Abstract
The worldwide impressive growth of metabolic disorders observed in the last decades, especially type 2 diabetes mellitus and obesity, has generated great interest in the potential benefits of early identification and management of patients at risk. In this view, prediabetes represents a high-risk condition for the development of type 2 diabetes mellitus and cardiovascular diseases, and an ideal target to intercept patients before they develop type 2 diabetes gaining a prominent role even in international guidelines. For prediabetic individuals, lifestyle modification is the cornerstone of diabetes prevention, with evidence of about 50% relative risk reduction. Accumulating data also show potential benefits from pharmacotherapy. In this context, the only available data pertain to metformin as a pharmaceutical drug and vitamin D and l-arginine as nutraceuticals. l-arginine appears to be a very interesting tool in the clinical management of patients with pre-diabetes. In this review we summarize the current knowledge on the role of l-arginine in prediabetes as a potentially useful preventive strategy against the progression to type 2 diabetes, with a particular focus on the underlying molecular mechanisms and the past and ongoing trials. In this article we also report the interesting data about the perception of the prediabetic condition and its therapeutic management in the clinical practice in Italy. An early identification and a prompt management of people with prediabetes appears to be of paramount importance to prevent the progression to diabetes and avoid its cardiovascular consequences.
Keywords: Prediabetes, l-Arginine, Type 2 diabetes mellitus, Cardiovascular risk factors, Cardiovascular disease, Cellular recovery, Endothelial function
Importance of Prediabetes: Definition and Therapeutic Perspectives
The worldwide impressive growth of metabolic disorders observed in the last decades, especially type 2 diabetes mellitus and obesity, has generated great interest in the potential benefits of early identification and management of patients at risk. Patients with type 2 diabetes are at increased risk of developing cardiovascular disease (CVD) such as coronary artery disease (CAD), heart failure (HF), atrial fibrillation (AF), stroke and aortic and peripheral artery diseases. Moreover, diabetes is a major risk factor for developing chronic kidney disease (CKD) [1].
In this setting, prediabetes represents an ideal target for early identification of people at high-risk for the development of type 2 diabetes and cardiovascular diseases and has gained a prominent role even in the most recent international guidelines. [2]. The World Health Organization (WHO) defines prediabetes as fasting glucose levels ranging between 6.1 and 6.9 mmol/L (110–125 mg/dL) with levels below 6.1 mmol/L (< 110 mg/dL) regarded as normal [3, 4]. However, the American Diabetes Association (ADA) has adopted more stringent criteria, considering prediabetes glucose levels 5.6–6.9 mmol/L (100–125 mg/dL) and only patients with glucose < 5.6 mmol/L (< 100 mg/dL) classified as having normal glucose metabolism [3].
Five to 10 percent (5–10%) of people per year with prediabetes will progress to diabetes, with approximately the same proportion converting back to normoglycaemia [5]. Prevalence of prediabetes is increasing worldwide and experts have projected that more than 470 million people will have prediabetes by 2030 [2].
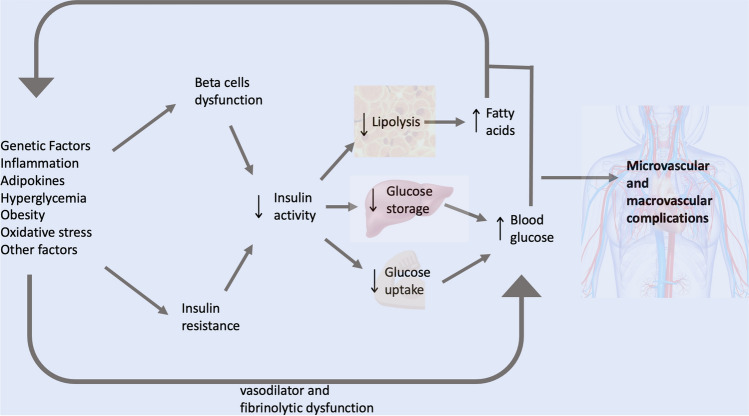
Individuals with prediabetes have usually already lost 30–40% of the volume of pancreatic beta cells, an alteration associated with insulin resistance progression and an increased demand for insulin production, leading to elevated circulating concentrations of intact proinsulin [6, 7]. This is indicative of reduced enzymatic cleavage capacity within intracellular processes. During this phase, pancreatic beta cells are particularly exposed to oxidative stress due to both increased production of oxygen-free radicals and reduced antioxidant production [8, 9]. This situation heavily interferes with intracellular signalling, leading to dysfunction and subsequent disruption of the beta cell [10].
Hyperglycemia, insulin resistance, inflammation, and metabolic dysfunctions lead to endothelial vasodilator and fibrinolytic dysfunction, determining an increased risk of microvascular and macrovascular complications [11–14]. The microvasculature influences insulin sensitivity by controlling the delivery of insulin and glucose to skeletal muscle and endothelial dysfunction and remodelling of the extracellular matrix (ECM) promoting the progression from normoglycemia to prediabetes and then to diabetes, suggesting so a continuum within a single pathophysiological process [14].
Prediabetes has also been associated with increased risks of cancer and dementia [14]. Several studies have demonstrated that prediabetic patients can be affected by coronary artery disease (CAD) and heart failure with preserved ejection fraction (HFpEF) even before progressing to overt diabetes [11]. Macrovascular complications are the major contributor to diabetes-related health care costs, and prediabetes contributes substantially to this expense. The main pathophysiological aspects of prediabetes are summarized in Fig. 1.
Fig. 1.
Pathophysiological aspects of prediabetes
Therefore, prediabetes beside its role as a major component of the “so called” metabolic syndrome represents a major but silent driver leading to overt diabetes and clinicians need to become aware of the key importance of recognizing and intercepting prediabetes and overcome any hesitance to initiate effective therapeutic measures.
For prediabetic individuals, lifestyle modifications represent the first intervention and the cornerstone of diabetes prevention, with evidence of about 50% relative risk reduction [15]. Accumulating data also show potential benefits from pharmacotherapy. In this context, the only available data pertain to metformin as a pharmaceutical drug and vitamin D and l-arginine as nutraceuticals. Regarding vitamin D, several studies have been conducted, and recently, two meta-analyses were published, demonstrating a reduction in the development of new-onset diabetes in individuals with prediabetes treated with vitamin D supplementation. However, these meta-analysis were not designed to assess the tolerability of this therapeutic strategy, and therefore FDA issued a cautionary note advising against exceeding 4000 IU/day of vitamin D [16, 17]. So, very high doses of vitamin D therapy might prevent diabetes in some patients but it may also cause harm [16]. Also Chromium supplementation has been shown to exert some beneficial effects in Type 2 Diabetes and a prevalence of Chromium deficiency has been reported in prediabetic patients [18]. Regarding metformin, many studies demonstrate that higher (850 mg twice daily) and lower (250 mg twice or three times daily) dosages of this drug decrease the rate of transition from prediabetes to diabetes [18–21]. However, metformin is not FDA and EMA-approved for prediabetes [22] and it can be prescribed only off-label for this condition. Moreover, metformin has some adverse side effects that cannot be overlooked [23].
In this context, l-arginine, a well-tolerated and economic molecule involved in various metabolic processes and a substrate for nitric oxide synthase enzymes that generate nitric oxide, as well as a key molecule in endothelial function and insulin sensitivity, has been proposed as a novel therapeutic option for the prevention of the development of diabetes in prediabetic patients.
In Table 1 we resume all the possible treatments for pre-diabetes and their pro and cons.
Table 1.
Potential treatment interventions in pre-diabetes.
| Related trials | Side effects | Recommendation | |
|---|---|---|---|
| Metformin | Performed | Reported | Present |
| Vitamin D | Not Performed | ReportedPresent | Present |
| Chromium | Not Performed | Not reported | Present |
| l-arginine | Performed | Not reported | Absent |
Our review aims to summarize the current literature on the role of l-arginine in prediabetes as a therapeutic agent for this condition and as a preventive strategy against progression to diabetes. The review explores l-arginine pharmacodynamics, pharmacokinetic aspects, and molecular mechanisms in prediabetes and past or ongoing trials about the role of this molecule in prediabetes.
In this review we also discuss the relatively low perception of prediabetes in different clinical practice settings. For this purpose, we considered particularly appropriate to include the results of a recent nationwide survey which was promoted by the Italian Society of Cardiovascular Prevention (SIPREC) to investigate the perception among Italian physicians about the prediabetic condition and its therapeutic management.
Methods
We comprehensively searched the literature for data on the role of l-arginine in the treatment of prediabetes. Particularly, we investigated its role in prediabetes for diabetes prevention. We also explored the perception among physicians about prediabetes and its therapeutic management.
We used “prediabetes” or “l-Arginine”, “type 2 diabetes mellitus”, “cardiovascular risk factors”, “cardiovascular disease”, “endothelial protection” as search terms.
Articles published from 1968 to 1st October 2023 in English on both PubMed and MEDLINE were included. Most recent and largest original articles and meta-analyses were selected. Reviews, consensus papers and guidelines were included if relevant. A search across the references of the selected reports helped to identify further additional relevant studies.
Pharmacodynamic and Pharmacokinetic of l-arginine and Its Potential Molecular Mechanisms Contributing to Prevent Development of Diabetes
l-arginine (2-Amino-5-guanidinovaleric acid-Arg) is a conditionally essential amino-acid, that is mainly formed in the urea cycle [24]. Dietary l-arginine is absorbed in the small intestine and transported to the liver. A small part of dietary l-arginine passes through the liver in the systemic circulation [25].
l-arginine plasma concentrations peaked after the 30 min of intravenous infusion and subsequently quickly decrease. Immediately following a 30 g intravenous infusion, about 5 g of l-arginine is eliminated in the urine. Urine excretion of l-arginine occurs only within the first 90 minutes, indicating that this is when the renal threshold for reabsorption is likely to be exceeded.
After an oral dose of 10 g of l-arginine, the peak plasma concentrations occur one hour after dosage [26]. The absolute bioavailability of a single oral 10 g dose of l-arginine is about 20% [26].
l-arginine is well tolerated and both intravenous and oral administrations have no significant effect on the vital signs [27]. Also in special or vulnerable populations, such as pregnant women, preterm infants, and individuals with cystic fibrosis, no severe adverse reactions after administration of l-arginine were reported in the literature [28].
l-arginine is involved in the synthesis of creatine, l-Ornithine, l-Glutamate, collagen, polyamines, and agmatine[29]. l-Arginine improves oxidative metabolism, via an enhanced mitochondrial function, finally promoting a better physical performance[30]. l-Arginine promotes the secretion of growth hormone from the pituitary gland [31] and is involved in T-cell proliferation enhancing the immune responses[32]. l-arginine is a precursor of nitric oxide (NO), an ubiquitous mediator that is produced by enzymes collectively defined NO synthases [33]. NO is a strong endogenous vasodilator which also has antiplatelet properties and other effects that may contribute to prevent atherogenesis [34, 35]. While impaired NO availability in the endothelium and platelets has been linked to cardiovascular risk factors and ageing [36], experimental and clinical studies have demonstrated that in some of these conditions, the attenuation of vascular and platelet NO activity can be reversed by oral or intravenous administration of l-arginine [37, 38]. Moreover, administration of l-arginine led to an improvement of endothelial function in animal models of atherosclerosis and hypercholesterolemia [33].
l-Arginine has a key role in diabetes and prediabetes; indeed, this molecule is a strong secretagogue of the endocrine system, as it induces insulin and glucagon secretion [39, 40].
In the experimental setting, Dubey et al. [41] showed that l-arginine is effective in lowering plasma glucose levels in rats, improving glucose tolerance.
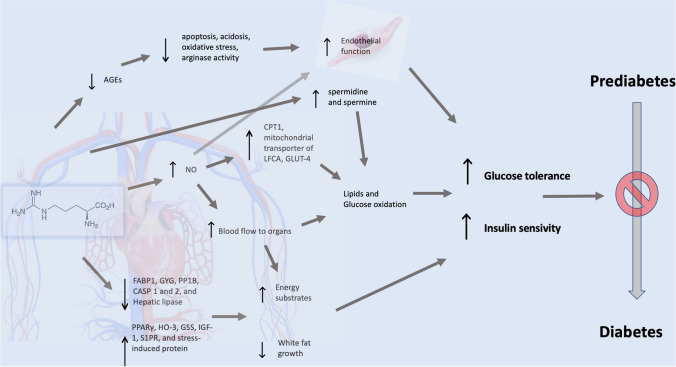
Moreover, l-arginine supplementation was also shown to reduce adiposity and to improve insulin sensitivity both in animal models of obesity and in patients with diabetes and obesity. Jobgen et al. [42] using microarray analysis technique in a rat model showed that dietary arginine supplementation was able to influence the expression of several genes in white adipose tissue, in particular, arginine (Arg) supplementation reduced mRNA levels for fatty acid binding-protein-1, glycogenin, protein-phosphates-1B, caspases 1 and 2, and hepatic lipase, but increased expression of PPARγ, heme-oxygenase-3, glutathione synthetase, insulin-like growth factor II, sphingosine-1-phosphate receptor, and stress-induced protein. To summarize, through these multiple actions of arginine supplementation, arginine can improve energy-substrates and it seems reasonable to suggest that they are improved, and that white fat growth in insulin-sensitive tissues is reduced.
l-arginine through the enhancement of NO synthesis improves blood flow to organs and allows a greater uptake of energy substrates for oxidation to CO2 and water [43]. Furthermore, NO upregulates the activity of carnitine palmitoyl-transferase-1, the mitochondrial transporter of long chain fatty acids, and the expression of glucose-transporter-4 in hepatocytes and skeletal muscle. This may result in increased oxidation of both lipids and glucose through Krebs cycle and electron transport system [44].
l-arginine also forms spermidine and spermine, two molecules that could increase oxidation of lipids and glucose by maintaining integrity and mitochondrial function [45]
Hayde et al. showed that oral high-dose of l-arginine has an immunomodulatory effect that may cause an improved clearance of advanced-stage non-enzymatic glycosylation products, thereby ameliorating glucose tolerance in diabetic patients [46].
Galluccio et al. [47] exposed cultured endothelial cells to pre-treatment with l-arginine for 24 hours, followed by nutritional stress with high concentrations of lipids, insulin, and glucose for the subsequent 24 h, and finally to physiological conditions for an additional 48 hours. Nutritional stress resulted in increased oxidative stress with elevated levels of AGEs (Advanced Glycation End Products) and arginase activity, leading to acidosis and cell death. Pre-treatment with arginine protected the cells by reducing apoptosis, acidosis, oxidative stress, arginase activity, and AGE accumulation. l-arginine pre-treatment reduced AGE production and accumulation by interfering with the expression of the AGE receptor gene and STAB1 gene. The latter effect, by acting as a scavenger of AGE receptors, hindered the binding of AGEs to their receptor, preventing intracellular signalling activation that leads to cellular damage. These protective effects of l-arginine persisted even after discontinuation of pre-treatment.
Overall, these mechanisms could play a contributory role in the prevention of diabetes in prediabetic subjects. The most important molecular mechanisms induced by l-arginine that could prevent diabetes in prediabetic patients are summarized in Fig. 2.
Fig. 2.
l-Arginine’s molecular mechanisms contributing to prevent development of diabetes. In the figure: AGEs Advanced Glycation End products, CASP Caspase, CPT1 Carnitine PalmitoylTransferase 1, FABP1 Fatty Acid Binding Protein 1, GLUT-4 Glucose Transporter Protein Type-4, GSS Glutathione Synthetase, GYG Glycogenin, HO-3 Heme Oxygenase-3, IGF-1 Insuline-like Growth Factor-1, LFCA Long Chain Fatty Acids, NO nitric oxide, PP1B protein phosphatase 1B, PPARy Peroxisome Proliferator-activated Receptor y, SP1R Sphingosine-1-phosphate receptor
l-arginine and Diabetes Prevention: Past and Future Trials
In recent years, the results of many trials investigating the role of l-arginine in the prevention of diabetes have been published. In particular, a first single-center, randomized, double-blind, parallel-group, placebo-controlled, phase III trial conducted on 144 individuals affected by impaired glucose tolerance and metabolic syndrome showed that daily administration of 6.4 g of l-arginine for 18 months compared to placebo increases the cumulative probability to switch to normal glucose tolerant (42.4% and 22.1%, respectively, Hazard Ratio (HR), 2.60; 95% CI 1.51–4.46, p < 0.001). The higher cumulative probability to become glucose tolerant was continued during an extended period (18 months) in patients previously treated with l-arginine (HR, 3.21; 95% CI 1.87–5.51; p < 0.001). [48]. In this study, l-arginine significantly ameliorated insulin sensitivity and beta cells function [48]; however, after 18 months of follow up, l-arginine as compared with placebo was not able to reduce new incidence of diabetes [21.4 and 20.8%, respectively, HR, 1.04; 95% confidence interval (CI), 0.58-1.86]. Interestingly, At the end of a 12- month extended period, the cumulative incidence of diabetes in subjects previously treated with l-arginine was reduced as compared with placebo (27.2% and 47.1%, respectively, HR, 0.42; 95% CI 0.24–0.75, p < 0.05). A subsequent study conducted with a 9-year follow-up on the 104 subjects who did not develop diabetes in the previous trial during the 18-month treatment with l-arginine or placebo showed that at the end of the follow-up, the cumulative incidence of diabetes was 40.6% in the l-arginine-treated group for 18 months and 57.4% in the placebo group, with a HR of 0.66 (p < 0.02). Simultaneously, a statistically significant improvement in the proinsulin/c-peptide ratio and other insulin sensitivity markers was reported. The authors of this study concluded that 18‐month oral L‐arginine treatment decreased diabetes risk over 9 years in middle‐aged subjects with impaired glucose tolerance and metabolic syndrome. [6]
The l-arginine dosage and treatment duration used in these studies were based on the excellent tolerability of this product to ensure a higher likelihood of success. Nonetheless, a study is currently underway to investigate the effects of the administration of a l-Arginine formulation without sucrose, a product specifically designed for patients with glycaemic metabolism disorders, at a reduced dosage (3.2 g/day divided into two administrations) with a planned duration of three months. Based on results obtained in other therapeutic application of l-Arginine this treatment regimen may yield favourable results in glycaemic metabolism while ensuring a better compliance.
Past and future trials on l-Arginine for diabetes prevention are summarized in Table 2.
Table 2.
l-Arginine and diabetes prevention: past and future trials
| Authors | Inclusion criteria | Dosage | Primary endpoint | Follow up | Results: Intervention vs Control |
|---|---|---|---|---|---|
| Monti et al. | Impaired glucose tolerance and metabolic syndrome | 3.2 g twice a day | Diagnosis of diabetes during the 108-month study period | 12 months | 21.4 % vs 20.8% HR 1.04 (95% CI 0.58–1.86]; p < 0.02) |
| Monti et al. | Middle-aged subjects with impaired glucose tolerance and metabolic syndrome | 3.2 g twice a day | Change from Baseline in LVEF at 6 months | 9 years | 40.6 % vs 57.4% HR 0.66 (95% CI 0.48, 0.91; p = 0.91) |
| Endocrinology Unit of IRCCS San Raffaele (Rome, Italy) | 20–70 years old patients with Body Mass Index (BMI) > 25 kg/m2 and pre-diabetes | 1.66 mg twice a day |
Change from baseline in HbA1c, HOMA-IR, HOMA-B. |
90 days | Projected starting date (first-patient-in): January 2024 |
Low Perception and Mis-management of Prediabetes: Results of the PreDiZero survey
The Italian Society of Cardiovascular Prevention (SIPREC) has recently promoted a survey to evaluate the perception among Italian physicians who manage patients with metabolic syndrome regarding the prediabetic condition and its therapeutic management. This cross-sectional nationwide survey was based on a web anonymized questionnaire composed by 10 questions. Five-hundred-thirty-four medical doctors from different regions of Italy were involved. Fifty-three percent of participants were general practitioners, 15% of contributors were dietologists, 13% of physicians were endocrinologists, 6% of participants were cardiologists, 6% were diabetologists while the remaining 7% have others specialty. All participants declared that they usually manage patients with prediabetes in their routine clinical practice. Forty-three percent of these reported that they visit on average more than 30 prediabetic patients in a year. About a half of the participants (47.5 %) reported that they do not usually recommend nutraceuticals in addition to lifestyle and dietary recommendation changes in prediabetic patients and only 25% of these 47.5% of physicians reported that they prescribe l-Arginine as a nutraceutical for the treatments of prediabetes. Overall, the data gathered from this survey indicate a low perception on the importance of prediabetes in their routine practice and suggest that there is probably room to implement the available therapeutic options in the management of patients with prediabetes.
Conclusions
In conclusion, prediabetes is a widespread and growing public health problem. This condition can be considered a manageable risk factor for diabetes and cardiovascular disease. Prediabetes is not a chronic disease but a reversible condition if it is intercepted early and effectively corrected if lifestyle changes are adopted (appropriate diet and physical activity) together with therapeutic strategies implementation. Unfortunately, prediabetic management is currently not systematically focused by physicians, with a limited use all the available therapeutic options.
l-Arginine is a well-tolerated molecule provided with beneficial effects in diabetes prevention when administered to prediabetic patients. l-Arginine can improve energy-substrate oxidation, it can reduce white fat growth in insulin sensitive tissues and oxidative stress, and finally it can ameliorate glucose tolerance. Several past trials have demonstrated that, most likely through these mechanism, l-Arginine may contribute to reduce the development of diabetes or induce regression of prediabetes to normal glucose metabolism. However, further larger studies and randomized controlled clinical trials are required to establish the potential preventive role of l-Arginine in prediabetes.
Acknowledgements
The present work has been endorsed by the Italian Society of Cardiovascular Prevention SIPREC). The Authors wish to thank for their contribution in sharing the preliminary results of the PreDiZero Survey in Italy: Annalisa Giandalia (Messina), Antonio Bossi (Bergamo). Giuseppe Marazzi (Rome), Marcello Orio (Salerno), Giancarlo Guarino (Tuscany), Antonello Diana (Savignano), Rosco Maura (Valenzano), Laura Montroni Monducci (Imola) and Gaetano Piccinocchi (Napoli).
Author contributions
AF and MV developed the concepts and the literature analysis reported in this article and the authors have given final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content and they agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Data Availability
Not applicable.
Declarations
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Conflict of Interest
None of the authors report a conflict of interest to disclose on the content of the present manuscript. Massimo Volpe is President of the Italian Society of Cardiovascular Prevention.
References
- 1.Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44(39). [DOI] [PubMed]
- 2.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet Lond Engl. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19–40. [DOI] [PMC free article] [PubMed]
- 4.Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S. Diagnosis and management of prediabetes: a review. JAMA. 2023;329:1206–1216. doi: 10.1001/jama.2023.4063. [DOI] [PubMed] [Google Scholar]
- 5.Weiner A, Zhang M, Ren S, Tchang B, Gandica R, Murillo J. Progression from prediabetes to type 2 diabetes mellitus in adolescents: a real world experience. Front Clin Diabetes Healthc [Internet]. 2023. Doi: 10.3389/fcdhc.2023.1181729 [DOI] [PMC free article] [PubMed]
- 6.Monti LD, Galluccio E, Villa V, Fontana B, Spadoni S, Piatti PM. Decreased diabetes risk over 9 year after 18-month oral l-arginine treatment in middle-aged subjects with impaired glucose tolerance and metabolic syndrome (extension evaluation of l-arginine study) Eur J Nutr. 2018;57:2805–2817. doi: 10.1007/s00394-017-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do OH, Gunton JE, Gaisano HY, Thorn P. Changes in beta cell function occur in prediabetes and early disease in the Leprdb mouse model of diabetes. Diabetologia. 2016;59:1222–1230. doi: 10.1007/s00125-016-3942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol Off J Pol Physiol Soc. 2019;70:809–24. [DOI] [PubMed]
- 9.Eguchi N, Vaziri ND, Dafoe DC, Ichii H. The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int J Mol Sci. 2021;22:1509. doi: 10.3390/ijms22041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, et al. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes. 2023;14:130–146. doi: 10.4239/wjd.v14.i3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zand A, Ibrahim K, Patham B. Prediabetes: why should we care? Methodist DeBakey Cardiovasc J. 2018;14:289–297. doi: 10.14797/mdcj-14-4-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cefalu WT. “Prediabetes”: are there problems with this label? no, we need heightened awareness of this condition! Diabetes Care. 2016;39:1472–1477. doi: 10.2337/dc16-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med Maywood NJ. 2016;241:1323–1331. doi: 10.1177/1535370216654227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserman DH, Wang TJ, Brown NJ. The VASCULATURE IN PREDIABETES. Circ Res. 2018;122:1135–1150. doi: 10.1161/CIRCRESAHA.118.311912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauguel-Moreau M, Hergault H, Cazabat L, Pépin M, Beauchet A, Aïdan V, et al. Prevalence of prediabetes and undiagnosed diabetes in a large urban middle-aged population: the CARVAR 92 cohort. Cardiovasc Diabetol. 2023;22:31. doi: 10.1186/s12933-023-01761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris E. Meta-analysis: vitamin D therapy reduced type 2 diabetes. JAMA. 2023;329:703. doi: 10.1001/jama.2023.1550. [DOI] [PubMed] [Google Scholar]
- 17.Pittas AG, Kawahara T, Jorde R, Dawson-Hughes B, Vickery EM, Angellotti E, et al. Vitamin D and risk for type 2 diabetes in people with prediabetes : a systematic review and meta-analysis of individual participant data from 3 randomized clinical trials. Ann Intern Med. 2023;176:355–363. doi: 10.7326/M22-3018. [DOI] [PubMed] [Google Scholar]
- 18.Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37:1705–1717. doi: 10.1080/03007995.2021.1955667. [DOI] [PubMed] [Google Scholar]
- 19.Lilly M, Godwin M. Treating prediabetes with metformin. Can Fam Physician. 2009;55:363–369. [PMC free article] [PubMed] [Google Scholar]
- 20.Hostalek U, Gwilt M, Hildemann S. Therapeutic USE OF METFORMIN in prediabetes and diabetes prevention. Drugs. 2015;75:1071–1094. doi: 10.1007/s40265-015-0416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beulens J, Rutters F, Rydén L, Schnell O, Mellbin L, Hart HE, et al. Risk and management of pre-diabetes. Eur J Prev Cardiol. 2019;26:47–54. doi: 10.1177/2047487319880041. [DOI] [PubMed] [Google Scholar]
- 22.Murillo JE, McNeil C, Van Nest K, Schaeffer J, Polingo L, Adjei S, et al. Abstract 9819: real world data: off-label metformin in patients with prediabetes is associated with improved cardiovascular outcomes. Circulation. 2021;144:A9819–A9819. [Google Scholar]
- 23.Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321:1926–1927. doi: 10.1001/jama.2019.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez MJ, Mohiuddin SS. Biochemistry, essential amino acids. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Sep 24]. http://www.ncbi.nlm.nih.gov/books/NBK557845/. Accessed 29 Sept 2023.
- 25.Böger RH. The Pharmacodynamics of L-Arginine123. J Nutr. 2007;137:1650S–1655S. doi: 10.1093/jn/137.6.1650S. [DOI] [PubMed] [Google Scholar]
- 26.Tangphao O, Grossmann M, Chalon S, Hoffman BB, Blaschke TF. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol. 1999;47:261–266. doi: 10.1046/j.1365-2125.1999.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langkamp-Henken B, Herrlinger-Garcia KA, Stechmiller JK, Nickerson-Troy JA, Lewis B, Moffatt L. Arginine supplementation is well tolerated but does not enhance mitogen-induced lymphocyte proliferation in elderly nursing home residents with pressure ulcers. JPEN J Parenter Enteral Nutr. 2000;24:280–287. doi: 10.1177/0148607100024005280. [DOI] [PubMed] [Google Scholar]
- 28.McNeal CJ, Meininger CJ, Reddy D, Wilborn CD, Wu G. Safety and Effectiveness of Arginine in Adults123. J Nutr. 2016;146:2587S–2593S. doi: 10.3945/jn.116.234740. [DOI] [PubMed] [Google Scholar]
- 29.Wu G, Morris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gambardella J, Fiordelisi A, Spigno L, Boldrini L, Lungonelli G, Di Vaia E, et al. Effects of chronic supplementation of L-arginine on physical fitness in water polo players. Oxid Med Cell Longev. 2021;2021:6684568. doi: 10.1155/2021/6684568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alba-Roth J, Müller OA, Schopohl J, von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab. 1988;67:1186–1189. doi: 10.1210/jcem-67-6-1186. [DOI] [PubMed] [Google Scholar]
- 32.Adebayo A, Varzideh F, Wilson S, Gambardella J, Eacobacci M, Jankauskas SS, et al. l-Arginine and COVID-19: An Update. Nutrients. 2021;13:3951. doi: 10.3390/nu13113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Böger RH, Bode-Böger SM. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol. 2001;41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79. [DOI] [PubMed] [Google Scholar]
- 34.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation. Circ Res. 1999;84:210–219. doi: 10.1161/01.RES.84.2.210. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li P-L, et al. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci [Internet]. 2018 [cited 2023 Sep 24];19. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164974/. Accessed 29 Sept 2023. [DOI] [PMC free article] [PubMed]
- 36.Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–333. doi: 10.1016/S0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 37.Wang BY, Candipan RC, Arjomandi M, Hsiun PT, Tsao PS, Cooke JP. Arginine restores nitric oxide activity and inhibits monocyte accumulation after vascular injury in hypercholesterolemic rabbits. J Am Coll Cardiol. 1996;28:1573–1579. doi: 10.1016/S0735-1097(96)00337-3. [DOI] [PubMed] [Google Scholar]
- 38.Hutchison SJ, Reitz MS, Sudhir K, Sievers RE, Zhu BQ, Sun YP, et al. Chronic dietary L-arginine prevents endothelial dysfunction secondary to environmental tobacco smoke in normocholesterolemic rabbits. Hypertens Dallas Tex. 1979;1997(29):1186–1191. doi: 10.1161/01.hyp.29.5.1186. [DOI] [PubMed] [Google Scholar]
- 39.Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969;257:415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Sener A, Lebrun P, Blachier F, Malaisse WJ. Stimulus-secretion coupling of arginine-induced insulin release. Insulinotropic action of agmatine. Biochem Pharmacol. 1989;38:327–330. doi: 10.1016/0006-2952(89)90044-0. [DOI] [PubMed] [Google Scholar]
- 41.Dubey H, Dubey A, Gulati K, Ray A. Protective effects of L-arginine on cognitive deficits and biochemical parameters in an experimental model of type-2 diabetes mellitus induced Alzheimer’s disease in rats. J Physiol Pharmacol Off J Pol Physiol Soc. 2022;73:3–17. [DOI] [PubMed]
- 42.Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, et al. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids. 2009;37:187–198. doi: 10.1007/s00726-009-0246-7. [DOI] [PubMed] [Google Scholar]
- 43.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 44.García-Villafranca J, Guillén A, Castro J. Involvement of nitric oxide/cyclic GMP signaling pathway in the regulation of fatty acid metabolism in rat hepatocytes. Biochem Pharmacol. 2003;65:807–812. doi: 10.1016/S0006-2952(02)01623-4. [DOI] [PubMed] [Google Scholar]
- 45.Madsen KL, Brockway PD, Johnson LR, Hardin JA, Gall DG. Role of ornithine decarboxylase in enterocyte mitochondrial function and integrity. Am J Physiol. 1996;270:G789–797. doi: 10.1152/ajpgi.1996.270.5.G789. [DOI] [PubMed] [Google Scholar]
- 46.Hayde M, Vierhapper H, Lubec B, Popow C, Weninger M, Xi Z, et al. Low-dose dietary L-arginine increases plasma interleukin 1 alpha but not interleukin 1 beta in patients with diabetes mellitus. Cytokine. 1994;6:79–82. doi: 10.1016/1043-4666(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 47.Galluccio E, Spadoni S, Fontana B, Bosi E, Piatti P, Monti LD. Long lasting protective effects of early l-arginine treatment on endothelium in an in vitro study. Clin Nutr Edinb Scotl. 2021;40:1519–1529. doi: 10.1016/j.clnu.2021.02.040. [DOI] [PubMed] [Google Scholar]
- 48.Monti LD, Setola E, Lucotti PCG, Marrocco-Trischitta MM, Comola M, Galluccio E, et al. Effect of a long-term oral l-arginine supplementation on glucose metabolism: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14:893–900. doi: 10.1111/j.1463-1326.2012.01615.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.