Abstract
The adenohypophysis is comprised of the anterior and intermediate lobes (AL and IL, respectively). Cluster of differentiation 9 (CD9)- and sex-determining region Y-box 2 (SOX2)-positive cells are stem/progenitor hormone-producing cells in the AL. They are located in the marginal cell layer (MCL) facing Rathke’s cleft between the AL and IL (primary niche) and the parenchyma of the AL (secondary niche). We previously showed that, in rats, CD9/SOX2-positive cells in the IL side of the MCL (IL-side MCL) migrate to the AL side (AL-side MCL) and differentiate into prolactin-producing cells (PRL cells) in the AL parenchyma during pregnancy, lactation, and diethylstilbestrol treatment, all of which increase PRL cell turnover. This study examined the changes in CD9/SOX2-positive stem/progenitor cell niches and their proportions by manipulating the turnover of growth hormone (GH)- and thyroid-stimulating hormone (TSH)-producing cells (GH and TSH cells, respectively), which are Pit1 lineage cells, as well as PRL cells. After induction, the isolated CD9/SOX2-positive cells from the IL-side MCL formed spheres and differentiated into GH and TSH cells. We also observed an increased GH cell proportion upon treatment with GH-releasing hormone and recovery from continuous stress and an increased TSH cell proportion upon propylthiouracil treatment, concomitant with alterations in the proportion of CD9/SOX2-positive cells in the primary and secondary niches. These findings suggest that CD9/SOX2-positive cells have the potential to supply GH and TSH when an increase in GH and TSH cell populations is required in the adult pituitary gland.
Keywords: Cluster of differentiation 9 (CD9), Growth hormone (GH), Pituitary, Sex-determining region Y-box 2 (SOX2), Thyroid-stimulating hormone (TSH)
The pituitary gland is an endocrine organ that produces hormones to maintain homeostasis, including growth, metabolism, stress response, and reproduction. The rodent pituitary gland comprises an adenohypophysis containing the anterior lobe (AL), intermediate lobes (IL), and a neurohypophysis containing the posterior lobe (PL). S100β-positive non-endocrine cells in the AL are generally known as folliculo-stellate cells [1, 2]. Since most S100β-positive cells express the stem/progenitor marker sex-determining region Y-box 2 (SOX2), they attract increasing attention as stem/progenitor cells of hormone-producing cells in the AL [3,4,5,6,7].
S100β/SOX2-positive cells are located along the IL side and AL side of the marginal cell layer (MCL) that faces Rathke’s cleft and form the primary stem/progenitor cell niche, while the cell clusters scattered throughout the parenchyma are proposed as the secondary niche [6,7,8,9]. We found that cluster of differentiation 9 (CD9) and CD81 are novel markers specific to SOX2-positive cells in the primary and secondary niches of the rodent pituitary gland [4, 5, 10, 11]. CD9 is a tetraspanin membrane protein that forms a complex with CD81. Using a monoclonal antibody targeting CD9 expressed on the cell surface, we successfully isolated CD9/CD81/S100β/SOX2-positive cells from the IL side of the MCL (IL-side MCL) [4] and found that they are core stem cells supplying stem/progenitor cells to the AL side of the MCL (AL-side MCL) via epithelial-mesenchymal transition during development of the pituitary gland [4, 12]. Additionally, these cells are also essential in sustaining endocrine functions in the adult AL by controlling the turnover of hormone-producing cells [4, 12]. We found that the number of the increase in the prolactin-producing cells (PRL cells) during pregnancy and lactation was due to the supply of CD9/CD81/S100β/SOX2-positive cells from the IL-side MCL and the parenchyma [13], and that PRL cell proliferation induced by diethylstilbestrol (DES) was spatially attributed to the supply of CD9/CD81/S100β/SOX2-positive cells from the AL-side MCL and the parenchyma [13]. Although growth hormone-producing cells (GH cells) and thyroid-stimulating hormone-producing cells (TSH cells) belong to the transcription factor Pit1 lineage along with PRL cells [14, 15], it is not known whether their populations are controlled by CD9/CD81/S100β/SOX2-positive cells and which niches contribute to the supply of these hormone-producing cells. In this study, we manipulated the population of GH cells via continuous stress loading (decrease in GH cells) and the intraperitoneal injection of growth hormone-releasing hormone (GHRH; increase in GH cells) [16], and the population of TSH cells upon propylthiouracil (PTU) exposure (increase in TSH cells) [16, 17] to evaluate the relationship between CD9/SOX2-positive cell differentiation and GH/TSH cell turnover in the adult AL.
Materials and Methods
Animals
Forty-two male Wistar rats aged 8–10 weeks (weighing 200–250 g) were purchased from Japan SLC (Shizuoka, Japan) and maintained on a 12-h light/dark cycle with conventional food and water ad libitum. Their birth day was designated as postnatal day 0 (P0). The room temperature was maintained at approximately 22°C. All animal experiments were approved by the Committee on Animal Experiments of Kyorin University and were conducted in accordance with the Institutional Regulations for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science, and Technology (I21-12-02).
Manipulation of GH cell and TSH cell populations
According to the protocols designed by Ogawa et al. [16] and Torres et al. [18], we manipulated the turnover of GH cell and TSH cell populations in adult rat anterior pituitary gland to examine the response of CD9/SOX2-positive cells in the primary and secondary niches.
GHRH treatment: Six rats were intraperitoneally injected with 3 μg of GHRH (Aviva Systems Biology, San Diego, CA, USA) in 100 μl of saline, twice daily (0900 and 1800 h) for 3 d. On day 4, the rats were sacrificed 1 h after the last GHRH injection. Saline was used as the vehicle control (n = 6) [16].
Continuous stress (CS) treatment: Eighteen rats were randomly selected for the CS experiments. As a CS model, 12 rats were transferred to cages filled with water at approximately 22°C to a height of 1.5 cm, and the rats were kept in the cages for 5 days (CS5d). Water was replaced every 24 h. After CS treatment, six rats were sacrificed for the experiment, and the remaining six rats were returned to their home cages for another 3 days (RC3d). The cages in the RC3d group were replaced every 24 h. As a control, six rats were kept in their home cages without CS treatment for 5 days (no continuous stress: NCS). The cages of the rats in the NCS group were replaced every 24 h [16].
Propylthiouracil (PTU) treatment: Six rats were treated with 0.1% PTU, a blocker of thyroxine-triiodothyronine conversion, in the drinking water for 14 days to induce hypothyroidism [18].
All rats were sacrificed via exsanguination from the right atrium under deep anesthesia with medetomidine (0.15 mg/kg, Zenyaku Kogyo, Tokyo, Japan), midazolam (2.0 mg/kg, SANDOZ, Tokyo, Japan), and butorphanol (2.5 mg/kg, Meiji Seika Pharma, Tokyo, Japan). Hanks’ balanced salt solution (Thermo Fisher Scientific, Waltham, MA, USA) and 4% paraformaldehyde in 0.05 M phosphate buffer (PB; pH 7.4) were then perfused from the aorta for cell culture and for histological analyses, respectively.
Immunohistochemistry and immunocytochemistry
Rats were deeply anesthetized with a combination of the three aforementioned anesthetics, and the pituitary glands were dissected. They were immediately immersed in a fixative consisting of 4% formaldehyde in 0.05 M PB (pH 7.4) for 20–24 h at 4°C. The tissues were incubated in PB (pH 7.2) containing 30% sucrose at 4°C for 2 days. They were then embedded in Tissue Tek O.C.T. compound (Sakura Finetek Japan, Tokyo, Japan) and rapidly frozen. Frozen frontal sections with a thickness of 8 μm were obtained using a cryostat (Tissue-tek Polar DM, Sakura Finetek Japan) and mounted on glass slides (Matsunami, Osaka, Japan). Immunohistochemistry and immunocytochemistry were performed as previously described [5]. Briefly, sections were incubated for 20 min at 30°C in phosphate-buffered saline (PBS; pH 7.2) containing 2% normal donkey serum and then incubated at 4°C with the corresponding primary and secondary antibodies (Supplementary Table 1). The sections were scanned using an epifluorescence microscope (BX61; Olympus, Tokyo, Japan) with a CellSens Dimension system (Olympus).
To verify the presence of anti-CD9 antibodies, a pre-absorption experiment was performed. Briefly, the antibodies were preincubated with a CD9 peptide (ORB217044; Biobyt, Cambridge, UK) at a molar ratio for 2 days at 4°C and then centrifuged at 8,000 g for 10 min at 4°C. The resulting supernatant was used as the preabsorbed antibody. The nuclei were counterstained with a VECTASHIELD Mounting Medium containing DAPI (Vector Laboratories, Newark, CA, USA). After immunohistochemistry, six random fields (157.5 × 210 μm rectangle) were captured per section using a 40× objective lens. The number of hormone-positive cells and the total number of cells (DAPI-positive) per area were counted using a CellSens Dimension system (Olympus). Cell counts were performed in triplicate for each experimental group. For immunocytochemistry, cultured cells were fixed with 4% paraformaldehyde in 0.025 M PB for 20 min at 21–23°C and then permeabilized with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 2 min at room temperature. Cells were incubated with PBS containing 2% normal donkey serum for 20 min at 30°C, followed by incubation with the corresponding primary and secondary antibodies (Supplementary Table 1).
Isolation of CD9-positive cells from the IL-side MCL or CD90-positive TSH cells from the AL
The PL, IL, and AL of 8–10-week-old Wistar male rats were dispersed as previously described [4, 12]. The dispersed cells were counted using a hemocytometer and separated using the pluriBead-cascade cell isolation system (Universal Mouse pluriBeads kit, pluriSelect Corp., San Diego, CA, USA), as described by Pierzchalski et al. (2013), using anti-mouse monoclonal CD9 or CD90 antibodies (BD Biosciences) according to the manufacturer’s instructions [4, 12, 19]. Isolated IL-side MCL-derived CD9-positive and AL-derived CD90-positive TSH cells were used for cell culture.
Primary culture
Dispersed AL cells and isolated CD90-positive TSH cells from the AL were plated onto 8-well glass chamber slides (1 cm2/well; Nalge Nunc International, Rochester, NY, USA) at a density of 1.0 × 105 cells/well in 500 μl of Medium 199 with Earle’s salts (Thermo Fisher Scientific), supplemented with 0.1% bovine serum albumin. The cells were then cultured for 48 h at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After the incubation, the cells were kept in the vehicle (medium with 0.1% volume of PBS) or GHRH-, PTU-, or thyrotropin-releasing hormone (TRH)-containing medium (1nM each) for 1 h or 24 h in an incubator.
Pituisphere formation
CD9-positive cells from the IL of Wistar rats were plated on untreated 35-mm dishes (AGC Techno Glass, Shizuoka, Japan) at a density of 2.5 × 104 cells/dish in DMEM/F-12 containing B27 supplement (1:50; Thermo Fisher Scientific), N2 supplement (1:100; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), BSA (0.5%; Sigma-Aldrich), bFGF (20 ng/ml), and EGF (20 ng/ml). The cells were incubated in a humidified chamber with 5% CO2 at 37°C. Pituitary gland growth was assessed as previously described [4]. The differentiation of pituispheres into GH-, PRL-, and TSH-producing cell lineages was induced using the induction procedure described by Suga et al. [20] with some modifications. Briefly, the pituispheres were cultured using the overlay 3D culture method on Matrigel-coated 16-well chamber slides (0.4 cm2/well) (Thermo Fisher Scientific) with 20 ng/ml bFGF, 20 ng/ml EGF, and 20% KnockOut Serum Replacement (Thermo Fisher Scientific) for 4 days. The spent medium was then replaced with a medium including 6-bromoindirubin-3′-oxime (BIO) (GSK3β-inhibitor, 250 nM; FUJIFILM Wako Pure Chemical Corp.), and the pituispheres were cultivated for another 10 days.
Quantification of mRNA levels using quantitative polymerase chain reaction (qPCR)
qPCR was performed as previously described [5]. Total RNA from each rat (n = 3) was extracted using an RNeasy Plus Mini Kit (Qiagen, Hilden, Germany), and contaminating DNA was removed via digestion using RNase-free DNase (Qiagen) for 15 min at 21–23°C. Next, cDNA was synthesized using a ReverTra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan). qPCR was performed in a Dice Real-Time System II thermal cycler (Takara Bio, Shiga, Japan) using gene-specific primers and Brilliant III Ultra-Fast SYBR Green qPCR Master Mix (Qiagen). The sequences of the gene-specific primers were as follows: Cd9 (NM_053018), 5′-GGCTATACCCACAAGGACGA-3′ and 5′-GCTATGCCACAGCAGTTCAA-3′ (product length: 140 bp); Sox2 (NM_001109181), 5′-CCATTTTCGTGGTCTTGTTT-3′ and 5′-TCAACCTGCATGGACATTTT-3′ (product length: 94 bp); Gh (NM_001034848) 5′-CAAGAGGCTGGTGCTTTACC-3′ and 5′-AATGTAGGCACGCTCGAACT-3′ (product length: 123 bp); Tshb (NM_013116) 5′-CGTGCTTTTCGCTCTTGCTT-3′ and 5′-TGGTCAGGCAGTAGGCACAC-3′ (product length: 101 bp); hypoxanthine phosphoribosyltransferase 1 (Hprt1) (NM_012583), 5′-CTCATGGACTGATTATGGACAGGAC-3′ and 5′-GCAGGTCAGCAAAGAACTTATAGCC-3′ (product length: 123 bp). Hprt1 expression was quantified for normalization [21]. The relative expression levels were calculated by comparing the cycle threshold (Ct) values for each target gene. Ct values were converted to relative gene expression levels using the 2-(ΔCt sample – ΔCt control) (2-ΔΔCT) method. The expression level of each target mRNA was analyzed in three different rats.
Statistical analysis
Data are presented as the mean ± SEM of the measured values for at least three preparations for each group of rats. The D’Agostino–Pearson test was used to assess normality and F-tests and Student’s t-tests were used for two-group comparisons. The Tukey–Kramer test was used for multiple group comparisons. Differences were considered significant at P-values < 0.05.
Results
Purification and characterization of CD9-positive cells from the IL
Supplementary Fig. 1 shows images of the hematoxylin and eosin staining of the pituitary gland treated with the vehicle, GHRH for 3 d (GHRH3d), PTU, no continuous stress (NCS), continuous stress (CS), and recovery for 3 days after CS (CS5d RC3d). Consistent with our previous observations [4, 13], cell bridges connecting the IL side and AL side of the MCL across Rathke’s cleft were found (Supplementary Fig. 1, arrowheads; Supplementary Fig. 2A, white arrowheads). In the adult AL, CD9/SOX2-positive cells were located in the MCL and parenchyma stem/progenitor cell niches (Supplementary Fig. 2A). We purified and isolated CD9-positive cells from the IL-side MCL to investigate their ability to differentiate. During smear preparation, we confirmed that most cells in the CD9-positive fraction were immunopositive for both CD9 and SOX2 (Supplementary Fig. 2B). Prior to induction, the pituispheres displayed immunoreactivity for both CD9 and SOX2 (Fig. 1A). After 14-day cultivation in the differentiation media, CD9/GH- or CD9/TSH-immunopositive cells (Fig. 1B, white arrowheads) and GH- or TSH-immunopositive cells were observed in the pituispheres (Fig. 1B, black arrowheads).
Fig. 1.
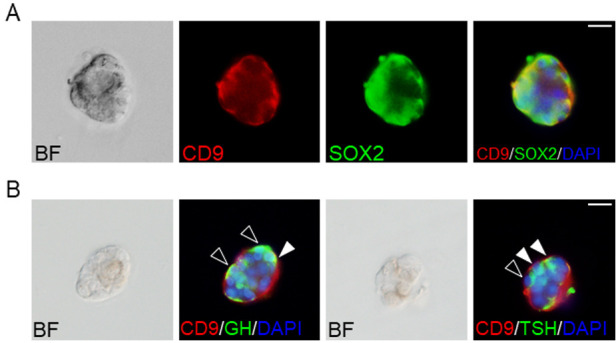
Pituispheres of CD9/SOX2-positive cells in the intermediate lobes (IL) side of the marginal cell layer (MCL). (A) Double immunofluorescence staining for CD9 (red) and SOX2 (green) using CD9-positive pituispheres. The left panel shows a bright field (BF) image of the pituisphere. The right panel shows the merged image with DAPI (blue). (B) Hormone-producing cells in the CD9-positive pituispheres after induction. BF images and merged images with DAPI (blue), CD9 (red), and GH (green), or TSH (green) are shown. Black and white arrowheads indicate GH or TSH single-positive cells and CD9/GH or CD9/TSH double-positive cells, respectively. Scale bars: 20 μm (A and B).
Changes in the proportion of GH or TSH cells in the AL
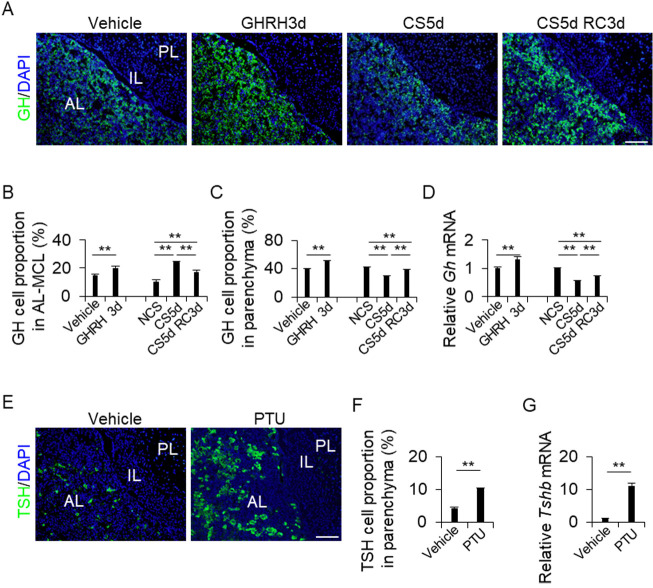
The pituitary glands of each treatment group (vehicle, GHRH3d, NCS, CS5d, and CS5d RC3d) were immunohistochemically stained for GH (Fig. 2A), and the GH-positive cells in the AL-side MCL and AL parenchyma were quantified (Figs. 2B and 2C). In both the AL-side MCL and AL parenchyma, GHRH3d treatment resulted in a higher proportion of GH cells than vehicle treatment (Figs. 2B and 2C). Intriguingly, the results of the CS5d and CS5d RC3d treatments differed between the AL-side MCL and AL parenchyma. The proportions were higher in the AL-side MCL (Fig. 2B) and lower in the AL parenchyma than those in the NCS control (Fig. 2C). In addition, the proportion in the CS5d RC3d treatment was lower than that in the CS5d treatment in the AL-MCL (Fig. 2B), but the opposite was observed in the AL parenchyma (Fig. 2C). Next, we analyzed the mRNA levels of Gh in the AL. The GHRH treatment group showed significantly higher Gh expression levels (P < 0.01) than the vehicle treatment group (Fig. 2D), whereas the CS5d and CS5d RC3d treatment groups showed lower Gh expression levels (Fig. 2D).
Fig. 2.
(A and E) Immunohistochemistry for GH (A) and TSH (E) in the vehicle, GHRH treatment for 3 d (GHRH3d), continuous stress for 5 days (CS5d), and recovery for 3 days after CS for 5 days (CS5d RC3d). DAPI (blue) was added to identify the nuclei. (B, C, and F) Proportions of GH cells or TSH cells from the DAPI-positive cells in each treatment. The cells were counted in the AL-side MCL (AL-MCL) and the AL parenchyma (Parenchyma). NCS: no continuous stress, PTU: 2-week propylthiouracil treatment. (D and G) mRNA levels of Gh (D) and Tshb (G) in the AL in each treatment (mean ± SEM, n = 5). Data were normalized to that of Hprt1. The Gh and Tshb mRNA levels were calculated as the ratios of the NCS and vehicle values. PL, posterior lobe; IL, intermediate lobe; AL, anterior lobe. Scale bars: 100 μm (A and E). ** P < 0.01.
The pituitary glands of the vehicle and PTU treatment groups were stained for TSH (Fig. 2E), and the TSH cells were counted in the AL-side MCL and AL parenchyma. TSH cells were not detected in the AL-side MCL (data not shown). The PTU-treated group showed a higher proportion of TSH cells than the vehicle control group (Fig. 2F). The PTU-treated group also showed higher Tshb expression in the parenchyma than the vehicle-treated group (Fig. 2G). The presence of the GHRH receptor (GHRHR) and TRH receptor (TR) in CD9-positive cells was also assessed immunohistochemically. The results showed that GHRHR and TR were expressed in CD9-positive cells in the IL-side MCL, AL-side MCL, and AL parenchyma (Supplementary Figs. 3A and 3B; white arrowheads).
Effect of GHRH and PTU on primary cultures of GH and TSH cells
To examine the effect of GHRH on GH cells, primary cultures of AL cells were incubated in the presence or absence of GHRH, and the proportion of CD9-positive cells and GH cells and the expression of Cd9, Sox2, and Gh were examined. Their proportions of CD9-positive cells and GH cells did not change after 24 h of GHRH treatment (Supplementary Figs. 4A, 4B). The expression of Cd9 and Sox2 in AL cells did not change after 1 and 24 h of GHRH treatment (Supplementary Figs. 4C and 4D), whereas Gh expression was upregulated after 1 h, but not 24 h, of GHRH treatment (Supplementary Fig. 4C). To examine the effect of PTU on TSH cells, we purified CD90-positive TSH cells [19] from the AL using a monoclonal anti-rat CD90 antibody and a pluriBead-cascade cell isolation system. Using a smear preparation of CD90-positive cells, the immunoreactivities for CD90 and TSH were verified (Supplementary Fig. 4E). Next, we analyzed the effects of PTU and TRH on TSH-positive cells. Neither 1 h nor 24 h of PTU treatment affected Cd9 and Tshb expression in TSH-positive cells (Supplementary Figs. 4F–4I). In contrast, 1 h, but not 24 h, of TRH treatment upregulated Tshb expression in TSH-positive cells (Supplementary Fig. 4H).
Changes in the proportion of CD9/SOX2-positive cells in the MCL and parenchyma of AL
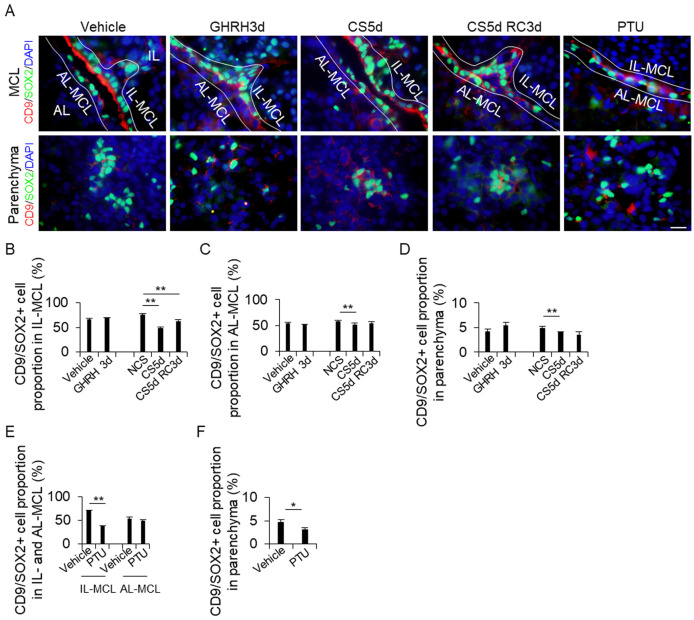
The pituitary glands of each treatment group were stained for CD9 and SOX2, and the number of double-positive cells was counted in the IL-side MCL, AL-side MCL, and AL parenchyma (Figs. 3A–3D). The proportion of CD9/SOX2-positive cells did not change after GHRH treatment (Figs. 3B–3D). However, the proportion of CD9/SOX2-positive cells in the CS5d treatment group was significantly lower than that in the NSC group in the IL side and AL side of the MCL and AL parenchyma (Figs. 3B–3D). The proportion of CD9/SOX2-positive cells in the PTU treatment group was lower than that in the vehicle treatment group in the IL-side MCL and AL parenchyma (Figs. 3E–3F).
Fig. 3.
Detection of CD9/SOX2-positive cells in the IL-side and AL-side MCL and the AL parenchyma. (A) Double immunohistochemistry for CD9 and SOX2 in the vehicle, GH treatment for 3 d (GHRH3d), continuous stress for 5 days (CS5d), recovery for 3 days after CS for 5 days (CS5d RC3d), and 2-week propylthiouracil (PTU) treatment. The first and second rows show the MCL and the parenchyma, respectively. DAPI (blue) was added to identify the nuclei. (B) Proportion of CD9/SOX2-positive cells from the DAPI-positive cells in each treatment. The cells were counted in the IL-side MCL (IL-MCL; B and E), AL-side MCL (AL-MCL; C and E), and AL parenchyma (Parenchyma; D and F). AL, anterior lobe; IL, intermediate lobe; MCL, marginal cell layer. Scale bars: 20 μm (A). ** P < 0.01. * P < 0.05.
Detection of CD9/GH- and CD9/TSH-positive cells in the MCL and AL parenchyma
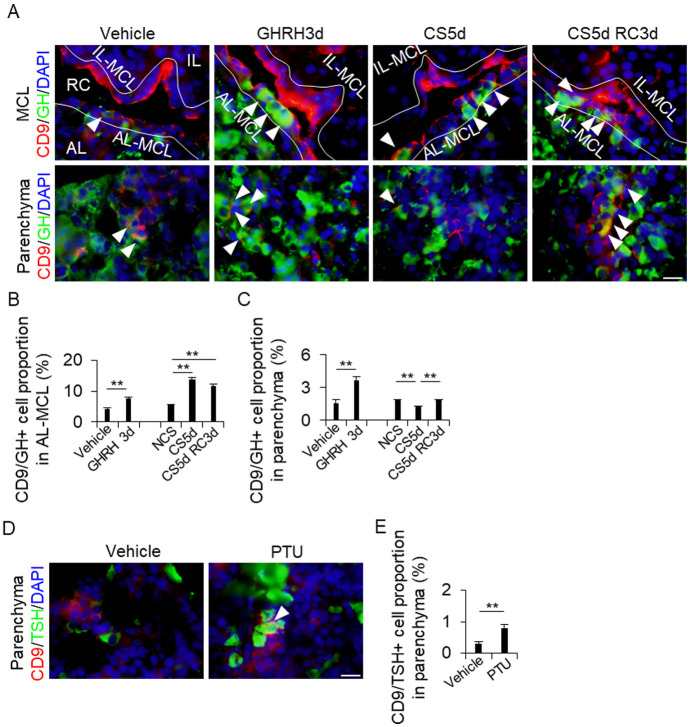
Since CD9/GH and CD9/TSH double-positive cells were observed in the CD9/SOX2-positive cells of the pituitary glands after induction (Fig. 1B, white arrowheads), we double-stained the pituitary glands of each treatment group for CD9 and GH or TSH to check whether the proportions of CD9/GH- and CD9/TSH-positive cells were altered by the treatments (Fig. 4A). In the AL-side MCL, the proportion of CD9/GH-positive cells increased in the GHRH3d, CS5d, and CS5d RC3d treatment groups compared to that in the vehicle and NCS control groups (Fig. 4B). In the AL parenchyma, the proportion of CD9/GH-positive cells also increased in the GHRH3d, CS5d, and RC3d treatment groups compared to that in the vehicle and CS5d groups (Fig. 4C). However, the number of CD9/GH-positive CS5d cells in the AL parenchyma was lower than that in the control parenchyma (Fig. 4C). CD9/TSH-positive cells were observed only in the AL parenchyma (Fig. 4D), and the proportion of CD9/TSH-positive cells in the PTU treatment group was higher than that in the vehicle treatment group (Fig. 4E).
Fig. 4.
Detection of CD9/GH- or CD9/TSH-positive cells in the AL-side MCL and the AL parenchyma. (A and D) Double immunohistochemistry for CD9 (red) and GH (green) in the vehicle, GH treatment for 3 days (GHRH3d), continuous stress for 5 days (CS5d), and recovery for 3 days after CS for 5 days (CS5d RC3d) (A), and for CD9 (red) and TSH (green) in the vehicle and 2-week propylthiouracil treatments (PTU) (D). DAPI (blue) was added to identify the nuclei. White arrowheads indicate CD9/GH- (A) or CD9/TSH- (D) positive cells. (B, C, and E) Proportion of CD9/GH- (B and C) or CD9/TSH-positive cells (E) from the DAPI-positive cells in each treatment. The cells were counted in the AL-side MCL (AL-MCL; B) and AL parenchyma (Parenchyma; C and E). AL, anterior lobe; IL, intermediate lobe; MCL, marginal cell layer; PL, posterior lobe; RC, Rathke’s cleft; IL-MCL, IL side of the MCL. Scale bars: 20 μm (right panel of A and D). ** P < 0.01.
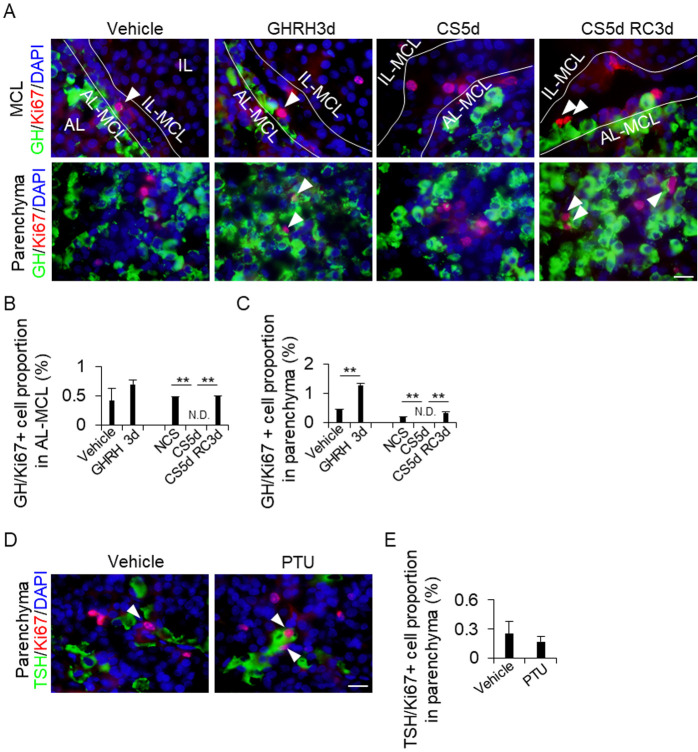
Detection of GH/Ki67- and TSH/Ki67-positive cells in the MCL and AL parenchyma
To examine whether the increase in the proportion of GH and TSH cells was due to the supply of CD9-positive cells or the self-proliferation of GH and TSH cells, the cells were stained for Ki67, a robust cell proliferation marker, and the number of GH/Ki67-positive (Fig. 5A) and TSH/Ki67-positive cells were counted (Fig. 5D). The proportion of GH/Ki67-positive cells in the AL-side MCL remained unchanged after GHRH treatment (Fig. 5A), whereas it increased in the AL parenchyma (Figs. 5B and 5C). In both the AL-side MCL and AL parenchyma, the proportion of GH/Ki67-positive cells decreased after CS5d treatment compared to that in the control treatment group. The number of these cells increased after CS5d RC3d treatment compared to that after CS5d treatment (Figs. 5B and 5C). We detected TSH/Ki67-positive cells in the AL parenchyma (Fig. 5C), but not in the AL-side MCL (data not shown). The proportion of these cells remained unchanged regardless of PTU treatment (Fig. 5E).
Fig. 5.
Proportion of GH/Ki67-positive cells in the IL-side and AL-side MCL and the AL parenchyma. (A and D) Double immunohistochemistry for GH (green) and Ki67 (red) in the vehicle, GH treatment for 3 days (GHRH3d), continuous stress for 5 days (CS5d), and recovery for 3 days after CS for 5 days (CS5d RC3d) (A), and for TSH (green) and Ki67 (red) in the vehicle and 2-week propylthiouracil treatments (PTU) (D). DAPI (blue) was added to identify the nuclei. White arrowheads indicate GH/Ki67- (A) or TSH/Ki67-positive cells (D). (B, C and E) Proportion of GH/Ki67-positive cells from the DAPI-positive cells in each treatment. The cells were counted in the AL-side MCL (AL-MCL; B) and AL parenchyma (Parenchyma; C and E). AL, anterior lobe; IL, intermediate lobe; MCL, marginal cell layer; IL-MCL, IL side of the MCL. Scale bars: 20 μm (A and B). ** P < 0.01.
Discussion
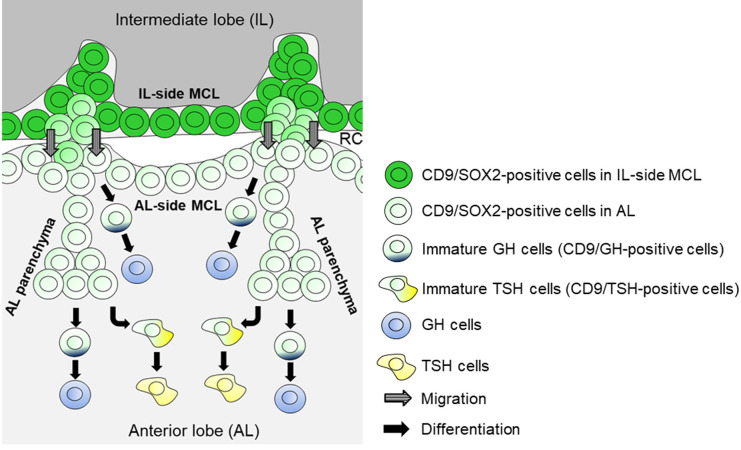
In the present study, we showed that the increase in the number of GH cells following GHRH treatment and after recovery from continuous stress may be attributable to the differentiation of CD9/SOX2-positive cells in the AL-side MCL and AL parenchyma (Fig. 6) and the proliferation of GH cells in the AL. In contrast, the increased proportion of TSH cells after PTU treatment may have been caused by the differentiation of CD9/SOX2-positive cells in the AL parenchyma (Fig. 6).
Fig. 6.
Schematic of GH or TSH cell turnover in the adult AL and the supply of CD9/SOX2-positive stem cells. CD9/SOX2-positive cells in the IL-side MCL migrate into the AL-side MCL and then into the AL parenchyma. GH cells are derived from CD9/SOX2-positive stem cells in the AL-side MCL and the AL parenchyma. TSH cells are derived from CD9/SOX2-positive stem cells in the AL parenchyma. RC, Rathke’s cleft.
CD9/SOX2-positive cells in the adult IL become tissue-resident stem/progenitor cells [4, 12]. With evidence of the presence of cell bridges across Rathke’s cleft between the IL-side- and AL-side MCL, we hypothesized that CD9/SOX2-positive cells migrate successively, starting from the IL-side MCL, the AL-side MCL, and then to the AL parenchyma, by changing their cellular properties towards more committed progenitor cells [4, 12]. In the present study, we isolated CD9/SOX2-positive cells from IL-side MCL and found that some of the cells were immunopositive for GH or TSH and expressed Cd9, which are supposedly immature hormone-producing cells, during differentiation in vitro. Similarly, we observed CD9/GH-positive cells in the AL-side MCL and AL parenchyma, and this proportion increased with GHRH treatment. Therefore, the change in the proportion of GH cells under the influence of GHRH may be partially due to the induction of Gh expression in CD9/SOX2-positive cells in addition to the self-proliferation of GH cells in the AL.
The proliferation of GH cells was promoted by GHRH treatment; however, it was almost entirely suppressed in rats that underwent 5 days of CS via suppression of ERK1⁄2 phosphorylation [16]. In the CS treatment group, the proportion of GH cells increased in the AL-side MCL. Concomitantly, we found that the proportion of CD9/SOX2-positive cells in the IL-side and AL-side MCL decreased, while that of CD9/GH-positive cells in the AL-side MCL increased. These results suggest that GH cells may be formed from CD9/SOX2-positive stem/progenitor cells in the IL-side and AL-side MCL (Fig. 6). In contrast, in the AL parenchyma, the proportions of GH-, CD9/SOX2-, CD9/GH-, and GH/Ki67-positive cells decreased after CS treatment. This evidence suggests that the supply of GH cells from CD9/SOX2-positive cells became insufficient after CS treatment, and GH cells self-proliferated in the AL to compensate for the loss of GH cell supply. However, it remains unclear why the proliferation of GH cells in the AL-side MCL and AL parenchyma is suppressed by CS treatment.
After CS recovery, the proportions of GH- and CD9/GH-positive cells in the AL-side MCL increased compared to those subjected to CS5d treatment, whereas the proportion of CD9/SOX2-positive cells decreased in the IL-side MCL. Furthermore, analysis of CS recovery analysis showed that the proportions of CD9/GH-positive cells in the AL-side MCL and parenchyma increased compared to those in the control and CS5d, respectively, while the GH/Ki67-positive cells in the AL-side MCL and parenchyma increased compared to those subjected to CS5d. This evidence suggests that CD9/SOX2-positive cells change their properties to more committed progenitor cells during migration from the IL-side and AL-side MCL to the AL parenchyma, eventually differentiate into GH cells, and then self-proliferate in the AL parenchyma. The proportion of GH/Ki67-positive cells in the AL-side MCL remained unchanged after GHRH treatment but increased after CS5d RC3d treatment compared to that after CS5d. This difference may reflect the degree of induction of proliferation (within the physiological range in CS5d and RC3d vs. the pathological range in GHRH treatment).
Regarding the effect of PTU on TSH cells, the number of CD9/SOX2-positive cells in the IL-side MCL and AL parenchyma decreased, while the number of TSH- and CD9/TSH-positive cells increased. Furthermore, PTU treatment did not alter the proliferative activity of TSH cells in the parenchyma. Interestingly, CD9/TSH-positive cells were not observed in the AL-side MCL regardless of PTU treatment. This evidence indicates that TSH cells differentiate from CD9/SOX2-positive cells in the AL parenchyma (Fig. 6). Our previous study showed that PRL cells in the rat pituitary gland were derived from CD9/SOX2-positive cells in the AL-side MCL and AL parenchyma during pregnancy and lactation (physiological) and after DES treatment (pathological) [13]. PRL, GH, and TSH cells belong to the Pit1 lineage [14, 15]; however, our results suggest that the differentiation of each Pit1 lineage-type cell from CD9/SOX2-positive cells occurs in different niches. In the AL parenchyma, hormone-producing cells were unevenly distributed. GH cells are primarily located in the lateral wings of the AL and are scattered throughout the median wedge [22, 23]. PRL cells are randomly distributed throughout the lobe while TSH cells are mostly found in the anteromedial portion of the AL [22, 23]. The CD9/SOX2-positive cells in the anteromedial portion of the AL may preferentially differentiate into TSH cells. Since the proliferation of GH and PRL cells [4, 12], but not TSH cells, was observed in the AL parenchyma in both pathological and physiological contexts, and since the percentages of hormone-producing cells in the AL were different between GH (~50%), PRL (~15%), and TSH cells (~5%) [22, 23], these differences may be attributed to their proliferative capacity.
In conclusion, CD9/SOX2-positive stem cells in the AL-side MCL have the potential to supply GH cells when an increase in the GH cell population is required, and cells in the AL parenchyma may respond to the demand for TSH cells in the adult pituitary gland. These findings may describe one of the mechanisms by which Pit1 lineage cells form from adult stem cells and may be relevant in future studies focusing on corticotroph and gonadotroph differentiation processes in the pituitary gland.
Conflict of interests
The authors have nothing to declare.
Supplementary
Acknowledgments
We thank the Joint Usage/Research Center for Endocrine/Metabolism, Institute for Molecular and Cellular Regulation, Gunma University (www.imcr.gunma-u.ac.jp/activity/activity3) for providing the antibodies. We thank H. Hirashima for the technical assistance in this study. This work was supported by a JSPS KAKENHI grants (No. 22K06798 to K.H. and No. 23K10968 to R.H.) and the Kyorin University Individual Investigator Award from the Faculty of Health Sciences, Kyorin University.
References
- 1.Kato Y, Yoshida S, Kato T. New insights into the role and origin of pituitary S100β-positive cells. Cell Tissue Res 2021; 386: 227–237. [DOI] [PubMed] [Google Scholar]
- 2.Vila-Porcile E. The network of the folliculo-stellate cells and the follicles of the adenohypophysis in the rat (pars distalis). Z Zellforsch Mikrosk Anat 1972; 129: 328–369 (in French). [PubMed] [Google Scholar]
- 3.Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 2008; 105: 2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horiguchi K, Fujiwara K, Takeda Y, Nakakura T, Tsukada T, Yoshida S, Hasegawa R, Takigami S, Ohsako S. CD9-positive cells in the intermediate lobe of the pituitary gland are important supplier for prolactin-producing cells in the anterior lobe. Cell Tissue Res 2021; 385: 713–726. [DOI] [PubMed] [Google Scholar]
- 5.Horiguchi K, Fujiwara K, Yoshida S, Nakakura T, Arae K, Tsukada T, Hasegawa R, Takigami S, Ohsako S, Yashiro T, Kato T, Kato Y. Isolation and characterisation of CD9-positive pituitary adult stem/progenitor cells in rats. Sci Rep 2018; 8: 5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vankelecom H, Chen J. Pituitary stem cells: where do we stand? Mol Cell Endocrinol 2014; 385: 2–17. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Nishimura N, Ueharu H, Kanno N, Higuchi M, Horiguchi K, Kato T, Kato Y. Isolation of adult pituitary stem/progenitor cell clusters located in the parenchyma of the rat anterior lobe. Stem Cell Res (Amst) 2016; 17: 318–329. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 2005; 146: 3985–3998. [DOI] [PubMed] [Google Scholar]
- 9.Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev 2012; 21: 801–813. [DOI] [PubMed] [Google Scholar]
- 10.Horiguchi K, Yoshida S, Tsukada T, Fujiwara K, Nakakura T, Hasegawa R, Takigami S, Ohsako S. Cluster of differentiation (CD) 9-positive mouse pituitary cells are adult stem/progenitor cells. Histochem Cell Biol 2021; 155: 391–404. [DOI] [PubMed] [Google Scholar]
- 11.Horiguchi K, Yoshida S, Tsukada T, Nakakura T, Fujiwara K, Hasegawa R, Takigami S, Ohsako S. Expression and functions of cluster of differentiation 9 and 81 in rat mammary epithelial cells. J Reprod Dev 2020; 66: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horiguchi K, Fujiwara K, Tsukada T, Nakakura T, Yoshida S, Hasegawa R, Takigami S, Ohsako S. CD9-positive cells in the intermediate lobe migrate into the anterior lobe to supply endocrine cells. Histochem Cell Biol 2021; 156: 301–313. [DOI] [PubMed] [Google Scholar]
- 13.Horiguchi K, Fujiwara K, Tsukada T, Nakakura T, Yoshida S, Hasegawa R, Takigami S. Differentiation of stem progenitor CD9/SOX2-positive cells is promoted with increased prolactin-producing and endothelial cells in the pituitary. J Reprod Dev 2022; 68: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camper SA, Saunders TL, Katz RW, Reeves RH. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics 1990; 8: 586–590. [DOI] [PubMed] [Google Scholar]
- 15.Lin SC, Li S, Drolet DW, Rosenfeld MG. Pituitary ontogeny of the Snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development 1994; 120: 515–522. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa T, Sei H, Konishi H, Shishioh-Ikejima N, Kiyama H. The absence of somatotroph proliferation during continuous stress is a result of the lack of extracellular signal-regulated kinase 1/2 activation. J Neuroendocrinol 2012; 24: 1335–1345. [DOI] [PubMed] [Google Scholar]
- 17.Charlton HM, Worth RW. The ultrastructure of the anterior pituitary gland of the vole, Microtus agrestis, in normal and experimentally manipulated animals. J Anat 1975; 120: 69–79. [PMC free article] [PubMed] [Google Scholar]
- 18.Torres AI, Pasolli HA, Maldonado CA, Aoki A. Changes in thyrotroph and somatotroph cell populations induced by stimulation and inhibition of their secretory activity. Histochem J 1995; 27: 370–379. [PubMed] [Google Scholar]
- 19.Horiguchi K, Nakakura T, Yoshida S, Tsukada T, Kanno N, Hasegawa R, Takigami S, Ohsako S, Kato T, Kato Y. Identification of THY1 as a novel thyrotrope marker and THY1 antibody-mediated thyrotrope isolation in the rat anterior pituitary gland. Biochem Biophys Res Commun 2016; 480: 273–279. [DOI] [PubMed] [Google Scholar]
- 20.Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 2011; 480: 57–62. [DOI] [PubMed] [Google Scholar]
- 21.Horiguchi K, Fujiwara K, Tsukada T, Nakakura T, Yoshida S, Hasegawa R, Takigami S. The multiciliated cells in Rathke’s cleft express CYP26A1 and respond to retinoic acid in the pituitary. Cell Tissue Res 2022; 388: 583–594. [DOI] [PubMed] [Google Scholar]
- 22.Horvath E, Kovacs K. Fine structural cytology of the adenohypophysis in rat and man. J Electron Microsc Tech 1988; 8: 401–432. [DOI] [PubMed] [Google Scholar]
- 23.Horvath E, Kovacs K. Pituitary gland. Pathol Res Pract 1988; 183: 129–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.