Abstract
Cabotegravir (CAB) and rilpivirine (RPV) is the first complete long-acting (LA) injectable regimen recommended by treatment guidelines for the maintenance of HIV-1 virologic suppression in people with HIV-1 who are virologically suppressed on a stable antiretroviral regimen that is administered monthly (Q1M) or every 2 months (Q2M). As an alternative regimen to lifelong daily oral antiretroviral therapy, Q1M or Q2M dosing schedules are associated with increased patient satisfaction and treatment preference. In addition, it may address challenges associated with daily oral dosing, including fear of treatment disclosure or stigma, anxiety related to oral dosing adherence, and the daily reminder of HIV disease status. Cabotegravir + RPV LA is administered by clinical staff as two intramuscular injections dosed Q1M or Q2M. In this review, we share practical dosing guidance for CAB+RPV LA injectable therapy, including how to initiate therapy, schedule injection visits, manage dosing interruptions due to missed or delayed injection visits, manage errors in dosing, and transition to alternative antiretroviral therapy after discontinuation. Practical guidance on the clinical management of CAB+RPV LA dosing, including a detailed discussion using case-based scenarios that may be encountered in clinical practice, is provided. The clinician-administered CAB+RPV LA regimen has dosing management considerations that are flexible and considerate of the patient and has the potential to provide a highly desirable and efficacious alternative to daily oral antiretroviral therapy for many people with HIV-1.
Keywords: antiretroviral therapy, cabotegravir, HIV-1, HIV infections, intramuscular injections, rilpivirine
Plain language summary
Guidance for clinicians on the management of long-acting Cabotegravir and Rilpivirine Injectable Therapy for HIV-1
Cabotegravir (CAB) and rilpivirine (RPV) is the first long-acting (LA) injectable therapy for people with HIV-1 who previously achieved undetectable virus levels using other HIV-1 medications. People with HIV-1 receive CAB+RPV LA as two injections given by their clinician every 1 month or every 2 months, providing an alternative treatment option to lifelong daily oral medications. People with HIV-1 receiving CAB+RPV LA every 1 or 2 months have higher levels of treatment satisfaction and often prefer CAB+RPV LA compared with daily oral medications. Cabotegravir+RPV LA may also address challenges associated with daily oral medications, including fear of inadvertently sharing HIV status, anxiety related to taking daily medications, and having a daily reminder of HIV. In this review, we provide guidance for clinicians on how to administer CAB+RPV LA injectable therapy, including how to start patients on CAB+RPV LA injections, schedule injection visits, manage missed or delayed injection visits, manage dosing errors, and switch patients to a different treatment if CAB+RPV LA is discontinued. This review also includes a detailed discussion of potential scenarios related to the administration and scheduling of CAB+RPV LA injections that may occur in clinical practice. Overall, this review serves as a practical guide for managing CAB+RPV LA injectable therapy in clinical practice that will be useful for HIV clinicians.
Introduction
Although antiretroviral therapy (ART) has transformed HIV into a treatable chronic condition, the requirement for lifelong daily oral therapy continues to pose challenges for people with HIV.1,2 These may include difficulties with daily adherence, fear of treatment disclosure or stigma, and pill burden or fatigue, which may affect treatment success.2 –5 Cabotegravir (CAB) + rilpivirine (RPV) long-acting (LA) is the first complete injectable regimen administered by a clinician monthly (Q1M) or every 2 months (Q2M); it is recommended by multiple treatment guidelines (US Department of Health and Human Services, International Antiviral Society-USA, European AIDS Clinical Society, etc.) as a preferred option for the maintenance of HIV-1 suppression in people who are virologically suppressed on a stable antiretroviral regimen and may address many of these challenges.6 –11
Available as prolonged-release suspensions in two separate vials for intramuscular administration, CAB and RPV LA are co-packaged as CABENUVA™ or individually as VOCABRIA™ and REKAMBYS™ in several markets.6 –8 Cabotegravir and RPV tablets for daily oral dosing are also available to optionally assess short-term tolerability during an oral lead-in (OLI) period before starting injections and for short-term (up to 2 months) oral treatment to manage interruptions in injectable therapy.
CAB+RPV LA requires intramuscular administration by a clinician. As a novel HIV-1 treatment, this innovative therapy will require a significant paradigm shift for both patients and clinicians. Herein, we share practical dosing guidance for CAB+RPV LA injectable therapy, including how to initiate therapy, schedule injection visits, manage interruptions in dosing due to missed or delayed injection visits, and transition to alternative ART following discontinuation.
Practical management of CAB+RPV LA dosing
CAB+RPV LA is a novel clinician-administered therapy with dosing management considerations that are flexible and considerate of the patient and their lifestyle. It is a highly effective ART regimen for which studies have shown that it is often preferred by people with HIV-1 over oral therapy.12 –14 With adherence to the LA dosing visit schedule, CAB+RPV LA has the potential to provide a highly acceptable and efficacious alternative to daily oral ART regimens for many people with HIV-1.
Initiation of CAB+RPV LA Therapy
OLI or starting injections directly
CAB+RPV LA is indicated as a complete regimen for the treatment of HIV-1 infection in adults (and adolescents aged ⩾12 years and weighing ⩾35 kg in the United States) to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA < 50 copies/mL) on a stable antiretroviral regimen. It is indicated if there is no history of treatment failure and no known or suspected resistance to either drug or drug class (depending on local prescribing information), with some guidelines recommending the switch after sustained suppression for at least 3–6 months.6,9,10,15 CAB+RPV LA injections may be initiated with or without an OLI at the discretion of the clinician and patient. Approval of switching from prior oral ART to CAB+RPV LA injections without an OLI was based on similar safety, efficacy, and CAB and RPV pharmacokinetic (PK) profiles between participants initiating CAB+RPV LA with (n = 121) or without (n = 111) an OLI phase. 16 An OLI involves the administration of CAB 30 mg + RPV 25 mg once daily with a meal for ~1 month (at least 28 days), with the last dose taken on the same day as the first CAB+RPV LA injections to ensure continuous maintenance of viral suppression. Without an OLI, the last current ART dose is taken on the same day that the first CAB+RPV LA injections are administered. Of note, with RPV LA injections, there are no concerns about timing of food intake or coadministration with acid-reducing agents, such as proton pumps inhibitors, as is observed with oral RPV administration. 17
Initiation and continuation of injections
Monthly and Q2M dosing schedules for CAB+RPV LA are shown in Table 1. Injections are administered intramuscularly in the ventrogluteal (recommended) or dorsogluteal muscle. 6 In the presence of buttock implants or dermal fillers, clinicians should assess the anatomical position of the implants or fillers and use their judgment to determine whether CAB+RPV LA is appropriate for the patient. Monthly dosing requires an initiation dose at Month 1 of CAB LA 600 mg and RPV LA 900 mg (3-mL injection each), followed by Q1M CAB LA 400 mg and RPV LA 600 mg (2-mL injection each) from Month 2 onward. Every-2-month dosing starts with a monthly dose for the first 2 months, to allow therapeutic plasma drug concentrations to be reached rapidly, using the 3-mL injections of CAB LA (600 mg) and RPV LA (900 mg). Therefore, initiation dosing comprises two doses of CAB LA 600 mg and RPV LA 900 mg (3-mL injection each) given 1 month apart (Months 1 and 2). These doses are then followed by continuation CAB LA 600 mg and RPV LA 900 mg (3-mL injection each) doses given Q2M from the third visit (Month 4) and onward.
Table 1.
Recommended CAB+RPV dosing schedules. 6
| Optional oral lead-in (at least 28 days) a | Intramuscular injections | ||
|---|---|---|---|
| Q1M dosing | Month before starting injections | Initiation injections: Month 1 (one-time dosing) b | Continuation injections: Month 2 and onward (Q1M dosing) |
| CAB | 30 mg once daily with a meal c | 600 mg (3 mL) | 400 mg (2 mL) |
| RPV | 25 mg once daily with a meal | 900 mg (3 mL) | 600 mg (2 mL) |
| Q2M dosing | Month before starting injections | Initiation injections: Months 1 b and 2 | Continuation injections: Month 4 and onward (Q2M dosing) |
| CAB | 30 mg once daily with a meal c | 600 mg (3 mL) | 600 mg (3 mL) |
| RPV | 25 mg once daily with a meal | 900 mg (3 mL) | 900 mg (3 mL) |
Optional oral therapy should be continued until the day the first injection is administered.
Given on the last day of current antiretroviral therapy or oral lead-in, if used.
Oral CAB does not require being taken with a meal, but it is recommended to be taken at the same time as RPV, which requires administration with a meal.
CAB, cabotegravir; Q1M, once monthly; Q2M, every 2 months; RPV, rilpivirine.
Switching LA dosing schedules
If preferred, and in countries where both dosing regimens are approved, patients may switch from the Q1M to Q2M dosing schedule or vice versa. To switch from Q1M to Q2M dosing, administer the first Q2M continuation dose of CAB LA 600 mg and RPV LA 900 mg (3-mL injection each) 1 month after patients receive their last Q1M continuation dose of CAB LA 400 mg and RPV LA 600 mg (2-mL injection each). Subsequent doses are administered Q2M thereafter. There is no need for an additional initiation dose when switching from Q1M to Q2M dosing.
When switching from Q2M (CAB LA 600 mg and RPV LA 900 mg) to Q1M dosing, patients should receive their first Q1M continuation dose of CAB LA 400 mg and RPV LA 600 mg 2 months after the last Q2M injections and then continue Q1M dosing thereafter. There is no need for an additional initiation dose.
Scheduling injection visits
Target treatment date concept
In phase III clinical trials, CAB+RPV LA was dosed every 4 weeks (28 days apart) or every 8 weeks (56 days apart). Modification from Q4W to Q1M and Q8W to Q2M dosing allows for a fixed target treatment date (TTD; e.g. the 15th of the calendar month) that can be easily remembered and conveniently chosen by patients to support adherence. Adhering to the TTD results in 12 Q1M dosing visits per year or 6 Q2M dosing visits per year. Patients should consider selecting a date between calendar days 1–28 and avoid days 29, 30, and 31 for scheduling ease. The TTD can be changed if a more convenient date is desired either by the patient or the clinic (see Injection dosing window section).
Injection dosing window (±7 days)
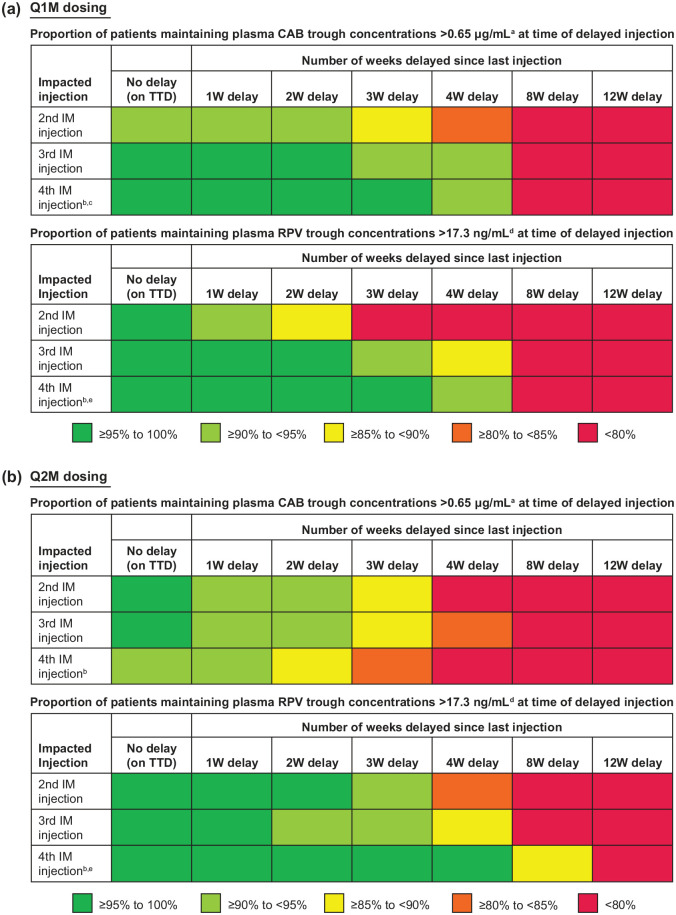
Adherence to scheduled injection visits is strongly recommended to maintaining virologic suppression and ensuring optimal drug concentrations. Clinical trials permitted a ±7-day injection dosing window for the fourth injection and beyond to provide flexibility with appointment scheduling (e.g. future treatment date falls on a weekend or holiday, the patient is out of town or has another life event).12,14 Although only a −7-day dosing window was permitted for the second and third LA injections in clinical trials, PK simulations based on clinical trial data indicated that a 1-week delay in injection dosing had no appreciable impact on CAB and RPV trough concentrations with Q1M and Q2M dosing, regardless of which injection was impacted (Figure 1). Injection delays of >1 week and outside of the +7-day dosing window are not recommended.
Figure 1.
Delayed CAB LA and RPV LA injection impact with (a) Q1M and (b) Q2M dosing. Tables show the simulated proportion of participants with plasma CAB and RPV trough concentrations above target concentrations at time of dosing delay by increasing the dosing delay length based on PK simulations.
aModel-predicted fifth percentile CAB trough concentration value after the first injection in phase III trials. 18
bTable is truncated after the fourth injection because guidance for later injections is the same.
cFourth injection for CAB LA represents near steady-state CAB concentrations.
dObserved fifth percentile of RPV trough concentrations 4 weeks after the first injection in ATLAS and FLAIR phase III trials.
eFourth injection for RPV LA represents when RPV concentrations are still accumulating.
CAB, cabotegravir; IM, intramuscular; LA, long-acting; PK, pharmacokinetics; RPV, rilpivirine; TTD, target treatment date; W, week.
By adhering to a fixed TTD, Q1M or Q2M, the flexible window dates remain the same for each visit (e.g. if the TTD is the 15th of the month, the dosing window consistently remains the 8th–22nd of the month).
Injections may be administered up to 7 days before (‘early’ window) or 7 days after (‘deferred’ window) the originally scheduled dosing visit date for both Q1M and Q2M dosing regimens. 6 If an injection was administered either before or after the TTD but within the ±7-day treatment window in a particular month, the patient should return to, or as close as possible to, their original TTD for their next injection visit to ensure consistent visit schedules. For example, if a Q1M injection dose is deferred by 1 week to the 22nd instead of the original TTD of the 15th, the next Q1M injection dose should be given ~3 weeks later, on or near the 15th of the following month. This ensures concentrations will not drift downward over time and risk loss of virologic control, especially if the 1-week deferred window is utilized several months in a row.
For both Q1M and Q2M dosing regimens, if the TTD needs to be moved to a significantly earlier or later date for improved convenience, subsequent visits should be scheduled using the ±7-day window until the injection dosing visit date falls on the new desired TTD. For example, if a Q1M injection is typically given on the 15th and the TTD needs to be changed to the 1st of the month, the next Q1M injection should be brought forward to the 8th of the following month and then subsequently the 1st of the month.
Dosing beyond the +7-day window must be avoided, which is especially important during the first 2–3 injections of the dosing schedule while CAB and RPV concentrations are still accumulating, to minimize risk of lower CAB and RPV concentrations potentially resulting in loss of virologic control and resistance development. Figure 1 shows the simulated impact on CAB and RPV concentrations with dosing delays of various lengths.
Managing interruptions in injection dosing
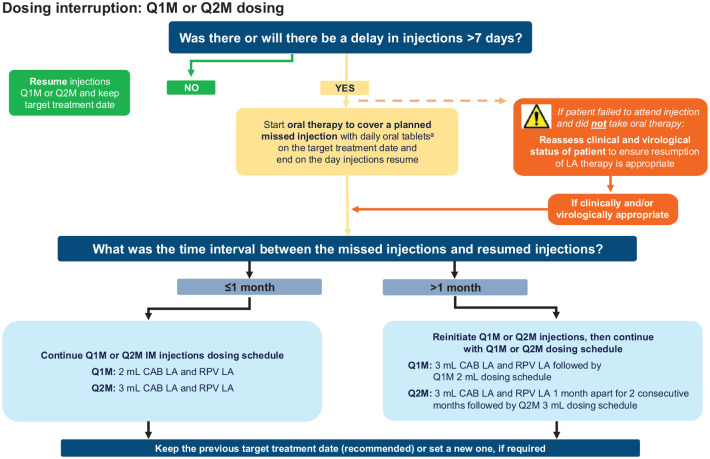
At times, patients may need to interrupt their Q1M or Q2M dosing schedule to accommodate life events, either planned (e.g. scheduled travel) or unforeseen (e.g. unexpected bereavement, accident, or loss to follow-up). Adherence to the injection schedule is strongly recommended for maintaining CAB and RPV concentrations. Patients should therefore be counseled by their healthcare providers about the importance of adhering to dosing visits and informed that oral therapy can be used to cover a planned missed visit (sometimes called oral bridging) and mitigate these risks. If an injection visit is missed, the patient’s clinical and virological status should be reassessed to ensure that the resumption of LA therapy is appropriate. A decision-making flowchart for managing dosing interruptions with Q1M or Q2M dosing is shown in Figure 2.
Figure 2.
Decision-making flowchart for the management of dosing interruptions with Q1M and Q2M dosing.
aIn the European Union, oral CAB+RPV is recommended for oral therapy to cover a planned missed injection.7,8 In the United States, oral therapy can be with oral CAB+RPV or any other fully suppressive oral antiretroviral regimen. 6 For oral therapy taken for >2 months, an alternative oral regimen, which may include RPV, is recommended.6 –8
CAB, cabotegravir; LA, long-acting; Q1M, once monthly; Q2M, every 2 months; RPV, rilpivirine.
Oral therapy for planned dosing interruptions
If a future injection visit cannot be rescheduled within the ±7-day window resulting in a possible missed Q1M or Q2M injection visit, oral CAB 30 mg + RPV 25 mg once daily (or, in some markets, any other appropriate oral ART regimen expected to have full antiretroviral activity) can be used to replace a scheduled injection visit for up to two consecutive months to ensure therapeutic drug coverage during the interval between injections.6 –8 For oral therapy durations longer than 2 months, an alternative oral regimen to CAB+RPV tablets, which can include RPV, is recommended.6 –8 The first dose of oral therapy should be taken on the original TTD (+7 days) and continued daily, with the last dose taken on the same day injections are restarted.
Unplanned dosing interruptions
There may be times when a patient attends the clinic late (outside of the +7-day window) for injections and will not have taken oral therapy between injection visits or notified the clinic. If a patient misses ⩾1 scheduled injection visit (e.g. unforeseen life event or loss to follow-up), there is a risk of virologic rebound and resistance development. If the patient remains clinically appropriate for LA therapy, injection dosing needs to be restarted as soon as possible using either the original or a new TTD, as described in the recommendations in the next section (see Restarting injection dosing section).
Restarting injection dosing
Recommendations for restarting LA therapy after a dosing interruption depend on the time elapsed since the last missed injection visit. Guidance is identical regardless of whether or not oral therapy was used to cover a planned interruption because the waning decline of CAB and RPV concentrations resulting from absorption from the injection site is the same regardless of which supplemental oral therapy was used. The recommendations aim to minimize the impact of dosing interruptions on the overall CAB and RPV PK profiles.
For Q1M or Q2M dosing, if ⩽1 month (28 days) has passed since the missed TTD, the Q1M or Q2M dosing schedule can be resumed (Table 2). If >1 month (28 days) has passed since the missed TTD, dosing needs to be reinitiated again, beginning with the initiation dose (3 mL) followed by Q1M continuation doses (2 mL) thereafter for the Q1M regimen and beginning with two initiation doses (3 mL) 1 month apart followed by Q2M dosing thereafter for the Q2M regimen. In both scenarios, keeping the original TTD is recommended, but establishing a new TTD is also an option, if required. Additionally, plasma HIV-1 RNA tests may be requested at both the first and second injection visits after a missed injection to ensure virologic suppression is maintained. For more details, see case 4 of the following scenarios.
Table 2.
Recommendations for resuming CAB+RPV LA injections after missed injections for Q1M and Q2M dosing.6 –8
| Time since last injection | Time since missed injection | Recommendations a |
|---|---|---|
| Q1M dosing b | ||
| ⩽2 months | ⩽1 month | Resume Q1M 2-mL CAB LA 400 mg + RPV LA 600 mg continuation injections |
| >2 months | >1 month | Reinitiate with 3-mL CAB LA 600 mg + RPV LA 900 mg then continue with Q1M 2-mL CAB LA 400 mg + RPV LA 600 mg continuation injections |
| Q2M dosing c | ||
| ⩽2 months (injection 2) | ⩽1 month | Resume 3-mL CAB LA 600 mg + RPV LA 900 mg injection then continue with 3-mL injections Q2M |
| >2 months (injection 2) | >1 month | Reinitiate with 3-mL CAB LA 600 mg + RPV LA 900 mg followed by a second 3-mL initiation dose 1 month later; then continue with 3-mL injections Q2M |
| ⩽3 months (injection 3 and beyond) | ⩽1 month | Resume 3-mL CAB LA 600 mg + RPV LA 900 mg injection then continue with 3-mL injections Q2M |
| >3 months (injection 3 and beyond) | >1 month | Reinitiate with 3-mL CAB LA 600 mg + RPV LA 900 mg followed by a second 3-mL initiation dose 1 month later; then continue with 3-mL injections Q2M |
aInjection dosing should resume as soon as possible if oral therapy was not taken and on the last day of oral therapy if oral therapy was used to cover missed injections.
Oral CAB 30 mg + RPV 25 mg once daily should be taken starting 1 month ±7 days after the last injection.
Oral CAB 30 mg + RPV 25 mg once daily should be taken starting ~2 months ±7 days after the last injection.
CAB, cabotegravir; LA, long-acting; Q1M, once monthly; Q2M, every 2 months; RPV, rilpivirine.
Managing errors in dosing or LA administration
Errors in dosing or LA administration were uncommon in the CAB+RPV LA clinical trials. Administering the wrong dose (e.g. 2-mL dose instead of 3-mL dose or vice versa) or leakage from the injection site may or may not be identified at the time of administration and may lead to underdosing or overdosing scenarios of one or both drug components of the treatment regimen. Careful injection preparation and administration techniques should be used, and corrective action should be taken in the event of a known or suspected dosing error.
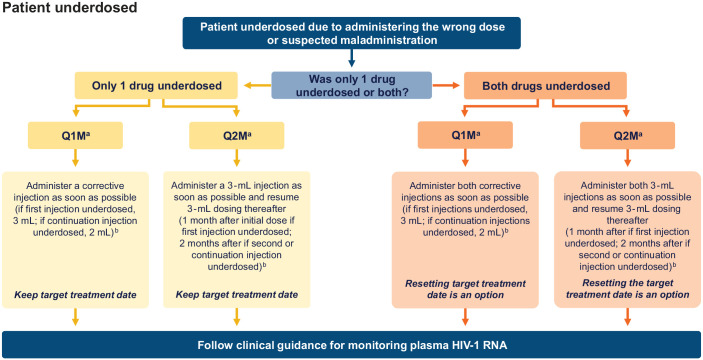
In an underdosing scenario for Q1M and Q2M dosing, it is advised that a corrective injection be administered as soon as practically possible after the dosing error is noticed (either a 2- or 3-mL dose, depending on the dosing regimen). Subsequent doses can resume either 1 or 2 months after the corrective dose, depending on the dosing schedule and whether initiation injections have been completed. For both dosing regimens, if only one drug (either CAB or RPV) was underdosed and corrected, the patient should keep the same TTD. If both medicines were underdosed and corrected, resetting the TTD to the date of the corrective dosing is an option. Plasma HIV-1 RNA tests may be requested at the first and second injection visits after a dosing error to ensure virologic suppression is maintained. As an alternative to administering a corrective dose, oral therapy with CAB+RPV may be prescribed as soon as the dosing error is noticed and continued until the next scheduled injection visit. A decision-making flowchart for the management of underdosing is shown in Figure 3.
Figure 3.
Decision-making flowchart for the management of underdosed injections.
aAs an alternative to administering a corrective dose, oral therapy with CAB+RPV may be prescribed as soon as the dosing error is noticed and continued until the next scheduled injection visit.
bTo date, no safety concerns have been identified with two 3-mL doses of the same drug in 1 day.
CAB, cabotegravir; Q1M, once monthly; Q2M, every 2 months; RPV, rilpivirine.
If a dose that is higher than planned (e.g. an additional dose of CAB LA 600 mg) is administered, PK simulations suggest that CAB concentrations should remain below steady-state CAB concentrations observed with once-daily dosing of oral CAB 60 mg and should not require any additional intervention.
Finally, for Q2M dosing, if the initiation injections are scheduled >1 month (+7 days) apart instead of 1 month because of scheduling error, 3-mL CAB LA and RPV LA injections should be administered as soon as it is realized, and the TTD should be reset to the day of the month that the second injection is administered. Planning the subsequent injections depends on the length of delay between the first and second initiation injections (⩽1-month delay: resume; >1-month delay: reinitiate), as described in Table 2. A plasma HIV-1 RNA test may be requested at the first and second injection visits after the scheduling error is discovered to ensure virologic suppression is maintained.
Discontinuation
Management of patients discontinuing CAB+RPV LA therapy must be carefully considered as the release of CAB and RPV concentrations from the injection site persist and continue to decrease for 1 year or longer in some patients, with a potential risk of resistance development if the patient is not virologically controlled with alternative ART. 6 After discontinuation due to non-virologic reasons, another fully suppressive oral ART regimen should be started no later than 1 month after the final Q1M injection or 2 months after the final Q2M injection to prevent loss of viral control and development of resistance.6 –8 If discontinuing due to virologic failure, another ART regimen should be started as soon as possible, with the choice being driven in part by resistance testing. There are no specific limitations to the selection of an alternative oral ART regimen from a drug–drug interaction perspective, as there are few drug–drug interactions with CAB or RPV and other oral antiretrovirals. 6 Alternative therapy should be chosen based on patient-specific factors in accordance with local guidelines and resistance testing.
Clinical management of CAB+RPV LA dosing: Case-based scenarios
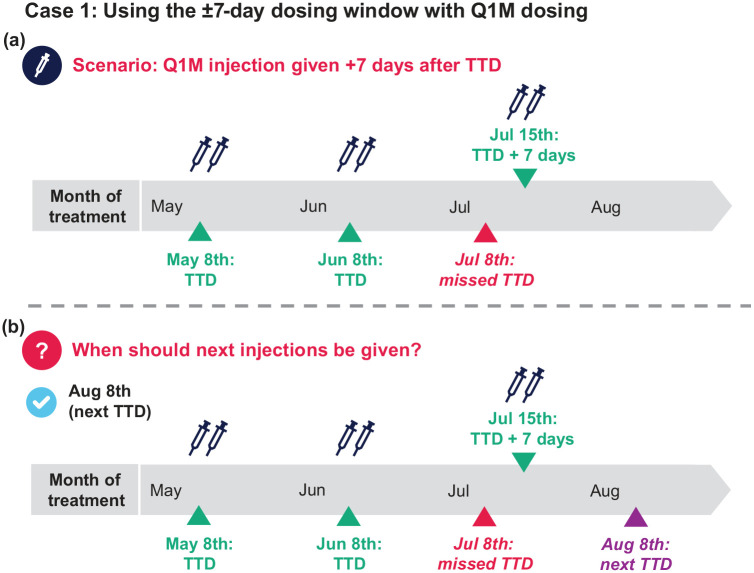
Case 1: Using the ±7-day dosing window with Q1M dosing
A patient starts Q1M CAB+RPV LA and has selected a TTD of the 8th of the month. They receive their initiation injections on May 8th (Month 1), their first continuation injections on June 8th (Month 2), and their second continuation injections on July 15th (Month 3 + 7 days; Figure 4).
Figure 4.
(a and b) Case-based scenario using the ±7-day dosing window with Q1M dosing.
Q1M, once monthly; TTD, target treatment date.
Q1: When should the patient’s next appointment be scheduled?
August 1st (1 week before TTD)
August 8th (next TTD as July dose was within the ±7-day dosing window)
August 15th (1 week after TTD)
Q1 answer: b [Figure 4(b)]
Additional considerations: When it is not possible to receive injections on the TTD, injection appointments can be scheduled up to 7 days before or 7 days after the TTD using the ±7-day window. Patients who use the ±7-day window are recommended to return to or as close as possible to their TTD for their next injection visit to prevent CAB and RPV levels from drifting downward in the event of subsequently using the ±7-day window option.
Managing interruptions in injection dosing
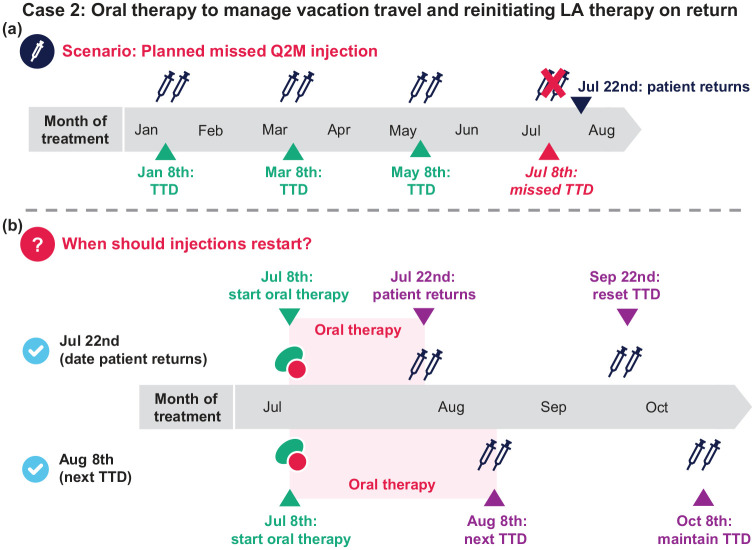
Case 2: Oral therapy to manage vacation travel and reinitiating LA therapy on return
A patient has been receiving CAB+RPV LA Q2M on the 8th of the month for a year. They receive their May injections but will be away on a trip until July 22nd, 2 weeks after their planned TTD. After counseling, you prescribe oral therapy to cover the interruption in LA dosing (sometimes called oral bridging). They start oral therapy on July 8th [next TTD; Figure 5(a)].
Figure 5.
(a and b) Case-based scenario for managing interruptions in injection dosing with oral therapy.
Q2M, every 2 months; TTD, target treatment date.
Q1: When should injections restart?
a. July 22nd (when the patient returns; patient takes 2 weeks of oral therapy)
b. August 8th (patient takes 1 month of oral therapy and keeps the 8th of the month as TTD)
c. Both (a) and (b) are reasonable options
Q1 answer: c [Figure 5(b)]
Additional considerations: Both a and b are reasonable, but option b may be preferable for some because (1) oral tablets are routinely dispensed in 30-day pill bottles packs and (2) maintaining an established TTD may be easier to manage. The last dose of oral therapy should be taken on the same day injections resume.
Q2: What is the appropriate dose and schedule for subsequent injections?
a. Reinitiate injections (3 mL, CAB LA 600 mg and RPV LA 900 mg): 2 initiation injections 1 month apart, then Q2M thereafter
b. Resume the Q2M injection dosing schedule (3 mL, CAB LA 600 mg and RPV LA 900 mg)
Q2 answer: b
Additional considerations. In this scenario, regardless of resumption date, it has been ⩽1 month since the planned missed injections, so resume Q2M scheduling, keeping the previous TTD or setting a new one if required. Options for TTD are as follows:
• Option 1: If electing to keep the TTD on the 8th of the month, continue oral therapy and resume injections on August 8th (e.g. followed by Q2M injection visits on October 8th, December 8th, etc)
• Option 2: If electing to resume injections on July 22nd, the TTD can be reset to the 22nd of the month (with caution in repeated changes of TTD)
• Option 3: Alternatively, if resuming on July 22nd but the patient would like to keep their established TTD, subsequent visits can be brought forward using the – 7-day window until injection visits return back to the 8th of the month (e.g. next injection visit on September 15th followed by November 8th)
In scenarios where it has been >1 month since the planned missed injection, reinitiate injections with two initiation injections 1 month apart then Q2M dosing thereafter, keeping the previous TTD or setting a new one if desired, as above.
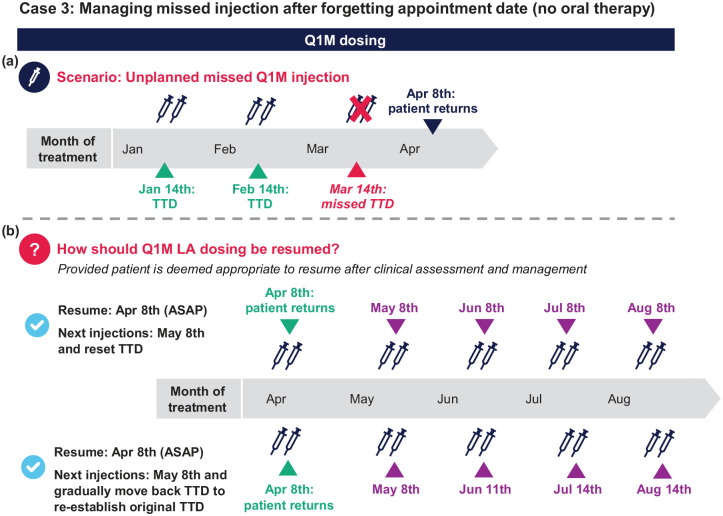
Case 3: Managing missed injection after forgetting appointment date (no oral therapy) on Q1M dosing
A patient has been stable on Q1M dosing for 1 year and receiving injections on the 14th of each month. They received their February injections on time but missed their next injection appointment on March 14th. They return on April 8th (3 weeks + 4 days late), and after clinical reassessment and determination that the patient is still an appropriate candidate for CAB+RPV LA (e.g. obtaining HIV-1 RNA viral load), you decide that injection dosing can be restarted [Figure 6(a)].
Figure 6.
(a and b) Case-based scenario for managing injection dosing interruptions with Q1M dosing without taking oral therapy.
ASAP, as soon as possible; LA, long-acting; Q1M, once monthly; TTD, target treatment date.
Q1. On April 8th, the patient is ⩽1 month past the March TTD. How do you re-commence LA dosing?
a. Resume Q1M dosing (2 mL) as soon as possible (⩽1 month from missed TTD)
b. Reinitiate dosing with first initiation dose (3 mL) as soon as possible and 2 mL Q1M thereafter
c. Do nothing and have the patient return to the clinic on the next TTD
Q1 answer: a [Figure 6(b)]
Additional considerations (assuming it is virologically appropriate): In this scenario, ⩽1 month has passed since the missed injection date, so the patient can continue with Q1M dosing schedule (2-mL injections) as soon as possible. In a scenario where >1 month has passed since the missed injection, the patient should be reinitiated with the 3-mL doses as soon as possible and continue the Q1M 2-mL dosing schedule thereafter.
Q2. When should the patient receive their next injection?
a. May 8th and change the TTD to the 8th of each month going forward
b. May 8th and gradually move backward subsequent injection visits until dosing is reestablished on the 14th of the month (original TTD)
c. April 14th; keep their scheduled TTD
d. Both (a) and (b) are reasonable options
Q2 answer: d [Figure 6(b)]
Additional considerations: The patient should return on May 8th. If desirable for the patient, subsequent injections can be gradually moved backward by a few days until dosing is reestablished on their original TTD (the 14th of the month).
In this and similar scenarios, it is important to consider how long the patient has been stable on LA therapy before the dosing delay. For example, a dosing delay in a patient who has been stable on CAB+RPV LA for >1 year may have less of an impact than in a patient who has been on LA therapy for 2–3 months due to greater accumulation of drug concentrations. Regardless of the time on LA therapy, patients should be clinically reassessed to ensure that resumption of LA therapy is clinically and virologically appropriate.
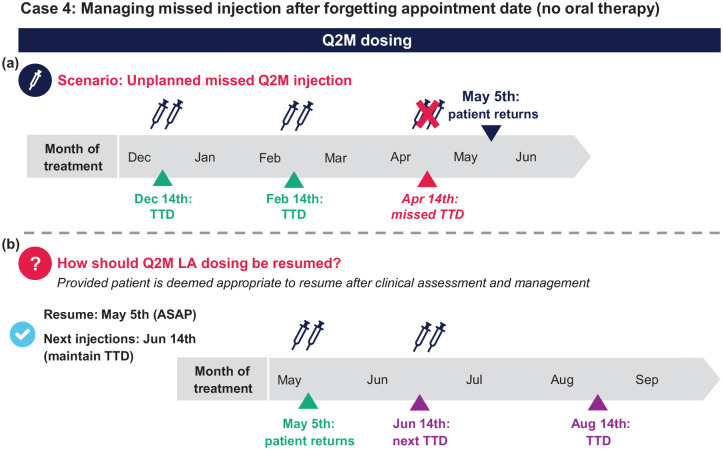
Case 4: Managing missed injection after forgetting appointment date (no oral therapy) on Q2M dosing
A patient has been stable on Q2M dosing for 1 year and receives injections on the 14th of each month. They received their February injections on time but missed their next injection appointment on April 14th. They return on May 5th (3 weeks late), and after clinical reassessment and management, you decide that injection dosing can be restarted [Figure 7(a)].
Figure 7.
(a and b) Case-based scenario for managing injection dosing interruptions with Q2M dosing without taking oral therapy.
ASAP, as soon as possible; LA, long-acting; Q2M, every 2 months; TTD, target treatment date.
Q1. The patient is 3 weeks past the TTD. How do you re-commence LA dosing?
a. Resume Q2M dosing as soon as possible (⩽1 month from missed TTD)
b. Reinitiate Q2M injections as soon as possible, with two initiation injections 1 month apart followed by Q2M thereafter
c. Do nothing and have the patient return to the clinic on the next TTD
Q1 answer: a [Figure 7(b)]
Additional considerations: After assessment, if the patient is considered clinically and virologically appropriate to continue LA dosing, Q2M dosing should be resumed as soon as possible. An HIV-1 RNA test may be requested upon the patient’s return, but options exist for how to proceed while awaiting the results. It may be more practical for the patient to resume LA dosing and complete an HIV-1 RNA test on the same day; if the HIV-1 RNA test results reveal viral breakthrough, the patient can be switched to another fully suppressive oral ART regimen. Alternatively, it may be preferred to prescribe a fully suppressive oral ART regimen on the day the patient returns and resume LA dosing after the clinic has received the HIV-1 RNA test results.
Q2. When should the patient receive their next injection?
a. July 5th; the TTD should be changed to the 5th going forward
b. June 14th; keep the next TTD
Q2 answer: b [Figure 7(b)]
Additional considerations: Patients should keep their TTD or as close as possible to their TTD for their next injection visit. If the TTD is no longer convenient, resetting the TTD is an option.
Managing errors in dosing or scheduling
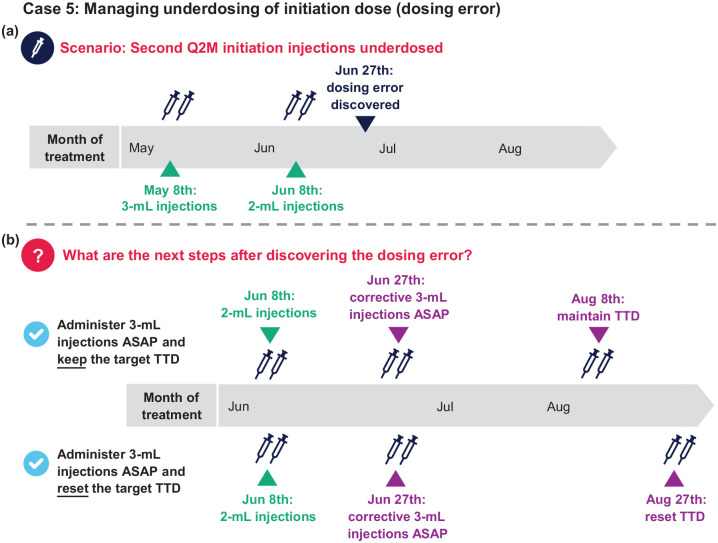
Case 5: Managing underdosing of initiation dose (dosing error)
A patient starting Q2M CAB+RPV LA selects a TTD on the 8th of the month. They receive their first initiation injections on May 8th and second initiation injections on June 8th. However, 3 weeks later on June 27th, it was discovered that the patient accidentally received 2 mL of both drugs instead of the 3-mL initiation doses for their second initiation injections on June 8th [Figure 8(a)].
Figure 8.
(a and b) Case-based scenario for managing dosing errors.
ASAP, as soon as possible; Q2M, every 2 months; TTD, target treatment date.
Q1: This error happened almost 3 weeks ago. What are the next steps?
a. Administer 3-mL CAB+RPV as soon as possible and keep the TTD (next injections on August 8th)
b. Administer 3-mL CAB+RPV as soon as possible and reset the TTD (next injections on August 27th)
c. Both (a) and (b) are reasonable options
Q1 answer: c [Figure 8(b)]
Additional considerations: After administering the corrective dose, Q2M dosing with the 3-mL injection doses are resumed thereafter. If the first injection was underdosed, dosing should be resumed 1 month after the initiation or corrective dose followed by Q2M injections; there is no need for reloading (i.e. a third initiation injection). If the second initiation injection or a continuation injection was underdosed, dosing should be resumed Q2M after the initiation injection or the corrective dose.
As both drugs were underdosed and corrected, resetting the TTD is an option, but maintaining the established TTD may be easier to manage. A plasma HIV-1 RNA test may be requested at the first (June 27th) and second (August 8th or 27th) injection visits after underdosing. In a scenario where the clinic is not able to obtain CAB LA and RPV LA injection doses as soon as the dosing error is realized, oral CAB + RPV or any other fully suppressive oral ART regimen could be prescribed to cover the period until the next injections can be administered.
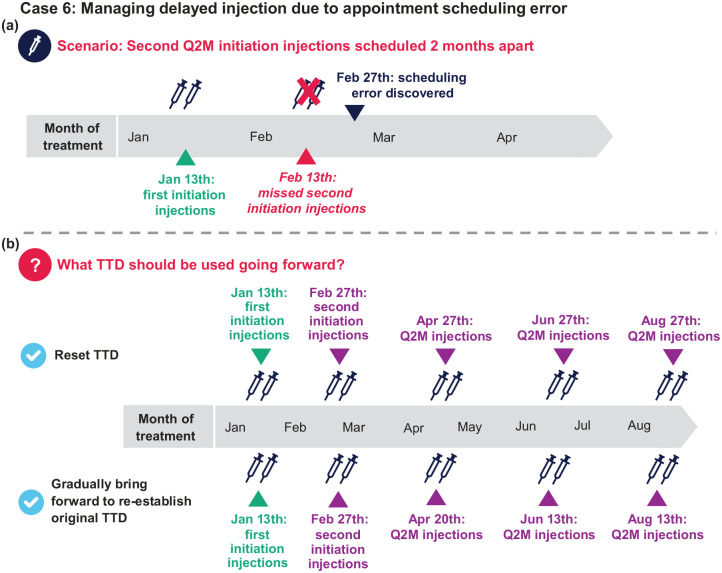
Case 6: Managing delayed injection due to appointment scheduling error
A patient starting Q2M CAB+RPV LA selected the 13th of the month as their TTD and received their first initiation doses on January 13th. The second initiation doses were mistakenly scheduled for March 13th (i.e. 2 months after the first dose) instead of 1 month later on February 13th. The scheduling error was discovered on February 27th, 2 weeks after the correct second injection visit should have occurred [Figure 9(a)].
Figure 9.
(a and b) Case-based scenario for managing scheduling errors.
Q2M, every 2 months; TTD, target treatment date.
Q1: The patient is 2 weeks past the correct second injection visit date. How do you proceed?
a. Do nothing; keep the next injections on March 13th and Q2M thereafter
b. Administer 3-mL injections as soon as possible (February 27th) and Q2M thereafter
c. Reinitiate dosing with the first injection dose (3 mL) today followed by 3-mL doses in 1 month and Q2M thereafter
Q1 answer: b
Additional considerations: In this scenario, the second initiation injections were <1 month late (⩽2 months after the first initiation dose), so subsequent injections should resume the Q2M dosing schedule. In a scenario where the Q2M initiation injections were given two or more months apart, injection dosing should be reinitiated with two 3-mL doses 1 month apart followed by 3-mL injections Q2M thereafter.
Q2: The patient received their second initiation injections on February 27th. When should the patient receive their third injections?
a. April 27th. Going forward, the TTD should be changed to the 27th of the month
b. April 13th. Keep the scheduled TTD where possible
c. It depends. If the 27th of the month is inconvenient, injection visits can be moved forward using the 7-day window until dosing is reestablished on the 13th of the month [April 20th, then June 23th (original TTD)]
d. Both (a) and (c) are reasonable options
Q2 answer: d [Figure 9(b)]
Additional considerations: A plasma HIV-1 RNA test may be requested at the first (February 27th) and second (April 27th) injection visits after dosing error.
Conclusion
As with any ART regimen, adherence to scheduled dosing visits is strongly recommended to maintain virologic suppression with CAB+RPV LA injectable therapy and to prevent loss of virologic control and possible resistance development. Individuals should be counseled on the importance of attending injection visits on their TTDs or to use the flexible ±7-day window. Patients should also be advised to inform their clinic as soon as possible before any known future interruptions in injection dosing (e.g. extended travel) as short-term oral therapy with CAB and RPV can be offered to maintain therapeutic plasma drug concentrations during delays in injections, thereby ensuring good virologic control. Recommendations for restarting LA therapy are based on the length of time since the missed injection and are independent of oral therapy use. In the case of an unplanned missed injection visit where oral therapy has not been taken (e.g. re-engaged in care after loss to follow-up), clinicians should determine the clinical appropriateness of resuming LA therapy. In cases of treatment discontinuation, an alternative ART regimen should be started to maintain virologic suppression.
Acknowledgments
This work was funded by ViiV Healthcare. The authors would like to thank Mark Shaefer, David Margolis, Stephen Piscitelli, and Joseph Piscitelli for their contributions. Editorial assistance was provided under the direction of the authors by Megan Schmidt, PhD, CMPP, and Lauren Bragg, ELS, MedThink SciCom, and was funded by ViiV Healthcare. Article submission assistance was provided under the authorization and approval of the authors by Larissa Tangeman, MedThink SciCom, and was funded by ViiV Healthcare.
Footnotes
Availability of data and materials: The datasets supporting the conclusions of this article can be requested for further research from www.clinicalstudydatarequest.com.
Contributor Information
Parul Patel, ViiV Healthcare, 410 Blackwell Street, Durham, NC 27701, USA.
Paula Teichner, ViiV Healthcare, Durham, NC, USA.
Emilie Elliot, ViiV Healthcare, Brentford, UK.
Marta Boffito, Chelsea and Westminster Hospital NHS Foundation Trust, London, UK; Imperial College London, London, UK.
Milena Murray, Merck & Co, Inc, Rahway, NJ, USA; Midwestern University and Northwestern Medicine, Chicago, IL, USA.
Joseph W. Polli, ViiV Healthcare, Durham, NC, USA
Mark Baker, ViiV Healthcare, Durham, NC, USA.
Susan L. Ford, GSK, Durham, NC, USA
Kelong Han, GSK, Collegeville, PA, USA.
Alberto Russu, Janssen Pharmaceutica NV, Beerse, Belgium.
Herta Crauwels, Janssen Pharmaceutica NV, Beerse, Belgium.
Ronald D. D’Amico, ViiV Healthcare, Durham, NC, USA
William R. Spreen, ViiV Healthcare, Durham, NC, USA
Jean van Wyk, ViiV Healthcare, Brentford, UK.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: All cases presented are fictionalized.
Author contributions: Parul Patel: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Paula Teichner: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Emilie Elliot: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Marta Boffito: Formal analysis; Writing – review & editing.
Milena Murray: Formal analysis; Writing – review & editing.
Joseph W. Polli: Formal analysis; Investigation; Writing – review & editing.
Mark Baker: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Susan L. Ford: Formal analysis; Investigation; Writing – review & editing.
Kelong Han: Formal analysis; Investigation; Writing – review & editing.
Alberto Russu: Formal analysis; Investigation; Writing – review & editing.
Herta Crauwels: Formal analysis; Investigation; Writing – review & editing.
Ronald D’Amico: Formal analysis; Investigation; Writing – review & editing.
William R. Spreen: Formal analysis; Investigation; Writing – review & editing.
Jean van Wyk: Formal analysis; Investigation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by ViiV Healthcare.
Competing interests: PP, PT, EE, JWP, MBa, RDD, WRS, and JvW are employees of ViiV Healthcare and own stock in GSK. MBo received travel grants, speaker/advisor fees, and research grants to the organization from Janssen, Roche, ViiV Healthcare, Bristol-Myers Squibb, Merck Sharpe & Dohme, Gilead Sciences, Mylan, Cipla, Novavax, Valneva, GSK, Teva Pharmaceuticals, Pfizer, and Theratechnologies. MM has served as a speaker for Merck, Gilead, and ViiV Healthcare and served on advisory boards for ViiV Healthcare, Janssen, and Theratechnologies. SF and KH are employees of and own stock in GSK. AR and HC are employees of Janssen Pharmaceutica NV and may hold stock in Johnson & Johnson.
References
- 1. Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis 2006; 194: 11–19. [DOI] [PubMed] [Google Scholar]
- 2. Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med 2012; 2: a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013; 16(3 Suppl 2): 18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001; 15: 1181–1183. [DOI] [PubMed] [Google Scholar]
- 5. Gardner EM, Sharma S, Peng G, et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS 2008; 22: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabenuva [prescribing information]. Durham, NC: ViiV Healthcare; 2022. [Google Scholar]
- 7. Vocabria [summary of product characteristics]. Amersfoort, Netherlands: ViiV Healthcare BV; 2022. [Google Scholar]
- 8. Rekambys [summary of product characteristics]. Beerse, Belgium: Janssen Pharmaceutica NV; 2022. [Google Scholar]
- 9. Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV, https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf (2023, accessed 13 January 2023).
- 10. Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA Panel. JAMA 2023; 329: 63–84. [DOI] [PubMed] [Google Scholar]
- 11. European AIDS Clinical Society. European AIDS Clinical Society guidelines version11.1 https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf (2022, accessed 13 January 2023).
- 12. Orkin C, Arasteh K, Gorgolas Hernandez-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382: 1124–1135. [DOI] [PubMed] [Google Scholar]
- 13. Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2021; 396: 1994–2005. [DOI] [PubMed] [Google Scholar]
- 14. Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382: 1112–1123. [DOI] [PubMed] [Google Scholar]
- 15. British HIV Association. BHIVA guidelines for the routine investigations and monitoring of adults HIV-1-positive individuals, https://www.bhiva.org/file/DqZbRxfzlYtLg/Monitoring-Guidelines.pdf (2019, accessed 28 April 2023).
- 16. Orkin C, Bernal Morell E, Tan DHS, et al. Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study. Lancet HIV 2021; 8: e668–e678. [DOI] [PubMed] [Google Scholar]
- 17. Vocabria [prescribing information]. Research Triangle Park, NC: ViiV Healthcare; 2022. [Google Scholar]
- 18. Han K, Baker M, Lovern M, et al. Population pharmacokinetics of cabotegravir following administration of oral tablet and long-acting intramuscular injection in adult HIV-1-infected and uninfected subjects. Br J Clin Pharmacol 2022; 88: 4607–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]