Abstract
Background
Conduction disturbances demanding permanent pacemaker implantation (PPI) remain a common complication after transcatheter aortic valve replacement (TAVR). Optimization of the implantation depth (ID) by introducing the cusp-overlap projection (COP) technique led to a reduced rate of PPI when self-expanding valves were used.
Objectives
The aim of the present study was to determine if using the novel COP view is applicable for all types of TAVR prosthesis and results in a higher ID and reduced incidence of new conduction disturbances and PPI.
Methods
In this prospective case-control study 586 consecutive patients undergoing TAVR with either balloon-expandable Edwards SAPIEN S3 (n = 280; 47.8%), or mechanically expandable Boston LOTUS Edge heart valve prostheses (n = 306; 52.2%) were included. ID as well as rates of periprocedural PPI and left bundle branch block (LBBB) were compared between the conventional three-cusp coplanar (TCC) projection and the COP view for implantation.
Results
Of 586 patients, 282 (48.1%) underwent TAVR using COP, whereas in 304 patients (51.9%) the TCC view was applied. Using COP a significantly higher ID was achieved in Edwards SAPIEN S3 TAVR procedures (ID mean difference −1.0 mm, 95%−CI −1.9 to −0.1 mm; P = 0.029), whereas the final platform position did not differ significantly between both techniques when a Boston LOTUS Edge valve was used (ID mean difference −0.1 mm, 95%-CI −1.1 to +0.9 mm; P = 0.890). In Edwards SAPIEN S3 valves, higher ID was associated with a numerically lower post-procedural PPI incidence (4.9% vs. 7.3%; P = 0.464). Moreover, ID was significantly deeper in patients requiring PPI post TAVR compared to those without PPI [8.7 mm (6.8–10.6 mm) vs. 6.5 mm (6.1–7.0 mm); P = 0.005]. In Boston LOTUS Edge devices, COP view significantly decreased the incidence of LBBB post procedure (28.1% vs. 47.9%; P < 0.001), while PPI rates were similar in both groups (21.6% vs. 25.7%; P = 0.396).
Conclusion
The present study demonstrates the safety, efficacy and reproducibility of the cusp-overlap view even in balloon-expandable and mechanically-expandable TAVR procedures. Application of COP leads to significantly less LBBB in repositionable Boston LOTUS Edge valves and a numerically lower PPI rate in Edwards SAPIEN S3 valves post TAVR compared to the standard TCC projection. The results should encourage to apply the COP view more widely in clinical practice.
Keywords: aortic stenosis, conduction disturbance, cusp-overlap projection, implantation depth, implantation technique, permanent pacemaker implantation, TAVR, transcatheter aortic valve replacement
Introduction
Within the last two decades, transcatheter aortic valve replacement (TAVR) has revolutionized the management of symptomatic severe aortic stenosis (1–6). Continuous enhancements in all areas have allowed expansion from high-risk and inoperable patients to patients at all levels of surgical risk (7, 8). However, conduction disturbances demanding permanent pacemaker implantation (PPI) remains a common finding following TAVR with a reported incidence up to 36% before discharge (9–14). The main reason is the anchoring mechanism of most transcatheter heart valves (radial force) and the proximity of the implantation site to the cardiac conduction system resulting in high-grade atrioventricular block and new onset left bundle branch block (LBBB) (Figure 1) (15). Previous studies highlight that conduction abnormalities and new pacemaker requirement were linked to worse clinical outcomes including increased mortality and heart-failure rehospitalization (16–20). In view of the younger TAVR population in recent years, long-term consequences of pacing will become increasingly important (16).
Figure 1.
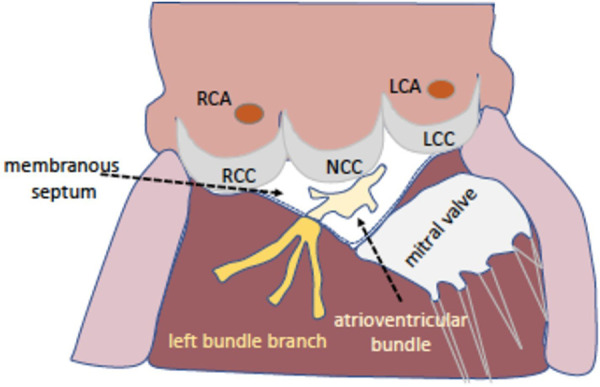
The aortic valve and the conduction system. The proximity of the aortic valve and the cardiac conduction system explains the occurrence of new conduction disturbances after transcatheter aortic valve replacement. Illustration showing the localization of the membranous septum and the left bundle branch between the right- and the non-coronary cusp. LCC/NCC/RCC, left-/non-/right-coronary cusp; LCA/RCA, left/right coronary artery.
Nowadays, several predictors associated with increased risk for post-procedural PPI are described in literature, however most of them are not modifiable (12, 13, 16, 21, 22). Optimization of the implantation depth (ID) by introducing the cusp-overlap projection (COP) technique might be a viable approach to lower the risk of interaction with the conduction system (23, 24). In TAVR using a self-expanding Evolut series prosthesis, the novel COP technique led to a significant higher prosthesis release and was associated with a reduced risk of PPI (25–27).
The aim of the present study was to determine if the rationale and practicalities of using the novel cusp-overlap view is applicable for all types of TAVR prosthesis and results in reduced incidence of new conduction disturbances and PPI.
Methods
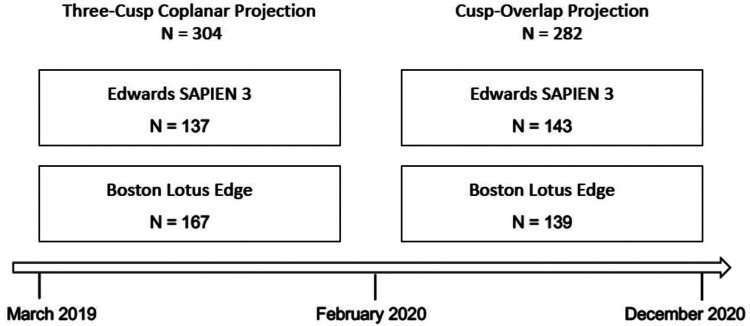
This prospective case-control study included consecutive patients undergoing transfemoral TAVR for aortic valve disease in our high-volume hospital heart center between March 2019 to December 2020. The decision for TAVR was made by the interdisciplinary heart team according to the 2017 European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines for the management of valvular heart disease (28).
TAVR was performed in a hybrid catheterization laboratory under conscious sedation by an experienced operator team of four interventionalists with a standardized procedure protocol according to current guidelines. The aortic valve prostheses were implanted under fluoroscopic guidance via the femoral access route. Patients received either a balloon-expandable Edwards SAPIEN 3 or a mechanically expandable Boston LOTUS Edge valve prosthesis. The decision for implanted device type was made by at least two experienced interventionalists and in accordance to current recommendations. Especially size (over-/undersizing), extent and morphology of calcification [particularly in the left ventricular out-flow tract (LVOT)] as well as access vessel situation were considered. There were no strict exclusion criteria for any of the prothesis types but the tendency to avoid balloon-expandable valves in case of severe LVOT-calcification (due to the risk of annulus rupture). Prostheses were sized using manufacturer recommendations, including annular and LVOT dimensions and location as well as severity of annular and LVOT calcification. Between March 2019 and January 2020 valve implantation was performed using the conventional three-cusp coplanar (TCC) view, whereas from February 2020 onwards the COP view was applied exclusively (Figure 2). The standard TCC view was reproduced based on computed tomography (CT) data. Thereafter, COP view was achieved by rotation around the center line. The proposed fluoroscopic angulations for optimal valve implantation were verified by angiography. The cusp-overlap view integrated a modified implantation technique according to the classic COP technique like wire management. For the sake of simplicity, only the term COP view will be used below.
Figure 2.
Study design. Between 03/2019 and 12/2020 consecutive patients without permanent pacemaker or implantable cardioverter defibrillator undergoing native valve transcatheter aortic valve replacement (TAVR) were included in the study. Prior to 02/2020 TAVR was performed in a three-cusp coplanar projection, and subsequently in a cusp-overlap projection.
Patients with pre-existing PPI as well as valve-in-valve procedures were excluded from this analysis.
The baseline characteristics and relevant periprocedural information of each patient were recorded. All patients received daily 12-lead electrocardiogram (ECG) to document serial changes in conduction as well as repeated laboratory testing and clinical examination. Transthoracic echocardiography was performed before and after the procedure to measure, amongst others, transvalvular aortic valve gradients and aortic regurgitation.
Furthermore, all patients underwent preprocedural ECG-gated 256 multislice contrast-enhanced CT, which was evaluated with a dedicated software (3mensio Structural Heart 9.1 software, Pie Medical Imaging B.V., Maastricht, The Netherlands). Besides the decision for the valve type and size, measurements of aortic annulus, LVOT, calcification and distance to coronary ostia, among others, were performed in accordance to the expert consensus document of the Society of Cardiovascular Computed Tomography (29). Moreover, the vascular access route was determined.
Primary clinical outcomes were new-onset of LBBB and PPI rates following TAVR as well as the measurement of ID comparing valve deployment with COP and TCC view. Furthermore, safety outcomes including device embolization, need for second valve implantation, coronary artery obstruction as well as moderate or severe aortic insufficiency post procedure were analyzed.
PPI was considered in patients with persistent complete high grade atrioventricular block after TAVR. Assessment of the prothesis implantation was based on post-procedural evaluation of aortography and was carried out using a dedicated software (CAAS 7.4., Pie Medical Imaging, Maastricht, the Netherlands). ID was expressed as the maximal distance of the native aortic annulus plane on the side of the non-coronary cusp (NCC) to the most proximal edge of the implanted valve in the left ventricle (Figure 3). ID was measured in COP as well as TCC projection in all patients, with the greater distance used as the true ID.
Figure 3.
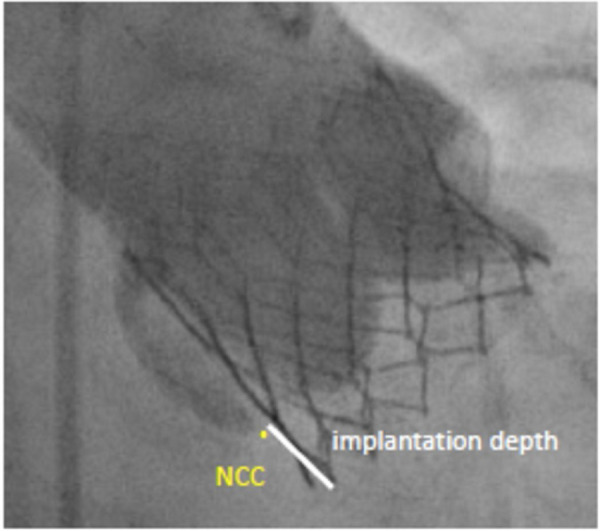
Implantation depth. Measurement from the non-coronary cusp (NCC) to the distal end of the intraventricular portion of the implanted valve.
All patients provided written informed consent to participate in the ULM TAVR-Registry. The study was approved by the local ethics committee and has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Statistical analysis
Categorical data are presented as counts and percentages (%). Comparison of proportions was carried out using the χ2-test. Continuous variables are expressed as mean ± one standard deviation (SD). Continuous variables for two groups were compared with the Student's t-test. A P-value of <0.05 was considered to indicate statistical significance. Statistical analyses were performed using MedCalc software (MedCalc Version 20.210, MedCalc Software Ltd, Ostend; Belgium).
Results
Patient characteristics
A total of 586 consecutive patients with severe aortic stenosis undergoing TAVR via the femoral access were included in this study. Of those, 282 patients (48.1%) underwent TAVR using COP, whereas in 304 patients (51.9%) the TCC projection was applied (Figure 4). Baseline characteristics of both groups are displayed in Table 1. Median age was around 80 years [79.9 (79.0–80.7) years vs. 79.9 (79.2–80.6) years; P = 0.924] and sex ratio was well balanced (male 60.9% vs. 57.8%; P = 0.727). Mean STS-score was 3.3 [3.1–3.6] [3.3 (2.9–3.7) vs. 3.4 (3.1–3.7); P = 0.857] and 12.4%/13.2% of patients had LBBB/right bundle branch block (RBBB) prior to TAVR (13.4% vs. 11.5%; P = 0.608 and 13.8% vs. 12.6%; P = 0.709; respectively). Besides slight differences in mean and maximum transaortic pressure gradient [44.4 (41.4–47.5) mmHg vs. 37.3 (35.6–39.0) mmHg, P < 0.001 and 69.9 (67.1–72.6) mmHg vs. 62.8 (60.1–65.4) mmHg; P < 0.001, respectively] as well as in left ventricular ejection fraction [52.2 (50.9–53.4) % vs. 48.3 (46.7–49.8) % P < 0.001] all other baseline characteristics were similar distributed in both populations.
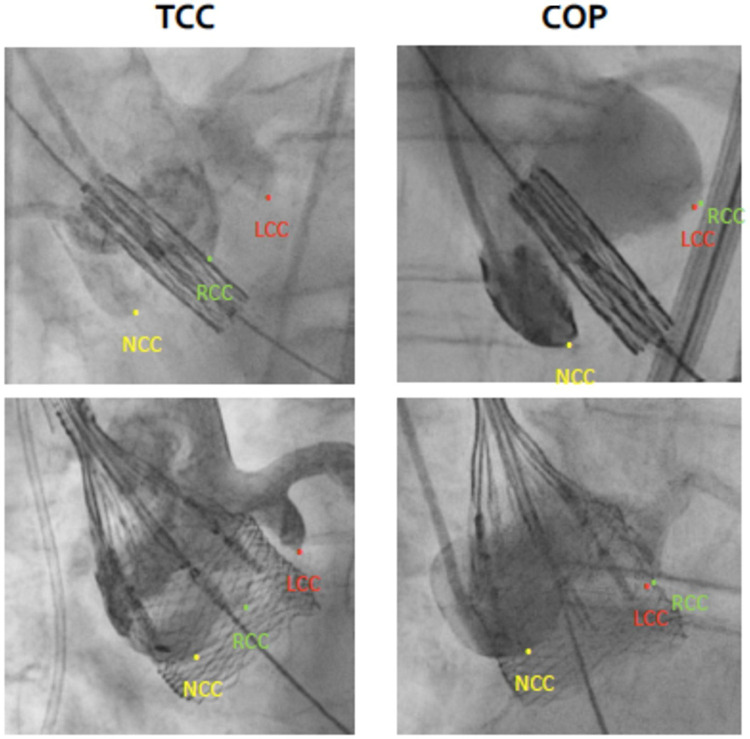
Figure 4.
Anatomical distinctions between tricuspid-coplanar (TCC) and cusp overlap (COP) projections. Angiographic images of implantation in TCC (left) versus COP (right) for Edwards SAPIEN 3 (upper panel) and Boston Lotus Edge valves (lower panel). In the COP view the left- (LCC) and right-coronary cusp (RCC) overlap while the non-coronary cusp (NCC) is isolated on the left side.
Table 1.
Patient characteristics.
| TCC | COP | P-value | |
|---|---|---|---|
| Total (n) | 304 | 282 | |
| Age (years) | 79.9 (79.2–80.6) | 79.9 (79.0–80.7) | 0.924 |
| Male | 57.8% | 60.9% | 0.727 |
| BMI (kg/m2) | 27.3 (26.8–27.9) | 27.0 (26.2–27.8) | 0.610 |
| STS-PROM | 3.4 (3.1–3.7) | 3.3 (2.9–3.7) | 0.767 |
| Diabetes mellitus | 28.1% | 29.1% | 0.857 |
| Chronic kidney disease | 61.6% | 49.0% | 0.167 |
| Arterial hypertension | 93.4% | 87.3% | 0.698 |
| Coronary artery disease | 60.0% | 55.9% | 0.725 |
| NYHA class III/IV | 67.7% | 66.5% | 0.945 |
| Left ventricular ejection fraction (%) | 48.3 (46.7–49.8) | 52.2 (50.9–53.4) | 0.0001 |
| AV max. PG (mmHg) | 62.8 (60.1–65.4) | 69.9 (67.1–72.6) | 0.0003 |
| AV mean (PG mmHg) | 37.3 (35.6–39.0) | 44.4 (41.4–47.5) | 0.0001 |
| Atrial fibrillation | 22.4% | 24.5% | 0.704 |
| LBBB | 11.5% | 13.4% | 0.608 |
| RBBB | 12.6% | 13.8% | 0.709 |
| Edwards SAPIEN 3 | 45.1% | 50.7% | 0.425 |
| Boston LOTUS Edge | 54.9% | 49.3% | 0.480 |
Data are presented as percentages, counts or mean ± SD. Significant p values are presented in bold.
AV mean/max PG, aortic valve mean/max pressure gradient; BMI, body mass index; COP, cusp-overlap projection; LBBB, left bundle branch block; NYHA, New York Heart Association; RBBB, right bundle branch block; STS-PROM, Society of Thoracic Surgeons—Predicted Risk of Mortality; TCC, three-cusp coplanar projection.
In 280 TAVR procedures (47.8%) a balloon-expandable Edwards SAPIEN S3 valve was implanted, while in 306 cases (52.2%) a mechanically expandable Boston LOTUS edge valve was used (Figure 4). Detailed baseline data of both valve subgroups are shown in Supplementary Table 1. Notably, in patients undergoing Edwards SAPIEN S3 TAVR procedure baseline incidence of LBBB was significantly more frequently in the COP-subgroup (10.9% vs. 16.4%, P-value < 0.0001).
Procedural and clinical outcome
Edwards SAPIEN S3
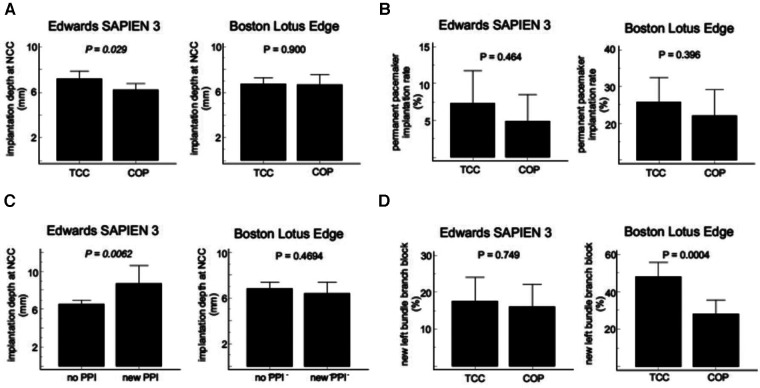
In Edwards SAPIEN S3 TAVR procedures the final absolute mean ID was significantly smaller using COP view compared with the standard TCC projection (mean difference −1.0 mm, 95%-CI −1.9 to −0.1 mm; P = 0.029) (Figure 5A). The rate of new PPI following TAVR was numerically lower in the COP group than in the TCC group (7.3% vs. 4.9%; P = 0.464) (Figure 5B). Mean ID was significantly deeper in patients needing PPI post TAVR compared to those without PPI [8.69 mm (95%-CI 6.80–10.58 mm) vs. 6.52 mm (95%-CI 6.08–6.97 mm); P = 0.0052] (Figure 5C). Incidence of new-onset of LBBB post TAVR was similar distributed between both implantation techniques (TCC 17.5% vs. COP 16.1%, P = 0.752) (Figure 5D). Residual mean transaortic pressure gradient was also similar in both groups [TCC 12.0 mmHg (95%-CI 11.1–12.9 mmHg) vs. COP 12.7 mmHg (95%-CI 11.8–13.6 mmHg); P = 0.275]. Moreover, there were no device embolization, need for second valve implantation, coronary artery obstruction or moderate or severe aortic insufficiency post procedure in both cohorts (Table 2).
Figure 5.
Comparison of cusp overlap projection (COP) and tricuspid coplanar projection (TCC) for TAVR with Edwards SAPIEN 3 and Boston LOTUS Edge valves. (A) implantation depth, (B) new permanent pacemaker implantation (PPI) rate, (C) implantation depth in patients with or without new PPI, (D) incidence of new-onset of left bundle branch block (LBBB).
Table 2.
Valve function and major complications.
| Edwards SAPIEN 3 | Boston Lotus Edge | |||||
|---|---|---|---|---|---|---|
| TCC | COP | P-value | TCC | COP | P-value | |
| AV max. PG (mmHg) | 22.3 (20.8–23.8) | 23.4 (21.9–25.0) | 0.303 | 23.5 (21.8–25.2) | 25.0 (23.2–26.9) | 0.213 |
| AV mean PG (mmHg) | 12.0 (11.1–12.9) | 12.7 (11.8–13.6) | 0.275 | 12.7 (11.7–13.6) | 14.1 (13.0–15.0) | 0.056 |
| Aortic regurgitation grade II/III | 0% | 0% | n/a | 0% | 0% | n/a |
| Device embolization | 0% | 0% | n/a | 0% | 0% | n/a |
| Coronary obstruction | 0% | 0% | n/a | 0% | 0% | n/a |
Data are presented as percentages or mean ± SD.
AV mean/max PG, aortic valve mean/max pressure gradient; COP, cusp-overlap projection; TCC, three-cusp coplanar projection.
Boston LOTUS edge
In patients receiving the Boston LOTUS Edge prothesis the final absolute mean ID did not differ significantly between both implantation views (mean difference −0.1 mm, 95%-CI −1.1 to +0.9 mm; P = 0.890) (Figure 5A). PPI rates following TAVR were 21.6% in the COP group and 25.7% in the TCC group (P = 0.396) (Figure 5B). Mean ID was also similar between patients with and without need for new PPI [6.38 mm (95% CI 5.38–7.39 mm) vs. 6.81 mm (95% CI 6.24–7.38 mm); P = 0.469] (Figure 5C). Post-procedure, the COP view was associated with a significant reduced incidence of LBBB (28.1% vs. 47.9%; P < 0.001) compared to the conventional TCC projection (Figure 5D). Residual mean transaortic pressure gradient was similar distributed in both groups [TCC 12.7 mmHg (95%-CI 11.7–13.6 mmHg) vs. COP 14.1 mmHg (95%-CI 13.0–15.0 mmHg); P = 0.056]. In both cohorts, device embolization, need for second valve implantation, coronary artery obstruction or moderate or severe aortic insufficiency did not occur (Table 2).
Discussion
This is the first study analyzing the impact of the novel cusp-overlap view on conduction disturbances and PPI rates when performing TAVR with both balloon-expandable and mechanically expandable heart valves. The main findings can be summarized as follows: (1) Compared with the standard TCC projection, the COP view was associated with a significantly higher ID resulting in numerical less PPI after balloon-expandable TAVR. Moreover, ID was significantly higher in patients requiring PPI post TAVI compared to those without. (2) In mechanically expandable heart valves ID and rate of PPI were unaltered by using COP, however, less LVOT interference led to significantly reduced incidence of new onset of LBBB post procedure compared to TCC. (3) The periprocedural risk was low and similar distributed between both implantation techniques irrespective of the used valve type.
Nowadays, TAVR offers a safe and viable alternative to surgery for the management of severe and symptomatic AS at all levels of surgical risk (1–8). However, despite major enhancements, conduction disturbances demanding PPI remain a common and challenging complication after TAVR with the potential for long-term patient harm including increased early and late all-cause mortality as well as higher risk of heart failure rehospitalization (9–11, 13, 16–18). Especially since the therapy is increasingly moving toward patients with lower surgical risk and longer life expectancy, reduction of the persistently high PPI rates is one of the key issues in the field of TAVR (1, 2, 16).
To date, numerous risk factors for PPI after TAVR are well known, including amongst others LVOT calcification, preexisting conduction abnormalities like RBBB, left anterior hemiblock or first-degree atrioventricular block as well as the choice of valve prosthesis, degree of prothesis oversizing, short membranous septum length and depth of implantation of the new valve (12, 13, 16, 21, 22, 30). Besides the right prosthesis choice and oversizing ratio in some extent, especially the optimization of the ID represents a promising approach to reduce the risk of PPI after TAVR (23, 31). Due to the location of the conduction system with its bundle of His and the left bundle branch just below the annulus plane, a higher valve ID in the LVOT can reduce the risk of interaction with the conduction tissue and consequently the PPI risk after TAVR (15, 32, 33). Since just a few millimeters in ID can cause a large difference in PPI rates, precise imaging of the aortic root during valve implantation is an essential but challenging step for optimal valve positioning (10, 24).
The novel cusp-overlap approach, which was firstly reported at a conference in 2016 and systematically introduced in 2018, was suggested to enable better visualization and more accurate guiding of the optimal prosthesis ID during valve deployment (24, 32, 34, 35). In contrast to the standard TCC view, in which the right coronary cusp (RCC) is in the middle of the left (LCC) and the non-coronary cusp (NCC), the technique is based on overlapping the RCC and LCC, and thus isolating NCC (32). The anticipated advantages provided by the COP view are amongst others the elimination of the parallax of the delivery system, a better visualization of the NCC, achievement of a true coplanar view, elongation of the LVOT and consequently an accurate evaluation of an optimal and higher ID of the valve prosthesis (24, 32, 33, 36).
Due to promising results from previous studies the COP view has been widely extended in clinical practice, however data predominantly exists for the self-expanding valves (25–27). In a large meta-analysis with 3,647 patients from 11 studies undergoing self-expanding TAVR COP was associated with a significantly reduced PPI rate and higher ID compared to the standard TCC view (23). In the recently published Optimize PRO study with 400 patients receiving Evolut PRO/PRO + (Medtronic) self-expanding valves Grubb et al. displayed that the use of TAVR care pathway and COP resulted in favorable clinical outcomes with low PPI rates of only 9.8% at 30 days (37). The present study corroborates and further extends these findings.
We demonstrated that utilizing the COP view for valve implantation in TAVR with the balloon-expandable Edwards SAPIEN S3 prosthesis also resulted in a significantly higher ID compared to the standard TCC projection. Furthermore, higher valve implantation was associated with a favorable trend toward lower PPI rates, however without reaching statistical significance. To test if more patients might have allowed for a statistically significant difference, we compared the ID of patients receiving a PPI post TAVR with patients who did not. The latter group had a significantly lower ID, which is in line with data from Sammour et al. showing a similar reduction in the PPI rate of Edwards SAPIEN S3 valves with an alternative implantation technique to achieve higher valve implantation (RAO-CAU fluoroscopic projection) (38). Nevertheless, besides the COP view there are certainly several other factors affecting the implantation height like wire management or the learning curve every operator has to complete when adopting the technique. Moreover, prothesis specific behavior during implantation (tendency to migrate into ventricle vs. the aorta) may have led to different implantation heights.
In contrast to the Edwards SAPIEN S3 prosthesis, in TAVR using a mechanically-expandable Boston LOTUS Edge valve final ID did not differ between the new COP and the standard TCC view. The most likely reason for the unchanged ID is the distinct technique used for releasing the LOTUS valve during deployment. Boston LOTUS Edge valves were simultaneously released from their aortic and ventricular ends during mechanical expansion. As a result, it was more challenging to target and center the stretched-out valve. Since the valve tended to be dragged down as soon as the ventricular portion of the valve made contact with the LVOT, the final ID was less predictable compared to Edwards SAPIEN valves. Furthermore, parts of the LOTUS valve had to be positioned beneath the annulus to securely anchor the valve to avoid embolization as well as to reduce paravalvular aortic regurgitation. Due to its unique mechanical expansion during implantation as well as high radial force LOTUS Edge valves had a considerably higher rate of PPI compared to Medtronic Evolut and Edwards SAPIEN valves (1, 2, 39, 40). The observed PPI rate of 23.7% following Boston Lotus Edge TAVR in the present study agrees with data of previous trials (39, 41).
Remarkably, although higher ID for Boston Lotus Edge TAVR could not be achieved by using the COP view, postprocedural LBBB was significantly reduced from 47.9% to 28.1% compared to the TCC projection. Previous studies have also shown a lower incidence of new-onset of LBBB post TAVR using COP for deployment of a Medtronic Evolut Pro prosthesis, while the LBBB rate did not changed for Edwards SAPIEN S3 valves in our study (25–27, 42). In contrast to the Edwards SAPIEN S3 valve, Boston LOTUS Edge and Medtronic Evolut Pro valves can be repositioned before their final release if the interventionalist is dissatisfied with the final platform position. As mentioned above, the COP view allowed for better visualization of the annular plane leading to less LVOT manipulation during valve deployment and thus less trauma to the conduction system for self-expanding valves.
LBBB is a known marker of poorer long-term survival and leads to an intraventricular desynchrony potentially resulting in an impairment of the left ventricular function. In former studies new-onset of LBBB post TAVR was associated with an increased risk of PPI at 1-year, higher risk of heart failure rehospitalization as well as increased cardiac death and early and late all-cause mortality (19, 20, 43–45). We note that Boston Scientific has withdrawn the Lotus Edge Aortic Valve System in 2020 due to complexities associated with its delivery system during the implantation procedure. Nevertheless, our results can hypothesize as ´proof of concept` for the implementation of the COP technique for other TAVR valves, when the application leads to improved outcomes even in this valve with the highest PPI rates post procedure. Moreover, our study indicates that PPI rate also depends on other factors like the radial force.
However, the benefit of lower conduction disturbances and PPI post TAVR needs to be carefully weighed against possible adverse events, which may result from higher final valve ID (15, 24). Although not observed in this study, a higher implant theoretically possesses a potential increased risk for device embolization, paravalvular leakage, coronary artery obstruction as well as a complicated coronary reaccess. However, even in previous studies, the mentioned adverse events occurred very rarely after the application of COP and to a comparable extent as with the TCC view, which finally emphasize the safety and effectiveness of the COP view even in balloon-expandable and mechanically-expandable valves (25, 36, 38, 42).
Limitations
The results of our study are to be interpreted with several confinements. First, this is a single-center observational study carrying all the inherent limitations ascribed to such type of design. Second, two non-contemporary cohorts were compared to evaluate differences between COP and TCC view. Furthermore, study cohorts were not matched on all baseline variables. Third, TAVR with the standard TCC projection was performed earlier than with the COP view. Therefore, PPI rates may have influenced by operatorś learning curve as well as the development of technique and devices over the years. Fourth, despite the prospective study design, the ID was measured retrospectively. Moreover, post TAVR ID was only measured angiographically and not by CT. Fifth, persistent LBBB and atrioventricular block after TAVR were assessed in ECGs until hospital discharge. Data on duration or resolution at 30 days or one year were not available. Sixth, the decision to implant a permanent pacemaker was ultimately at the discretion of the local heart team. However, except for class I indication the threshold for choosing a permanent pacemaker may differ among physicians and even institutions. Seventh, the Lotus Edge Aortic Valve System has been withdrawn from the market in 2020. Therefore, our implications have to be interpreted as hypothesis generating. Lastly, we observed no relevant safety endpoint in the present analysis for both implant techniques, possibly caused by a too small number of included patients. Further large-scale and multicenter studies are needed to further evaluate the safety and effectiveness of the COP and to confirm our results.
Conclusion
The present study demonstrates the safety, efficacy and reproducibility of the cusp-overlap view even in balloon-expandable and mechanically-expandable TAVR procedures. Compared to the standard three-cusp coplanar view, application of cusp-overlap view leads to higher implantation depth in Edwards SAPIEN S3 TAVR resulting in numerically lower rate of permanent pacemaker implantation as well as to less LVOT interference and trauma in repositionable Boston LOTUS Edge valves coming across with lower incidences of new-onset of LBBB. The results should encourage to apply the cusp-overlap view more widely in clinical practice irrespective of the used valve type.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by local Ethics Committee of Ulm University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. MK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. AL: Data curation, Investigation, Methodology, Writing – original draft. CB: Data curation, Investigation, Validation, Writing – review & editing. MB: Data curation, Investigation, Validation, Writing – review & editing. JM: Data curation, Investigation, Validation, Writing – review & editing. BG: Conceptualization, Data curation, Investigation, Supervision, Validation, Writing – review & editing. WR: Conceptualization, Supervision, Validation, Writing – review & editing. DB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1269833/full#supplementary-material
References
- 1.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380(18):1695–705. 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 2.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380(18):1706–15. 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374(17):1609–20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 4.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376(14):1321–31. 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364(23):2187–98. 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363(17):1597–607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 7.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77(4):450–500. 10.1016/j.jacc.2020.11.035 [DOI] [PubMed] [Google Scholar]
- 8.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 9.Sammour Y, Krishnaswamy A, Kumar A, Puri R, Tarakji KG, Bazarbashi N, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2021; 14(2):115–34. 10.1016/j.jcin.2020.09.063 [DOI] [PubMed] [Google Scholar]
- 10.van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: a systematic review. Eur Heart J. (2018) 39(21):2003–13. 10.1093/eurheartj/ehx785 [DOI] [PubMed] [Google Scholar]
- 11.Levack MM, Kapadia SR, Soltesz EG, Gillinov AM, Houghtaling PL, Navia JL, et al. Prevalence of and risk factors for permanent pacemaker implantation after aortic valve replacement. Ann Thorac Surg. (2019) 108(3):700–7. 10.1016/j.athoracsur.2019.03.056 [DOI] [PubMed] [Google Scholar]
- 12.Rodés-Cabau J, Ellenbogen KA, Krahn AD, Latib A, Mack M, Mittal S, et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: jACC scientific expert panel. J Am Coll Cardiol. (2019) 74(8):1086–106. 10.1016/j.jacc.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 13.Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. (2017) 136(11):1049–69. 10.1161/CIRCULATIONAHA.117.028352 [DOI] [PubMed] [Google Scholar]
- 14.Nazif TM, Williams MR, Hahn RT, Kapadia S, Babaliaros V, Rodés-Cabau J, et al. Clinical implications of new-onset left bundle branch block after transcatheter aortic valve replacement: analysis of the PARTNER experience. Eur Heart J. (2014) 35(24):1599–607. 10.1093/eurheartj/eht376 [DOI] [PubMed] [Google Scholar]
- 15.Armario X, Rosseel L, Mylotte D. Cusp overlap technique in TAVR: tAVR implantation optimization—CT analysis and practical aspects of the cusp overlap technique. Cardiac Interventions Today. (2021) 15(Supplement). [Google Scholar]
- 16.Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard L, et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J. (2020) 41(29):2771–81. 10.1093/eurheartj/ehz924 [DOI] [PubMed] [Google Scholar]
- 17.Chamandi C, Barbanti M, Munoz-Garcia A, Latib A, Nombela-Franco L, Gutiérrez-Ibanez E, et al. Long-term outcomes in patients with new permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2018) 11(3):301–10. 10.1016/j.jcin.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 18.Costa G, Zappulla P, Barbanti M, Cirasa A, Todaro D, Rapisarda G, et al. Pacemaker dependency after transcatheter aortic valve implantation: incidence, predictors and long-term outcomes. EuroIntervention. (2019) 15(10):875–83. 10.4244/EIJ-D-18-01060 [DOI] [PubMed] [Google Scholar]
- 19.Giustino G, van der Boon RMA, Molina-Martin de Nicolas J, Dumonteil N, Chieffo A. de Jaegere PPT, et al. Impact of permanent pacemaker on mortality after transcatheter aortic valve implantation: the PRAGMATIC (pooled rotterdam-milan-toulouse in collaboration) pacemaker substudy. EuroIntervention. (2016) 12(9):1185–93. 10.4244/EIJV12I9A192 [DOI] [PubMed] [Google Scholar]
- 20.Regueiro A, Abdul-Jawad Altisent O, Del Trigo M, Campelo-Parada F, Puri R, Urena M, et al. Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. Circ Cardiovasc Interv. (2016) 9(5):e003635. 10.1161/CIRCINTERVENTIONS.115.003635 [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Zhou D, Dang M, He Y, Zhang S, Fang J, et al. The predictors of conduction disturbances following transcatheter aortic valve replacement in patients with bicuspid aortic valve: a multicenter study. Front Cardiovasc Med. (2021) 8:757190. 10.3389/fcvm.2021.757190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeno Y, Abramowitz Y, Kawamori H, Kazuno Y, Kubo S, Takahashi N, et al. A highly predictive risk model for pacemaker implantation after TAVR. JACC Cardiovasc Imaging. (2017) 10(10 Pt A):1139–47. 10.1016/j.jcmg.2016.11.020 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Zhu G, Liu X, Wu W, Chai H, Tao M, et al. Comparison of cusp-overlap projection and standard three-cusp coplanar view during self-expanding transcatheter aortic valve replacement: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:927642. 10.3389/fcvm.2022.927642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengupta A, Alexis SL, Lee T, Zaid S, Krishnamoorthy PM, Khera S, et al. Cusp overlap technique: should it become the standard implantation technique for self-expanding valves? Curr Cardiol Rep. (2021) 23(11):154. 10.1007/s11886-021-01583-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendiz OA, Noč M, Fava CM, Gutiérrez Jaikel LA, Sztejfman M, Pleskovič A, et al. Impact of cusp-overlap view for TAVR with self-expandable valves on 30-day conduction disturbances. J Interv Cardiol. (2021) 2021:9991528. 10.1155/2021/9991528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doldi PM, Stolz L, Escher F, Steffen J, Gmeiner J, Roden D, et al. Transcatheter aortic valve replacement with the self-expandable core valve evolut prosthesis using the cusp-overlap vs. tricusp-view. J Clin Med. (2022) 11(6):1561. 10.3390/jcm11061561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascual I, Hernández-Vaquero D, Alperi A, Almendarez M, Avanzas P, Kalavrouziotis D, et al. Permanent pacemaker reduction using cusp-overlapping projection in TAVR: a propensity score analysis. JACC Cardiovasc Interv. (2022) 15(2):150–61. 10.1016/j.jcin.2021.10.002 [DOI] [PubMed] [Google Scholar]
- 28.Falk V, Baumgartner H, Bax JJ, de Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. (2017) 52(4):616–64. 10.1093/ejcts/ezx324 [DOI] [PubMed] [Google Scholar]
- 29.Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular computed tomography. J Cardiovasc Comput Tomogr. (2019) 13(1):1–20. 10.1016/j.jcct.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 30.Siontis GCM, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. (2014) 64(2):129–40. 10.1016/j.jacc.2014.04.033 [DOI] [PubMed] [Google Scholar]
- 31.Zhou D, Yidilisi A, Fan J, Zhang Y, Dai H, Zhu G, et al. Three-year outcomes of transcatheter aortic valve implantation for bicuspid versus tricuspid aortic stenosis. EuroIntervention. (2022) 18(3):193–202. 10.4244/EIJ-D-21-00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang GHL, Zaid S, Michev I, Ahmad H, Kaple R, Undemir C, et al. “Cusp-overlap” view simplifies fluoroscopy-guided implantation of self-expanding valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2018) 11(16):1663–5. 10.1016/j.jcin.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 33.Wong I, Bieliauskas G, De Backer O, Søndergaard L. Technical considerations for transcatheter aortic valve replacement with ACURATE neo2. JACC Cardiovasc Interv. (2021) 14(2):224–6. 10.1016/j.jcin.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 34.Zaid S, Raza A, Michev I, Ahmad H, Kaple R, Undemir C, et al. “Cusp overlap” view facilitates accurate fluoro-guided implantation of self-expanding valve in TAVR. J Am Coll Cardiol. (2016) 68(18_Supplement):B288–9. 10.1016/j.jacc.2016.09.125 [DOI] [Google Scholar]
- 35.Tchétché D, Siddiqui S. Optimizing fluoroscopic projections for TAVR: any difference between the double S-curve and the cusp-overlap technique? JACC Cardiovasc Interv. (2021) 14(2):195–7. 10.1016/j.jcin.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 36.Pascual I, Almendárez M, Avanzas P, Álvarez R, Arboine LA, Del Valle R, et al. Cusp-overlapping TAVI technique with a self-expanding device optimizes implantation depth and reduces permanent pacemaker requirement. Rev Esp Cardiol (Engl Ed). (2022) 75(5):412–20. 10.1016/j.recesp.2021.05.014 [DOI] [PubMed] [Google Scholar]
- 37.Grubb KJ, Gada H, Mittal S, Nazif T, Rodés-Cabau J, Fraser DGW, et al. Clinical impact of standardized TAVR technique and care pathway: insights from the optimize PRO study. JACC Cardiovasc Interv. (2023) 16(5):558–70. 10.1016/j.jcin.2023.01.016 [DOI] [PubMed] [Google Scholar]
- 38.Sammour Y, Banerjee K, Kumar A, Lak H, Chawla S, Incognito C, et al. Systematic approach to high implantation of SAPIEN-3 valve achieves a lower rate of conduction abnormalities including pacemaker implantation. Circ Cardiovasc Interv. (2021) 14(1):e009407. 10.1161/CIRCINTERVENTIONS.120.009407 [DOI] [PubMed] [Google Scholar]
- 39.Meredith Am IT, Walters DL, Dumonteil N, Worthley SG, Tchétché D, Manoharan G, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary endpoint results from the REPRISE II study. J Am Coll Cardiol. (2014) 64(13):1339–48. 10.1016/j.jacc.2014.05.067 [DOI] [PubMed] [Google Scholar]
- 40.Rampat R, Khawaja MZ, Hilling-Smith R, Byrne J, MacCarthy P, Blackman DJ, et al. Conduction abnormalities and permanent pacemaker implantation after transcatheter aortic valve replacement using the repositionable LOTUS device: the United Kingdom experience. JACC Cardiovasc Interv. (2017) 10(12):1247–53. 10.1016/j.jcin.2017.03.044 [DOI] [PubMed] [Google Scholar]
- 41.Falk V, Wöhrle J, Hildick-Smith D, Bleiziffer S, Blackman DJ, Abdel-Wahab M, et al. Safety and efficacy of a repositionable and fully retrievable aortic valve used in routine clinical practice: the RESPOND study. Eur Heart J. (2017) 38(45):3359–66. 10.1093/eurheartj/ehx297 [DOI] [PubMed] [Google Scholar]
- 42.Maier O, Piayda K, Binnebößel S, Berisha N, Afzal S, Polzin A, et al. Real-world experience with the cusp-overlap deployment technique in transcatheter aortic valve replacement: a propensity-matched analysis. Front Cardiovasc Med. (2022) 9:847568. 10.3389/fcvm.2022.847568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mailey JA, Brennan PF, Kearney A, Hogg MC, McNeice AH, Jeganathan R, et al. Reframing optimal implantation of the sapien 3 transcatheter heart valve. J Invasive Cardiol. (2022) 34(5):E380–9. [DOI] [PubMed] [Google Scholar]
- 44.Nazif TM, Chen S, George I, Dizon JM, Hahn RT, Crowley A, et al. New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: an analysis from the PARTNER II trial. Eur Heart J. (2019) 40(27):2218–27. 10.1093/eurheartj/ehz227 [DOI] [PubMed] [Google Scholar]
- 45.Xiao HB, Lee CH, Gibson DG. Effect of left bundle branch block on diastolic function in dilated cardiomyopathy. Br Heart J. (1991) 66(6):443–7. 10.1136/hrt.66.6.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.