Abstract
Introduction
Osteoporosis is a debilitating silent disease with a huge socio-economic impact. Prevention strategies and early detection of osteoporosis need to be carried out in every health care unit to substantially reduce the fracture rates. Indian studies have indicated a knowledge gap on diagnosis and management of osteoporosis amongst medical professionals and consumers.
Areas Covered
This article reviews the evidences available on searches from PubMed and The National Library of Medicine, author's opinions based on clinical experience. There is a need for escalating the efforts to bridge the knowledge gap regarding various aspects of osteoporosis amongst professionals and consumers. Three indications for postmenopausal hormone therapy (HT), which have constantly withstood the test of time, are symptom relief, urogenital atrophy, and bone health. This article specifically focuses on management of postmenopausal osteoporosis by HT alone or in combinations.
Expert Opinion
Early menopause is within 10 years of menopause and late menopause is considered beyond 10 years of menopause. HT is a cost-effective therapy in the early post menopause especially in symptomatic women at risk for osteoporosis unless contraindicated. HT prevents all osteoporotic fractures even in low-risk population. All HT preparations including low dose and non-oral routes of estrogen are effective for bone health. The bone protective effect lasts while on HT. Extended use of HT in women after 10 years of menopause with reduced bone mass is an option after detailed counselling of the risk benefit analysis compared with the other available therapies for osteoporosis. The primary therapy to prevent bone loss in women with premature menopause and secondary amenorrhea is HT. HT work up and annual follow-up is essential before prescribing HT.
Keywords: Postmenopausal osteoporosis, Hormone therapy, Selective estrogen receptor modulators, Individualize therapy
Introduction
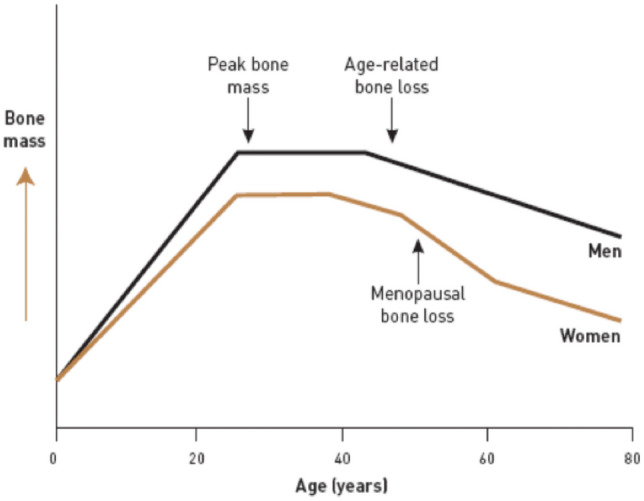
The most prevalent systemic skeletal disease associated with ageing and estrogen deficiency is osteoporosis. Refer to Fig. 1, osteoporosis presents with decreased bone mineral density and there are changes in structure and strength of bone tissue, resulting in high risk for osteoporotic or fragility fracture [1]. Fragility fracture is defined as a fracture due to fall from a standing height. Osteoporosis is a silent disease and its first symptom—osteoporotic fracture is devastating for the patient and the caregivers. At and beyond 50 years of age, there is 40% lifetime risk for osteoporotic fracture. Women have 2–4 times greater risk for an osteoporotic fracture than in men [2]. In simple terms 1 out of 2 women and 1 out of 5 men older than 50 years will suffer from osteoporotic fractures [3]. Hip fracture is more frequent than the combined risk of breast, uterus and cervical malignancy. Mortality rate is 12–20% within 1 year of hip fracture, more than 50% becomes dependent and more than 30% are left with permanent disability [4]. With spinal fracture there is shortening of height, crowding of internal organs, back pain, kyphosis, prolonged disability, and increased mortality [5].
Fig. 1.
Graphical representaion of menopause and age-related bone loss in men and women
There is rapid increase in number of elderly women in Asia, Latin America, the Middle East, and Africa. It has been projected that these parts of world will account for more than 70% of the estimated 6.26 million fractures which is expected in the year 2050 [6]. The prevalence of postmenopausal osteoporosis as reported in Indian studies has a wide range of 8–80% [7]. There is insufficient population-based epidemiological data on fractures. Indian data from hospital regarding fractures in hip indicate that they are common in women between 60 and 70 years of age. A study from North India, Rohtak district reported rate of hip fracture as 159 per 1 lac women above age of 50 and this hospital-based study has being used to define interventional thresholds in Fracture Risk Assessment Tool (FRAX) for India [8]. Risk of vertebral fracture in Indians is not very different from the Western and other Asian populations and tends to be lesser in overweight population [9]. More than 50 million people in India had low bone mass [1]. Age wise stratification of the risk shows more than 40% prevalence of low bone mass from the age of 40 years, which increases by more than 62% by age 60 years and 80% by the age of 65 years in women [7]. In addition, when compared to Caucasians, Indians suffer from osteoporotic fractures 10–20 years earlier than them [11, 12].
Considering the magnitude of the problem and its socio-economic impact, all health care units should work upon prevention and early detection of osteoporosis. Indian studies have indicated a knowledge gap in diagnosing and managing osteoporosis amongst medical professionals and consumers [13, 14]. These studies emphasize the need for escalating the efforts to bridge the gap regarding various aspects of osteoporosis amongst professionals. In continuum, there is a need to work on awareness campaigns on prevention and management of osteoporosis at a community level.
Estrogen and Bone
Postmenopausal women experience primary osteoporosis; estrogen deficiency is the leading cause of increased risk (Fig. 2). In 1882, Bruns first demonstrated that proximal femur fracture was more common in women [15]. Later in 1940, Albright et al. noted that vertebral fractures were common amongst women with surgical menopause [16]. An association and hypothesis were laid, and today, there is enough evidence to establish the association between estrogen and bone health.
Fig. 2.
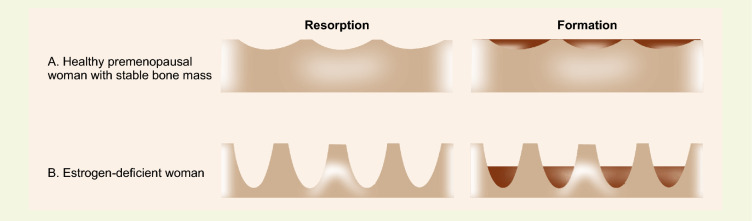
A Pictorial representation of resorption and formation with a stable bone mass. B Increased resorption in estrogen deficiency leading to decreased bone mass
Estrogen-deficient states, such as menopause, premature menopause, persistent anovulation, and amenorrhea, and medications such as gonadotropin-releasing hormone agonists are associated with bone loss [17, 18]. Aromatase inhibitors (AIs) used in treatment of hormone-responsive breast cancer, effectively block estradiol biosynthesis in peripheral tissues [19]. AIs suppress circulating estrogen levels beyond those observed after natural menopause, and rates of AI-associated bone loss exceed the bone mineral density (BMD) loss rate after menopause by more than twofold [20]. This has been observed as increased fracture rates in clinical trials comparing AIs with placebo in women with early breast cancer [21].
The acceptance of the entity postmenopausal osteoporosis (PMO) indicates the role of ovarian steroid hormones in metabolism of bone. At menopause, the rate of bone loss is accelerated in the background of the slow rate of cortical bone loss occurring with the ageing process. There is decrease in the trabecular bone width after menopause, followed by decrease in thickness of cortical bone beyond age 70.
Estrogen deficiency leads to increased bone turnover marked by accelerated osteoclast-mediated bone reabsorption and deficient bone formation, resulting in decreased bone mass [22]. Rate of bone loss is 1–5% per year due to declining estrogen levels in the first 5–7 years of menopause [23]. Women’s response to the declining ovarian steroid levels is labelled as fast losers [> 3% change in BMD per year] and average losers (< 3% change in BMD per year) [24]. The collagen matrix is responsible for the strength of bone which constitutes 90% of the organic matrix of bone. There is decline in collagen content of bone with ageing but it decreases significantly more in the postmenopausal period owing to estrogen deficiency [25].
Cortical bone collagen levels correlate with both estradiol levels and BMD [25]. Cummings et al. observed that the BMD is higher for serum estradiol greater than 10 pg/ml and lower for the same being < 5 pg/ml. For serum estradiol less than 5 pg/ml, fractures are increased by eight times [26].
It is well-known that bone resorption is very fast in the initial 3–4 years of menopause, so consideration should be given to begin hormone therapy soon after menopause, given the window of opportunity. This is the period during which the response to treatment can be maximum, as stopping resorption leads to instant filling and remodelling, thereby increasing bone formation and hence a significant increase in BMD [7].
Hormone Therapy
It includes many hormonal products—estrogens, progestins, androgens, tissue-selective estrogen complex as tibolone and selective estrogen receptor modulator. They can be given by different routes with different side effect profile. The risks and benefits vary for different age groups.
The terminology used in this review for hormone therapy is—HT—hormone therapy; MHT—menopausal hormone therapy; HRT—hormone replacement therapy; ET—estrogen therapy; EPT estrogen–progesterone therapy; and AT-androgen therapy.
Role in Postmenopausal Osteoporosis
Based on the evidences regarding effectiveness, safety and cost, standard MHT should be given as one of the first-line therapies for preventing and treating postmenopausal fractures women younger than 60 years unless there is a contraindication [7].
On the other hand, as per available evidences, MHT initiation after 60 years for prevention of fracture is not recommended. However, in women already on MHT, its continuation beyond age of 60 should be considered for possible beneficial long-term compared to other non-hormonal therapies [7].
Using a high dose of HT/ET for initial 6 months followed by reducing the dose in subsequent years will treat bone loss effectively in postmenopausal women by rapidly reducing bone resorption in initial estrogen deficiency phase.
HT and ET were commonly used in the last 30 years, but their use declined following the publication of the Women's Health Initiative (WHI) study in 2001.
Evidence for Hormone Therapy in Osteoporosis
Albright's original observational open-labelled studies demonstrated that estrogen-treated osteoporotic women maintained their height, unlike untreated women [27].
Estrogen was being used for 30 years as hormone therapy in postmenopausal osteoporosis prior to the 1st release of Women’s Health Initiative (WHI) study with evidence backing its use in all age groups. A lower risk of fractures in hip was demonstrated in post-menopausal women with higher circulating levels of 17-beta estradiol in the study of Osteoporotic Fractures [26].
Dual-energy X-ray absorptiometry (DXA) showed an increase in BMD of hip and spine over a period of 2 years in a double-blind study named The Postmenopausal Estrogen Progestin Intervention study (PEPI); and a decline in biochemical markers indicating that HT reduces the rate of remodelling. It also demonstrated that there was a bone loss when HT was discontinued [28].
The meta-analysis done on observational and randomized controlled trials further supports estrogen replacement therapy to be used as first line treatment to prevent and treat fractures due to bone loss. It showed that postmenopausal HT increases bone mass by decreasing turnover of bone thereby reducing osteoporotic fracture rate and also extra-skeletal beneficial effects by reducing 30–50% and 15–20% chances of coronary heart disease (CHD) and cerebrovascular accidents, respectively. This positive effect on bone was also seen when ET was started in late menopause. HT when initiated early in the phase of transition to menopause showed the most significant benefit [29].
The best data to date on HT is the significant reduction of 24–39% in osteoporotic fracture, as seen from WHI, the most prominent landmark randomized trial. There was significant decline in the incidence of fractures in women on long-term HT (5.6 years of combined HT: absolute rate was 86 per thousand, 95% confidence interval being 79–84; after 7.1 year usage of estrogen only HT: absolute rate was 102 per one thousand, 95% confidence interval being 91–112). The only clinical outcome where benefit was seen substantially from HT was decrease in risk of fracture [30].
This is in tune with the observational trials and meta-analysis by Torgerson et al., where use of HT indicated a significant reduction in all fragility fractures [31].
The first randomized trial which demonstrated a decline in the incidence of all osteoporotic-related fractures in unselected population compared to placebo is the WHI. No difference in effect was found when stratification of women was done by body mass index (BMI), age and time since menopause. It also showed that HT started in older women could prevent osteoporosis at different selected sites. No significant reduction was found in fracture after treatment with HT for postmenopausal women with CHD in The HERS (Heart and Estrogen/progestin Replacement) trial.
Cochrane Review 2012 has shown a significant fall in the risk of fractures in women using continuous combined HT or estrogen-only HT when followed up over near 8 years. Despite HT being considered sufficient for preventing osteoporosis in postmenopausal women, it has been recommended only for those women with significant risk, where non-estrogen treatment seems unsuitable [32].
A fall in use of estrogen therapy was seen for a decade after 2002 when WHI result was released in hurry. WHI data were re-analyzed which mentioned that HT within 10 years of menopause was safe unless contraindicated and this gave the theory of the “window of opportunity”.
Compared to placebo MHT was shown to decrease fracture risk in the combined WHI trials, irrespective of FRAX probability and history of falls in postmenopausal women [33].
A systematic review which included the WHI results, randomized controlled trials (RCT) and clinical trials of duration at least 1 year looked at different aspects of hormone therapy and bone. BMD measurement showed similar results when comparing prevention and treatment trials, opposed and unopposed HT, oral and transdermal estrogen and various types of progestogens [34].
Low-Dose HT
HT showed a dose-dependent effect on bone. Post WHI, the thought was to lose an effective dose of HT for a minimum duration. This led to research on low-dose HT(LD-HT) and ultra-dose HT based on the concept that the required dose of estrogen to protect bone is much lower than previously thought. 1 mg Estradiol, 0.45 mg conjugated equine estrogen (CEE), or 25 μg estradiol via transdermal patch are protective, and some evidence exists for even lower doses. Ultralow doses of oral CEE 0.3 mg, 17 E estradiol 0.5 mg and 14 ug estradiol transdermal route have been studied in both early (mean age 51–52) and late postmenopausal (mean age 67–74) with significant increases in BMD in both populations [35]. Furthermore, older postmenopausal women may achieve similar skeletal effects with lower estrogen doses than younger women. Lees et al. demonstrated that 1 mg estradiol in women over 58 years achieved the same increase in bone density as 2 mg in those under 51 years.
The efficacy of LD-HT to prevent osteoporosis has been assessed in several studies. It can reduce turnover rate of bone by 30% approximately thereby increasing bone density. Younger postmenopausal women (age 50–53 years, 3–5 years after menopause) have also been benefitted by LD-HT in preventing bone loss in addition to other menopausal symptoms, such as hot flushes, mood change and sexual dysfunction. LD-HT has added benefit of personalizing the doses as per each patients’ needs. The first one large randomized placebo control trial which evaluated BMD after lower doses of CEE alone or in combination with low dose of medroxyprogesterone acetate (MPA) was the women’s HOPE (Health, Osteoporosis, Progestin, Estrogen) study. This study demonstrated lower doses of CEE (0.45 mg and 0.3 mg per day), with or without a low dose of MPA (1.5 mg per day) helped in preventing BMD loss in hip and spine. It reduced turnover of bone in early postmenopausal women in addition to relieving vaginal atrophy, vasomotor symptoms, endometrial hyperplasia and had better bleeding profiles [35].
Tibolone
Tibolone is a synthetic steroid which belongs to selective tissue estrogenic activity regulator (STEAR) group. It has estrogenic, androgenic and progestogenic effects. It reduces osteoclastic activity in bone and thus inhibits bone resorption which is estrogenic effect. It has been approved to prevent osteoporosis and to treat symptoms of menopause 1 year after amenorrhea. The LIFT study, which was a trial by Cummings et al. in 2008, to evaluate tibolone in treatment of osteoporosis in older menopausal women having mean age of 68 years examined the use of 1.25 mg of tibolone. A significant decline in both fractures of vertebra and non-vertebral sites was observed in comparison with placebo, but an increase in rate of stroke was also found [36].
Selective Estrogen Receptor Modulators
SERMs are Selective Estrogen Receptor Modulators which lack steroid structure of estrogen but they can act at estrogen receptor as agonist or antagonist in different tissues. Initially SERMs were developed to prevent and treat breast cancer, such as tamoxifen and raloxifene, but later they were found to conserve bone mass. Tamoxifen had been used as adjuvant in treatment and prevention (in high-risk women) of breast cancer for about last 30 years. While tamoxifen had a significant bone-sparing effect, its unwanted effects were high risk of endometrial malignancy, pulmonary embolism, deep-vein thrombosis, stroke and cataracts. Therefore, it’s not used to prevent or treat osteoporosis in menopausal women [37].
60 mg raloxifene per day helps to preserve and improve the density of bone at spine (2.6%) and hip (2.1%) at 4 years of use and it also reduces risk of invasive breast cancer by 76%. It lacks efficacy in reducing hip fracture. Risk reduction of new fracture in vertebra is seen by the tune of 69% in postmenopausal osteoporotic women and by 47% in postmenopausal women with osteopenia over 3 years [38]. While raloxifene can prevent and treat osteoporosis especially in women having increased risk of breast cancer, its side effects are increase in risk of venous thromboembolism (VTE) as with estrogen, hot flushes being more likely in perimenopausal time and cramps in legs.
Bazedoxifene
Bazedoxifene is one SERM which was synthesized to improve skeletal symptoms and lipid profile specifically without having any effect on hot flushes. The first FDA approved combination of conjugated estrogen and estrogen agonist/antagonist is CE/BZA (conjugated estrogen and bazedoxifene) which relieves vasomotor symptoms in addition to preventing osteoporosis. This combination of CE/BZA is called tissue selective estrogen complex (TSEC) that is not available in India [40].
Combination Therapy with Hormone Therapy
Not all but some studies have shown combination of estrogen plus bisphosphonates being more effective in increasing BMD than either drug alone. Addition of calcium supplement to ET was found in a study to increase BMD of spine by 2% and that of femoral neck by 1.5% when compared to ET/HT alone. When alendronate was combined with estradiol/norethisterone acetate, no increase in BMD of hip was found when compared to either medication alone [41]. Alendronate, risedronate and calcitriol can be combined with 0.625 mg CEE. Combining two agents have shown 2% higher BMD in spine and 1–2% in hip. Combination therapy when compared to single therapy showed continued suppression of the biochemical markers of bone resorption. Enough data are lacking on further fracture reduction.
Diagnosis of Osteoporosis
BMD defines the diagnosis of osteoporosis. It is reported as T-Score measured by DXA based on reference data obtained by DXA according to WHO. This WHO diagnostic criteria can be applied to women in transition phase of menopause. Indications for BMD measurement in women during the transition of menopause are for those having clinical risk factors for fracture, such as low body weight, prior fracture or high-risk medications [42].
Indications for DXA (Grade B) given in Table 2.
Table 2.
Indications for DXA
| Indications for DXA (Grade B) | More than 5 years since menopause |
|---|---|
| Less than 5 years of menopause with risk factors | |
| Women in menopause transition with secondary causes | |
| Osteopenia evidenced by radiology and presence of vertebral compression fracture | |
| Fragility fractures seen in women on radiology or DXA | |
| Emerging indications are to measure total body fat and lean tissue mass | |
| This suggestion is based on the following: |
Natural menopause seen at early age in Indian women, which is 46.5 years [43] The life expectancy of an Indian woman being 70.3 years Accrual of low peak bone mass [44] Clinical presentation of fracture at an early age Accelerated bone loss within 5 years of menopause [44] More than 40% of women beyond age 40 years have low bone mass which increases to more than 80% after 65 years Screening can reduce the hip fracture risk by 20% as shown by a meta-analysis of the three trials [45] |
Definition of Osteoporotic Fracture Risk
BMD defines fracture risk (both primary and secondary causes) which is classified as low-, moderate- and very high-risk.
Management Plan as given in Table 3.
Table 3.
Management plan
| Management plan includes | Categorizing risk by clinical factors alone or by BMD |
| Confirmation of diagnosis by DXA | |
| Excluding secondary causes—Complete Blood picture, serum 25 hydroxy vitamin D, serum calcium and serum alkaline phosphatase | |
| Counselling all about lifestyle changes | |
| Giving appropriate pharmacotherapy and follow-up | |
| Therapeutic Lifestyle management | For low bone mass and lower risk of fracture: reassurance, enforce lifestyle measures |
| Nutrition: an adequate ingestion of calcium (1–1.5 g/day), Vitamin D (> 1000–2000 IU/day), Vitamin K (1 mg/day), and magnesium | |
| Habits: avoid alcohol and tobacco | |
| Exercise: weight-bearing, strength training and balance exercise tailored to the need under supervision | |
| Fall Prevention: 30% of persons 65 years and older fall, 50% of persons over 80 years and older fall, 5% of them will have a fracture, and 1% will have a hip fracture | |
| Whom and when to treat | Treatment is decided by the severity of the fracture risk, the cost of intervention, risk benefit analysis, the active participation of the patient and the correct guidance by the physician |
| Low bone mass with more than two risk factors have high risk of fractures and osteoporosis without fracture are candidates for HT | |
| Premenopausal: hormone therapy, others if estrogen therapy is contraindicated | |
| Perimenopausal: hormone therapy, bisphosphonates | |
|
Postmenopausal: Less than 10 years: hormone therapy, raloxifene, tibolone, bisphosphonates, calcitonin, denosumab, parathyroid hormone More than 10 years: bisphosphonates, calcitonin, denosumab, parathyroid hormone |
How Long to Treat
BMD testing every 1 year after starting therapy and every 2 years after that.
Current Indications for Hormone Therapy
There are 3 indications for postmenopausal HT, which have been time-tested and these are symptom relief, urogenital atrophy, and bone [7]. Early menopause: within 10 years of menopause. Late menopause: beyond 10 years of menopause.
Indications in Bone Health
1. EPT/ET is a cost-effective treatment in the early period of menopause, especially in women with symptoms of osteoporosis, unless contraindicated. HT prevents the osteoporotic fractures, also in low-risk populations [46, 47].
2. All preparations of estrogen, including low-dose and non-oral routes, are adequate for bone health. Transdermal route should be the preferred in women at high risk for deep vein thrombosis (DVT), stroke, and CVD [48].
3. Stoppage of HT leads to loss of bone protective effect. Women with reduced bone mass after 10 years of menopause can opt for extended use of HT after detailed counselling of the risk and benefit and also comparing the other available options. [49, 50].
4. For prevention of bone loss in women with premature menopause and secondary amenorrhea HT should be given as primary therapy.
5. Primary HT workups, counselling and compliance for annual follow-ups are mandatory before prescribing HT.
Contraindications of Hormone Therapy are given in Table 4.
Table 4.
Contraindications of hormone therapy
| Contraindications of Hormone Therapy | Active endometrial and female hormone-dependent cancers |
| Active breast cancer and any estrogen/progesterone receptor-positive cancers | |
| Pregnancy | |
| Vaginal bleeding under evaluation | |
| Impaired liver function result or any active disease in liver | |
| History of venous thrombosis provoked by estrogen inherent risk of thromboembolism | |
| Relative contraindications | Migraine |
| Thrombophlebitis in superficial veins | |
| Family history of breast cancer in first degree relative | |
| Leiomyoma | |
| Endometriosis | |
| Disease of gall bladder |
Hormone Therapy in Women with Uterus
Addition of progestins to estrogen reduces the risk of endometrial hyperplasia and malignancy, which can occur with unopposed estrogen. For women who underwent endometrial ablation, progestogen must be given as some endometrial tissue can be present in uterus, even with prolonged amenorrea. Progestins, with androgenic properties can be preferred to protect the hip.
Hormone Therapy in Hysterectomized Women
Only estrogen is given to women without uterus and there is no need for a progestogen. Those who had history of endometriosis may need estrogen–progestin therapy.
Risk–Benefit Analysis
There is slight increase in the risk of invasive breast cancer with EPT; the absolute increase being less than 0.1% per year. WHI has shown reduction in the risk of breast cancer with ET.
The risk of venous thromboembolism (VTE) is 2 per 1000 per year in women aged 50–60 years. Risk is maximum in the first year of treatment which increases with advancing age, obesity, and previous history of VTE. HT should be discontinued temporarily during surgery or any immobilization, including travel.
The risk of stroke is increased with ET and EPT (hazard ratio is 1.39) in the WHI study, similar to the Nurses Health Study; which is contrary to the Danish Nurses Study. In this study, unopposed 1 mg estradiol per day was given to 13,122 healthy postmenopausal women who were followed for 5 years and had a protective hazard ratio of 0.80; this indicates that outcome is influenced by the type and dose of estrogen and also the chosen population.
HT when started within 10 years of menopause may benefit cognition and Alzheimer’s disease but when started beyond age 65 it can worsen cognitive function and found to have no benefit on Alzheimer's disease.
WHI and Nurses Health Studies had shown a cardioprotective effect when HT was started close to menopause with a relative risk of 0.66.
There is 2–5-fold increased risk of endometrial cancer in women using estrogen without adding progesterone.
EPT reduces the risk of colorectal cancer.
Hormone Therapy Regimens
Several types of regimens are available while prescribing ET. Clinical goal is to maintain benefits of estrogen with protection of uterine endometrium and reducing unscheduled uterine bleeding, which affects compliance.
Several types of regimens are: cyclic, cyclic-combined, continuous-cyclic, continuous long-cycle, continuous-combined, and intermittent combine (Table 1).
Table 1.
Regimes of hormone therapy
| Regimen | Estrogen | Progestogen |
|---|---|---|
| Cyclic | Days 1–25 | Last 10–14 days of the cycle |
| Cyclic-combined | Days 1–25 | Days 1–25 |
| Continuous-cyclic (sequential) | Daily | 10–14 days every month |
| Continuous long-cycle | Daily | 14 days every 3–6 months |
| Continuous-combined | Daily | Daily |
Perimenopausal Woman
Monthly or trimonthly cyclic regimens are options for this population. Women having infrequent menstruation and progestogenic side effects should be given a trimonthly preparation. As there is high risk of irregular bleeding, so continuous combined regimens should not be used in perimenopausal women [51].
Postmenopausal Woman
Continuous combined treatment may reduce risk of endometrial cancer when compared to sequential regimens. Continuous combined regimens induce endometrial atrophy (see Table 2).
Women can present with irregular bleeding or spotting during the first 4–6 months of continuous combined therapy, so it does not warrant investigation. Endometrial assessment must be considered if the bleeding becomes heavier rather than lighter, persists beyond 6 months, or occurs after a significant time of amenorrhea. The incidence of irregular bleeding may be reduced by increasing the ratio of the progestogen to the estrogen [51] (see Table 3).
A systematic review assessing changes in BMD over a 2 year trials in the past decade showed no obvious difference between the various estrogen compounds (oral, non-oral, human, and non-human estrogen) [51] (see Table 4).
Duration of Therapy
ET and EPT are recommended for different duration of treatment. ET has a better safety profile, so it can be given for a longer time in the absence of adverse effects and other risk factors. For ET, more benefit was observed during a mean of 7 years of use and 4 years of follow-up. For EPT, duration is limited by the increased risk of breast cancer and breast cancer mortality associated with 3–5 years of use (see Table 5).
Table 5.
Work-up and follow-up for hormone therapy
| Clinical History and Examination | Evaluation of woman’s needs, risk profile |
| Risk assessment for Diabetes Mellitus, CVD, Alzheimer’s disease, DVT, Osteoporosis and cancers | |
| Family history of fractures, CVD and breast cancer | |
| Physical examination: BMI, pulse, blood pressure, breast examination, abdominal and pelvic examination | |
| Investigations | Complete Blood Count |
| Urine Routine analysis | |
| Fasting blood glucose level | |
| Lipid profile | |
| Pap smear/cervical cytology | |
| Transvaginal sonography of uterus and adnexa |
Essential Factors to Lower the Risks and Side Effects of HT
Age of initiation: ideally, HT should begin within 10 years of menopause or by 60 years of age.
Low dose regimen should be used as appropriate.
Route of administration: transdermal route has a less risk of thrombosis compared with oral administration which has a high risk for DVT, CVD, or stroke.
Type of progestin: side-effect profile varies with different progestogens. The newer progestin drospirenone (a derivative of spironolactone) should be considered in hypertensive women; natural progesterone is also a good choice. Ethindrone derivatives do not have a preferential bone protective effect.
Individualized benefit–risk profile should be constructed for every woman planning any HT regimen. It depends on women’s situation, severity of symptoms and their effect on her quality of life. The absolute risks are low for the use of HT in healthy women ages 50–59 years. Whereas, long-term standard dose HT, or HT initiation in older age is associated with greater risks.
HT workup and follow-up please see Table 5.
Follow-up
Initially, 3 months and later yearly check-ups.
Conclusion
Today, we have strong evidence for role of MHT as a first line therapy in early postmenopausal women for a low risk for fractures, low bone mass and management of osteoporosis without fragility fractures. Estrogen replacement therapy, if recommended by an orthopedician, should be prescribed with the joint consultation of a gynecologist or an endocrinologist.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.(1991). Consensus development conference: prophylaxis and treatment of osteoporosis. The American Journal of Medicine, 90, 107-110. [DOI] [PubMed]
- 2.Siris ES, Miller PD, Barret-Conner E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: Results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 3.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available from: http://www.nof.org/professionals/clinical-guidelines. Accessed 13 Jan 2011.
- 4.Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: A world-wide projection. Osteoporosis International. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 5.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: A prospective study. Study of Osteoporotic Fractures Research Group. Archives of Internal Medicine. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 6.Melton LJ., III Hip fractures: A worldwide problem today and tomorrow. Bone. 1993;14:1–8. doi: 10.1016/8756-3282(93)90341-7. [DOI] [PubMed] [Google Scholar]
- 7.Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis: Indian menopause society. Journal of Mid-life Health. 2020;11:96. doi: 10.4103/jmh.JMH_143_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhanwal DK, Siwach R, Dixit V, Mithal A, Jameson K, Cooper C. Incidence of hip fracture in Rohtak district, North India. Archives of Osteoporosis. 2013;8(1–2):135. doi: 10.1007/s11657-013-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marwaha RK, Tandon N, Gupta Y, Bhadra K, Narang A, Mani K, Mithal A, Kukreja S. The prevalence of and risk factors for radiographic vertebral fractures in older Indian women and men: Delhi Vertebral Osteoporosis Study (DeVOS) Archives of Osteoporosis. 2012;7:201–207. doi: 10.1007/s11657-012-0098-8. [DOI] [PubMed] [Google Scholar]
- 10.Mithal A, Kaur P. Osteoporosis in Asia: A call to action. Current Osteoporosis Reports. 2012;10(4):245–247. doi: 10.1007/s11914-012-0114-3. [DOI] [PubMed] [Google Scholar]
- 11.Shatrugna V, KulkarniKumar BPA, et al. Bone status of Indian Women from a low income group and it’s relationship to the nutritional status. Osteoporosis International. 2005;16:1827. doi: 10.1007/s00198-005-1933-1. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A. Osteoporosis in India—the nutritional hypothesis. In: Mithal A, Rao DS, Zaidi M, editors. Metabolic bone disorders. Hindustani Book Depot; 1998. p. 115. [Google Scholar]
- 13.Thakur P, Kuriakose C, Cherian KE, Asha HS, Kapoor N, Paul TV. Knowledge gap regarding osteoporosis among medical professionals in Southern India. Journal of Evaluation in Clinical Practice. 2020;26:272–280. doi: 10.1111/jep.13164. [DOI] [PubMed] [Google Scholar]
- 14.Senthilraja M, Cherian KE, Jebasingh FK, Kapoor N, Paul TV, Asha HS. Osteoporosis knowledge and beliefs among postmenopausal women: A cross-sectional study from a teaching hospital in southern India. J Family Med Prim Care. 2019;8:1374–1378. doi: 10.4103/jfmpc.jfmpc_95_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocrine Reviews. 2004;25:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 16.Albright F, Smith PH, Richardson AM. Postmenopausal osteoporosis: Its clinical features. JAMA. 1941;116:2465–2474. doi: 10.1001/jama.1941.02820220007002. [DOI] [Google Scholar]
- 17.Gambacciani M, Spinetti A, Taponeco F, Cappagli L, Fioretti P. Longitudinal evaluation of perimenopaausal vertebral bone loss: Effects of a low-dose oral contraceptive preparation on bone mineral density and metabolism. Obstetrics and Gynecology. 1994;83:392–396. [PubMed] [Google Scholar]
- 18.Gambacciani M, Spinetti A, Taponeo F, Taponeco F, Manetti P, Piaggesi L, et al. Bone loss in perimenopusal women: a longitudinal study. Maturitas. 1994;18:191–197. doi: 10.1016/0378-5122(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 19.Aebi S, Davidson T, Gruber G, Castiglione M, ESMO Guidelines Working Group Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21(Suppl 5):v9–14. doi: 10.1093/annonc/mdq159. [DOI] [PubMed] [Google Scholar]
- 20.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Critical Reviews in Oncology Hematology. 2009;69:73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE, Gnant M, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: Practical guidance for prevention and treatment. Annals of Oncology. 2011;22:2546–2555. doi: 10.1093/annonc/mdr017. [DOI] [PubMed] [Google Scholar]
- 22.Seifert-Klauss V, Mueller JE, Luppa P, Probst R, Wilker J, Höss C, , et al. Bone metabolism during the perimenopausal transition: a prospective study. Maturitas. 2002;41:23–33. doi: 10.1016/S0378-5122(01)00248-1. [DOI] [PubMed] [Google Scholar]
- 23.Guthrie JR, Ebeling PR, Hopper JL, Barrett-Connor E, Dennerstein L, Dudley EC, et al. A prospective study of bone loss in menopausal Australian-born women. Osteoporosis International. 1998;8:282–290. doi: 10.1007/s001980050066. [DOI] [PubMed] [Google Scholar]
- 24.Tanko LB, Christianson C. Hormone replacement therapy. In: Genazzani AR, editor. Postmenopausal osteoporosis: hormones and other therapies. Controversial issues in climacteric medicine series. CRC Press; 2006. pp. 186–187. [Google Scholar]
- 25.Khastgir G, Studd J, Holland N, Alaghband-Zadeh J, Fox S, Chow J. Anabolic effects of estrogen replacement on bone replacement in postmenopausal women with osteoporosis: Histomorphometric evidence in a longitudinal study. Journal of Clinical Endocrinology and Metabolism. 2001;86:289–295. doi: 10.1210/jcem.86.1.7161. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. New England Journal of Medicine. 1998;339(11):733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 27.Forbes AP. Fuller Albright. His concept of postmenopausal osteoporosis and what came of it. Clinical Orthopaedics and Related Research. 1991;269:128–141. doi: 10.1097/00003086-199108000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Marcus R, Holloway L, Wells B, Greendale G, James MK, Wasilauskas C, et al. The relationship of biochemical markers of bone turnover to bone density changes in postmenopausal women: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. Journal of Bone and Mineral Research. 1999;14:1583–1595. doi: 10.1359/jbmr.1999.14.9.1583. [DOI] [PubMed] [Google Scholar]
- 29.Wells G, Tugwell P, Shea B, et al. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocrine Review. 2002;23:529–539. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- 30.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Torgerson DJ, Bell-Syer SEM. Hormone replacement therapy and prevention of nonvertebral fractures: A meta-analysis of randomized trials. JAMA. 2001;285(22):2891–2897. doi: 10.1001/jama.285.22.2891. [DOI] [PubMed] [Google Scholar]
- 32.Marjoribanks, J., Farquhar, C., Roberts, H., & Lethaby, A. (2012). Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews (7), CD004143 [DOI] [PubMed]
- 33.Lorentzon M, Johansson H, Harvey NC. Menopausal hormone therapy reduces the risk of fracture regardless of falls risk or baseline FRAX probability—results from the Women’s Health Initiative hormone therapy trials. Osteoporosis International. 2023 doi: 10.1002/14651858.CD004143.pub4.Accessed24June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldštajn MŠ, Mikuš M, Ferrari FA, Bosco M, U, Effects of transdermal versus oral hormone replacement therapy in postmenopause: A systematic review. Archives of Gynecology and Obstetrics. 2023;307(6):1727–1745. doi: 10.1007/s00404-022-06647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsay R, Gallagher C, Kleerekoper M, et al. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668–2676. doi: 10.1001/jama.287.20.2668. [DOI] [PubMed] [Google Scholar]
- 36.Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, Kloosterboer L, Mosca L, Christiansen C, Bilezikian J, Kerzberg EM, Johnson S, Zanchetta J, Grobbee DE, Seifert W, Eastell R. LIFT Trial Investigators: The effects of tibolone in older postmenopausal women. New England Journal of Medicine. 2008;359:697–708. doi: 10.1056/NEJMoa0800743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. (1998). Lancet, 351, 1451–1467. [PubMed]
- 38.Grady D, Ettinger B, Moscarelli E, Multiple Outcomes of Raloxifene Evaluation Investigators et al. Safety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluation. Obstetrics & Gynecology. 2004;104:837–844. doi: 10.1097/01.AOG.0000137349.79204.b8. [DOI] [PubMed] [Google Scholar]
- 39.Lobo RA, Pinkerton JV, Gass MLS, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic bone parameters and overall safety profile. Fertility and Sterility. 2009;92:1025–1038. doi: 10.1016/j.fertnstert.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 40.Genant HK. Bazedoxifene: A new selective estrogen receptor modulator for postmenopausal osteoporosis. Menopause International. 2011;17:44–49. doi: 10.1258/mi.2011.011011. [DOI] [PubMed] [Google Scholar]
- 41.Yoon BK, Lee DY, Park MC, Cho SH, Park HM, Choi YM. Effects of combination therapy of alendronate and hormonal therapy on bone mineral density in postmenopausal Korean women: Multicenter, randomized controlled clinical trial. Journal of Korean Medical Science. 2017;32(6):992–998. doi: 10.3346/jkms.2017.32.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonelli C, Adler RA, Blake GM, Caudill JP, Khan A, Leib E, Maricic M, Prior JC, Eis SR, Rosen C, Kendler DL. Dual-energy X-Ray absorptiometry technical issues: The 2007 ISCD Official Positions. Journal of Clinical Densitometry. 2008;11(1):109–122. doi: 10.1016/j.jocd.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Singh M. Early age of natural menopause in India, a biological marker for early preventive health programs. Climacteric. 2012;15:581–586. doi: 10.3109/13697137.2011.643514. [DOI] [PubMed] [Google Scholar]
- 44.Shatrugna V, Kulkarni B, Kumar PA, Rani KU, Balakrishna N. Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporosis International. 2020;31(2):251–257. doi: 10.1007/s00198-005-1933-1. [DOI] [PubMed] [Google Scholar]
- 45.Merlijn T, Swart KMA, van der Horst HE, et al. Fracture prevention by screening for high fracture risk: a systematic review and meta- analysis. Osteoporosis International. 2020;31(2):251–257. doi: 10.1007/s00198-019-05226-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Women’s Health Initiative Investigators et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the women’s health initiative randomized trial. Journal of Bone and Mineral Research. 2006;21:817–828. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 47.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, Women’s Health Initiative Investigators et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 48.Huang AJ, Ettinger B, Vitinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra- low-dose Transdermal estradiol on bone turnover and BMD in postmenopausal women. Journal of Bone and Mineral Research. 2007;22:1791–1797. doi: 10.1359/jbmr.070707. [DOI] [PubMed] [Google Scholar]
- 49.Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, WHI Investigators et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 50.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, WHI Investigators et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dören M, Nilsson JA, Johnell O. Effects of specific post-menopausal hormone therapies on bone mineral density in post-menopausal women: A meta-analysis. Human Reproduction. 2003;18:1737–1746. doi: 10.1093/humrep/deg315. [DOI] [PubMed] [Google Scholar]