Abstract
C-repeat binding factors (CBFs) are well-known transcription factors (TFs) that regulate plant cold acclimation. RNA sequencing (RNA-seq) data from diverse plant species provide opportunities to identify other TFs involved in the cold response. However, this task is challenging because gene gain and loss has led to an intertwined community of co-orthologs and in-paralogs between and within species. Using orthogroup (closely related homologs) analysis, we identified 10,549 orthogroups in five representative eudicots. A phylotranscriptomic analysis of cold-treated seedlings from eudicots identified 35 high-confidence conserved cold-responsive transcription factor orthogroups (CoCoFos). These 35 CoCoFos included the well-known cold-responsive regulators CBFs, HSFC1, ZAT6/10, and CZF1 among others. We used Arabidopsis BBX29 for experimental validation. Expression and genetic analyses showed that cold-induction of BBX29 is CBF- and abscisic acid-independent, and BBX29 is a negative regulator of cold tolerance. Integrative RNA-seq and Cleavage Under Targets and Tagmentation followed by sequencing analyses revealed that BBX29 represses a set of cold-induced TFs (ZAT12, PRR9, RVE1, MYB96, etc.). Altogether, our analysis yielded a library of eudicot CoCoFos and demonstrated that BBX29 is a negative regulator of cold tolerance in Arabidopsis.
Key words: phylogenomics, phylotranscriptomics, orthogroup, cold acclimation, BBX29, CBF-independent pathway
By integrating orthogroup and phylotranscriptomic analyses, this study identifies a library of high-confidence conserved cold-responsive transcription factor orthogroups (CoCoFos) in eudicots and experimentally demonstrates that BBX29 is a negative regulator of cold tolerance in Arabidopsis.
Introduction
Despite a sessile lifestyle, plants are not as completely passive as they seem. Many temperate plants exhibit increased freezing tolerance when they are first exposed to nonfreezing but low temperature conditions through an adaptive process known as cold acclimation (CA) (Thomashow, 1999). Accumulating evidence suggests that many of the physiological and metabolic changes caused by low temperatures are due to the expression of cold-responsive (COR) genes induced by CA (Hannah et al., 2005; Maruyama et al., 2009).
Dehydration-responsive elements (DREs) containing a core cis-regulatory element of CCGAC are responsible for regulating the expression of dehydration- and cold-inducible genes, such as Arabidopsis COR15A, RD29A, and RD29B (Baker et al., 1994; Yamaguchi-Shinozaki and Shinozaki, 1994). Yeast one-hybrid screening revealed that C-repeat binding factor 1 (CBF1) and CBF3 specifically bind to DREs (Stockinger et al., 1997; Liu et al., 1998). Transgenic CBF1 overexpression in Arabidopsis induces the expression of COR genes and results in increased freezing tolerance in non-cold-acclimated plants (Jaglo-Ottosen et al., 1998). Additional studies have confirmed that CBF genes such as the Arabidopsis CBF1/2/3 (also referred to as DREB1B/C/A) genes are important regulators of cold tolerance conferred by CA.
Numerous studies over the past 25 years have shed light on how CBF genes are regulated. Many transcription factors (TFs) that bind to cis-regulatory elements in the promoters of Arabidopsis CBF genes have been identified (Kidokoro et al., 2022; Qi et al., 2022). An ice1-1 mutant that impairs cold induction of a CBF3 promoter-luciferase reporter gene, INDUCER OF CBF EXPRESSION 1 (ICE1), was identified as a potential TF that regulates CBF3 expression (Chinnusamy et al., 2003). Electrophoretic mobility shift , chromatin immunoprecipitation (ChIP)-qPCR, and ChIP sequencing (ChIP-seq) assays demonstrated that ICE1 binds directly to the MYC recognition sites (CANNTG, also referred to as an E-box) in the CBF3 promoter (Chinnusamy et al., 2003; Kim et al., 2015). Promoter fusion and mutant analyses demonstrated that CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR TFs bind to the CGCG boxes in the promoters of CBF genes and activate CBF expression (Doherty et al., 2009; Kim et al., 2013). CBF expression is also regulated by the circadian clock components CIRCADIAN CLOCK-ASSOCIATED 1, LATE ELONGATED HYPOCOTYL, REVEILLE4 (RVE4), and RVE8 (Dong et al., 2011; Kidokoro et al., 2021). Phytochrome-interacting factors (e.g., PIF3/7) also regulate cold tolerance by binding to the G-box (CACGTG) and/or E-box (CANNTG) in CBF promoters (Lee and Thomashow, 2012; Jiang et al., 2017). These studies demonstrate that a complex regulatory network modulates CBF expression during CA.
In addition to studies on the upstream regulators of CBFs, the COR genes have been identified as downstream target genes regulated by CBFs (i.e., CBF regulons). Three independent studies using CBF overexpression or cbfs mutant lines identified hundreds of CBF regulons (Park et al., 2015; Jia et al., 2016; Zhao et al., 2016). Another recent study identified over 1000 promoters bound by CBFs by using ChIP-seq and further identified 146 CBF regulons (Song et al., 2021). Despite a key role for CBFs in CA, only approximately 10%–20% of COR genes are regulated by CBFs (Park et al., 2015; Jia et al., 2016; Zhao et al., 2016; Song et al., 2021), which suggests other TFs are also involved in cold tolerance. Park et al. (2015) employed hierarchical and k-means clustering with the Arabidopsis low-temperature ATH1 GeneChip dataset (Kilian et al., 2007) to screen for genome-wide “first-wave” TFs that were cold-induced in-parallel with CBFs. Among the “first-wave” TFs, HSFC1, ZAT12, ZF, ZAT10, and CZF1 were found to cooperatively regulate many CBF regulons (Park et al., 2015). Two independent studies identified that expression of HSFC1, ZAT12, and CZF1 was independent of CBFs and suggested that these genes are involved in responses to low temperature in CBF-independent pathways (Jia et al., 2016; Zhao et al., 2016).
In addition to Arabidopsis, molecular responses to cold stress have been studied in many other plant species, such as Oryza sativa (Yang, 2022), Zea mays (Guo et al., 2021), Ocimum americanum (Zhan et al., 2016), Nicotiana tabacum (Jin et al., 2017), Hevea brasiliensis (Gong et al., 2018), Phyllostachys edulis (Liu et al., 2020), Populus trichocarpa (Zhao et al., 2021; Wang et al., 2022), and Malus sieversii (Zhou et al., 2021).
These prior studies focused on one or two species, and the molecular mechanisms underlying cold responses among different species are generally restricted to the well-studied CBF pathway genes. An abundance of RNA sequencing (RNA-seq) datasets from diverse species provides an unparalleled opportunity for comparative transcriptomics to identify conserved TFs involved in regulating cold tolerance across species. However, orthologous genes across species or even ecotypes often exhibit differential responses to abiotic stresses such as low temperature (Zhang et al., 2017; Park et al., 2018; Meng et al., 2021). Biased retention and loss of gene duplicates in different species after independent whole-genome and/or small-scale duplications have led to complex and intertwined evolution of co-orthologs and in-paralogs between and within plant species (Koonin, 2005; Guo et al., 2022). The situation is further complicated by gene expression divergence. It is thus inadequate to perform gene-by-gene comparisons when trying to identify orthologous TFs involved in cold tolerance regulation.
In this study, we employed orthogroup analysis to avoid gene complexity from co-orthologs and in-paralogs between and within species. An orthogroup is a set of genes from multiple species that are all descended from a single gene in the last common ancestor (Emms and Kelly, 2019). We selected five representative eudicot species for comparative genomics and identified 10,549 orthogroups. RNA-seq analysis was then performed on these five species to determine how each of the orthogroups responded to cold non-freezing temperatures. This analysis identified 35 Conserved COR TF orthogroups (CoCoFos). From this group of CoCoFos, we selected and further characterized the role of B-BOX DOMAIN PROTEIN 29 (BBX29) in cold stress tolerance in Arabidopsis. We examined the dependence of BBX29 cold-induced expression on the CBF or abscisic acid (ABA) response pathway. To explore the function of BBX29, we compared the freezing tolerances of BBX29 overexpression and bbx29 mutants with those of wild-type plants (WT; Col-0). We identified COR genes regulated by BBX29 through comparative transcriptomics of BBX29 overexpression and bbx29 mutant lines. Cleavage Under Targets and Tagmentation followed by sequencing (CUT&Tag-seq) was performed and identified 137 COR genes from Arabidopsis targeted by BBX29.
Results
Identification of 35 CoCoFos in eudicots
We searched for universal plant COR TFs in five species of diverse eudicot lineages: Arabidopsis thaliana, Carya illinoinensis, Glycine max, Betula pendula, and P. trichocarpa. Genes from these diverse species were classified into 10,549 orthogroups using OrthoFinder (Emms and Kelly, 2019) (Supplemental Table 1). We performed RNA-seq on each of the five species before and after cold stress treatments lasting 0, 2, 24, and 168 h to identify genes regulated by the cold response in the orthogroups. The 10,549 orthogroups were divided into six clusters based on how the expression of their genes responded to cold treatment (Figure 1A and Supplemental Table 1). Cluster 1 contained 238 orthogroups that were upregulated during cold stress in all five species (Supplemental Figure 1). Thirty-five of the orthogroups in Cluster 1 were universal COR TF orthogroups (TFOGs), which were designated as CoCoFos (Figure 1A and Supplemental Table 2).
Figure 1.
Conserved cold-responsive transcription factor orthogroups (CoCoFos) in eudicots and their expression compared with CBFs.
(A) Orthogroup identification and gene expression clustering under cold stress. A total of 10,549 conserved orthogroups were identified and divided into six clusters based on the cold-induced level of gene expression in each orthogroup. Clusters one through six consist of orthogroups that are cold-induced in five, four, three, two, one, and none of the five species, respectively. The number before the slash indicates the total number of orthogroups in the corresponding cluster, and the number after the slash indicates the number of TF orthogroups (TFOGs).
(B) Cold-induced expression heatmap of the 35 CoCoFos. The orthogroup expression value is represented by the average trimmed mean of M value of genes in each orthogroup. Gene expression after cold stress (4°C) treatments lasting 0, 2, 24, and 168 h were determined from our RNA-seq experiments.
(C) Transcription factors (TFs) shared and unique between CoCoFos and CBFs-regulated COR TFs in Arabidopsis. Statistical significance was determined using Fisher’s exact test.
(D) GO enrichment analysis of 95 Arabidopsis CoCoFo TFs.
CoCoFos include members from diverse stress-related TF families such as APETALA2/Ethylene Responsive Factor (AP2/ERF), WRKY, NAM, ATAF and CUC (NAC), MYB, CONSTANS-like (COL), basic leucine zipper (bZIP) and basic-helix-loop-helix (bHLH) among others. They are significantly induced after cold stress treatments of 2, 24, and/or 168 h in all five species (Figure 1B). The AP2/ERF family includes CBF genes and other closely related homologs, which are well-studied TFs involved in plant CA (Mizoi et al., 2012; Ritonga et al., 2021).
Cold induction of a minority of CoCoFos is dependent on CBFs in Arabidopsis
Given the major role of CBFs in cold tolerance, we wanted to determine which CoCoFos are regulated by CBFs in Arabidopsis. We compared 112 Arabidopsis TFs taken from the 35 CoCoFos (Supplemental Table 3) with 81 validated Arabidopsis CBF-regulated COR TFs (Song et al., 2021). We found that 17 Arabidopsis CoCoFo TFs are also CBF-regulated COR TFs (P = 1.1e-8) (Figure 1C). This significant overlap suggests the CBF-dependent regulatory network plays a key role in the cold response of eudicots. However, we also identified 95 Arabidopsis CoCoFo TFs that are likely cold-regulated in a CBF-independent manner (Figure 1C). Some of these CoCoFos have been experimentally validated to be CBF- independent, such as HSFC1, ZAT12, and CZF1 (Park et al., 2015; Zhao et al., 2016). Gene Ontology (GO) enrichment analysis revealed that the 95 CBF-independent CoCoFo TFs are enriched in nucleic acid binding, transcription regulator activity, and regulation of gene expression (Figure 1D). Other notable GO terms related to stress responses, such as response to cold, heat, salt, water, wounding, and light stimulus, are also among the most enriched terms. These findings demonstrate that only 17 of 112 Arabidopsis CoCoFo TFs are cold-regulated in a CBF-dependent manner, and the majority are likely cold-regulated in a CBF-independent manner. These results strongly suggest that many other cold response pathways remain to be identified.
Cold induction of BBX29 is CBF-independent
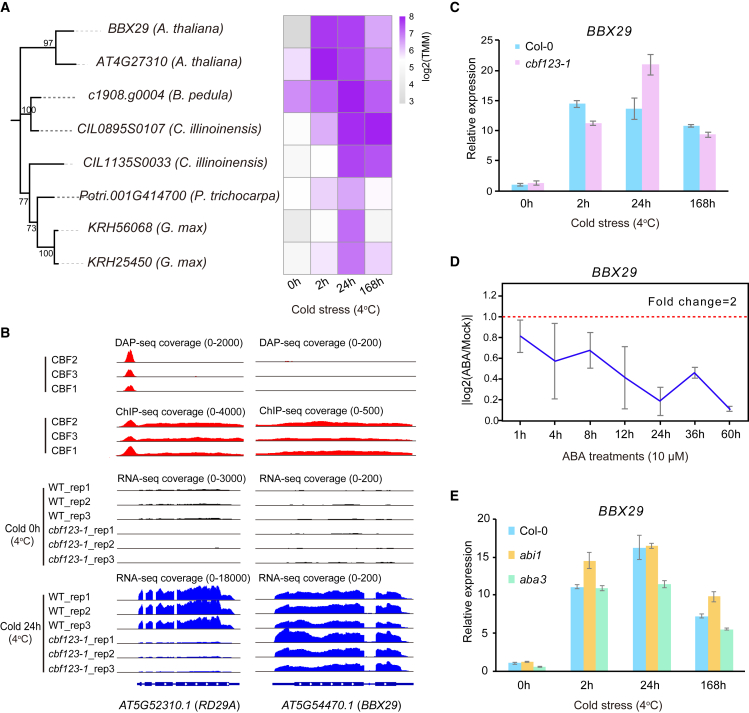
Most of the CoCoFo genes are part of “typical” stress-related TF families, such as the AP2/ERF, WRKY, NAC, MYB, COL, bZIP, and bHLH families, that have well-known roles in plant responses to cold stress and other abiotic stresses (Supplemental Table 3). These results provide further confidence that the 35 CoCoFos are involved in regulating cold responses, and overexpression of the validated TFs, or their close homologs, induce phenotypic changes such as increased plant freezing tolerance. To further validate the CoCoFos, we characterized Arabidopsis BBX29 in the BBX V orthogroup, which is an “atypical” stress-related TF family. BBX29 is a cold-induced gene (Mikkelsen and Thomashow, 2009) that is involved in regulating photomorphogenesis (Song et al., 2020). BBX29 co-orthologs in other eudicot species are also upregulated in response to cold temperatures (Figure 2A). However, a functional role in cold stress tolerance for BBX29 has yet to be determined.
Figure 2.
BBX29 cold-induction is CBF- and ABA-independent.
(A) Cold-induced expression of BBX29 orthologs in five eudicots.
(B) CBF binding profiles across BBX29 loci and BBX29 expression in the wild-type (WT) and cbf123-1 mutant lines under cold stress. The Arabidopsis DAP-seq and ChIP-seq data for CBFs, and the RNA-seq data for the WT and cbf123-1 lines under cold stress, were obtained and analyzed using publicly available data (O’Malley et al., 2016; Song et al., 2021; Zhao et al., 2016). RD29A is regulated by CBFs during cold stress and served as a positive control.
(C) Quantitative real-time PCR validation of cold-induced expression of BBX29 in the WT and cbf123-1 lines.
(D)BBX29 expression after ABA treatments at different time points.
(E)BBX29 expression under cold stress in Col-0, abi1, and aba3.
The mean values are calculated from three biological replicates. The error bars represent the SD. Significant differences in gene expression between the two treatments or samples is defined as a two-fold or greater change in gene expression with a P value of < 0.01 calculated using Student’s t-test.
To determine if BBX29 cold-induced expression is CBF-dependent, we examined CBF1/2/3 genome-wide binding profiles from ChIP-seq (Song et al., 2021) and DNA affinity purification sequencing (DAP-seq) experiments (O’Malley et al., 2016) but did not observe any CBF-binding peaks at the BBX29 locus (Figure 2B). We also compared the expression of BBX29 between WT and cbf123-1 mutant line under cold stress using data from RNA-seq experiments (Zhao et al., 2016). The results revealed that BBX29 exhibits very similar patterns of cold-induced expression in both the cbf123-1 mutant and WT lines (Figure 2B). Quantitative real-time PCR analysis provided further support for these findings (Figure 2C). Taken together, these results demonstrate that cold induction of Arabidopsis BBX29 is independent of CBFs.
Cold induction of BBX29 is predominantly ABA-independent
Prior studies have shown that many cold-related genes are induced through ABA signaling (Ishitani et al., 1997; Tähtiharju and Palva, 2001; Zhang et al., 2016). We used public transcriptomic data to analyze BBX29 expression at multiple time points after ABA treatment (Song et al., 2016). BBX29 expression was only slightly affected (fold change < 2) at 1-60 h post-ABA treatment (Figure 2D). Our own quantitative real-time PCR results confirmed that BBX29 expression changes very little in aba3 (ABA-deficient) and abi1 (ABA-insensitive) mutants compared with WT plants (Figure 2E). Together, these findings demonstrate that expression of BBX29 induced by cold stress is predominantly regulated by ABA- and CBF-independent pathways.
A negative role for BBX29 in cold tolerance
We obtained bbx29 mutants (bbx29-1 and bbx29-2) (Song et al., 2020) and generated 35S::BBX29-Myc overexpression (OE) lines to further characterize the role of BBX29 in the cold stress response (Supplemental Figure 2). The 35S::BBX29-Myc OE lines (OE3 and OE5) and bbx29 mutants displayed similar growth phenotypes to WT and cbf123-1 under non-stress conditions (Song et al., 2020) (Figure 3A). Under freezing conditions with CA, cbf123-1 exhibited hypersensitive phenotypes described previously by Zhao et al. (2016). In contrast, the two independent bbx29 mutants exhibited an increased tolerance towards freezing with and without CA, whereas the two independent OE lines exhibited hypersensitive phenotypes (Figure 3A). The bbx29 mutants had the highest survival rates following freezing followed by the WT and OE lines (Figure 3B). Ion leakage from the OE lines following freezing was significantly higher than that of WT plants, whereas ion leakage from the bbx29 mutants was significantly lower than that of WT plants (Figure 3C). These findings suggest that BBX29 plays a negative role in cold tolerance.
Figure 3.
BBX29 negatively regulates cold tolerance in Arabidopsis.
(A) Freezing tolerance phenotypes of Col-0, bbx29 mutants (bbx29-1 and bbx29-2), 35S::BBX29-Myc OE lines (OE3 and OE5), and cbf123-1 with or without CA.
(B) Survival rate of each line after freezing treatment.
(C) Ion leakage from leaves of Col-0, bbx29, and 35S::BBX29-Myc OE lines during the freezing treatment.
The mean values are calculated from three biological replicates. Asterisks represent a significant difference using Student’s T-test (∗P < 0.05, ∗∗P < 0.01).
Thousands of COR genes are regulated by BBX29
We performed an RNA-seq analysis on WT, 35S::BBX29-Myc (OE5), and bbx29-2 lines subjected to cold stress to identify COR genes differentially regulated in BBX29 OE or loss- of-function lines (Supplemental Table 4). BBX29-regulated COR genes were determined using two criteria: (1) differentially expressed following cold treatment in WT and (2) differentially expressed (fold change ≥2) in the BBX29 OE or mutant lines. We identified 1547 BBX29-regulated COR genes that satisfied these two criteria (Figure 4A). Fuzzy c-means clustering of the 1547 genes identified 10 clusters with differential expression patterns between the WT, bbx29-2, and OE5 lines (Supplemental Table 5 and Supplemental Figure 3). The COR genes in clusters 3 and 10 are significantly induced by cold stress (2 or 24 h) treatment in the WT and bbx29-2 lines but are unaffected or slightly upregulated in the OE5 line. In contrast, COR genes in clusters 6 and 9 are significantly repressed following cold stress (2 or 24 h) treatment in the WT and bbx29-2 lines but also have low expression under non-stress conditions in the OE5 line (Figure 4B). These findings suggest that thousands of genes are differentially regulated by the OE and loss-of-function of BBX29.
Figure 4.
Over one thousand COR genes are regulated by BBX29.
(A) A Venn diagram of differentially expressed genes in Col-0, bbx29-2, and 35S::BBX29-Myc (OE5) lines after cold stress.
(B) Four gene clusters differentially expressed in the BBX29 OE line. Relative expression is the difference between gene expression at each time point and the expression at the time point with the highest gene expression.
(C) Functional GO term networks of BBX29-regulated COR genes. The network was constructed and visualized using ClueGO (Bindea et al., 2009).
A GO term enrichment analysis of BBX29-regulated COR genes revealed an enrichment in light-related categories such as response to light stimulus, response to light intensity, and response to UV light that are in general agreement with previous reports (Song et al., 2020) (Figure 4C). Also significantly enriched were GO terms for stress and hormone-related categories including response to cold, temperature stimulus, ABA, jasmonic acid (JA), and salicylic acid (SA) (Figure 4C). The findings suggest that BBX29 could regulate genes involved in many biological processes, including responses to light, temperature changes, and hormones.
Genome-wide sites and motifs bound by BBX29
To determine the genes directly regulated by BBX29, we performed CUT&Tag-seq on the BBX29 OE line (35S::BBX29-Myc) and identified the BBX29-binding profile of the Arabidopsis genome. We discovered a total of 1093 binding peaks in 994 genes (Supplemental Table 6). More than 80% of these binding peaks are present in promoter regions (<2 kb) of Arabidopsis genes with 73.7% within 1-kb of the start codon (Figure 5A). We selected a subset of genes with BBX29-binding peaks and performed CUT&Tag-qPCR analysis. The qPCR results revealed a significant enrichment in BBX29 binding to these genes (Figure 5B), which supports the CUT&Tag-seq analysis.
Figure 5.
Genome-wide binding profile of BBX29 and its direct COR targets.
(A) Distribution of BBX29 binding sites in the Arabidopsis genome. The pie chart shows the proportion of BBX29-binding peaks distributed among different annotated regions of genes. The UpSet plot shows intersections among BBX29-binding regions across genes.
(B) CUT&Tag-qPCR validation of BBX29 binding. The CUT&Tag-qPCR results were normalized to ACTIN2 (AT3G18780) and ACTIN8 (AT1G49240). The mean values are calculated from three biological replicates. Asterisks represent a significant difference using Student’s T-test (∗∗P < 0.01).
(C) Two enriched motifs in BBX29-binding regions. MEME-ChIP was used to discover the motifs (Machanick and Bailey, 2011).
(D) A Venn diagram showing the overlap between BBX29-targeted genes and BBX29-regulated COR genes.
(E) GO enrichment of BBX29-targeted COR genes.
We extracted the sequences around BBX29-binding peaks and performed motif enrichment analysis using MEME-ChIP to determine the sequence of BBX29-binding motifs (Machanick and Bailey, 2011). Two significantly enriched motifs were obtained: a well-known G-box (CACGTG) and a novel motif (Figure 5C). The G-box motif is bound by other B-box domain proteins such as BBX21, OsBBX14, and SlBBX20 (Xu et al., 2018; Bai et al., 2019; Xiong et al., 2019). The novel motif contains a core GTG, which constitutes half of the G-box motif (Figure 5C). These observations indicate that BBX29 may regulate gene expression by recognizing the G-box and/or related elements in the promoters of target genes.
BBX29 directly targets 137 COR genes
As described above, the Arabidopsis genome contains 1547 COR genes that are differentially expressed in BBX29 OE or loss-of-function lines (Figure 4A). We identified 137 differentially expressed COR genes that also contain BBX29-binding peaks in their promoters, indicating they are directly targeted by BBX29 (Figure 5D, Supplemental Table 6, and Supplemental Table 8). A GO enrichment analysis indicated that these genes are significantly enriched in terms related to response to abiotic stresses such as cold, heat, water, salt, hypoxia, hormone, and light stimulus (Figure 5E). These findings demonstrate that BBX29 can directly bind to COR genes and regulate their expression.
BBX29 regulates the expression of COR genes primarily independent of CBFs
Although cold induction of BBX29 is independent of CBFs and ABA signaling (Figure 2), it is still possible that BBX29 might co-regulate the expression of COR genes with CBFs or ABA signaling after cold induction. We tested this hypothesis by checking the expression levels of 146 CBF-targeted COR genes and 3060 ABA-responsive genes curated from previous studies (Song et al., 2016, 2021). Our RNA-seq analysis of Arabidopsis plants treated with and without cold conditions (Supplemental Table 4) revealed 1217 differentially expressed COR genes from the 3060 ABA-responsive genes. This finding supports previous studies that demonstrated ABA-responsive genes are often cold regulated (Lee and Seo, 2015; Wang et al., 2016; Lv et al., 2018). We then compared BBX29-targeted COR genes with CBF-targeted COR genes and ABA-responsive COR genes. Half of the CBF-targeted COR genes are also regulated by ABA signaling, which is consistent with previous studies (Wilhelm and Thomashow, 1993; Wang et al., 1995; Narusaka et al., 2003). Interestingly, nearly 40% of BBX29-targeted CORs are regulated by ABA signaling, whereas only 7 (<5%) of BBX29-targeted CORs overlap with CBF-targeted CORs (Figure 6A). These findings indicate that BBX29 regulates COR genes mostly independent of the CBF pathway.
Figure 6.
BBX29 regulates cold responses primarily via CBF-independent pathways.
(A) A Venn diagram showing the overlap between BBX29-targeted CORs, CBF-targeted CORs, and ABA-responsive CORs.
(B) BBX29-binding profiles across a set of COR TFs that are differentially expressed in the BBX29 OE line. The RNA-seq data for WT and cbfs lines treated with cold stress were obtained and analyzed using publicly available data (Jia et al., 2016), whereas CUT&Tag-seq and RNA-seq experiments with BBX29 were performed in this study.
BBX29 directly regulates a set of COR TF genes
Within the dataset of COR genes targeted by BBX29 were some TFs known to be involved in cold and/or other abiotic stress responses (Supplemental Table 8). For example, ZAT12 is a zinc-finger TF that is induced by cold temperatures and coexpressed with CBFs. Overexpression of ZAT12 enhances plant freezing tolerance (Vogel et al., 2005; Park et al., 2015). PRR9 is a component of temperature responsiveness in the Arabidopsis circadian clock system (Salomé and McClung, 2005). RVE1 is a Myb-family TF involved in regulating freezing tolerance (Meissner et al., 2013), and MYB96 can integrate ABA and cold signaling to activate the CBF-COR pathway (Lee and Seo, 2015). The loci of these COR TFs have BBX29-binding peaks, and their expression is reduced in the BBX29 OE line (Figure 6B and Supplemental Figure 4). Additionally, RNA-seq analysis of WT and cbfs (Jia et al., 2016) lines confirmed that the expression of these COR TFs is independent of CBFs (Figure 6B). Together, our results suggest that BBX29 functions in a negative feedback loop in cold tolerance by repressing a set of COR TF genes primarily independent of CBFs.
Discussion
Since it was first discovered that CBFs play an important role in CA, the CBF-dependent cold-regulatory pathway has been studied extensively (Ding et al., 2020; Kidokoro et al., 2022). Recent molecular evidence indicates that CBFs evolved in angiosperms after splitting from the sister clade of gymnosperms and have expanded stepwise in eudicots and monocots primarily through independent whole-genome duplication and/or tandem duplication events (Guo et al., 2022; Nie et al., 2022). The CBF-dependent cold-regulatory pathway may be conserved in angiosperms. We identified 35 high-confidence CoCoFos by comparing the genomes and transcriptomes of five eudicots. Included within these CoCoFos were CBFs, but we demonstrate that the majority of CoCoFos are likely regulated in a CBF-independent manner. As anticipated, the CoCoFos contain well-studied cold-regulated genes from stress-related TF families such as AP2/ERF, WRKY, NAC, MYB, COL, bZIP and bHLH (Figure 1B and Supplemental Table 3). Overexpressing or knocking out these genes is known to affect freezing tolerance and other abiotic stress responses in plants (Supplemental Table 3). We experimentally validated the role of Arabidopsis BBX29 from the BBX V orthogroup in the cold stress response. As described previously, we found that BBX29 cold-induction is CBF- and ABA-independent (Figure 2B-2E) (Mikkelsen and Thomashow, 2009). In contrast to a positive role for the BBX29 homologs BBX7/8 (Li et al., 2021), BBX29 is a negative regulator of cold tolerance in Arabidopsis (Figure 3). Only ∼7.2% of BBX29-regulated genes are also regulated by BBX7/8 (Supplemental Figure 5A), which implies BBX29 and BBX7/8 regulate divergent cold regulatory pathways. An evolutionary analysis of the BBX family shows that BBX29 and BBX7/8 are grouped into cluster V and cluster II, respectively. This further supports a divergence in function between BBX29 and BBX7/8 (Supplemental Figure 5B).
A comparison of genes downstream of BBX29 and CBFs revealed only seven (less than 5%) overlapping genes between BBX29 and CBFs (Figure 6A). This indicates that most BBX29-targeted COR genes are regulated independently of CBFs. Additionally, results from a yeast two-hybrid (Y2H) assay suggest that BBX29 does not associate with CBFs (Supplemental Figure 6). These findings strongly suggest that BBX29 regulates cold tolerance primarily through a CBF-independent pathway. We found that BBX29 target genes are involved in plant responses to other types of abiotic stress (salt, osmotic, and wounding), light (blue and red), hormones (SA, JA, and cytokinin (CK)), and other signals (Figure 7). Thus, BBX29 may act as an important integrator of diverse signaling pathways, including external environmental cues (light and abiotic stresses) as well as internal hormonal signals to regulate plant stress tolerance, growth, and development.
Figure 7.
35 eudicot CoCoFos and functional categories of BBX29-targeted COR genes in Arabidopsis.
The 35 CoCoFos are listed in Supplemental Table 2. Whether or not their expression is directly regulated by CBFs under cold stress in Arabidopsis was determined in a previous publication (Song et al., 2021). BBX29-targeted COR genes are involved in plant responses to abiotic stresses (cold, salt, osmotic, and wounding), light (blue and red), hormones (ABA, SA, JA, and CK), and other signals. The COR genes are grouped by their biological functions, and genes in each category are partially selected based on gene annotations. Detailed information of the genes is listed in Supplemental Table 8.
BBX29 also has a close homolog in Arabidopsis called BBX28 (AT4G27310) (Figure 2A). To determine if BBX28 shares a redundant role with BBX29 under cold stress, we compared the freezing tolerance of WT, bbx29 (bbx29-1 and bbx29-2), and bbx28-1 bbx29-1 double mutant lines. The bbx29 and bbx28-1 bbx29-1 mutants exhibited increased freezing tolerance compared to WT plants, and the survival rate of the bbx28-1 bbx29-1 line was considerably higher than that of the bbx29 line (Supplemental Figure 7). These results suggest that BBX28 and BBX29 share partially redundant roles in regulating cold tolerance in Arabidopsis. Interestingly, the BBX29 homologs BBX28 and BBX22, are likely transcriptionally repressed by BBX29 (Figure 7 and Supplementary Table 8). Such a regulatory relationship also occurs between the Arabidopsis CBF homologs CBF1, CBF2, and CBF3. CBF2 negatively regulates the expression of CBF1 and CBF3, ensuring that their expression is tightly controlled (Novillo et al., 2004). These results suggest that the low-temperature regulatory network is highly interconnected and dynamically regulated, and further experiments are required to demonstrate the spatiotemporal expression and protein interactions between BBX29 and its homologs (e.g., BBX28 and BBX22). As demonstrated in Figure 2A, BBX29 co-orthologs in other eudicots are cold-induced, so we decided to transiently expressed PtrBBX29 (Potri.001G414700) in P. trichocarpa. Although OE of PtrBBX29 affects the expression of some COR genes (e.g., PtrZAT12, PtrMYB96, and PtrPRR9) (Supplemental Figure 8), the role of conserved BBX29 orthologs in other eudicots is still unknown. In summary, we identified a group of eudicot CoCoFos that will serve as a valuable resource for the identification of additional conserved and cold-regulated TFs in plants. We also demonstrate that the Arabidopsis CoCoFo BBX29 is a negative regulator of cold tolerance.
Methods
Orthogroup analysis
We selected five eudicot species (A. thaliana, C. illinoinensis, G. max, B. pendula, and P. trichocarpa) and downloaded their genomes and protein sequences from the public Phytozome and Ensembl Plants databases. All 119,836 coding genes were classified into different orthogroups using OrthoFinder 2.3.8 (Emms and Kelly, 2019) with default parameters. After filtering out the orthogroups that did not cover the five species, we obtained 10,549 orthogroups. We then classified the orthogroups into TF or non-TF orthogroups based on the presence or absence of a DNA-binding domain, respectively (Supplemental Table 1).
Identification of universal COR genes and orthogroups in five species
RNA-seq was performed on tissue collected from the five plant species mentioned above at 0, 2, 24, and 168 h of cold stress treatments to identify universal COR genes and orthogroups. For A. thaliana, 3-week-old seedlings were used for cold stress treatments. For the other species, young seedlings of ∼30 cm in height were used. RNA-seq experiments and subsequent analyses were performed as described previously (Guo et al., 2023; Wang et al., 2023). Briefly, all plant seedlings were cultured in an artificial climate chamber (25°C, 16/8 h light/dark). Four seedling groups (1-4) were classified based on their size relative to each other. To ensure that plants from each of the four cold treatment time points (0, 2, 24, and 168 h) could be harvested at the same time, plants need to be collected after a 168 h cold treatment were first incubated at 4°C a week before harvest. These were followed by seedlings treated for 24, 2, and 0 h in that order. After the treatments, the fourth expanded leaves were collected, and total RNA was collected for RNA-seq experiments. Three replicate experiments were performed on each sample at each time point.
Cold-induced genes with expression levels (trimmed mean of M value) above 20 after 2, 24, or 168 h and genes with a greater than 2-fold change between any of the time points relative to time 0 h were identified and considered to be COR genes. Orthogroups with at least one cold-induced gene were considered to be COR orthogroups. Orthogroups that were cold-induced in all five eudicot species were designated as conserved COR orthogroups. Among the conserved COR orthogroups, we identified 35 TF orthogroups (i.e., CoCoFos).
Plant materials and growth conditions
Cbf123-1 (Zhao et al., 2016), aba3, abi1 (Cui et al., 2016), bbx29-1, bbx29-2 (Song et al., 2020), and 35S::BBX29-Myc (this study) lines were generated in the A. thaliana ecotype Columbia (Col-0) background. Prior to planting, seeds were surface sterilized with 70% ethanol containing 2% Triton X-100 for 5 min. This was followed by two quick wash steps with 75% ethanol. Anhydrous ethanol was used to adhere the seeds onto sterile filter paper. Seeds were sown on 1/2 Murashige and Skoog medium supplemented with 0.8% agar and 1% sucrose and cultured at 22°C under a 12/12 h light/dark photoperiod.
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. cDNA was synthesized using the HiScript III First Strand cDNA Synthesis Kit (+gDNA Wiper) (Vazyme, Nanjing, China). Quantitative real-time PCR was performed using a CFX96 real-time PCR system with ChamQ SYBR qPCR Master Mix (Vazyme). PCR was performed using the following conditions: 95°C for 10 min, 45 cycles of 95°C for 30 s, and 60°C for 10 s. The relative expression level of each gene in Arabidopsis was normalized to ACTIN2 (AT3G18780) and ACTIN8 (AT1G49240), while the expression of genes in P. trichocarpa were normalized to PtrHIS (Potri.005G072300) and PtrACTIN (Potri.019G010400). The resulting data were analyzed using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Freezing tolerance and ion leakage assays
Two-week-old seedlings raised on 1/2 Murashige and Skoog medium under a 12/12 h light/dark photoperiod were treated with or without CA (4°C for 3 d). The freezing tolerance assay was then performed as described by Jia et al. (2016). Briefly, the treatment program was set at 4°C for 10 min and followed by 0°C for 20 min. Then, the temperature was further dropped 1°C / h until reached to the desired freezing treatment temperature as described in the figure legends. After the freezing treatment, plants were grown at 4°C in the dark for 12 h before being transferred to 22°C for an additional 3 d. The freezing tolerance phenotype of each line was observed, and the survival rate of each line was quantified. For the ion leakage assay, 3-week-old seedlings grown in the soil were first treated with or without CA (4°C for 3 d) and then treated with freezing for 1 h at different desired temperatures. The rosette leaves of the injured seedlings from each freezing treatment were collected to perform an ion leakage assay as described by Ding et al. (2018). At least three independent experiments were performed, and each experiment was performed with three technical replicates.
RNA-seq analysis
Two-week-old Col-0, bbx29-2, and 35S::BBX29-Myc (OE5) seedlings raised under a 12/12 h light/dark photoperiod were treated with cold (4°C) for 0, 2, and 24 h. Total RNA was extracted from the leaves of the seedlings using the TRIzol method. RNA-seq was performed using an Illumina NovaSeq PE150 platform by Novogene (Tianjin, China). Two biological replicates were included in each experiment and RNA-seq analysis was performed as described in our previous publications (Zhao et al., 2021; Guo et al., 2022). GO enrichment analysis of differentially expressed genes was performed and visualized using ClueGO (Bindea et al., 2009).
CUT&Tag-seq and data analysis
To identify genes directly targeted by BBX29, CUT&Tag (Kaya-Okur et al., 2019) was performed using the NovoNGS CUT&Tag 3.0 High-Sensitivity Kit (for Illumina) (Novoprotein, Suzhou, China) following the manufacturer’s protocol. Briefly, protoplasts of 2-week-old 35S::BBX29-Myc (OE5) and WT (CUT&Tag control) seedlings were isolated and incubated with NovoNGS ConA beads. The bead-bound protoplasts were incubated in primary antibody buffer containing an anti-MYC antibody at 4°C by rotating overnight. The primary antibody buffer was removed the following day, and the protoplasts were incubated with secondary antibody buffer for 1 h. Bead-bound protoplasts were then incubated in ChiTag buffer with NovoNGS ChiTag 3.0 Transposome (pAG-Tn5), followed by tagmentation with tagmentation buffer at 37°C for 1 h. After tagmentation, genomic DNA was extracted and amplified with 5×AmpliMix and i5/i7 primers to generate a library. The PCR products were purified and sequenced using an Illumina NovaSeq PE150 platform by Novogene. For each of the CUT&Tag-seq experiments, two biological replicates were performed. The primers used can be found in Supplemental Table 7.
After initial quality filtering, we aligned the sequencing reads to the A. thaliana genome (TAIR10) using Bowtie2 with default parameters (Langmead and Salzberg, 2012). Peak calling was performed on Col-0 (CUT&Tag control) and 35S::BBX29-Myc samples using MACS v.2.7.1 (Zhang et al., 2008). Each peak was annotated with the name of the nearest gene using ChIPseeker v.1.20 (Yu et al., 2015). Genes with at least one binding peak in the promoter sequence upstream of the translation initiation site (2 kb) were likely directly targeted by BBX29.
Y2H assay
We performed a Y2H assay according to the Matchmaker Gold Y2H System User Manual (Clontech). Briefly, full-length coding sequences of BBX29 and CBFs (CBF1, CBF2, and CBF3) were cloned and inserted into the pGBKT7 and pGADT7 vectors, respectively. Combinations of pGBKT7-BBX29 with pGADT7-CBF1/2/3 were co-transformed into the Y2HGold yeast strain. The combination of pGBKT7-p53 and pGADT7-T was used as a positive control, and the combination of pGBKT7-Lam and pGADT7-T was used as a negative control. The transformants were separately cultured on synthetic dropout nutriant mediums, including synthetic defined (SD) medium/−Trp/−Leu, SD/−Trp/−Leu/−His/−Ade, and SD/−Trp/−Leu/−His/−Ade containing with Aurcobasidin A and X-α-gal.
Transient transformation of PtrBBX29 into P. trichocarpa
The full-length coding sequence of PtrBBX29 was cloned and inserted into the pCAMBIA1300 vector. Agrobacterium tumefaciens strains carrying pCAMBIA1300-PtrBBX29 or the empty vector pCAMBIA1300 were used to transiently transform the leaves of P. trichocarpa using the leaf infiltration method described previously (Ye et al., 2022). After 48 h of co-cultivation, the transformed P. trichocarpa seedlings were transferred into artificial climate chambers set to 4°C (cold stress treatment) and 25°C (cold stress control). Co-cultivation was continued for another 24 h before the infiltrated leaves were collected for RNA extraction. The expression of PtrBBX29 and COR genes were quantified using quantitative real-time PCR.
Data and code availability
The original contributions presented in this study are included in the article and its supplementary files. The RNA-seq datasets from the five representative eudicot species treated with cold stress have been submitted to the NCBI BioProject (PRJNA767196). The RNA-seq data for Col-0, bbx29-2, and 35S::BBX29-Myc (OE5) lines and CUT&Tag-seq data for Col-0 and 35S::BBX29-Myc (OE5) lines have been submitted to the NCBI BioProject (PRJNA881642) and National Genomics Data Center (PRJCA019407).
Funding
This work is supported by the National Natural Science Foundation of China (31871233), Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02070-1), and the Scientific Research Development Fund of Zhejiang A&F University (2022LFR082).
Author contributions
W.W., J.-K.Z., H.H., and J.H. designed the research. S.W., Y.S., Y.Z., Y.D., and X.Z. performed the experiments. L.G. and X.Y. performed comparative genomes and cold transcriptomes in five eudicots. D.D., L.G., and Y.N. analyzed RNA-seq and CUT&Tag-seq data. S.W., W.W., J.-K.Z., and H.H. wrote and revised the manuscript.
Acknowledgments
We greatly appreciate Dr. Dongqing Xu for the bbx29-1, bbx29-2, and bbx28-1 bbx29-1 seeds and Dr. Fuqiang Cui for the abi1 and aba3 seeds. No conflict of interest is declared.
Published: September 9, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Contributor Information
Huahong Huang, Email: huanghh@zafu.edu.cn.
Jian-Kang Zhu, Email: zhujk@sustech.edu.cn.
Wenwu Wu, Email: wwwu@zafu.edu.cn.
Supplemental information
References
- Bai B., Lu N., Li Y., Guo S., Yin H., He Y., Sun W., Li W., Xie X. OsBBX14 promotes photomorphogenesis in rice by activating OsHY5L1 expression under blue light conditions. Plant Sci. 2019;284:192–202. doi: 10.1016/j.plantsci.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Baker S.S., Wilhelm K.S., Thomashow M.F. The 5'-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol. Biol. 1994;24:701–713. doi: 10.1007/BF00029852. [DOI] [PubMed] [Google Scholar]
- Bindea G., Mlecnik B., Hackl H., Charoentong P., Tosolini M., Kirilovsky A., Fridman W.H., Pagès F., Trajanoski Z., Galon J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V., Ohta M., Kanrar S., Lee B.H., Hong X., Agarwal M., Zhu J.K. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F., Brosché M., Lehtonen M.T., Amiryousefi A., Xu E., Punkkinen M., Valkonen J.P.T., Fujii H., Overmyer K. Dissecting Abscisic Acid Signaling Pathways Involved in Cuticle Formation. Mol. Plant. 2016;9:926–938. doi: 10.1016/j.molp.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Ding Y., Shi Y., Yang S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant. 2020;13:544–564. doi: 10.1016/j.molp.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Ding Y., Jia Y., Shi Y., Zhang X., Song C., Gong Z., Yang S. OST1-mediated BTF3L phosphorylation positively regulates CBFs during plant cold responses. EMBO J. 2018;37 doi: 10.15252/embj.201798228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty C.J., Van Buskirk H.A., Myers S.J., Thomashow M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M.A., Farré E.M., Thomashow M.F. Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:7241–7246. doi: 10.1073/pnas.1103741108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms D.M., Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20:238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X.X., Yan B.Y., Hu J., Yang C.P., Li Y.J., Liu J.P., Liao W.B. Transcriptome profiling of rubber tree (Hevea brasiliensis) discovers candidate regulators of the cold stress response. Genes Genomics. 2018;40:1181–1197. doi: 10.1007/s13258-018-0681-5. [DOI] [PubMed] [Google Scholar]
- Guo Q., Li X., Niu L., Jameson P.E., Zhou W. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol. 2021;186:677–695. doi: 10.1093/plphys/kiab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Wang S., Nie Y., Shen Y., Ye X., Wu W. Convergent evolution of AP2/ERF III and IX subfamilies through recurrent polyploidization and tandem duplication during eudicot adaptation to paleoenvironmental changes. Plant Commun. 2022;3 doi: 10.1016/j.xplc.2022.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Xu Z., Wang S., Nie Y., Ye X., Jin X., Zhu J., Wu W. Integrative multi-omics analysis of three early diverged rosid species reveals an ancient hierarchical cold-responsive regulatory network. Physiol. Plantarum. 2023;175 doi: 10.1111/ppl.13892. [DOI] [PubMed] [Google Scholar]
- Hannah M.A., Heyer A.G., Hincha D.K. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet. 2005;1:e26. doi: 10.1371/journal.pgen.0010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M., Xiong L., Stevenson B., Zhu J.K. Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell. 1997;9:1935–1949. doi: 10.1105/tpc.9.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen K.R., Gilmour S.J., Zarka D.G., Schabenberger O., Thomashow M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Jia Y., Ding Y., Shi Y., Zhang X., Gong Z., Yang S. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345–353. doi: 10.1111/nph.14088. [DOI] [PubMed] [Google Scholar]
- Jiang B., Shi Y., Zhang X., Xin X., Qi L., Guo H., Li J., Yang S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2017;114 doi: 10.1073/pnas.1706226114. E6695-e6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Zhang H., Zhang J., Liu P., Chen X., Li Z., Xu Y., Lu P., Cao P. Integrated transcriptomics and metabolomics analysis to characterize cold stress responses in Nicotiana tabacum. BMC Genom. 2017;18:496. doi: 10.1186/s12864-017-3871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya-Okur H.S., Wu S.J., Codomo C.A., Pledger E.S., Bryson T.D., Henikoff J.G., Ahmad K., Henikoff S. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 2019;10:1930. doi: 10.1038/s41467-019-09982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro S., Shinozaki K., Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022;27:922–935. doi: 10.1016/j.tplants.2022.01.008. [DOI] [PubMed] [Google Scholar]
- Kidokoro S., Hayashi K., Haraguchi H., Ishikawa T., Soma F., Konoura I., Toda S., Mizoi J., Suzuki T., Shinozaki K., Yamaguchi-Shinozaki K. Posttranslational regulation of multiple clock-related transcription factors triggers cold-inducible gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2021048118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J., Whitehead D., Horak J., Wanke D., Weinl S., Batistic O., D'Angelo C., Bornberg-Bauer E., Kudla J., Harter K. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007;50:347–363. doi: 10.1111/j.1365-313X.2007.03052.x. [DOI] [PubMed] [Google Scholar]
- Kim Y., Park S., Gilmour S.J., Thomashow M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013;75:364–376. doi: 10.1111/tpj.12205. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Lee M., Lee J.H., Lee H.J., Park C.M. The unified ICE-CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis. Plant Mol. Biol. 2015;89:187–201. doi: 10.1007/s11103-015-0365-3. [DOI] [PubMed] [Google Scholar]
- Koonin E.V. Orthologs, paralogs, and evolutionary genomics. Annu. Rev. Genet. 2005;39:309–338. doi: 10.1146/annurev.genet.39.073003.114725. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.M., Thomashow M.F. Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2012;109:15054–15059. doi: 10.1073/pnas.1211295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.G., Seo P.J. The MYB96-HHP module integrates cold and abscisic acid signaling to activate the CBF-COR pathway in Arabidopsis. Plant J. 2015;82:962–977. doi: 10.1111/tpj.12866. [DOI] [PubMed] [Google Scholar]
- Li Y., Shi Y., Li M., Fu D., Wu S., Li J., Gong Z., Liu H., Yang S. The CRY2-COP1-HY5-BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell. 2021;33:3555–3573. doi: 10.1093/plcell/koab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wu C., Hu X., Gao H., Wang Y., Luo H., Cai S., Li G., Zheng Y., Lin C., Zhu Q. Transcriptome profiling reveals the crucial biological pathways involved in cold response in Moso bamboo (Phyllostachys edulis) Tree Physiol. 2020;40:538–556. doi: 10.1093/treephys/tpz133. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lv X., Li H., Chen X., Xiang X., Guo Z., Yu J., Zhou Y. The role of calcium-dependent protein kinase in hydrogen peroxide, nitric oxide and ABA-dependent cold acclimation. J. Exp. Bot. 2018;69:4127–4139. doi: 10.1093/jxb/ery212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P., Bailey T.L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Takeda M., Kidokoro S., Yamada K., Sakuma Y., Urano K., Fujita M., Yoshiwara K., Matsukura S., Morishita Y., et al. Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcripts regulated by DREB1A and DREB2A. Plant Physiol. 2009;150:1972–1980. doi: 10.1104/pp.109.135327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M., Orsini E., Ruschhaupt M., Melchinger A.E., Hincha D.K., Heyer A.G. Mapping quantitative trait loci for freezing tolerance in a recombinant inbred line population of Arabidopsis thaliana accessions Tenela and C24 reveals REVEILLE1 as negative regulator of cold acclimation. Plant Cell Environ. 2013;36:1256–1267. doi: 10.1111/pce.12054. [DOI] [PubMed] [Google Scholar]
- Meng X., Liang Z., Dai X., Zhang Y., Mahboub S., Ngu D.W., Roston R.L., Schnable J.C. Predicting transcriptional responses to cold stress across plant species. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2026330118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M.D., Thomashow M.F. A role for circadian evening elements in cold-regulated gene expression in Arabidopsis. Plant J. 2009;60:328–339. doi: 10.1111/j.1365-313X.2009.03957.x. [DOI] [PubMed] [Google Scholar]
- Mizoi J., Shinozaki K., Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta. 2012;1819:86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Narusaka Y., Nakashima K., Shinwari Z.K., Sakuma Y., Furihata T., Abe H., Narusaka M., Shinozaki K., Yamaguchi-Shinozaki K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003;34:137–148. doi: 10.1046/j.1365-313x.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- Nie Y., Guo L., Cui F., Shen Y., Ye X., Deng D., Wang S., Zhu J., Wu W. Innovations and Stepwise Evolution of CBFs/DREB1s and Their Regulatory Networks in Angiosperms. J. Integr. Plant Biol. 2022;64:2111–2125. doi: 10.1111/jipb.13357. [DOI] [PubMed] [Google Scholar]
- Novillo F., Alonso J.M., Ecker J.R., Salinas J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2004;101:3985–3990. doi: 10.1073/pnas.0303029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley R.C., Huang S.S.C., Song L., Lewsey M.G., Bartlett A., Nery J.R., Galli M., Gallavotti A., Ecker J.R. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell. 2016;165:1280–1292. doi: 10.1016/j.cell.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Gilmour S.J., Grumet R., Thomashow M.F. CBF-dependent and CBF-independent regulatory pathways contribute to the differences in freezing tolerance and cold-regulated gene expression of two Arabidopsis ecotypes locally adapted to sites in Sweden and Italy. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee C.M., Doherty C.J., Gilmour S.J., Kim Y., Thomashow M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015;82:193–207. doi: 10.1111/tpj.12796. [DOI] [PubMed] [Google Scholar]
- Qi L., Shi Y., Terzaghi W., Yang S., Li J. Integration of light and temperature signaling pathways in plants. J. Integr. Plant Biol. 2022;64:393–411. doi: 10.1111/jipb.13216. [DOI] [PubMed] [Google Scholar]
- Ritonga F.N., Ngatia J.N., Wang Y., Khoso M.A., Farooq U., Chen S. AP2/ERF, an important cold stress-related transcription factor family in plants: A review. Physiol. Mol. Biol. Plants. 2021;27:1953–1968. doi: 10.1007/s12298-021-01061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé P.A., McClung C.R. PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell. 2005;17:791–803. doi: 10.1105/tpc.104.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Huang S.S.C., Wise A., Castanon R., Nery J.R., Chen H., Watanabe M., Thomas J., Bar-Joseph Z., Ecker J.R. A transcription factor hierarchy defines an environmental stress response network. Science. 2016;354:aag1550. doi: 10.1126/science.aag1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhang X., Li M., Yang H., Fu D., Lv J., Ding Y., Gong Z., Shi Y., Yang S. The direct targets of CBFs: In cold stress response and beyond. J. Integr. Plant Biol. 2021;63:1874–1887. doi: 10.1111/jipb.13161. [DOI] [PubMed] [Google Scholar]
- Song Z., Yan T., Liu J., Bian Y., Heng Y., Lin F., Jiang Y., Wang Deng X., Xu D. BBX28/BBX29, HY5 and BBX30/31 form a feedback loop to fine-tune photomorphogenic development. Plant J. 2020;104:377–390. doi: 10.1111/tpj.14929. [DOI] [PubMed] [Google Scholar]
- Stockinger E.J., Gilmour S.J., Thomashow M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tähtiharju S., Palva T. Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in Arabidopsis thaliana. Plant J. 2001;26:461–470. doi: 10.1046/j.1365-313x.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- Thomashow M.F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Vogel J.T., Zarka D.G., Van Buskirk H.A., Fowler S.G., Thomashow M.F. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005;41:195–211. doi: 10.1111/j.1365-313X.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- Wang F., Guo Z., Li H., Wang M., Onac E., Zhou J., Xia X., Shi K., Yu J., Zhou Y. Phytochrome A and B Function Antagonistically to Regulate Cold Tolerance via Abscisic Acid-Dependent Jasmonate Signaling. Plant Physiol. 2016;170:459–471. doi: 10.1104/pp.15.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Datla R., Georges F., Loewen M., Cutler A.J. Promoters from kin1 and cor6.6, two homologous Arabidopsis thaliana genes: transcriptional regulation and gene expression induced by low temperature, ABA, osmoticum and dehydration. Plant Mol. Biol. 1995;28:605–617. doi: 10.1007/BF00021187. [DOI] [PubMed] [Google Scholar]
- Wang S., Shen Y., Guo L., Tan L., Ye X., Yang Y., Zhao X., Nie Y., Deng D., Liu S., Wu W. Innovation and Emerging Roles of Populus trichocarpa TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR Transcription Factors in Abiotic Stresses by Whole-Genome Duplication. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.850064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang Y., Ye X., Shen Y., Liu H., Zhao X., Guo L., Cao L., Du Y., Wu W. A phylotranscriptomic dataset of angiosperm species under cold stress. Sci. Data. 2023;10:399. doi: 10.1038/s41597-023-02307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm K.S., Thomashow M.F. Arabidopsis thaliana cor15b, an apparent homologue of cor15a, is strongly responsive to cold and ABA, but not drought. Plant Mol. Biol. 1993;23:1073–1077. doi: 10.1007/BF00021822. [DOI] [PubMed] [Google Scholar]
- Xiong C., Luo D., Lin A., Zhang C., Shan L., He P., Li B., Zhang Q., Hua B., Yuan Z., et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2019;221:279–294. doi: 10.1111/nph.15373. [DOI] [PubMed] [Google Scholar]
- Xu D., Jiang Y., Li J., Holm M., Deng X.W. The B-Box Domain Protein BBX21 Promotes Photomorphogenesis. Plant Physiol. 2018;176:2365–2375. doi: 10.1104/pp.17.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Cold responses in rice: From physiology to molecular biology. J. Plant Physiol. 2022;269 doi: 10.1016/j.jplph.2021.153602. [DOI] [PubMed] [Google Scholar]
- Ye X., Wang S., Zhao X., Gao N., Wang Y., Yang Y., Wu E., Jiang C., Cheng Y., Wu W., Liu S. Role of lncRNAs in cis- and trans-regulatory responses to salt in Populus trichocarpa. Plant J. 2022;110:978–993. doi: 10.1111/tpj.15714. [DOI] [PubMed] [Google Scholar]
- Yu G., Wang L.G., He Q.Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- Zhan X., Yang L., Wang D., Zhu J.K., Lang Z. De novo assembly and analysis of the transcriptome of Ocimum americanum var. pilosum under cold stress. BMC Genom. 2016;17:209. doi: 10.1186/s12864-016-2507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ngu D.W., Carvalho D., Liang Z., Qiu Y., Roston R.L., Schnable J.C. Differentially Regulated Orthologs in Sorghum and the Subgenomes of Maize. Plant Cell. 2017;29:1938–1951. doi: 10.1105/tpc.17.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yu H., Yang X., Li Q., Ling J., Wang H., Gu X., Huang S., Jiang W. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol. Biochem. 2016;108:478–487. doi: 10.1016/j.plaphy.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhang Z., Xie S., Si T., Li Y., Zhu J.K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016;171:2744–2759. doi: 10.1104/pp.16.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Tan L., Wang S., Shen Y., Guo L., Ye X., Liu S., Feng Y., Wu W. The SR Splicing Factors: Providing Perspectives on Their Evolution, Expression, Alternative Splicing, and Function in Populus trichocarpa. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Li X., Liu X., Wen X., Zhang Y., Zhang D. Transcriptome profiling of Malus sieversii under freezing stress after being cold-acclimated. BMC Genom. 2021;22:681. doi: 10.1186/s12864-021-07998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article and its supplementary files. The RNA-seq datasets from the five representative eudicot species treated with cold stress have been submitted to the NCBI BioProject (PRJNA767196). The RNA-seq data for Col-0, bbx29-2, and 35S::BBX29-Myc (OE5) lines and CUT&Tag-seq data for Col-0 and 35S::BBX29-Myc (OE5) lines have been submitted to the NCBI BioProject (PRJNA881642) and National Genomics Data Center (PRJCA019407).