Abstract
This study analyzes the use and timing of terminal disclaimers in all biologic patents involved in litigation from 2010 to 2023.
Spending on biologics in 2021 represented $260 billion or 46% of all spending on prescription drugs in the US.1 Biosimilar competition can lower prices, but biosimilar entry is often delayed by expansive patent thickets (dozens or hundreds of patents directed toward the same product). Many of these patents claim innovations that are closely related to one another.2,3 Although the US Patent and Trademark Office is required to reject patents that are obvious follow-ons from earlier versions, they may grant such patents when applicants file terminal disclaimers.
A terminal disclaimer is a stipulation that the follow-on patent will expire at the same time as the original patent. This tool, which is not permitted in other countries, allows drug manufacturers to quickly obtain new patents that offer trivial changes over existing innovations.4 These patents can trigger lengthy and costly litigation and add uncertainty for biosimilar manufacturers who must invalidate or design around all patents in a biologic’s portfolio.
In recognition of this problem, a bipartisan group of US senators recently urged the US Patent and Trademark Office to limit or eliminate the use of terminal disclaimers in pharmaceutical patents.5 Even though biologic patent thickets have received increased scrutiny, the role of terminal disclaimers in these patent thickets has not been systematically examined. We analyzed the use and timing of terminal disclaimers in all biologic patents involved in litigation from 2010 to 2023.
Methods
We used the Legal Analytics Platform (Lex Machina) to identify patents involved in biosimilar litigation beginning in 2010 (when the modern biosimilar pathway was established) until April 2023. We focus on litigated patents because, unlike the manufacturers of small-molecule drugs, the manufacturers of biologics are only required to list their patents with the US Food and Drug Administration (FDA) when they face litigation.
Claims define the legal scope of patents, and we determined whether each claim was related to the drug’s manufacturing technique, method of treatment, formulation, composition of matter, or delivery device (Table). Because patents often contain many claims, each patent could be associated with multiple subject matter claims. We used data from the US Patent and Trademark Office prosecution history to determine if patents had terminal disclaimers and when patents were issued relative to FDA approval of the relevant biologic.6 The analyses were completed using Excel version 16 (Microsoft).
Table. Type of Claims in Patents With vs Without Terminal Disclaimers.
| No. of patents litigated | Patents by terminal disclaimer status, No. (%) | ||
|---|---|---|---|
| With a terminal disclaimer | Without a terminal disclaimer | ||
| Unique patents | 271 | 129 (48) | 142 (52) |
| Subject matter of patent claima | |||
| Manufacturing techniqueb | 130 | 46 (36) | 84 (52) |
| Method of treatmentc | 84 | 42 (33) | 42 (26) |
| Formulationd | 55 | 39 (30) | 16 (10) |
| Composition of mattere | 18 | 5 (4) | 13 (8) |
| Delivery devicef | 7 | 1 (1) | 6 (4) |
Some of the patents include claims that cover more than 1 subject matter.
Covers the methods for producing the biologic (eg, purification method).
Covers the use of the biologic to treat a specific disease or population.
Covers the biologic’s active ingredient along with the excipients (eg, buffers or carriers) that make up the dosage form that is administered to the patient.
Covers the biologic’s active ingredient (eg, the amino acid backbone of an antibody).
Covers the delivery devices used for the administration of a biologic (eg, the injector pen).
Results
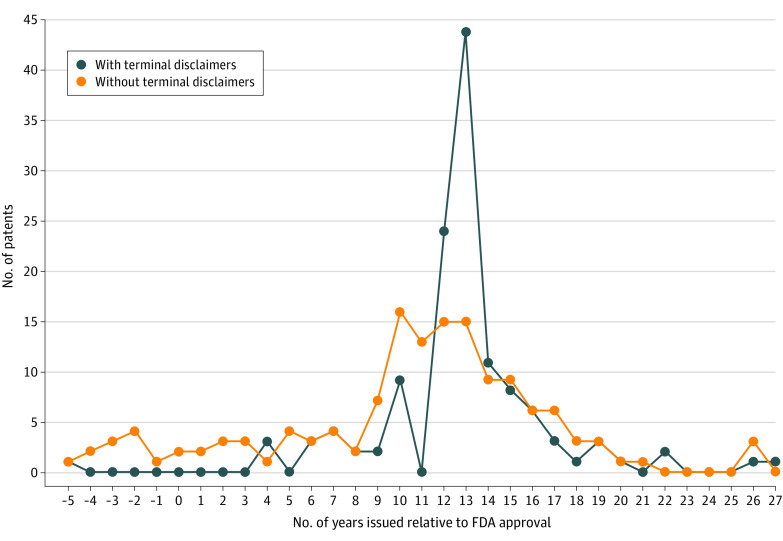
Manufacturers of 12 biologics brought litigation against manufacturers of 48 biosimilars from 2010 to 2023. Of the 271 patents involved in litigation, 129 (48%) contained terminal disclaimers. Patents with terminal disclaimers had fewer claims on composition of matter compared with patents without terminal disclaimers (4% vs 8%) and more claims on method of treatment (33% v 26%) and formulation (30% vs 10%) (Table). The number of patents with terminal disclaimers spiked 12 years after product approval, coinciding with the end of FDA-granted statutory biologic exclusivity (Figure). During year 13, there were 44 patents issued with terminal disclaimers compared with 15 patents without terminal disclaimers.
Figure. Number of Patents With vs Without Terminal Disclaimers Involved in Litigation Relative to Drug Approval From the US Food and Drug Administration (FDA).
This figure shows when litigated patents with vs without terminal disclaimers were granted relative to the approval of biologics by the FDA. Both types of patents experienced sharp increases during the study period followed by declines through year 20. The pronounced peak for patents with terminal disclaimers occurred in years 12 and 13, coinciding with the end of FDA-granted statutory exclusivity.
Discussion
Almost half of all biologic patents involved in litigation from 2010 to 2023 had terminal disclaimers. These patents spiked just as 12-year statutory exclusivity periods were ending. The scale and timing of these patenting practices suggest that biologic firms may be using patents with terminal disclaimers to strengthen barriers to biosimilar entry.
By adding patents with terminal disclaimers beginning in year 12, biologic manufacturers introduce uncertainty precisely when biosimilar challenges begin. Biosimilar firms must then contest or design around a wave of new patents containing claims that become enforceable just as statutory exclusivity periods are ending.
A limitation of this study is that it only examined litigated patents; however, these are the key patents that impede biosimilar entry. Congress could reduce the barriers to biosimilar entry by capping the number of patents that brand-name manufacturers can assert against biosimilar firms (eg, just 1 patent among those linked by terminal disclaimers). Minimizing the use and effect of terminal disclaimers could help improve patient access to lower-cost biosimilar drugs and reduce health care spending.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Karen Lasser, MD, and Kristin Walter, MD, Senior Editors.
Data sharing statement
References
- 1.IQVIA Institute . Biosimilars in the United States 2023-2027. Published January 2023. Accessed June 5, 2023. https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-united-states-2023-2027
- 2.Tu SS, Kesselheim AS, Wetherbee K, Feldman WB. Changes in the number of continuation patents on drugs approved by the FDA. JAMA. 2023;330(5):469-470. doi: 10.1001/jama.2023.11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goode R, Feldman WB, Tu SS. Ancillary product patents to extend biologic patent life. JAMA. 2023;330(21):2117-2119. doi: 10.1001/jama.2023.19547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu SS. Patenting fast and slow: examiner rejections and applicant traversals to non-prior art rejections. Michigan State Law Rev. 2021;411:1-57. doi: 10.2139/ssrn.3546944 [DOI] [Google Scholar]
- 5.Signed by US Senators Patrick Leahy, John Cornyn, Richard Blumenthal, Susan M. Colins, Amy Klobuchar, and Mike Braun . Letter to Kathi Vidal, director of the US Patent and Trademark Office on June 8, 2022. Accessed October 3, 2023. https://www.collins.senate.gov/imo/media/doc/patent_letter.pdf
- 6.US Patent and Trademark Office . Patent Center. Accessed October 4, 2023. https://patentcenter.uspto.gov/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data sharing statement