Abstract
Background
Assisted reproduction technology (ART) has advanced significantly, raising concerns regarding its impact on the secondary sex ratio (SSR), which is the sex ratio at birth in offspring. This study aimed to explore factors affecting SSR in singletons, singletons from twin gestation, and twins from twin gestation within the context of ART.
Methods
A retrospective analysis was conducted on data from 8335 births involving 6,223 couples undergoing ART. Binary logistic regression assessed relationships between parental and embryonic factors and SSR in singletons and singletons from twin gestation. Multinomial logistic regression models were utilized to identify factors influencing SSR in twins from twin gestation.
Results
Secondary infertility (OR = 1.164, 95% CI: 1.009–1.342), advanced paternal age (OR = 1.261, 95% CI: 1.038–1.534), and blastocyst embryo transfer (OR = 1.339, 95% CI: 1.030–1.742) were associated with an increased SSR, while frozen embryo transfer (FET) showed a negative association with SSR (OR = 0.738, 95% CI: 0.597–0.912) in singletons. A longer duration of gonadotropin (Gn) usage reduced SSR in singletons (OR = 0.961, 95% CI: 0.932–0.990) and singletons from twin gestation (OR = 0.906, 95% CI: 0.838–0.980). In singletons from twin gestation, male-induced infertility (OR = 2.208, 95% CI: 1.120–4.348) and higher Gn dosage (OR = 1.250, 95% CI: 1.010–1.548) were significantly associated with an increased SSR. Women aged > 35 years and intracytoplasmic sperm injection (ICSI) were associated with lower SSR (OR = 0.539, 95% CI: 0.293–0.990 and OR = 0.331, 95% CI: 0.158–0.690, respectively). In twins from twin gestation, paternal age exceeded maternal age (OR = 0.682, 95% CI: 0.492–0.945) and higher Gn dosage (OR = 0.837, 95% CI: 0.715–0.980) were associated with a higher proportion of male twins. Cleavage stage transfer (OR = 1.754, 95% CI: 1.133–2.716) resulted in a higher percentage of boy-girl twins compared to blastocyst transfer.
Conclusion
This study demonstrates the complex interplay of various factors in determining the SSR in ART, highlighting the importance of considering infertility type, paternal age, fertilization method, embryo transfer stage, and Gn use duration when assessing SSR. Nevertheless, further research with a large sample size is necessary to confirm and expand upon the findings of this study.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12978-023-01723-8.
Keywords: Assisted reproductive technology, Infertility, Logistic regression, Secondary sex ratio
Background
Assisted reproductive technology (ART) has evolved from a marvel innovation to a conventional medical treatment for infertility. Since the first successful in vitro fertilization (IVF) treatment in 1978, it was estimated that at least 5 million infants worldwide had been born by ART [1], making IVF the principal treatment for infertile women [2]. Intracytoplasmic sperm injection (ICSI), introduced in 1992, allows the direct injection of a single spermatozoon into the oocyte and has become a valuable alternative for couples suffering infertility, especially those affected by poor sperm quality and unsuccessful IVF attempts [3].
The human sex ratio, critical for gender balance, consists of primary sex ratio (PSR) and secondary sex ratio (SSR). The PSR can deviate from 1:1 in theory, sometimes reaching as high as 170 males to 100 female [4]. However, SSR affected by spontaneous abortion, premature delivery [5], sex preference [6] and many other factors, is generally around 105 males per 100 female births [7]. ART procedures may influence SSR due to embryo handling. Researchers have tried to identify factors affecting SSR during ART [8, 9]. Dean J H et al. performed a population-based retrospective study indicating that ART procedures significantly influenced SSR [10]. They found that ICSI was related to a decrease in SSR compared to IVF, consistent with other studies [11, 12]. Frozen embryo transfer (FET) was also linked to a lower proportion of male babies in a study [13], although conflicting results were reported in others [14, 15]. Furthermore, Dean J H et al. [10] found that blastocyst stage transfer was associated with a higher proportion of male infants compared to cleavage stage transfer. A study by Maalouf, Walid E et al. [16] involving 85,511 treatment cycles and 106,066 babies in the United Kingdom, demonstrated that the neonatal SSR obtained from embryos transferred at the blastocyst stage is 6% higher than that of embryos transferred at the cleavage stage. Maternal age was considered an independent factor that influenced SSR, with women < 35 years more likely to have male births and women ≥ 35 years tending to have female infants [17]. However, Rueness, Janne et al. [18] found no evidence of a connection between maternal age and human sex ratio. Frattarelli, John L et al. [19] demonstrated that paternal age might have indirectly affect on SSR through blastocyst formation rates. Various factors, such as the type of infertility [20], specific infertility factor [11], and body mass index (BMI) [21], have been suggested as potential risk factors. Obviously, factors affecting SSR are multifaceted.
Even though numerous studies have explored potential factors of SSR in the ART population and have observed skewed gender distribution in the ART population compared to naturally conceived population, conclusive findings about the influence of ART on SSR remain elusive. Additionally, few studies have investigated SSR in the context of singletons and multiple births based on the premise that all mothers received double embryo transfer. Furthermore, there has been no investigation into the SSR of singletons born from twin pregnancies. Thus, this retrospective study, including 6223 cycles and 8335 babies, was conducted to investigate potential factors influencing the SSR of singletons, singletons from twin gestation, and twins from twin gestation. Singletons denote infants born from a single pregnancy where the mother carried only one child. Singletons from twin gestation refer to cases in which mothers with twin pregnancies successfully delivered only one child, while twins from twin gestation pertain to situations in which mothers with twin pregnancies successfully delivered two children.
Methods
Study subjects
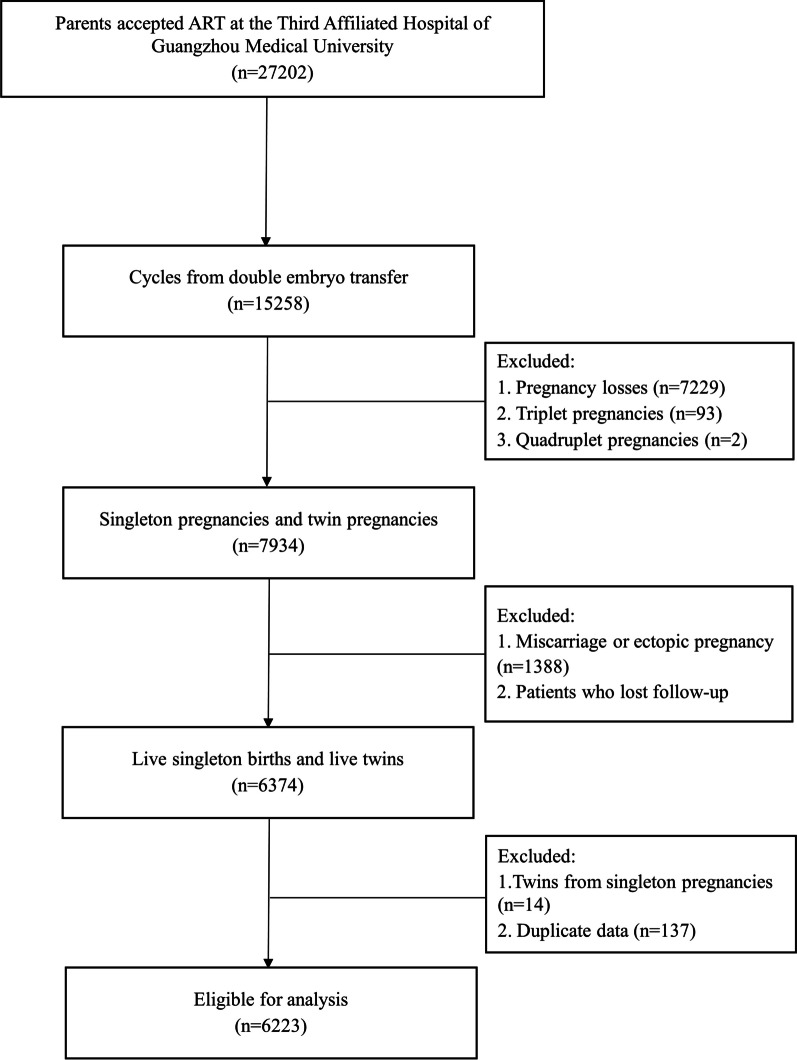
A total of 27,202 couples who underwent assisted reproductive technology (ART) at the Centre for Reproductive Medicine of the Third Affiliated Hospital of Guangzhou Medical University, China, between January 2010 and January 2015, were recruited in the study. The inclusion criteria were as follows: (1) couples who received ART treatment at the Third Affiliated Hospital of Guangzhou Medical University; (2) ART cycles from double embryo transfer; (3) singleton pregnancies and twin pregnancies; (4) live births of singleton or twins; (5) subjects who completed the study follow-up. Moreover, for singleton pregnancies, only singleton births were included. The exclusion criteria are detailed in Fig. 1. Finally, a total of 6223 couples who underwent ART were selected as study subjects in the retrospective observational study.
Fig. 1.
Flow diagram for the study selection. ART Assisted reproductive technology
Data collection
In this research, various variables were examined, including the type of infertility, infertility factor, maternal age group, paternal age group, age disparities between partners, fertilization method, maternal body mass index (BMI), embryo transfer stage, dosage of gonadotropin (Gn), and days of Gn use. These variables were extracted from the hospital electronic medical records system of the Third Affiliated Hospital of Guangzhou Medical University. Besides, data regarding the gender of newborns was collected from the hospital’s electronic medical records system and telephonic follow-up.
The study received ethical approval from the ethics committee of the third Affiliated Hospital of Guangzhou Medical University. Informed consent was provided by all study participants.
Definition and measurements
The secondary sex ratio (SSR) was defined as the ratio of male to female births in this study. Maternal age was classified into either < 35 or ≥ 35 years [17]. Paternal age was divided into groups based on interquartile ranges. The maternal BMI was divided into three groups in accordance with Chinese population standards: BMI < 18.5 kg/m2, 18.5 < BMI < 23.9 kg/m2, and BMI ≥ 24 kg/m2, according to the Chinese criteria[22]. Age differences referred to the difference between the mother's age and the father's age (maternal age minus paternal age). Embryos from in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) were all fresh at the time of transfer, while any remaining embryos were from frozen embryo transfer (FET). Infertility was categorized into primary and secondary infertility, according to whether a woman had previously experienced a pregnancy. Secondary infertile was characterized by women who had previously given a live birth and were now experiencing difficulty conceiving again [23].
Data analysis
Shapiro–Wilk test was used to assess the normal distribution of continuous variables. For normally distributed continuous variables, t-tests were used, and the results were expressed as means with standard deviations. In cases where continuous variables did not meet a normal distribution, the Mann–Whitney U test was used, and continuous variables were presented as medians (25th percentile, 75th percentile). Categorical variables were presented as numbers and proportions. Based on the expectation value, the Pearson χ2 test or Fisher’s exact test would be used to compare differences between groups in categorical variables. Logistic regression models, adjusted for selected characteristics, were used to estimate the associations between parental and embryonic factors with SSR. Odds ratios (ORs) with a 95% confidence interval (CI) were calculated. All statistical analyses were conducted using SPSS software (version 25, IBM). A two-tailed P value < 0.05 was considered statistically significant.
Results
Baseline characteristics
In this study, a total of 8335 babies were included from 6223 cycles. The secondary sex ratio (SSR) of singletons, singletons from twin gestation, and twins from twin gestation were 124.1: 100, 134.2: 100 and 109.7: 100, respectively. For singletons, a significantly higher SSR was observed in births where parents had experienced secondary infertility compared to those with primary infertility (SSR: 136.5: 100 vs. 114.4: 100, P = 0.009). Additionally, paternal age ≥ 37 years was associated with a higher likelihood of live male births compared to other paternal age groups (SSR = 145.4: 100, P = 0.019). The median gonadotropin (Gn) usage was 2100 IU/L (25th-75th percentile: 1500–2775), and the median days of Gn use were 12 days (25th-75th percentile: 11–13). In singletons from twin gestation, the median Gn was 2025 IU/L (25th-75th percentile: 1462.4–2850), and the median days of Gn use were 12 days (25th-75th percentile: 11–13). For twins from twin gestation, there was a significantly higher proportion of males with blastocyst stage embryo transfer compared to cleavage stage transfers (SSR:138.6: 100 vs. 103.3: 100, P = 0.002). In addition, the median Gn usage for this group was 2062.5 IU/L (25th-75th percentile: 1575–2700), with a median duration of Gn use of 12 days (25th-75th percentile: 11–13) (Table 1).
Table 1.
Basic characteristics of the patients and embryos undergoing ART treatment
| Variables | Singletons | Singletons from twin gestation | Twins from twin gestation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P | Male | Female | P | Male | Female | P | |
| Infertility type (n, %) | 0.009 | 0.524 | 0.959 | ||||||
| Primary | 1018(53.4) | 890(46.6) | 163(56.0) | 128(44.0) | 1182(52.1) | 1086(47.9) | |||
| Secondary | 947(57.7) | 694(42.3) | 159(58.7) | 112(41.3) | 1028(52.6) | 928(47.4) | |||
| Infertility factor (n, %) | 0.463 | 0.791 | 0.278 | ||||||
| Male | 1324(56.0) | 1042(44.0) | 225(57.7) | 165(42.3) | 1564(53.0) | 1388(47.0) | |||
| Female | 314(55.3) | 254(44.7) | 46(59.0) | 32(41.0) | 296(48.7) | 312(51.3) | |||
| Both male and female | 327(53.2) | 288(46.8) | 51(54.3) | 43(45.7) | 350(52.7) | 314(47.3) | |||
| Maternal age group (n, %) | 0.085 | 0.230 | 0.823 | ||||||
| ≤35 | 1748(54.9) | 1437(45.1) | 286(58.2) | 205(41.8) | 2102(52.3) | 1914(47.7) | |||
| >35 | 217(59.6) | 147(40.4) | 36(50.7) | 35(49.3) | 108(51.9) | 100(48.1) | |||
| Paternal age group (n, %) | 0.019 | 0.968 | 0.864 | ||||||
| ≤30 | 519(52.5) | 470(47.5) | 85(55.9) | 67(44.1) | 717(52.0) | 663(48.0) | |||
| 31–331 | 545(56.6) | 418(43.4) | 83(58.5) | 59(41.5) | 418(51.2) | 398(48.8) | |||
| 34–362 | 424(53.5) | 368(46.5) | 86(58.1) | 62(41.9) | 547(53.4) | 477(46.6) | |||
| ≥373 | 477(59.3) | 328(40.7) | 68(56.7) | 52(43.3) | 528(52.7) | 476(47.3) | |||
| Age difference (n, %) | 0.675 | 0.330 | 0.093 | ||||||
| Older mother | 302(53.7) | 260(46.3) | 46(55.4) | 37(44.6) | 304(49.4) | 312(50.6) | |||
| Older father | 1400(55.8) | 1110(44.2) | 239(59.0) | 166(41.0) | 1601(53.2) | 1411(46.8) | |||
| None | 263(55.1) | 214(44.9) | 37(50.0) | 37(50.0) | 305(51.2) | 291(48.8) | |||
| Fertilization method (n, %) | 0.526 | 0.186 | 0.238 | ||||||
| IVF | 1178(56.2) | 919(43.8) | 201(59.3) | 138(40.7) | 1394(52.1) | 1280(47.9) | |||
| ICSI | 298(55.1) | 243(44.9) | 29(46.8) | 33(53.2) | 252(48.5) | 268(51.5) | |||
| FET | 455(53.9) | 389(46.1) | 89(57.8) | 65(42.2) | 522(54.7) | 432(45.3) | |||
| Maternal BMI (n, %) | 0.284 | 0.805 | 0.359 | ||||||
| <18.5 | 275(52.5) | 249(47.5) | 37(54.4) | 31(45.6) | 308(50.5) | 302(49.5) | |||
| 18.5–23.9 | 1351(56.2) | 1054(43.8) | 228(58.2) | 164(41.8) | 1530(52.4) | 1390(47.6) | |||
| ≥24 | 339(54.7) | 281(45.3) | 57(55.9) | 45(44.1) | 372(53.6) | 322(46.4) | |||
| Type of cycle (n, %) | 0.332 | 0.884 | 0.240 | ||||||
| Fresh | 1510(55.8) | 1195(44.2) | 233(57.1) | 175(42.9) | 1688(51.6) | 1582(48.4) | |||
| Frozen | 454(53.9) | 388(46.1) | 89(57.8) | 65(42.2) | 522(54.7) | 432(45.3) | |||
| Embryo transfer stage (n, %) | 0.378 | 0.638 | 0.002 | ||||||
| Cleavage stage | 1694(55.0) | 1387(45.0) | 255(58.1) | 184(41.9) | 1741(50.8) | 1685(49.2) | |||
| Blastocyst | 227(57.3) | 169(42.7) | 64(55.7) | 51(44.3) | 424(58.1) | 306(41.9) | |||
| Usage of Gn (IU/L, M (P25, P75)) | 2100(1500, 2775) | 2025(1462.4, 2850) | 2062.5(1575, 2700) | ||||||
| Days of Gn use (day, M (P25, P75)) | 12(11, 13) | 12(11, 13) | 12(11, 13) | ||||||
ART Assisted reproductive technology, ICSI Intracytoplasmic sperm injection, IVF In vitro fertilization, FET Frozen embryo transfer, Gn Gonadotropin, BMI Body mass index
1Singletons from twin gestation and twins from twin gestation were classified as 31–33 and 31–32, respectively
2Singletons from twin gestation and twins from twin gestation were classified as 34–37 and 33–35, respectively
3Singletons from twin gestation and twins from twin gestation were classified as ≥ 38 and ≥ 36, respectively
Factors associated with SSR in singletons
For singletons, univariable logistic regression analyses revealed associations between SSR and factors such as infertility type, paternal age, and days of Gn (Additional file 1). Following mutual adjustment for infertility type, infertility factors, paternal age, fertilization methods, embryo transfer stages and days of Gn use, higher SSR was associated with patients experiencing secondary infertility (OR = 1.164, 95% CI:1.009–1.342) and fathers aged 37 years or older (OR = 1.261, 95% CI: 1.038–1.534); Blastocyst embryo transfer exhibited a higher SSR compared to cleavage stage embryo transfer (OR = 1.339, 95% CI: 1.030–1.742). Lower SSR (OR = 0.738, 95% CI: 0.597–0.912) was observed in patients undergoing frozen embryo transfer (FET) treatment compared to vitro fertilization. A slight decline in SSR was noted with an increase in the duration of Gn use (in days) (OR = 0.961, 95% CI: 0.932–0.990) (Table 2).
Table 2.
Multivariable logistic regression analysis with SSR in singletons
| Variables | OR | 95% CI |
|---|---|---|
| Infertility type | ||
| Primary | 1.000 | – |
| Secondary | 1.164 | 1.009–1.342 |
| Infertility factor | ||
| Female | 1.000 | – |
| Male | 1.031 | 0.800–1.330 |
| Both male and female | 0.896 | 0.739–1.087 |
| Paternal age group (year) | ||
| ≤30 | 1.000 | – |
| 31–33 | 1.143 | 0.951–1.372 |
| 34–36 | 1.048 | 0.865–1.271 |
| ≥37 | 1.261 | 1.038–1.534 |
| Fertilization method | ||
| IVF | 1.000 | – |
| ICSI | 0.996 | 0.772–1.285 |
| FET | 0.738 | 0.597–0.912 |
| Embryo transfer stage | ||
| Cleavage stage | 1.000 | – |
| Blastocyst | 1.339 | 1.030–1.742 |
| Days of Gn use (day) | 0.961 | 0.932–0.990 |
Male singletons selected as the reference category
SSR Secondary sex ratio, IVF In vitro fertilization, ICSI Intracytoplasmic sperm injection, FET Frozen embryo transfer, Gn Gonadotropin
Factors associated with SSR in singletons from twin gestation
For singletons from twin gestation, initial univariable logistic regression analyses did not reveal any significant difference between SSR and potential factors in (Additional file 2). However, subsequent multivariable regression analysis, after adjusting for confounding factors, showed that infertility attributed to male factors was positively associated with an increased SSR (OR = 2.208, 95% CI: 1.120–4.348) in the model. Moreover, SSR was significantly different between the reference category (age ≤ 35 years) and women aged > 35 years (OR = 0.539, 95% CI: 0.293–0.990). Besides, intracytoplasmic sperm injection (ICSI) accounted for a decreased SSR (OR = 0.331, 95% CI: 0.158–0.690) compared to in vitro fertilization (IVF). Increased duration of Gn use also significantly linked to a decreased the SSR (OR = 0.906, 95% CI: 0.838–0.980). Moreover, a higher dosage of Gn was related to an increased SSR (OR = 1.250, 95% CI: 1.010–1.548) (Table 3).
Table 3.
Multivariable logistic regression analysis from twin gestation
| Variables | OR | 95% CI |
|---|---|---|
| Infertility factor | ||
| Female | 1.000 | |
| Male | 2.208 | 1.120–4.348 |
| Both male and female | 0.989 | 0.612–1.597 |
| Maternal age group (year) | ||
| ≤35 | 1.000 | |
| >35 | 0.539 | 0.293–0.990 |
| Paternal age group (year) | ||
| ≤30 | 1.000 | |
| 31–33 | 1.135 | 0.706–1.821 |
| 34–37 | 1.235 | 0.764–1.996 |
| ≥38 | 1.330 | 0.762–2.326 |
| Fertilization method | ||
| IVF | 1.000 | |
| ICSI | 0.331 | 0.158–0.690 |
| FET | 0.938 | 0.595–1.479 |
| Dosage of Gn (× 103 IU/L) | 1.250 | 1.010–1.548 |
| Days of Gn use (day) | 0.906 | 0.838–0.980 |
Male births selected as the reference category
IVF In vitro fertilization, ICSI Intracytoplasmic sperm injection, FET Frozen embryo transfer, Gn Gonadotropin
Factors associated with SSR in twins from twin gestation
In the case of twins born from twin gestation, only age difference and embryo transfer stage were found to be associated with SSR (Additional file 3). The results of the multinomial logistic regression modeling of the combined effect of dosage of Gn, days of Gn use, age difference, paternal age group, maternal body mass index (BMI), and embryo transfer stage of SSR among twins from twin gestation, are shown in Table 4. In comparison to groups with no age difference, groups in which paternal age exceeded maternal age exhibited a higher proportion of male twins relative to boy-girl twins (OR = 0.682, 95% CI: 0.492–0.945). Cleavage stage transfer had a higher probability of having boy-girl twins compared to blastocyst transfer (OR = 1.754, 95% CI: 1.133–2.716). In addition, a higher dosage of Gn was associated with a greater percentage of male twins (OR = 0.837, 95% CI: 0.715–0.980).
Table 4.
Multinomial logistic regression analysis with SSR in twins from twin gestation
| Birth* | Variables | OR | 95% CI |
|---|---|---|---|
| Female twins | Dosage of Gn (× 103 IU/L) | 0.931 | 0.805–1.076 |
| Days of Gn use (day) | 1.021 | 0.977–1.067 | |
| Age difference | |||
| Older mother | 0.815 | 0.546–1.215 | |
| Older father | 0.864 | 0.629–1.187 | |
| None | 1.000 | ||
| Embryo transfer stage | |||
| Cleavage stage | 0.783 | 0.530–1.158 | |
| Blastocyst | 1.000 | ||
| Paternal age group (year) | |||
| ≤30 | 1.056 | 0.773–1.444 | |
| 31–32 | 0.988 | 0.712–1.371 | |
| 33–35 | 1.072 | 0.794–1.448 | |
| ≥36 | 1.000 | ||
| Maternal BMI | |||
| <18.5 | 1.129 | 0.770–1.654 | |
| 18.5–23.9 | 1.048 | 0.786–1.396 | |
| ≥24 | 1.000 | ||
| Fertilization method | |||
| IVF | 1.061 | 0.703–1.600 | |
| ICSI | 0.844 | 0.514–1.386 | |
| FET | 1.000 | ||
| Boy-girl twins | Dosage of Gn (× 103 IU/L) | 0.837 | 0.715–0.980 |
| Days of Gn use (day) | 1.032 | 0.985–1.081 | |
| Age difference | |||
| Older mother | 0.917 | 0.616–1.366 | |
| Older father | 0.682 | 0.492–0.945 | |
| None | 1.000 | ||
| Embryo transfer stage | |||
| Cleavage stage | 1.754 | 1.133–2.716 | |
| Blastocyst | 1.000 | ||
| Paternal age group (year) | |||
| ≤30 | 0.939 | 0.672–1.312 | |
| 31–32 | 0.972 | 0.686–1.377 | |
| 33–35 | 0.995 | 0.719–1.377 | |
| ≥36 | 1.000 | ||
| Maternal BMI | |||
| <18.5 | 1.340 | 0.892–2.012 | |
| 18.5–23.9 | 1.160 | 0.845–1.591 | |
| ≥24 | 1.000 | ||
| Fertilization method | |||
| IVF | 0.846 | 0.546–1.312 | |
| ICSI | 0.882 | 0.531–1.464 | |
| FET | 1.000 |
*Male twins selected as the reference category
SSR Secondary sex ratio, IVF In vitro fertilization, ICSI Intracytoplasmic sperm injection, FET Frozen embryo transfer, Gn Gonadotropin, BMI Body mass index
Discussion
The secondary sex ratio (SSR) is an important implication for population health and fertility. In this hospital-based retrospective study, we investigated the factors related to the sex ratio of newborns born to mothers who underwent assisted reproductive technology (ART) and achieved singleton or twin deliveries. Our findings shed light on various factors that influence SSR in singletons and twins, underscoring the impact of a specific ART protocol on sex ration of newborns. These factors included infertility type, paternal age group, fertilization method, embryo transfer stage, course of gonadotropin (Gn), infertility factor, maternal age group, and age difference.
In terms of the infertility type, our findings suggest that, in singleton pregnancies, secondary infertility is more prone to having male offspring through ART compared to primary infertility. Primary infertility typically affects young patients who have never experienced a pregnancy. The causes of infertility in this group are often complex and diverse, making them more susceptible to sperm-ovum fertilization failure and low fertilization rates during their first in vitro fertilization (IVF)-assisted pregnancy [20]. Our research also indicates an association between SSR and paternal age. Studies have shown that, in a singleton pregnancy, boys are more likely to be born to older fathers than in the control group [15]. As society progresses and reproductive technology advances, the number of older couples seeking ART is on the rise. Our research indicates that fathers aged 37 or above have a higher probability of having male babies compared to fathers aged 30 or less. However, Jacobsen R [24] found that a lower SSR was associated with an increase in paternal age, possible due to a significant reduction in blastocyst formation rate over paternal age [25]. This reduction may lead to a higher proportion of male embryos in blastocysts [26], ultimately affecting the sex ratio at birth among older men. Thus, the relationship between SSR and paternal age warrants further study. In addition, in singletons from twin gestation, maternal age was also significantly associated with SSR. Those maternal ages > 35 were related to a reduced SSR compared to those aged 35 or younger. Tarín J J et al. [17] suggested a significant shift toward females (71.4%) in the sex ratio in mothers aged 35 or older, while the neonatal sex ratio was significantly shifted toward males (62.7%) in women under 35. The mechanism behind this may be linked with higher levels of stress in older populations, with female embryos exhibiting greater stress tolerance, leading to the elimination of more male embryos [18].
In addition, the characteristics of the ART treatment can alter the proportion of male infants in our research. Previous studies show that the use of BT is associated with an increase in SSR, while intracytoplasmic sperm injection (ICSI) is related to a reduction in SSR [27]. However, only a few studies have reported the difference between SSR in fresh embryo transfer and frozen embryo transfer (FET). In particular, our research shows that the use of FET significantly increases SSR compared to the use of IVF. Several biologically plausible mechanisms may explain the selective survival of male embryos in multiple embryo transfer, including immunologic factors, genetic survival advantages encoded on the Y chromosome, and imprinting errors on the X chromosomes of female embryos [13]. Most Chinese reproductive medicine centers use blastocyst transfers in FET cycles. It is suspected that the alteration of SSR toward males is due to the blastocyst transfer itself rather than FET [14].
Our study revealed that prolonged gonadotropin use was linked to a higher proportion of female infants in both singletons and singletons from twin gestation. The underlying reasons for the discrepancy remain unclear. However, it is unlikely that prolonged gonadotropin use would cause sex-selective abortions by adversely affecting male-fertilized eggs [28]. Thus, further studies are warranted to explore the potential association between SSR and Gn.
Consistent with existing literature, blastocyst embryos were found to be correlated with a higher proportion of male offspring in contrast with cleavage stage embryos. Male embryos tend to develop faster than female embryos do in IVF or ICSI, and as a result, operators are more inclined to select embryos with fast embryonic development and favorable morphology for transfer [29, 30]. Moreover, the male embryo may be likely to achieve the blastocyst embryo transfer [31]. Another explanation for the elevated SSR following blastocyst stage transplantation could be the atypical inactivation of one of the two X chromosomes in female embryos [32]. Preferential female mortality at early post-implantation stages may be due to in-vitro-culture-induced precocious X-chromosome inactivation, combined with a decrease induced by ICSI in the number of trophectoderm cells in female blastocysts [33]. In addition, the difference in sex ratio may also be attributed to the fact that cleavage-stage embryo transfer results in a lower percentage of biochemical pregnancy losses per embryo transfer compared to blastocyst transfer [27].
For singletons from twin gestation, except for the same impact of gonadotropin days on the sex ratio of the newborn, there are additional factors on the sex ratio of the newborn. Our research aligns with several studies [10, 34] that have highlighted the connection between using ICSI and a reduction in SSR. ICSI is typically performed to address male factor infertility, often related to poor semen quality or morphological defects of semen [12] and spermatozoa that carried the Y-chromosome suffered from morphological alterations as well as the reduction of the number [35]. As a result, the use of ICSI could lead to selection bias because operators were more likely to select sperm that exhibit normal morphology and healthy [35, 36]. Additionally, relevant researches have demonstrated that oocytes are more likely to accept Y-bearing spermatozoa for fertilization in IVF as opposed to ICSI [33, 37]. This could account for the observed lower SSR in the ICSI treatment group compared to IVF.
Regarding infertility factors, our study identified a higher percentage of male babies born to ART patients solely attributed to male factors compared to those with other infertility factors. However, Dean J H et al. [10] claimed no association between infertility factors and SSR. Likewise, Luke, Barbara et al. [11] found that male factor subfertility did not influence SSR after adjusting for potential confounders. Thus, further studies are still needed to explore the relationship between infertility factors and SSR.
For twins from twin gestation, our results suggest that older fathers tend to have a higher chance of having twin boys. Nevertheless, further data is needed to support our findings. Several factors may be associated with SSR in this context. Firstly, older fathers are more likely to have boys. A cross-sectional study conducted in China supported this observation, revealing that the SSR in the oldest group of fathers was higher than that of the youngest age group, which was consistent with our results [34]. However, a retrospective study in China shows that, consistent with spontaneous pregnancy, paternal age over 32 has an imbalance towards lower SSR [27]. Previous study has shown a decrease in the blastocyst formation rate with an increase in parental age [19]. Secondly, younger mothers are more likely to give birth to boys. Rueness et al. pointed out in their study that there was no statistical difference between maternal age and SSR in the general pregnant population. However, in pregnant women diagnosed with preeclampsia, as the age of pregnant women increases, the proportion of male infants born decreases. This suggests that the sex ratio shifts when older pregnant women are affected by complex pregnancy conditions [18].
Furthermore, this study is the first time to suggest that cleavage stage transfer may have a positive impact on the birth of boy-girl twins, an interesting finding as this phenomenon has not been previously reported in the literature. However, this finding might be due to chance, given the relatively small sample size of the study. A larger sample size and more rigorous multi-center collaboration are needed to further investigate the reasons for this result.
At present, many parents undergoing ART treatments hold preferences for gender of their offspring. However, Chinese law prohibits gender selection through ART. Nonetheless, clinicians can provide patients with an expected probability based on this study. Moreover, it is essential to recognize that the global fertility rate is continuously decreasing, and an increasing number of infertile individuals are opting for ART treatment. Consequently, the impact of the SSR on population gender balance will become increasingly pronounced. Clinicians can play a key role in mitigating any gender imbalances by considering to the potential influencing factors discussed in this study.
Conclusions
Three models were constructed to explore the potential association between SSR and various factors related to both parents and embryos. Several factors were found that had a significant effect on SSR. In singletons, secondary infertility, paternal age ≥ 37 years and blastocyst transfer were linked with a higher likelihood of male births compared to primary infertility, paternal age < 37 years, and cleavage stage transfer, respectively, Conversely, FET and increased duration of Gn use were linked to a decreased SSR. For singletons from twin gestation, male-induced infertility, and a higher dosage of Gn were positively correlated with SSR, while maternal age > 35 years, ICSI, and the duration of Gn use were associated with a decrease of SSR compared to maternal age ≤ 35 years and IVF, respectively. In twins from twin gestation, groups where paternal age exceeded maternal age were more likely to have male twins than to have boy-girl twins compared to groups with no age difference. Cleavage stage transfer had a higher probability of resulting in boy-girl twins compared to blastocyst transfer. While the study acknowledges the need for more extensive sample size for future research, the impacts of infertility factors, age difference and duration of Gn use on SSR remain important considerations. Understanding which factors within ART process can affect SSR holds significance for maintaining gender balance in the population.
Limitations
This study has several limitations. Firstly, our samples were derived from a single reproductive center, and our sample size is relatively small. Thus, to validate present findings, it’s necessary to expand the sample size in future analyses. Secondly, due to the retrospective nature of this analysis, there are inherent limitations concerning the integrity and homogeneity of the data. Lastly, the data was extracted based on the hospital's system, without the use of a detailed questionnaire survey. This method may lead to the missing of certain important information. In the future, we plan to perform multicenter research to satisfy sample diversity and meet sample capacity requirements, enhancing the richness and credibility of the research evidence. Besides, we intend to conduct laboratory research to figure out whether infertility factors, age difference and duration of Gn use indeed impact SSR and to explore the relevant mechanisms. With the improvement of our research, we will perform a more comprehensive analysis of the factors influencing SSR.
Supplementary Information
Additional file 1: Table S1. Univariate logistic regression analyses of different variables with SSR in Singletons.
Additional file 2: Table S2. Univariate logistic regression analyses of different variables with SSR in Singletons from twin gestation.
Additional file 3: Table S3. Univariate logistic regression analyses of different variables with SSR in twins from twin gestation.
Acknowledgements
We thank Jing Zheng (5Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, Wisconsin, U.S.A) for his critical reading and editing of the manuscript.
Abbreviations
- SSR
Secondary sex ratio
- PSR
Primary sex ratio
- ART
Assisted reproductive technology
- ICSI
Intracytoplasmic sperm injection
- IVF
In vitro fertilization
- FET
Frozen embryo transfer
- Gn
Gonadotropin
Author contributions
XZ and TL conceived and designed the study, and revised the manuscript. JZ drafted the article, performed the statistical analyses, and assisted in revising the article. QZ assisted with data analysis and data cleaning. JL, RY and JL provided assistance with article drafting and article revising. HS and JH assisted with data analysis and article drafting. YL assisted with the study design and provided the data. All authors approved the final version and contributed to agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the research were appropriately investigated and resolved.
Funding
This research was supported by the Natural Science Fund of Guangdong Province (No. 2022A1515010040 to X.Q.Z), National Natural Science Foundation of China (42175181) and Foreign Expert Program of the Ministry of Science and Technology (G2022199006L).
Availability of data and materials
The data will be made available on request to the corresponding author.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving patients were in accordance with the ethical standards of the Third Affiliated Hospital of Guangzhou Medical University Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiansen Zhao, Haoran Shen and Qijiong Zhu contributed equally to this work.
Contributor Information
Yanshan Lin, Email: 81948918@qq.com.
Tao Liu, Email: gztt_2002@163.com.
Xinqi Zhong, Email: zhongxq2016@gzhmu.edu.cn.
References
- 1.Kissin DM, Jamieson DJ, Barfield WD. Monitoring health outcomes of assisted reproductive technology. N Engl J Med. 2014;371(1):91–93. doi: 10.1056/NEJMc1404371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Geyter C. Assisted reproductive technology: Impact on society and need for surveillance. Best Pract Res Clin Endocrinol Metab. 2019;33(1):3–8. doi: 10.1016/j.beem.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet (London, England) 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 4.Pergament E, Toydemir PB, Fiddler M. Sex ratio: a biological perspective of 'Sex and the City'. Reprod Biomed Online. 2002;5(1):43–46. doi: 10.1016/S1472-6483(10)61596-9. [DOI] [PubMed] [Google Scholar]
- 5.McMillen MM. Differential mortality by sex in fetal and neonatal deaths. Science (New York, NY) 1979;204(4388):89–91. doi: 10.1126/science.571144. [DOI] [PubMed] [Google Scholar]
- 6.Hamoudi A, Nobles J. Do daughters really cause divorce? Stress, pregnancy, and family composition. Demography. 2014;51(4):1423–1449. doi: 10.1007/s13524-014-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James WH. Hypotheses on the stability and variation of human sex ratios at birth. J Theor Biol. 2012;310:183–186. doi: 10.1016/j.jtbi.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Yin T, Zhang Y, Zhou D, Yang J. The effect of blastocyst transfer on newborn sex ratio and monozygotic twinning rate: an updated systematic review and meta-analysis. Reprod Biomed Online. 2018;37(3):292–303. doi: 10.1016/j.rbmo.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Cirkel C, König IR, Schultze-Mosgau A, Beck E, Neumann K, Griesinger G. The use of intracytoplasmic sperm injection is associated with a shift in the secondary sex ratio. Reprod Biomed Online. 2018;37(6):703–708. doi: 10.1016/j.rbmo.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Dean JH, Chapman MG, Sullivan EA. The effect on human sex ratio at birth by assisted reproductive technology (ART) procedures–an assessment of babies born following single embryo transfers, Australia and New Zealand, 2002–2006. BJOG. 2010;117(13):1628–1634. doi: 10.1111/j.1471-0528.2010.02731.x. [DOI] [PubMed] [Google Scholar]
- 11.Luke B, Brown MB, Grainger DA, Baker VL, Ginsburg E, Stern JE. The sex ratio of singleton offspring in assisted-conception pregnancies. Fertil Steril. 2009;92(5):1579–1585. doi: 10.1016/j.fertnstert.2008.08.107. [DOI] [PubMed] [Google Scholar]
- 12.Hentemann MA, Briskemyr S, Bertheussen K. Blastocyst transfer and gender: IVF versus ICSI. J Assist Reprod Genet. 2009;26(8):433–436. doi: 10.1007/s10815-009-9337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin P-Y, Huang F-J, Kung F-T, Wang L-J, Chang SY, Lan K-C. Comparison of the offspring sex ratio between fresh and vitrification-thawed blastocyst transfer. Fertil Steril. 2009;92(5):1764–1766. doi: 10.1016/j.fertnstert.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Bu Z, Chen Z-J, Huang G, Zhang H, Wu Q, Ma Y, et al. Live birth sex ratio after in vitro fertilization and embryo transfer in China–an analysis of 121,247 babies from 18 centers. PLoS ONE. 2014;9(11):e113522. doi: 10.1371/journal.pone.0113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang LJ, Quan S. Factors affecting live birth sex ratio in assisted reproductive technology procedures. J Southern Med Univ. 2015;35(07):977–984. [PubMed] [Google Scholar]
- 16.Maalouf WE, Mincheva MN, Campbell BK, Hardy ICW. Effects of assisted reproductive technologies on human sex ratio at birth. Fertil Steril. 2014;101(5):1321–1325. doi: 10.1016/j.fertnstert.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Tarín JJ, Bernabeu R, Baviera A, Bonada M, Cano A. Sex selection may be inadvertently performed in in-vitro fertilization-embryo transfer programmes. Hum Reprod. 1995;10(11):2992–2998. doi: 10.1093/oxfordjournals.humrep.a135835. [DOI] [PubMed] [Google Scholar]
- 18.Rueness J, Vatten L, Eskild A. The human sex ratio: effects of maternal age. Hum Reprod. 2012;27(1):283–287. doi: 10.1093/humrep/der347. [DOI] [PubMed] [Google Scholar]
- 19.Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott RT. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Wang LM, Li P, Qiu PP, Ji H, Ding L, Zhang Q, et al. Analysis of the outcome of assisted reproductive therapy for primary infertility and secondary infertility. Syst Med. 2018;3(23):7–10. [Google Scholar]
- 21.Zhu J, Tang W, Mao J, Li J, Zhuang X, Liu P, et al. Effect of male body mass index on live-birth sex ratio of singletons after assisted reproduction technology. Fertil Steril. 2015 doi: 10.1016/j.fertnstert.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B-F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 23.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen R. Parental ages and the secondary sex ratio. Hum Reprod. 2001;16(10):2244. doi: 10.1093/humrep/16.10.2244. [DOI] [PubMed] [Google Scholar]
- 25.Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 26.Ray PF, Conaghan J, Winston RM, Handyside AH. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J Reprod Fertil. 1995;104(1):165–171. doi: 10.1530/jrf.0.1040165. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Du J, Zhao J, Lv H, Wang Y, Chen X, et al. The sex ratio of singleton and twin delivery offspring in assisted reproductive technology in China. Sci Rep. 2017;7(1):7754. doi: 10.1038/s41598-017-06152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James WH. Gonadotrophin and the human secondary sex ratio. Br Med J. 1980;281(6242):711–712. doi: 10.1136/bmj.281.6242.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pergament E, Fiddler M, Cho N, Johnson D, Holmgren WJ. Sexual differentiation and preimplantation cell growth. Hum Reprod. 1994;9(9):1730–1732. doi: 10.1093/oxfordjournals.humrep.a138783. [DOI] [PubMed] [Google Scholar]
- 30.Dumoulin JCM, Derhaag JG, Bras M, Van Montfoort APA, Kester ADM, Evers JLH, et al. Growth rate of human preimplantation embryos is sex dependent after ICSI but not after IVF. Hum Reprod. 2005;20(2):484–491. doi: 10.1093/humrep/deh614. [DOI] [PubMed] [Google Scholar]
- 31.Narvaez JL, Chang J, Boulet SL, Davies MJ, Kissin DM. Trends and correlates of the sex distribution among U.S. assisted reproductive technology births. Fertil Steril. 2019;112(2):305–314. doi: 10.1016/j.fertnstert.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Wu LM, Gao M, Wang XH, Sang MY, Xu B, Zhou GX, et al. Secondary sex ratio analysis of singleton babies born following assisted reproductive technology. Chin J Reprod Contracep. 2022;06:615–620. [Google Scholar]
- 33.Tarín JJ, García-Pérez MA, Hermenegildo C, Cano A. Changes in sex ratio from fertilization to birth in assisted-reproductive-treatment cycles. Reprod Biol Endocrinol. 2014;12:56. doi: 10.1186/1477-7827-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Liu X, Zhang H, Li L, Liu R, Zhang H, et al. Associated factors of secondary sex ratio of offspring in assisted reproductive technology: a cross-sectional study in Jilin Province, China. BMC Pregnancy Childbirth. 2020;20(1):666. doi: 10.1186/s12884-020-03373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ménézo YJR. Paternal and maternal factors in preimplantation embryogenesis: interaction with the biochemical environment. Reprod Biomed Online. 2006;12(5):616–621. doi: 10.1016/S1472-6483(10)61188-1. [DOI] [PubMed] [Google Scholar]
- 36.Setti AS, Figueira RCS, Braga DPAF, Iaconelli A, Borges E. Gender incidence of intracytoplasmic morphologically selected sperm injection-derived embryos: a prospective randomized study. Reprod Biomed Online. 2012;24(4):420–423. doi: 10.1016/j.rbmo.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Griffin DK, Handyside AH, Harper JC, Wilton LJ, Atkinson G, Soussis I, et al. Clinical experience with preimplantation diagnosis of sex by dual fluorescent in situ hybridization. J Assist Reprod Genet. 1994;11(3):132–143. doi: 10.1007/BF02332090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Univariate logistic regression analyses of different variables with SSR in Singletons.
Additional file 2: Table S2. Univariate logistic regression analyses of different variables with SSR in Singletons from twin gestation.
Additional file 3: Table S3. Univariate logistic regression analyses of different variables with SSR in twins from twin gestation.
Data Availability Statement
The data will be made available on request to the corresponding author.