Abstract
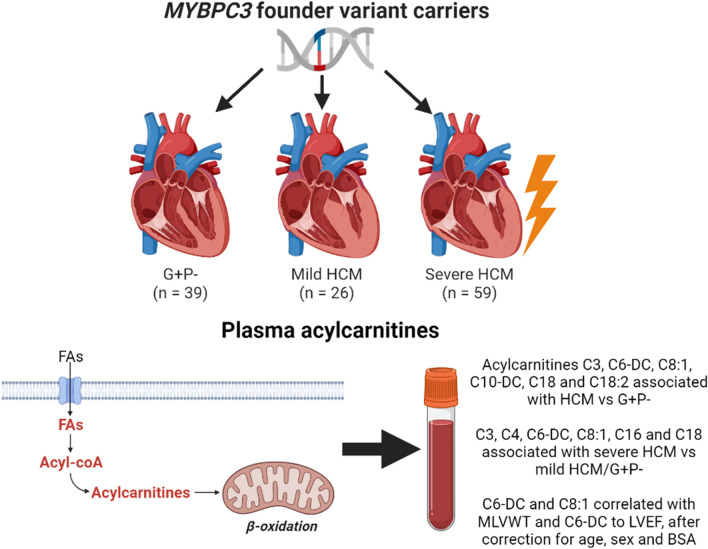
Hypertrophic cardiomyopathy (HCM) is a relatively common genetic heart disease characterised by myocardial hypertrophy. HCM can cause outflow tract obstruction, sudden cardiac death and heart failure, but severity is highly variable. In this exploratory cross-sectional study, circulating acylcarnitines were assessed as potential biomarkers in 124 MYBPC3 founder variant carriers (59 with severe HCM, 26 with mild HCM and 39 phenotype-negative [G + P-]). Elastic net logistic regression identified eight acylcarnitines associated with HCM severity. C3, C4, C6-DC, C8:1, C16, C18 and C18:2 were significantly increased in severe HCM compared to G + P-, and C3, C6-DC, C8:1 and C18 in mild HCM compared to G + P-. In multivariable linear regression, C6-DC and C8:1 correlated to log-transformed maximum wall thickness (coefficient 5.01, p = 0.005 and coefficient 0.803, p = 0.007, respectively), and C6-DC to log-transformed ejection fraction (coefficient -2.50, p = 0.004). Acylcarnitines seem promising biomarkers for HCM severity, however prospective studies are required to determine their prognostic value.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12265-023-10398-2.
Keywords: Hypertrophic Cardiomyopathy, MYBPC3, Biomarker, Acylcarnitine, Metabolism
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common Mendelian cardiac disease and an important cause of sudden cardiac death [1–3]. While many patients exhibit little to no symptoms, HCM may present with left ventricular outflow tract obstruction (LVOTO) requiring septal reduction therapy (SRT), and a small proportion of patients experiences malignant ventricular arrhythmia (MVA) and/or debilitating heart failure (HF) [4, 5]. Inheritance is typically autosomal dominant with incomplete penetrance [6]. Pathogenic variants are predominantly found in genes encoding cardiac sarcomere proteins, with (likely) pathogenic genetic variants identified in about 50% of patients in historical, well-characterised cohorts [7, 8]. Based on contemporary, more heterogeneous cohorts, the yield of genetic testing in HCM is estimated at 33% [9]. The most frequently affected gene is MYBPC3, which encodes cardiac myosin-binding protein C, a key regulator of cardiomyocyte contractility [10]. Founder variants in MYBPC3, including c.2373dupG, c.2827C > T, c.2864_2865delCT or c.3776delA, are identified in 20–35% of HCM cases in the Netherlands [11]. These variants lead to nonsense mediated mRNA decay of the mutant allele, resulting in haploinsufficiency [12].
Impaired energy metabolism has been proposed as a key pathomechanism in HCM [13]. In particular, studies have shown impaired myocardial fatty acid metabolism, including changes in myocardial acylcarnitines [14–16]. Acylcarnitines are conjugations of acyl groups, primarily derived from fatty acids, with carnitine, which transport the acyl groups from the cytosol into mitochondria for energy production [17]. Changes in plasma acylcarnitines reflect the dysregulation of fatty acid metabolism in tissue, especially the heart [18, 19]. Consistently, untargeted (non-quantitative) metabolomics studies suggested myocardial changes in acylcarnitines are mirrored in circulating acylcarnitines in HCM [20–22]. However, these findings still require confirmation using quantitative methods to assess whether circulating acylcarnitines can function as minimally-invasive biomarkers for HCM severity.
In this exploratory study, we quantitatively measured plasma acylcarnitines and employed a feature selection algorithm to assess possible associations between plasma acylcarnitines and HCM severity, as well as echocardiographic surrogates, in 124 carriers of MYBPC3 founder variants across the phenotypic spectrum.
Methods
Subject Inclusion
This study consisted of a cross-sectional analysis of the BIO FOr CARe study (Identification of biomarkers of hypertrophic cardiomyopathy development and progression in Dutch MYBPC3 founder variant carriers), which included carriers of the c.2373dupG, c.2827C > T, c.2864_2865delCT and c.3776delA MYBPC3 founder variants aged ≥ 18 years from three Dutch University Medical Centres (Utrecht, Groningen and Amsterdam). The design of this study and patient inclusion was previously published [23]. Subjects underwent prospective blood collection for biomarker assessment. Patients with prior heart transplantation were excluded. The study was approved by the institutional review board of the University Medical Centre Utrecht and all subjects provided informed consent.
Acylcarnitine Profiling
Acylcarnitine profiles were measured in plasma at the metabolic diagnostics laboratory of the University Medical Centre Utrecht. Plasma was derived from heparinised blood samples, collected under non-fasting, resting conditions and centrifuged for 10 min at 2000 g, and subsequently stored at -80 °C until analysis [23].
Samples were butylated and evaporated (using nitrogen) at 40 °C, after which concentrations of 42 acylcarnitines were analysed using tandem liquid chromatography-mass spectrometry (LC–MS; Acquity UPLC system [serial numbers of components: E12UPAB979A, F12UPA794M, C12UPM597G and E12UPO328M] & Xevo TQ MS [serial number VBA 760], Waters, Milford, Massachusetts), as previously published [24]. Calibration curves were used for quantification. Missing acylcarnitine values were assumed to reflect concentrations below the detection limit of LC–MS, and were replaced by half the minimum observed value of each acylcarnitine.
Acylcarnitines are referred to by their acyl-chain carbon chain length (C0-C18), the presence and number of unsaturated bonds (indicated as counts after colons [:]), and the presence of hydroxyl- (-OH) or dicarboxyl (-DC) groups.
Study Outcomes & Definitions
Subjects were classified into severe HCM, mild HCM and genotype-positive phenotype-negative (G + P-). Severe HCM phenotype was defined as a composite endpoint composed of a documented maximum left ventricular wall thickness (MLVWT) ≥ 20 mm, LVOTO necessitating SRT, HF (clinical diagnosis of congestive HF, requiring treatment with loop diuretics, or left ventricular ejection fraction [LVEF] < 50%) or MVA (sustained ventricular tachycardia, ventricular fibrillation, appropriate implantable cardioverter-defibrillator intervention, resuscitated cardiac arrest, or sudden cardiac death). Sustained ventricular tachycardia was defined as > 30 s, with haemodynamic instability or requiring earlier termination. Sudden cardiac death was defined as death due to documented ventricular arrhythmia or within 1 h of symptom onset, in absence of other identified causes. Subjects fulfilling HCM criteria but no criteria for severe HCM were classified as mild HCM. HCM was defined as a MLVWT ≥ 13 mm, not explained by abnormal loading conditions [1]. Subjects that did not fulfil HCM criteria were classified as G + P-.
Additionally, MLVWT, indexed left atrial volume (LAVi) and LVEF were assessed as continuous variables.
Statistical Analysis
Baseline characteristics were presented as counts with percentages for dichotomous and categorical variables and medians with interquartile ranges for continuous variables, and compared using Chi-square tests (or Fisher-Freeman Halton tests when observed counts were < 10) and Kruskal–Wallis tests, respectively. Correlations between acylcarnitines were assessed using Spearman’s correlation coefficient and visualised using a heatmap.
To identify acylcarnitines associated with HCM severity, feature selection was performed using elastic net logistic regression with the “glmnet” and “caret” packages [25, 26]. Age and sex were additionally included as candidate predictors. Continuous variables were mean-centred and scaled to one standard deviation. Model hyperparameters were selected using 10-times repeated fivefold cross validation. Boxplots were used to visualise associations of acylcarnitines with HCM severity. Associations were tested using Mann Whitney U and Kruskal–Wallis tests.
Acylcarnitines were correlated to echocardiographic parameters using linear regression. Multivariable linear regression was used to correct for age, sex and body surface area (BSA). Logarithmic transformations were explored to improve model fit, specifically exploring potential deviations from a standard normal distribution, linearity or homoscedasticity. Missing values in BSA and echocardiographic parameters were imputed using multiple imputation by chained equations [27]. Based on Von Hippol’s two-stage calculation, 125 imputations were used [28].
Sensitivity analyses were performed stratified by HF and SRT, using the composite endpoint for severe HCM without the MLVWT ≥ 20 mm endpoint, using a complete-case analysis, removing participants with missing data instead of imputing these values, and excluding subjects with SRT from linear regression analysis for MLVWT.
In this exploratory analysis, p-values < 0.05 were considered statistically significant. All analyses were performed in R version 4.1.2 (R Development Core Team, 2020).
Results
A total of 124 subjects were included. Severe HCM was identified in 59 subjects (47.6%). Specifically, 47 subjects had a documented MLVWT ≥ 20 mm, 10 underwent SRT, 11 experienced MVA and 27 experienced HF (congestive HF in 14, LVEF < 50% in 16). The overlap between the constituent endpoints is shown in Supplemental Figure 1. Mild HCM was identified in 26 subjects (21.0%). The remaining 39 subjects were G + P- (31.5%). Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics
| G + P- | Mild HCM | Severe HCM | P-value | |
|---|---|---|---|---|
| (n = 39) | (n = 26) | (n = 59) | ||
| Age (years) | 47.3 [32.9, 56.1] | 66.2 [52.8, 69.8] | 56.8 [47.0, 68.6] | 0.001 |
| Male sex | 12 (30.8) | 12 (46.2) | 40 (67.8) | 0.001 |
| Body surface area (m2) | 1.9 [1.8, 2.0] | 1.9 [1.7, 2.0] | 2.0 [1.9, 2.2] | 0.008 |
| Family history of SCD | 11 (28.2) | 16 (61.5) | 22 (38.6) | 0.028 |
| Unexplained non-vasovagal syncope | 1 (2.6) | 3 (11.5) | 11 (19.0) | 0.042 |
| NYHA class III/IV | 0 (0.0) | 0 (0.0) | 8 (20.0) | 0.002 |
| Non-sustained VT | 6 (28.6) | 13 (61.9) | 35 (64.8) | 0.017 |
| MLVWT (mm) | 10 [9, 11] | 14 [13,16] | 20 [17, 23] | < 0.001 |
| LVEF (%) | 60 [60, 64] | 60 [58, 65] | 60 [50, 61] | 0.004 |
| LVOT gradient (mmHg) | 5 [3, 6] | 5 [4, 7] | 5 [4, 12] | 0.22 |
| LAVi (ml/m2) | 26 [22, 29] | 36 [28, 42] | 48 [36, 63] | < 0.001 |
| Atrial fibrillation | 2 (5.1) | 3 (11.5) | 27 (46.6) | < 0.001 |
| Concomitant hypertension | 8 (20.5) | 8 (30.8) | 18 (31.0) | 0.51 |
Data are shown as counts (%), means (standard deviation) or medians [interquartile range]. P-values were determined across all three groups; p-values < 0.05 are shown in bold. G + P- Genotype-positive phenotype-negative; HCM Hypertrophic cardiomyopathy; LAVi Indexed left atrial volume; LVEF Left ventricular ejection fraction; LVOT Left ventricular outflow tract; MLVWT Maximum left ventricular wall thickness; NYHA New York Heart Association; SCD Sudden cardiac death; VT Ventricular tachycardia
Acylcarnitine concentrations are provided in Supplemental Table 1. Correlations between acylcarnitines are shown in Supplemental Figure 2. Correlations ranged from -0.14 to 0.95. The median absolute correlation was 0.26 (interquartile range 0.15–0.39). The median of the strongest absolute correlation per acylcarnitine pair was 0.64 (interquartile range 0.50–0.71), with absolute correlations > 0.8 in six pairs of acylcarnitines (C8-C10, C10-C12:1, C12-C12:1, C12-C14, C12-C14:1, C12:1-C14:1).
Acylcarnitines Associated with HCM Severity
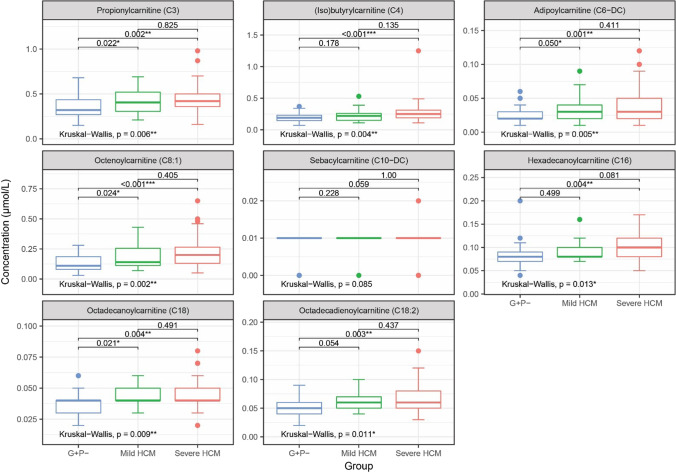
Acylcarnitines C3, C4, C6-DC, C8:1, C16, C18 and sex associated with severe HCM compared to mild HCM and G + P-. Acylcarnitines C3, C6-DC, C8:1, C10-DC, C18, C18:2, sex and age associated with HCM (both mild and severe) compared to G + P-. Odds ratios are provided in Table 2.
Table 2.
Acylcarnitine selection
| Severe HCM vs G + P-/mild HCM |
Mild/severe HCM vs G + P- |
|
|---|---|---|
| Male sex | 2.124 | 1.827 |
| Age | Not selected | 1.371 |
| C3 | 1.001 | 1.224 |
| C4 | 1.075 | Not selected |
| C6-DC | 1.291 | 1.222 |
| C8:1 | 1.095 | 1.311 |
| C10-DC | Not selected | 1.029 |
| C16 | 1.105 | Not selected |
| C18 | 1.028 | 1.108 |
| C18:2 | Not selected | 1.045 |
Odds ratios for the acylcarnitines selected by the elastic net logistic regression models for severe HCM versus G + P-/mild HCM and mild/severe HCM versus G + P-. Continuous variables were scaled and centred. Acylcarnitines C0, C2, C3-DC, C4-DC, C4:3-OH, C5, C5-DC, C5-OH, C5:1, C6, C6-OH, C6:1, C7, C8, C8-DC, C10, C10:1, C10:2, C12, C12-DC, C12-OH, C12:1, C14, C14-OH, C14:1, C14:2, C16-DC, C16-OH, C16:1, C16:1-OH, C18-OH, C18:1, C18:1-DC, C18:1-OH, and C18:2-OH were not selected by either model. G + P- genotype-positive phenotype negative; HCM Hypertrophic cardiomyopathy; vs Versus
Boxplots for the selected acylcarnitines are shown in Fig. 1. Acylcarnitines C3, C4, C6-DC, C8:1, C16, C18 and C18:2 were significantly increased in subjects with severe HCM compared to G + P-. Additionally, C3, C6-DC, C8:1 and C18 were significantly increased in subjects with mild HCM compared to G + P-.
Fig. 1.
Boxplots of selected acylcarnitines. Boxplots showing concentrations of the acylcarnitines selected by elastic net logistic regression, with pairwise and overall p-values from Mann–Whitney U and Kruskal–Wallis tests, respectively. *p < 0.05, **p < 0.01, ***p < 0.001. G + P-, genotype-positive phenotype-negative; HCM, hypertrophic cardiomyopathy
Correlations with Clinical Variables
Results from linear regression analyses are shown in Table 3. In univariable analysis, acylcarnitines C3, C4, C6-DC, C8:1 and C18:2 were significantly correlated with MLVWT. C6-DC additionally correlated with LAVi and LVEF. After correction for age, sex and BSA in multivariable analyses, C6-DC and C8:1 remained significantly correlated with MLVWT, and C6-DC with LVEF.
Table 3.
Correlations of acylcarnitines with clinical variables
| Univariable | log(MLVWT) | log(LAVi) | log(LVEF) | |||
| Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | |
| log(C3) | 0.251 | 0.010 | 0.219 | 0.161 | -0.036 | 0.509 |
| log(C4) | 0.208 | 0.015 | 0.263 | 0.463 | -0.067 | 0.117 |
| C6-DC | 3.61 | 0.024 | 5.88 | 0.024 | -2.35 | 0.002 |
| C8:1 | 0.823 | 0.007 | 0.497 | 0.313 | -0.224 | 0.140 |
| C10-DC | 19.3 | 0.086 | 12.5 | 0.445 | -9.11 | 0.094 |
| log(C16) | 0.155 | 0.215 | 0.209 | 0.272 | -0.129 | 0.043 |
| log(C18) | 0.125 | 0.316 | 0.141 | 0.513 | -0.035 | 0.592 |
| log(C18:2) | 0.199 | 0.043 | 0.097 | 0.515 | -0.111 | 0.024 |
| Multivariable | log(MLVWT) | log(LAVi) | log(LVEF) | |||
| Adjusted coefficient | P-value | Adjusted coefficient | P-value | Adjusted coefficient | P-value | |
| log(C3) | 0.124 | 0.223 | 0.033 | 0.835 | 0.029 | 0.582 |
| log(C4) | 0.129 | 0.141 | 0.125 | 0.366 | -0.024 | 0.575 |
| C6-DC | 5.01 | 0.005 | 5.13 | 0.054 | -2.50 | 0.004 |
| C8:1 | 0.803 | 0.007 | 0.178 | 0.697 | -0.158 | 0.293 |
| C10-DC | 12.4 | 0.257 | 2.79 | 0.862 | -5.99 | 0.256 |
| log(C16) | 0.060 | 0.629 | 0.040 | 0.842 | -0.083 | 0.181 |
| log(C18) | -0.009 | 0.946 | -0.118 | 0.621 | 0.043 | 0.497 |
| log(C18:2) | 0.096 | 0.359 | -0.077 | 0.633 | -0.064 | 0.201 |
Results from univariable and multivariable linear regression (adjusted for age, sex and body surface area). P-values < 0.05 are shown in bold. LAVi Indexed left atrial volume; LVEF Left ventricular ejection fraction; LVOT Left ventricular outflow gradient; MLVWT Maximum wall thickness
Sensitivity Analyses
Subject characteristics stratified by HF are shown in Supplemental Table 2, and analyses stratified by HF are shown in Supplemental Table 3. Acylcarnitines C3, C4 and C6-DC remained associated with severe HCM (versus mild HCM/G + P-) in the non-HF stratum, and C3, C6-DC, C8:1, C10-DC, C18 and C18:2 all remained associated with HCM (mild and severe together versus G + P-) in the non-HF stratum.
Subject characteristics stratified by SRT are shown in Supplemental Table 4, and analyses stratified by SRT are shown in Supplemental Table 5. Acylcarnitines C3, C4, C6-DC, C16 and C18 remained associated with severe HCM in the non-SRT stratum, and C3, C6-DC, C8:1, C10-DC, C18 and C18:2 all remained associated with HCM in the non-SRT stratum.
Subject characteristics stratified by severe HCM without the MLVWT ≥ 20 mm endpoint are shown in Supplemental Table 6. Results of elastic net analysis are shown in Supplemental Table 7, with sex and acylcarnitines C6-DC, C8:1 and C16 remaining associated with the outcome.
As shown in Supplemental Table 8, point estimates were similarly directed in linear regression analyses using complete-case analysis. In multivariable analyses, C8:1 remained significantly correlated to MLVWT (p = 0.023) and C6-DC to LVEF (p = 0.003). Likewise, point estimates for MLVWT remained similar after exclusion of subjects with SRT, as shown in Supplemental Table 9. In multivariable analysis, C6-DC remained significantly correlated to MLVWT (p = 0.004).
Discussion
In order to explore potential biomarkers for HCM development and/or progression, we determined plasma acylcarnitine profiles in 124 carriers of MYBPC3 founder variants, including 59 severe HCM patients, 26 with mild HCM and 39 G + P- individuals. We identified profiles encompassing eight acylcarnitines that associated with HCM severity. Seven of these acylcarnitines (C3, C4, C6-DC, C8:1, C16, C18 and C18:2) were significantly increased in severe HCM compared to G + P, and four (C3, C6-DC, C8:1 and C18) were additionally increased in mild HCM compared to G + P-. After correction for age, sex and BSA, C6-DC was significantly correlated with MLVWT and LVEF and C8:1 with MLVWT.
Fatty Acid Oxidation and Metabolic Treatment in HCM
Impaired energy metabolism has been proposed as a key pathomechanism in HCM [13]. HCM-causing genetic variants can lead to reductions in the energy-conserving super relaxed myosin state, which induces changes in cardiac metabolism [29, 30]. Particularly, cardiomyocytes shift away from fatty acid oxidation to utilise other energy sources [14–16].
Currently, two fatty acid metabolism modulators are being studied as treatment of HCM. The METAL-HCM phase 2 trial assessed perhexiline, a mitochondrial carnitine palmitoyltransferase-1 inhibitor, in symptomatic HCM patients, showing energetic and modest functional improvement [31]. However, further studies were delayed due to concerns regarding systemic toxicity and a phase 2 trial in patients with HCM and HF with preserved ejection fraction (NCT02862600) was terminated early due to lack of efficacy. RESOLVE-HCM, a phase 2 trial assessing the effects of perhexiline on MLVWT, is currently recruiting [32]. Trimetazidine, a mitochondrial long-chain 3‑ketoacyl coenzyme A thiolase inhibitor, was associated with a decline in functional parameters in a phase 2 trial on symptomatic patients with non-obstructive HCM [33]. The ENERGY trial, assessing whether trimetazidine may restore cardiac efficiency in G + P-, is currently underway [34].
Additionally, the cardiac myosin inhibitor mavacamten reduced LVOTO, symptoms and guideline-eligibility for SRT in obstructive HCM in two phase 3 trials, and was well-tolerated in a phase 2 trial in symptomatic non-obstructive HCM [35–37]. Another myosin inhibitor, aficamten, was shown to reduce LVOTO in patients with obstructive HCM a phase 2 trial [38]. These drugs likely ameliorate metabolic dysregulation in HCM by promoting the super-relaxed state of myosin and thereby reducing energetic demand, as treatment with mavacamten restored cellular oxygen consumption rates in induced pluripotent stem cell-derived cardiomyocytes carrying HCM-causing MYH7 missense variants, and improved the number of dysregulated genes encoding mitochondrial proteins in mouse models of HCM [30, 39, 40].
Myocardial and Circulating Acylcarnitines in HCM
Three multi-omics studies comparing tissue obtained from HCM patients undergoing septal myectomy to non-failing donor hearts confirmed changes in fatty acid oxidation [14–16]. Myocardial acylcarnitines were studied in two of these. Acylcarnitines transport acyl groups, primarily derived from fatty acids, from the cytosol into mitochondria for beta-oxidation [15]. Consistent with the changes in fatty acid oxidation, almost all of the determined acylcarnitines were significantly decreased [15, 16].
Several untargeted metabolomics studies suggested that the differences in myocardial acylcarnitines were mirrored in circulating acylcarnitines in HCM patients. Shimada et al. identified various acylcarnitines among the most important metabolites in plasma that discriminated exercise response between age-, sex- and body mass index-matched patients with HCM, left ventricular hypertrophy secondary to hypertension and other cardiovascular diseases [20]. Schuldt et al. found serum C5-DC among the most important metabolites in discriminating patients with LVOTO from unmatched asymptomatic carriers of HCM-associated genetic variants, with higher C5-DC in patients with LVOTO [21]. In our previous study, acylcarnitines C8:1, C16:2 and C20 were identified among the top metabolites in plasma to discriminate between severe HCM patients and age- and sex-matched patients with mild HCM and G + P-, with higher C8:1 and C20 and lower C16:2 in severely affected patients [22]. However, these studies used case–control designs and non-quantitative methods, therefore still requiring confirmation in studies with more robust designs using quantitative methods.
Nakamura et al. quantified acylcarnitines C0 and C2 in serum of HCM patients, showing that C0 was significantly increased and C2 significantly decreased compared to subjects with left ventricular hypertrophy secondary to hypertension and healthy controls [41, 42]. In HCM patients, C0 correlated to myocardial fatty acid metabolism assessed by [123I]-β-Methyl iodophenyl-pentadecanoic acid-imaging, which was significantly reduced compared to either control group.
In the present study, we performed a more comprehensive quantitative assessment of acylcarnitines using LC–MS, in a larger, genetically homogeneous cohort of HCM patients including G + P- relatives. This revealed several additional acylcarnitines associated with HCM severity. Consistent with the studies in myocardium, this included acylcarnitines of various acyl-chain lengths. The mechanisms connecting decreased myocardial levels of acylcarnitines to increased plasma levels remain unclear, however similarly directed changes in myocardial and plasma acylcarnitines have been described in dilated cardiomyopathy [43, 44].
The acylcarnitines identified in this study retained their associations with HCM after stratification for HF and SRT. However, the associations of C8:1, C16 and C18 with severe HCM were no longer observed after stratification for HF, suggesting that these associations were driven by HF. Additionally, we explored correlations with echocardiographic parameters for HCM, identifying correlations of C6-DC with MLVWT and LVEF and C8:1 with MLVWT independent of age, sex and BSA. Based on our data, acylcarnitines appear to be promising biomarkers for HCM severity.
Study Limitations
Despite this being the largest study to assess circulating acylcarnitines in HCM, including G + P-, thus far, sample size was still insufficient to correct for additional potential confounders, including diabetes mellitus, renal function and medication usage. Additionally, this study included a relatively large number of subjects with end-stage HCM, characterised by HF, and subjects with previous SRT, which may limit generalisability. Previous positron emission tomography suggested attenuated myocardial energy demands after alcohol septal ablation, however effects of SRT on acylcarnitines have not yet been studied [45, 46]. In our study, most associations of acylcarnitines with HCM and many of the associations with severe HCM remained after stratification for HF and SRT, as well as after removing the MLVWT endpoint from the composite endpoint for severe HCM, suggesting robust associations with HCM severity.
The cross-sectional design of this study limits inference. Multiple testing may have resulted in type I error. Large prospective cohort studies are required to confirm our findings and assess the prognostic value of acylcarnitines in predicting specific clinical effects, on top of known predictors and other potentially predictive circulating biomarkers, such as natriuretic peptides, troponins, high-sensitivity C-reactive protein and uric acid [47].
Furthermore, our study cannot exclude extracardiac production as the source of the perturbed acylcarnitines, as we did not acquire tissue samples or invasive measurements. However, the heart was previously identified as the main contributor to plasma concentrations of acylcarnitines, particularly medium- and long-chain acylcarnitines (C6-C20), and six out of the eight acylcarnitines indicated by our study were previously shown to be significantly different in myocardium of HCM patients compared to healthy donor hearts [15, 17, 19]. Additionally, samples were obtained under non-fasting conditions, while a fasted state is likely more appropriate to assess myocardial mitochondrial dysfunction [17]. However, biomarkers should ideally remain predictive regardless of sampling conditions, and our data suggest acylcarnitines are associated to HCM severity when obtained in non-fasting conditions.
Conclusion
The identification of HCM biomarkers is crucial to improve individualised risk prediction and thereby treatment of patients with this clinically heterogeneous disease. In this study of 124 carriers of MYBPC3 founder variants across the phenotypic spectrum of HCM, profiles of eight circulating acylcarnitines were associated with HCM severity. Seven acylcarnitines (C3, C4, C6-DC, C8:1, C16, C18 and C18:2) were significantly increased in severe HCM compared to G + P, and four (C3, C6-DC, C8:1 and C18) were additionally increased in mild HCM compared to G + P-. C6-DC was significantly correlated with MLVWT and LVEF and C8:1 with MLVWT, after correction for age and sex. Acylcarnitines appear to be promising biomarkers for HCM severity, however further studies are required to assess their prognostic value.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the subjects for their participation.
Abbreviations
- G + P-
Genotype-positive, phenotype-negative
- HCM
Hypertrophic cardiomyopathy
- HF
Heart failure
- LAVi
Indexed left atrial volume
- LC-MS
Liquid chromatography-mass spectrometry
- LVEF
Left ventricular ejection fraction
- LVOTO
Left ventricular outflow tract obstruction
- MLVWT
Maximum left ventricular wall thickness
- MVA
Malignant ventricular arrhythmia
- SRT
Septal reduction therapy
Funding
This work was supported by the Netherlands Cardiovascular Research Initiative with the support of the Dutch Heart Foundation (CVON2014-40 DOSIS, Dutch Cardiovascular Alliance 2020B005 DoubleDose to M.J., A.F.B, F.W.A., J.P.v.T., R.A.d.B. and J.v.d.V.; CVON2015-12 e-Detect to F.W.A, J.P.v.T. and I.C.), the Dutch Heart Foundation (Dekker 2015T041 to A.F.B. and M.J.), University College London Hospitals National Institute for Health and Care Research Biomedical Research Centre (to F.W.A.), and British Heart Foundation (PG/18/5033837, PG/22/10989 and University College London/British Heart Foundation Research Accelerator AA/18/6/34223 to A.F.S.).
Data Availability
Data are available upon request from the authors.
Declarations
Human Subjects/Informed Consent Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. This study was approved by the institutional review board of the UMC Utrecht. Informed consent was obtained from all patients for being included in the study. No animal studies were carried out by the authors for this article.
Disclosures
No conflicts of interest relevant to this manuscript.
Footnotes
M. Jansen, A.F. Schmidt, J.J.M. Jans, S.N. van der Crabben, D. Dooijes, R.H. Lekanne Deprez, A.A.M. Wilde, R.A. de Boer, J.P. van Tintelen, F.W. Asselbergs and A.F. Baas are Members of the European Reference Network for rare, low prevalence and complex diseases of the heart: ERN GUARD-Heart
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 2.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2020;142:e558–e631. doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 3.Tseng ZH, Olgin JE, Vittinghoff E, et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD sTUDY. Circulation. 2018;137:2689–2700. doi: 10.1161/CIRCULATIONAHA.117.033427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron BJ, Rowin EJ, Casey SA, et al. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol. 2015;65:1915–1928. doi: 10.1016/j.jacc.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 5.Marstrand P, Han L, Day SM, et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe registry. Circulation. 2020;141:1371–1383. doi: 10.1161/CIRCULATIONAHA.119.044366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiaans I, Birnie E, van Langen IM, et al. The yield of risk stratification for sudden cardiac death in hypertrophic cardiomyopathy myosin-binding protein C gene mutation carriers: focus on predictive screening. Eur Heart J. 2010;31:842–848. doi: 10.1093/eurheartj/ehp539. [DOI] [PubMed] [Google Scholar]
- 7.Richard P, Charron P, Carrier L, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 8.van Velzen HG, Schinkel AFL, Baart SJ, et al. Outcomes of contemporary family screening in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2018;11:e001896. doi: 10.1161/CIRCGEN.117.001896. [DOI] [PubMed] [Google Scholar]
- 9.Butters A, Bagnall RD, Ingles J. Revisiting the diagnostic yield of hypertrophic cardiomyopathy genetic testing. Circ Genom Precis Med. 2020;13:e002930. doi: 10.1161/CIRCGEN.120.002930. [DOI] [PubMed] [Google Scholar]
- 10.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- 11.Christiaans I, Nannenberg EA, Dooijes D, et al. Founder mutations in hypertrophic cardiomyopathy patients in the Netherlands. Neth Heart J. 2010;18:248–254. doi: 10.1007/BF03091771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk SJ, Dooijes D, dos Remedios C, et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119:1473–1483. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 13.van der Velden J, Tocchetti CG, Varricchi G, et al. Metabolic changes in hypertrophic cardiomyopathies: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc Res. 2018;114:1273–1280. doi: 10.1093/cvr/cvy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei J, Schuldt M, Nagyova E, et al. Multi-omics integration identifies key upstream regulators of pathomechanisms in hypertrophic cardiomyopathy due to truncating MYBPC3 mutations. Clin Epigenetics. 2021;13:61. doi: 10.1186/s13148-021-01043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranjbarvaziri S, Kooiker KB, Ellenberger M, et al. Altered cardiac energetics and mitochondrial dysfunction in hypertrophic cardiomyopathy. Circulation. 2021;144:1714–1731. doi: 10.1161/CIRCULATIONAHA.121.053575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Previs MJ, O'Leary TS, Morley MP, et al. Defects in the proteome and metabolome in human hypertrophic cardiomyopathy. Circ Heart Fail. 2022;15:e009521. doi: 10.1161/CIRCHEARTFAILURE.121.009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dambrova M, Makrecka-Kuka M, Kuka J, et al. Acylcarnitines: nomenclature, biomarkers, therapeutic potential, drug targets, and clinical trials. Pharmacol Rev. 2022;74:506–551. doi: 10.1124/pharmrev.121.000408. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz M, Labarthe F, Fortier A, et al. Circulating acylcarnitine profile in human heart failure: a surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am J Physiol Heart Circ Physiol. 2017;313:H768–h781. doi: 10.1152/ajpheart.00820.2016. [DOI] [PubMed] [Google Scholar]
- 19.Makrecka-Kuka M, Sevostjanovs E, Vilks K, et al. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci Rep. 2017;7:17528. doi: 10.1038/s41598-017-17797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada YJ, Batra J, Kochav SM, et al. Difference in metabolomic response to exercise between patients with and without hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2021;14:246–255. doi: 10.1007/s12265-020-10051-2. [DOI] [PubMed] [Google Scholar]
- 21.Schuldt M, van Driel B, Algül S, et al. Distinct metabolomic signatures in preclinical and obstructive hypertrophic cardiomyopathy. Cells. 2021;10:2950. doi: 10.3390/cells10112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen M, Schuldt M, van Driel BO, et al. Untargeted metabolomics identifies potential hypertrophic cardiomyopathy biomarkers in carriers of MYBPC3 founder variants. Int J Mol Sci. 2023;24:4031. doi: 10.3390/ijms24044031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen M, Christiaans I, van der Crabben SN, et al. BIO FOr CARE: biomarkers of hypertrophic cardiomyopathy development and progression in carriers of Dutch founder truncating MYBPC3 variants-design and status. Neth Heart J. 2021;29:318–329. doi: 10.1007/s12471-021-01539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Sain-van der Velden MG, Diekman EF, Jans JJ, et al. Differences between acylcarnitine profiles in plasma and bloodspots. Mol Genet Metab. 2013;110:116–21. doi: 10.1016/j.ymgme.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28:26. [Google Scholar]
- 27.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Soft. 2011;45:1–67. [Google Scholar]
- 28.von Hippel PT. How many imputations do you need? A two-stage calculation using a quadratic rule. Sociol Methods Res. 2020;49:699–718. doi: 10.1177/0049124117747303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamara JW, Li A, Lal S, et al. MYBPC3 mutations are associated with a reduced super-relaxed state in patients with hypertrophic cardiomyopathy. PLoS ONE. 2017;12:e0180064. doi: 10.1371/journal.pone.0180064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toepfer CN, Garfinkel AC, Venturini G, et al. Myosin sequestration regulates sarcomere function, cardiomyocyte energetics, and metabolism, informing the pathogenesis of hypertrophic cardiomyopathy. Circulation. 2020;141:828–842. doi: 10.1161/CIRCULATIONAHA.119.042339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abozguia K, Elliott P, McKenna W, et al. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122:1562–1569. doi: 10.1161/CIRCULATIONAHA.109.934059. [DOI] [PubMed] [Google Scholar]
- 32.Ananthakrishna R, Lee SL, Foote J, et al. Randomized controlled trial of perhexiline on regression of left ventricular hypertrophy in patients with symptomatic hypertrophic cardiomyopathy (RESOLVE-HCM trial) Am Heart J. 2021;240:101–113. doi: 10.1016/j.ahj.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Coats CJ, Pavlou M, Watkinson OT, et al. Effect of trimetazidine dihydrochloride therapy on exercise capacity in patients with nonobstructive hypertrophic cardiomyopathy: a randomized clinical trial. JAMA Cardiol. 2019;4:230–235. doi: 10.1001/jamacardio.2018.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Driel BO, van Rossum AC, Michels M, Huurman R, van der Velden J. Extra energy for hearts with a genetic defect: ENERGY trial. Neth Heart J. 2019;27:200–205. doi: 10.1007/s12471-019-1239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivotto I, Oreziak A, Barriales-Villa R, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X. [DOI] [PubMed] [Google Scholar]
- 36.Desai MY, Owens A, Geske JB, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol. 2022;80:95–108. doi: 10.1016/j.jacc.2022.04.048. [DOI] [PubMed] [Google Scholar]
- 37.Ho CY, Mealiffe ME, Bach RG, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;75:2649–2660. doi: 10.1016/j.jacc.2020.03.064. [DOI] [PubMed] [Google Scholar]
- 38.Maron MS, Masri A, Choudhury L, et al. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2023;81:34–45. doi: 10.1016/j.jacc.2022.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Anderson RL, Trivedi DV, Sarkar SS, et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115:E8143–e8152. doi: 10.1073/pnas.1809540115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green EM, Wakimoto H, Anderson RL, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351:617–621. doi: 10.1126/science.aad3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T, Sugihara H, Kinoshita N, et al. Serum carnitine concentrations in patients with idiopathic hypertrophic cardiomyopathy: relationship with impaired myocardial fatty acid metabolism. Clin Sci (Lond) 1999;97:493–501. [PubMed] [Google Scholar]
- 42.Nakamura T, Sugihara H, Kinoshita N, Yoneyama S, Azuma A, Nakagawa M. Can serum carnitine levels distinguish hypertrophic cardiomyopathy from hypertensive hearts? Hypertension. 2000;36:215–219. doi: 10.1161/01.hyp.36.2.215. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Parker BL, Pearson E, et al. Core functional nodes and sex-specific pathways in human ischaemic and dilated cardiomyopathy. Nat Commun. 2020;11:2843. doi: 10.1038/s41467-020-16584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verdonschot JAJ, Wang P, Van Bilsen M, et al. Metabolic profiling associates with disease severity in nonischemic dilated cardiomyopathy. J Card Fail. 2020;26:212–222. doi: 10.1016/j.cardfail.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Timmer SA, Knaapen P, Germans T, et al. Effects of alcohol septal ablation on coronary microvascular function and myocardial energetics in hypertrophic obstructive cardiomyopathy. Am J Physiol Heart Circ Physiol. 2011;301:H129–H137. doi: 10.1152/ajpheart.00077.2011. [DOI] [PubMed] [Google Scholar]
- 46.Aoyama R, Takano H, Kobayashi Y, et al. Evaluation of myocardial glucose metabolism in hypertrophic cardiomyopathy using 18F-fluorodeoxyglucose positron emission tomography. PLoS ONE. 2017;12:e0188479. doi: 10.1371/journal.pone.0188479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansen M, Algül S, Bosman LP, et al. Blood-based biomarkers for the prediction of hypertrophic cardiomyopathy prognosis: a systematic review and meta-analysis. ESC Heart Fail. 2022;9:3418–3434. doi: 10.1002/ehf2.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the authors.