Abstract
Introduction
Immunocompromised (IC) adults are at increased risk of developing herpes zoster (HZ) and HZ-related complications due to therapy or underlying disease. This study evaluated the cost effectiveness of recombinant zoster vaccine (RZV) versus no vaccine for the prevention of HZ in hematopoietic stem cell transplant (HSCT) recipients and other IC adults aged ≥ 18 years in the United States (US).
Methods
A static Markov model simulated cohorts of IC individuals using a 1-year cycle length and 30-year time horizon to estimate the cost effectiveness of RZV. Inputs were sourced from clinical trial results and publicly available sources/literature. Modeled populations included US adult HSCT recipients (base case), patients with human immunodeficiency virus (HIV), patients with breast cancer, patients with Hodgkin’s lymphoma, and renal transplant recipients. The model reported societal costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). Sensitivity and threshold analyses were conducted.
Results
In the base case of 19,671 US adult HSCT recipients, RZV resulted in total societal cost savings of US$0.1 million and 109 incremental QALYs versus no vaccine. RZV was a ‘dominant strategy’ versus no vaccine because vaccination resulted in cost savings with QALY gains. RZV was also cost saving in renal transplant recipients, and cost effective at a willingness-to-pay threshold of US$100,000 per QALY gained in patients with HIV, breast cancer, and Hodgkin’s lymphoma, with ICERs of US$33,268, US$67,682, and US$95,972 per QALY gained, respectively, versus no vaccine.
Conclusions
Model results show RZV is potentially cost saving for the prevention of HZ in US adult HSCT recipients and US adults with selected immunocompromising conditions, and cost effective for others, supporting the use of RZV to prevent HZ and HZ-related complications in IC adults.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-023-00438-7.
Key Points for Decision Makers
| In the base case of US adult hematopoietic stem cell transplant recipients, recombinant zoster vaccine was considered a ‘dominant strategy’ versus no vaccine because vaccination resulted in cost savings with quality-adjusted life-year (QALY) gains over 30 years. |
| Analyses of selected immunocompromised adult populations showed that vaccination with recombinant zoster vaccine is likely cost saving (renal transplant recipients) or cost effective (patients with HIV, breast cancer, and Hodgkin’s lymphoma) at a hypothetical willingness-to-pay threshold of US$100,000 per QALY gained. |
Introduction
Almost one out of every three individuals in the United States (US) will develop herpes zoster (HZ) in their lifetime, accounting for 1 million cases each year nationwide [1]. HZ is caused by a reactivation of the varicella-zoster virus, which persists latently in host neurons following initial infection [1]. While older adults are at an elevated risk of HZ due to natural waning of immune function, the incidence and burden of HZ increases sharply in populations that are immunodeficient or immunosuppressed (hereafter referred to collectively as ‘immunocompromised’ [IC]) [2–5]. Individuals may be IC due to disease, such as human immunodeficiency virus (HIV) infection, or due to therapy, such as in hematopoietic stem cell transplant (HSCT) recipients. Approximately 2.7% of US adults, or 6.44 million individuals, are estimated to be IC based on a 2013 study [6, 7]. HZ cases in IC populations also result in greater healthcare resource use and costs than HZ cases in immunocompetent populations in the US [4].
In 2017, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) preferentially recommended recombinant zoster vaccine (RZV, Shingrix) for HZ prevention among all immunocompetent adults aged 50 years and older, including those previously vaccinated with zoster vaccine live (ZVL, Zostavax) [8]. In July 2021, the US Food and Drug Administration (FDA) expanded the indication for RZV to include adults aged 18 years and older who are or will be at increased risk for HZ due to immunodeficiency or immunosuppression caused by known disease or therapy; and in October 2021, the ACIP recommendation for RZV was expanded to include adults aged 19 years and older who are or will be at increased risk for HZ [9, 10].
This study evaluated the cost effectiveness of RZV versus no vaccine when used for the prevention of HZ in US adults aged 18 years and older who are IC due to HSCT or other selected IC conditions, with a focus on adults aged 18–49 years.
Methods
Model Overview
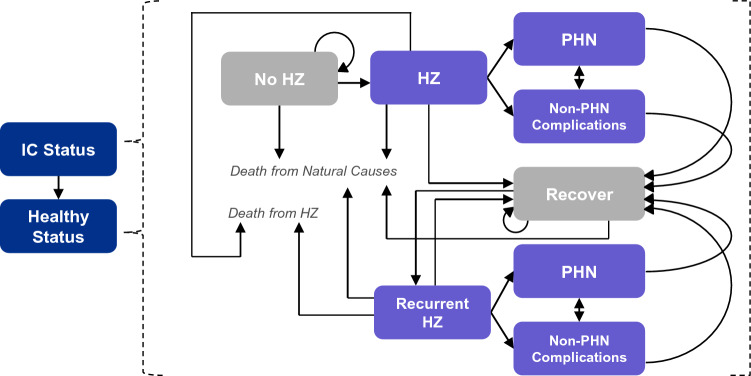
Outcomes in this study were estimated using the ZOster ecoNomic Analysis IC (ZONA IC) Model (not publicly available), an adaptation of the ZONA 50 + Model for populations aged 50 years and older [11, 12]. The model structure and analysis framework for the ZONA IC model is illustrated in Fig. 1. The model estimates the outcomes resulting from different inputs for vaccinating against HZ for selected IC populations who are or will be at increased risk of HZ. This deterministic, static Markov model built in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) has a 1-year cycle length and follows a hypothetical cohort of adults for 30 years. Given the uncertainty around long-term survival in various IC populations, 30 years was selected as a conservative time horizon to capture all relevant outcomes of HZ and HZ vaccination while balancing the uncertainty of extrapolating beyond 30 years.
Fig. 1.
Structure of the ZONA IC model. Vaccination may or may not occur in the no HZ health status, depending on the modeled strategy. HZ herpes zoster, IC immunocompromised, PHN postherpetic neuralgia, ZONA Zoster Economic Analysis.
In the ZONA IC Model, outcomes were observed for a selected set of populations of US adults who were IC. There were two strategies: RZV (vaccination of the entire cohort at the simulation start) and no vaccine (vaccination does not occur). After an initial duration where individuals were assumed to have reduced immune function for a discrete number of years (IC status), individuals transitioned to an immunocompetent (healthy) status for the remainder of the time horizon. This reflects the temporary nature of some IC conditions and allowed for IC- and immunocompetent-specific model inputs to avoid overestimation of the benefits of RZV. In the healthy status, individuals were assumed to have the same HZ epidemiology, costs, utilities, and all-cause mortality rates as those for the immunocompetent population observed in the ZONA Model, for the respective ages modeled (the ZONA Model is described by Curran et al.) [11]. An annual discount rate of 3% was applied for all costs, life-years, and quality-adjusted life-years (QALYs), aligning with recommendations from the Second Panel on Cost-Effectiveness in Health and Medicine [13]. All cost inputs were inflated to 2019 US dollars (US$) using the medical care component of the US Consumer Price Index for direct medical costs and the general US Consumer Price Index for indirect costs [14, 15].
Modeled Populations
An autologous HSCT population of 19,671 patients, the estimated number of HSCT procedures conducted in the US in 2017, was modeled in the base case [16]. HSCT recipients are one of the most immunodeficient populations and have a higher incidence of HZ than individuals in the overall IC population; therefore, protecting against HZ would address a critical need [5, 17, 18]. This population is also well-studied and data for RZV efficacy, safety, and differential utility loss in HSCT patients were readily available. Although one study reported a post hoc analysis of trial data to estimate RZV efficacy among adults with hematologic malignancies, RZV efficacy and safety in IC populations have only been formally assessed prospectively in a phase III clinical trial in adult HSCT recipients. Data from this phase III clinical trial were used to estimate RZV efficacy against burden of illness and HZ-related utility loss in HSCT recipients [19–21]. In the model, the population was assumed to be 35 years of age (the approximate midpoint of the assumed base-case cohort age range of 18–49 years), to have received autologous HSCT immediately prior to the start of the model time horizon, and not to have previously received any vaccination against HZ.
Patients in other IC populations were also modeled in Scenario Analyses A–D, specifically: (A) patients with HIV infection; (B) patients with solid tumors, using breast cancer as an example subpopulation; (C) patients with hematological malignancies, using Hodgkin’s lymphoma as an example subpopulation; and (D) recipients of a renal transplant.
Model Inputs and Outputs
All analyses were conducted from the societal perspective with both direct and indirect costs considered. Economic outcomes included vaccination costs, and direct and indirect costs due to HZ resulting from each strategy. Vaccination costs consisted of vaccine acquisition costs, vaccine administration costs, and direct costs of adverse events. Direct costs due to HZ captured medical costs to treat a case of HZ with and without postherpetic neuralgia (PHN), and the costs of other complications due to HZ. Indirect costs consisted of costs arising from working hours lost due to vaccine-related adverse events and HZ (absenteeism), including those hours lost due to PHN, as reported by Eriksson et al. [22]. The outcomes from the two strategies were then compared to calculate differences resulting from RZV versus no vaccine. The key outcome measure for all analyses was the incremental cost-effectiveness ratio (ICER), as measured by the incremental cost per QALY gained. Health outcomes included HZ and PHN cases.
Model input values were identified through a targeted literature review focused on publications from 2005 to 2020 (Table 1, electronic supplementary material [ESM] Tables A1–A7). Some model inputs had values that varied by age. As a cohort aged annually, the inputs corresponding to the cohort’s age were applied. Some model input values also depended on whether they were being applied to individuals with IC or a healthy status. The duration of the IC status before individuals transitioned to a healthy status differed between the populations modeled in different scenarios. The base-case analysis assumed HSCT patients were IC for 5 years, based on an epidemiological study of HZ among autologous HSCT recipients [23]. The IC status duration and associated rationale for the other selected IC populations are detailed in Table 1. The input values applied to individuals aged 50 years and older with a healthy status have been previously described; the input values applied to individuals aged 18–49 years with a healthy status are presented in ESM Table A4 [11].
Table 1.
ZONA IC Model settings varied in scenario analyses, considering RZV versus no vaccine for United States adults with different immunocompromised conditions
| Input | Input value | ||||
|---|---|---|---|---|---|
| Base-case analysis (HSCT population)a | HIV population (Scenario A)b | Breast cancer population (Scenario B)c | Hodgkin’s lymphoma population (Scenario C)d | Renal transplant population (Scenario D)e | |
| Population size | 19,671 | 1,018,846 | 279,100 | 8480 | 22,106 |
| Population starting age (years) | 35 | 35 | 45 | 25 | 40 |
| IC status duration (years) | 5 | 30 | 2 | 2 | 30 |
| Annual incidence of initial and recurrent HZ (per person-year) | 0.0600 | 0.0093 | 0.0171 | 0.0336 | 0.0244 |
| Annual probability all-cause mortality | 0.0994 | 0.0347 | 0.0217 | 0.0284 |
Ages 18–49 years: 0.0030 Ages 50–59 years: 0.0090 Ages 60+ years: 0.0470 |
| Initial RZV efficacy against HZf | |||||
| One dose | 58.0%g | 78.9%h | 77.2%h | 69.8%h | 75.6%h |
| Two doses | 72.5% | 98.6% | 96.5% | 87.2% | 94.5% |
| Initial RZV efficacy against PHNf | |||||
| One dose | 75.9%h | 78.0%h | 77.8%h | 77.4%h | 77.6%h |
| Two doses | 94.8% | 97.5% | 97.2% | 96.7% | 97.0% |
| Annual waning of RZV efficacyf | |||||
| One dose | 18.2%i | 4.6%i | 8.2%i | 12.2%i | 10.2%i |
| Two doses | 9.1% | 2.3% | 4.1% | 6.1% | 5.1% |
HIV human immunodeficiency virus, HSCT hematopoietic stem cell transplant, HZ herpes zoster, IC immunocompromised, PHN postherpetic neuralgia, RZV recombinant zoster vaccine, US United States, ZONA Zoster Economic Analysis
aThe population size was the total number of HSCTs performed on individuals aged 21 years and older in 2017 in the US, as reported by the Center for International Blood and Marrow Transplant Research [16]
bThe population size was the estimated number of individuals aged 20 years and older living with diagnosed HIV in 2018 in the US, as reported by the Centers for Disease Control and Prevention [28]. The starting age of the population was approximately the mean age of individuals living with HIV in an epidemiological study of HZ in individuals with HIV [38]. The IC status duration and annual incidence of HZ were from the same study, which reported the annual incidence of HZ among HIV patients at an urban HIV clinic between 2002 and 2009, calculated from data from The Johns Hopkins University AIDS database. The annual probability of all-cause death was derived from the study by Siddiqi et al. [39] which reported a 28.86-year estimated average life expectancy in 2011 after diagnosis of HIV
cThe population size was the total number of individuals of all ages projected to be diagnosed with breast cancer in 2020, as reported by Siegel et al. [27]. The population reflects a younger breast cancer population; National Cancer Institute data [39], which reported that 19.7% of breast cancer diagnoses occurred in patients between the ages of 45 and 54 years, were used. The IC status duration and annual incidence of HZ were from the study by Habel et al. [18], who reported the annual incidence of HZ among adults with solid tumor malignancies and by level of immunosuppression. The annual probability of all-cause death was set to the 5-year survival probability for patients with breast cancer reported by Noone et al. [41]
dThe population size was the total number of individuals of all ages projected to be diagnosed with Hodgkin’s lymphoma in 2020, as reported by Siegel et al. [27]. The starting age of the population reflects the age at which adults are most frequently diagnosed, i.e. 20–34 years per National Cancer Institute data [40]. The IC status duration and annual incidence of HZ were estimated from the findings reported by Habel et al. [18], who reported the annual incidence of HZ among adults with hematologic malignancies and by level of immunosuppression. The annual probability of all-cause death was set to the 5-year survival probability for patients with Hodgkin’s lymphoma reported by Noone et al. [41].
eThe population size was the total number of kidney transplants performed in 2020 in the US among individuals aged 18 years and older. [26] The starting age of the population was assumed, based on Organ Procurement and Transplantation Network data [24], which showed that approximately 57% of the more than 50 transplants studied were in adults aged 35–49 years. The IC status duration was based on the assumption of lifetime maintenance use of immunosuppressants for renal transplant recipients, per clinical expert opinion. The annual incidence of HZ was obtained from the study by Pergam et al. [42], set to the annual incidence rate of HZ among 500 patients who received kidney transplants between 1995 and 2007. The annual probability of all-cause death was set to annual death rates for recipients of deceased donor kidney transplants reported by Kaballo et al. [43].
fThe initial efficacy of RZV and annual waning of RZV in each population was estimated from regression equations based on the association between placebo HZ incidence and RZV efficacy and waning in immunocompetent and IC populations from RZV clinical trials [19, 21, 24, 25]; the placebo HZ incidences used to estimate efficacy and waning from the regression equations for the IC conditions were derived from the study by Blank et al. [38] (HIV), Pergam et al. [42] (renal transplant), and Habel et al. [18] (Hodgkin’s lymphoma and breast cancer)
gWe assumed a 20% relative reduction from two-dose efficacy, due to a lack of data for one-dose RZV (i.e., high second-dose compliance) in the clinical trials. We assumed a ± 20% relative change from the base-case value for the uncertainty range
hWe assumed a 100% relative increase from two-dose waning of efficacy, due to a lack of data for one-dose RZV (i.e., high second-dose compliance) in the clinical trials. Assumed ± 50% relative change from the base-case value for the uncertainty range
iThe value shown was applied until the population returned to healthy status or until the population reached an age at which all-cause mortality was greater than the annual probability of death shown
For the scenario analyses, we conducted simple linear regression analyses that linked HZ incidence with RZV efficacy, using the results from the placebo arm of a phase III clinical trial and efficacy and waning estimates from immunocompetent and IC cohorts in the same phase III trial and other RZV clinical trials [19, 20, 24, 25]. For each selected IC population, the regression analyses estimated potential differences in RZV efficacy and waning as a function of HZ risk while IC. Further details about the values, sources, derivations, and standard errors for all inputs used in the analyses can be found in the electronic supplementary Appendix.
Deterministic sensitivity and threshold analyses were conducted to estimate the sensitivity of ICERs to each of the models’ input values. A probabilistic sensitivity analysis was conducted to explore uncertainty around the model’s outcomes, given the uncertainty around the model’s inputs, including 5000 Monte Carlo simulations.
Scenario analyses A–D modeled patients with HIV, patients with solid tumors (using breast cancer as an example subpopulation), patients with hematological malignancies (using Hodgkin’s lymphoma as an example subpopulation), and recipients of a renal transplant, respectively. Their starting ages varied but were all between 18 and 49 years, aligning with the research and health policy question. Cohort sizes were determined as either the estimated yearly incidence of a condition in the US (breast cancer, 279,100; Hodgkin’s lymphoma, 8480; renal transplant, 22,106) or its prevalence (HIV; 1,018,846) [16, 26–28]. Epidemiology, duration of IC status, and the efficacy and waning of RZV applied in the scenario analyses were varied.
Scenario Analysis E was conducted to vary the following three parameters: starting age, IC duration, and HZ incidence. For starting age 35 years, IC status duration (1–30 years) and annual HZ incidence (10–80 per 1000 person-years) were simultaneously varied. Additionally, for the population cohorts with starting ages of 25, 35, and 45 years, each combination of IC status duration (15 or 30 years) and annual HZ incidence (6–10 per 1000 person-years) was also explored. Age-specific annual probabilities of all-cause mortality were assumed to be twice the probability of immunocompetent individuals, to reflect IC populations having an underlying risk for death that is greater than the general US population but less than the HSCT population modeled in the base-case analysis. All other ZONA IC Model input values applied in Scenario E were consistent with the values applied in the base-case analysis for US HSCT adults aged 35 years.
Results
Base Case: Hematopoietic Stem Cell Transplant (HSCT) Recipients
A plain language summary of the results of this study is presented in ESM Fig. A1. For the base-case cohort of 19,671 HSCT recipients, RZV resulted in 2297 and 422 fewer HZ and PHN cases, respectively, versus no vaccine. When the cohort was vaccinated with RZV, discounted direct costs and indirect costs due to HZ (US$9.4 million and US$2.0 million, respectively) were lower than with no vaccine, while the associated vaccination costs were US$10.0 million. This amounted to a total discounted societal cost of US$21.4 million (Table 2). Thus, total discounted societal cost savings of US$0.1 million were observed for RZV versus no vaccine over a time horizon of 30 years. RZV also resulted in a gain of 109 discounted QALYs versus no vaccine. RZV was therefore a dominant strategy relative to no vaccine (i.e., more QALYs gained with a lower total cost).
Table 2.
Cost and QALY outcomes for RZV versus no vaccine for US adults IC due to HSCT (base case) and other conditions (Scenarios A–D)
| Base casea | |||
|---|---|---|---|
| RZV | No vaccine | Incremental (RZV vs. no vaccine) | |
| Total societal cost (US$)a | 21,355,313 | 21,461,730 | −106,417 |
| Direct costs due to HZ (US$) | 9,360,330 | 18,956,241 | 9,595,9110 |
| Indirect costs due to HZ (US$) | 2,016,822 | 2,505,489 | 488,666 |
| Vaccination costs (US$) | 9,978,160 | NA | NA |
| QALYs | 213,289 | 213,180 | 109 |
| ICER (US$ per QALY gained) | NA | NA | Cost saving |
| Scenario analysesb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Scenario A: HIV population | Scenario B: Breast cancer population | Scenario C: Hodgkin’s lymphoma population | Scenario D: Renal transplant population | |||||
| RZV | No vaccine | RZV | No vaccine | RZV | No vaccine | RZV | No vaccine | |
| Total societal cost (US$, millions) | 663.4 | 551.2 | 196.7 | 162.4 | 5.5 | 3.8 | 37.3 | 43.0 |
| Direct costs due to HZ (US$, millions) | 140.0 | 526.2 | 33.2 | 103.7 | 0.8 | 3.0 | 24.9 | 41.1 |
| Indirect costs due to HZ (US$, millions) | 6.6 | 24.9 | 21.9 | 58.8 | 0.4 | 0.7 | 1.2 | 1.9 |
| Vaccination costs (US$, millions) | 516.8 | 0 | 141.6 | 0 | 4.3 | 0 | 11.2 | 0 |
| Total societal cost difference [RZV vs. no vaccine] (US$, millions) | 112.2 | 34.2 | 1.7 | −5.8 | ||||
| Incremental QALYs gained (RZV vs. no vaccine) | 3373 | 506 | 18 | 156 | ||||
| ICER (US$ per QALY gained) | 33,268 | 67,682 | 95,972 | Cost saving | ||||
HIV human immunodeficiency virus, HSCT hematopoietic stem cell transplant, HZ herpes zoster, IC immunocompromised, ICER incremental cost-effectiveness ratio, NA not applicable, QALY quality-adjusted life year, RZV recombinant zoster vaccine, US United States
aAll outcomes are discounted. Population size: 19,671
bHIV scenario population size: 1,018,846; breast cancer scenario population size: 279,100; Hodgkin’s lymphoma scenario population size: 8480; renal transplant scenario population size: 22,106. See electronic supplementary Table A8 for more detail
Sensitivity and Threshold Analyses
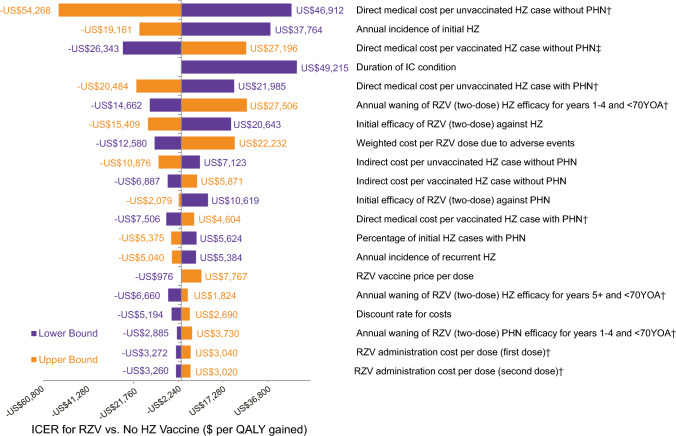
The results of the deterministic sensitivity analysis are presented in Fig. 2. The ICER for the HSCT population was most sensitive to the following inputs based on their defined uncertainty ranges: direct medical costs per HZ case, annual incidence of initial HZ (i.e., first occurrence of HZ), IC status duration, annual waning of RZV efficacy, and initial efficacy of RZV against HZ. The maximum ICER (US$49,215 per QALY gained) was observed when the IC status duration was assumed to be 2 years. Only the results for the top 20 inputs are presented in Fig. 2. The majority (82%, most of which are not shown) of input variations did not increase or decrease the ICER by more than US$5000 per QALY gained.
Fig. 2.
Deterministic sensitivity analysis results for the cost effectiveness of RZV versus no vaccine strategy for US HSCT adults aged 35 years. †Individual variation of an input that is also varied in this deterministic sensitivity analysis grouped with other potentially correlated inputs. HSCT hematopoietic stem cell transplant, HZ herpes zoster, ICER incremental cost-effectiveness ratio, PHN postherpetic neuralgia, QALY quality-adjusted life-year, RZV recombinant zoster vaccine, US United States, YOA years of age
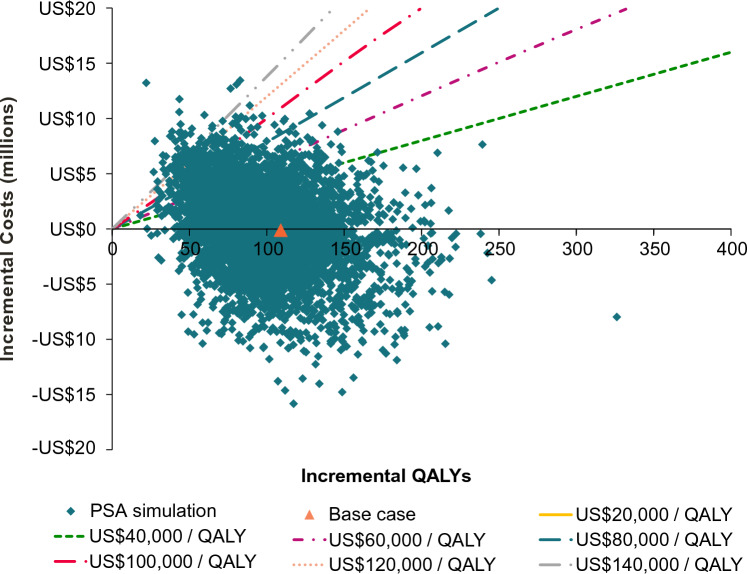
The probabilistic sensitivity analysis showed RZV in the HSCT population had a 44.5% probability of being a cost-saving strategy versus no vaccine (Fig. 3). At a hypothetical willingness-to-pay threshold of US$100,000 per QALY gained, vaccinating with RZV would be considered cost effective in 97.1% of simulations.
Fig. 3.
Incremental costs versus incremental QALYs from 5000 probabilistic sensitivity analysis simulations of RZV versus no vaccine for US HSCT adults aged 35 years. HSCT hematopoietic stem cell transplant, PSA probabilistic sensitivity analysis, QALYs quality-adjusted life-year, RZV recombinant zoster vaccine, US United States
Threshold analyses assessed how much change in key parameters would render RZV no longer cost effective in the HSCT population (i.e., result in an ICER higher than US$100,000 per QALY gained). RZV would not be cost effective if the annual incidence of initial and recurrent HZ per person-year was ≤ 0.024, or 60% lower than the base-case value; the IC status duration was 1 year or less (−80%); or the initial RZV efficacy was ≤ 36.3% for HZ or ≤ 47.4% for PHN (− 50%) (Fig. A2).
Scenario Analyses: Other Immunocompromised Populations
For the population of 1,018,846 individuals with HIV (Scenario A), RZV resulted in 118,583 and 15,026 fewer HZ and PHN cases, versus no vaccine. RZV also resulted in a total societal cost increase of US$112.2 million and 3373 incremental QALYs gained relative to no vaccine. This translated to an ICER of US$33,268 per QALY gained. Vaccination with RZV of 279,100 patients with breast cancer (Scenario B) resulted in 38,069 and 3184 fewer HZ and PHN cases, versus no vaccine. RZV also resulted in a total societal cost increase of US$34.2 million and 506 incremental QALYs gained, compared with no vaccine, translating to an ICER of US$67,682 per QALY gained. For the population of 8480 patients with Hodgkin’s lymphoma (Scenario C), RZV resulted in 849 and 94 fewer HZ and PHN cases, respectively, versus no vaccine. RZV also resulted in a total societal cost increase of US$1.7 million and 18 incremental QALYs gained, compared with no vaccine. This translated to an ICER of US$95,972 per QALY gained. When the population of 22,106 renal transplant recipients was vaccinated with RZV (Scenario D), there were 4453 and 603 fewer HZ and PHN cases, respectively. Additionally, the total societal costs decreased by US$5.8 million, there were 156 incremental QALYs gained, and RZV was found to be cost saving versus no vaccine.
In Scenario E, when the IC status duration and the annual HZ incidence during IC status were simultaneously varied, the majority of ICERs were cost saving (Table 3). ICERs were highest with the shortest IC durations and lowest annual HZ incidence.
Table 3.
Scenario E: ICERs for RZV versus no vaccine for US IC adults, across ranges of values for starting age, IC status duration, and annual herpes zoster incidence
| IC status duration (years) | Annual incidence of HZ (per 1000 person-years)a, based on a starting age of 35 years | ||||
|---|---|---|---|---|---|
| 10 | 20 | 40 | 60 | 80 | |
| 1 | US$548,377 | US$312,732 | US$146,083 | US$82,966 | US$51,646 |
| 2 | US$300,961 | US$133,185 | US$33,046 | Cost saving | Cost saving |
| 3 | US$199,578 | US$70,612 | Cost saving | Cost saving | Cost saving |
| 4 | US$143,150 | US$38,106 | Cost saving | Cost saving | Cost saving |
| 5 | US$104,716 | US$17,323 | Cost saving | Cost saving | Cost saving |
| 30 | Cost saving | Cost saving | Cost saving | Cost saving | Cost saving |
| Annual incidence of HZ (per 1000 person-years)a | ||||||
|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | ||
| Starting age (years) | 25 | |||||
| IC status duration (years) | 15 | US$66,285 | US$47,555 | US$33,256 | US$21,985 | US$12,878 |
| 30 | US$18,192 | US$8549 | US$480 | Cost saving | Cost saving | |
| Starting age | 35 | |||||
| IC status duration | 15 | US$31,638 | US$21,137 | US$12,565 | US$5,455 | Cost saving |
| 30 | US$12,694 | US$7989 | US$1211 | Cost saving | Cost saving | |
| Starting age | 45 | |||||
| IC status duration | 15 | US$12,828 | US$13,225 | US$7028 | US$1569 | Cost saving |
| 30 | US$3533 | US$6251 | US$3327 | US$304 | Cost saving | |
All ZONA IC Model input values other than those varied in this scenario were consistent with the base-case analysis for US HSCT adults (starting age 35 years)
HSCT hematopoietic stem cell transplantation, HZ herpes zoster, IC immunocompromised, ICER incremental cost-effectiveness ratio, RZV recombinant zoster vaccine, US United States, ZONA Zoster Economic Analysis
aRZV efficacy and waning values were estimated based on the annual HZ incidence value considered. For more detail, please see the ‘Data Sources’ subsection in the manuscript, and electronic supplementary Figs. A3 and A4
Discussion
Results for the base-case analysis in HSCT recipients and adults with other selected IC conditions suggest RZV is likely cost effective for the prevention of HZ in US IC adults aged 18 years and older in selected IC populations, and may be cost saving for some IC populations. RZV is also effective in reducing HZ and PHN cases in selected IC populations. These results were robust to a wide range of uncertainties in key parameters that influenced the health outcomes and the overall costs of the vaccine program.
To our knowledge, our study is only the second to comprehensively evaluate the cost effectiveness of an HZ vaccine in IC cohorts. The CDC has developed a similar model, the results of which informed the 2021 ACIP recommendation for RZV in adults aged 19 years and older who are or will be at increased risk for HZ because of immunodeficiency or immunosuppression caused by known disease or therapy [10]. Results from the CDC model showed vaccination with RZV was cost saving for the base case of adult HSCT recipients aged 18–49 years, aligning with results of the present analysis [29].
Previous analyses found RZV was a cost-effective intervention for immunocompetent adults aged 50 years and older in the US [11, 30–32]. Estimates of RZV’s efficacy and durability in the prevention of HZ and associated complications were lower in an HSCT clinical trial study than in trials with immunocompetent adults, as would be expected due to the HSCT cohort’s IC status, which results in a decreased responsiveness to vaccination [19, 33]. However, our analysis demonstrated that the increased risk, utility impact, and cost impact of HZ cases in HSCT and the other modeled IC cohorts were high, making the cost-effectiveness estimates for RZV versus no vaccine in IC cohorts similar to those for older adults.
In our base case of US adult HSCT recipients, RZV resulted in total societal cost savings of US$0.1 million and 109 incremental QALYs versus no vaccine, and RZV was also cost saving in scenario analysis of recipients of a renal transplant. At a hypothetical willingness-to-pay threshold of US$100,000 per QALY gained, a common threshold reported in US cost-effectiveness analyses [34], RZV was cost effective in the scenario analyses of patients with HIV, breast cancer, and Hodgkin’s lymphoma, with ICERs of US$33,268, US$67,682, and US$95,972 per QALY gained, respectively, versus no vaccine. Our findings were comparable with ICERs reported by studies focusing on immunocompetent adults, which ranged from approximately US$10,000 to US$91,000 per QALY gained [11, 30–32]. The probabilistic sensitivity analysis results emphasize the robustness of the base-case results, and the conclusions drawn from them. Overall, this study provides valuable cost-effectiveness information that can be considered by policy makers, alongside other criteria deemed relevant for decision making (e.g., feasibility of implementation and impact on health equity).
While the base-case analysis included HSCT recipients as a well-studied, highly IC population that has a higher incidence of HZ than individuals in the overall IC population, the scenario analyses investigated RZV cost effectiveness in other IC conditions to address the heterogeneity of IC patients in real-world settings. Model input values were based on clinical trial results and data from a targeted literature review that was conducted to ascertain realistic input values. Our analysis made simplifying assumptions that the IC status duration was a discrete number of years, and that the cohort maintained a healthy status for the remainder of the time horizon. The IC duration and resulting HZ risk are likely more dynamic in real-world IC cohorts. However, in some simulations (e.g., HIV) the cohort remained IC for the full time horizon, aligning with the disease pathology. Notably, analyses showed that RZV cost effectiveness was more favorable when HZ incidence and vaccine efficacy were high, IC duration was long, and vaccine efficacy waning and IC condition-specific mortality were low. When individuals are highly immunosuppressed, generally HZ incidence, disease burden, HZ costs, and natural mortality will be high, while starting vaccine efficacy will be lower and vaccine efficacy waning will be higher (as compared with those who are less immunosuppressed).
Our base-case analysis assumed HSCT patients were IC for 5 years based on an epidemiological study of HZ among autologous HSCT recipients [23]. We assumed that the period observed in that study covered the relevant period of elevated HZ risk for HSCT patients. We considered briefer durations of IC status in the deterministic sensitivity and threshold analyses, but those analyses applied the 5-year annual incidence estimate from that epidemiological study. Had we assumed a 2-year IC status duration in the base-case analysis, we also would have applied higher annual HZ incidence during IC status, closer to 80–90 cases per 1000 person-years, as multiple studies have observed in the first 2 years following HSCT [19, 23, 35].
Although our base-case analysis starting age was 35 years, the available vaccine efficacy and HZ incidence data were taken from trials with older populations [19, 23]. In the phase III clinical trial, the vaccine efficacy among individuals aged 18–49 years was 72%, compared with 67% in those aged ≥ 50 years; the overall efficacy was 68% [19]. To address potential bias, we conservatively used the overall vaccine efficacy estimate in the model, as opposed to the 72% efficacy observed for ages 18–49 years where better results for RZV would have been observed. The annual HZ incidence estimated from Sahoo et al. was applied because it had the longest follow-up (median 39.7 months) among the studies considered, and provided a 5-year annual incidence estimate following HSCT [17, 19, 23, 35, 36]. Although the 35-year-old starting age for the HSCT population was selected to focus analyses on adults aged 18–49 years, and it is not reflective of the mean age of the HSCT population in the studies considered (median age 55.5 years in the study by Sahoo et al.), the age-related relationship for HZ incidence in this population was not statistically significant, with an incidence rate ratio of 1.08 (0.91–1.27) for ages 50–59 years versus 18–49 years [17, 23].
Limited data were available to estimate RZV effectiveness and durability in IC cohorts other than HSCT, therefore regression analyses were conducted using data from RZV clinical trials in immunocompetent and IC cohorts to estimate these values [19, 20, 24, 25]. The regression calculated the relationship between HZ incidence and RZV efficacy (i.e., higher incidence, lower efficacy, and vice versa). A key limitation of this approach is that it assumes that differences in RZV efficacy across populations are entirely explained by differences in HZ incidence; other immunological factors and age may also be relevant.
Different all-cause mortality rates were used for the healthy (immunocompetent) and IC cohorts. Due to the lack of data, we conservatively applied HZ case-fatality estimates for an immunocompetent population to both the healthy and IC status [37]. Although death is uncommon in HZ infections, immunosuppression is recognized as a risk factor. Our estimate of HZ-related mortality is therefore likely an underestimate.
Conclusions
The results of this study show RZV may be cost saving for the prevention of HZ in US adults aged 18 years and older with selected IC conditions and cost effective for others. Furthermore, these results support the use of RZV to prevent HZ and its associated complications in IC adults. Our findings may inform clinicians and policy makers in decision making around the efficacy and value of RZV in IC adults, where the absence of a vaccine indicated for prevention of HZ has resulted in high unmet medical need. The present analysis assessed the cost effectiveness of RZV in selected IC populations. Further research is necessary to better understand the impact of RZV across the highly heterogeneous IC population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the investigators and their teams who took part in this study. The authors also thank Seri Anderson from RTI Health Solutions for providing assistance with the literature review, and documentation of sources and assumptions for the model inputs. The authors also thank Costello Medical for editorial assistance and publication coordination, on behalf of GSK, and acknowledge Isabel Katz from Costello Medical, USA, for medical writing and editorial assistance based on the authors’ input and direction. This study was funded by GlaxoSmithKline Biologicals SA.
Declarations
Conflict of interest
Ahmed Salem, Elizabeth M. La, Desmond Curran, and Sara Poston are employees of the GSK group of companies and report holding shares in the GSK group of companies. Justin Carrico and Katherine A. Hicks report payments to RTI Heath Solutions from the GSK group of companies for the conduct of this study. Brandon J. Patterson reports holding shares in the GSK group of companies and is a former employee of the GSK group of companies. Stéphane Lorenc and Christopher F. Carpenter declare to have received consulting fees from the GSK group of companies. All authors declare no other financial and non-financial relationships and activities.
Funding
This study was sponsored by GlaxoSmithKline Biologicals SA (HO-19-19750). Support for third-party writing assistance for this article was funded by GlaxoSmithKline Biologicals SA in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability (software application or custom code)
The Microsoft Excel-based model used in this study is proprietary property of GSK and cannot be shared.
Authors’ contributions
Substantial contributions to study conception and design: AS, EML, DC, BJP, JC, SL, KAH, SP, CFC. Substantial contributions to analysis and interpretation of the data: AS, EML, DC, BJP, JC, SL, KAH, SP, CFC. Drafting the article or revising it critically for important intellectual content: AS, EML, DC, BJP, JC, SL, KAH, SP, CFC. Final approval of the version of the article to be published: AS, EML, DC, BJP, JC, SL, KAH, SP, CFC.
Data sharing statement
The Microsoft Excel-based model used in this study is proprietary property of GSK and cannot be shared. All data generated or analyzed during this study are included in this published article or as supplementary information files.
References
- 1.Centers for Disease Control and Prevention . Shingles (herpes zoster): clinical overview. Atlanta: Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 2.Johnson BH, Palmer L, Gatwood J, et al. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15:502. doi: 10.1186/s12879-015-1262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 4.Yawn BP, Itzler RF, Wollan PC, et al. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009;84:787–794. doi: 10.4065/84.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay SL, Guo A, Pergam SA, et al. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71:e125–e134. doi: 10.1093/cid/ciz1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316:2547–2548. doi: 10.1001/jama.2016.16477. [DOI] [PubMed] [Google Scholar]
- 7.United States Census Bureau . Age and sex composition in the United States: 2013. Suitland: United States Government; 2016. [Google Scholar]
- 8.Dooling KLGA, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–108. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shingrix [package insert], revised: 07/2021. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021.
- 10.Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥ 19 years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36:5037–5045. doi: 10.1016/j.vaccine.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Curran D, Patterson BJ, Van Oorschot D, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States who have been previously vaccinated with zoster vaccine live. Hum Vaccin Immunother. 2019;15:765–771. doi: 10.1080/21645515.2018.1558689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 14.United States Bureau of Labor Statistics. Consumer Price Index for all urban consumers, 2020. Accessed 7 Feb 2020.
- 15.United States Bureau of Labor Statistics. Consumer Price Index for medical care. Washington, DC: United States Bureau of Labor Statistics; 2020. Accessed 7 Feb 2020.
- 16.Center for International Blood and Marrow Transplant Research. Transplant activity report, 2019. Milwaukee: Center for International Blood and Marrow Transplant Research
- 17.Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42:325–334. doi: 10.1007/s15010-013-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habel LA, Ray GT, Silverberg MJ, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomark Prev. 2013;22:82–90. doi: 10.1158/1055-9965.EPI-12-0815. [DOI] [PubMed] [Google Scholar]
- 19.Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322:123–133. doi: 10.1001/jama.2019.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19:988–1000. doi: 10.1016/S1473-3099(19)30163-X. [DOI] [PubMed] [Google Scholar]
- 21.Curran D, Matthews S, Rowley SD, et al. Recombinant zoster vaccine significantly reduces the impact on quality of life caused by herpes zoster in adult autologous hematopoietic stem cell transplant recipients: a randomized placebo-controlled trial (ZOE-HSCT) Biol Blood Marrow Transplant. 2019;25:2474–2481. doi: 10.1016/j.bbmt.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson J, Hunger M, Bourhis F, et al. Cost and utility in immunocompromised subjects who developed herpes zoster during the randomized V212 Inactivated Varicella-Zoster Vaccine (ZVIN) trial. Exp Rev Pharmacoecon Outcomes Res. 2020;20(6):613–621. doi: 10.1080/14737167.2020.1693267. [DOI] [PubMed] [Google Scholar]
- 23.Sahoo F, Hill JA, Xie H, et al. Herpes zoster in autologous hematopoietic cell transplant recipients in the era of acyclovir or valacyclovir prophylaxis and novel treatment and maintenance therapies. Biol Blood Marrow Transplant. 2017;23:505–511. doi: 10.1016/j.bbmt.2016.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 25.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 26.Organ Procurement and Transplantation Network. National data, 2021. US Department of Health and Human Services. Accessed 12 Jan 2021.
- 27.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention . HIV surveillance report, 2018 (updated) Atlanta: Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 29.Leidner AJ, Anderson TC, Hong K, et al. Cost-effectiveness analysis of vaccination with recombinant zoster vaccine among hematopoietic cell transplant recipients and persons with other immunocompromising conditions aged 19 to 49 years. Value Health. 2023;26(2):204–215. doi: 10.1016/j.jval.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prosser LA, Harpaz R, Rose AM, et al. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med. 2019;170:380–388. doi: 10.7326/M18-2347. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter CF, Aljassem A, Stassinopoulos J, et al. A cost-effectiveness analysis of an adjuvanted subunit vaccine for the prevention of herpes zoster and post-herpetic neuralgia. Open Forum Infect Dis. 2019;6:ofz219. doi: 10.1093/ofid/ofz219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le P, Rothberg MB. Cost-effectiveness of the adjuvanted herpes zoster subunit vaccine in older adults. JAMA Intern Med. 2018;178:248–258. doi: 10.1001/jamainternmed.2017.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo H-MKY, Bang CH, et al. Antiviral prophylaxis for preventing herpes zoster in hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Antivir Res. 2017;140:106–115. doi: 10.1016/j.antiviral.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Neumann PJ, Kim DD. Cost-effectiveness thresholds used by study authors, 1990–2021. JAMA. 2023;329(15):1312–1314. doi: 10.1001/jama.2023.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winston DJ, Mullane KM, Cornely OA, et al. Inactivated varicella zoster vaccine in autologous haemopoietic stem cell transplant recipients: an international, multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:2116–2127. doi: 10.1016/S0140-6736(18)30631-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D, Weiss T, Feng Y, et al. Duration of antiviral prophylaxis and risk of herpes zoster among patients receiving autologous hematopoietic stem cell transplants: a retrospective, observational study. Adv Ther. 2017;34:1610–1621. doi: 10.1007/s12325-017-0553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163:489–497. doi: 10.7326/M15-0093. [DOI] [PubMed] [Google Scholar]
- 38.Blank LJ, Polydefkis MJ, Moore RD, et al. Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr. 2012;61:203–207. doi: 10.1097/QAI.0b013e318266cd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqi AE, Hall HI, Hu X, et al. Population-based estimates of life expectancy after HIV diagnosis: United States 2008–2011. J Acquir Immune Defic Syndr. 2016;72:230–236. doi: 10.1097/QAI.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Cancer Institute . SEER cancer stat facts. Bethesda: National Cancer Institute; 2020. [Google Scholar]
- 41.Noone A, Howlader N, Krapcho M, et al. SEER cancer statistics review, 1975–2015. Updated 10 Sep 2018. Bethesda: National Cancer Institute; 2018. [Google Scholar]
- 42.Pergam SA, Forsberg CW, Boeckh MJ, et al. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis. 2011;13:15–23. doi: 10.1111/j.1399-3062.2010.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaballo MA, Canney M, O'Kelly P, et al. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin Kidney J. 2018;11:389–393. doi: 10.1093/ckj/sfx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arias E, Xu J. United States life tables: 2017. National vital statistics reports. 2019:68(7). [PubMed]
- 45.GSK. ZOSTER-002 annex tables: vaccine efficacy by year [data on file]. GSK; 2020.
- 46.GSK. ZOSTER-002 annex tables: frequency of medically attended visit types by time period post vaccination and age group: combined doses (total vaccinated cohort) [data on file]. GSK; 2020.
- 47.GSK. ZOSTER-002 clinical study report [data on file]. GSK; 2020.
- 48.Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125:1301–1312. doi: 10.1002/cncr.31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tseng HF, Smith N, Harpaz R, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–166. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 50.Tseng HF, Chi M, Smith N, et al. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis. 2012;206:190–196. doi: 10.1093/infdis/jis334. [DOI] [PubMed] [Google Scholar]
- 51.Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore L, Remy V, Martin M, et al. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc. 2010;8:7. doi: 10.1186/1478-7547-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 54.Lieu TA, Ortega-Sanchez I, Ray GT, et al. Community and patient values for preventing herpes zoster. Pharmacoeconomics. 2008;26:235–249. doi: 10.2165/00019053-200826030-00006. [DOI] [PubMed] [Google Scholar]
- 55.Micromedex . Red book online. Englewood: Micromedex; 2021. [Google Scholar]
- 56.Tsai Y, Zhou F, Lindley MC. Insurance reimbursements for routinely recommended adult vaccines in the private sector. Am J Prev Med. 2019;57:180–190. doi: 10.1016/j.amepre.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortega-Sanchez I. Decision and cost-effectiveness analyses of herpes zoster vaccination in adults 50 years of age and older. Atlanta: Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 58.Agency for Healthcare Research and Quality. Mean expenses per person with care for selected conditions by type of service: United States, 2014. Medical Expenditure Panel Survey Household Component Data. Rockville: Agency for Healthcare Research and Quality; 2017.
- 59.Healthcare Cost and Utilization Project. HCUPnet–hospital inpatient national statistics 2014. Rockville: Agency for Healthcare Research and Quality; 2014.
- 60.United States Bureau of Labor Statistics . Median usual weekly earnings of full-time wage and salary workers by age, race, Hispanic or Latino ethnicity, and sex, fourth quarter 2019 averages, not seasonally adjusted. Washington, DC: United States Bureau of Labor Statistics; 2020. [Google Scholar]
- 61.United States Bureau of Labor Statistics . Employment status of the civilian noninstitutional population by age, sex, and race, 2019. Washington, DC: United States Bureau of Labor Statistics; 2020. [Google Scholar]
- 62.Kirchhoff AC, Leisenring W, Syrjala KL. Prospective predictors of return to work in the 5 years after hematopoietic cell transplantation. J Cancer Surviv. 2010;4:33–44. doi: 10.1007/s11764-009-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison EJ, Ehlers SL, Bronars CA, et al. Employment status as an indicator of recovery and function one year after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:1690–1695. doi: 10.1016/j.bbmt.2016.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.