Abstract
Changes in sensory afferent activity contribute to the transition from acute to chronic pain. However, it is unlikely that a single sensory receptor is entirely responsible for persistent pain. It is more probable that extended changes to multiple receptor proteins expressed by afferent neurons support persistent pain. A-Kinase Anchoring Protein 79/150 (AKAP) is an intracellular scaffolding protein expressed in sensory neurons that spatially and temporally coordinates signaling events. Since AKAP scaffolds biochemical modifications of multiple TRP receptors linked to pain phenotypes, we probed for other ionotropic receptors that may be mediated by AKAP and contribute to persistent pain. Here, we identify a role for AKAP modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptor (AMPA-R) functionality in sensory neurons. Pharmacological manipulation of distinct AMPA-R subunits significantly reduces persistent mechanical hypersensitivity observed during hyperalgesic priming. Stimulation of both protein kinases C and A (PKC, PKA, respectively) modulate AMPA-R subunit GluR1 and GluR2 phosphorylation and surface expression in an AKAP-dependent manner in primary cultures of DRG neurons. Furthermore, AKAP knock out reduces sensitized AMPA-R responsivity in DRG neurons. Collectively, these data indicate that AKAP scaffolds AMPA-R subunit organization in DRG neurons that may contribute to the transition from acute-to-chronic pain.
Keywords: AKAP, AMPA, glutamate, pain
Introduction
Biochemical modifications to receptors in afferent neurons support persistent pain. The coordination of these modifications is foundational to the generation and maintenance of peripheral hypersensitivity. Relative to changes in translational control1–3 and down-regulation of inhibitory metabotropic receptors, 4 kinase sensitization of ionotropic receptors plays a significant role in the maintenance of persistent pain. Yet, as peripheral sensory neurons undergo dynamic neuroplastic changes in response to inflammation and injury, there are many unknowns concerning the coordination of these dynamic changes in persistent/chronic pain.
A-Kinase Anchoring Protein 79/150 (AKAP) is a scaffolding protein that coordinates kinase association with plasma membrane substrates, 5 including several ligand gated channels, such as NMDA and glutamate receptor complexes.6–8 More recent work has also identified a role for AKAP in coordinating kinase phosphorylation of sensory TRPA1 and TRPV1 receptors and feed-forward sensitization of afferent neurons.9–12 Interestingly, AKAP KO animals demonstrate less persistent pain (thermal/mechanical) behavior than either TRPA1 or TRPV1 KO animals,9,13,14 indicating that AKAP could be controlling another signal transducer that contributes to persistent sensory hypersensitivity.
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA-R) is a tetrameric ionotropic receptor expressed throughout the nervous system. As AMPA GluR1/2 heteromeric tetramers compose the majority of AMPA-Rs in naïve neurons, 15 GluR1/2 heteromers at the surface are not Ca+2-permeable, while GluR1 homomers are. However, well-accepted findings related to metabotropic glutamate sensitization counter disagreement on the presence of an ionotropic glutamate response in nociceptors. Previous studies indicate AMPA-R subunit expression in DRG neurons,16–18 with more recent evidence demonstrating physiological AMPA responses.19,20 Controversy surrounding a potential role for AMPA-R in pain phenotypes was abated by Gangadharan et al., using molecular deletion of AMPA-R subunits in SNS-Cre mice to demonstrate subunit-specific roles and functional expression in chronic inflammatory pain. 21 Importantly, results from this work confirm previous findings demonstrating that AMPA-R contribution to afferent drive in non-pathologic conditions is relatively low-to-none compared to injury models. However, the explanation for this phenomenon, coupled with a mechanism for receptor sensitization remains unknown. Moreover, it is unclear how the molecular re-organization of AMPA-R subunits is managed in afferent sensory neurons and whether it is associated with persistent pain behavior. Herein, we provide evidence to identify that AKAP coordinates AMPA-R functionality in sensory neurons in a role that contributes to the transition from acute-to-chronic pain.
Materials and methods
Animals
Procedures using animals were approved by the Institutional Animal Care and Use Committees of University of Texas Health San Antonio (UTHSA). Moreover, these procedures were conducted in accordance with policies for the ethical treatment of animals established by the National Institutes of Health and International Association for the Study of Pain. Efforts were made to limit animal discomfort and reduce the number of animals used. Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 176–199 g were used in this study. AKAP KO animals were developed in the lab of John D. Scott, PhD (University of Washington) and maintained in house.
Tissue culturing
Male Sprague-Dawley rats (175–200 g, Charles River Laboratories) were used. L4-L6 dorsal root ganglia (DRG) were removed bilaterally from male rats, and dissociated by collagenase treatment (30 min, Worthington), followed by trypsin treatment (15 min, SigmaAldrich). Cells were centrifuged and re-suspended between each treatment with Pasteur pipettes. DRG were then centrifuged, aspirated, and re-suspended in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) with 10% fetal bovine serum (FBS, Gibco), 100 ng/mL nerve growth factor (NGF, Harlan), 1% 5-fluoro deoxyuridine (SigmaAldrich), 1% penicillin/streptomycin (GIBCO), and 1% L-glutamine, and then placed on plates coated with poly-D lysine (Corning), or glass coverslips coated with poly-D lysine and laminin (SigmaAldrich). Cultures for biochemistry experiments were maintained at 37°C, 5% CO2, and grown in 48-well or six-well plates for 5–7 days. Cultures for calcium imaging were maintained similarly for 24–28 h.
Hyperalgesic priming behavior
Mechanical withdrawal threshold of the rodent paw was measured with a plantar electronic von Frey Anesthesiometer (ALMEMO 2450 Ahlborn, IITC). Briefly, animals were placed in plastic boxes with a mesh floor surface for a 30 min habituation period. For rats, the “rigid tip” was used with a 0.8 mm uniform tip diameter. The amount of force generated that elicited hind paw withdrawal was automatically measured and recorded as paw withdrawal threshold in grams (g). Measurements were taken in triplicate at least 30 s apart and the average was used for statistical analysis. Data are presented as mean paw withdrawal threshold ± SEM paw withdrawal thresholds. Observers were blinded to the treatment conditions.
All injections were given intraplantarly in 50 μL volumes via a 28-gauge needle inserted through the lateral footpad just under the skin to minimize tissue damage. 22 Drug stocks were dissolved in Phosphate Buffered Saline (PBS). For rat priming experiments, rats were tested for baseline responses prior to injections, and then injected with Complete Freund’s Adjuvant (CFA, 50%) or vehicle (sterile saline) on day 1. Rats were tested again on day 5 for baseline responses, and then injected with PGE2 (100 ng) followed by repeated mechanical threshold testing at 15 min, 30 min, 2 h, and 4 h post-PGE2 injection.
Co-immunoprecipitation and Western blotting
DRG cultures in 10-cm plates were treated as described and prepared for homogenization and/or isolation of crude plasma membrane fractions, as described previously. 10 Crude plasma membrane or whole cell homogenates are quantified for protein concentration by Bradford analysis. 23 Equal samples underwent co-immunoprecipitation (100 μg total protein) or gross homogenate WB analysis (10 μg total protein). Samples isolated for co-immunoprecipitation were diluted to 500 μL with homogenization buffer 10 and incubated with antibodies specific to AKAP150 (1 μg, Santa Cruz Biotechnology, clone E-1), pulled down with protein agarose-A (SigmaAldrich), and resolved by Sodium Dodecyl Sulfate -Polyacrylamide Gel Electrophoresis (SDS-PAGE). Gels were transferred to polyvinyldifluoride (PVDF, EMD Millipore), blocked in 5% non-fat milk in Tris buffered saline with 0.1% Tween-20 non-ionic detergent, and incubated 18 h at 4°C with the following primary antibodies: AMPA GluR1 (Abcam ab19522), AMPA GluR2 (Abcam ab133477), AKAP150 (SantaCruz Biotechnology, clone E-1), SAP97 (Abcam, ab3437), β-arrestin (SigmaAldrich, clone 1B15), p-S831 AMPA GluR1 (Abcam, ab109464), p-S845 AMPA GluR1 (Abcam, ab76321), p-S880 AMPA GluR2 (Abcam, ab52180), β1 integrin (SantaCruz Biotechnology, sc-374429). Anti-rabbit secondary antibodies (GE Healthcare Life Sciences) were applied to rinsed blots, incubated at RT for 1 h, and then blots were rinsed again. Blots were incubated with enhanced chemiluminescence solution (GE Healthcare Life Sciences), exposed to X-ray film and developed for analysis. Films were scanned and immunoreactive bands were quantified using NIH Image 1.62 shareware.
Plasma membrane fractionation
Following homogenization by 20 strokes in a Potter-Elvehjem homogenizer in a hypotonic homogenization buffer (25 mM HEPES, 25 mM sucrose, 1.5 mM MgCl2, 50 mM NaCl, pH 7.2), cell extract was incubated on ice for 15 min and then centrifuged at 1000 g for 1 min at 4°C to remove nuclei and intact cells from the homogenate. The resulting supernatant was centrifuged at 16,000 × g for 30 min at 4°C, separating cytosolic proteins from cell membrane proteins. The pellet (crude membrane fraction) was then re-suspended in homogenization buffer containing 1% Triton.
Biotinylation
Cultured DRG cells were biotinylated with EZ-Link Biotin (0.5 mg/mL; ThermoFisher), harvested, and precipitated as previously described. 24
Calcium imaging
DRG neurons were incubated for 30 min at 37°, 5% CO2, with Fura2-AM (2 µM, Invitrogen Life Technologies, Grand Island, NY) in the presence of 0.05% Pluronic (Invitrogen Life Technologies) for 30 min or 1 h, respectively. Imaging was performed on an inverted Nikon Eclipse TE-2000-U microscope, equipped with 20X/0.75 numerical aperture Fluor objective. Cultured cells were excited (340 and 380 nm wavelength) from a Lambda LS Fluorescent light source (Sutter Instruments), with a Hamatasu digital CCD Camera detecting emissions at 510 nm. Collected data were analyzed with MetaFluor Software (Molecular Devices) with ratiometric 340/380 nm values recorded.
Experiments were performed at room temperature (RT) with use of a PM2000 Microinjector (MicroData Instruments, Inc.), perfusion valve controller (Warner Instrument Corporation), and multi-barrel glass pipette, which allowed for continuous exchange of bath solution during drug application. The micropipette, which delivered drug-containing solutions, was precisely placed in the optic field that includes our cells of interests. Both bath and pipet solution were under an automatically controlled pressurized system that, in pairing with pneumatic pump, supported precise, fast and consistent fluid flow and exchange. Recordings were performed in standard extracellular solution (SES, in mM: 140 NaCl, 5 KCl, 2 MgCl2, 2CaCl2, 10 HEPES, 10 Glucose, pH 7.4 [NaOH], which also served as a vehicle for all drugs used for Ca2+ imaging. Neuronal responsiveness to AMPA (30 μM) was determined by recording cellular responses >0.05 Δ340/380 nm from neurons producing a positive response to 50 mM KCl. All treatments were conducted in the presence of 100 μM cyclothiazide (CTZ) as a positive allosteric modulator of GluR1 AMPA receptors that inhibits rapid desensitization. 25
Results
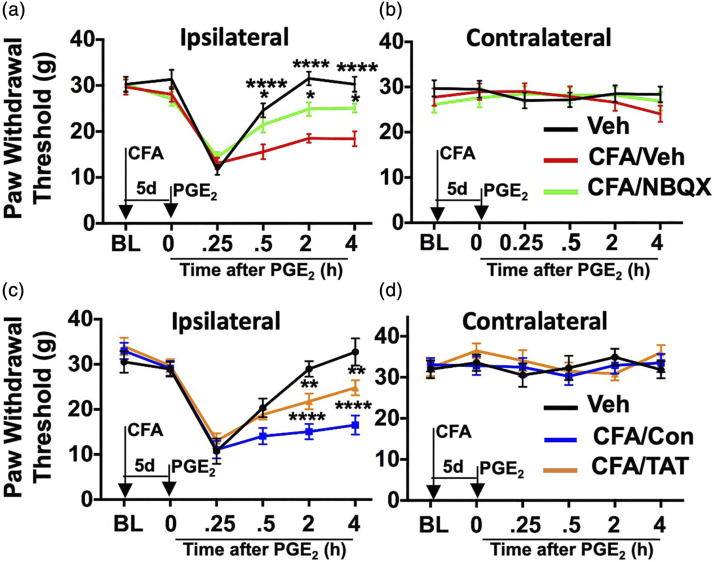
Many patients experience persistent pain that cannot be explained by central sensitization alone, 26 indicating that peripheral nociceptor activation plays a crucial role in long-term pain phenotypes. To determine whether peripheral AMPA-R contributes to persistent pain, we employed a hyperalgesic priming model originally devised by Levine and colleagues.27,28 In this model, rats are treated i.pl. with a “priming” stimulus such as CFA to simulate peripheral injury that sensitizes nociceptor populations into persistent pain phenotypes to a later chemical stimulus, such as PGE2. This model has been validated rigorously to dissect the transition from acute-to-chronic pain by tracking biochemical and physiological correlates of behavioral output measures.1,29–34 In Figure 1, we used this model with CFA administered i.pl. to one hind paw of a rat, followed by sub-optimal PGE2 (100 ng in 50 μL sterile saline) injected i.pl. with paw withdrawal threshold to a von Frey mechanical stimulus monitored for 4 h post-PGE2. Although typical periods of behavioral observation can exceed days-to-weeks, we observed a significant reduction in mechanical hyperalgesic priming in rats that received CFA and i.pl. 2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX, 200 nmol in 50 μL sterile saline, AMPA-R GluR1 antagonist, Figure 1(a), *p = .0167, ****p < .0001, two-way ANOVA with Sidak’s multiple Comparisons test) or TAT-GluA2-3γ peptide (TAT, 50 nmol/50 μL sterile saline, blocks GluR2 subunit internalization,35,36 Figure 1(c), **p = .0028, ****p < .0001, two-way ANOVA with Sidak’s multiple Comparisons test) over vehicle or control peptide, respectively. Importantly, i.pl. injections with GluR subunit-targeting agents occurred for 4 days following the CFA treatment (during the priming period), and did not affect contralateral behavior (Figure 1(b) and (d)). Taken together, these behavioral results indicate that peripheral ionotropic glutamate receptors contribute to CFA-induced hyperalgesic priming.
Figure 1.
Pharmacological Manipulation of AMPA Subunits Reduce Mechanical Hyperalgesic Priming. Male rats were injected i.pl. with vehicle or Complete Freund’s Adjuvant (CFA, 50% in 50 μL saline). Over the next 4 days, rats were injected daily with vehicle or NBQX (AMPA GluR1 antagonist, 200 nmol/50 μL, i.pl., (a and b), or control peptide (Con) or TAT-GluA2-3g (TAT, GluR2 internalization inhibitor, 50 nmol/50 μL, i.pl., (c and d). Rats were tested for paw withdrawal threshold (PWT, g) on Day 5, then injected with PGE2 (100 ng in 50 μL saline, i.pl.), and again tested for PWT 0.25, 0.5, 2, and 4 h. n = 24 rats/treatment, triplicate readings per time point; compared to Veh/PGE2 (a, *p = .0167, ****p < .0001, c, **p = .0028, ****p < .0001), two-way ANOVA with Sidak’s multiple Comparisons test.
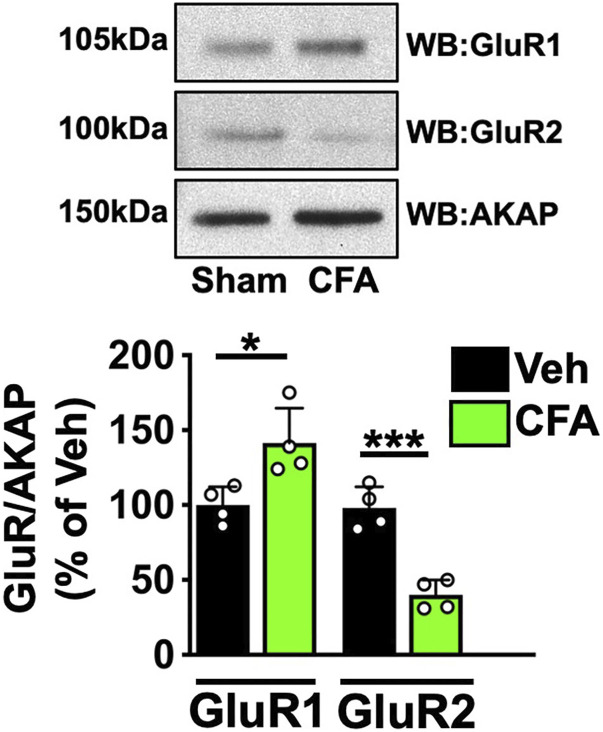
AMPA GluR1/2 heteromeric tetramers compose the majority of AMPA-R in naïve neurons. 15 However, AMPA-R sensitization requires the internalization of GluR2 subunits from the plasma membrane and the translocation of GluR1 subunits to the plasma membrane to form Ca+2-permeable homomers.6,37 We sought to identify whether CFA could stimulate the reorganization of AMPA-R subunits in afferent DRG neurons. Rats were treated as in Figure 1 with CFA or vehicle, and L4-L6 DRG were extracted, homogenized, and processed for plasma membrane fractionation. Western blot analysis of samples from these plasma membrane fractions in Figure 2 indicate that CFA treatment significantly shifts GluR1/2 expression profiles. As indicated in both representative images and cumulative densitometry, CFA increased plasma membrane GluR1 expression (*p = .0195, unpaired t test, n = 4) while reducing GluR2 (***p = .0005, paired t test, n = 4), supporting a shift towards increased GluR1 homomer formation and subsequent Ca+2 permeability upon activation.
Figure 2.
AMPA GluR Subunit PM Expression in DRG tissue following Hind Paw Incision treatment. Right hind paw injection with Complete Freund’s Adjuvant (CFA, 50% in 50 μL saline, i.pl.) compared to left sham (sham). Respective side DRG were homogenized and fractionated for plasma membrane fractions. Aliquots resolved by SDS-PAGE and probed for GluR1, GluR2, and AKAP. Representative WB results, n = 4 trials. Quantitative analysis of densitometry normalized to AKAP, *p = .0195, ***p = .0005, unpaired t test, n = 4.
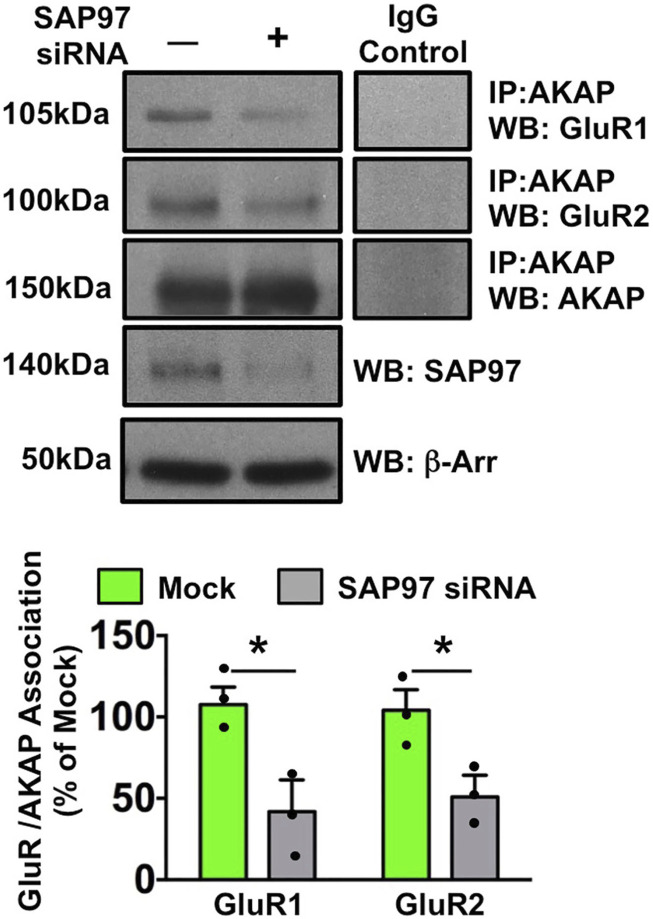
AMPA-R subunit re-organization is dependent on post-translational signaling mechanisms. Protein Kinase C (PKC) phosphorylation of GluR2 stimulates internalization of the Ca+2-impermeable GluR1/2 heteromer, 38 while Protein Kinase A (PKA) phosphorylation of GluR1 stimulates formation of the Ca+2-permeable GluR1 homomer. 39 However, little information is available as to what controls the re-organization of subunits by coordinating phosphorylation events. We first investigated the scaffolding potential for AKAP based on previous studies by our group in which AKAP regulates TRPV1 and TRPA1 function in injured/inflamed neurons,9–11,13,14 coupled with previous work that characterized AKAP regulation of AMPA-R GluR1 in CNS neurons.40,41 In Figure 3, co-immunoprecipitation experiments from cultured DRG sensory neurons identify a unique arrangement between AKAP and AMPA-R: association exists between both AMPA GluR1 and GluR2 subunits with AKAP. Importantly, this association is interrupted in neurons treated with siRNA specific for SAP97, a MAGUK protein that anchors AKAP to GluR1. 6 We utilize siRNA here since SAP97 knock-out is embryonically lethal. 42 These data provide the first evidence for AKAP/AMPA-R association in sensory neurons.
Figure 3.
SAP97 knockdown reduces AMPA GluR/AKAP Association. Sensory neurons were transfected in mock fashion or with SAP97 siRNA. Equal aliquots of crude plasma membrane preps were immunoprecipitated with AKAP antibody and probed for GluR1, GluR2, and AKAP immunoreactivities. Densitometries normalized to mock. Whole cell lysates were probed for SAP97 and β-arrestin to demonstrate siRNA specificity and efficacy. Representative WB results, n = 3 trials, means ± SEM shown, GluR1 **p = .040, GluR2 *p = .031, unpaired t test.
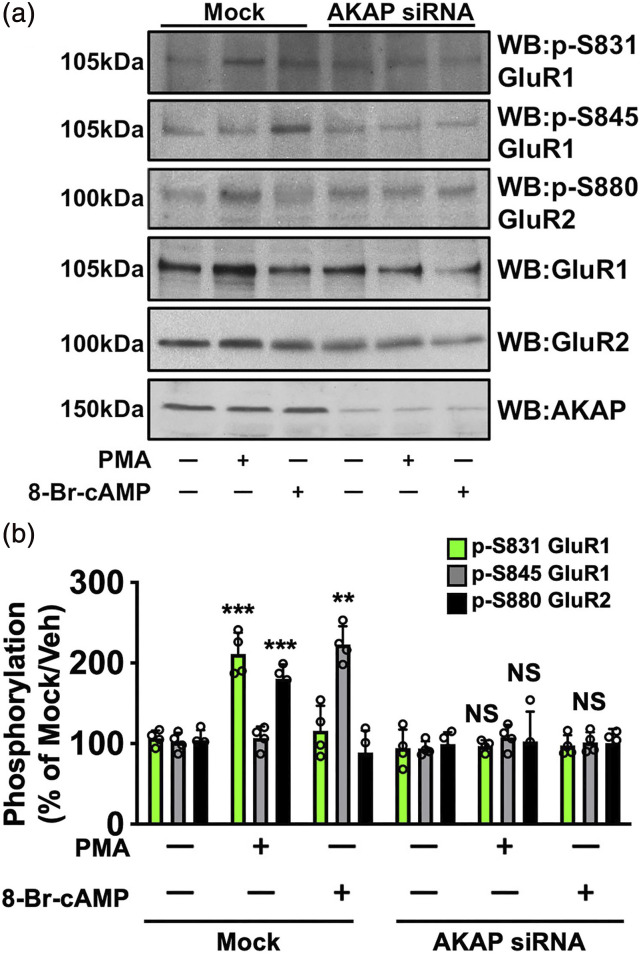
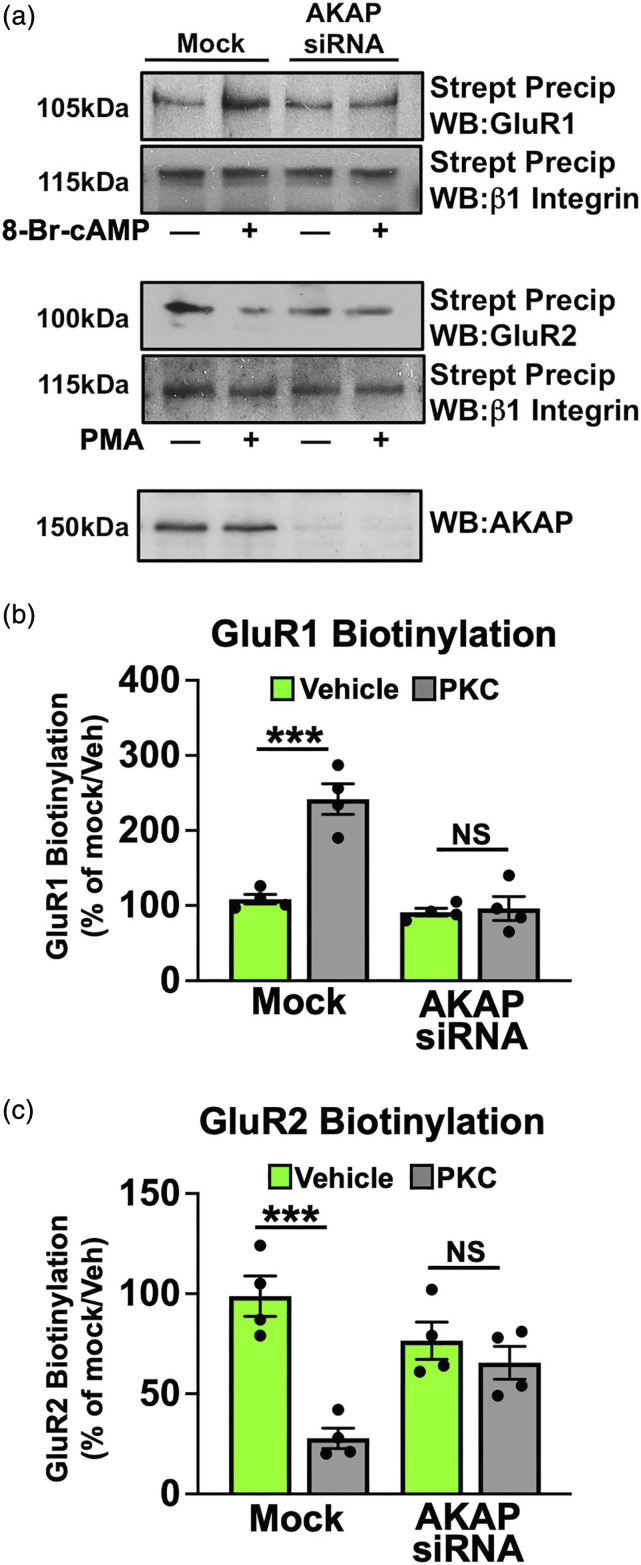
We next investigated whether AKAP coordinates AMPA-R subunit phosphorylation in DRG neurons. PKC phosphorylation of GluR2 at S880 drives internalization of the Ca+2-impermeable subunit from the plasma membrane, promoting GluR1 phosphorylation, homomerization and AMPA receptor Ca+2-permeability. 43 Sensory neurons were transfected with missense or AKAP-specific siRNA (previously validated 10 ), serum-starved for 18 h, and then treated with vehicle, phorbol 12-myristate 13-acetate (PMA, PKC activator, 1 μM) or 8-Br-cAMP (PKA activator, 10 μM) for 15 min. Cleared whole cell lysates were collected and analyzed using phospho-specific antibodies for GluR1 S831, GluR1 S845, and GluR2 S880. These sites regulate internalization of GluR2 (S880 37 ), GluR1 translocation to the membrane (S845 6 ) and increased GluR1 Ca+2 conductance (S831 44 ). In Figure 4, PKC-phosphorylation of GluR2 S880 requires AKAP expression in sensory neurons. This is an important and unique finding, as previous work in other neuronal models do not implicate AKAP as an important regulator of this phenomenon. Furthermore, PKC-stimulated phosphorylation of GluR1 S845 and PKA-stimulated phosphorylation of GluR1 S831 also require AKAP. These data indicate that AKAP scaffolds AMPA-R phosphorylation in sensory neurons.
Figure 4.
PKC/PKA phosphorylation of AMPA Requires AKAP. Sensory neurons cultured, transfected in mock fashion or with AKAP150 siRNA, serum-starved, then treated with PMA (1 μM, 15 min, PKC activator) or 8-Br-cAMP (10 μM, 15 min, PKA activator), and harvested for WB analysis. (a) Representative WB results, n = 4 trials. (b) Quantitative analysis of immunoreactive densitometry, means ± SEM, p-S831 PMA versus Veh ***p = .0003, p-S845 PMA versus Veh ***p = .0001, p-S880 8-Br-cAMP versus Veh **p = .009, NS = no significance, unpaired t test.
AMPA-R phosphorylation stimulates differential sub-cellular targeting of its subunits. PKC phosphorylation of GluR2 S880 stimulates internalization of the subunit from the plasma membrane, 37 while PKA phosphorylation of GluR1 S845 stimulates homomerization and translocation to the plasma membrane. 6 Cultured sensory neurons were transfected with missense or AKAP-specific siRNA, serum-starved for 18 h, and treated with vehicle, PMA (1 μM) or 8-Br-cAMP (10 μM) for 10 min, prior to surface biotinylation. Streptavidin precipitates were resolved by SDS-PAGE and plasma membrane-localized GluR1 and GluR2 were quantified by densitometry and normalized to β1 integrin. In Figure 5, PKA activation increases GluR1 surface biotinylation (***p = .0008, unpaired t test), while PKC activation reduces GluR2 on the cell surface (***p = .0007, unpaired t test), both in a manner dependent upon AKAP expression. Importantly, the dependency of GluR2 internalization on AKAP is unique to sensory neurons and is reported here for the first time.
Figure 5.
AKAP Mediates AMPA GluR1/2 Surface Expression. Sensory neurons cultured, transfected in mock fashion or with AKAP150 siRNA, serum-starved, then treated with vehicle, 8-Br-cAMP (10 μM, 10 min, PKA activator) or PMA (1 μM, 10 min, PKC activator), surface biotinylated, streptavidin precipitated, and harvested for WB analysis. (a) Representative WB results, n = 4 trials, means ± SEM shown. Densitometric quantification of AMPA GluR1 biotinylation (b), ***p = .0008, GluR2 biotinylation (c) ***p = .0007, NS = no significance, unpaired t test.
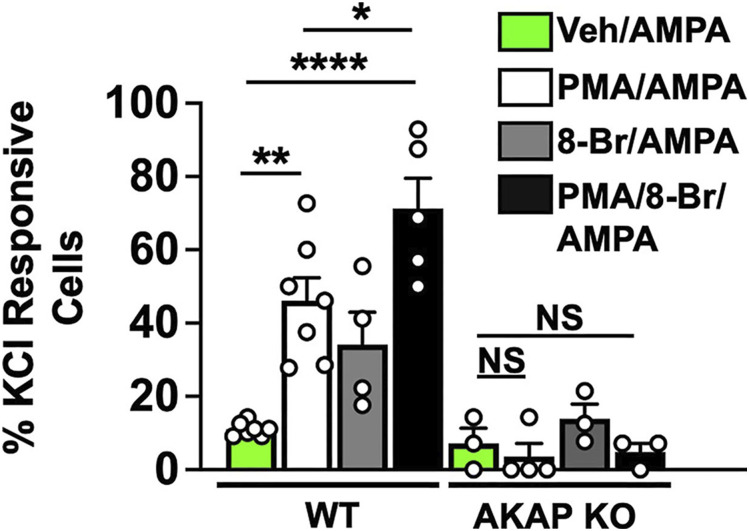
AMPA-R GluR1/2 subunit re-distribution to/from the plasma membrane changes the Ca+2-permeability of the channel. GluR1/2 heteromers at the surface are not Ca+2-permeable, while GluR1 homomers are. Given that AMPA-R subunit subcellular localization is dependent on phosphorylation, which we found to be dependent on AKAP (Figure 5), we sought to quantify Ca+2-responsivity of sensory neurons following PKA/PKC activation. Dorsal root ganglia (DRG) neurons from AKAP WT and KO mice were cultured for 18 h and serum-starved for 2 h prior to 15 min treatment with vehicle, PMA (1 μM), 8-Br-cAMP (10 μM), or PMA and 8-Br-cAMP. We then quantified neuronal responses to AMPA (100 nM) in KCl (+) cells using real-time Ca+2-imaging. With a threshold of 0.05 ΔF340/380 from baseline, we observed an increase in AMPA-responsive neurons following PKC activation in Figure 6. Although PKA activation by 8-Br-cAMP alone did not increase Ca+2-responsivity, combined PKC/PKA activation elicited the highest percentage of AMPA-responsive sensory neurons. This is important as it indicates that PKC-mediated internalization of GluR2 facilitates PKA-mediated translocation of GluR1 to form Ca+2-permeable homomers. Of equal importance, sensory neurons isolated from AKAP KO mice failed to produce similar results. Taken together, these results indicate that AKAP coordinates AMPA neuroplasticity in sensory neurons.
Figure 6.
PKA/PKC Activation Increase AMPA Responsive Neurons from AKAP WT Only. DRG neurons cultured from male AKAP WT or KO male mice, pre-treated for 15 min with vehicle, PMA (1 μM), 8-Br-cAMP (8-Br, 10 μM), or PMA and 8-Br-cAMP (15 min) before AMPA (30 μM). Responder threshold set at 0.05 ΔF340/380 above baseline, from n = 37–90 KCl (+) cells/treatment, with each point representing the percentage of AMPA-responsive neurons/total KCl-responsive neurons/coverslip, *p = .0335, **p = .0012, ****p < .0001, NS = no significance, one-way ANOVA with Tukey post-hoc.
Discussion
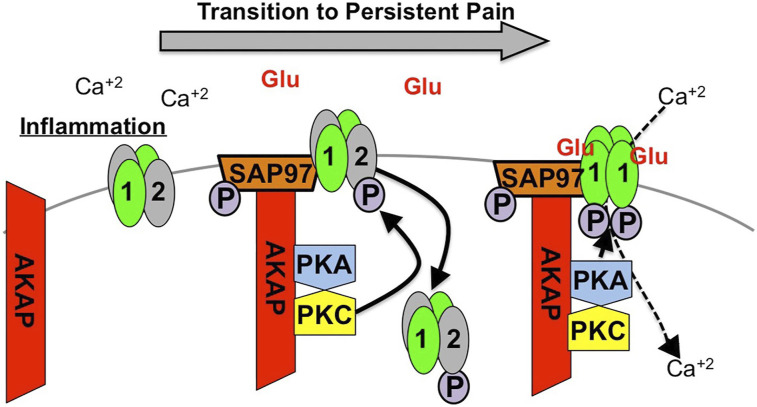
Changes in sensory afferent receptor activity contribute to the transition from acute to chronic pain. However, it is unlikely that a single receptor is entirely responsible for persistent pain and that extended changes to multiple receptor proteins at the afferent terminal are responsible. AKAP scaffolds biochemical modifications to multiple receptors linked to persistent pain phenotypes. Studies presented here indicate that sensitization of AMPA-R in DRG neurons requires AKAP. Knockdown of AKAP expression in DRG cultures inhibits PKA- and PKC-dependent phosphorylation of AMPA GluR1/2 that controls subunit internalization, plasma membrane expression profiles and calcium permeability. Furthermore, knock-out of AKAP inhibits increased AMPA responsiveness observed following PKA- and PKC activation that occurs during inflammatory insult and in the transition from acute-to-chronic pain. 45 These studies add to existing knowledge to support the overarching identification of AKAP as an integral coordinator of persistent pain neuroplasticity (Figure 7).
Figure 7.
Mechanism of AMPA Modulation in Persistent Pain. Inflammation drives AKAP association with AMAP GluR1/2 heteromer. AKAP Scaffolds PKC phosphorylation of GluR2 (causing internalization of the subunit) and PKA phosphorylation of GluR1 (causing subunit tetramerization and glutamate-dependent Ca+2 influx.
AKAP directly scaffolds multiple kinases to TRP receptors to manage post-translational modifications that affect ionotropic activity.10–12,46 However, AKAP association with AMPA-R in CNS neurons is considered indirect as it requires SAP97 intermediation.40,47 Here, we probed the possibility that this indirect association could still effectively scaffold post-translational modifications to AMPA-R subunits to direct ionotropic receptor function in DRG sensory neurons. Indeed, the knockdown of SAP97 expression in DRG cultures reduced AKAP association with both AMPA-R subunits GluR1/2 (Figure 3), with multiple figures indicating the importance of AKAP to AMPA-R GluR1/2 plasma membrane expression and function. These findings aren’t necessarily unique in AMPA-R neuronal biochemistry yet identify that AMPA-R is modulated in DRG neurons similar to higher order CNS neurons. This innovative conclusion intimates that AMPA-R may participate in peripheral “learning” neuroplasticity similar to CNS roles for the receptor. 48
AMPA-R expression in peripheral afferent neurons has been demonstrated previously 17 with expression observed on central, pre-synaptic terminals. 20 However, results reported here indicate a potential role for AKAP scaffolding of AMPA-R subunits on innervating terminals. One drawback of this study is the lack of peripheral tissue immunohistochemistry demonstrating AKAP co-expression with AMPA-R in peripheral afferent endings. However, previous accounts of peripheral AMPA-R subunit expression in the periphery, 49 coupled with documented changes in peripheral GluA1 expression following CFA 50 and reports of increased glutamate content in stimulated peripheral tissues51,52 supports a role for peripherally-expressed AMPA-R. Furthermore, our previous work documenting AKAP scaffolding events in DRG neurons confirms peripheral expression.9–11,13,53,54 Although presumptive, it’s possible that AKAP may scaffold both acute and chronic AMPA subunit reorganization scenarios in response to inflammatory insult. Importantly, results presented here identify the organizational mechanisms utilized by DRG neurons to mediate AMPA-R subunit re-organization to facilitate receptor activity and contribute to persistent pain.
AMPA-R serves as an important target for pharmaceutical development. Negative allosteric inhibitor Perampanel (FYCOMPA®) has been studied in four clinical trials to treat epilepsy and depressive disorders (clinicaltrials.gov). Previous work on non-specific AMPA-R antagonist NS1209 demonstrated both pre-clinical efficacy in mouse models of pain, 55 and clinical efficacy in patients suffering from nerve injury. 56 However, multiple side effects associated with systemic AMPA-R antagonism depress clinical utility in certain patient populations. For this reason, groups have studied local drug delivery methods to minimize potential systemic side effects associated with oral or i.v. drug administration.57,58 This is important considering that many patients experience persistent pain that cannot be explained by central sensitization alone, 26 indicating that peripheral nociceptor activation plays a crucial role in long-term pain phenotypes. Therefore, targeting peripheral AMPA-R sensitization and scaffolding mechanisms that regulate it could provide pain relief via localized treatment and reduce the transition from acute-to-chronic pain.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH NINDs grant number NS128574.
ORCID iD
Nathaniel A Jeske https://orcid.org/0000-0001-6033-0616
References
- 1.Ferrari LF, Araldi D, Levine JD. Distinct terminal and cell body mechanisms in the nociceptor mediate hyperalgesic priming. J Neurosci 2015; 35: 6107–6116. DOI: 10.1523/JNEUROSCI.5085-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari LF, Bogen O, Chu C, Levine JD. Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain 2013; 14: 731–738. DOI: 10.1016/j.jpain.2013.01.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoutorsky A, Price TJ. Translational control mechanisms in persistent pain. Trends Neurosci 2018; 41: 100–114. DOI: 10.1016/j.tins.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araldi D, Ferrari LF, Levine JD. Repeated Mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci 2015; 35: 12502–12517. DOI: 10.1523/JNEUROSCI.1673-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theurkauf WE, Vallee RB. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem 1982; 257: 3284–3290. [PubMed] [Google Scholar]
- 6.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 2000; 27: 107–119. [DOI] [PubMed] [Google Scholar]
- 7.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol 2005; 7: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem 2005; 280: 16962–16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brackley AD, Gomez R, Guerrero KA, Akopian AN, Glucksman MJ, Du J, Carlton SM, Jeske NA. A-kinase anchoring protein 79/150 scaffolds transient receptor potential A 1 phosphorylation and sensitization by metabotropic glutamate receptor activation. Sci Rep 2017; 7: 1842. DOI: 10.1038/s41598-017-01999-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008; 138: 604–616. DOI: 10.1016/j.pain.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain 2009; 146: 301–307. DOI: 10.1016/j.pain.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008; 28: 4904–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeske NA, Por ED, Belugin S, Chaudhury S, Berg KA, Akopian AN, Henry MA, Gomez R. A-kinase anchoring protein 150 mediates transient receptor potential family V type 1 sensitivity to phosphatidylinositol-4,5-bisphosphate. J Neurosci: The Official Journal of the Society for Neuroscience 2011; 31: 8681–8688. DOI: 10.1523/JNEUROSCI.0020-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szteyn K, Rowan MP, Gomez R, Du J, Carlton SM, Jeske NA. A-kinase anchoring protein 79/150 coordinates metabotropic glutamate receptor sensitization of peripheral sensory neurons. Pain 2015; 156(11): 2364–2372. DOI: 10.1097/j.pain.0000000000000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009; 62: 254–268. DOI: 10.1016/j.neuron.2009.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol 1998; 391: 78–86. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport 1993; 4: 1263–1265. DOI: 10.1097/00001756-199309000-00013 [DOI] [PubMed] [Google Scholar]
- 18.Tachibana M, Wenthold RJ, Morioka H, Petralia RS. Light and electron microscopic immunocytochemical localization of AMPA-selective glutamate receptors in the rat spinal cord. J Comp Neurol 1994; 344: 431–454. DOI: 10.1002/cne.903440307 [DOI] [PubMed] [Google Scholar]
- 19.Gong K, Kung LH, Magni G, Bhargava A, Jasmin L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One 2014; 9: e95491. DOI: 10.1371/journal.pone.0095491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron 2002; 35: 135–146. DOI: 10.1016/s0896-6273(02)00729-8 [DOI] [PubMed] [Google Scholar]
- 21.Gangadharan V, Wang R, Ulzhofer B, Luo C, Bardoni R, Bali KK, Agarwal N, Tegeder I, Hildebrandt U, Nagy GG, Todd AJ, Ghirri A, Haussler A, Sprengel R, Seeburg PH, MacDermott AB, Lewin GR, Kuner R. Peripheral calcium-permeable AMPA receptors regulate chronic inflammatory pain in mice. J Clin Invest 2011; 121: 1608–1623. DOI: 10.1172/JCI44911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci: The Official Journal of the Society for Neuroscience 2000; 20: 4680–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 24.Jeske NA, Patwardhan AM, Henry MA, Milam SB. Fibronectin stimulates TRPV1 translocation in primary sensory neurons. J Neurochem 2009; 108: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fucile S, Miledi R, Eusebi F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc Natl Acad Sci U S A 2006; 103: 2943–2947. DOI: 10.1073/pnas.0511063103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 1965; 206: 97–99. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience 2010; 165: 896–901. DOI: 10.1016/j.neuroscience.2009.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain 2005; 113: 185–190. DOI: 10.1016/j.pain.2004.10.021 [DOI] [PubMed] [Google Scholar]
- 29.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience 2008; 152: 521–525. DOI: 10.1016/j.neuroscience.2008.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari LF, Araldi D, Levine JD. Regulation of expression of hyperalgesic priming by estrogen receptor alpha in the rat. J Pain 2017; 18: 574–582. DOI: 10.1016/j.jpain.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari LF, Bogen O, Levine JD. Role of nociceptor αCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci 2013; 33: 11002–11011. DOI: 10.1523/JNEUROSCI.1785-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inyang KE, Burton MD, Szabo-Pardi T, Wentworth E, McDougal TA, Ramirez ED, Pradhan G, Dussor G, Price TJ. Indirect AMP-activated protein kinase activators prevent incision-induced hyperalgesia and block hyperalgesic priming, whereas positive allosteric modulators block only priming in mice. J Pharmacol Exp Ther 2019; 371: 138–150. DOI: 10.1124/jpet.119.258400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kandasamy R, Price TJ. The pharmacology of nociceptor priming. Handb Exp Pharmacol 2015; 227: 15–37. DOI: 10.1007/978-3-662-46450-2_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paige C, Barba-Escobedo PA, Mecklenburg J, Patil M, Goffin V, Grattan DR, Dussor G, Akopian AN, Price TJ. Neuroendocrine mechanisms governing sex differences in hyperalgesic priming involve prolactin receptor sensory neuron signaling. J Neurosci 2020; 40: 7080–7090. DOI: 10.1523/JNEUROSCI.1499-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 2005; 310: 1340–1343. DOI: 10.1126/science.1116894 [DOI] [PubMed] [Google Scholar]
- 36.Liu TY, Cheng Y, Qin XY, Yu LC. Pharmacologically inhibiting GluR2 internalization alleviates neuropathic pain. Neurosci Bull 2015; 31: 611–616. DOI: 10.1007/s12264-015-1556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 2000; 20: 7258–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci 2009; 29: 3206–3219. DOI: 10.1523/JNEUROSCI.4514-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 1996; 16: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat Neurosci 2009; 12: 172–181. DOI: 10.1038/nn.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson JL, Gorski JA, Dell’Acqua ML. NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca2+-Permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron 2016; 89: 1000–1015. DOI: 10.1016/j.neuron.2016.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol 2001; 21: 1475–1483. DOI: 10.1128/MCB.21.5.1475-1483.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 2000; 3: 1291–1300. DOI: 10.1038/81823 [DOI] [PubMed] [Google Scholar]
- 44.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 2003; 112: 631–643. [DOI] [PubMed] [Google Scholar]
- 45.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009; 32: 611–618. DOI: 10.1016/j.tins.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008; 59: 450–461. [DOI] [PubMed] [Google Scholar]
- 47.Nikandrova YA, Jiao Y, Baucum AJ, Tavalin SJ, Colbran RJ. Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J Biol Chem 2010; 285: 923–934. DOI: 10.1074/jbc.M109.033985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choquet D. Linking nanoscale dynamics of AMPA receptor organization to plasticity of excitatory synapses and learning. J Neurosci 2018; 38: 9318–9329. DOI: 10.1523/JNEUROSCI.2119-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett 1995; 197: 25–28. [DOI] [PubMed] [Google Scholar]
- 50.Carlton SM, Coggeshall RE. Inflammation-induced changes in peripheral glutamate receptor populations. Brain Res 1999; 820: 63–70. DOI: 10.1016/s0006-8993(98)01328-6 [DOI] [PubMed] [Google Scholar]
- 51.deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport 2000; 11: 497–502. [DOI] [PubMed] [Google Scholar]
- 52.Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res 1998; 787: 161–164. DOI: 10.1016/s0006-8993(97)01568-0 [DOI] [PubMed] [Google Scholar]
- 53.Chaudhury S, Bal M, Belugin S, Shapiro MS, Jeske NA. AKAP150-mediated TRPV1 sensitization is disrupted by calcium/calmodulin. Mol Pain 2011; 7: 34. DOI: 10.1186/1744-8069-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szteyn K, Rowan MP, Gomez R, Du J, Carlton SM, Jeske NA. A-kinase anchoring protein 79/150 coordinates metabotropic glutamate receptor sensitization of peripheral sensory neurons. Pain 2015; 156: 2364–2372. DOI: 10.1097/j.pain.0000000000000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blackburn-Munro G, Bomholt SF, Erichsen HK. Behavioural effects of the novel AMPA/GluR5 selective receptor antagonist NS1209 after systemic administration in animal models of experimental pain. Neuropharmacology 2004; 47: 351–362. DOI: 10.1016/j.neuropharm.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 56.Gormsen L, Finnerup NB, Almqvist PM, Jensen TS. The efficacy of the AMPA receptor antagonist NS1209 and lidocaine in nerve injury pain: a randomized, double-blind, placebo-controlled, three-way crossover study. Anesth Analg 2009; 108: 1311–1319. DOI: 10.1213/ane.0b013e318198317b [DOI] [PubMed] [Google Scholar]
- 57.Tadicherla S, Berman B. Percutaneous dermal drug delivery for local pain control. Ther Clin Risk Manag 2006; 2: 99–113. [PMC free article] [PubMed] [Google Scholar]
- 58.Stearns LM, Abd-Elsayed A, Perruchoud C, Spencer R, Hammond K, Stromberg K, Weaver T. Intrathecal drug delivery systems for cancer pain: an analysis of a prospective, multicenter product surveillance registry. Anesth Analg 2020; 130: 289–297. DOI: 10.1213/ANE.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]