Abstract
Plant mineral nutrition is essential for crop yields and human health. However, the uneven distribution of mineral elements over time and space leads to a lack or excess of available mineral elements in plants. Among the essential nutrients, calcium (Ca2+) stands out as a prominent second messenger that plays crucial roles in response to extracellular stimuli in all eukaryotes. Distinct Ca2+ signatures with unique parameters are induced by different stresses and deciphered by various Ca2+ sensors. Recent research on the participation of Ca2+ signaling in regulation of mineral elements has made great progress. In this review, we focus on the impact of Ca2+ signaling on plant mineral uptake and detoxification. Specifically, we emphasize the significance of Ca2+ signaling for regulation of plant mineral nutrition and delve into key points and novel avenues for future investigations, aiming to offer new insights into plant ion homeostasis.
Key words: Ca2+ signaling, mineral nutrition, ion channels and transporters, uptake and transport
This review provides a comprehensive overview of Ca2+ signaling in plant mineral uptake and detoxification. The role of Ca2+ as a second messenger in response to extracellular stimuli and the distinct Ca2+ signatures induced by different stresses are highlighted. The review also offers insights into future investigations of plant ion homeostasis.
Introduction
Plant growth and development are affected by changing environmental conditions and various stress factors, including soil mineral content and accumulation of harmful elements. The soil provides plants with 14 essential mineral nutrients, which are categorized into macronutrients and micronutrients on the basis of their dry biomass percentage (< or ≥ 0.1%). Macronutrients consist of nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), and magnesium (Mg), and micronutrients include iron (Fe), manganese, copper, zinc, molybdenum, boron (B), chloride, and nickel (Maathuis and Diatloff, 2013; Vatansever et al., 2017). Besides the essential elements, some elements in the soil are not essential for plant growth and reproduction but are conducive to plant growth; these include sodium, silicon, cobalt, and selenium (Pilon-Smits et al., 2009; Vatansever et al., 2017). However, a class of trace metals or metalloid elements, including cadmium, lead, chromium, arsenic (As), and aluminum, can be severely toxic to plants (Ghori et al., 2019).
Ca2+ is an essential nutrient and the most prominent second messenger, playing a crucial role in response to extracellular stimuli in all eukaryotes (Lee and Seo, 2021). Different stresses cause distinct Ca2+ signatures (also called stimulus-specific Ca2+ patterns): Ca2+ signals with different parameters such as transient or repetitive oscillation, duration, amplitude, frequency, and spatial distribution (Kudla et al., 2018b). Ca2+ influx via Ca2+ channels and Ca2+ efflux via Ca2+ transporters comprise an orchestrated balanced system. Ca2+ channels include cyclic nucleotide-gated channels (CNGCs), glutamate receptor-like channels, the mechanosensitive channel of small conductance (MscS)-like channels, Mid1-complementing activity channels, reduced hyperosmolality-induced [Ca2+]i increase (hyperosmolality-gated calcium-permeable channels [OSCA]) channels, two-pore channels, annexins, and MILDEW RESISTANCE LOCUS O proteins (Kudla et al., 2018a; Luan and Wang, 2021; Guichard et al., 2022; Gao et al., 2023). Ca2+ transporters include Ca2+-ATPases and Ca2+/H+ exchangers (CAXs). Ca2+-ATPases are classified into two groups: P2A type (endoplasmic reticulum [ER]-type Ca2+-ATPase: ECA1–ECA4) and P2B type (autoinhibited Ca2+-ATPases: ACA1, ACA2, ACA4, and ACA7–ACA13) (García Bossi et al., 2019). CAX genes are identified as CAX1–CAX6 (Shigaki et al., 2006). The protagonists involved in the process of decoding Ca signals are Ca2+-binding proteins that act as sensors. They bind to Ca2+ in response to elevated Ca2+ levels in the cell and include calcineurin B-like proteins (CBLs), calmodulins (CaMs), CaM-like proteins (CMLs), Ca-dependent protein kinases (CPKs), and Ca- and CaM-dependent protein kinases. The roles of Ca2+ sensors and Ca2+ channels in Ca2+ signaling have been studied extensively over the past few decades and have recently been described in detail (Tian et al., 2020; Luan and Wang, 2021; Dong et al., 2022a). Here, we focused on the role of Ca2+ signaling in regulating plant mineral uptake and transport.
Ca2+ signaling in absorption and utilization of macronutrients
Ca2+ signaling modulates N-regulatory networks
Nitrate (NO3−) serves not only as an N source but also as a signaling molecule that regulates numerous processes, including gene expression, root architecture, shoot development, seed germination, and flowering (Lin and Tsay, 2017; O'Brien et al., 2016). Several players in the NO3− signaling pathway have been identified, including NO3− sensors, NO3− transporters, Ca signaling components, protein kinases, and transcription factors.
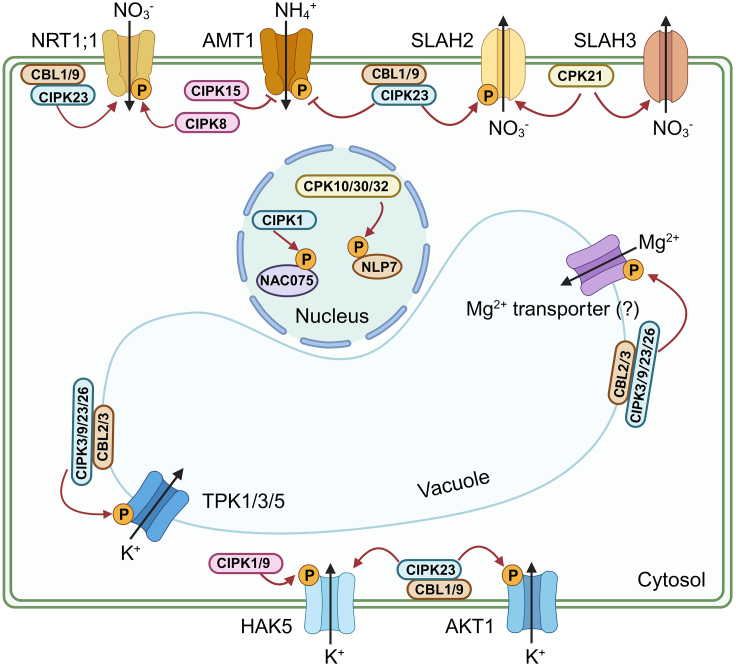
Ca2+ signaling is involved in regulation of various plant growth and developmental processes, including regulation of N. NO3− treatments rapidly and transiently increase [Ca2+]cyt in Arabidopsis roots (Riveras et al., 2015). Distinct Ca2+ dynamics are triggered by NO3− in the tip, pericycle, and stele of intact roots. Likewise, a gradual increase in subcellular Ca2+ levels attributed to NO3− can be observed over several minutes using GCaMP6-based imaging in leaf cells co-expressing the highly sensitive Ca2+ biosensor GCaMP6 and a nuclear mCherry marker (Liu et al., 2017). The NITRATE TRANSPORTER 1 (NRT1) family member NRT1;1, also known as CHLORATE-RESISTANT 1 (CHL1), acts as a NO3− sensor to perceive external NO3− (Tsay et al., 1993; Liu et al., 1999; Remans et al., 2006). Importantly, complexes of CNGC15 and NRT1;1 dynamically regulate Ca2+ channel activity by sensing the external NO3− concentration (Wang et al., 2021a). Two CBL-INTERACTING PROTEIN KINASES (CIPKs), CIPK8 and CIPK23, have been found to differentially regulate NO3− signaling. CIPK8 plays a positive role in NO3−-induced expression of primary NO3− response genes and acts as a positive regulator of the low-affinity response. On the other hand, CIPK23 serves as a negative regulator of the high-affinity response. In the presence of low NO3− concentrations, CHL1 binds to NO3− and directly interacts with CIPK23, leading to phosphorylation of CHL1 at Thr101, thereby maintaining a low-level primary response (Ho et al., 2009; Hu et al., 2009; Figure 1). Dynamic regulation of the dual-affinity system by Ca2+ signaling enables CHL1 to sense a wide range of NO3− concentrations in plants and trigger different responses. Furthermore, expression of the NRT2 family members NRT2;4 and NRT2;5 is inhibited in the cbl7 mutant under N starvation stress, indicating that CBL7 may be involved in modulation of high-affinity NO3− uptake under NO3− starvation conditions (Ma et al., 2015). In the plant NO3− transport system, SLAC-ASSOCIATED 1 HOMOLOG2 (SLAH2), a homolog of slow-type anion channel-associated 1 (SLAC1), can be phosphorylated by the CBL1/9–CIPK23 module, improving its NO3− transport function, and CPK21 can also activate SLAH2/3 to promote their NO3− transport (Maierhofer et al., 2014; Cubero-Font et al., 2016).
Figure 1.
Ca2+ signaling regulatory network in macronutrient absorption and utilization.
Under low-nitrogen (N) conditions, the CBL1/9–CIPK23 module phosphorylates NRT1;1 to enhance NO3− uptake. Conversely, under high-N conditions, CIPK8 phosphorylates NRT1;1, facilitating NO3− absorption. In addition, CBL1/9–CIPK23 and CIPK15 can phosphorylate AMT1, inhibiting its ammonium (NH4+) transport function and mitigating NH4+ toxicity. SLAH2 can be phosphorylated by CBL1/9–CIPK23, promoting NO3− uptake, and CPK21 can also phosphorylate SLAH2/3 to promote NO3− transport. NLP7, another NO3− sensor, undergoes phosphorylation by CPK10, CPK30, and CPK32 to regulate its nucleoplasmic shuttle. CIPK1 regulates expression of downstream target genes by phosphorylating and activating NAC075 under low-N conditions. Regarding potassium (K) transport, CBL1/9 recruit their interacting kinase CIPK23 to the root cell PM. CIPK23 then phosphorylates the K transporters AKT1 and HAK5, promoting K+ absorption. CIPK1 and CIPK9 regulate root K uptake by phosphorylating HAK5. Under low-K+ conditions, the CBL2/3–CIPK3/9/23/26 module activates the tandem-pore K+ channels TPK1/3/5 on the tonoplast, releasing vacuolar K+ into the cytoplasm. The CBL2/3–CIPK3/9/23/26 module recruits magnesium (Mg2+) to the tonoplast and regulates downstream target transporters that mediate efficient sequestration of Mg2+ in vacuoles, maintaining a non-toxic level of Mg2+ in the cytoplasm. CBL, calcineurin B-like protein; CIPK, CBL-interacting protein kinase; NRT, NO3− transporter; AMT1, NH4+ transporter 1; SLAH, homolog of slow type anion channel-associated 1 (SLAC1); NLP7, NIN-LIKE PROTEIN 7; CPK, Ca-dependent protein kinase; NAC075, NAC transcription factor; AKT, K+ transporter; HAK5, high-affinity K+ transporter 5; TPK, two-pore K+ channel.
To transmit N signals to intracellular and downstream signaling molecules, intracellular signal-sensing mechanisms are also required. NIN-LIKE PROTEIN 7 (NLP7) has been proposed to act as a ligand-dependent transcriptional activator and an intracellular NO3− sensor (Alvarez et al., 2020; Liu et al., 2022). The subgroup III Ca2+-sensor protein kinases CPK10, CPK30, and CPK32 have been found to affect nucleoplasmic localization of NLP7 by phosphorylating its Ser205 residue (Liu et al., 2017). Two types of NO3− sensors in the plasma membrane (PM) and cytoplasm ensure that N signals are transmitted rapidly and respond in a timely manner to different N concentrations. Expression of the basic region/leucine zipper motif (bZIP) transcription factor family members TGA1 and TGA4 is upregulated in a Ca2+-dependent manner and regulates expression of NRT2;1 and NRT2;2, which mediate NO3− transport (Alvarez et al., 2014; Zhong et al., 2015). Under low-NO3− conditions, CIPK1 is activated and phosphorylates the NAC (NAM/ATAF/CUC) transcription factor NAC075, regulating expression of the downstream target WRKY53 (Xiao et al., 2022) (Figure 1). A series of transcription factors, including NLP7, are regulated by Ca2+ signaling, forming an elaborate regulatory network.
Ammonium (NH4+) is the primary source of N in many species, but excessive NH4+ can lead to NH4+ toxicity (Loqué and von Wirén, 2004). To prevent NH4+ toxicity, two members of the NH4+ transport (AMT) family, AMT1;1 and AMT1;2, are inhibited by the CBL1–CIPK23 complex (Straub et al., 2017). Expression of CIPK23 is upregulated by STOP1 when NH4+ is present in excess (Tian et al., 2021). Dynamic regulation of NRTs and AMTs by Ca2+ signaling components, such as the CBL1/9–CIPK23 module, helps to maintain the balance between N absorption and NH4+ toxicity. In addition, CIPK15 inhibits the activity of AMT1 isoforms by phosphorylating their C terminus (Chen et al., 2020; Figure 1). However, whether Ca2+ channels and other CBL–CIPK or CPK proteins are involved in regulation of AMTs requires further investigation.
Ca2+ signaling regulates the response to P deficiency
P, an essential mineral nutrient for plant growth and development, is a critical component of many metabolites and macromolecules, including proteins, phospholipids, and nucleic acids (Lopez-Arredondo et al., 2014). Previous studies have demonstrated a correlation between cytosolic Ca and phosphate levels in plants. Phosphate (Pi) deficiency induces a rapid decrease in [Ca2+]cyt in Arabidopsis roots (Matthus et al., 2019b, 2020) (Figure 1). A recent study found that At1g62420 (RXR3) reduces root hair growth by encoding tip-focused [Ca2+]cyt oscillations through ROOT HAIR DEFECTIVE 6-LIKE 4 interaction with CaM under low-Pi stress (Ying and Scheible, 2022). CAX1, a vacuolar Ca2+/H+ transporter, is required for systemic Pi homeostasis involving shoot-to-root signaling in Arabidopsis (Liu et al., 2011). However, further investigation is necessary to determine whether Ca signaling is directly involved in regulation of P transporters and to characterize its specific regulatory mechanism. It would be interesting to investigate how Ca2+ channels generate Ca2+ signals under varying P concentrations and to determine whether CBL–CIPK modules and CPKs directly participate in regulating P signaling networks. In addition, it would be worthwhile to examine the impact of P availability on the expression and activity of Ca2+ channels, as well as the potential crosstalk between Ca and P signaling pathways.
Ca2+ signaling adjusts K homeostasis
K is present as a soluble ion (K+) in plants, where it plays essential roles in many physiological processes, such as osmotic balance, stomatal regulation, protein biosynthesis, water and nutrient absorption, and enzyme activation (Wang et al., 2021b).
Studies have shown that K+ deficiency triggers two successive and distinct Ca2+ signals in roots, which exhibit spatial and temporal specificity. Ca2+ channels located in the root epidermis and root hair zone can be activated by hyperpolarization of the PM under K+ deficiency conditions (Véry and Davies, 2000; Demidchik et al., 2002). Moreover, the increase in reactive oxygen species levels induced by K+ deficiency can lead to Ca2+ signaling via reactive oxygen species–activated Ca2+ channels (Shin and Schachtman, 2004; Demidchik and Maathuis, 2007). The CBL–CIPK network plays a vital role in the K+ deficiency response. K+ transporter 1 (AKT1) and high-affinity K+ transporter 5 (HAK5), a K+/H+ symporter, are considered to be the major components involved in K+ uptake in Arabidopsis root cells under low-K+ conditions (Nieves-Cordones et al., 2014). CBL1/9 recruit their interacting kinase CIPK23 to the root cell PM, and CIPK23 then phosphorylates AKT1 and HAK5 to promote plant K+ uptake (Li et al., 2006; Xu et al., 2006; Cheong et al., 2007; Ragel et al., 2015; Lara et al., 2020). In addition, CBL10 negatively modulates AKT1 activity by competing for binding of CIPK23 to AKT1 (Ren et al., 2013). In addition to increasing their K+ uptake, plant cells mobilize K reserves in the vacuoles. Under low-K+ stress, the CBL2/3–CIPK3/9/23/26 module activates the tandem-pore K+ channels TPK1/3/5 on the vacuolar membrane (VM), releasing vacuolar K+ into the cytoplasm (Tang et al., 2020a). The PM–CBL1/9–CIPK23 and VM–CBL2/3–CIPK3/9/23/26 signaling modules play a crucial role in connecting low-K+ stress with activation of K+ channels, thereby maintaining K+ homeostasis. Recent studies have revealed that early occurrence of K+-induced activation of the vacuolar Ca2+ sensors CBL2/3 contributes to activation of the CBL1/9 pathway under K+ deficiency. A recent study showed that the protein abundance and phosphorylation status of CBL–CIPK–channel modules are influenced by external K+ status (Li et al., 2023b), providing unique insights into the coordinated regulation of K+ homeostasis by VM and PM CBL–CIPK–channel modules. Furthermore, CIPK1 and CIPK9 regulate root K+ uptake by phosphorylating HAK5 (Lara et al., 2020). Raf-like mitogen-activated protein kinase kinase (MAPKK) kinase (ILK1) directly interacts with HAK5 in conjunction with CML9, promoting HAK5 accumulation on the PM (Brauer et al., 2016). Together with CIPK6, CBL4 regulates the activity and PM targeting of the K+ channel AKT2 in a kinase interaction–dependent manner (Held et al., 2011; Figure 1). Ca2+ signaling has been found to participate in regulating the different localizations of K+ channels and transporters in different physiological processes. However, further investigations are needed to explore the potential roles of other Ca2+ sensors, such as CPK, in regulating K homeostasis.
Ca2+ signaling and Ca nutrition
Ca is essential for plant growth and development under non-stressed and adverse conditions. Ca2+ not only acts as an important structural component to maintain cell wall stiffness and cell membrane stability but also plays a key role as a Ca2+ signal in many physiological processes, such as development and stress response (Hepler, 2005).
High levels of Ca2+ are harmful to plant cells (Li et al., 2014). When cytoplasmic Ca2+ levels become excessive, proteins such as CAX and ACAs localize to the PM or tonoplast and decrease the cytoplasmic Ca2+ concentration by exporting excess Ca2+ to the apoplast or vacuolar lumen. Furthermore, Ca2+ channels for influx and pumps or antiporters for efflux produce Ca2+ oscillators (Harper, 2001). CNGC2-mediated Ca influx and tonoplast-localized CAX1/3 jointly regulate the distribution of Ca2+ in plant cells, preventing excessive accumulation of Ca2+ in the cytoplasm and apoplastic space (Wang et al., 2017). CNGC is regulated by Ca2+, CaM, and regulatory motifs that bind to CaM in the CAX promoter (Martins et al., 2017). This suggests that Ca2+ signaling is also involved in sensing and regulation of Ca2+ as a nutrient. However, research in this field may be hindered by the vast majority of Ca2+ sensors that are typically present in cells but have little relevance to sensing of Ca2+ as a nutrient.
Mg transport requires the involvement of Ca2+ signaling
Mg is an essential plant nutrient and a cofactor for many enzymes. It is also involved in photosynthesis and synthesis of nucleic acids and proteins. Deficiency and excess of Mg2+ in the soil can adversely affect plant growth and crop yields (Verbruggen and Hermans, 2013).
Ca2+ signaling plays a critical role in regulating the dynamic homeostasis of Mg2+. The CBL2/3–CIPK3/9/23/26 complex recruits Mg2+ to the tonoplast and further regulates downstream target transporters that mediate efficient sequestration of Mg2+ in vacuoles, thereby maintaining a non-toxic level of Mg2+ in the cytoplasm (Tang et al., 2015; Figure 1). However, the specific Mg2+ transporters regulated by the CBL–CIPK module remain unclear. Future efforts will focus on outstanding questions regarding the generation of specific Ca2+ signals in plant cells during high/low Mg2+ stress, clarifying the regulation mechanism of Ca2+ signaling on the PM and other membrane systems and identifying unknown downstream transporters or channels.
Ca2+ signaling in the uptake of micronutrients
Ca2+ signals under Fe deficiency
Fe is an essential micronutrient for all organisms and an important regulator of various cellular processes involved in intracellular respiration, photosynthesis, and many other functions, such as DNA synthesis and N fixation (Vert et al., 2002).
Fe deficiency has been reported to elicit an increase in [Ca2+]cyt in the elongation and root-hair zone, which is the main region for Fe mobilization and absorption (Tian et al., 2016). When plants are challenged with different Fe and Pi availabilities, Ca2+ signals also show different characteristics (Matthus et al., 2019a, 2019b). The characteristic Ca2+ signals detected upon external application of purine nucleotides under sufficient Fe and Pi conditions are significantly altered when plants experience Pi starvation and are restored after Fe exclusion (Matthus et al., 2019b). Under sufficient Pi conditions, Fe deficiency leads to a third, different Ca characteristic (Matthus et al., 2019a).
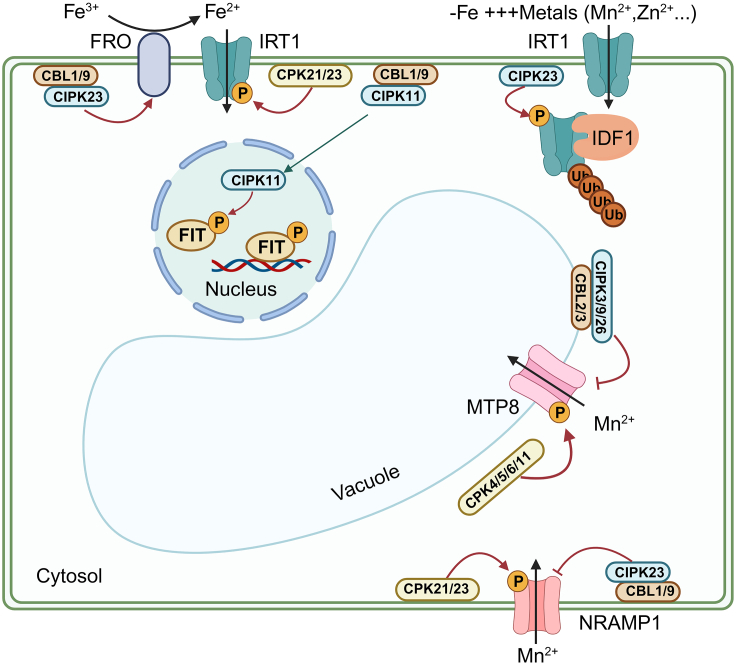
Fe(III) in the soil is reduced to Fe(II), which can be absorbed and utilized by plants, via Fe(III) chelate reductase (FRO) (Khan et al., 2019). Under Fe-deficient conditions, Fe-regulated transporter 1 (IRT1) is responsible for absorption of Fe(II), while ENHANCED BENDING 1, as an Ca2+-dependent inhibitor, prevents Fe absorption by binding to IRT1 (Khan et al., 2019). A recent study found that constitutively active CPK21 and CPK23 enhance plant tolerance to Fe deficiency through their interaction with and phosphorylation of IRT1 at the Ser149 residue, providing evidence that Ca2+ signaling directly mediates Fe absorption by regulating IRT1 (Wang et al., 2023). In addition, involvement of CBL1/9–CIPK23 in the process of Fe deficiency has been identified, and the cipk23 mutant exhibits sensitivity to Fe deficiency because of reduced ferric chelate reductase activity (Tian et al., 2016). When Fe deficiency was accompanied by increased availability of non-Fe metals in the soil, CIPK23 phosphorylated IRT1 at the S/T residues to facilitate recruitment of the E3 ubiquitin (Ub) ligase IRT1 degradation factor 1 (IDF1) for efficient endosomal sorting and subsequent degradation, thereby preventing IRT1 from transporting non-Fe metals such as Zn, Cu, and Mn (Dubeaux et al., 2018). CPK21/23 promote Fe absorption by phosphorylating IRT1 under low-Fe conditions, whereas phosphorylation of IRT1 by CIPK23 promotes its efficient sorting and subsequent degradation under low-Fe and high non-Fe metal stress, preventing plants from absorbing excess non-Fe metals while compromising Fe absorption. These two processes achieve precise regulation of plant metal stability by regulating IRT1. In addition, under Fe deficiency, activation of CIPK11, mediated by Ca2+-triggered CBL1/9, and subsequent phosphorylation of the basic-helix-loop-helix transcription factor fer-like Fe deficiency-induced transcription factor (FIT) convert inactive FIT into active FIT, further contributing to plant adaptation to Fe deficiency (Gratz et al., 2019; Figure 2).
Figure 2.
Regulation of Ca2+ signaling in plant uptake and transport of micronutrients.
An increase in [Ca2+]cyt induces the CBL1/9–CIPK23 module to enhance the activity of FRO, which is essential for conversion of soil Fe3+ into the transportable form Fe2+. Constitutively active CPK21 and CPK23 enhance plant tolerance to Fe deficiency by interacting with and phosphorylating IRT1. Under Fe deficiency, Ca2+–CBL1/9–CIPK11 phosphorylates the basic-helix-loop-helix transcription factor FIT, converting it from an inactive form to an active form. This promotes expression of downstream Fe-responsive genes and increases Fe absorption. The Fe deficiency–induced Ca2+ signature activates CIPK23 to phosphorylate IRT1 at the S/T residues to facilitate recruitment of IRT1 degradation factor 1 (IDF1) E3 Ub ligase in the presence of excess non-Fe metals, preventing IRT1 from transporting non-Fe metals, such as Zn and Mn. Phosphorylated IRT1 then transports other bivalent metals, such as Mn2+ and Zn2+. Four activated Ca sensors (CPK4, CPK5, CPK6, and CPK11) interact with MTP8 and phosphorylate its Ser31/32 residues, facilitating transport of excess Mn2+ into vacuoles. CBL2/3 recruit CIPK3/9/26 to form a complex that phosphorylates MTP8. This ultimately inhibits its activity and functions as a braking mechanism. Under Mn-deficient conditions, CPK21 and CPK23 interact with and phosphorylate NRAMP1 to enhance its transport activity. Conversely, under high Mn stress, the CBL1/9–CIPK23 complex senses Ca2+ signals and phosphorylates NRAMP1 at the Ser20/22 residues, triggering clathrin-mediated endocytosis of NRAMP1 and reducing Mn absorption. FRO, ferric chelate reductase; FIT, fer-like Fe deficiency-induced transcription factor; IRT1, Fe-regulated transporter 1; MTP8, metal tolerance protein 8; NRAMP1, natural resistance-associated macrophage protein 1.
Ca2+ signaling maintains Mn transport and homeostasis
Mn is an important cofactor of more than 30 enzymes, an essential element in the metalloenzyme cluster of the photosystem II oxygen-evolving complex, and a requirement for multiple steps in the biosynthesis of carbohydrates, lipids, and lignin in plants (Schmidt et al., 2016; Alejandro et al., 2020; Xie et al., 2023). It is therefore important to maintain plant Mn homeostasis through Mn uptake and transport.
A series of recent studies has elucidated the regulatory mechanism of Ca2+ signaling in Mn uptake and transport in plants. Mn deficiency induces a pattern of long-lasting multicellular Ca2+ oscillations, with maximum concentrations spatially confined to specific cell groups in the root elongation zone. CPK21 and CPK23 interact with and phosphorylate the PM-localized, high-affinity Mn transporter NATURAL RESISTANCE-ASSOCIATED MACROPHAGE PROTEIN 1 (NRAMP1) at the Thr498 residue, enhancing the transport activity of NRAMP1 and facilitating Mn2+ absorption under conditions of Mn depletion (Fu et al., 2022; Huang, 2022; Figure 2). High-Mn stress also leads to an increase in cytoplasmic Ca2+ concentration and the generation of distinct Ca2+ signals, which differ in time, frequency, and amplitude from those observed under low-Mn stress (Zhang et al., 2021; Fu et al., 2022). The CBL1/9–CIPK23 complex senses Ca2+ signals and phosphorylates NRAMP1 at the Ser20/22 residues, promoting clathrin-mediated endocytosis of NRAMP1 and reducing Mn absorption by plants under high-Mn stress (Zhang et al., 2023). Metal tolerance protein 8 (MTP8), a member of the cation diffusion facilitator (CDF) family, functions as a vacuolar Mn/Fe transporter and plays an important role in Mn detoxification in plants (Eroglu et al., 2016, 2017). In the cytoplasm, four activated Ca sensors (CPK4, CPK5, CPK6, and CPK11) interact with MTP8 and phosphorylate its Ser31/32 residues to facilitate transport of excess Mn2+ to the vacuoles for segregation, ultimately improving tolerance to Mn toxicity (Zhang et al., 2021). Intriguingly, after prolonged exposure to Mn toxicity, other Ca sensors, CBL2/3, recruit CIPK3/9/26 to form a complex that phosphorylates MTP8 primarily at Ser35, ultimately inhibiting its activity and acting as a braking mechanism (Ju et al., 2022) (Figure 2). These processes synergistically regulate Mn homeostasis in plants under fluctuating environmental Mn conditions.
Although the molecular mechanisms underlying the Ca2+ signals that regulate plant Mn homeostasis have been partially elucidated, the mechanisms by which Ca2+ signals are generated and regulate Mn homeostasis in other subcellular structures, such as the Golgi apparatus and ER, remain unclear. Therefore, future work will focus on elucidating mechanisms of Ca2+ signal generation and regulation in these subcellular structures under Mn stress. In addition, plants often encounter multiple, simultaneous element stresses in the soil under natural conditions. For example, when plants are subjected to both low Fe and low Mn stress, the resulting Ca2+ signals differ in duration, amplitude, and frequency. CPK21/23 detect these different Ca2+ signals and then phosphorylate and modify different substrates, such as IRT1 or NRAMP1, to transmit the Ca2+ signals. However, how plants recognize and accurately transmit signals to produce specific responses in the face of complex environmental changes remains a focal point and a challenge for future research.
Role of Ca2+ signaling in Cu metabolism
Cu is an essential micronutrient for plant development and a cofactor for various enzymes (Burkhead et al., 2009). In Ulva compressa (a marine alga), excessive Cu induces Ca2+ release from the ER, and the ryanodine-sensitive and IP3-sensitive Ca channels in the ER are activated in response to excess Cu (González et al., 2010). Cu-induced activation of L-type voltage-dependent Ca channels and transient receptor potential (TRP) channels leads to intracellular Ca2+ release, which requires extracellular Ca entry (González et al., 2012b; Gómez et al., 2015). Increases in Ca2+ induced the activation of defense genes via CaMs and CDPKs under conditions of Cu excess (González et al., 2012a). In addition, activation of CaMs and CDPKs leads to Cu entry and membrane depolarization (Gómez et al., 2015). To date, TRP has been observed in mammals, insects, nematodes, and macroalgae but not in plants (González et al., 2018). Further investigation is needed to determine whether similar mechanisms exist in plants and whether Ca2+ sensors, including CBL–CIPK and CPK, directly regulate Cu transporters.
B starvation causes Ca2+ influx
B is an essential element for plant growth, and B deficiency induces various physiological and metabolic alterations in plant cells (Brdar-Jokanović, 2020; Dong et al., 2022b). Cells subjected to short-term B deprivation show increased Ca2+ uptake, likely via Ca2+ channels (Koshiba et al., 2010). B starvation enhances cytosolic Ca2+ levels and expression of CNGC19, ACA and CAX efflux, and Ca2+ sensor genes in Arabidopsis roots (Quiles-Pando et al., 2013; González-Fontes et al., 2014). Upon B resupply, Ca2+ levels are restored, and CAX3 plays a major role in maintaining Ca2+ homeostasis (Quiles-Pando et al., 2019). It would be interesting to explore the direct involvement of Ca2+ sensors in regulation of B transporters.
Ca2+ signaling helps to regulate plant absorption of beneficial elements and detoxification of toxic elements
As attention to crop quality and human health has increased, the study of beneficial elements has become important. There is a class of elements that promote plant growth at suitable concentrations, but are not necessary for plant growth, such as Si, Na, Co, and Se (Pilon-Smits et al., 2009; Vatansever et al., 2017). Studying the growth regulation of beneficial elements in plants can not only improve plant yield and quality but also increase human intake of beneficial elements through food.
As a direct source of mineral nutrients for plants, the soil may also contain heavy metal elements that are toxic to plants. To attenuate the toxic effects of these metals, plants must develop interpretative mechanisms. Evidence suggests that the Ca–CaM pathway is involved in the response to Cd, Pb, Cr(VI), As, and Al toxicity (Tang et al., 2023).
Ca2+ signaling is involved in Na homeostasis
Na in the soil is an important nutrient for plant growth and development. However, at high concentrations, it disturbs and inhibits various physiological processes and plant growth (Zhu, 2016).
As an Na+ sensor, glycosyl inositol phosphorylceramide senses high salinity and triggers Ca2+ influx, producing a rapid and transient increase in cytosolic Ca2+ levels (Jiang et al., 2019). Salinity stress triggers several responses, including Ca2+ oscillations, which play a multifaceted role in eliminating detrimental effects (Schmöckel et al., 2015). In addition, FERONIA, a PM local receptor kinase, plays a key role in maintaining plant cell wall structure under salinity stress and is mainly associated with Ca2+ signaling cascades by regulating Ca2+ channel activity (Okubo-Kurihara et al., 2016; Feng et al., 2018). Furthermore, Na+ influx into cells can be sensed by non-selective cation channels (Wu, 2018), and elevation of cytosolic Ca2+ content is also regulated by two-pore channels, Ca2+-ATPases, and CAXs (Wilkins et al., 2016). These regulatory modules enable plants to rapidly sense and produce specific Ca2+ signals under salt stress.
Under normal physiological conditions, plants generally maintain low Na+ concentrations ranging from 1–10 mM (Binzel et al., 1988). Studies have shown that cngc3 null mutations result in decreased salt tolerance, whereas knockout of CNGC10 leads to increased tolerance of salt stress. This suggests that CNGC3 and CNGC10, which are located on the PM, function as channels for Na+ influx in Arabidopsis (Gobert et al., 2006; Guo et al., 2008; Jin et al., 2015).
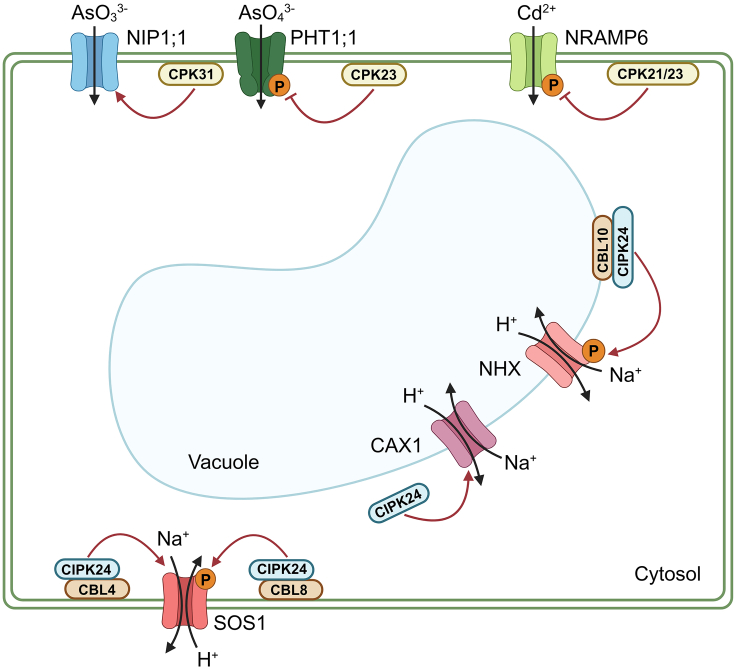
Na+ is an abundant element in soils and soil solutions, and salinity limits plant growth and impairs agricultural productivity. Na+/H+ antiporters (NHXs) such as PM salt overly sensitive 1 (SOS1) and vacuolar NHX1 have been shown to enhance salt tolerance in plants (Khan et al., 2015). The SOS pathway is the classic mechanism by which plants export excess Na+. NHX SOS1 is localized in the PM and closely associated with the CBL–CIPK network (Luan et al., 2009). In the classic SOS pathway, Ca2+ signals are sensed by CBL4 (SOS3), which binds to and activates the kinase activity of CIPK24 (SOS2), and SOS1 is phosphorylated by CIPK24, which enhances Na+ efflux (Liu and Zhu, 1998; Liu et al., 2000; Shi et al., 2000; Qiu et al., 2002; Figure 3). In addition to the classic pathway, CBL8 in Arabidopsis can activate CIPK24 under high salinity stress, further enhancing the function of the SOS pathway in Na+ efflux (Steinhorst et al., 2022). Recently, a study found that phosphatidic acid binds to the Lys57 residue in CIPK24, which activates SOS1 to promote Na+ efflux under salt stress. Interestingly, phosphatidic acid promotes phosphorylation of SOS3-like Ca-binding protein 8 (SCaBP8/CBL10) by CIPK24 under salt stress, which attenuates the SCaBP8-mediated inhibition of AKT1 (Li et al., 2023a).
Figure 3.
Ca2+ signaling regulates absorption of beneficial elements and detoxification of toxic elements in plants.
In the classic SOS pathway, Ca2+ signals are sensed by CBL4/SOS3, which binds to and activates the kinase activity of CIPK24/SOS2. CIPK24, in turn, phosphorylates and enhances the Na+ efflux activity of SOS1. Under high-salinity stress, CBL8 in Arabidopsis can also activate CIPK24, further enhancing the function of the SOS pathway in Na+ efflux. In addition, tonoplast-localized CBL10 is required for salt tolerance and activates an NHX together with CIPK24, facilitating compartmentalization of Na+ in the vacuole. Apart from its involvement in the SOS pathway, CIPK24 also plays a role in regulating the vacuolar H+/Ca2+ antiporter CAX1. Regarding arsenic (As) stress responses, CPK31 has been shown to interact with the As(III) transporter NIP1;1, influencing As(III) uptake and tolerance. In addition, CPK23 phosphorylates PHT1;1 and regulates its subcellular localization under As(V) stress. For cadmium stress responses, CPK21/23 phosphorylate NRAMP6, inhibiting its Cd transport activity and ultimately enhancing Cd tolerance. SOS, salt overly sensitive; NHX, Na+/H+ antiporter; CAX, H+/Ca2+ antiporter; NIP1;1, nodulin 26-like intrinsic protein 1;1; PHT1;1, Pi transporter 1;1; NRAMP6, natural resistance-associated macrophage protein 6.
As an independent component of kinase activity, CIPK24 has also been found to regulate the vacuolar H+/Ca2+ antiporter CAX1 (Cheng et al., 2004). CBL10 is required for salt tolerance, presumably by activating a vacuolar NHX together with CIPK24, enabling compartmentalization of Na+ into the vacuole (Kim et al., 2007). However, SOS2 has been shown to phosphorylate CBL10 to stabilize the CBL10–SOS2 complex and enhance PM Na+/H+ exchange activity to promote Na+ efflux (Quan et al., 2007; Lin et al., 2009; Figure 3). In conclusion, the Ca2+–CBL–CIPK signaling pathway plays a significant role in regulation of salt stress, and it remains of great interest to explore the functions and molecular mechanisms of other Ca2+ sensors and Ca2+ channels under salt stress.
Ca2+ signaling is involved in plant heavy metal detoxification
Cd is a nonessential metal that can be transported into plants through Ca2+ channels, causing Cd toxicity (Perfus-Barbeoch et al., 2002; Haider et al., 2021). Fluorescence imaging with the Ca2+-specific fluorescent probe 4-AM showed that Ca2+ signals were stimulated by exogenous Cd in duckweed (Lemna turionifera) rhizoids (Yang et al., 2020), and transgenic duckweed expressing a Ca2+-sensing fluorescent sensor GCaMP3 showed a Ca2+ signal response during Cd stress (Ren et al., 2022). Likewise, transgenic Arabidopsis expressing GCaMP6, a novel ultrasensitive Ca2+ sensor, exhibited obvious Ca2+ signals in the root meristematic zone under high-Cd stress. Furthermore, CPK21/23 phosphorylate NRAMP6 primarily at Ser489 and Thr505 to inhibit its Cd transport activity, thereby improving plant Cd tolerance (Zhang et al., 2022; Figure 3).
As is a metalloid with heavy-metal properties that is ubiquitous in many environments (Chen et al., 2019). Dietary intake of arsenate-contaminated plant-derived food represents a major fraction of potentially health-threatening human exposure to As. Recently, As(V) stress was shown to induce a significant Ca2+ signal in Arabidopsis roots that appeared in the root maturation zone and gradually increased in the middle column zone. CPK23 phosphorylates Pi transporter 1;1 (PHT1;1) at Ser514 and regulates PHT1;1 subcellular localization under As(V) stress (Liu et al., 2023), and CPK activity is markedly enhanced under As(V) stress in rice (Huang et al., 2012). In this regard, CPK31 has been found to interact with nodulin 26-like intrinsic protein 1;1 (NIP1;1) and determine As(III) uptake and tolerance in Arabidopsis (Ji et al., 2017; Figure 3).
Significant progress has been made in understanding Ca2+ signaling in heavy metal regulation, and it is clear that Ca2+ signaling is involved in heavy metal detoxification in plants. Nonetheless, there are still gaps in our understanding of the involvement of Ca2+ signaling in heavy metal toxicity.
Regulation of Ca2+ signaling in response to other essential mineral nutrients, beneficial elements, and toxic elements
S, Zn, Cl, Mo, and Ni are essential mineral nutrients for plants (Maathuis and Diatloff, 2013), and many studies have confirmed that Si, Co, and Se are beneficial for plant growth and development (Gui et al., 2022; Hu et al., 2021; Wiese et al., 2007). Under natural conditions, heavy metals such as Al, Pb, and Cr in soil can significantly hinder plant growth and disrupt normal development of roots, stems, and other tissues (Yadav et al., 2021). However, little has been reported about the involvement of Ca2+ signaling in regulating the absorption and transport of these elements.
Exogenous supplementation with Ca2+ and NO efficiently mitigates Ni toxicity and regulates growth and development of the cyanobacterium Nostoc muscorum ATCC 27893, implying a signaling role for Ca2+ and NO in response to Ni stress (Verma et al., 2021). In addition, some effects of Ca2+ on Ni tolerance have been reported to be related to triggering of Ca2+ signaling in Cucurbita pepo L. (Valivand and Amooaghaie, 2021). Under Zn deficiency, transcript levels of some genes in the CAM, CML, CPK, and CBL–CIPK families of Ca2+ sensors change when Zn is resupplied, suggesting that these Ca2+ sensors may respond to Zn deficiency (Arsova et al., 2019). Previous studies have shown that CML24 regulates ALMT1-dependent resistance to Al (Zhu et al., 2022). A CaM-binding protein (NtCBP4) of tobacco decreases Ni accumulation and increases Pb accumulation (Arazi et al., 1999).
The mechanism by which Ca2+ signaling participates in regulation of these essential mineral nutrients and beneficial elements is not well understood, and this part of the regulatory network requires further analysis. The functions of Ca2+ channels in generation of specific Ca2+ signals during S, Zn, Cl, Mo, and Ni stress, as well as the functions of CBL–CIPK modules and CPKs in absorption and transport of these elements, will be a major focus of research on Ca2+ signal influence on essential mineral nutrient regulation. A thorough understanding of how Ca2+ channels generate specific Ca2+ signals during absorption of beneficial elements and detoxification of different metals or metalloids, and how these signals are transmitted through Ca2+ sensors, will greatly enhance our understanding of the mechanisms that underlie the involvement of Ca2+ signals in absorption of beneficial elements and heavy metal toxicity.
Concluding remarks and future prospects
Ca2+ signal generation in response to different elements
A considerable number of studies have indicated that Ca2+ signaling plays a crucial role in regulating plant nutrient uptake, nutrient transport, and various nutrient stresses. It has been observed that different nutrient element stresses can induce changes in cytoplasmic Ca2+ concentration, thus generating specific Ca2+ signals. These nutrient elements encompass macronutrients such as N, P, and K; micronutrients including Fe and Mn; and beneficial and toxic elements such as Na, Cd, and As (Tian et al., 2016; Riveras et al., 2015; Jiang et al., 2019; Zhang et al., 2021, 2022; Fu et al., 2022; Ying and Scheible, 2022; Liu et al., 2023). Although Ca2+ signals primarily manifest in the roots under nutrient stress, their occurrence and characteristics in shoot tissues have received limited attention to date. Furthermore, it will be important to elucidate the encoding and decoding mechanisms for specific Ca2+ signals under different nutrient element stresses, as they are inherently stimulus-specific in terms of their magnitude, location, and duration. These stimulus-induced changes in cytoplasmic Ca2+ concentration produce unique spatial and temporal patterns known as Ca2+ signatures (Tian et al., 2020).
Taking Mn as an example, Mn depletion triggers spatiotemporally distinct, long-lasting multicellular Ca2+ oscillations in Arabidopsis roots. These Ca2+ signals initially emerge in individual cells before spreading intercellularly, gradually intensifying, and finally transforming into higher-order multicellular oscillations. In response to high Mn exposure, a transient Ca2+ signal begins to rise approximately 17 min after the initiation of stress, steadily reaching its peak at around 18 min, and declining thereafter (Fu et al., 2022). Further exploration of similar cases under different nutrient stresses will provide valuable insights into the specific mechanisms that underlie Ca2+ signal generation, propagation, and decoding. Additional insight into these processes will make a significant contribution to our understanding of plant responses to nutrient stresses and heavy metal detoxification.
Intracellular Ca2+ imaging in plant abiotic stress research
Ca2+ indicators are essential for studying the concentrations of Ca2+ in various cells or tissues and are considered indispensable tools in this field. They can be broadly categorized into two types based on their fluorescence spectra, Ca2+ affinity, and chemical characteristics: chemical indicators and genetically encoded Ca indicators (GECIs).
Chemical indicators, such as fura-2, indo-1, fluo-3, and fluo-4, have long been used as traditional tools for detecting Ca2+ levels in the cytoplasm. These indicators rely on the binding of Ca2+ to specific fluorescent molecules, enabling measurement of Ca2+ concentrations in real time (Paredes et al., 2008). On the other hand, GECIs represent a novel class of Ca2+ indicators that have emerged with advances in genetic engineering technology. They enable long-term and real-time monitoring of Ca2+ levels in vivo. GECIs can also provide insights into changes in Ca2+ in specific subcellular structures through the use of organelle-specific localization signals. These remarkable features of GECIs have contributed to their widespread use for in vivo Ca imaging experiments. Precise measurement and visualization of Ca2+ dynamics using these indicators have become essential tools for understanding the complex and diverse functions of Ca2+ signaling in living systems.
According to the principle of luminescence, GECIs can be divided into two categories: GECIs based on a single fluorescent protein and GECIs composed of fluorescent protein pairs that undergo fluorescence resonance energy transfer (Miyawaki et al., 1997; Nakai et al., 2001).
In plant research, Yellow Cameleon 3.6 is a commonly used fluorescence resonance energy transfer–based fluorescent Ca2+ indicator. It has been used extensively to monitor plant Ca2+ kinetics and investigate the relationships between Ca2+ signaling and various physiological processes such as root hair growth, pollen tube tip growth, and stomatal response (Monshausen et al., 2008; Swanson and Gilroy, 2013; Thor and Peiter, 2014; Zhang et al., 2020). Another popular Ca2+ indicator in plants is GCaMP, which is based on a single fluorescent protein. GCaMP6, in particular, has shown high sensitivity and is suitable for detecting low-frequency signals (Nakai et al., 2001; Chen et al., 2013). Advances have also been made in producing multifunctional Ca indicators based on GCaMP. For instance, the ratiometric Ca2+ indicator R-GECO1-mTurquoise and MatryoshCaMP6s have proven to be effective tools for mapping absolute Ca2+ concentration changes under different elemental stresses (Ast et al., 2017; Waadt et al., 2017). The Ca2+ sensor GCaMP6f-mCherry combines the superior dynamic range and temporal accuracy of GCaMP6f with ratiometric data acquisition via mCherry emission monitoring standardization. This method has been used to detect Ca2+ signals under Mn deficiency, demonstrating its potential for investigating trace element–induced Ca2+ signaling (Fu et al., 2022). These advances and applications help to facilitate the detection of Ca2+ signals under nutrient stress, enabling further research in this area.
Role of the Ca2+ signal regulatory network in regulation of mineral elements
In previous reports, the CBL1/9–CIPK23 module has been shown to participate in regulating the absorption and transport of nutrients such as N, K, and Fe. Similarly, the CBL2/3–CIPK3/9/23/26 module is involved in regulation of Mn and Mg, and CPK21/23 have been found to play a role in absorption and transport of Mn, Fe, As, and Cd (Tang et al., 2020b; Ju et al., 2022; Fu et al., 2022; Wang et al., 2023; Zhang et al., 2022; Liu et al., 2023). An interesting question is how the same CBL–CIPK or CPK modules perceive and differentiate specific environmental stimuli, enabling them to accurately regulate different downstream effectors. Previous studies have shown that Ca2+ signals generated by plants under various stresses are distinct, exhibiting differences in timing, spatial distribution, and amplitude (Luan and Wang, 2021; Dong et al., 2022a). As a result, the same CBL–CIPK or CPK modules are thought to recognize specific Ca2+ signals and elicit unique responses to different stresses.
Ca2+ sensors are found in various locations in the plant cell, including the PM and VM (Sanyal et al., 2015). However, the synergistic regulation of Ca2+ signal transduction networks mediated by different plant Ca2+ sensors, particularly those in the PM, VM, or other membrane systems, remains to be further investigated. This is an area in which future research is needed to elucidate the intricate mechanisms that underlie coordination of Ca2+ signaling pathways in response to different stimuli.
Under natural conditions, the environment is characterized by variability and complexity, necessitating further investigation of the crosstalk among Ca2+ signal transduction networks under different nutrient stresses. One area of interest is the Ca2+-binding affinity of different Ca2+ sensors. It is important to understand whether different Ca2+ sensors can perceive distinct ranges of Ca2+ concentrations to initiate specific signaling pathways. Overall, there are still significant gaps in our understanding of the temporal, spatial, and intensity changes in Ca2+ signal production under different stress conditions, as well as the differential responses of different Ca2+ channels to environmental signals. Moreover, the molecular mechanisms by which CaM and CML participate in different nutrient stress responses remain to be elucidated. In addition to further exploring the biological processes by which Ca2+ signaling regulates plant nutrient-stress responses, future research will also focus on using biotechnological applications to cultivate stress-tolerant crops.
Funding
This work was supported by the National Natural Science Foundation of China (32222008 to C.W.) and the China Postdoctoral Science Foundation (2023M732883 to C.J.).
Author contributions
All work was performed in collaboration. T.W. and C.W. wrote the original draft and prepared the figures. T.W., X.C., C.J., and C.W. designed and finalized the manuscript. The authors have read and agreed to the published version of the article.
Acknowledgments
No conflict of interest is declared.
Published: August 26, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Contributor Information
Chuanfeng Ju, Email: chuanfengju@nwafu.edu.cn.
Cun Wang, Email: cunwang@nwafu.edu.cn.
References
- Alejandro S., Höller S., Meier B., Peiter E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020;11:300. doi: 10.3389/fpls.2020.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J.M., Riveras E., Vidal E.A., Gras D.E., Contreras-López O., Tamayo K.P., Aceituno F., Gómez I., Ruffel S., Lejay L., et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. 2014;80:1–13. doi: 10.1111/tpj.12618. [DOI] [PubMed] [Google Scholar]
- Alvarez J.M., Schinke A.L., Brooks M.D., Pasquino A., Leonelli L., Varala K., Safi A., Krouk G., Krapp A., Coruzzi G.M. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 2020;11:1157. doi: 10.1038/s41467-020-14979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arazi T., Sunkar R., Kaplan B., Fromm H. A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 1999;20:171–182. doi: 10.1046/j.1365-313x.1999.00588.x. [DOI] [PubMed] [Google Scholar]
- Arsova B., Amini S., Scheepers M., Baiwir D., Hanikenne M. Resolution of the proteome, transcript and ionome dynamics upon Zn re-supply in Zn-deficient Arabidopsis. bioRxiv. 2019 doi: 10.1101/600569. Preprint at. [DOI] [Google Scholar]
- Ast C., Foret J., Oltrogge L.M., De Michele R., Kleist T.J., Ho C.H., Frommer W.B. Ratiometric Matryoshka biosensors from a nested cassette of green- and orange-emitting fluorescent proteins. Nat. Commun. 2017;8:431. doi: 10.1038/s41467-017-00400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M.L., Hess F.D., Bressan R.A., Hasegawa P.M. Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol. 1988;86:607–614. doi: 10.1104/pp.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer E.K., Ahsan N., Dale R., Kato N., Coluccio A.E., Piñeros M.A., Kochian L.V., Thelen J.J., Popescu S.C. The Raf-like kinase ILK1 and the high affinity K+ transporter HAK5 are required for innate immunity and abiotic stress response. Plant Physiol. 2016;171:1470–1484. doi: 10.1104/pp.16.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdar-Jokanović M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhead J.L., Gogolin Reynolds K.A., Abdel-Ghany S.E., Cohu C.M., Pilon M. Copper homeostasis. New Phytol. 2009;182:799–816. doi: 10.1111/j.1469-8137.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- Chen H.Y., Chen Y.N., Wang H.Y., Liu Z.T., Frommer W.B., Ho C.H. Feedback inhibition of AMT1 NH4+-transporters mediated by CIPK15 kinase. BMC Biol. 2020;18:196. doi: 10.1186/s12915-020-00934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.Y., DesMarais T., Costa M. Metals and Mechanisms of Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2019;59:537–554. doi: 10.1146/annurev-pharmtox-010818-021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N.-H., Pittman J.K., Zhu J.-K., Hirschi K.D. The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 2004;279:2922–2926. doi: 10.1074/jbc.M309084200. [DOI] [PubMed] [Google Scholar]
- Cheong Y.H., Pandey G.K., Grant J.J., Batistic O., Li L., Kim B.G., Lee S.C., Kudla J., Luan S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007;52:223–239. doi: 10.1111/j.1365-313X.2007.03236.x. [DOI] [PubMed] [Google Scholar]
- Cubero-Font P., Maierhofer T., Jaslan J., Rosales M.A., Espartero J., Díaz-Rueda P., Müller H.M., Hürter A.L., Al-Rasheid K.A.S., Marten I., et al. Silent S-Type Anion Channel Subunit SLAH1 Gates SLAH3 Open for Chloride Root-to-Shoot Translocation. Curr. Biol. 2016;26:2213–2220. doi: 10.1016/j.cub.2016.06.045. [DOI] [PubMed] [Google Scholar]
- Demidchik V., Maathuis F.J.M. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol. 2007;175:387–404. doi: 10.1111/j.1469-8137.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- Demidchik V., Davenport R.J., Tester M. Nonselective cation channels in plants. Annu. Rev. Plant Biol. 2002;53:67–107. doi: 10.1146/annurev.arplant.53.091901.161540. [DOI] [PubMed] [Google Scholar]
- Dong Q., Wallrad L., Almutairi B.O., Kudla J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022;64:287–300. doi: 10.1111/jipb.13228. [DOI] [PubMed] [Google Scholar]
- Dong X., Jiang C., Wei S., Jiao H., Ran K., Dong R., Wang S. The regulation of plant lignin biosynthesis under boron deficiency conditions. Physiol. Plantarum. 2022;174 doi: 10.1111/ppl.13815. [DOI] [PubMed] [Google Scholar]
- Dubeaux G., Neveu J., Zelazny E., Vert G. Metal Sensing by the IRT1 Transporter-Receptor Orchestrates Its Own Degradation and Plant Metal Nutrition. Mol. Cell. 2018;69:953–964.e5. doi: 10.1016/j.molcel.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Eroglu S., Meier B., von Wirén N., Peiter E. The Vacuolar Manganese Transporter MTP8 Determines Tolerance to Iron Deficiency-Induced Chlorosis in Arabidopsis. Plant Physiol. 2016;170:1030–1045. doi: 10.1104/pp.15.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu S., Giehl R.F.H., Meier B., Takahashi M., Terada Y., Ignatyev K., Andresen E., Küpper H., Peiter E., von Wirén N. Metal Tolerance Protein 8 Mediates Manganese Homeostasis and Iron Reallocation during Seed Development and Germination. Plant Physiol. 2017;174:1633–1647. doi: 10.1104/pp.16.01646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Kita D., Peaucelle A., Cartwright H.N., Doan V., Duan Q., Liu M.-C., Maman J., Steinhorst L., Schmitz-Thom I., et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018;28:666–675.e5. doi: 10.1016/j.cub.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D., Zhang Z., Wallrad L., Wang Z., Höller S., Ju C., Schmitz-Thom I., Huang P., Wang L., Peiter E., et al. Ca2+-dependent phosphorylation of NRAMP1 by CPK21 and CPK23 facilitates manganese uptake and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2204574119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Wang C., Xi Y., Shao Q., Hou C., Li L., Luan S. RALF signaling pathway activates MLO calcium channels to maintain pollen tube integrity. Cell Res. 2023;33:71–79. doi: 10.1038/s41422-022-00754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Bossi J., Kumar K., Barberini M.L., Domínguez G.D., Rondón Guerrero Y.D.C., Marino-Buslje C., Obertello M., Muschietti J.P., Estevez J.M. The role of P-type IIA and P-type IIB Ca2+-ATPases in plant development and growth. J. Exp. Bot. 2019;71:1239–1248. doi: 10.1093/jxb/erz521. [DOI] [PubMed] [Google Scholar]
- Ghori N.H., Ghori T., Hayat M.Q., Imadi S.R., Gul A., Altay V., Ozturk M. Heavy metal stress and responses in plants. Int J Environ Sci Te. 2019;16:1807–1828. [Google Scholar]
- Gobert A., Park G., Amtmann A., Sanders D., Maathuis F.J. Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J. Exp. Bot. 2006;57:791–800. doi: 10.1093/jxb/erj064. [DOI] [PubMed] [Google Scholar]
- Gómez M., González A., Sáez C.A., Morales B., Moenne A. Copper-induced activation of TRP channels promotes extracellular calcium entry, activation of CaMs and CDPKs, copper entry and membrane depolarization in Ulva compressa. Front. Plant Sci. 2015;6:182. doi: 10.3389/fpls.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fontes A., Navarro-Gochicoa M.T., Camacho-Cristóbal J.J., Herrera-Rodríguez M.B., Quiles-Pando C., Rexach J. Is Ca2+ involved in the signal transduction pathway of boron deficiency? New hypotheses for sensing boron deprivation. Plant Sci. 2014;217–218:135–139. doi: 10.1016/j.plantsci.2013.12.011. [DOI] [PubMed] [Google Scholar]
- González A., Sáez C.A., Moenne A. Copper-induced activation of TRPs and VDCCs triggers a calcium signature response regulating gene expression in Ectocarpus siliculosus. PeerJ. 2018;6 doi: 10.7717/peerj.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Trebotich J., Vergara E., Medina C., Morales B., Moenne A. Copper-induced calcium release from ER involves the activation of ryanodine-sensitive and IP3-sensitive channels in Ulva compressa. Plant Signal. Behav. 2010;5:1647–1649. doi: 10.4161/psb.5.12.13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Cabrera M.d.L.Á., Henríquez M.J., Contreras R.A., Morales B., Moenne A. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol. 2012;158:1451–1462. doi: 10.1104/pp.111.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Cabrera M.d.l.A., Mellado M., Cabello S., Márquez S., Morales B., Moenne A. Copper-induced intracellular calcium release requires extracellular calcium entry and activation of L-type voltage-dependent calcium channels in Ulva compressa. Plant Signal. Behav. 2012;7:728–732. doi: 10.4161/psb.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz R., Manishankar P., Ivanov R., Köster P., Mohr I., Trofimov K., Steinhorst L., Meiser J., Mai H.J., Drerup M., et al. CIPK11-Dependent Phosphorylation Modulates FIT Activity to Promote Arabidopsis Iron Acquisition in Response to Calcium Signaling. Dev. Cell. 2019;48:726–740.e10. doi: 10.1016/j.devcel.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Gui J.-Y., Rao S., Huang X., Liu X., Cheng S., Xu F. Interaction between selenium and essential micronutrient elements in plants: a systematic review. Sci. Total Environ. 2022;853:158673. doi: 10.1016/j.scitotenv.2022.158673. [DOI] [PubMed] [Google Scholar]
- Guichard M., Thomine S., Frachisse J.-M. Mechanotransduction in the spotlight of mechano-sensitive channels. Curr. Opin. Plant Biol. 2022;68 doi: 10.1016/j.pbi.2022.102252. [DOI] [PubMed] [Google Scholar]
- Guo K.M., Babourina O., Christopher D.A., Borsics T., Rengel Z. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol. Plant. 2008;134:499–507. doi: 10.1111/j.1399-3054.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- Haider F.U., Liqun C., Coulter J.A., Cheema S.A., Wu J., Zhang R., Wenjun M., Farooq M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021;211 doi: 10.1016/j.ecoenv.2020.111887. [DOI] [PubMed] [Google Scholar]
- Harper J.F. Dissecting calcium oscillators in plant cells. Trends Plant Sci. 2001;6:395–397. doi: 10.1016/s1360-1385(01)02023-4. [DOI] [PubMed] [Google Scholar]
- Held K., Pascaud F., Eckert C., Gajdanowicz P., Hashimoto K., Corratgé-Faillie C., Offenborn J.N., Lacombe B., Dreyer I., Thibaud J.-B., et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011;21:1116–1130. doi: 10.1038/cr.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P.K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Huang C.F. Ca2+ signaling in plant manganese uptake: CPK21/23 kinases phosphorylate and activate manganese transporter NRAMP1. Stress Biology. 2022;2:43. doi: 10.1007/s44154-022-00067-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.C., Wang Y.Y., Tsay Y.F. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009;57:264–278. doi: 10.1111/j.1365-313X.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- Hu X., Wei X., Ling J., Chen J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.768523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.L., Nguyen Q.T.T., Fu S.F., Lin C.Y., Chen Y.C., Huang H.J. Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol. Biol. 2012;80:587–608. doi: 10.1007/s11103-012-9969-z. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Zhou X., Tao M., Yuan F., Liu L., Wu F., Wu X., Xiang Y., Niu Y., Liu F., et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature. 2019;572:341–346. doi: 10.1038/s41586-019-1449-z. [DOI] [PubMed] [Google Scholar]
- Ji R., Zhou L., Liu J., Wang Y., Yang L., Zheng Q., Zhang C., Zhang B., Ge H., Yang Y., et al. Calcium-dependent protein kinase CPK31 interacts with arsenic transporter AtNIP1;1 and regulates arsenite uptake in Arabidopsis thaliana. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Jing W., Zhang Q., Zhang W. Cyclic nucleotide gated channel 10 negatively regulates salt tolerance by mediating Na+ transport in Arabidopsis. J. Plant Res. 2015;128:211–220. doi: 10.1007/s10265-014-0679-2. [DOI] [PubMed] [Google Scholar]
- Ju C., Zhang Z., Deng J., Miao C., Wang Z., Wallrad L., Javed L., Fu D., Zhang T., Kudla J., et al. Ca2+-dependent successive phosphorylation of vacuolar transporter MTP8 by CBL2/3-CIPK3/9/26 and CPK5 is critical for manganese homeostasis in Arabidopsis. Mol. Plant. 2022;15:419–437. doi: 10.1016/j.molp.2021.11.012. [DOI] [PubMed] [Google Scholar]
- Khan I., Gratz R., Denezhkin P., Schott-Verdugo S.N., Angrand K., Genders L., Basgaran R.M., Fink-Straube C., Brumbarova T., Gohlke H., et al. Calcium-Promoted Interaction between the C2-Domain Protein EHB1 and Metal Transporter IRT1 Inhibits Arabidopsis Iron Acquisition. Plant Physiol. 2019;180:1564–1581. doi: 10.1104/pp.19.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.S., Ahmad D., Khan M.A. Trends in genetic engineering of plants with Na+/H+ antiporters for salt stress tolerance. Biotechnol. Biotechnol. Equip. 2015;29:815–825. [Google Scholar]
- Kim B.G., Waadt R., Cheong Y.H., Pandey G.K., Dominguez-Solis J.R., Schültke S., Lee S.C., Kudla J., Luan S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007;52:473–484. doi: 10.1111/j.1365-313X.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- Koshiba T., Kobayashi M., Ishihara A., Matoh T. Boron nutrition of cultured tobacco BY-2 cells. VI. Calcium is involved in early responses to boron deprivation. Plant Cell Physiol. 2010;51:323–327. doi: 10.1093/pcp/pcp179. [DOI] [PubMed] [Google Scholar]
- Kudla J., Becker D., Grill E., Hedrich R., Hippler M., Kummer U., Parniske M., Romeis T., Schumacher K. Advances and current challenges in calcium signaling. New Phytol. 2018;218:414–431. doi: 10.1111/nph.14966. [DOI] [PubMed] [Google Scholar]
- Kudla J., Becker D., Grill E., Hedrich R., Hippler M., Kummer U., Parniske M., Romeis T., Schumacher K. Advances and current challenges in calcium signaling. New Phytol. 2018;218:414–431. doi: 10.1111/nph.14966. [DOI] [PubMed] [Google Scholar]
- Lara A., Ródenas R., Andrés Z., Martínez V., Quintero F.J., Nieves-Cordones M., Botella M.A., Rubio F. Arabidopsis K+ transporter HAK5-mediated high-affinity root K+ uptake is regulated by protein kinases CIPK1 and CIPK9. J. Exp. Bot. 2020;71:5053–5060. doi: 10.1093/jxb/eraa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Seo P.J. Ca2+ talyzing Initial Responses to Environmental Stresses. Trends Plant Sci. 2021;26:849–870. doi: 10.1016/j.tplants.2021.02.007. [DOI] [PubMed] [Google Scholar]
- Li J., Shen L., Han X., He G., Fan W., Li Y., Yang S., Zhang Z., Yang Y., Jin W., et al. Phosphatidic acid-regulated SOS2 controls sodium and potassium homeostasis in Arabidopsis under salt stress. EMBO J. 2023;42 doi: 10.15252/embj.2022112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.-L., Tang R.-J., Wang C., Luan S. Potassium nutrient status drives posttranslational regulation of a low-K response network in Arabidopsis. Nat. Commun. 2023;14:360–413. doi: 10.1038/s41467-023-35906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Kim B.G., Cheong Y.H., Pandey G.K., Luan S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:12625–12630. doi: 10.1073/pnas.0605129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Duan H., Chen F., Wang Z., Huang X., Deng X., Liu Y. Identification of quantitative trait loci controlling high Calcium response in Arabidopsis thaliana. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Yang Y., Quan R., Mendoza I., Wu Y., Du W., Zhao S., Schumaker K.S., Pardo J.M., Guo Y. Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell. 2009;21:1607–1619. doi: 10.1105/tpc.109.066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.L., Tsay Y.F. Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis. J. Exp. Bot. 2017;68:2603–2609. doi: 10.1093/jxb/erx053. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhu J.-K. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- Liu J., Ishitani M., Halfter U., Kim C.-S., Zhu J.-K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA. 2000;97:3730–3734. doi: 10.1073/pnas.060034197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.H., Huang C.Y., Tsay Y.F. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell. 1999;11:865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.H., Liu M., Lin Z., Wang Z.F., Chen B., Liu C., Guo A., Konishi M., Yanagisawa S., Wagner G., Sheen J. NIN-like protein 7 transcription factor is a plant nitrate sensor. Science. 2022;377:1419–1425. doi: 10.1126/science.add1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.H., Niu Y., Konishi M., Wu Y., Du H., Sun Chung H., Li L., Boudsocq M., McCormack M., Maekawa S., et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature. 2017;545:311–316. doi: 10.1038/nature22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.Y., Aung K., Tseng C.Y., Chang T.Y., Chen Y.S., Chiou T.J. Vacuolar Ca2+/H+ transport activity is required for systemic phosphate homeostasis involving shoot-to-root signaling in Arabidopsis. Plant Physiol. 2011;156:1176–1189. doi: 10.1104/pp.111.175257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Wang Z., Guo S., Fang Y., Zhang Z., Gao H., Ren H., Wang C. Plasma membrane-associated calcium signaling regulates arsenate tolerance in Arabidopsis. Plant Physiol. 2023;192:910–926. doi: 10.1093/plphys/kiad171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arredondo D.L., Leyva-González M.A., González-Morales S.I., López-Bucio J., Herrera-Estrella L. Phosphate nutrition: improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014;65:95–123. doi: 10.1146/annurev-arplant-050213-035949. [DOI] [PubMed] [Google Scholar]
- Loqué D., von Wirén N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004;55:1293–1305. doi: 10.1093/jxb/erh147. [DOI] [PubMed] [Google Scholar]
- Luan S., Wang C. Calcium Signaling Mechanisms Across Kingdoms. Annu. Rev. Cell Dev. Biol. 2021;37:311–340. doi: 10.1146/annurev-cellbio-120219-035210. [DOI] [PubMed] [Google Scholar]
- Luan S., Lan W., Chul Lee S. Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL–CIPK network. Curr. Opin. Plant Biol. 2009;12:339–346. doi: 10.1016/j.pbi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Ma Q., Tang R.J., Zheng X.J., Wang S.M., Luan S. The calcium sensor CBL7 modulates plant responses to low nitrate in Arabidopsis. Biochem Bioph Res Co. 2015;468:59–65. doi: 10.1016/j.bbrc.2015.10.164. [DOI] [PubMed] [Google Scholar]
- Maathuis F.J.M., Diatloff E. Roles and functions of plant mineral nutrients. Methods Mol. Biol. 2013;953:1–21. doi: 10.1007/978-1-62703-152-3_1. [DOI] [PubMed] [Google Scholar]
- Maierhofer T., Lind C., Hüttl S., Scherzer S., Papenfuß M., Simon J., Al-Rasheid K.A.S., Ache P., Rennenberg H., Hedrich R., et al. A Single-Pore Residue Renders the Arabidopsis Root Anion Channel SLAH2 Highly Nitrate Selective. Plant Cell. 2014;26:2554–2567. doi: 10.1105/tpc.114.125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V., Carneiro F., Conde C., Sottomayor M., Gerós H. The grapevine VvCAX3 is a cation/H+ exchanger involved in vacuolar Ca2+ homeostasis. Planta. 2017;246:1083–1096. doi: 10.1007/s00425-017-2754-0. [DOI] [PubMed] [Google Scholar]
- Matthus E., Wilkins K.A., Davies J.M. Iron availability modulates the Arabidopsis thaliana root calcium signature evoked by exogenous ATP. Plant Signal. Behav. 2019;14 doi: 10.1080/15592324.2019.1640563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthus E., Wilkins K.A., Swarbreck S.M., Doddrell N.H., Doccula F.G., Costa A., Davies J.M. Phosphate Starvation Alters Abiotic-Stress-Induced Cytosolic Free Calcium Increases in Roots. Plant Physiol. 2019;179:1754–1767. doi: 10.1104/pp.18.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthus E., Doddrell N.H., Guillaume G., Mohammad-Sidik A.B., Wilkins K.A., Swarbreck S.M., Davies J.M. Phosphate Deprivation Can Impair Mechano-Stimulated Cytosolic Free Calcium Elevation in Arabidopsis Roots. Plants. 2020;9 doi: 10.3390/plants9091205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A., Llopis J., Heim R., McCaffery J.M., Adams J.A., Ikura M., Tsien R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Monshausen G.B., Messerli M.A., Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147:1690–1698. doi: 10.1104/pp.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J., Ohkura M., Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Nieves-Cordones M., Alemán F., Martínez V., Rubio F. K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms. J. Plant Physiol. 2014;171:688–695. doi: 10.1016/j.jplph.2013.09.021. [DOI] [PubMed] [Google Scholar]
- O'Brien J.A., Vega A., Bouguyon E., Krouk G., Gojon A., Coruzzi G., Gutiérrez R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant. 2016;9:837–856. doi: 10.1016/j.molp.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Okubo-Kurihara E., Ohtani M., Kurihara Y., Kakegawa K., Kobayashi M., Nagata N., Komatsu T., Kikuchi J., Cutler S., Demura T., et al. Modification of plant cell wall structure accompanied by enhancement of saccharification efficiency using a chemical, lasalocid sodium. Sci. Rep. 2016;6:34602–34610. doi: 10.1038/srep34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes R.M., Etzler J.C., Watts L.T., Zheng W., Lechleiter J.D. Chemical calcium indicators. Methods. 2008;46:143–151. doi: 10.1016/j.ymeth.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus-Barbeoch L., Leonhardt N., Vavasseur A., Forestier C. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32:539–548. doi: 10.1046/j.1365-313x.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits E.A.H., Quinn C.F., Tapken W., Malagoli M., Schiavon M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009;12:267–274. doi: 10.1016/j.pbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Qiu Q.-S., Guo Y., Dietrich M.A., Schumaker K.S., Zhu J.-K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan R., Lin H., Mendoza I., Zhang Y., Cao W., Yang Y., Shang M., Chen S., Pardo J.M., Guo Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell. 2007;19:1415–1431. doi: 10.1105/tpc.106.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles-Pando C., Rexach J., Navarro-Gochicoa M.T., Camacho-Cristóbal J.J., Herrera-Rodríguez M.B., González-Fontes A. Boron deficiency increases the levels of cytosolic Ca2+ and expression of Ca2+-related genes in Arabidopsis thaliana roots. Plant Physiol. Biochem. (Issy les Moulineaux, Fr.) 2013;65:55–60. doi: 10.1016/j.plaphy.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Quiles-Pando C., Navarro-Gochicoa M.T., Herrera-Rodríguez M.B., Camacho-Cristóbal J.J., González-Fontes A., Rexach J. Boron Deficiency Increases Cytosolic Ca2+ Levels Mainly via Ca2+ Influx from the Apoplast in Arabidopsis thaliana Roots. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20092297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragel P., Ródenas R., García-Martín E., Andrés Z., Villalta I., Nieves-Cordones M., Rivero R.M., Martínez V., Pardo J.M., Quintero F.J., et al. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol. 2015;169:2863–2873. doi: 10.1104/pp.15.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T., Nacry P., Pervent M., Filleur S., Diatloff E., Mounier E., Tillard P., Forde B.G., Gojon A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc. Natl. Acad. Sci. USA. 2006;103:19206–19211. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q., Xu Z., Xue Y., Yang R., Ma X., Sun J., Wang J., Lin S., Wang W., Yang L., Sun Z. Mechanism of calcium signal response to cadmium stress in duckweed. Plant Signal. Behav. 2022;17 doi: 10.1080/15592324.2022.2119340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X.L., Qi G.N., Feng H.Q., Zhao S., Zhao S.S., Wang Y., Wu W.H. Calcineurin B-like protein CBL 10 directly interacts with AKT 1 and modulates K+ homeostasis in Arabidopsis. Plant J. 2013;74:258–266. doi: 10.1111/tpj.12123. [DOI] [PubMed] [Google Scholar]
- Riveras E., Alvarez J.M., Vidal E.A., Oses C., Vega A., Gutiérrez R.A. The calcium ion Is a second messenger in the nitrate signaling pathway of Arabidopsis. Plant Physiol. 2015;169:1397–1404. doi: 10.1104/pp.15.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S.K., Pandey A., Pandey G.K. The CBL–CIPK signaling module in plants: a mechanistic perspective. Physiol. Plantarum. 2015;155:89–108. doi: 10.1111/ppl.12344. [DOI] [PubMed] [Google Scholar]
- Schmidt S.B., Jensen P.E., Husted S. Manganese Deficiency in Plants: The Impact on Photosystem II. Trends Plant Sci. 2016;21:622–632. doi: 10.1016/j.tplants.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Schmöckel S.M., Garcia A.F., Berger B., Tester M., Webb A.A.R., Roy S.J. Different NaCl-induced calcium signatures in the Arabidopsis thaliana ecotypes Col-0 and C24. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigaki T., Rees I., Nakhleh L., Hirschi K.D. Identification of Three Distinct Phylogenetic Groups of CAX Cation/Proton Antiporters. J. Mol. Evol. 2006;63:815–825. doi: 10.1007/s00239-006-0048-4. [DOI] [PubMed] [Google Scholar]
- Shi H., Ishitani M., Kim C., Zhu J.-K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R., Schachtman D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorst L., He G., Moore L.K., Schültke S., Schmitz-Thom I., Cao Y., Hashimoto K., Andrés Z., Piepenburg K., Ragel P., et al. A Ca2+-sensor switch for tolerance to elevated salt stress in Arabidopsis. Dev. Cell. 2022;57:2081–2094.e7. doi: 10.1016/j.devcel.2022.08.001. [DOI] [PubMed] [Google Scholar]
- Straub T., Ludewig U., Neuhäuser B. The Kinase CIPK23 Inhibits Ammonium Transport in Arabidopsis thaliana. Plant Cell. 2017;29:409–422. doi: 10.1105/tpc.16.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S.J., Gilroy S. Imaging changes in cytoplasmic calcium using the Yellow Cameleon 3.6 biosensor and confocal microscopy. Methods Mol. Biol. 2013;1009:291–302. doi: 10.1007/978-1-62703-401-2_27. [DOI] [PubMed] [Google Scholar]
- Tang R.-J., Zhao F.-G., Yang Y., Wang C., Li K., Kleist T.J., Lemaux P.G., Luan S. A calcium signalling network activates vacuolar K+ remobilization to enable plant adaptation to low-K environments. Nat. Plants. 2020;6:384–393. doi: 10.1038/s41477-020-0621-7. [DOI] [PubMed] [Google Scholar]
- Tang R.J., Wang C., Li K., Luan S. The CBL–CIPK Calcium Signaling Network: Unified Paradigm from 20 Years of Discoveries. Trends Plant Sci. 2020;25:604–617. doi: 10.1016/j.tplants.2020.01.009. [DOI] [PubMed] [Google Scholar]
- Tang R.J., Zhao F.G., Garcia V.J., Kleist T.J., Yang L., Zhang H.X., Luan S. Tonoplast CBL–CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2015;112:3134–3139. doi: 10.1073/pnas.1420944112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Wang H.Q., Chen J., Chang J.D., Zhao F.J. Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J. Integr. Plant Biol. 2023;65:570–593. doi: 10.1111/jipb.13440. [DOI] [PubMed] [Google Scholar]
- Thor K., Peiter E. Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol. 2014;204:873–881. doi: 10.1111/nph.13064. [DOI] [PubMed] [Google Scholar]
- Tian Q., Zhang X., Yang A., Wang T., Zhang W.H. CIPK23 is involved in iron acquisition of Arabidopsis by affecting ferric chelate reductase activity. Plant Sci. 2016;246:70–79. doi: 10.1016/j.plantsci.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Tian W., Wang C., Gao Q., Li L., Luan S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants. 2020;6:750–759. doi: 10.1038/s41477-020-0667-6. [DOI] [PubMed] [Google Scholar]
- Tian W.H., Ye J.Y., Cui M.Q., Chang J.B., Liu Y., Li G.X., Wu Y.R., Xu J.M., Harberd N.P., Mao C.Z., et al. A transcription factor STOP1-centered pathway coordinates ammonium and phosphate acquisition in Arabidopsis. Mol. Plant. 2021;14:1554–1568. doi: 10.1016/j.molp.2021.06.024. [DOI] [PubMed] [Google Scholar]
- Tsay Y.F., Schroeder J.I., Feldmann K.A., Crawford N.M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993;72:705–713. doi: 10.1016/0092-8674(93)90399-b. [DOI] [PubMed] [Google Scholar]
- Valivand M., Amooaghaie R. Calcium signaling confers nickel tolerance in Cucurbita pepo L. Int. J. Phytoremediation. 2021;23:362–373. doi: 10.1080/15226514.2020.1814992. [DOI] [PubMed] [Google Scholar]
- Vatansever R., Ozyigit I.I., Filiz E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: a Review. Appl. Biochem. Biotechnol. 2017;181:464–482. doi: 10.1007/s12010-016-2224-3. [DOI] [PubMed] [Google Scholar]
- Verbruggen N., Hermans C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil. 2013;368:87–99. doi: 10.1007/s11104-013-1589-0. [DOI] [Google Scholar]
- Verma N., Pandey A., Tiwari S., Prasad S.M. Calcium mediated nitric oxide responses: Acquisition of nickel stress tolerance in cyanobacterium Nostoc muscorum ATCC 27893. Biochem. Biophys. Rep. 2021;26 doi: 10.1016/j.bbrep.2021.100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G., Grotz N., Dédaldéchamp F., Gaymard F., Guerinot M.L., Briat J.F., Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A.A., Davies J.M. Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA. 2000;97:9801–9806. doi: 10.1073/pnas.160250397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R., Krebs M., Kudla J., Schumacher K. Multiparameter imaging of calcium and abscisic acid and high-resolution quantitative calcium measurements using R-GECO1-mTurquoise in Arabidopsis. New Phytol. 2017;216:303–320. doi: 10.1111/nph.14706. [DOI] [PubMed] [Google Scholar]
- Wang X., Feng C., Tian L., Hou C., Tian W., Hu B., Zhang Q., Ren Z., Niu Q., Song J., et al. A transceptor-channel complex couples nitrate sensing to calcium signaling in Arabidopsis. Mol. Plant. 2021;14:774–786. doi: 10.1016/j.molp.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen Y.F., Wu W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021;63:34–52. doi: 10.1111/jipb.13053. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kang Y., Ma C., Miao R., Wu C., Long Y., Ge T., Wu Z., Hou X., Zhang J., Qi Z. CNGC2 Is a Ca2+ Influx Channel That Prevents Accumulation of Apoplastic Ca2+ in the Leaf. Plant Physiol. 2017;173:1342–1354. doi: 10.1104/pp.16.01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhang Y., Liu Y., Fu D., You Z., Huang P., Gao H., Zhang Z., Wang C. Calcium-dependent protein kinases CPK21 and CPK23 phosphorylate and activate the iron-regulated transporter IRT1 to regulate iron deficiency in Arabidopsis. Sci. China Life Sci. 2023 doi: 10.1007/s11427-022-2330-4. [DOI] [PubMed] [Google Scholar]
- Wiese H., Nikolic M., Römheld V. In: The Apoplast of Higher Plants: Compartment of Storage, Transport and Reactions: The Significance of the Apoplast for the Mineral Nutrition of Higher Plants. Sattelmacher B., Horst W.J., editors. Springer Netherlands; 2007. Silicon in Plant Nutrition; pp. 33–47. [DOI] [Google Scholar]
- Wilkins K.A., Matthus E., Swarbreck S.M., Davies J.M. Calcium-mediated abiotic stress signaling in roots. Front. Plant Sci. 2016;7:1296. doi: 10.3389/fpls.2016.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Plant salt tolerance and Na+ sensing and transport. Crop J. 2018;6:215–225. [Google Scholar]
- Xiao H., Hu Y., Wang Y., Cheng J., Wang J., Chen G., Li Q., Wang S., Wang Y., Wang S.S., et al. Nitrate availability controls translocation of the transcription factor NAC075 for cell-type-specific reprogramming of root growth. Dev. Cell. 2022;57:2638–2651.e6. doi: 10.1016/j.devcel.2022.11.006. [DOI] [PubMed] [Google Scholar]
- Xie D., Ma X., Zhao Y., Li J., Fu D., Zhang Z., Ju C., Wang C. SCIENCE CHINA Vitae. 2023. Absorption, transport and regulation of manganese in plants. (in Chinese) [Google Scholar]
- Xu J., Li H.-D., Chen L.-Q., Wang Y., Liu L.-L., He L., Wu W.-H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Yadav V., Arif N., Kováč J., Singh V.P., Tripathi D.K., Chauhan D.K., Vaculík M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021;159:100–112. doi: 10.1016/j.plaphy.2020.11.047. [DOI] [PubMed] [Google Scholar]
- Yang L., Yao J., Sun J., Shi L., Chen Y., Sun J. The Ca2+ signaling, Glu, and GABA responds to Cd stress in duckweed. Aquat. Toxicol. 2020;218 doi: 10.1016/j.aquatox.2019.105352. [DOI] [PubMed] [Google Scholar]
- Ying S., Scheible W.R. A novel calmodulin-interacting Domain of Unknown Function 506 protein represses root hair elongation in Arabidopsis. Plant Cell Environ. 2022;45:1796–1812. doi: 10.1111/pce.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Steinhorst L., Kudla J. Analyzing the Impact of Protein Overexpression on Ca2+ Dynamics and Development in Tobacco Pollen Tubes. Methods Mol. Biol. 2020;2160:223–231. doi: 10.1007/978-1-0716-0672-8_16. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Z., Liu Y., Zhang T., Liu J., You Z., Huang P., Zhang Z., Wang C. Plasma membrane-associated calcium signaling modulates cadmium transport. New Phytol. 2022;238:313–331. doi: 10.1111/nph.18698. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Fu D., Sun Z., Ju C., Miao C., Wang Z., Xie D., Ma L., Gong Z., Wang C. Tonoplast-associated calcium signaling regulates manganese homeostasis in Arabidopsis. Mol. Plant. 2021;14:805–819. doi: 10.1016/j.molp.2021.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Fu D., Xie D., Wang Z., Zhao Y., Ma X., Huang P., Ju C., Wang C. CBL1/9-CIPK23-NRAMP1 axis regulates manganese toxicity. New Phytol. 2023;239:660–672. doi: 10.1111/nph.18968. [DOI] [PubMed] [Google Scholar]
- Zhong L., Chen D., Min D., Li W., Xu Z., Zhou Y., Li L., Chen M., Ma Y. AtTGA4, a bZIP transcription factor, confers drought resistance by enhancing nitrate transport and assimilation in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015;457:433–439. doi: 10.1016/j.bbrc.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Zhu J.K. Abiotic Stress Signaling and Responses in Plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang P., Bai Z., Herde M., Ma Y., Li N., Liu S., Huang C.F., Cui R., Ma H., et al. Calmodulin-like protein CML24 interacts with CAMTA2 and WRKY46 to regulate ALMT1-dependent Al resistance in Arabidopsis thaliana. New Phytol. 2022;233:2471–2487. doi: 10.1111/nph.17812. [DOI] [PubMed] [Google Scholar]