Abstract
Our objective was to convene interdisciplinary experts from government, academia, and industry to develop a Research Roadmap to identify research priorities about processed food intake and risk for obesity and cardiometabolic diseases (CMD) among United States populations. We convened attendees at various career stages with diverse viewpoints in the field. We held a “Food Processing Primer” to build foundational knowledge of how and why foods are processed, followed by presentations about how processed foods may affect energy intake, obesity, and CMD risk. Breakout groups discussed potential mechanistic and confounding explanations for associations between processed foods and obesity and CMD risk. Facilitators created research questions (RQs) based on key themes from discussions. Different breakout groups convened to discuss what is known and unknown for each RQ and to develop sub-RQs to address gaps. Workshop attendees focused on ultra-processed foods (UPFs; Nova Group 4) because the preponderance of evidence is based on this classification system. Yet, heterogeneity and subjectivity in UPF classification was a challenge for RQ development. The 6 RQs were: 1) What objective methods or measures could further categorize UPFs, considering food processing, formulation, and the interaction of the two? 2) How can exposure assessment of UPF intake be improved? 3) Does UPF intake influence risk for obesity or CMDs, independent of diet quality? 4) What, if any, attributes of UPFs influence ingestive behavior and contribute to excess energy intake? 5) What, if any, attributes of UPFs contribute to clinically meaningful metabolic responses? 6) What, if any, external environmental factors lead people to consume high amounts of UPFs? Uncertainty and complexity around UPF intake warrant further complementary and interdisciplinary causal, mechanistic, and methodological research related to obesity and CMD risk to understand the utility of applying classification by degree of processing to foods in the United States.
Keywords: ultra-processed food, Nova classification system, dietary assessment, ingestive behavior, cardiometabolic health, food environment, built environment, food science, food technology, food engineering
Statement of Significance.
By incorporating perspectives across various disciplines and sectors, this Research Roadmap outlines research gaps and priority research questions to help advance the understanding of whether and how intake of ultra-processed foods causally influences risk for obesity and cardiometabolic health to better improve the evidence base for future dietary guidance.
Introduction
Modern food processing is vital to United States and global food systems to increase food safety, food and nutrition security, nutrient needs, and serves to decrease food waste from farm to table [1]. More recently, high intake of foods classified as “ultra-processed foods (UPFs),” a food group and terminology developed for the Nova food processing classification system [2], may negatively contribute to the United States dietary pattern in ways that may increase risk for obesity and cardiometabolic diseases (CMD) [3,4]. UPFs contribute to ∼60% of total energy intake in the United States [[3], [4], [5]] and are defined by Nova as industrial formulations of processed food substances (e.g., oils, solid fats, sugars, starch, protein isolates) that contain little or no whole foods and typically include flavorings, colorings, emulsifiers, and other cosmetic additives [2]. Recently, there has been an influx of observational research suggesting associations between high intakes of UPFs and increased risk for obesity [6] and CMDs [7,8]. Dietary guidelines in some countries, such as Brazil [9] and Canada [10], recommend limiting UPFs and consuming mainly unprocessed/minimally processed foods. It is debated whether future United States dietary guidance about UPF intake, as defined by Nova, is feasible, adds value beyond conventional diet quality metrics that do not consider food processing, and whether a negative connotation around food processing may be counter-productive for public health and consumer messaging [11,12].
The Dietary Guidelines for Americans (DGA) is the foundation of dietary recommendations for the United States population and federal nutrition policies and programs [13]. The 2025 Dietary Guidelines Advisory Committee (DGAC) is currently reviewing the evidence related to UPF intake and risk for obesity for potential consideration for the 2025–2030 DGA. This body of evidence is largely observational, and public policy is best informed by the intersection of multiple evidence types. There are several commentaries and thoughtful review papers proposing mechanisms or confounders to explain associations with obesity and the common comorbidities of CMDs [[14], [15], [16], [17]] but only a few randomized controlled trials provide causal or mechanistic evidence for select risk factors [18,19]. To promote generation of a stronger and more diverse evidence base to inform future dietary guidance in the United States, an interdisciplinary group from government, academia, and industry were convened to develop a Research Roadmap consisting of research questions (RQ) to guide causal, mechanistic, and methodological research investigating if and how UPFs impact risk for obesity and CMDs in the United States. The objective of this manuscript is to 1) summarize the workshop proceedings, including relevant presentations and panel discussions, and 2) present a narrative review of available evidence supporting each of the RQs of the Research Roadmap.
Workshop Description
Attendees
The workshop planning committee invited participants from academia, government agencies, consumer-packaged goods companies, ingredient processing companies, and commodity groups. Invitees were selected on the basis of expertise, engagement in the research community, and prior and ongoing research or interest on the topic of processed foods or food processing. Names, affiliations, and a brief biography of all attendees or planning committee members are provided in Supplemental Table 1. There was a broad mix of expertise in nutrition science, nutrition epidemiology, food science and technology, dietary assessment, ingestive behavior, sensory science, economics, biostatistics, biochemistry, physiology, metabolism, and public health. Sixteen individuals were from academia, 10 from government agencies, and 6 from the private sector; 11 attendees were considered early in their career, including graduate students, post-doctoral fellows, and early career investigators (i.e., <10 y from their terminal degree). Working under the Adversarial Collaborations Framework [20], participants had “disagreeing” views on the topic, many with prior publications both supporting or critiquing the topic of categorizing foods based on processing, and expertise on processed foods more generally. Common foundational knowledge and vocabulary were established via recommended pre-reads (Supplemental Table 2).
Workshop objective
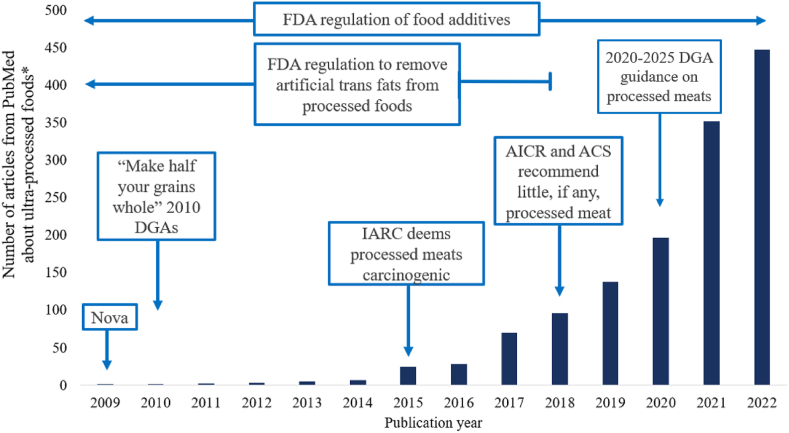
The workshop was held in Hot Springs, Arkansas, United States on March 1–2, 2023. Dr. Lauren O’Connor opened the workshop by describing how concerns about processed food intake pre-dates conception of the Nova classification system (e.g., processed meats, artificial trans fats, refined grains; Figure 1 [21,22]). More recently, research on processed foods has consisted of an influx of observational studies assessing associations between UPF intakes, as classified by Nova [2], and health outcomes (Figure 1). This large body of evidence has established a clear correlation between high UPF intake and risk for obesity and CMDs. High intakes of processed foods and UPF may have implications for other health outcomes as well, but that was out of scope for this workshop. Thus, the objective of the workshop was described as an effort to identify where more causal, mechanistic, or methodological research can advance the understanding of associations between intake of processed foods and risk for obesity and CMDs. The intention was not to discount the current observational evidence base, but to identify research priority areas where causal, mechanistic, or methodological research is needed to create a more balanced evidence base to best inform future dietary guidance in the United States. There was a general agreement among attendees that the focus should be on UPFs (Nova Group 4) because the preponderance of evidence and common discourse is based on Nova classification opposed to other classification systems. However, heterogeneity and subjectivity in UPF classification was noted as a challenge.
FIGURE 1.
Timeline of select policies related to processed foods mapped onto the influx of research related to ultra-processed foods, as defined by Nova. Nova was first introduced to the research community in 2009 and then again in 2010, at the beginning of this timeline [21,22]. "Make half your grains whole" was first introducted by the 2005 DGA, but falls outside of this timeline, ∗Terms used for PudMed search: “ultra-processed food” OR ultra-processed foods” OR “ultra-processed food” OR “ultra-processed foods.” ACS, American Cancer Society; AICR, American Institute for Cancer Research; DGA, Dietary Guidelines for Americans; FDA, United States Food and Drug Administration; IARC, International Agency for Research on Cancer.
Food processing primer
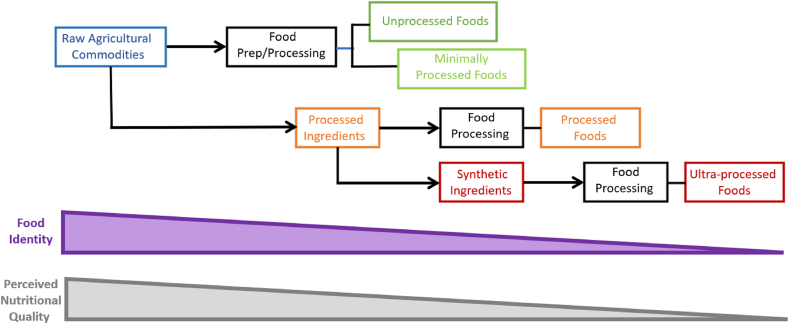
Dr. Mario Ferruzzi presented a "Food Processing Primer" to establish foundational knowledge of why and how foods are processed, using definitions described in Table 1 [[23], [24], [25], [26], [27], [28], [29]], followed by a panel discussion that included Dr. Bruce Hamaker, Dr. Alyson Mitchell, and Dr. John Coupland. Dr. Ferruzzi described that commercially available foods are formulated and processed for preservation, safety, quality (sensory and nutritional), reduction of post-harvest losses, longer shelf-life, and to increase food availability. Food formulation, including the combination of food ingredients and food additives for specific functionalities, is an integral part of food processing that affects taste, aroma, texture, safety, shelf-life, and nutritional content. Processed foods are made by a combination of formulation and processing that includes many unit operations (e.g., ingredient cleaning, concentration, blending, size reduction, forming, and/or heating or cooling) combined in a specific sequence to define a process from raw materials to a finished product. The example of cow’s milk (considered minimally processed by Nova) and plant-based alternatives (considered ultraprocessed by Nova) each undergo >8 unit operations including formulation with ingredients and additives (including synthetic fortificants) [15]. Dr. Ferruzzi closed by highlighting commonalities among ultra- or highly processed foods from existing classification systems [30]: 1) type and extent of processing of a raw agricultural product; 2) high degree of formulation, using multiple ingredients or “synthetic” food additives of both natural and synthetic origin, and 3) “industrialization” of the process. Therefore, UPFs are increasingly identified based on the product of raw agricultural commodities successively losing identity as the extent of post-harvest food processing increases as they are formulated into new products (Figure 2).
TABLE 1.
Definitions of terms used in creation of the Research Roadmap
| Food processing | The use of methods and techniques involving equipment, energy, and tools to transform agricultural products such as grains, meats, vegetables, fruits, and milk into food ingredients or finished food products [23]. |
| Processed food | A food material has been changed in some way through a combination of ingredients together with processing steps to make the food safe to eat, shelf-stable for future use, convenient to use (e.g., microwaveable dinners), tasty/palatable, (e.g., a milk chocolate bar), and/or more nutritious (e.g., breakfast cereals fortified with vitamins) [23]. |
| Nova Classification System of Food Processing | Nova classifies foods into the following 4 main groups: Group 1 includes unprocessed/minimally processed foods, Group 2 includes processed culinary ingredients, Group 3 includes processed foods, and Group 4 includes UPFs [2]. |
| Ultra-processed foods according to the Nova Classification System of Food Processing | Foods and beverages classified as ultra-processed by the Nova system (i.e., UPFs) are described as formulations of several ingredients including sugar, oils, fats, and salt and food substances of no or rare culinary use as well as food additives with cosmetic functions (e.g., colorings, artificial sweeteners) used to improve sensory properties of foods or beverages [2,64]. Note this is the definition of UPFs as provided by the Nova Classification System of Food Processing. There may be alternative definitions or classification systems using this term, but are not described here. |
| Formulation | The combination of ingredients and additives added and prepared according to prescribed methods to produce a product intended for further processing or ready for consumption. |
| Ingredient | Any material, crude or purified, that is added to food to produce a formulation and a desired effect on product quality and safety. |
| Additive | A food additive is a subclass of food ingredients as defined in Section 201(s) of the Federal Food, Drug, and Cosmetic (FD&C) Act as any substance the intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristic of any food (including any substance intended for use in producing, manufacturing, packing, processing, preparing, treating, packaging, transporting, or holding food; and including any source of radiation intended for any such use); if such substance is not GRAS or sanctioned before 1958 or otherwise excluded from the definition of food additives [24]. |
| Food matrix | The chemical and physical components of food and the molecular relationships that affect how food is digested and metabolized [25]. |
| Unit operations | Groups of basic steps in processing of ingredients and food, for example, drying, filtration, extrusion [26]. |
| Palatability, hyperpalatability | Palatability is the positive hedonic evaluation of a food’s sensory characteristics (i.e., taste, smell, texture, temperature, visual appearance, sound, and trigeminal input). Palatability is not a static feature of a food/beverage; it changes in response to sensory monotony and metabolic need state [27,28]. There is no agreed upon definition of hyper-palatability or recognition of the concept. Attempts to define hyper-palatability tend to focus on sensory properties of the foods/beverages or their nutrient combinations (e.g., fat and sugar, fat and sodium, carbohydrates, and sodium) crossing defined thresholds [57,102]. |
| Liking1 | The pleasantness of taste of food in the mouth. Note that this is different from the palatability [29]. |
| Food reward | The momentary value of a food to the individual at the time of ingestion [29]. |
| Satiation | Process that leads to the termination of eating; therefore, controls meal size. Also known as intra-meal satiety [110]. |
| Satiety | Process that leads to inhibition of further eating Also known as postingestive satiety or intermeal satiety. |
| Food environment | Includes the physical, social, and person-centered environments that play a role in what people choose to eat [112]. |
Abbreviations: FD&C, Food, Drug, and Cosmetic; GRAS, generally recognized as safe; UPF, ultra-processed food.
Liking in regard to ingestive behavior.
FIGURE 2.
Generalized summary of how food processing categorizations capture both formulation and processes. Ultra-processed foods are created by transforming raw agricultural commodities into a final product that successively lose identity (Food Identity, purple bar) as the extent of post-harvest food processing increases (from left to right). Nutritional value is perceived to decrease in a similar fashion (Perceived Nutritional Quality, gray bar). Blue boxes represent raw agricultural commodities or foods; green boxes foods are unprocessed or minimally processed foods that would be categorized as Nova Group 1; orange boxes represent processed foods that would be categorized as Nova Group 3; and red boxes represent foods that would be categorized as Nova Group 4, ultra-processed foods, and associated ingredients.
Processed foods and health flash talks
The "Food Processing Primer" was followed by flash talks focused on recent or ongoing clinical and epidemiological research evaluating the association between processed food intake and various health outcomes. Dr. Bruce Hamaker presented human clinical research studies and experiments with rodent models demonstrating how carbohydrate structure and processing affect glycemic response. For example, extrusion increases carbohydrate availability; starch retrogradation reduces carbohydrate availability; and particle size, food form, and viscosity affect glycemic response differentially. He also presented data showing minimal differences in glycemic response, gastric emptying, and appetite ratings after consumption of whole and refined grains that were matched for these properties as well as for wheat source [31]. Dr. Fang Fang Zhang followed with a presentation of the growing body of evidence, mostly from large prospective cohort studies, that consistently demonstrates a positive association with all-cause mortality [14,[32], [33], [34], [35], [36], [37]], cardiovascular disease risk and mortality [[38], [39], [40], [41]], hypertension [[42], [43], [44]], type 2 diabetes [[45], [46], [47], [48]], and obesity [[49], [50], [51], [52], [53]] in adults who consumed higher compared with lower amounts of UPFs, as defined by Nova. Evidence is limited and less consistent for cancer risk and mortality and childhood obesity [14]. Dr. Ciarán Forde then described the RESTRUCTURE project [54,55] that investigates whether texture-based differences in meal eating rate influence energy intake from UPFs over 2 wk [19]. Ongoing experiments are designed to address how complex meals, multiple meals, and dietary patterns that differ in selected properties and processing may influence ad libitum energy intake. Collectively, these examples provide evidence of how the physical, structural, and sensory properties of processed foods may influence habitual energy intakes and metabolic responses, related to risk for obesity and CMDs.
Critical review of potential mechanisms of how processed foods impact health
Drs Rick Mattes and Kevin Hall presented perspectives on potential mechanistic pathways linking UPF intake with negative health outcomes. Dr. Mattes described how proposed mechanisms fall into 3 categories: 1) food choice (e.g., low cost, prolonged shelf-life, packaging, hyper-palatability, and hunger stimulation/fullness suppression), 2) food composition (e.g., macronutrients, food texture, added sugars/salt/fat, energy density, low calorie sweeteners, and additives), and 3) digestive processes (e.g., oral processing effort, eating rate, gastric emptying time, gastrointestinal transit time, and microbiome). These are described in detail elsewhere [17]. However, no mechanism has directly or sufficiently explained associations between UPF intake and health outcomes in human studies, as previously described [17]. He noted that one limitation is the lack of clear and standardized definitions for exposures and outcomes of interest. For example: 1) an evolving definition of a UPF up until 2016 [56], 2) lack of agreement on the definition of hyper-palatable [57], 3) whether the definition of “energy density” should include beverages and high-water foods [58], and 4) ill-defined outcomes related to impacts on microbiota. Dr. Mattes concluded by stating that mechanisms and causality remain to be established for associations between UPF intake and health outcomes and it is important to start by clearly defining the concepts under consideration to efficiently guide research efforts.
Dr. Hall described a previous study conducted in his lab [18] which was a crossover, randomized, domiciled feeding study. Participants consumed 2 dietary patterns for 2 weeks each: one that contained ∼80% of total energy from UPFs and the other that contained ∼80% of total energy from minimally processed foods (that is, Nova Group 1). The dietary patterns were matched for presented energy, fiber, fat, sodium, sugar, carbohydrates, glycemic load, and overall energy density when including energy from beverages served with meals. When excluding beverages served with meals, the UPF diet had a substantially higher energy density than the minimally processed diet. The UPF meals were consumed more quickly (grams per minute) than the non-UPF meals when meal beverages were included in the calculation, but more slowly when excluding beverages [59]. Whether including or excluding beverages, the eating rate in terms of energy consumed per minute was significantly greater with the UPF dietary pattern. In a secondary analysis of 2 domiciled controlled feeding studies, Dr. Hall showed that non-beverage energy density and proportion of hyper-palatable foods (using a previously proposed definition of the term [57]) each explained ∼40% of the meal energy intake differences between the high UPF and minimally processed dietary patterns [59]. Dr. Hall closed by describing an ongoing study that is designed to determine the relative roles of non-beverage energy density and hyper-palatable foods on excess energy intake of dietary patterns high in UPFs [60].
March madness
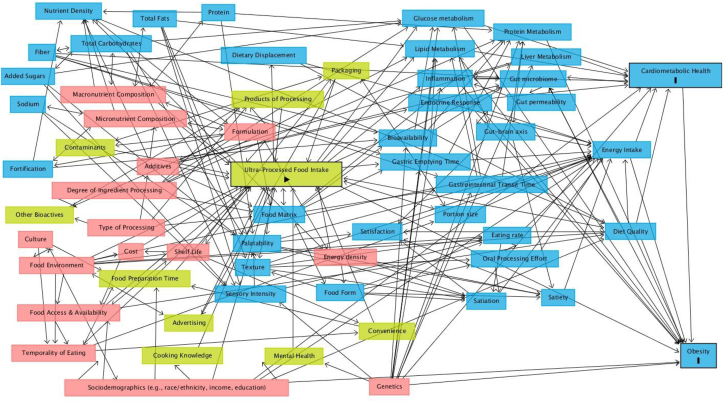
Attendees participated in a brainstorming session coined “March Madness,” in reference to a United States college basketball tournament ongoing near the time of the workshop. The objective of “March Madness” was to 1) develop a conceptual model of whether and how UPF intake may causally influence risk for obesity and CMDs and 2) identify priority RQs to guide future research on this topic. Five to 6 attendees with varied perspectives and expertise were assigned to groups to brainstorm variables that may explain associations between UPF intake (primary exposure) and risk for obesity and CMDs (primary outcomes), including antecedent, predictor, modifying, mediating, and confounding variables. After the workshop, the brainstormed variables were incorporated into a directed acyclic graph (DAG) using DAGitty (v3.0, http://www.dagitty.net/) to depict the proposed direct and indirect pathways that may explain observed associations between UPF intake and risk for obesity and CMDs (see Figure 3). The DAG was developed based on the variables and relationships discussed at the workshop; therefore, it is not an exhaustive list nor intended to depict the weight of available evidence.
FIGURE 3.
A directed acyclic graph (DAG) depicting discussed potential direct and indirect pathways that may explain observed associations between ultra-processed food intake and increased risk for obesity and cardiometabolic disease within the United States. Created using DAGitty (v3.0, http://www.dagitty.net/). The directionality of the arrows depicts a potential relationship between variables discussed at the workshop, not necessarily consensus that a causal relationship exists or the strength of the available evidence. The yellow boxes represent exposure variables; the blue boxes represent outcomes variables or ancestors of outcome variables; and the pink boxes represent ancestor of exposure and outcome variables. The arrow in the “Ulta-Processed Food Intake” box represents the primary exposure, and the dashes in the “Cardiometabolic Health” and “Obesity” boxes represent primary outcomes.
Research Roadmap
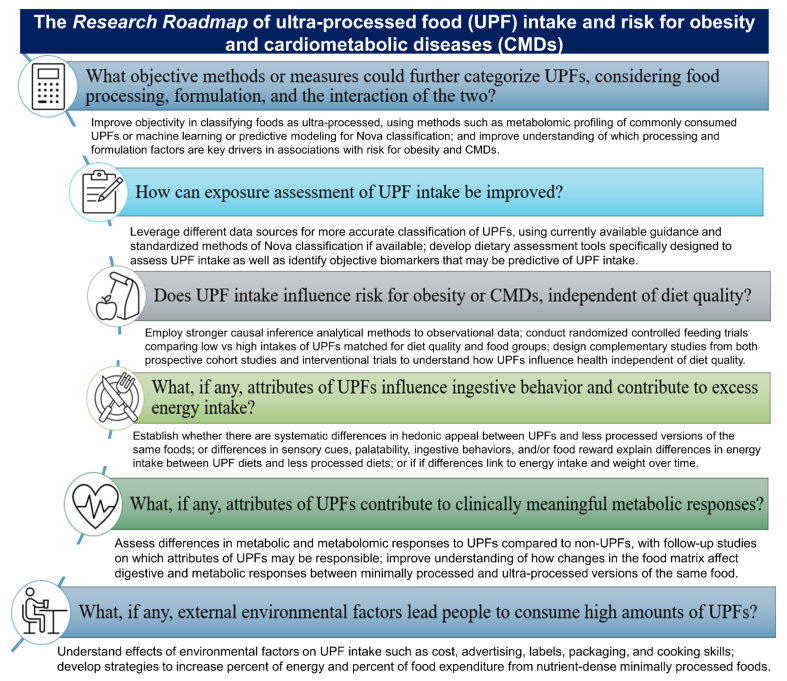
The Research Roadmap consists of the prominent themes from the breakout groups and the DAG, described above. The main RQs that constitute the Research Roadmap are presented in Figure 4. Breakout groups were convened to discuss what is known and unknown for each RQ and develop sub-RQs that could help address research gaps. After the workshop, the RQ summaries were further developed by the breakout groups to include relevant publications from ongoing and upcoming research related to each RQ as well as consultation with relevant experts to ensure a balanced representative of the available evidence; systematic literature searches were not conducted. We did not assign priority or order to the RQs included in the Research Roadmap because they cover a diverse array of topics and are highly interrelated.
FIGURE 4.
Research Roadmap about ultra-processed foods, food processing, and human health in the context of the United States food system. UPF, ultra-processed food according to the Nova Classification System for Food Processing.
What objective methods or measures could further categorize UPFs, considering food processing, formulation, and the interaction of the two?
Because of the heterogeneity of UPFs and complexity of food processing, there is a pressing need to improve objective categorization. One possible approach is stratification of UPFs by factors such as number of unit operations used to create the product, presence of additives and/or components of concern (e.g., added sugars, sodium), nutrient ratios (e.g., fiber: carbohydrate, potassium: sodium, energy: volume), and total number of ingredients. Similar schemes have been proposed previously to improve classification and account for ingredient quality, degree of transformation, food matrix effects, and nutrients of concern [61,62]. Predictive modeling approaches (e.g., principal component analysis, partial least-squares discriminant analysis) could explore common UPF characteristics or predictors of UPF designation. A recent effort provided a machine learning algorithm that predicted Nova classification for food and beverages included in the USDA’s Food and Nutrient Database for Dietary Studies (FNDDS) with 73% accuracy [63]. However, this algorithm relies only on the nutrient composition of the foods provided in the database. Although nutrient data are correlated with Nova classification, ingredient and food label information, not nutrient composition, is the main driver for determining Nova Group [64]. Thus, more research with novel approaches is needed to incorporate additional processing characteristics into processed food classification algorithms.
It is necessary to consider which processing and formulation factors of UPFs are key drivers in the observed associations with risk for obesity and CMDs. For example, do certain UPFs cluster together based on processing, formulation, and metabolomic profiles (nutritional "dark matter" beyond nutrient composition) and what characteristics do they share? Which processing and formulation factors correlate with or predict metabolomic profiles of UPFs? Combining information on processing and formulation factors (e.g., water activity, components of concern, thermal history, chemical interactions, pH) with targeted and untargeted metabolomics of UPFs may allow for refinement of UPF categorization. The identification of UPF clusters based on this information may reveal UPF subtypes that can be studied as exposures in future epidemiological datasets and feeding studies. Identified metabolomic signatures of UPFs may also be compared with in vivo metabolomic profiles of human participants after consumption of a dietary pattern high in UPFs (as demonstrated previously with compounds from salmon [65]). This approach could be used to determine whether risk for obesity or CMD outcomes could be predicted based on intake of specific types of UPFs or UPFs with certain characteristics, beyond nutrient composition alone. Ultimately, this research will facilitate more detailed investigation of the etiology of UPF intake and risk for obesity and CMD, and help identify whether specific UPF characteristics or components are mechanistically driving these associations. This type of research could also provide insightful information of how re-formulation or advancements in food processing technologies could lead to UPFs that are higher quality and more health-promoting.
How can exposure assessment of UPF intake be improved?
An integral piece in any dietary assessment method is the food composition table or database that is used to assign reported foods or beverages to corresponding food codes and converting to nutrients and food groups of interest. For example, in the United States, the data collected as part of What We Eat in America, the dietary component of the National Health And Nutrition Examination Survey (NHANES), is the basis for the foods and beverages included in the USDA's Food and Nutrient Database for Dietary Studies (FNDDS) [66,67]. FNDDS does not include data on characteristic components of UPFs, such as flavorings, colorings, emulsifiers, and other cosmetic additives. There are public and commercial data sources [68] (e.g., Scantrack [69], Homescan [69], and Total Store Advantage [70]) that could potentially be linked to FNDDS to obtain this information (e.g., ingredient lists of the most commonly purchased brand name) to aid in assigning a Nova group. However, NHANES data capture individual-level intake, and these commercial data sources capture store- or household-level purchase data, causing linking of databases to be a challenge. Where linking is not feasible, these datasets can also be used to corroborate results across different sources. If the same themes emerge, then that increases confidence in the results. Because of noted limitations in dietary intake and food composition databases and continuous changes to the food supply [71], researchers need to make assumptions to apply Nova to their datasets. A recent paper explored that various classification decisions or assumptions may change estimates of percent energy from UPFs in the United States population aged 2+ y old by up to 10% [64]. Standardization of Nova use was a common concern among workshop attendees. There is evolving guidance for the research community on how to apply the Nova classification system to dietary databases [72]. For example, there is a standardized method available for public use of applying Nova to the NHANES data, as well as data collected via dietary assessment tools that link to the same underlying database such as the National Cancer Institute's Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool [64].
Beyond database limitations, current dietary assessment tools do not probe respondents for information needed to make accurate Nova classifications. This is particularly a concern for food-frequency questionnaire (FFQ), but 24-hour (24-h) recalls, which provide food-level information, often also lack needed detail (e.g., whether foods are prepared at home or brand names) [64,72]. In addition to retro-fitting existing tools, new FFQs are available [73,74], and 24-h dietary recall instruments are in development, that specifically assess level of processing according to Nova. Novel technologies such as sensor-based technologies or continuous passive monitoring could better capture intake, and provide more detail on ingredients, packaging, composition, and amount consumed [75]. These novel approaches could be used within existing cohorts or for newly developed studies. However, it is important to note that any tool for estimating dietary intake is still reliant on the quality of the data included in the dietary databases, as discussed above, as well as participant recall and reporting. Objective measures of intake, such as metabolites that are predictive of UPF intake [[76], [77], [78]], could also help corroborate self-reported data to reduce measurement error. In addition, cross-pollination between existing food processing classification systems [30] within the same datasets may help refine UPF definitions [[79], [80], [81], [82]] and identify specific UPF characteristics to support mechanistic health research.
Does UPF intake influence risk for obesity or CMDs, independent of diet quality?
A point of contention is whether classifying foods based on processing adds values beyond convention measures of diet quality that do not consider food processing [11,12]. It is unclear if associations between UPF intake and health outcomes are independent of the overall quality of the dietary pattern in which high amounts of UPFs are often consumed. It is common for epidemiological studies that assess associations between UPF intake and health outcomes to adjust for diet quality using various metrics that do not consider processing directly [40,41,[83], [84], [85]], such as the Healthy Eating Index (HEI), Nutri-Score, Dietary Approaches to Stop Hypertension, Mediterranean-Dietary Approaches to Stop Hypertension diet intervention for neurodegenerative delay, or the alternative-HEI. A systematic review found that most studies that reported associations between UPFs and health outcomes remained statistically significant and similar in magnitude after adjustment for diet quality or certain types of dietary patterns [86]. However, the impact of overall diet quality on these associations may be overshadowed in United States datasets because of low diet quality among most of the population [13] as well as high measurement error associated with self-reported dietary intake data [87]. Comparing effect estimates before and after adjustment using multiple diet quality metrics that do not consider processing can help determine the confounding effect of background diet quality. In addition, UPFs encompass a heterogenous group of foods, including both nutrient-dense and nutrient-poor options. To determine whether associations are driven by food processing or nutrient characteristics of UPFs, it is necessary to further differentiate associations with health outcomes among various UPF subtypes that have different nutritional quality. For example, several nutrient-dense foods such as whole grain cereals or lightly sweetened yogurts are considered UPFs. Disease risk associated with these foods likely differs from other types of UPFs, such as processed meats or sugar-sweetened beverages, although current findings are mixed [40,41]. Finally, epidemiological approaches using stronger analytical methods could be employed to infer causality [88], yet still rely on improvements proposed in the two previous RQs described above and the limitations inherent to observational evidence.
Dietary guidance is strongest when there is corroboration across multiple evidence types. Epidemiological studies that use CMD endpoints (e.g., cardiac events, incident diabetes) could be used to inform design of randomized controlled trials using intermediate health outcomes (for example, LDL cholesterol, apolipoprotein B, glycemic response). Leveraging complementary studies of this nature can better inform causal inference on complex topics that cannot be studied directly [89,90]. A randomized controlled trial matching dietary patterns for overall quality and food group intake is needed. Ongoing modeling experiments show that it is possible to achieve a high HEI diet with ≥80% energy from UPFs, but some inherent properties of UPFs, such as higher sodium or added sugars, make this challenging [91,92]. Studies that are matched for all or most nutritional factors can help isolate effects of processing [93]. However, there is also value in studies that allow characteristics of UPFs to vary (for example, letting added sugars or sodium vary) to isolate other potential mechanisms not necessarilty specific to Nova Group assignment. Furthermore, UPFs are (by definition) not home-made in domestic settings; thus, intervention studies are needed to investigate how consuming foods prepared at home using conventional cooking methods compared with pre-prepared/packaged differentially affects health outcomes. Moving forward, there is a need for simultaneous research from both well-conducted non-randomized studies and interventional trials to understand how UPFs influence health outcomes independent of overall diet quality.
What, if any, attributes of UPFs influence ingestive behavior and contribute to excess energy intake?
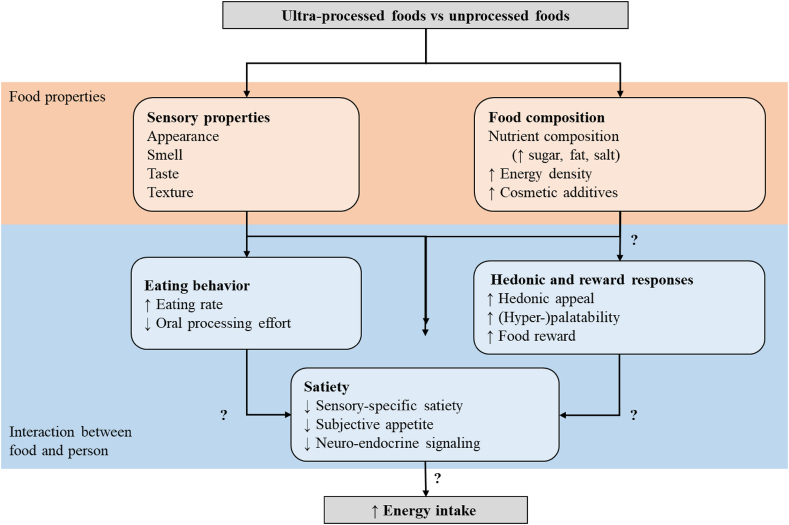
Associations between UPFs and obesity have led to speculation that the sensory, hedonic, reward, and satiety properties of UPF promote excess energy intake and positive energy balance (Figure 5). “Heightened product appeal” is a correlate of UPFs [2,94]. There is speculation that the unique composition of UPF (that is, the ratio of salt/fat, sugar/fat and carbohydrate/salt) make them “hyper-palatable” relative to less-processed versions of the same product, increasing the drive to consume and reward value [59,95]. However, specific UPF properties (e.g., sensory properties, hedonic appeal) need to be linked to distinct mechanisms (e.g., meal initiation, satiation, satiety, food reward, behavioral reinforcement learning) that influence energy intake. Palatability is determined by many factors, including sensory properties (e.g., appearance, smell, taste, texture) and metabolic need state (Table 1). Food intake increases when a food is more liked [[96], [97], [98], [99], [100], [101], [102]]. UPFs are proposed to have a level of appeal that is higher than traditionally liked foods but is not formally tested. Even the most appealing food decreases in liking during consumption via “sensory-specific satiety.” One hypothesis is that UPFs promote sustained intake and delayed onset of sensory-specific satiety compared with less-processed foods [103], but this has not been demonstrated. Taste (particularly sweetness and saltiness) outside of the context of palatability is also a proposed mechanism of how UPFs increase energy intake. However, there is limited evidence to show that differences in UPFs with high levels of sugar, fat, salt, or cosmetic additives contribute in a novel way to the hedonic drive to consume quantities above other liked non-UPFs. Food texture, which is influenced by processing and formulation, influences meal eating rate and acute and prolonged energy consumption [104,105] and UPFs tend to be consumed faster than less-processed foods [106], but the evidence is mixed [59]. Therefore, food texture may be a better predictor of acute meal energy intake than degree of processing [19,107]. Although sensory properties and palatability are important for food selection and portion size, they only encompass a portion of factors that influence food choice, appetitive sensations, and total energy intake [14,19] and tend to only be evaluated in short-term studies. It remains unclear whether differences in energy intake based on hedonics or degree of processing are sustained long term (that is, ≥4 wk), contributing to prolonged positive energy balance, and weight gain. Such studies would ideally account for individual differences in preferred sensory properties of foods and how they change in different contexts and over the lifespan. It is possible that reinforcement associated with UPFs increases subconscious wanting, which is a distinct reward construct underlying motivated behavior and incentive salience [108,109]. Research is needed to test whether UPFs differ from isoenergetic, equi-palatable minimally processed foods in post-prandial subjective appetitive responses, neuroendocrine satiety signaling (e.g., ghrelin, cholecystokinin), neural reward responses, and acute and long-term energy intake.
FIGURE 5.
Potential pathways that the sensory, hedonic, reward, and satiety properties of ultra-processed foods promote excess energy intake and positive energy balance.
Future research is needed to test whether unique hedonics, sensory properties, or other aspects of UPFs result in consistent and sustained differences in appetite on a calorie for calorie basis. Research is needed to establish 1) whether there is a clear and systematic difference in hedonic appeal and palatability between UPF and less-processed versions of the same foods, meals, and diets; 2) whether differences in sensory cues, palatability, ingestive behaviors, and/or food reward explain observed differences in energy intake between UPF diets and less-processed diets; and 3) whether these differences are linked to sustained positive energy balance and weight gain over relevant time periods (that is, ≥6 mo). These mechanisms should be tested in clinical trials with appropriate controls, ideally separately from other known effects (e.g., portion size, energy density), using best practices for measuring appetite and energy intake [110]. When these mechanisms have been formally tested, and their relative contribution to energy intakes and health outcomes has been quantified, it will be possible to prioritize re-formulation targets and processing techniques for future food products.
What, if any, attributes of UPFs contribute to clinically meaningful metabolic responses?
Certain characteristics of UPFs (e.g., high sodium, saturated fat, and added sugars and low fiber content) are established public health concerns that increase risk for obesity and CMDs when consumed above or below recommended amounts [13]. High intake of UPFs may also displace intake of minimally processed foods, nutrient-dense foods, including fruits, vegetables, and other foods associated with positive health outcomes, but that are often under-consumed in the United States [16]. However, knowledge gaps remain in how UPFs may mechanistically influence metabolic health beyond nutrient composition and diet quality. Research is needed to understand differences in metabolic and metabolomic response to UPFs compared with non-UPFs. This would include factors such as absorption, digestion, metabolism, and excretion; microbiota composition and function; glycemic response; insulin sensitivity; hemodynamics; vascular function; inflammation; gut hormone secretion; inflammation; and blood lipid profile. Multi-omic approaches need to be understood to identify acute and longer-term changes in physiological response. If evidence is supportive of a differential response, then follow-up studies are needed to investigate which attributes of UPFs may be responsible, potentially using approaches described in other RQs. These attributes may include presence of specific ingredients (e.g., additives, emulsifiers, stabilizers, flavors, modified starches, fibers) or processing-induced compounds (e.g., acrylamide, advanced glycation end products). Finally, research is needed to understand if changes in the food matrix affect digestive and metabolic responses as a food moves along the continuum from minimally processed to ultra-processed [111]. It would first be appropriate to conduct a systematic or scoping review on these questions, because research on these topics exists but may not have been viewed through the UPF lens. Leveraging preclinical and clinical models is needed but exposures of interest (e.g., additives, emulsifiers) must be reflective of realistic doses in the food or food supply; thus, supraphysiological levels in assays or trials will not help advance understanding on this topic.
What, if any, external environmental factors lead people to consume high amounts of UPFs?
The food environment includes the physical, social, and person-centered environments that play a role in what people can or choose to eat [112]. Aside from taste and liking, food intake is largely driven by environmental factors such as accessibility, availability, cost, convenience, storage, access to cooking facilities, and consumers’ nutrition knowledge and beliefs [[113], [114], [115]]. It is critical to understand how food environment characteristics across the United States contribute to high UPF selection and consumption. Investigation of the effect of environmental factors on UPF intake should consider cost, advertising, labels, and packaging as characteristics that are highly correlated with UPF designation [56]. In addition, high UPF intake is correlated with low cooking skills [116]. Future research should include clearly defined outcomes that are related to the food environment [112] and specifically contrast effects across and within high intake of Nova categories. Percent of energy intake and percent of food expenditure from UPFs are examples of relevant outcomes. However, the inverse also needs to be considered, that is, strategies to increase the percent of energy and percent of food expenditure from nutrient-dense minimally processed foods, such as fresh fruits and vegetables. Dimensions of the food environment and consumer knowledge/understanding that are relevant for examination include cost, convenience, storage, packaging, availability, accessibility (presence/absence of stores, presence/absence of foods in stores), health information, labels, and desirability to purchase or consume such foods. Mechanistic analyses should consider covariates or confounders such as age, body mass index, ethnicity, geography, educational, income, and sex. The National Collaborative on Childhood Obesity Research [112] provides detailed definitions of food environment concepts as well as metrics, tools, and guidance which can be applied to investigate this topic.
Multiple research approaches can be leveraged to evaluate how the food environment affects selection and consumption of foods across Nova categories, including 1) observational data analysis (e.g., NHANES; Corsana Consumer Network, and Nielsen Homescan); 2) controlled experiments and randomized controlled trials on food choice and retail environments [117]; and 3) evaluations of interventions with free-living individuals. Meaningful and important work on food environments and food choice is being conducted by many researchers (see reference list in National Collaboration on Childhood Obesity Research [112] for examples), and this work has been increasingly supported by major research funders in the United States [e.g., the National Institutes of Health and the USDA’s National Institute of Food and Agriculture (NIFA)]. However, there is a need for proper metrics of measuring type and degree of processing in the context of food environment research, as discussed in first two RQs. It is common for food environment research to use brief dietary screeners rather than more detailed instruments, such as 24-h recalls, that can be used to estimate UPF intake with more granularity and less misclassification [118], as discussed in previous RQs. In addition, sub-categorizations of UPFs, such as the 41 pre-defined Nova subgroups [64] or additional novel gradations within Nova Group 4, would be beneficial to food environment research. However, this would require use of more detailed and time-consuming dietary assessment tools. It is important to note that incorporating dietary assessment of UPFs in food environment research will still be limited by the concerns outlined about proper classification of UPFs and dietary assessment methods to estimate UPF intake.
Additional Considerations
A panel was convened at the workshop to address additional considerations of UPF intake beyond health outcomes. The panel consisted of professionals from academia, government, and industry (Drs Kirsten A Herrick, Andrew Brown, Sean Cash, Lydia Kaume, Janice Rueda, Kevin Miller). Concerns were raised about assessing UPF intake with our currently available tools, particularly FFQs and databases that pre-date Nova. This was such a predominant and shared concern that it became an RQ as described in detail above. These concerns are in addition to the existing well-characterized limitations of self-reported nutrition and dietary exposures [87]. Consumer communication was also a common theme. The term “packaged foods” was posed as a potential alternative due to more consumer familiarity with the term than “processed food.” However, it was noted that UPFs are not necessarily synonymous with packaged foods, using packaged fresh fruits as an example. It was also noted that there is an opportunity for all sectors to better communicate about processed foods and food processing and address terms that are used beyond their technical definition, such as “processing” or “formulation.” Formulation and processing, although inherently linked, present different concepts that are communicated under the term “processing.” Formulation and processing are driven by multiple factors including, but not limited to, nutritional targets, ingredient properties, and price point for products [119]. It was noted that the lack of transparency regarding food processing steps and process-derived compounds such phthalates, bisphenols, mineral oils, and microplastics that can migrate from contact packaging to foods, should also be considered in future discussions.
Panelists were asked about the question being reviewed by the current DGAC: “What is the relationship between consumption of dietary patterns with varying amounts of ultraprocessed foods and growth, size, body composition, risk of overweight and obesity, and weight loss and maintenance?” Attendees largely agreed that the evidence will likely be weak because of a paucity of experimental or mechanistic studies related to UPFs. Dietary guidance is best informed by corroboration across various evidence types. Therefore, continual cross-discipline efforts are needed to design complementary high-quality non-randomized studies, randomized controlled trials, as well as mechanistic research to best inform this question for the DGAC. With international attendees, it was noted that a number of countries are considering guidance around UPF consumption [e.g., Qatar, the United States, the Netherlands, Nordic countries, and the UK (although there is opposition in the UK [120])]. In addition to established guidance around UPF intake in Brazil [9] and Canada [10], a municipality in Brazil recently prohibited the sale, marketing, and distribution of UPFs in schools [121]. Guidance around UPF intake in the United States would pose challenges given that UPFs constitute ∼60% of energy intake in the United States [5], compared with ∼30% in other countries that have implemented guidance [122]. It is currently possible to model dietary patterns that have high adherence to the DGAs (as measured by the HEI-2015) as well as high percent of total energy intake from UPFs (≥80%) [91,92]. There was discussion that implementing UPF guidance in the DGA may pose additional challenges for the government food and nutrition programs that the DGA guide, such as the National School Lunch Program, Supplemental Nutrition Assistance Program, and the Women, Infants, and Children Program [123]. For example, replacing ready-to-heat or ready-to-eat foods that are considered ultra-processed according to Nova with nutritionally comparable but less-processed versions from scratch may increase food cost, staffing, equipment needs, as well as waste, as noted by others [120,124,125].
Public-private partnerships (PPPs) were discussed as means to fund research related to the Research Roadmap. Guardrails are needed to ensure appropriate funding allocation, scientific rigor, and integrity. For example, the USDA’s NIFA abides by established federal regulations managed by the Office of Management and Budget Guidance for Grants and Agreements, as well as peer review practices. Examples of successful PPPs are the USDA Global Branded Food Product Database where industry provides nutrient composition of their branded food products to be included in the USDA FoodData Central for public access [66], and the Small Business Innovation Research program by the United States Small Business Administration for enabling research and development through researcher partnerships with small businesses. A PPP directly related to UPFs is the RESTRUCTURE Project [54], described above, which has an academic steering committee, an advisory committee including industry experts, and an independent party that handles project communications. The importance of optics and transparency of industry partnerships cannot be overstated for legitimacy and public trust [126].
Conclusion
In conclusion, this Research Roadmap outlines future directions to develop a stronger, more balanced evidence base to advance the understanding of how UPF consumption influences risk for obesity and CMDs. The RQs highlight the need for advances in methodological and mechanistic research to better understand whether observed associations between UPFs and risk for obesity and CMDs are causal, and if so, by what mechanisms. Subjectivity in UPF classification and heterogeneity of foods and beverages classified as UPFs remains a challenge, and advancements in classification and intake assessments are foundational to support future research. The described RQs are highly interrelated, dependent on one another, and will require diverse and interdisciplinary teams to conduct complementary studies across a variety of methodologies to advance knowledge on how UPF intake influences health outcomes. These advances can help better identify and prioritize public health strategies to optimize the quality of processed foods to improve overall diet quality and reduce the burden of chronic disease in United States populations.
Acknowledgments
We thank all workshop participants for their engagement and enthusiasm during the development of this research roadmap. We also thank Lydia Kaume, administrator of the grant at NIFA, for her perspective and participation at the workshop. The planning committee who obtained the NIFA planning grant included individuals from the USDA-ARS (Lauren E O’Connor, Kelly A Higgins, David Baer, Janet Novotny) and ADM (Robert Bergia, Katarina Smiljanec). Janice Rueda (ADM), Kevin Miller (General Mills), Cindy Davis (USDA-ARS), Andrew Brown (Arkansas Children’s Research Institute and University of Arkansas for Medical Sciences), Mario Ferruzzi (Arkansas Children’s Nutrition Center), and Sylvia Rowe (SR Strategy) provided special input and logistical guidance. All workshop costs were supported by USDA-NIFA grant #2022-07671. The workshop was held at the Hotel Hot Springs in Hot Springs, Arkansas on March 1–2, 2023. Attendees from academic institutions were provided an honorarium from the grant to subsidize travel expenses. Attendees from government or food industry paid their own travel expenses.
Author contributions
The authors’ responsibilities were as follows—LEO, KAH, KS, RB: designed and conducted the workshop; LEO, KAH: wrote the paper with contributions from all coauthors; LEO: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Conflict of interest
Disclosures at the time of the workshop were collected using the American Society for Nutrition’s disclosure form that asks for relevant disclosures within the prior 3 y. Noted disclosures from all attendees were compiled into a table and are included as online supplementary material.
Funding
This work is supported by the USDA National Institute of Food and Agriculture #2022-07671.
Disclaimer
CD is on the Advances in Nutrition editorial board and played no role in the journal's evaluation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.09.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Weaver C.M., Dwyer J., Fulgoni V.L., King J.C., Leveille G.A., MacDonald R.S., et al. Processed foods: contributions to nutrition. Am. J. Clin. Nutr. 2014;99(6):1525–1542. doi: 10.3945/ajcn.114.089284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro C.A., Cannon G., Levy R.B., Moubarac J.-C., Lc Louzada M., Rauber F., et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–941. doi: 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Martínez Steele E., Du M., Pomeranz J.L., O'Connor L.E., Herrick K.A., et al. Trends in consumption of ultraprocessed foods among US youths aged 2–19 years. JAMA. 2021;326(6):519–530. doi: 10.1001/jama.2021.10238. 1999–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.L.E. O’Connor, E. Martinez-Steele, L. Wang, F. Zhang, K.A. Herrick, Food processing, according to the Nova classification system, and dietary intake of US infants and toddlers, J. Nutr. 153, 2413–2420, 10.1016/j.tjnut.2023.06.020. [DOI] [PMC free article] [PubMed]
- 5.Juul F., Parekh N., Martinez-Steele E., Monteiro C.A., Chang V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022;115(1):211–221. doi: 10.1093/ajcn/nqab305. [DOI] [PubMed] [Google Scholar]
- 6.Askari M., Heshmati J., Shahinfar H., Tripathi N., Daneshzad E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int. J. Obes. (Lond). 2020;44(10):2080–2091. doi: 10.1038/s41366-020-00650-z. [DOI] [PubMed] [Google Scholar]
- 7.Moradi S., Ali Hojjati Kermani M., Bagheri R., Mohammadi H., Jayedi A., Lane M.M., et al. Ultra-processed food consumption and adult diabetes risk: a systematic review and dose-response meta-analysis. Nutrients. 2021;13(12):4410. doi: 10.3390/nu13124410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Zhang Z., Yang H., Qiu P., Wang H., Wang F., et al. Consumption of ultra-processed foods and health outcomes: a systematic review of epidemiological studies. Nutr. J. 2020;19(1):86. doi: 10.1186/s12937-020-00604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Agriculture Organization of the United Nations Food-Based Dietary Guidelines – Brazil. https://www.fao.org/nutrition/education/food-based-dietary-guidelines/regions/countries/brazil/en/ [Internet] [cited May 20, 2023]. Available from:
- 10.Government of Canada Canada’s Dietary Guidelines. https://food-guide.canada.ca/en/guidelines/ [Internet] [cited May 20, 2023]. Available from:
- 11.Monteiro C.A., Astrup A. Does the concept of "ultra-processed foods" help inform dietary guidelines, beyond conventional classification systems? YES. Am. J. Clin. Nutr. 2022:116 1476–1481. doi: 10.1093/ajcn/nqac122. [DOI] [PubMed] [Google Scholar]
- 12.Astrup A., Monteiro C.A. Does the concept of "ultra-processed foods" help inform dietary guidelines, beyond conventional classification systems? NO. Am. J. Clin. Nutr. 2022;116:1476–1481. doi: 10.1093/ajcn/nqac122. [DOI] [PubMed] [Google Scholar]
- 13.United States Department of Agriculture and Department of Health and Human Services 2020-2025 Dietary Guidelines for Americans. https://www.dietaryguidelines.gov/sites/default/files/202012/Dietary_Guidelines_for_Americans_2020-2025.pdf [Internet] [cited May 20, 2023]. Available from:
- 14.Srour B., Kordahi M.C., Bonazzi E., Deschasaux-Tanguy M., Touvier M., Chassaing B., et al. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022;7(12):1128–1140. doi: 10.1016/S2468-1253(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 15.Forde C.G., Decker E.A. The importance of food processing and eating behavior in promoting healthy and sustainable diets. Annu. Rev. Nutr. 2022;42:377–399. doi: 10.1146/annurev-nutr-062220-030123. [DOI] [PubMed] [Google Scholar]
- 16.Juul F., Deierlein A.L., Vaidean G., Quatromoni P.A., Parekh N. Ultra-processed foods and cardiometabolic health outcomes: from evidence to practice. Curr. Atheroscler. Rep. 2022;24(11):849–860. doi: 10.1007/s11883-022-01061-3. [DOI] [PubMed] [Google Scholar]
- 17.Valicente V.M., Peng C.-H., Pacheco K.N., Lin L., Kielb E.I., Dawoodani E., et al. Ultra-processed foods and obesity risk: a critical review of reported mechanisms. Adv. Nutr. 2023:718–738. doi: 10.1016/j.advnut.2023.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall K.D., Ayuketah A., Brychta R., Cai H., Cassimatis T., Chen K.Y., et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77.e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teo P.S., JiaYing Lim A., Ting Goh A., J. R, Michelle Choy J.Y., McCrickerd K., et al. Texture-based differences in eating rate influence energy intake for minimally processed and ultra-processed meals. Am. J. Clin. Nutr. 2022;116(1):244–254. doi: 10.1093/ajcn/nqac068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark C., Thomas C., Mitchell G., Tetlock P.E. Keep your enemies close: adversarial collaborations will improve behavioral science. J. Appl. Res. Mem. Cogn. 2022;11(1):1–18. [Google Scholar]
- 21.Monteiro C.A., Bertazzi Levy R., Moreira Claro R., Rugani Ribeiro de Castro I., Cannon G. A new classification of foods based on the extent and purpose of their processing. Cad Saude Publica. 2010;26(11):2039–2049. doi: 10.1590/s0102-311x2010001100005. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009;12(5):729–731. doi: 10.1017/S1368980009005291. [DOI] [PubMed] [Google Scholar]
- 23.Institute for Food Technologists . 2022. Get the Facts: Food Processing.https://www.ift.org/-/media/policy-advocacy/ift-comments/efsa/ift-food-processing-toolkit.pdf [Internet] [cited May 20, 2023]. Available from: [Google Scholar]
- 24.United States Food and Drug Administration . 2010. Overview of Food Ingredients, Additives & Colors.https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors#introduction [Internet] [cited May 20, 2023]. Available from: [Google Scholar]
- 25.Capuano E., Oliviero T., van Boekel M. Modeling food matrix effects on chemical reactivity: challenges and perspectives. Crit. Rev. Food Sci. Nutr. 2018;58(16):2814–2828. doi: 10.1080/10408398.2017.1342595. [DOI] [PubMed] [Google Scholar]
- 26.Eale R.L. 1983. Unit of Operations in Food Processing. CHAPTER 1: Basic Principles of Food Process Engineering. [Google Scholar]
- 27.Sorensen L.B., Møller P., Flint A., Martens M., Raben A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int. J. Obes. Relat. Metab. Disord. 2003;27(10):1152–1166. doi: 10.1038/sj.ijo.0802391. [DOI] [PubMed] [Google Scholar]
- 28.McCrickerd K., Forde C.G. Sensory influences on food intake control: moving beyond palatability. Obes. Rev. 2016;17(1):18–29. doi: 10.1111/obr.12340. [DOI] [PubMed] [Google Scholar]
- 29.Rogers P.J., Hardman C.A. Food reward. What it is and how to measure it. Appetite. 2015;90:1–15. doi: 10.1016/j.appet.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 30.de Araujo T.P., de Moraes M.M., Afonso C., Santos C., Rodrigues S.S.P. Food processing: comparison of different food classification systems. Nutrients. 2022;14(4):729. doi: 10.3390/nu14040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pletsch E.A., Hayes A.M.R., Chegeni M., Hamaker B.R. Matched whole grain wheat and refined wheat milled products do not differ in glycemic response or gastric emptying in a randomized, crossover trial. Am. J. Clin. Nutr. 2022;115(4):1013–1026. doi: 10.1093/ajcn/nqab434. [DOI] [PubMed] [Google Scholar]
- 32.Bonaccio M., Di Castelnuovo A., Costanzo S., De Curtis A., Persichillo M., Sofi F., et al. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study. Am. J. Clin. Nutr. 2021;113(2):446–455. doi: 10.1093/ajcn/nqaa299. [DOI] [PubMed] [Google Scholar]
- 33.Schnabel L., Kesse-Guyot E., Allès B., Touvier M., Srour B., Hercberg S., et al. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern. Med. 2019;179(4):490–498. doi: 10.1001/jamainternmed.2018.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rico-Campa A., Martínez-González M.A., Alvarez-Alvarez I., de Deus Mendonça R., de la Fuente-Arrillaga C., Gómez-Donoso C., et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. doi: 10.1136/bmj.l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero Ferreiro C., Martín-Arriscado Arroba C., Cancelas Navia P., Lora Pablos D., Gómez de la Cámara A. Ultra-processed food intake and all-cause mortality: DRECE cohort study. Public Health Nutr. 2021;25:1–10. doi: 10.1017/S1368980021003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanco-Rojo R., Sandoval-Insausti H., López-Garcia E., Graciani A., Ordovás J.M., Banegas J.R., et al. Consumption of ultra-processed foods and mortality: a National prospective cohort in Spain. Mayo Clin. Proc. 2019;94(11):2178–2188. doi: 10.1016/j.mayocp.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Dehghan M., Mente A., Rangarajan S., Mohan V., Swaminathan S., Avezum A., et al. Ultra-processed foods and mortality: analysis from the prospective urban and rural epidemiology study. Am. J. Clin. Nutr. 2023;117(1):55–63. doi: 10.1016/j.ajcnut.2022.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Chen X., Chu J., Hu W., Sun N., He Q., Liu S., et al. Associations of ultra-processed food consumption with cardiovascular disease and all-cause mortality: UK Biobank. Eur. J. Public Health. 2022;32(5):779–785. doi: 10.1093/eurpub/ckac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du S., Kim H., Rebholz C.M. Higher ultra-processed food consumption is associated with increased risk of incident coronary artery disease in the atherosclerosis risk in communities study. J. Nutr. 2021;151(12):3746–3754. doi: 10.1093/jn/nxab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juul F., Vaidean G., Lin Y., Deierlein A.L., Parekh N. Ultra-processed foods and incident cardiovascular disease in the Framingham offspring study. J. Am. Coll. Cardiol. 2021;77(12):1520–1531. doi: 10.1016/j.jacc.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 41.Srour B., Fezeu L.K., Kesse-Guyot E., Allès B., Méjean C., Andrianasolo R.M., et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante) BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scaranni P., de Oliveira Cardoso L., Chor D., Caetano Prates Melo E., Maria Alvim Matos S., Giatti L., et al. Ultra-processed foods, changes in blood pressure and incidence of hypertension: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Public Health Nutr. 2021;24(11):3352–3360. doi: 10.1017/S136898002100094X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezende-Alves K., Hermana Miranda Hermsdorff H., Elizabeth da Silva Miranda A., Cristine Souza Lopes A., Bressan J., Marçal Pimenta A., et al. Food processing and risk of hypertension: Cohort of Universities of Minas Gerais, Brazil (CUME Project) Public Health Nutr. 2021;24(13):4071–4079. doi: 10.1017/S1368980020002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monge A., Canella D.S., López-Olmedo N., Lajous M., Cortés-Valencia A., Stern D., et al. Ultraprocessed beverages and processed meats increase the incidence of hypertension in Mexican women. Br. J. Nutr. 2021;126(4):600–611. doi: 10.1017/S0007114520004432. [DOI] [PubMed] [Google Scholar]
- 45.Srour B., Fezeu L.K., Kesse-Guyot E., Allès B., Debras C., Druesne-Pecollo N., et al. Ultraprocessed food consumption and risk of Type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern. Med. 2020;180(2):283–291. doi: 10.1001/jamainternmed.2019.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llavero-Valero M., Escalada-San Martín J., Martínez-González M.A., Javier Basterra-Gortari F., de la Fuente-Arrillaga C., Bes-Rastrollo M., et al. Ultra-processed foods and type-2 diabetes risk in the SUN project: a prospective cohort study. Clin. Nutr. 2021;40(5):2817–2824. doi: 10.1016/j.clnu.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 47.Levy R.B., Rauber F., Chang K., Laura da C Louzada M., Monteiro C.A., Millett C., et al. Ultra-processed food consumption and type 2 diabetes incidence: a prospective cohort study. Clin. Nutr. 2021;40(5):3608–3614. doi: 10.1016/j.clnu.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Duan M.J., Vinke P.C., Navis G., Corpeleijn E., Dekker L.H. Ultra-processed food and incident type 2 diabetes: studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective Lifelines cohort. BMC Med. 2022;20(1):7. doi: 10.1186/s12916-021-02200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordova R., Kliemann N., Huybrechts I., Rauber F., Vamos E.P., Bertazzi Levy R., et al. Consumption of ultra-processed foods associated with weight gain and obesity in adults: a multi-national cohort study. Clin. Nutr. 2021;40(9):5079–5088. doi: 10.1016/j.clnu.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Rauber F., Chang K., Vamos E.P., Laura da Costa Louzada M., Augusto Monteiro C., Millett C., et al. Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur. J. Nutr. 2021;60(4):2169–2180. doi: 10.1007/s00394-020-02367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M., Shi Z. Ultra-processed food consumption associated with overweight/obesity among Chinese adults-results from China health and nutrition survey 1997–2011. Nutrients. 2021;13(8):2796. doi: 10.3390/nu13082796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beslay M., Srour B., Méjean C., Allès B., Fiolet T., Debras C., et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Santé cohort. PLOS Med. 2020;17(8) doi: 10.1371/journal.pmed.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendonca R.D., Marçal Pimenta A., Gea A., de la Fuente-Arrillaga C., Angel Martinez-Gonzalez M., et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016;104(5):1433–1440. doi: 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- 54.Restructure. https://restructureproject.org/ [Internet] [cited May 20, 2023]. Available from:
- 55.Clinicaltrials.gov Sustained Effect of Food Texture of Ultra-processed Foods on Energy Intake. https://clinicaltrials.gov/ct2/show/NCT05561426 [Internet] [cited May 20, 2023]. Available from:
- 56.Gibney M.J. Ultra-processed foods: definitions and policy issues. Curr. Dev. Nutr. 2019;3(2):nzy077. doi: 10.1093/cdn/nzy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fazzino T.L., Rohde K., Sullivan D.K. Hyper-palatable foods: development of a quantitative definition and application to the US food system database. Obesity (Silver Spring). 2019;27(11):1761–1768. doi: 10.1002/oby.22639. [DOI] [PubMed] [Google Scholar]
- 58.Mattes R. Fluid calories and energy balance: the good, the bad, and the uncertain. Physiol. Behav. 2006;89(1):66–70. doi: 10.1016/j.physbeh.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 59.Fazzino T.L., Courville A.B., Guo J., Hall K.D. Ad libitum meal energy intake is positively influenced by energy density, eating rate and hyper-palatable food across four dietary patterns. Nat. Food. 2023;4:144–147. doi: 10.1038/s43016-022-00688-4. [DOI] [PubMed] [Google Scholar]
- 60.Clinicaltrials.gov Effect of Ultra-processed Versus Unprocessed Diets on Energy Metabolism. https://clinicaltrials.gov/ct2/show/NCT05290064 [Internet] [cited May 20, 2023]. Available from:
- 61.Davidou S., Christodoulou A., Fardet A., Frank K. The holistico-reductionist Siga classification according to the degree of food processing: an evaluation of ultra-processed foods in French supermarkets. Food Funct. 2020;11(3):2026–2039. doi: 10.1039/c9fo02271f. [DOI] [PubMed] [Google Scholar]
- 62.Fardet A. Characterization of the degree of food processing in relation with its health potential and effects. Adv. Food Nutr. Res. 2018;85:79–129. doi: 10.1016/bs.afnr.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Menichetti G., Ravandi B., Mozaffarian D., Barabási A.-L. Machine learning prediction of the degree of food processing. Nat. Commun. 2023;14(1):2312. doi: 10.1038/s41467-023-37457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez Steele E., O’Connor L.E., Juul F., Khandpur N., Galastri Baraldi L., Montiero C.A., et al. Identifying and estimating ultraprocessed food intake in the US NHANES according to the NOVA classification system of food processing. J. Nutr. 2022;153:225–241. doi: 10.1016/j.tjnut.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill E., Khajeh-Sharafabadi M., Rasolofomanana-Rajery S., Weaver N., Reisdorph R., Quinn K., et al. Unique-to-salmon compounds increase in plasma and are associated with cardiovascular health following a Mediterranean diet intervention. Curr. Develop. Nutr. 2022;6:286. doi: 10.1016/j.tjnut.2023.10.024. [DOI] [PubMed] [Google Scholar]
- 66.Fukagawa N.K., McKillop K., Pehrsson P.R., Moshfegh A., Harnly J., Finley J., et al. USDA's FoodData Central: what is it and why is it needed today? Am. J. Clin. Nutr. 2022;115(3):619–624. doi: 10.1093/ajcn/nqab397. [DOI] [PubMed] [Google Scholar]
- 67.Moshfegh A.J., Rhodes D.G., Martin L. National food intake assessment: technologies to advance traditional methods. Annu. Rev. Nutr. 2022;42:401–422. doi: 10.1146/annurev-nutr-062320-110636. [DOI] [PubMed] [Google Scholar]
- 68.Ng S.W., Popkin B.M. Monitoring foods and nutrients sold and consumed in the United States: dynamics and challenges. J. Acad. Nutr. Diet. 2012;112(1):41–45 e4. doi: 10.1016/j.jada.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The Neilson Company. http://en-us.nielsen.com/ [Internet] [cited May 20, 2023]. Available from:
- 70.Total Store Advantage. http://symphonyiri.com/ProductsSolutions/AllProducts/AllProductsDetail/tabid/159/productid/115/Default.aspx [Internet] [cited May 20, 2023]. Available from:
- 71.Pennington J.A., Stumbo P.J., Murphy S.P., McNutt S.W., Eldridge A.L., McCabe-Sellers B.J., et al. Food composition data: the foundation of dietetic practice and research. J. Am. Diet. Assoc. 2007;107(12) doi: 10.1016/j.jada.2007.09.004. 2105–2013. [DOI] [PubMed] [Google Scholar]
- 72.Martinez-Steele E., Khandpur N., Batis C., Bes-Rastrollo M., Bonaccio M., Cediel G., et al. Best practices for applying the Nova food classification system. Nat. Food. 2023;4:445–448. doi: 10.1038/s43016-023-00779-w. [DOI] [PubMed] [Google Scholar]
- 73.Sarbagili-Shabat C., Zelber-Sagi S., Fliss Isakov N., Ron Y., Hirsch A., Maharshak N., et al. Development and validation of processed foods questionnaire (PFQ) in adult inflammatory bowel diseases patients. Eur. J. Clin. Nutr. 2020;74(12):1653–1660. doi: 10.1038/s41430-020-0632-5. [DOI] [PubMed] [Google Scholar]
- 74.Dinu M., Bonaccio M., Martini D., Pia Madarena M., Vitale M., Pagliai G., et al. Reproducibility and validity of a food-frequency questionnaire (NFFQ) to assess food consumption based on the NOVA classification in adults. Int. J. Food Sci. Nutr. 2021;72(6):861–869. doi: 10.1080/09637486.2021.1880552. [DOI] [PubMed] [Google Scholar]
- 75.Wang L., Allman-Farinelli M., Yang J.-A., Taylor J.C., Gemming L., Hekler E., et al. Enhancing nutrition care through real-time, sensor-based capture of eating occasions: a scoping review. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.852984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Connor L.E., Kevin D.H., Herrick K.A., Reedy J., Chung S.T., Stagliano M., et al. Metabolomic profiling of an ultraprocessed dietary pattern in a domiciled randomized controlled crossover feeding trial. J. Nutr. 2023;153:2181–2192. doi: 10.1016/j.tjnut.2023.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huybrechts I., Rauber F., Nicolas G., Casagrande C., Kliemann N., Wedekind R., et al. Characterization of the degree of food processing in the European Prospective Investigation into Cancer and Nutrition: application of the Nova classification and validation using selected biomarkers of food processing. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1035580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su D., Chen J., Du S., Kim H., Yu B., Wong K.E., et al. Metabolomic markers of ultra-processed food and incident CKD. Clin. J. Am. Soc. Nephrol. 2023;18(3):327–336. doi: 10.2215/CJN.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eicher-Miller H.A., Fulgoni V.L., Keast D.R. Contributions of processed foods to dietary intake in the US from 2003–2008: a report of the Food and Nutrition Science Solutions Joint Task Force of the Academy of Nutrition and Dietetics, American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. J. Nutr. 2012;142(11):2065S–2072S. doi: 10.3945/jn.112.164442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eicher-Miller H.A., Fulgoni V.L., Keast D.R. Processed food contributions to energy and nutrient intake differ among US children by race/ethnicity. Nutrients. 2015;7(12):10076–10088. doi: 10.3390/nu7125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poti J.M., Mendez M.A., Ng S.W., Popkin B.M. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am. J. Clin. Nutr. 2015;101(6):1251–1262. doi: 10.3945/ajcn.114.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borghoff L., Strassner C., Richter T. 2021. A ProOrg Report, The role of organic processed food in food baskets, the role of processing technologies in the marketing of organic food and market trends in Europe for perception of processing technologies.https://orgprints.org/43434/1/PROORG_Organic_Processed_Food_in_Europe_2021_Borghoff_Strassner_Richter.pdf [Internet] [cited May 20, 2023]. Available from: [Google Scholar]
- 83.Bonaccio M., Costanzo S., Di Castelnuovo A., Persichillo M., Magnacca S., De Curtis A., et al. Ultra-processed food intake and all-cause and cause-specific mortality in individuals with cardiovascular disease: the Moli-sani Study. Eur. Heart J. 2022;43(3):213–224. doi: 10.1093/eurheartj/ehab783. [DOI] [PubMed] [Google Scholar]
- 84.Kliemann N., Rauber F., Bertazzi Levy R., Viallon V., Vamos E.P., Cordova R., et al. Food processing and cancer risk in Europe: results from the prospective EPIC cohort study. Lancet Planet Health. 2023;7(3):e219–e232. doi: 10.1016/S2542-5196(23)00021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Canhada S.L., Vigo Á., Cristine Luft V., Bertazzi Levy R., Maria Alvim Matos S., Del Carmen Molina M., et al. Ultra-processed food consumption and increased risk of metabolic syndrome in adults: the ELSA-Brasil. Diabetes Care. 2023;46(2):369–376. doi: 10.2337/dc22-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dicken S.J., Batterham R.L. The role of diet quality in mediating the association between ultra-processed food intake, obesity and health-related outcomes: a review of prospective cohort studies. Nutrients. 2021;14(1):23. doi: 10.3390/nu14010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirkpatrick S.I., Guenther P.M., Subar A.F., Krebs-Smith S.M., Herrick K.A., Freedman L.S., et al. Using short-term dietary intake data to address research questions related to usual dietary intake among populations and subpopulations: assumptions, statistical techniques, and considerations. J. Acad. Nutr. Diet. 2022;122(7):1246–1262. doi: 10.1016/j.jand.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 88.Richardson M.B., Williams M.S., Fontaine K.R., Allison D.B. The development of scientific evidence for health policies for obesity: why and how? Int. J. Obes. (Lond). 2017;41(6):840–848. doi: 10.1038/ijo.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hill E.R., O'Connor L.E., Wang Y., Clark C.M., McGowan B.S., Forman M.R., et al. Red and processed meat intakes and cardiovascular disease and type 2 diabetes mellitus: an umbrella systematic review and assessment of causal relations using Bradford Hill's criteria. Crit. Rev. Food Sci. Nutr. 2022:1–18. doi: 10.1080/10408398.2022.2123778. [DOI] [PubMed] [Google Scholar]
- 90.Livesey G., Taylor R., Livesey H.F., Buyken A.E., Jenkins D.J.A., Augustin L.S.A., et al. Dietary glycemic index and load and the risk of Type 2 diabetes: assessment of causal relations. Nutrients. 2019;11(6):1436. doi: 10.3390/nu11061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Juul F., dos Santos Simões B., Litvak J., Martinez-Steele E., Deierlein A., Vadiveloo M., et al. Processing level and diet quality of the US grocery cart: is there an association? Public Health Nutr. 2019;22(13):2357–2366. doi: 10.1017/S1368980019001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hess J.M., Comeau M., Casperson S., Slavin J.L., Johnson G.H., Messina M., et al. Dietary Guidance Meet NOVA: developing a menu for a healthy dietary pattern using ultra-processed foods [abstract] J. Nutr. 2023;153:2472–2481. doi: 10.1016/j.tjnut.2023.06.028. [DOI] [PubMed] [Google Scholar]
- 93.NIH Reporter Vascular Consequences of Ultra-Processed Foods in Middle-Aged Adults, Project Number 1R21AG075930-01A1. https://reporter.nih.gov/search/lbWdUo7kD0iOI3T6LL9wOw/project-details/10532576 [Internet] [cited May 20, 2023]. Available from:
- 94.Monteiro C.A., Cannon G., Moubarac J.-C., Levy R.B., Louzada M.L.C., Jaime P.C., et al. Freshly prepared meals and not ultra-processed foods. Cell Metab. 2019;30(1):5–6. doi: 10.1016/j.cmet.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 95.Small D.M., DiFeliceantonio A.G. Processed foods and food reward. Science. 2019;363(6425):346–347. doi: 10.1126/science.aav0556. [DOI] [PubMed] [Google Scholar]
- 96.Yeomans M., Symes T. Individual differences in the use of pleasantness and palatability ratings. Appetite. 1999;32(3):383–394. doi: 10.1006/appe.1999.0224. [DOI] [PubMed] [Google Scholar]
- 97.Yeomans M.R., Gray R.W., Mitchell C.J., True S. Independent effects of palatability and within-meal pauses on intake and appetite ratings in human volunteers. Appetite. 1997;29(1):61–76. doi: 10.1006/appe.1997.0092. [DOI] [PubMed] [Google Scholar]
- 98.Zandstra E.H., De Graaf C., Mela D.J., Van Staveren W.A. Short-and long-term effects of changes in pleasantness on food intake. Appetite. 2000;34(3):253–260. doi: 10.1006/appe.1999.0304. [DOI] [PubMed] [Google Scholar]
- 99.Bellisle F., Tournier A., Louis-Sylvestre J. Monosodium glutamate and the acquisition of food preferences in a European context. Food Qual. Pref. 1989;1(3):103–108. [Google Scholar]
- 100.Bobroff E.M., Kissileff H.R. Effects of changes in palatability on food intake and the cumulative food intake curve in man. Appetite. 1986;7(1):85–96. doi: 10.1016/s0195-6663(86)80044-7. [DOI] [PubMed] [Google Scholar]
- 101.De Graaf C., De Jong L.S., Lambers A.C. Palatability affects satiation but not satiety. Physiol. Behav. 1999;66(4):681–688. doi: 10.1016/s0031-9384(98)00335-7. [DOI] [PubMed] [Google Scholar]
- 102.Yeomans M.R. Taste, palatability and the control of appetite. Proc. Nutr. Soc. 1998;57(4):609–615. doi: 10.1079/pns19980089. [DOI] [PubMed] [Google Scholar]
- 103.Gibney M.J., Forde C.G. Nutrition research challenges for processed food and health. Nat. Food. 2022;3(2):104–109. doi: 10.1038/s43016-021-00457-9. [DOI] [PubMed] [Google Scholar]
- 104.Forde C.G., Bolhuis D. Interrelations between food form, texture, and matrix influence energy intake and metabolic responses. Curr. Nutr. Rep. 2022;11(2):p124–132. doi: 10.1007/s13668-022-00413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Teo P.S., van Dam R.M., Whitton C., Wei Lin Tan L., Forde C.G., et al. Consumption of foods with higher energy intake rates is associated with greater energy intake, adiposity, and cardiovascular risk factors in adults. J. Nutr. 2021;151(2):370–378. doi: 10.1093/jn/nxaa344. [DOI] [PubMed] [Google Scholar]
- 106.Forde C.G., Mars M., De Graaf K. Ultra-processing or oral processing? A role for energy density and eating rate in moderating energy intake from processed foods. Curr. Devel. Nutr. 2020;4(3) doi: 10.1093/cdn/nzaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gibney M.J. Food texture trumps food processing in the regulation of energy intake. Am. J. Clin. Nutr. 2022;116:9–10. doi: 10.1093/ajcn/nqac104. [DOI] [PubMed] [Google Scholar]
- 108.de Araujo I.E., Schatzker M., Small D.M. Rethinking food reward. Annu. Rev. Psychol. 2020;71:139–164. doi: 10.1146/annurev-psych-122216-011643. [DOI] [PubMed] [Google Scholar]
- 109.Berridge K.C., Robinson T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016;71(8):670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blundell J., de Graaf C., Hulshof T., Jebb S., Livingstone B. Appetite control: methodological aspects of the evaluation of foods. Obes. Rev. 2010;11(3):251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miller G.D., Ragalie-Carr J., Torres-Gonzalez M. Seeing the forest through the trees: the importance of the food matrix in diet quality and human health. Adv. Nutr. 2023;14:363–365. doi: 10.1016/j.advnut.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.National Collaboration on Childhood Obesity Research Measures Registry User Guides. Section 2: Measuring Food Environments. https://www.nccor.org/tools-mruserguides/food-environment/measuring-food-environments/ [Internet] [cited May 20, 2023]. Available from:
- 113.International Food Information Council . 2022. Food and Health Survey.https://ific.org/media-information/press-releases/2022-food-health-survey/ 2022 [Internet] [cited May 20, 2023]. Available from: [Google Scholar]
- 114.Sawyer A.D.M., van Lenthe F., Kamphuis C.B.M., Terragni L., Roos G. Dynamics of the complex food environment underlying dietary intake in low-income groups: a systems map of associations extracted from a systematic umbrella literature review. Int. J. Behav. Nutr. Phys. Act. 2021;18(1):96. doi: 10.1186/s12966-021-01164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beydoun M.A., Wang Y. Do nutrition knowledge and beliefs modify the association of socio-economic factors and diet quality among US adults? Prev. Med. 2008;46(2):145–153. doi: 10.1016/j.ypmed.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 116.Lam M.C.L., Adams J. Association between home food preparation skills and behaviour, and consumption of ultra-processed foods: cross-sectional analysis of the UK National Diet and nutrition survey (2008-2009) Int. J. Behav. Nutr. Phys. Act. 2017;14(1):68. doi: 10.1186/s12966-017-0524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mah C.L., Luongo G., Hasdell R., Taylor N.G.A., Lo B.K. A systematic review of the effect of retail food environment interventions on diet and health with a focus on the enabling role of public policies. Curr. Nutr. Rep. 2019;8(4):411–428. doi: 10.1007/s13668-019-00295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kirkpatrick S.I., Reedy J., Butler E.N., Dodd K.W., Subar A.F., Thompson F.E., et al. Dietary assessment in food environment research: a systematic review. Am. J. Prev. Med. 2014;46(1):94–102. doi: 10.1016/j.amepre.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Botelho R., Araujo W., Pineli L. Food formulation and not processing level: conceptual divergences between public health and food science and technology sectors. Crit. Rev. Food Sci. Nutr. 2018;58(4):639–650. doi: 10.1080/10408398.2016.1209159. [DOI] [PubMed] [Google Scholar]
- 120.British Nutrition Foundation Position Statement on the Concept of Ultra-Processed Foods (UPF) https://www.nutrition.org.uk/news/2023/position-statement-on-the-concept-of-ultra-processed-foods-upf/ [Internet] [cited May 26, 2023]. Available from:
- 121.Global Health Advocacy Incubator Breaking the Cycle of Unhealthy Eating: Niterói's Victory in Banning Ultraprocessed Products in Schools. https://www.advocacyincubator.org/featured-stories/2023-04-17-breaking-the-cycle-of-unhealthy-eating-niterois-victory-in-banning-ultra-processed-products-in-schools [Internet] [cited May 20, 2023]. Available from:
- 122.Louzada M., Zancheta Ricardo C., Martinez Steele E., Bertazzi Levy R., Cannon G. The share of ultra-processed foods determines the overall nutritional quality of diets in Brazil. Public Health Nutr. 2018;21(1):94–102. doi: 10.1017/S1368980017001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ralston K., Newman C., Clauson A., Guthrie J., Buzby J. USDA. Economic Research Service; 2008. The National School Lunch Program: Background, Trends, and Issues. [Google Scholar]
- 124.American Society for Nutrition . 2022. RE: Request for Comments on Scientific Questions to Be Examined to Support the Development of the Dietary Guidelines for Americans.https://media.nutrition.org/wp-content/uploads/2022/05/ASN-Comments-on-Dietary-Guidelines-Topics.Questions_May-2022.pdf 2025-2030 (Docket No. HHSOASH-2022-0005-0001) [Internet] [cited May 20, 2023]. Available from: [Google Scholar]
- 125.Institute for Food Technologists . 2022. RE: Request for Comments on Scientific Questions to Be Examined to Support the Development of the Dietary Guidelines for Americans.https://www.ift.org/-/media/policy-advocacy/ift-comments/dga-scientific-questions-_ift-comments_051622.pdf 2025-2030 (Docket No. HHS-OASH-2022-0005-0001) [Internet] [cited May 20, 2023]. Available from: [Google Scholar]
- 126.Garza C., Stover P.J., Ohlhorst S.D., Field M.S., Steinbrook R., Rowe S., et al. Best practices in nutrition science to earn and keep the public's trust. Am. J. Clin. Nutr. 2019;109(1):225–243. doi: 10.1093/ajcn/nqy337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.