defined as all subjects who received at least one dose of the GEF and underwent a minimum of one safety assessment after the baseline evaluation. Notably, the absence of AEs served as a safety assessment. Analysis was performed based on the treatment received, and all participants who received the GEF were included in the GEF treatment group. Continuous variables were analyzed using analysis of variance (ANOVA) and presented as means with their corresponding standard deviations. Frequencies were used to describe categorical data, and the chi-square test or Fisher's exact test was used to compare variables. For continuous variables with unknown or non-normal distributions, the Wilcoxon rank-sum test was employed, with statistical significance determined at a test level of P < 0.05. Changes in scores from baseline to four and eight weeks after treatment were assessed using the paired t-test or Wilcoxon rank-sum test. Repeated-Measures Analysis of Variance (RM-ANOVA) was used to compare changes in scores between the groups.
3. Results
3.1. Baseline characteristics and participant's flow
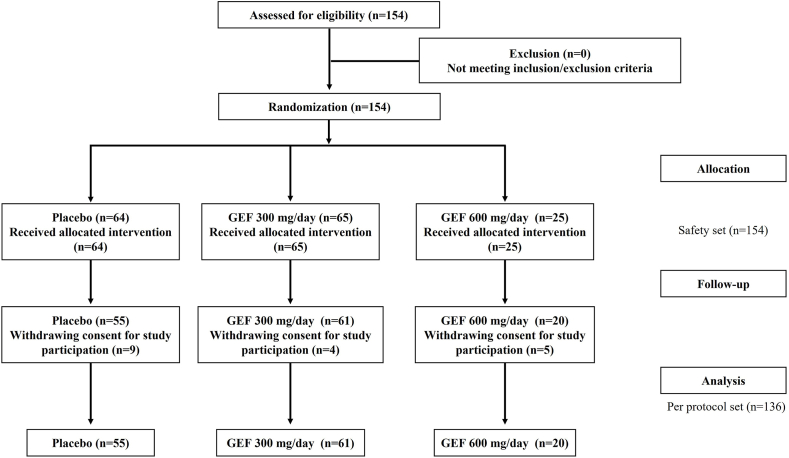
A total of 154 participants were enrolled between October 2018 and December 2022. Of the 154 participants, 90 were randomly assigned to receive the GEF at a dose of 300 mg/day or 600 mg/day, whereas the remaining 64 were assigned to the placebo group (safety set). During the follow-up period, 18 subjects withdrew from the study (9 in the GEF group and 9 in the placebo group) because of a lack of consent to continue participation. Finally, 136 participants (61 in the GEF 300 mg/day group, 20 in the GEF 600 mg/day group, and 55 in the placebo group) completed the study (Fig. 1). The demographic and baseline clinical characteristics were similar among the groups (Table 2).
Fig. 1.
A flow chart illustrating the study process.
Table 2.
Demographic and Baseline Clinical Characteristics
| Placebo (n = 55) | GEF 300 mg/day (n = 61) | GEF 600 mg/day (n = 20) | |
|---|---|---|---|
| Female sex (%) | 41 (74.55%) | 41 (67.21%) | 15 (75.00%) |
| Age (years) | 69.38 ± 9.14 | 69.21 ± 9.02 | 69.74 ± 7.08 |
| Education level (%) | 12.04 ± 3.98 | 11.00 ± 3.23 | 12.00 ± 3.82 |
| Hypertension (%) | 15 (27.28%) | 13 (21.31%) | 3 (15.00%) |
| Regular alcohol drinker | 1 (1.82%) | 10 (16.39%) | 12 (60.00%) |
| Smoking | |||
| Non-Smoker (%) | 49 (89.09%) | 55 (90.16%) | 19 (95.00%) |
| Ex-Smoker (≥ 6 months %) | 3 (5.45%) | 2 (3.28%) | 0 (0.00%) |
| Current Smoker (%) | 4 (7.27%) | 4 (6.56%) | 0 (0.00%) |
Data are reported as mean ± standard deviation.
3.2. Outcome measures
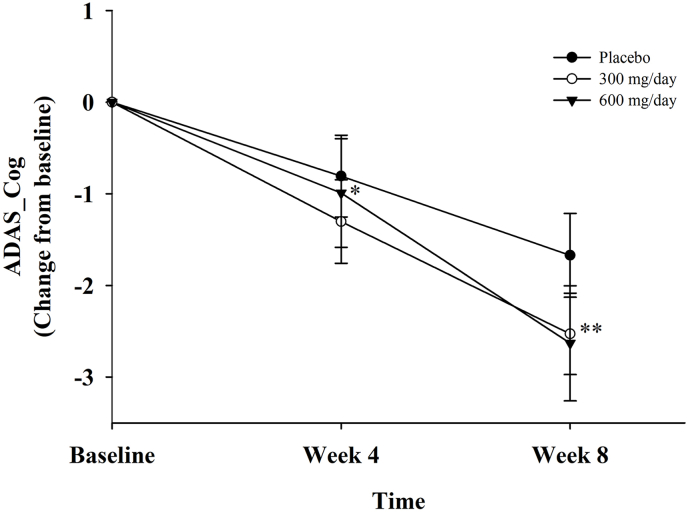
In terms of the primary and secondary efficacy outcomes, our observations indicated a statistically significant difference after GEF intake in the changes in K-ADAS-Cog total scores at every time point (from four to eight weeks) compared to the placebo group, as illustrated in Fig. 2 and Table 3. However, there were no significant differences after GEF intake in the changes of K-ADAS-noncog scores compared to the placebo groups (Table 3). For the primary outcome measure, there was no significant difference in the K-MMSE score changes between the gintonin (GEF 300 mg/day and GEF 600 mg/day) and placebo groups. The changes in K-MMSE scores between baseline and at four and eight weeks were not statistically significant in either the GEF or placebo groups (P = 0.419 and 0.223 at four and eight weeks in the GEF 300 mg/day group and P = 0.325 and 0.387 at four and eight weeks in the GEF 600 mg/day group).
Fig. 2.
The change in the mean ADAS-cog score from baseline to week 8 was calculated using an observed case analysis, and the SD was represented by the error bars. The endpoint carried forward at week eight showed a statistically significant difference (P < 0.05). GEF 600 mg/day administration showed a significant improvement of K-ADAS-cog at week four and GEF 300 mg/day administration presented a significant improvement of K-ADAS-cog at week eight, both compared to placebo group. The symbols depicted are as follows: the black circle for placebo group, the white circle for GEF 300 mg/day group, and the black triangle for GEF 600 mg/day group. ∗Placebo vs GEF 600 mg/day at week four, P < 0.05 by Wilcoxon Signed Rank Test, ∗∗Placebo vs GEF 300 mg/day at week eight, P < 0.05 by Wilcoxon Signed Rank Test.
Table 3.
Primary and Secondary Outcomes at Baseline, Four Weeks, and Eight Weeks
| Placebo (n = 55) | GEF 300 mg/day (n = 61) | Inter-group difference | P value† | GEF 600 mg/day (n = 20) | Inter-group difference | P value† | ||
|---|---|---|---|---|---|---|---|---|
| K-MMSE | Baseline | 27.66 ± 1.89 | 27.67 ± 1.62 | 27.05 ± 1.93 | ||||
| 4 week | 27.64 ± 2.45 | 27.58 ± 2.20 | 27.89 ± 1.76 | |||||
| Change 1 | 0.84 | −0.083 | 0.21 (0.08∼0.35) | 0.42 | 0.842 | 0.28 (0.06∼0.50) | 0.325 | |
| 8 week | 27.89 ± 1.86 | 27.97 ± 1.93 | 27.89 ± 1.76 | |||||
| Change 2 | 0.84 | 0.3 | −0.29 (−0.32∼-0.25) | 0.22 | 0.842 | −0.22 (−0.25∼-0.20) | 0.387 | |
| ADAS-cog | Baseline | 10.5 ± 4.62 | 10.40 ± 0.56 | 12.17 ± 1.39 | ||||
| 4 week | 9.74 ± 4.45 | 9.10 ± 0.46 | 11.18 ± 1.18 | |||||
| Change 1 | −0.806 | −1.302 | −1.54 (−2.05∼-1.03) | 0.16 | −0.99 | 10.74 (8.73∼12.75) | 0.03§ | |
| 8 week | 8.82 ± 4.55 | 7.99 ± 0.46 | 9.54 ± 1.35 | |||||
| Change 2 | −1.73 | −2.41 | −1.22 (−1.63∼-0.81) | 0.04§ | −2.63 | 9.64 (7.30∼11.98) | 0.68 | |
| ADAS-non cog | Baseline | 4.93 ± 4.27 | 4.89 ± 4.09 | 8.62 ± 8.28 | ||||
| 4 week | 5.34 ± 4.00 | 5.07 ± 3.83 | 8.59 ± 7.77 | |||||
| Change 1 | 0.41 | 0.18 | 0.05 (−0.16∼-0.27) | 0.41 | −0.03 | 8.19 (6.02∼10.37) | 0.80 | |
| 8 week | 5.22 ± 3.90 | 4.80 ± 3.94 | 8.06 ± 8.46 | |||||
| Change 2 | 0.29 | −0.09 | 0.20 (−0.09∼-0.48) | 0.21 | −0.56 | 7.84 (5.78∼9.90) | 0.43 | |
| BDI-II | Baseline | 15.27 ± 8.01 | 15.25 ± 8.76 | 15.89 ± 10.55 | ||||
| 4 week | 15.53 ± 8.4 | 15.07 ± 8.74 | 14.37 ± 9.39 | |||||
| Change 1 | −1.53 | 0.27 | 2.25 (−0.10∼-4.60) | 0.32 | −0.18 | 13.94 (11.64∼16.25) | 0.45 | |
| 8 week | 14.23 ± 8.15 | 14.68 ± 10.16 | 15.11 ± 9.88 | |||||
| Change 2 | −1.04 | −0.57 | 2.72 (1.38∼4.05) | 0.47 | −0.79 | 14.33 (10.91∼17.76) | 0.46 | |
| K-SCWT correct answer in word-reading domain (number) |
Baseline | 110.9 ± 3.63 | 109.61 ± 5.64 | 111.7 ± 0.97 | ||||
| 4 week | 110.9 ± 2.73 | 110.98 ± 3.93 | 110.6 ± 5.25 | |||||
| Change 1 | 0.07 | 1.37 | −1.72 (−5.67∼2.23) | 0.87 | −1.07 | −0.64 (−1.15∼-0.14) | 0.30 | |
| 8 week | 111.1 ± 3.59 | 110.9 ± 4.02 | 110.9 ± 5.38 | |||||
| Change 2 | 0.23 | 1.25 | −1.33 (−3.54∼0.89) | 0.475 | −0.66 | 0.73 (−0.47∼1.93) | 0.63 | |
| K-SCWT evaluation time in word-reading domain (sec) |
Baseline | 99.05 ± 51.67 | 98.75 ± 43.92 | 112.3 ± 53.59 | ||||
| 4 week | 86.63 ± 27.34 | 90.96 ± 42.24 | 106.5 ± 42.47 | |||||
| Change 1 | −12.43 | −7.79 | 24.7 (11.02∼38.36) | 0.011∗ | 5.17 | 25.73 (9.55∼41.92) | 0.18 | |
| 8 week | 91.56 ± 37.97 | 89.33 ± 40.95 | 109.3 ± 34.53 | |||||
| Change 2 | −7.5 | −9.41 | 17.9 (−3.65∼39.4) | 0.001∗∗ | 1.79 | 11.31 (10.08∼12.53) | 0.09 | |
| K-SCWT correct answer in color-reading domain (number) | Baseline | 111.4 ± 0.98 | 112 ± 0.00 | 109.3 ± 6.20 | ||||
| 4 week | 108.8 ± 7.52 | 107.8 ± 10.37 | 108.2 ± 9.46 | |||||
| Change 1 | −2.61 | −4.21 | −1.67 (−3.81∼0.47) | 0.52 | −1.04 | −3.72 (−8.26∼0.81) | 0.04∗ | |
| 8 week | 109.7 ± 5.63 | 110.0 ± 3.09 | 109.9 ± 4.03 | |||||
| Change 2 | −1.76 | −1.98 | −0.16 (−0.60∼0.29) | 0.98 | 0.64 | −1.78 (−3.47∼-0.08) | 0.03∗ | |
| K-SCWT evaluation time in color-reading domain (sec) |
Baseline | 150.7 ± 55.17 | 165.7 ± 47.54 | 232.8 ± 114.3 | ||||
| 4 week | 167.1 ± 85.21 | 152 ± 43.50 | 202.9 ± 104.2 | |||||
| Change 1 | 16.34 | −13.7 | −28.58 (−72.86∼15.70) | 0.48 | −29.80 | 4.78 (−4.79∼14.35) | 0.18 | |
| 8 week | 153.9 ± 65.11 | 144.3 ± 49.57 | 198.5 ± 94.13 | |||||
| Change 2 | 3.21 | −21.36 | −11.87 (−30.66∼6.93) | 0.181 | −34.28 | 26 (25.52∼26.48) | 0.14 | |
Data are reported as mean ± standard deviation.
ADAS-K: Korean version of the Alzheimer's disease assessment scale; ADAS-cog/noncog: cognitive/non-cognitive domains of ADAS-K; BDI-II: Beck Depression Inventory-II; CI: confidence interval; K-SCWT: Korean version of the color-word Strop Test; K-MMSE: Korean version of the Mini-Mental Status Examination. Change 1:4 weeks – baseline; change 2:8-weeks-baseline.
§P < 0.05, Wilcoxon rank sum test by % of inter-group difference vs placebo group.
∗P < 0.05, Wilcoxon rank sum test.
∗∗P < 0.01, P value for the change from placebo, by Paired t-test or Wilcoxon rank sum test.
†P value for the test group and the placebo group, by repeated-measure ANOVA.
However, we observed significant differences in K-SCWT scores between the two groups. The evaluation time in the word-reading domain of the K-SCWT in the GEF group (GEF 300 mg/day) decreased significantly compared to that in the placebo group at four and eight weeks (90.96 ± 42.24, P = 0.011 and 89.33 ± 40.95, P = 0.001, respectively). This change was not observed in the placebo group (P = 0.098 and P = 0.570, respectively). There was no significant difference in the number of correct answers in the word-reading domain of the K-SCWT between the two groups (P = 1).
The number of correct answers in the color-reading domain of the K-SCWT increased significantly compared to the placebo group at four and eight weeks in the GEF 600 mg/day group (108.21 ± 9.46, P = 0.037 and 109.90 ± 4.03, P = 0.031, respectively). However, there was no statistically significant difference observed in the evaluation time for the color-reading domain of the K-SCWT between the two groups (P = 0.393, Table 3).
In the SAF analysis, ten AEs were reported: three in the GEF group and seven in the placebo group. However, there was no statistically significant difference in the incidence of AEs between the two groups (P = 1.000; Table 4). Every AE was of mild or moderate degree, and none of the events had a definite or probable causal relationship with drug administration. One of the reported AEs was severe; however, this did not show any relationship with gintonin administration in the placebo group.
Table 4.
Profiles of Adverse Events
| Placebo (n = 55) | Type (Number of occurrence) | GEF 300 mg/day (n = 61) | Type (Number of occurrence) | GEF 600 mg/day (n = 20) | Type (Number of occurrence) | ||
|---|---|---|---|---|---|---|---|
| Severity | Mild | 5 | tinnitus (1) | 2 | dyspepsia (1) | 0 | |
| dizziness (1) | chest pain (1) | ||||||
| pruritus (1) | |||||||
| facial edema (1) | N/A | ||||||
| dyspepsia (1) | |||||||
| Moderate | 1 | plantar pain (1) | 1 | hyperprolactinemia (1) | 0 | ||
| Severe | 1 | dyspepsia (1) | 0 | 0 | |||
| Causality | Definite | 0 | 0 | 0 | |||
| Probable | 0 | 0 | 0 | ||||
| Possible | 4 | 1 | 0 | ||||
| Unlikely | 1 | 1 | 0 | ||||
| Unrelated | 2 | 1 | 0 | ||||
Placebo vs GEF 300 mg/day; P value = 0.5.
Placebo vs GEF 600 mg/day; P value = 0.5.
N/A: Not Applicable.
4. Discussion
We previously reported the results of a randomized, assessor- and participant-blinded, placebo-controlled trial that investigated the effect of the GEF on cognitive function in subjects with SMI [18]. However, due to the limited number of participants, the previous study did not show a significant improvement in cognitive functions after eight weeks of GEF administration compared to the placebo group, although GEF intake itself for eight weeks showed significant improvements in the K-MMSE and K-SCWT [18]. In the present study, we increased the number of subjects with SMI and administered two doses of gintonin (300 and 600 mg/day) for eight weeks. Therefore, this is the second randomized, assessor- and participant-blinded, placebo-controlled study to investigate the effect of GEF intake on cognitive function in patients with SMI. Our findings showed that the GEF significantly improved cognitive function compared with the placebo group, as measured by the K-ADAS-Cog and K-SCWT scores at four and eight weeks. However, the primary outcome analyses, such as the K-MMSE, returned negative results compared with the placebo group. In addition, GEF administration for eight weeks did not result in any AEs throughout the study period, indicating that no serious AEs occurred during the intake of the GEF (Table 4). This result is consistent with previous studies that have evaluated the effect of various candidate drugs on cognitive improvement in individuals with mild cognitive impairment (MCI) [[14], [15], [16],25].
It is not yet understood why GEF intake showed a significant improvement in ADAS-Cog and K-SCWT, but not in K-MMSE. This may be due to the characteristics of the three types of cognitive tests. The K-ADAS-Cog is a cognitive assessment tool with raw scores ranging from 0 to 2.5, with higher scores indicating greater cognitive dysfunctions [26]. The ADAS-Cog test is specifically used to screen patients with early-stage dementia, as it has few false positives and is comprehensive in several cognitive domains, such as attention, memory, language ability, visuospatial composition ability, praxis, and frontal lobe function. Therefore, it may be helpful for diagnosing dementia. The ADAS-Cog is a useful tool for sensitively detecting responses to treatment effects. Interestingly, at week four, high-dose GEF (600 mg/day) administration resulted in a significant improvement in ADAS-Cog scores, whereas low-dose GEF (300 mg/day) administration showed improvement at week eight (Fig. 2 and Table 3). This suggests a dose-dependent effect in the short term and a time-dependent effect over a longer period of GEF administration.
However, the K-MMSE was primarily designed to screen for moderate Alzheimer's disease (AD) or dementia [21]. As many SMI subjects have high baseline K-MMSE scores, it may be difficult to confirm statistically significant differences in K-MMSE scores even if there were some improvements, as shown in Table 3. This is supported by the fact that GEF intake by SMI improved K-MMSE scores [27]. Thus, the K-MMSE test may be less sensitive and may not be suitable for assessing cognitive improvement in SMI subjects, because SMI subjects are not yet diagnosed with AD. This may be one reason why GEF intake in subjects with SMI did not show significant cognitive improvement in the K-MMSE compared with the control placebo group.
In addition, we found that GEF intake for four and eight weeks (300 and 600 mg/day) improved the K-SCWT test (Table 3). The Stroop color-word test is a commonly used cognitive assessment tool that measures selective attention and inhibition in the frontal lobes [23]. Our present study using the GEF with the K-SCWT test showed significant improvements in subjects' cognitive abilities to form and change cognitive sets, inhibit automatic responses, respond appropriately to situational cues, and spontaneously generate thoughts. These improvements may be attributed to the ability of the GEF to increase brain acetylcholine levels, inhibit acetylcholinesterase activity, and enhance hippocampal neurogenesis via gintonin-induced LPA receptor activation [3]. The activation of LPA receptors by gintonin has been identified as the primary mechanism responsible for the observed cognitive improvements [27]. Recent studies have suggested that gintonin enhances synaptic transmission in the hippocampus through the release of glutamate, which, in turn, is linked to long-term potentiation. These findings were confirmed in hippocampal slices, where treatment with an LPA receptor antagonist blocked the enhancement of synaptic transmission [7]. Oral administration of gintonin also increases the expression of the brain-derived neurotrophic factor (BDNF) in the hippocampus, which is a key molecule for cognitive function in the brain [6]. The GEF's numerous beneficial properties that are related with brain functions may contribute cognitive function in elderly populations over 65 and make it a promising dietary supplement for preventing SMI and/or dementia.
Besides gintonin, several other natural products have been studied for their potential benefits in treating or preventing dementia in China and Japan [6,[28], [29], [30]]. Meta-analyses have shown that several Chinese herbal medicines are effective in improving cognitive function in AD patients [28]. Natural medicines are particularly effective in combination with conventional AD drugs. For example, the combination of Ginkgo biloba and donepezil had a greater anti-amnestic effect than either drug alone in a scopolamine-induced AD rat model without any changes in systemic or brain exposure [31]. Clinical trials have also shown that long-term intake of Ginkgo biloba improves cognitive function in patients with AD and mild dementia [32]. Among Japanese herbal medicines, the combination administration of Kami-Untan-To and donepezil was also found to be more effective than treatment with donepezil alone in improving cognitive functions [29]. Traditional Korean medicines, such as ginseng, have demonstrated varying degrees of memory benefits. Given the favorable safety profile of gintonin, combining the GEF with conventional drugs may increase its cognitive improvement effects, as reported in a previous study [33]. However, a larger study involving patients with AD might be required to confirm this hypothesis [33].
Several issues need to be addressed before the GEF can be considered for clinical use as a treatment for AD and to establish ADAS-Cog and/or K-MMSE as clinically meaningful primary outcomes. First, previous researches of medicinal interventions on individuals with MCI have demonstrated that alterations in cognitive scores slowly progress over time, indicating that the longer follow-up studies may be required for accurate assess of the potential disease-modifying effects of GEF. Second, previous studies included a large number of patients (ranging from to 250-2000) to evaluate the clinical effects of candidate drugs [15,[34], [35], [36], [37]]. Given the promising trend observed in this study, future studies should include a larger number of subjects to precisely evaluate the effects of the GEF. Finally, this study only evaluated the effect of the GEF alone; therefore, future studies are needed to investigate the additive or synergistic effects of the GEF in combination with conventional drugs on cognition in patients with AD [27].
5. Conclusion
The GEF is safe and was effective in the K-ADAS and evaluation time in the word-reading and color-reading domains of the K-SCWT in people with SMI within the current study setting. Thus, the GEF intake was effective in improving both global cognitive performance and frontal executive functions. Larger randomized controlled trials with longer durations are needed to further explore the efficacy of the GEF on cognition and to confirm the findings of the current study. Further research is warranted in more vulnerable populations such as those with mild cognitive impairment and advanced age.
Author contributions
RL analyzed the data, developed the figures, and wrote the manuscript. HSL designed the study, performed the arrangements, conducted the trials according to the protocol and data collection, and revised the manuscript. WWK analyzed the data and revised the manuscript. MK and SYN designed the study, and wrote and revised the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests
Acknowledgement
This work was supported by a grant from the Ministry of Health and Welfare (HU22C0168) to M. Kim and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2023R1A2C1003481) to S.Y. Nah.
Contributor Information
Rami Lee, Email: rmlee12@konkuk.ac.kr.
Han Sang Lee, Email: hansanglee1127@gmail.com.
Won-Woo Kim, Email: kww@gintoninku.co.kr.
Manho Kim, Email: kimmanho@snu.ac.kr.
Seung-Yeol Nah, Email: synah@konkuk.ac.kr.
References
- 1.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W., et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates g protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33(2):151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi S.H., Jung S.W., Lee B.H., Kim H.J., Hwang S.H., Kim H.K., Nah S.Y. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015:6. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H.J., Shin E.J., Lee B.H., Choi S.H., Jung S.W., Cho I.H., Hwang S.H., Kim J.Y., Han J.S., Chung C., et al. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-beta protein, and mouse model of Alzheimer's disease. Mol Cells. 2015;38(9):796–805. doi: 10.14348/molcells.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.J., Kwon S.H., Jang C.G., Lee J.H., et al. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31(1):207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 5.Park H., Kim S., Rhee J., Kim H.J., Han J.S., Nah S.Y., Chung C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113(5):1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 6.Kim S., Kim M.S., Park K., Kim H.J., Jung S.W., Nah S.Y., Han J.S., Chung C. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40(1):55–61. doi: 10.1016/j.jgr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin T.J., Kim H.J., Kwon B.J., Choi S.H., Kim H.B., Hwang S.H., Lee B.H., Lee S.M., Zukin R.S., Park J.H., et al. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through n-methyl-d-aspartic acid receptor activation: involvement of lpa receptors. Mol Cells. 2012;34(6):563–572. doi: 10.1007/s10059-012-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75 e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Jr., Kaye J., Montine T.J., et al. Toward defining the preclinical stages of alzheimer's disease: recommendations from the national institute on aging-alzheimer's association workgroups on diagnostic guidelines for alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weimer D.L., Sager M.A. Early identification and treatment of alzheimer's disease: social and fiscal outcomes. Alzheimers Dement. 2009;5(3):215–226. doi: 10.1016/j.jalz.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jessen F., Wolfsgruber S., Wiese B., Bickel H., Mosch E., Kaduszkiewicz H., Pentzek M., Riedel-Heller S.G., Luck T., Fuchs A., et al. Ad dementia risk in late mci, in early mci, and in subjective memory impairment. Alzheimers Dement. 2014;10(1):76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Jessen F., Wiese B., Bachmann C., Eifflaender-Gorfer S., Haller F., Kolsch H., Luck T., Mosch E., van den Bussche H., Wagner M., et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 14.Birks J., Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;3:CD006104. doi: 10.1002/14651858.CD006104. [DOI] [PubMed] [Google Scholar]
- 15.Doody R.S., Ferris S.H., Salloway S., Sun Y., Goldman R., Watkins W.E., Xu Y., Murthy A.K. Donepezil treatment of patients with mci: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 16.Feldman H.H., Ferris S., Winblad B., Sfikas N., Mancione L., He Y., Tekin S., Burns A., Cummings J., del Ser T., et al. Effect of rivastigmine on delay to diagnosis of alzheimer's disease from mild cognitive impairment: the inddex study. Lancet Neurol. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 17.Barnard N.D., Bush A.I., Ceccarelli A., Cooper J., de Jager C.A., Erickson K.I., Fraser G., Kesler S., Levin S.M., Lucey B., et al. Dietary and lifestyle guidelines for the prevention of alzheimer's disease. Neurobiol Aging. 2014;35(Suppl 2):S74–S78. doi: 10.1016/j.neurobiolaging.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Lee W.J., Shin Y.W., Chang H., Shin H.R., Kim W.W., Jung S.W., Kim M., Nah S.Y. Safety and efficacy of dietary supplement (gintonin-enriched fraction from ginseng) in subjective memory impairment: a randomized placebo-controlled trial. Integr Med Res. 2022;11(1) doi: 10.1016/j.imr.2021.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee W.J., Shin Y.W., Kim D.E., Kweon M.H., Kim M. Effect of desalted salicornia europaea l. Ethanol extract (pm-ee) on the subjects complaining memory dysfunction without dementia: a 12 week, randomized, double-blind, placebo-controlled clinical trial. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-76938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Yn D.L., Hahn S. A validity study on the Korean mini-mental state examination (k-mmse) in dementia patients. J Korean Neurol Assoc. 1997;15(2):300–308. [Google Scholar]
- 22.Youn J.C., Lee D.Y., Kim K.W., Lee J.H., Jhoo J.H., Lee K.U., Ha J., Woo J.I. Development of the Korean version of alzheimer's disease assessment scale (adas-k) Int J Geriatr Psychiatry. 2002;17(9):797–803. doi: 10.1002/gps.699. [DOI] [PubMed] [Google Scholar]
- 23.Kim Ty Kss J.E., Lee E.A., Yoo B.G., Lee S.C., Hong T.Y., Kim M.J. Development of the Korean stroop test and study of the validity and the reliability. J Korean Geriatr Soc. 2004;8(4):233–240. [Google Scholar]
- 24.Brown E.G., Wood L., Wood S. The medical dictionary for regulatory activities (meddra) Drug Safety. 1999;20(2):109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 25.Salloway S., Ferris S., Kluger A., Goldman R., Griesing T., Kumar D., Richardson S. Donepezil 401 Study G. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 26.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.J., Jung S.W., Kim S.Y., Cho I.H., Kim H.C., Rhim H., Kim M., Nah S.Y. Panax ginseng as an adjuvant treatment for alzheimer's disease. J Gin Res. 2018;42(4):401–411. doi: 10.1016/j.jgr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong L., May B.H., Feng M., Hyde A.J., Tan H.Y., Guo X., Zhang A.L., Lu C., Xue C.C. Chinese herbal medicine for mild cognitive impairment: a systematic review and meta-analysis of cognitive outcomes. Phytother Res. 2016;30(10):1592–1604. doi: 10.1002/ptr.5679. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama M., Tomita N., Iwasaki K., Ootsuki M., Matsui T., Nemoto M., Okamura N., Higuchi M., Tsutsui M., Suzuki T., et al. Benefits of combining donepezil plus traditional Japanese herbal medicine on cognition and brain perfusion in alzheimer's disease: a 12-week observer-blind, donepezil monotherapy controlled trial. J Am Geriatr Soc. 2006;54(5):869–871. doi: 10.1111/j.1532-5415.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 30.Shi J., Ni J., Lu T., Zhang X., Wei M., Li T., Liu W., Wang Y., Shi Y., Tian J. Adding Chinese herbal medicine to conventional therapy brings cognitive benefits to patients with alzheimer's disease: a retrospective analysis. BMC Complement Altern Med. 2017;17(1):533. doi: 10.1186/s12906-017-2040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J., Li K., Wang Y., Li D., Wang Q., Xie S., Wang J., Zuo Z. Enhanced anti-amnestic effect of donepezil by ginkgo biloba extract (egb 761) via further improvement in pro-cholinergic and antioxidative activities. J Ethnopharmacol. 2021;269 doi: 10.1016/j.jep.2020.113711. [DOI] [PubMed] [Google Scholar]
- 32.Liu H., Ye M., Guo H. An updated review of randomized clinical trials testing the improvement of cognitive function of ginkgo biloba extract in healthy people and alzheimer's patients. Front Pharmacol. 2019;10:1688. doi: 10.3389/fphar.2019.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon J., Choi S.H., Shim J.Y., Park H.J., Oh M.J., Kim M., Nah S.Y. Gintonin administration is safe and potentially beneficial in cognitively impaired elderly. Alzheimer Dis Assoc Disord. 2018 Jan-Mar;32(1):85–87. doi: 10.1097/WAD.0000000000000213. PMID: 29028648. [DOI] [PubMed] [Google Scholar]
- 34.Petersen R.C., Thomas R.G., Grundman M., Bennett D., Doody R., Ferris S., Galasko D., Jin S., Kaye J., Levey A., et al. Vitamin e and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 35.Sheen Y.S., Huang H.Y., Liao Y.H. The efficacy and safety of an antiaging topical serum containing hesperetin and sodium cyclic lysophosphatidic acid: a single-center clinical trial. J Cosmet Dermatol. 2021;20(12):3960–3967. doi: 10.1111/jocd.14063. [DOI] [PubMed] [Google Scholar]
- 36.Tricco A.C., Soobiah C., Berliner S., Ho J.M., Ng C.H., Ashoor H.M., Chen M.H., Hemmelgarn B., Straus S.E. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013;185(16):1393–1401. doi: 10.1503/cmaj.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winblad B., Gauthier S., Scinto L., Feldman H., Wilcock G.K., Truyen L., Mayorga A.J., Wang D., Brashear H.R., Nye J.S., et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]