Abstract
Background
In the 2022 European Society of Cardiology (ESC) and the European Respiratory Society (ERS) guidelines, the diagnostic criteria for pulmonary hypertension (PH) included a reduced mean pulmonary artery pressure (mPAP) of 20 mmHg (mPAP >20 mmHg). This study aimed to reassess cardiovascular metrics on computed tomography pulmonary angiography (CTPA) for chronic thromboembolic pulmonary hypertension (CTEPH) to optimize the timely diagnosis of patients with suspected PH.
Methods
Patients with suspected CTEPH who underwent CTPA and right heart catheterization (RHC) between January 2019 and December 2022 in China-Japan Friendship Hospital were retrospectively included. They were grouped into CTEPH and non-PH groups according to the new and old criteria (2022 and 2015 ESC/ERS guidelines) for the diagnosis of PH. Cardiovascular metrics including the main pulmonary artery diameter (MPAd), Cobb angle, and right ventricular free wall thickness (RVWT), among others, were measured. The correlation of these metrics with hemodynamic data was analyzed with Spearman rank correlation analysis, while the differences in cardiovascular metrics between the updated (mPAP >20 mmHg) and old PH criteria (mPAP ≥25 mmHg) were compared with independent samples t-test or the Mann-Whitney test. Receiver operator characteristic (ROC) curve analysis was performed for the prediction model.
Results
The study enrolled 180 patients (males n=86; age 55.5±12.0 years old). According to the old guidelines, 119 patients were placed into the PH group (mPAP ≥25 mmHg) , while according to the new guidelines, 130 patients were placed into the PH group (mPAP >20 mmHg). Cardiovascular metrics on CTPA between the updated and old guidelines were comparable (P>0.05). Compared to other metrics, an MPAd of 30.4 mm exhibited the highest area under the curve (AUC: 0.934±0.021), with a sensitivity of 0.88 and specificity of 0.90. MPAd [odds ratio (OR) =1.271], transverse diameter of the right ventricle (RVtd; OR =1.176), Cobb angle (OR =1.108), and RVWT (OR =3.655) were independent factors for diagnosing CTEPH (P<0.05). Cobb angle, right and left ventricular transverse diameter ratio, and right and left ventricular area ratio moderately correlated with mPAP (r=0.586, r=0.583, r=0.629) and pulmonary vascular resistance (PVR) (r=0.613, r=0.593, r=0.642).
Conclusions
Cardiovascular metrics on CTPA were comparable between the new and old guidelines for CTEPH diagnosis. Cardiovascular metrics on CTPA can noninvasively assess the hemodynamics of patients with CTEPH.
Keywords: Pulmonary hypertension (PH), chronic thromboembolic pulmonary hypertension (CTEPH), computed tomography pulmonary angiography (CTPA), hemodynamics
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a common and important cause of pulmonary hypertension (PH). It is characterized by pulmonary artery occlusion from organized thromboembolic material, causing progressive elevation of pulmonary vascular resistance (PVR) and mean pulmonary artery pressure (mPAP) (1,2). Over time, this pathological process can culminate in right heart failure and even death (1,2). CTEPH can be near-cured with pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA), and medical treatment (1). However, its nonspecific symptoms during the early stage cause a median diagnostic delay of 14 months from the onset of symptoms (3). Computed tomography pulmonary angiography (CTPA) is widely employed for the assessment in CTEPH, and an increased main pulmonary artery diameter (MPAd) and the ratio of MPAd and ascending aorta diameter (MPAd/AAd) along with right heart enlargement on CTPA may indicate the presence of PH (4-18).
In the 2015 European Society of Cardiology (ESC) and the European Respiratory Society (ERS) guidelines, mPAP and PVR for the diagnosis of PH were defined as ≥25 mmHg and ≥3 Wood units (WU), respectively (2,19), a standard that has long been used in clinical work and research. In 2022, the ESC and ERS updated the hemodynamics of PH by lowering the thresholds for mPAP and PVR in healthy individuals to 20 mmHg and 2 WU, respectively (1). This aims to enable patients with suspected PH to receive a timely diagnosis. A meta-analysis reported CTPA had high sensitivity and specificity in the detection of CTEPH when evaluated by expert radiologists (16). However, at present, no research has been conducted to investigate whether the threshold of cardiovascular metrics on CTPA for diagnosing PH is affected by changes in diagnostic criteria. Thus, we aimed to compare cardiovascular metrics on CTPA in the prediction of PH under the updated and old criteria and to reevaluate the metrics that are capable of detecting PH in patients at the early stage of CTEPH. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-250/rc).
Methods
Population and study design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Ethics Board of China-Japan Friendship Hospital (No. 2022-KY-048). Individual consent for this retrospective analysis was waived.
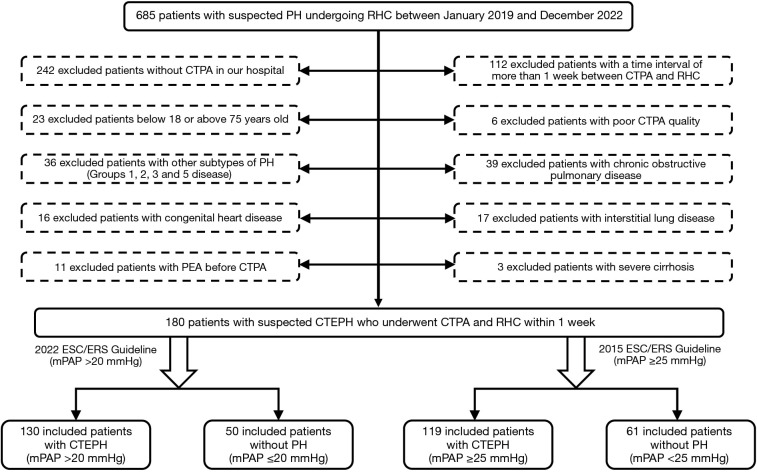
For this investigation, 685 participants who underwent right heart catheterization (RHC) between January 2019 and December 2022 were identified from the China-Japan Friendship Hospital. Figure 1 illustrates the process of selecting participants who had CTPA with fully visible lung fields within 1 week after RHC. From this group, 4 cohorts were formed based on the 2015 ESC/ERS guidelines (old) and 2022 ESC/ERS guidelines (updated), and included patients were respectively divided into the following groups: CTEPH patients with mPAP >20 mmHg and control patients with mPAP ≤20 mmHg; and CTEPH patients with mPAP ≥25 mmHg and control patients with mPAP <25 mmHg.
Figure 1.
Flowchart detailing participant selection. PH, pulmonary hypertension; RHC, right heart catheterization; CTEPH, chronic thromboembolic PH; CTPA, computed tomography pulmonary angiography; PEA, pulmonary endarterectomy; ESC, European Society of Cardiology; ERS, European Respiratory Society; mPAP, mean pulmonary arterial pressure.
The exclusion criteria were as follows: (I) patients younger than 18 years old or older than 75 years old; (II) patients without CTPA or with poor CTPA quality or incomplete RHC data in our hospital; (III) patients with evidence of chronic obstructive pulmonary disease or interstitial lung; (IV) patients with congenital heart disease or severe cirrhosis; and (V) patients who had undergone PEA before CTPA.
CTPA scan protocol
All patients underwent supine CTPA imaging with either a 256-row CT (GE Revolution CT, GE HealthCare, Chicago, IL, USA) or a 320-row CT (Aquilion ONE, Canon Medical Systems, Otawara, Japan) at the end of expiration, with the lung base to apex being covered. The specific scan parameters for the GE Revolution CT were as follows: a tube rotation speed of 0.28 s/rotation; tube voltage determined via kilovolt (KV) intelligent decision technology (KV assist; 100 and 120 KV), tube current determined via 3D automatic tube current modulation (Smart-milliampere), a pitch of 0.992:1, the slices × collimator width is 256×0.625 mm, and a reconstruction image slice thickness and spacing of 0.625 mm. Meanwhile, the specific scan parameters for the Aquilion ONE were as follows: a tube rotation speed of 0.35 s/rotation, a tube voltage of 120 kVp, a tube current determined via automatic tube current modulation, the Slices × collimator width is 320×0.5 mm, and a reconstruction image slice thickness and spacing of 0.5 mm.
A high-pressure injector was used to administer either iodinated contrast agents (350 mg I/mL) or iodopropamide (370 mg I/mL) through the antebrachial vein. A double-barrel syringe containing contrast agent and saline solution was used, with a flow rate of 5 mL/s, a total contrast agent volume of 60–75 mL, and 30 mL of saline solution. The contrast agent detection method used was automatically triggered, with a trigger threshold of 80 Hounsfield units (HU) in the main pulmonary artery.
RHC
All patients underwent RHC through the right internal jugular or femoral vein with a 6F Swan-Ganz thermodilution catheter (Bioptimal International, Tokyo, Japan). Hemodynamic data, including mPAP, ratio of systolic to diastolic PAP (sPAP/dPAP), mean right atrial pressure (mRAP), mean pulmonary artery wedge pressure (PAWP), ratio of systolic to diastolic blood pressure (sBP/dBP), cardiac output (CO), and cardiac index were obtained during the procedure. PVR was calculated using the following formula (20): PVR = (mPAP – mean PAWP)/CO WU.
Image analysis
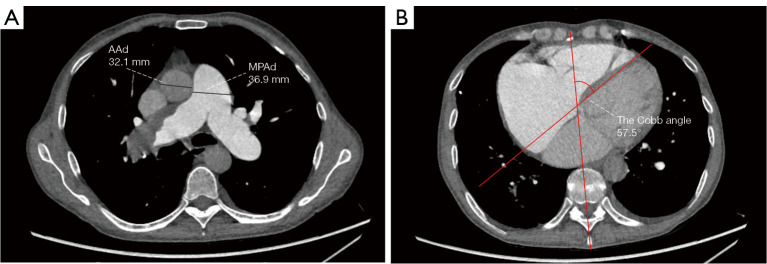
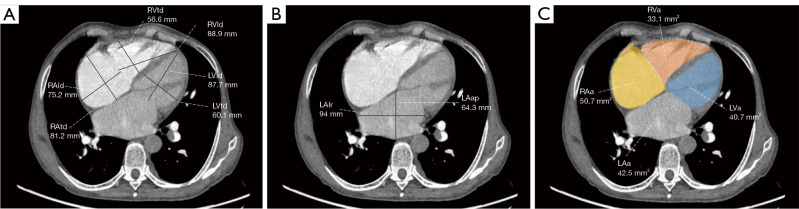
Two radiologists with 5 years of experience in chest imaging measured the cardiovascular metrics on CTPA and reached a consensus, with discussion being used to resolve any differences. On the transversal images of CTPA (Figure 2A), the widest diameter of the main pulmonary artery (MPAd) and the widest diameter of the ascending aorta (AAd) at the same level were measured. The Cobb angle (Figure 2B) was considered to be the rotation angle between the interventricular septum and the line connecting the midpoint of the sternum and the thoracic vertebral spinous process. Meanwhile, a radiologist with 15 years of experience completed multiplane reconstruction and measured the following: the longest longitudinal diameter of the right ventricle (RVld); the longest longitudinal diameter of the left ventricle (LVld); the transverse diameter of the right ventricle (RVtd); the transverse diameter of left ventricle (LVtd) (Figure 3A); the longest longitudinal diameter of the right atrium (RAld) and the transverse diameter of the right atrium (RAtd) (Figure 3A); the longest anteroposterior dimension of the left atrium (LAap) (Figure 3B); the widest left-right dimension of the left atrium (LAlr) (Figure 3B); and the maximum area of the right ventricle (RVa), left ventricle (LVa), right atrium (RAa), and left atrium (LAa) (Figure 3C) on 4-chamber views. The right ventricular free wall thickness (RVWT) and interventricular septal thickness (IVST) at CTPA sagittal position images are also measured and shown in Figure 4. The final measurement result was the average of 3 repeated measurements.
Figure 2.
The vascular diameters and Cobb angle measurement from transversal computed tomography pulmonary angiography images. (A) The widest MPAd and the widest AAd at the same level were measured at standard axial images. (B) The Cobb angle, the angle between the interventricular septum and the line connecting the midpoint of the sternum and the thoracic vertebral spinous process, was measured during diastole in the transversal image. MPAd, diameter of the main pulmonary artery; AAd, diameter of the ascending aorta.
Figure 3.
Measurements of the diameters and areas in 4-chamber-view computed tomography pulmonary angiography. (A) The longest longitudinal and transverse diameters of the biventricles and right atrium are illustrated for reformatted 4-chamber views. The transverse axis is parallel to the line connecting the heart valves. The ventricle longitudinal line is the midpoint of the heart valve and the line connecting the apex of the heart, and the right atrium longitudinal line is perpendicular to the transverse axis. (B) The longest LAap and the widest LAlr are illustrated for reformatted 4-chamber views. The LAap is the largest straight-line distance connecting the front and back walls of the left atrium, and the LAlr is perpendicular to the LAap. (C) The maximum 4-chamber heart area was measured on the reformatted 4-chamber position. RVtd, transverse diameter of the right ventricle; LVtd, transverse diameter of the left ventricle; RVld, longitudinal diameter of the right ventricle; LVld, longitudinal diameter of the left ventricle; RAld, longitudinal diameter of the right atrium; RAtd, transverse diameter of the right atrium; LAlr, left-right dimension of the left atrium; LAap, anteroposterior dimension of the left atrium; RVa, area of the right ventricle; LVa, area of the left ventricle; RAa, right atrial area; LAa, left atrial area.
Figure 4.
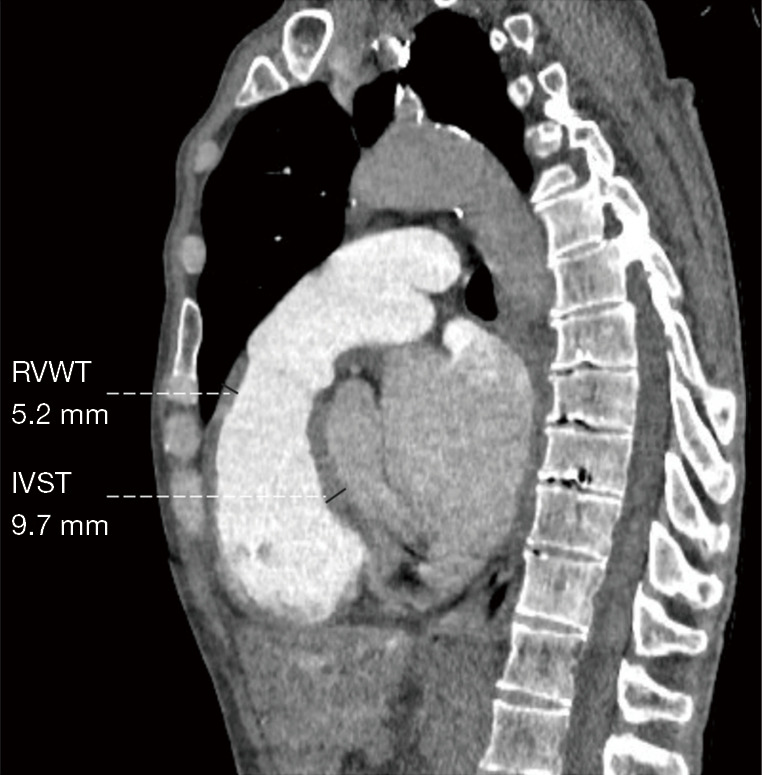
Measurement of the RVWT and IVST on the sagittal position obtained through the multiplanar reconstruction method. RVWT, right ventricular free wall thickness; IVST, interventricular septal thickness.
Statistical analysis
Statistical analysis was performed using IBM 27.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test and Shapiro-Wilks test were used to assess the normality of the variables. Normal data are expressed as mean ± standard deviation (SD), and the independent samples t-test was used for comparison in different groups. Nonnormal data are expressed as the median [interquartile range (IQR)], and the Mann-Whitney test was used for multiple comparisons among 3 groups. Count data are expressed as frequency (percentage), and the chi-squared test was used for the comparison of different groups. Univariate and stepwise multivariate binary logistic regression analysis was used to evaluate the independent predictors for CTEPH, and receiver operator characteristic (ROC) curve analysis was performed for cardiovascular parameters and prediction models, with the area under the curve (AUC), sensitivity, and specificity being calculated. The correlations between CTPA cardiovascular metrics, hemodynamics, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were analyzed using Spearman rank correlation analysis. The classification of correlation coefficient (r) was as follows: r≤0.3 indicated no or a very weak correlation, 0.3<r≤0.5 indicated low correlation, 0.5<r≤0.8 indicated moderate correlation, and r>0.8 indicated high correlation. Interobserver consistency was evaluated using intraclass correlation coefficient (ICC), with an ICC greater than 0.8 indicating high consistency. A 2-sided P value of less than 0.05 indicated statistical significance.
Results
Patient characteristics
This study included 180 patients (males n=86; age 55.5±12.0 years old) (Figure 1). Table 1 indicates the demographics, hemodynamics, and clinical characteristics of our study cohort. Patients were placed into groups of mPAP ≤20 mmHg (n=50) and mPAP >20 mmHg (n=130) according to the 2022 ESC/ERS guidelines (1). Among the 50 patients without PH (mPAP =14.7±3.3 mmHg; PVR =1.3 WU), there were 43 patients with chronic thromboembolic pulmonary disease (CTEPD), 4 patients with Takayasu arteritis, 2 patients with fibrosing mediastinitis, and 1 patient with Behcet syndrome. All 130 patients with PH (mPAP >20 mmHg) were diagnosed with CTEPH. Table 1 shows that there were no significant differences in age, gender, or body mass index (BMI) between patients with and without PH (P>0.05). According to the 2015 ESC/ERS guidelines, there were 61 patients with mPAP <25 mmHg and 119 patients with mPAP ≥25 mmHg who were diagnosed as CTEPH. Table 1 also indicates that the age, gender, and BMI between the patients with CTEPH and mPAP >20 mmHg and those with mPAP ≥25 mmHg were comparable (P>0.05).
Table 1. Demographic characteristics and hemodynamics of all included patients.
| Characteristics | Total patients (n=180) | mPAP ≤20 mmHg group (n=50) | mPAP > 20 mmHg group (n=130) | mPAP ≥25 mmHg group (n=119) | P valuea | P valueb |
|---|---|---|---|---|---|---|
| Age (years) | 55.5±12.0 | 53.2±12.7 | 56.4±11.6 | 56.3 ±11.2 | 0.101 | 0.916 |
| Gender (male/female) | 86/94 | 26/24 | 60/70 | 54/65 | 0.485 | 0.902 |
| BMI (kg/m2) | 24.5±3.4 | 24.9±3.5 | 24.4±3.3 | 24.3±3.2 | 0.291 | 0.803 |
| NT-proBNP (pg/mL) | 203 [64–876] | 51 [20–84] | 469 [128–1,177] | 518 [170–1,256] | <0.001** | 0.634 |
| 6MWD (m) | 410±116 | 498±89 | 387±112 | 377±111 | <0.001** | 0.529 |
| WHO FC I/II/III/IV | 48/64/53/15 | 34/9/6/1 | 14/55/47/14 | 10/50/45/14 | <0.001** | 0.927 |
| Resting hemodynamics | ||||||
| mPAP (mmHg) | 32.0±14.2 | 14.7±3.3 | 38.6±10.8 | 40.3±10.1 | <0.001** | 0.275 |
| sPAP (mmHg) | 57.0±26.3 | 26.5±6.9 | 68.8±21.0 | 71.7±19.4 | <0.001** | 0.253 |
| dPAP (mmHg) | 18.6±9.0 | 7.8±3.2 | 22.8±6.7 | 23.5±6.5 | <0.001** | 0.345 |
| mRAP (mmHg) | 4 [2–6] | 2 [0.8–4] | 5 [2–7] | 5 [2–7] | <0.001** | 0.774 |
| PAWP (mmHg) | 9.4±2.9 | 9.2±3.0 | 9.5±2.8 | 9.4±2.8 | 0.504 | 0.665 |
| sBP (mmHg) | 131.3±19.8 | 133.2±20.3 | 130.5±19.6 | 130.5±19.8 | 0.434 | 0.985 |
| dBP (mmHg) | 82.9±13.5 | 81.3±12.7 | 83.6±13.8 | 83.9±13.9 | 0.323 | 0.878 |
| PVR (Wood units) | 6.3 [2.1–11.2] | 1.3 [0.7–1.8] | 8.8 [5.8–13.5] | 9.6 [6.3–13.8] | <0.001** | 0.408 |
| CO (L/min) | 3.7±1.3 | 4.6±1.3 | 3.4±1.1 | 3.3±1.1 | <0.001** | 0.721 |
| Cardiac index (L/min/m2) | 2.2±0.7 | 2.6±0.7 | 2.0±0.6 | 1.95±0.6 | <0.001** | 0.751 |
Data are presented as mean ± standard deviation, median [interquartile range], or number (frequency). a, statistical difference between the mPAP >20 mmHg group and the mPAP ≤20 mmHg group; b, statistical difference between the mPAP >20 mmHg group and the mPAP ≥25 mmHg group. **, P<0.001 is considered statistically significant. mPAP, mean pulmonary arterial pressure; BMI, body mass index; NT-proBNP, N-terminal pro-brain natriuretic peptide; 6MWD, 6-minute walking distance; WHO FC, World Health Organization functional class; sPAP, systolic pulmonary arterial pressure; dPAP, diastolic pulmonary arterial pressure; mRAP, mean right atrial pressure; PAWP, pulmonary artery wedge pressure; sBP, systolic blood pressure; dBP, diastolic blood pressure; PVR, pulmonary vascular resistance; CO, cardiac output.
Cardiovascular metrics on CTPA
Table 2 shows the interobserver ICC for cardiovascular metrics from CTPA in all patients, which ranged from 0.818 to 0.977 (P<0.01). Based on the new guidelines, patients with CTEPH had a higher Cobb angle, MPAd, RVtd, RVld, RAtd, RAld, RVa, RAa, RVWT, MPAd/AAd, RVtd/LVtd, RVa/LVa, and RVWT/IVST compared to the patients without PH, as shown in Table 2 (P<0.05). However, Table 3 demonstrates that these cardiovascular metrics including Cobb angle, MPAd, RVtd, RVld, RAtd, RAld, RVa, RAa, RVWT, MPAd/AAd, RVtd/LVtd, RVa/LVa, and RVWT/IVST were comparable between the 2022 and 2015 ESC/ERS criteria (P>0.05).
Table 2. The cardiovascular parameters of CTPA in patients with chronic thromboembolic pulmonary hypertension (mPAP >20 mmHg) and without pulmonary hypertension (mPAP ≤20 mmHg) according to the 2022 ESC/ERS guidelines.
| Variable | Interobserver agreement(ICC, N=180) | Group without PH, mPAP ≤20 mmHg (N=50) | CTEPH group, mPAP >20 mmHg (N=130) |
P value |
|---|---|---|---|---|
| Cobb angle (degree) | 0.970 | 36.7±8.1 | 54.2±13.4 | <0.001** |
| MPAd (mm) | 0.961 | 26.7±3.5 | 35.6±5.4 | <0.001** |
| AAd (mm) | 0.960 | 32.8±5.3 | 32.2±4.8 | 0.447 |
| RVtd (mm) | 0.963 | 35.8±4.0 | 47.7±9.7 | <0.001** |
| RVld (mm) | 0.974 | 67.2±10.4 | 75.0±9.5 | <0.001** |
| RAtd (mm) | 0.912 | 43.3±6.4 | 54.9±11.7 | <0.001** |
| RAld (mm) | 0.904 | 39.4±8.0 | 50.4±11.6 | <0.001** |
| LVtd (mm) | 0.974 | 39.4±6.9 | 36.0±8.0 | 0.009* |
| LVld (mm) | 0.929 | 70.0±8.9 | 67.4±9.7 | 0.096 |
| LAlr (mm) | 0.870 | 55.2±8.4 | 56.3±9.2 | 0.462 |
| LAap (mm) | 0.874 | 39.2±7.3 | 39.0±7.0 | 0.90 |
| RVa (mm2) | 0.957 | 18.7±5.3 | 29.0±9.3 | <0.001** |
| RAa (mm2) | 0.947 | 15.0±4.7 | 24.4±10.5 | <0.001** |
| LVa (mm2) | 0.961 | 24.2±6.5 | 21.6±6.8 | 0.029* |
| LAa (mm2) | 0.928 | 17.5±4.9 | 16.6±5.3 | 0.172 |
| RVWT (mm) | 0.818 | 3.1±1.1 | 5.5±1.6 | <0.001** |
| IVST (mm) | 0.942 | 9.9±2.1 | 9.2±2.1 | 0.056 |
| MPAd/AAd ratio | 0.971 | 0.83±0.15 | 1.13±0.24 | <0.001** |
| RVtd/LVtd ratio | 0.974 | 0.93±0.14 | 1.41±0.50 | <0.001** |
| RVa/LVa ratio | 0.977 | 0.79±0.16 | 1.5±0.80 | <0.001** |
| RVWT/IVST ratio | 0.866 | 0.32±0.11 | 0.63±0.22 | <0.001** |
Continuous variables are expressed as mean value ± standard deviation. *, P<0.05 and **, P<0.001 are considered statistically significant. CTPA, computed tomography pulmonary angiography; mPAP, mean pulmonary arterial pressure; ESC, European Society of Cardiology; ERS, European Respiratory Society; ICC, intraclass correlation coefficient; PH, pulmonary hypertension; CTEPH, chronic thromboembolic pulmonary hypertension; MPAd, diameter of the main pulmonary artery; AAd, diameter of the ascending aorta; RVtd, transverse diameter of the right ventricle; RVld, longitudinal diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RAld, longitudinal diameter of the right atrium; LVtd, transverse diameter of the left ventricle; LVld, longitudinal diameter of the left ventricle; LAlr, left-right diameter of the left atrium; LAap, anteroposterior diameter of the left atrium; RVa, area of the right ventricle; RAa, area of the right atrium; LVa, area of the left ventricle; LAa, area of the left atrium; RVWT, right ventricular free wall thickness; IVST, interventricular septal thickness.
Table 3. Cardiovascular metrics on CTPA in patients with chronic thromboembolic pulmonary hypertension according to the 2015 (mPAP ≥25 mmHg) and 2022 (mPAP >20 mmHg) ESC/ERS guidelines.
| Metric on CTPA | CTEPH group, mPAP >20 mmHg (N=130) | CTEPH group, mPAP ≥25 mmHg (N=119) | P value |
|---|---|---|---|
| Cobb angle (degree) | 54.2±13.4 | 55.6±12.9 | 0.417 |
| MPAd (mm) | 35.6±5.4 | 36.1±5.2 | 0.542 |
| AAd (mm) | 32.2±4.8 | 32.1±4.7 | 0.916 |
| RVtd (mm) | 47.7±9.7 | 48.7±9.3 | 0.405 |
| RVld (mm) | 75.0±9.5 | 75.6±9.2 | 0.581 |
| RAtd (mm) | 54.9±11.7 | 55.7±11.6 | 0.575 |
| RAld (mm) | 50.4±11.6 | 51.0±11.8 | 0.665 |
| LVtd (mm) | 36.0±8.0 | 35.7±8.0 | 0.734 |
| LVld (mm) | 67.4±9.7 | 67.2±9.8 | 0.862 |
| LAlr (mm) | 56.3±9.2 | 56.6±9.1 | 0.789 |
| LAap (mm) | 39.0±7.0 | 38.7±6.8 | 0.765 |
| RVa (mm2) | 29.0±9.3 | 30.0±9.0 | 0.411 |
| RAa (mm2) | 24.4±10.5 | 25.1±10.7 | 0.614 |
| LVa (mm2) | 21.6±6.8 | 21.4±6.8 | 0.762 |
| LAa (mm2) | 16.6±5.3 | 16.5±5.2 | 0.945 |
| RVWT (mm) | 5.5±1.6 | 5.5±1.6 | 0.921 |
| IVST (mm) | 9.2±2.1 | 9.1±2.1 | 0.600 |
| MPAd/AAd ratio | 1.13±0.24 | 1.15±0.24 | 0.652 |
| RVtd/LVtd ratio | 1.41±0.50 | 1.45±0.50 | 0.439 |
| RVa/LVa ratio | 1.5±0.80 | 1.57±0.81 | 0.401 |
| RVWT/IVST ratio | 0.63±0.22 | 0.64±0.23 | 0.608 |
Continuous variables are expressed as mean value ± standard deviation. CTPA, computed tomography pulmonary angiography; mPAP, mean pulmonary arterial pressure; ESC, European Society of Cardiology; ERS, European Respiratory Society; CTEPH, chronic thromboembolic pulmonary hypertension; MPAd, diameter of the main pulmonary artery; AAd, diameter of the ascending aorta; RVtd, transverse diameter of the right ventricle; RVld, longitudinal diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RAld, longitudinal diameter of the right atrium; LVtd, transverse diameter of the left ventricle; LVld, longitudinal diameter of the left ventricle; LAlr, left-right diameter of the left atrium; LAap, anteroposterior diameter of the left atrium; RVa, area of the right ventricle; RAa, area of the right atrium; LVa, area of the left ventricle; LAa, area of the left atrium; RVWT, right ventricular free wall thickness; IVST, interventricular septal thickness.
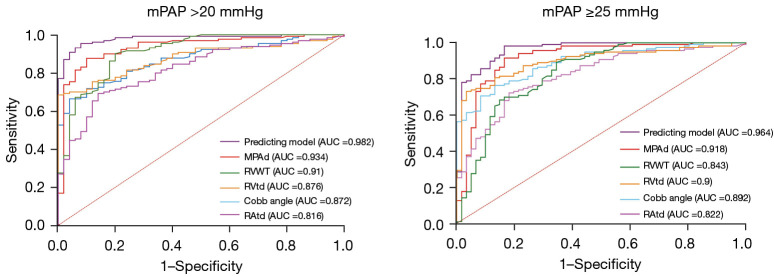
Univariate and forward multivariate binary logistic regression indicated that in both the old and new criteria, MPAd, RVtd, Cobb angle, and RVWT were independent predictors of CTPA for mPAP >20 mmHg (P<0.01) (Table 4). Table 5 shows the ROC analysis of the cardiovascular metrics in the prediction of PH under the old and new criteria. Compared to the other metrics, the MPAd of 30.4 mm exhibited the highest AUC (0.934±0.021) in the diagnosis of mPAP >20 mmHg, with a sensitivity of 0.88 and a specificity of 0.90 (Figure 5).
Table 4. Binary stepwise logistic regression analysis for cardiovascular imaging predictors of pulmonary hypertension under the 2015 and 2022 diagnostic criteria.
| Variable | Nonstandardized coefficient | Walt value | P value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| β | SE | Lower limit | Upper limit | ||||
| mPAP >20 mmHg | |||||||
| Cobb angle | 0.102 | 0.044 | 5.478 | 0.019* | 1.108 | 1.017 | 1.207 |
| MPAd | 0.240 | 0.095 | 6.354 | 0.012* | 1.271 | 1.055 | 1.532 |
| RVtd | 0.162 | 0.063 | 6.575 | 0.01* | 1.176 | 1.039 | 1.331 |
| RVWT | 1.296 | 0.397 | 10.664 | 0.001** | 3.655 | 1.679 | 7.955 |
| mPAP ≥25 mmHg | |||||||
| Cobb angle | 0.121 | 0.038 | 9.916 | 0.002** | 1.129 | 1.047 | 1.217 |
| MPAd | 0.246 | 0.083 | 8.793 | 0.003** | 1.279 | 1.087 | 1.505 |
| RVtd | 0.185 | 0.057 | 10.415 | 0.001** | 1.203 | 1.075 | 1.345 |
| RVWT | 0.703 | 0.246 | 8.205 | 0.004** | 2.020 | 1.249 | 3.269 |
*, P<0.05 and **, P<0.001 are considered statistically significant. OR, odds ratio; CI confidence interval; SE, standard error; mPAP, mean pulmonary arterial pressure; MPAd, diameter of the main pulmonary artery; RVtd, transverse diameter of the right ventricle; RVWT, the right ventricular free wall thickness.
Table 5. The ROC curve of cardiovascular metrics on CTPA under the 2015 and 2022 ESC/ERS diagnostic criteria.
| Cardiovascular metric on CTPA | Cutoff value | AUC | 95% CI | P value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| CTPA metric for mPAP >20 mmHg | |||||||
| Cobb angle (degree) | 48.3 | 0.872±0.026 | 0.822 | 0.922 | <0.001** | 0.67 | 1.00 |
| MPAd (mm) | 30.4 | 0.934±0.021 | 0.892 | 0.976 | <0.001** | 0.88 | 0.90 |
| RVtd (mm) | 43.3 | 0.876±0.025 | 0.827 | 0.925 | <0.001** | 0.69 | 1.00 |
| RAtd (mm) | 49.7 | 0.819±0.033 | 0.755 | 0.883 | <0.001** | 0.69 | 0.86 |
| RVa (mm2) | 24.5 | 0.842±0.031 | 0.781 | 0.904 | <0.001** | 0.72 | 0.86 |
| RAa (mm2) | 15.4 | 0.808±0.034 | 0.742 | 0.875 | <0.001** | 0.83 | 0.66 |
| RVWT (mm) | 3.8 | 0.910±0.025 | 0.861 | 0.959 | <0.001** | 0.90 | 0.80 |
| MPAd/AAd ratio | 0.98 | 0.873±0.027 | 0.821 | 0.925 | <0.001** | 0.76 | 0.84 |
| RVtd/LVtd ratio | 0.99 | 0.860±0.028 | 0.806 | 0.914 | <0.001** | 0.84 | 0.80 |
| RVa/LVa ratio | 0.93 | 0.870±0.026 | 0.819 | 0.920 | <0.001** | 0.79 | 0.84 |
| RVWT/IVST ratio | 0.40 | 0.925±0.020 | 0.886 | 0.964 | <0.001** | 0.90 | 0.80 |
| Prediction model | – | 0.979±0.009 | 0.961 | 0.996 | <0.001** | – | – |
| CTPA metric for mPAP ≥25 mmHg | |||||||
| Cobb angle (degree) | 46.2 | 0.892±0.023 | 0.847 | 0.937 | <0.001** | 0.77 | 0.87 |
| MPAd (mm) | 30.4 | 0.918±0.025 | 0.869 | 0.967 | <0.001** | 0.92 | 0.84 |
| RVtd (mm) | 43.3 | 0.900±0.023 | 0.854 | 0.946 | <0.001** | 0.73 | 0.97 |
| RAtd (mm) | 49.7 | 0.822±0.032 | 0.760 | 0.885 | <0.001** | 0.72 | 0.82 |
| RVa (mm2) | 24.5 | 0.868±0.028 | 0.813 | 0.924 | <0.001** | 0.77 | 0.87 |
| RAa (mm2) | 15.8 | 0.802±0.033 | 0.737 | 0.868 | <0.001** | 0.86 | 0.66 |
| RVWT (mm) | 3.8 | 0.843±0.032 | 0.780 | 0.906 | <0.001** | 0.89 | 0.66 |
| MPAd/AAd ratio | 0.98 | 0.856±0.030 | 0.798 | 0.915 | <0.001** | 0.78 | 0.77 |
| RVtd/LVtd ratio | 0.99 | 0.882±0.025 | 0.833 | 0.930 | <0.001** | 0.87 | 0.85 |
| RVa/LVa ratio | 0.92 | 0.898±0.022 | 0.854 | 0.942 | <0.001** | 0.85 | 0.80 |
| RVWT/IVST ratio | 0.45 | 0.891±0.024 | 0.844 | 0.938 | <0.001** | 0.85 | 0.77 |
| Prediction model | – | 0.964±0.014 | 0.936 | 0.992 | <0.001** | – | – |
Prediction model = Cobb angle + MPAd + RVtd + RVWT. Continuous variables are expressed as mean value ± standard deviation. **, P<0.001 is considered statistically significant. ROC, receiver operator characteristic curve; CTPA, computed tomography pulmonary angiography; ESC, European Society of Cardiology; ERS, European Respiratory Society; AUC, area under ROC curve; CI, confidence interval; mPAP, mean pulmonary arterial pressure; MPAd, diameter of the main pulmonary artery; RVtd, transverse diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RVa, area of the right ventricle; RAa, area of the right atrium; RVWT, right ventricular free wall thickness; AAd, diameter of the ascending aorta; LVtd, transverse diameter of the left ventricle; LVa, area of the left ventricle; IVST, interventricular septal thickness.
Figure 5.
The ROC of cardiovascular metrics and prediction models for diagnosing chronic thromboembolic pulmonary hypertension under both the 2015 and 2022 ESC and the ERS guidelines. (A) ROC of cardiovascular metrics and prediction models for diagnosing CTEPH under the new 2022 criteria (mean pulmonary artery pressure >20 mmHg). (B) ROC of the cardiovascular metrics and prediction models for diagnosing CTEPH under the old 2015 criteria (mPAP ≥25 mmHg). ROC, receiver operating characteristic; ESC, European Society of Cardiology; ERS, European Respiratory Society; mPAP, mean pulmonary artery pressure; AUC, area under the curve; CTEPH, chronic thromboembolic pulmonary hypertension; MPAd, diameter of the main pulmonary artery; RVWT, right ventricular free wall thickness; RVtd, transverse dimension of right ventricle; RAtd, transverse dimension of right atrium.
Correlation of cardiovascular metrics and hemodynamics with clinical data
Table 6 shows that the Cobb angle (r=0.586 to 0.693), RVtd/LVtd (r=0.583 to 0.596), and RVa/LVa (r=0.629 to 0.652) had a moderate correlation with mPAP, PVR, and NT-proBNP, respectively. Additionally, RVtd (r=0.368 to 0.570), RAtd (r=0.466 to 0.639), RAld (r=0.409 to 0.603), RVa (r=0.319 to 0.581), and RAa (r=0.432 to 0.629) also rspectively exhibited low to moderate correlations with mPAP, mRAP, PVR, and NT-proBNP. MPAd (r=0.482) and MPAd/AAd (r=0.391) had low correlations with mPAP and only weak correlations with PVR, NT-proBNP, and mRAP (r=0.143 to 0.262). There was a low negative correlation (r=−0.45) between CO and Cobb angle, while left ventricular metrics (LVld, LVtd, LVa) showed a low positive correlation (r=0.376 to 0.471) with CO.
Table 6. The correlation of cardiovascular metrics on CTPA with hemodynamics and serum NT-proBNP.
| Cardiovascular metric on CTPA | mPAP (mmHg) | mRAP (mmHg) | PVR (WU) | PAWP (mmHg) | CO (L/min) | NT-proBNP (pg/mL) |
|---|---|---|---|---|---|---|
| Cobb angle (degree) | 0.586** | 0.328** | 0.613** | −0.114 | −0.450** | 0.693** |
| MPAd (mm) | 0.482** | 0.218* | 0.262** | 0.037 | −0.042 | 0.230 ** |
| AAd (mm) | −0.074 | −0.005 | −0.058 | 0.041 | −0.080 | −0.021 |
| RVtd (mm) | 0.518** | 0.368** | 0.428** | −0.067 | −0.191* | 0.570** |
| RVld (mm) | 0.182** | 0.174* | 0.080 | 0.064 | 0.046 | 0.223** |
| RAtd (mm) | 0.558** | 0.466** | 0.478** | 0.011 | −0.258** | 0.639** |
| RAld (mm) | 0.467** | 0.409** | 0.425** | 0.030 | −0.289** | 0.603** |
| LVtd (mm) | −0.333** | −0.032 | −0.43** | 0.194* | 0.376** | −0.307** |
| LVld (mm) | −0.312** | −0.068 | −0.444** | 0.088 | 0.471** | −0.346** |
| LAlr (mm) | 0.151 | 0.186* | 0.090 | 0.091 | 0.019 | 0.082 |
| LAap (mm) | −0.079 | 0.111 | −0.211* | 0.115 | 0.257* | −0.146 |
| RVa (mm2) | 0.487** | 0.319** | 0.410** | −0.068 | −0.190* | 0.581** |
| RAa (mm2) | 0.495** | 0.432** | 0.442** | −0.002 | 0.270** | 0.629** |
| LVa (mm2) | −0.335** | −0.035 | −0.438** | 0.138 | 0.430** | −0.294** |
| LAa (mm2) | −0.023 | −0.118 | −0.164* | 0.209* | 0.248** | −0.115 |
| RVWT (mm) | 0.129 | 0.041 | −0.013 | 0.148 | 0.119 | −0.014 |
| IVST (mm) | −0.177* | −0.081 | −0.259** | 0.159 | 0.218* | −0.257** |
| MPAd/AAd ratio | 0.391** | 0.143* | 0.222* | 0.035 | 0.042 | 0.200* |
| RVtd/LVtd ratio | 0.583** | 0.248* | 0.593** | −0.174 | −0.382** | 0.596** |
| RVa/LVa ratio | 0.629** | 0.265** | 0.642** | −0.146 | −0.442** | 0.652** |
| RVWT/IVST ratio | 0.266** | 0.121 | 0.197* | −0.024 | −0.069 | 0.205* |
*, P<0.05 and **, P<0.001 are considered statistically significant. CTPA, computed tomography pulmonary angiography; NT-proBNP, N-terminal pro-brain natriuretic peptide; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PVR, pulmonary vascular resistance; WU, Wood units; PAWP, pulmonary artery wedge pressure; CO, cardiac output; MPAd, diameter of the main pulmonary artery; AAd, diameter of the ascending aorta; RVtd, transverse diameter of the right ventricle; RVld, longitudinal diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RAld, longitudinal diameter of the right atrium; LVtd, transverse diameter of the vleft ventricle; LVld, longitudinal diameter of the left ventricle; LAlr, left-right diameter of the left atrium; LAap, anteroposterior diameter of the left atrium; RVa, area of the right ventricle; RAa, area of the right atrium; LVa, area of the left ventricle; LAa, area of the left atrium; RVWT, right ventricular free wall thickness; IVST, interventricular septal thickness.
Discussion
Due to the changes in the mPAP criteria for diagnosing PH, we comprehensively compared the cardiovascular metrics from CTPA in CTEPH between the 2022 ESC/ERS guidelines (mPAP >20 mmHg) (1) and the 2015 ESC/ERS guidelines (mPAP ≥25 mmHg) (2). To our knowledge, this is the first study to analyze the difference in cardiovascular metrics on CTPA under the old and updated criteria. Our study produced several major findings: (I) there was no statistically significant difference in cardiovascular metrics for the diagnosis of PH between new and old guidelines; (II) MPAd, RVtd, Cobb angle, and RVWT on CTPA were independent predictors for mPAP >20 mmHg in patients with CTEPH; (III) compared to the other metrics, an MPAd of 30.4 mm exhibited the highest AUC, with a sensitivity of 0.88 and specificity of 0.90; (IV) cardiovascular metrics, especially metrics from the 4-chamber view of CTPA images moderately correlated with mPAP.
CTPA is a promising choice as the primary imaging method for suspected CTEPH due to its speed, wide availability, excellent spatial and temporal resolution, and ability to directly visualize chronic pulmonary embolism (16,17). Its high sensitivity and specificity for CTEPH allow for early diagnosis and help avoid advanced disease stages in patients with CTEPH (16). According to the 2022 ERS/ERS guidelines for the diagnosis of PH, cardiovascular metrics from CTPA, including Cobb angle, MPAd, RVtd, RVld, RAtd, RAld, RVa, RAa, RVWT, MPAd/AAd, RVtd/LVtd, RVa/LVa, and RVWT/IVST were significantly higher in patients with CTEPH than in patients without PH. These results are similar to those reported by Charters et al. (13) and Liu et al. (21), indicating that cardiovascular metrics on CTPA can predict mPAP >20 mmHg. Cardiovascular metrics from CTPA have been applied to assess PH (1,2,12,18). For instance, a cutoff value of 29 mm for MPAd has been used as an indicator of mPAP ≥25 mmHg. Corson et al. (22) reported an MPAd/AAd >1 as being an imaging marker of PH. Recently, a meta-analysis indicated that CT measurement MPAd/AAd ratio ≥1 has a combined sensitivity of 0.652 [95% confidence interval (CI): 0.579–0.719] and a combined specificity of 0.830 (95% CI: 0.796–0.880) in predicting mPAP ≥25 mmHg (12). In our study, according to the 2022 ERS/ERS guidelines, the sensitivity and specificity of a 30.4 mm cutoff value for MPAd in predicting mPAP >20 mmHg, respectively, was 0.88 and 0.90, whereas the sensitivity and specificity of a 0.98 cutoff value for MPAd/AAd in predicting mPAP >20 mmHg, respectively, was 0.76 and 0.84 in Table 5. Notably, both RVWT and RVWT/IVST ratio had a sensitivity of 0.90 and specificity of 0.80 in diagnosing mPAP >20 mmHg. Similarly, Swift et al. proposed that an MPAd of 30 mm represents a compromise threshold for identifying patients with mPAP >20 mmHg (5). In our study, Table 5 shows the cutoff value of MPAd/AAd and other parameters indicating RV enlargement under the new guidelines were also similar to those of previous studies (12,23,24).
In current study, there was no statistically significant differences in cardiovascular metrics between patients with mPAP >20 mmHg and mPAP ≥25 mmHg. The lack of differences in the cardiovascular parameters of CTPA between the old and new criteria for PH may be attributed to the following: (I) the difference of 4 mmHg in mPAP may have a limited impact on the morphological changes in the pulmonary artery and right heart; (II) the lower mPAP criterion may not fully capture the clinical condition or reflect the underlying pathological process itself (19); (III) the few cases with mPAP between 21 and 24 mmHg might have limited the significant differences in cardiovascular parameters between the old and new diagnostic criteria; (IV) the accuracy of cutoff values for cardiovascular parameters may be influenced by various factors, such as patient selection representing different subtypes of PH. Further investigations with larger cohorts are needed to confirm these findings.
In addition, our study demonstrated that Cobb angle, MPAd, RVtd, and RVWT on CTPA were independent predictors of PH for patients with suspected CETPH regardless of whether the new or old diagnostic criteria were used. Previous studies have focused on the impact of right ventricular changes on PH (14,25,26), while the right atrium in CTPA has been largely overlooked. Our study found that the RAtd in the 4-chamber view moderately correlated with mPAP. This indicates that RAtd may reflect mPAP and aid in PH evaluation (4). The Cobb angle also showed a moderate correlation with mPAP and PVR, and its cutoff value of 48.3° for predicting PH with mPAP >20 mmHg had high specificity and low sensitivity.
Limitations
There are some limitations in this study. First, we employed a single-center retrospective study design. As there were few cases of other subtypes of PH cases, they were excluded, with only patients with CTEPH being analyzed; this inevitably limits the generalizability of these findings to other types of PH. Therefore, future studies will include cases with precapillary and postcapillary PH. Second, only mPAP was used as the grouping criteria, and thus the alterations of cardiovascular metrics on CTPA under the different PVRs (2 and 3 WU) remain unknown. Third, RHC is not a routine procedure for healthy individuals, so only a small number of patients with normal and mild PH were included. Whether cardiovascular metrics in patients with mPAP ranging from 21 to 24 mmHg are different from those of patients with mPAP ≤20 mmHg is unclear, and the optimal cutoff values of cardiovascular metrics on CTPA in PH patients need to be further investigated.
Conclusions
Cardiovascular metrics on CTPA were similar between the updated and old PH guidelines. MPAd, Cobb angle, RVtd, and RVWT on CTPA were independent predictors of mPAP >20 mmHg. An MPAd of 30.4 mm represents a compromise threshold for identifying mPAP >20 mmHg in patients with CTEPH.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National High-Level Hospital Clinical Research Funding & Elite Medical Professionals Project of China-Japan Friendship Hospital (Nos. 2022-NHLHCRF-LX-01& ZRJY2021-BJ02); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2021-I2M-1-049); and the National Natural Science Foundation of China (No. 81871328).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional ethics board of China-Japan Friendship Hospital (No. 2022-KY-048). Individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-250/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-250/coif). The authors have no conflicts of interest to declare.
References
- 1.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, ESC/ERS Scientific Document Group . 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618-731. Erratum in: Eur Heart J 2023;44:1312. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 3.Klok FA, Barco S, Konstantinides SV, Dartevelle P, Fadel E, Jenkins D, Kim NH, Madani M, Matsubara H, Mayer E, Pepke-Zaba J, Delcroix M, Lang IM. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: results from the European CTEPH Registry. Eur Respir J 2018;52:1801687. 10.1183/13993003.01687-2018 [DOI] [PubMed] [Google Scholar]
- 4.Tsukada J, Yamada Y, Kawakami T, Matsumoto S, Inoue M, Nakatsuka S, Okada M, Fukuda K, Jinzaki M. Treatment effect prediction using CT after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Radiol 2021;31:5524-32. 10.1007/s00330-021-07711-5 [DOI] [PubMed] [Google Scholar]
- 5.Swift AJ, Dwivedi K, Johns C, Garg P, Chin M, Currie BJ, Rothman AM, Capener D, Shahin Y, Elliot CA, Charalampopolous T, Sabroe I, Rajaram S, Hill C, Wild JM, Condliffe R, Kiely DG. Diagnostic accuracy of CT pulmonary angiography in suspected pulmonary hypertension. Eur Radiol 2020;30:4918-29. 10.1007/s00330-020-06846-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M, Ma Z, Guo X, Chen X, Yang Y, Wang C. Cardiovascular parameters of computed tomographic pulmonary angiography to assess pulmonary vascular resistance in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol 2013;164:295-300. 10.1016/j.ijcard.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 7.Wittenberg R, van Vliet JW, Ghaye B, Peters JF, Schaefer-Prokop CM, Coche E. Comparison of automated 4-chamber cardiac views versus axial views for measuring right ventricular enlargement in patients with suspected pulmonary embolism. Eur J Radiol 2012;81:218-22. 10.1016/j.ejrad.2011.01.041 [DOI] [PubMed] [Google Scholar]
- 8.Lu MT, Demehri S, Cai T, Parast L, Hunsaker AR, Goldhaber SZ, Rybicki FJ. Axial and reformatted four-chamber right ventricle-to-left ventricle diameter ratios on pulmonary CT angiography as predictors of death after acute pulmonary embolism. AJR Am J Roentgenol 2012;198:1353-60. 10.2214/AJR.11.7439 [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Ma Z, Guo X, Zhang H, Yang Y, Wang C. Computed tomographic pulmonary angiography in the assessment of severity of chronic thromboembolic pulmonary hypertension and right ventricular dysfunction. Eur J Radiol 2011;80:e462-9. 10.1016/j.ejrad.2010.08.035 [DOI] [PubMed] [Google Scholar]
- 10.Stein PD, Matta F, Yaekoub AY, Goodman LR, Sostman HD, Weg JG, Hales CA, Hull RD, Leeper KV, Jr, Beemath A, Saeed IM, Woodard PK. Reconstructed 4-chamber views compared with axial imaging for assessment of right ventricular enlargement on CT pulmonary angiograms. J Thromb Thrombolysis 2009;28:342-7. 10.1007/s11239-009-0331-5 [DOI] [PubMed] [Google Scholar]
- 11.Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021;57:2002828. 10.1183/13993003.02828-2020 [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Liao H, Deng Z, He Z, Zheng Z, Lu J, Jiang M, Wu X, Guo W, Huang Z, Chen H, Hong C, Zhong N. Efficacy of computed tomography in diagnosing pulmonary hypertension: A systematic review and meta-analysis. Front Cardiovasc Med 2022;9:966257. 10.3389/fcvm.2022.966257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charters PFP, Rossdale J, Brown W, Burnett TA, Komber HMEI, Thompson C, Robinson G, MacKenzie Ross R, Suntharalingam J, Rodrigues JCL. Diagnostic accuracy of an automated artificial intelligence derived right ventricular to left ventricular diameter ratio tool on CT pulmonary angiography to predict pulmonary hypertension at right heart catheterisation. Clin Radiol 2022;77:e500-8. 10.1016/j.crad.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 14.Freed BH, Collins JD, François CJ, Barker AJ, Cuttica MJ, Chesler NC, Markl M, Shah SJ. MR. and CT Imaging for the Evaluation of Pulmonary Hypertension. JACC Cardiovasc Imaging 2016;9:715-32. 10.1016/j.jcmg.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.François CJ, Schiebler ML. Imaging of Pulmonary Hypertension. Radiol Clin North Am 2016;54:1133-49. 10.1016/j.rcl.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 16.Lambert L, Michalek P, Burgetova A. The diagnostic performance of CT pulmonary angiography in the detection of chronic thromboembolic pulmonary hypertension-systematic review and meta-analysis. Eur Radiol 2022;32:7927-35. 10.1007/s00330-022-08804-5 [DOI] [PubMed] [Google Scholar]
- 17.McInnis M. Imaging Advances in Chronic Thromboembolic Pulmonary Hypertension. Semin Roentgenol 2022;57:324-34. 10.1053/j.ro.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 18.Maschke SK, Werncke T, Dewald CLA, Becker LS, Meine TC, Olsson KM, Hoeper MM, Wacker FK, Meyer BC, Hinrichs JB. Depiction of mosaic perfusion in chronic thromboembolic pulmonary hypertension (CTEPH) on C-arm computed tomography compared to computed tomography pulmonary angiogram (CTPA). Sci Rep 2021;11:20042. 10.1038/s41598-021-99658-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maron BA, Kovacs G, Vaidya A, Bhatt DL, Nishimura RA, Mak S, Guazzi M, Tedford RJ. Cardiopulmonary Hemodynamics in Pulmonary Hypertension and Heart Failure: JACC Review Topic of the Week. J Am Coll Cardiol 2020;76:2671-81. 10.1016/j.jacc.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Ma ZH, Guo XJ, Wang SK, Chen XY, Yang YH, Wang C. A septal angle measured on computed tomographic pulmonary angiography can noninvasively estimate pulmonary vascular resistance in patients with chronic thromboembolic pulmonary hypertension. J Thorac Imaging 2012;27:325-30. 10.1097/RTI.0b013e3182541142 [DOI] [PubMed] [Google Scholar]
- 22.Corson N, Armato SG, 3rd, Labby ZE, Straus C, Starkey A, Gomberg-Maitland M. CT-based pulmonary artery measurements for the assessment of pulmonary hypertension. Acad Radiol 2014;21:523-30. 10.1016/j.acra.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest 2014;145:824-32. 10.1378/chest.13-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajaram S, Swift AJ, Capener D, Elliot CA, Condliffe R, Davies C, Hill C, Hurdman J, Kidling R, Akil M, Wild JM, Kiely DG. Comparison of the diagnostic utility of cardiac magnetic resonance imaging, computed tomography, and echocardiography in assessment of suspected pulmonary arterial hypertension in patients with connective tissue disease. J Rheumatol 2012;39:1265-74. 10.3899/jrheum.110987 [DOI] [PubMed] [Google Scholar]
- 25.Beyar R, Dong SJ, Smith ER, Belenkie I, Tyberg JV. Ventricular interaction and septal deformation: a model compared with experimental data. Am J Physiol 1993;265:H2044-56. 10.1152/ajpheart.1993.265.6.H2044 [DOI] [PubMed] [Google Scholar]
- 26.Tsujimoto Y, Kumasawa J, Shimizu S, Nakano Y, Kataoka Y, Tsujimoto H, Kono M, Okabayashi S, Imura H, Mizuta T. Doppler trans-thoracic echocardiography for detection of pulmonary hypertension in adults. Cochrane Database Syst Rev 2022;5:CD012809. 10.1002/14651858.CD012809.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as