Abstract
Objective
Mechanical thrombectomy (MT) has become standard treatment in acute ischemic stroke due to large vessel occlusion (LVO). However, optimal blood pressure (BP) management following successful recanalization remains unclear. We aim to investigate the association of strictly achieving BP targets of ≤160/90 mmHg with the extent of neuronal loss and functional outcome.
Methods
In patients prospectively enrolled in the Gutenberg‐Stroke‐Study (May 2018–November 2019), BP was measured half‐hourly for 24 h following MT. Based on achieving BP target of ≤160/90 mmHg, patients with successful recanalization of LVO were divided into “low‐BP” group (BP ≤ 160/90 mmHg) or “high‐BP” group (BP > 160/90 mmHg). Neuronal loss was quantified by serum‐based measurement of neurofilament light chain (sNfL) after three days. BP groups and association of BP parameters with sNfL were investigated by correlation analyses and multiple regression modeling.
Results
Of 253 enrolled patients (mean age 73.1 ± 12.9 years, 53.4% female), 165 met inclusion criteria. 21.2% (n = 35) strictly achieved “low‐BP” target. “low‐BP” was associated with unfavorable functional outcome at 90‐day follow‐up (aOR [95%CI]: 5.88 [1.88–18.32], p = 0.002) and decreased health‐related quality of life (mean EQ‐5D‐index 0.45 ± 0.28 vs 0.63 ± 0.31, p = 0.009). sNfL levels were increased in “low‐BP” patients (median [IQR] 239.7 [168.4–303.4] vs 118.8 [52.5–220.5] pg/mL, p = 0.026). Hypotensive episodes were more frequent in the “low‐BP” group (48.6% vs 29.2%, p = 0.031). sNfL level could identify patients who had experienced hypotensive episodes with high discriminative ability (AUC [95%CI]: 0.68 [0.56–0.78], p = 0.007).
Interpretation
Strict BP control (≤160/90 mmHg) within 24 h following successful recanalization of LVO by MT is associated with increased neuronal injury, displayed by higher sNfL levels, and poorer functional outcome, potentially indicating hypotension‐induced neuronal loss during post‐MT phase.
Introduction
Mechanical thrombectomy (MT) has become a standard treatment for patients with acute ischemic stroke due to large vessel occlusion (LVO). 1 Stroke‐associated impairment of cerebral autoregulation renders perfusion of ischemic tissue directly dependent on systemic blood pressure (BP). 2 However, optimal BP management following successful MT is under ongoing debate, with effects of BP targets on stroke‐associated neuronal loss and functional outcome remaining largely unclear.
Current international guidelines of the European Stroke Organization and the American Stroke Association advocate a BP target < 180/105 mmHg for the first 24 h following successful recanalization of LVO by MT, aiming to reduce reperfusion hemorrhages associated with elevated blood pressure levels. 1 , 3 National guidelines of the German neurological society additionally recommend a patient‐individual approach with possible intensified systolic BP target ≤160 mmHg, depending on pre‐stroke hypertensive disease and stroke characteristics. 4 Recommendations of specific blood pressure targets are also supported by a meta‐analysis of observational studies, recently reporting worse functional outcome, higher mortality and increased symptomatic intracranial hemorrhage rate with higher mean arterial pressure during the first 24 post‐procedural hours. 5
Nevertheless, effectiveness and safety of more intensified post‐recanalization BP targets on functional outcome in patients treated with MT remain unclear and are evaluated in several ongoing randomized controlled trials. 6 Historical observational data suggest a U‐shaped relationship between BP level and functional outcome in acute ischemic stroke with both, high and low‐BP values associated with worse functional outcome. Contrary, in patients with successful recanalization, lower BP levels have suggested to be associated with better functional outcome, depicted by a linear relationship of functional outcome and BP level. 7 At the same time, there is increasing evidence that in patients treated with MT, post‐procedural BP variability might be linked to worse functional outcome, 8 calling for investigation of personalized blood pressure management strategies. Peri‐interventional BP levels, hypotensive episodes and BP drops have been linked to worse functional outcome in observational studies. 9 However, their consequences in the context of post‐procedural BP targets are widely understudied.
Blood based biomarkers might contribute to further understanding hypo‐ and hyperperfusion related effects in the dilemma of balancing patient‐individual BP targets following MT. Neurofilament light chains (NfL), a marker of neuronal injury, has recently been shown to be associated with infarct volume in cerebral ischemia, 10 and might potentially serve to quantify differences in neuronal damage associated with distinct BP regimens.
With the current study, we aim to investigate the association of functional outcome 90 days after MT with strictly achieving routine care post‐procedural BP targets of ≤160/90 mmHg within the first 24 h following MT. We furthermore aim to investigate the association of post‐procedural BP parameters with neuronal loss, as measured by serum NfL (sNfL) levels.
Methods
Standard protocol approvals
Study protocols and procedures were conducted in compliance with the Declaration of Helsinki and in accordance with local ethical guidelines. The Gutenberg‐Stroke‐Study was approved by the responsible ethics committee of the Landesärztekammer Rheinland‐Pfalz (approval number: 2018‐13335‐Epidemiologie). Written informed consent was obtained from all participants (or guardians of participants). The Gutenberg‐Stroke‐Study is registered in the German Clinical Trial Registry (DRKS00017253). The manuscript follows the STROBE guideline.
Study population
The Gutenberg‐Stroke‐Study is an ongoing monocentric, prospective, observational study in our certified German university hospital stroke center consecutively enrolling adult patients diagnosed with acute ischemic stroke. Within the Gutenberg‐Stroke‐Study, 253 patients with LVO treated by MT were enrolled from May 2018 to November 2019. Our research question was retrospectively addressed to the prospectively collected data. For the current study, we included patients with acute ischemic stroke due to LVO treated by MT and excluded patients with unsuccessful reperfusion (n = 45), defined as Thrombolysis in Cerebral Infarction (TICI) scale of 2a or worse. We also excluded patients with incomplete BP data (n = 26) and patients who were lost to clinical follow‐up at day 90 (n = 17), resulting in a total cohort of n = 165 patients for further analyses. Figure 1 displays the study cohort. Analyses were carried out on the basis of complete datasets for the respective outcome parameter.
Figure 1.
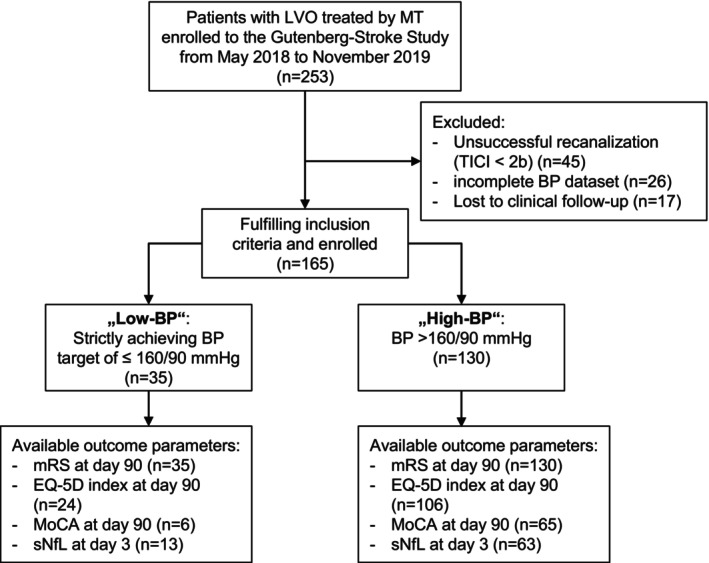
Study flowchart. Flowchart demonstrates derivation of study groups and availability of outcome parameters. BP, blood pressure; EQ‐5D, European quality of life index version 5D‐3L; LVO, large vessel occlusion; MoCA, Montreal Cognitive Assessment; mRS, modified Rankin Scale; MT, mechanical thrombectomy; sNfL, serum neurofilament light chain.
Baseline characteristics and outcomes
Baseline characteristics were reviewed by individual patient charts. The functional outcome was obtained by treating physician at discharge and by trained personal at 90‐day follow‐up via structured telephone interview. Poor functional outcome was defined as mRS 3–6. For calculation of health‐related quality of life, patient self (or proxy) reported answers to the German language version of EQ‐5D‐3L were used to calculate an EQ‐5d‐Index value. Value attached to each health state validated for the German population by time trade off were used according to Greiner et al. 11 Deceased patients were imputed with 0. Cognitive outcome was evaluated by telephone‐based Montreal cognitive assessment (MoCA) 3 months after MT. Serum‐samples for sNfL‐measurement were collected three days after MT and sNfL measured by single molecule array as previously described. 12
Blood pressure management and comparison groups
Following the institutional protocol on BP management after successful recanalization of LVO by MT, BP was targeted at 140/80 mmHg with a threshold of ≤160/90 mmHg. The standard approach to BP lowering in case of repeated overstepping of thresholds within 10–15 min was intravenous continuous application of urapidil (4–20 mg per hour) and/or clonidin (0.03–0.12 mg per hour). Alternative treatments were applied on a patient‐individual approach of the treating physician and could include use of intravenous metoprolol (2.5–5 mg) or nitroglycerin (1–8 mg per hour). Medications were then adjusted according to close meshed repeated BP measurement. For the current study, the cohort was retrospectively analyzed with respect to achieving post‐procedural BP targets of ≤160/90 mmHg. BP following MT was extracted half‐hourly for 24 h. BP values (systolic/diastolic, MAP) were automatically recorded by IntelliSpace Critical Care and Anesthesia information system (Koninklijke Philips IntelliVue X2). We divided the cohort into two comparison groups: patients who strictly met BP target of ≤160/90 mmHg for all measurements within the first 24‐h post‐MT were classified as “low‐BP” group. Patients were classified into the “high‐BP” group, when either systolic (>160 mmHg) or diastolic (>90 mmHg) or both systolic and diastolic BP parameters exceeded the above defined limits. One single measurement of systolic BP up to 180 mmHg was tolerated in the “low‐BP” group to allow for initiation of antihypertensive treatment. Hypotensive episodes were defined by mean arterial pressure (MAP) < 60 mmHg. Duration of hypotensive episodes were estimated by multiplying the number of BP measurements with MAP < 60 mmHg by the measurement interval of 30 min.
Quantification of infarct volume
The native cranial CT‐scan, routinely performed approximately 24 h after MT, was analyzed with regard to post‐MT ischemic lesion volume. Therefore, the hypodense ischemic area was quantified on 2D plane images and multiplied by the slice‐thickness of the respective CT‐scan.
Statistical analysis
Groups were compared on univariate level using the unpaired Student's t test for normally distributed continuous variables. Otherwise, the Mann–Whitney U test was used. Chi‐square test or Fisher's exact test was applied for categorical variables. Multiple linear regression analysis was conducted to assess association of BP group with EQ‐5D and sNfL as dependent variable. Multiple binary logistic regression analysis was conducted to determine the independent association of achieving BP targets of ≤160/90 mmHg and functional outcome at 90‐day follow‐up, adjusting for potential confounders (variables unbalanced between groups at univariate level with a threshold of p < 0.1 and variables known to influence functional outcome). Regression analyses were conducted on the basis of complete datasets. Pearson correlation coefficients were calculated to assess the association of BP parameters (maximum and minimum systolic BP, diastolic BP and MAP) with logarithmized sNfL levels. Receiver operating characteristics (ROC) curve analyses and the respective AUC (area under ROC curve) were calculated to assess predictive capability of sNfl to identify patients who experienced hypotensive episodes with MAP drops < 60 mmHg. Youden‐index was calculated to identify the sNfL cut‐off with highest discriminative ability.
Results
Baseline comparison of study groups
A total of 253 patients with LVO were enrolled into the Gutenberg‐Stroke‐Study from May 2018 to November 2019. 165 patients with LVO treated successfully with MT (mean age 72.8 ± 12.6 years, 49.1% male) met inclusion criteria, of which 35 patients were categorized into the “low‐BP” group (≤160/90 mmHg). 130 patients were categorized in the “high‐BP” group (>160/90 mmHg). Baseline clinical characteristics (age, sex, cardiovascular risk factors) were balanced in both groups. Similarly, stroke characteristics (NIHSS on admission, location of occlusion, ASPECTS score) and treatment characteristics (MT alone vs MT following bridging IVT, pre‐ and intra‐hospital procedural times) were evenly distributed between both groups (for details, see Table 1). We observed no significant difference in systolic BP values between our study groups (144.0 ± 28.7 vs 152.3 ± 22.7, p = 0.169). Diastolic (74.3 ± 18.4 vs 83.5 ± 15.2, p = 0.01) and mean arterial pressure (MAP) values on admission (97.5 ± 19.9 vs 106 ± 15.5, p = 0.03) were lower in the “low‐BP” group. Mean MAP was lower in the low‐BP group (mean ± SD: 80.6 ± 8.9 mmHg vs. 93.4 ± 11.0 mmHg, p < 0.001). In the low‐BP group, hypotensive episodes (MAP < 60 mmHg) were more frequent (48.6% [17/35] vs. 29.2% [4238/130], p = 0.031). Detailed information on BP parameters (minimal and maximal systolic/diastolic BP and MAP) within the study groups are stated in Table 2. Ischemic lesion volume, measured in follow‐up cranial CT after MT did not significantly differ between both BP groups (median volume [IQR] low‐BP: 13.7 mm3 [0.4–30.1] vs. 4.9 mm3 [0.0–20.2], p = 0.297).
Table 1.
Baseline characteristics and outcome stratified by strictly achieving blood pressure target of ≤160/90 mmHg during the first 24 h after MT.
| Variable | BP ≤ 160/90 mmHg “low‐BP” (n = 35) | BP > 160/90 mmHg “high BP” (n = 130) | p‐value |
|---|---|---|---|
| Patient characteristics | |||
| Age | 73.0 ± 13.1 | 72.8 ± 12.5 | 0.924 |
| Male sex | 21/35 (60%) | 60/130 (46.2%) | 0.146 |
| Arterial hypertension | 27/35 (77.1%) | 103/130 (79.2%) | 0.789 |
| Diabetes mellitus | 11/35 (31.4%) | 30/130 (23.1%) | 0.310 |
| Dyslipidemia | 17/35 (48.6%) | 65/130 (50.0%) | 0.881 |
| Atrial fibrillation | 13/35 (27.1%) | 58/130 (44.6%) | 0.428 |
| Active smoking | 4/35 (11.4%) | 16/130 (12.3%) | 0.888 |
| Heart failure | 6/35 (17.1%) | 19/130 (14.6%) | 0.711 |
| Coronary artery disease | 9/35 (25.7%) | 17/130 (13.1%) | 0.069 |
| Admission blood pressure (syst/diast) | 144.0 ± 28.7/74.3 ± 18.4 | 152.3 ± 22.7/83.5 ± 15.2 | 0.169/0.010 |
| 145 [124–165.5]/80 [60.5–84] (n = 123) | 150 [139–163]/82 [70–92] (n = 33) | ||
| Admission MAP | 97.5 ± 19.9 | 106.4 ± 15.5 | 0.028 |
| 100.3 [83.3–110.5] | 106.7 [96.7–116.7] | ||
| Antihypertensive baseline medication | |||
| Any antihypertensive medication | 23/35 (65.7%) | 87/130 (66.9%) | 0.893 |
| ACE‐inhibitor | 16/35 (45.7%) | 63/130 (48.5%) | 0.773 |
| Beta‐receptor blocker | 13/35 (37.1%) | 67/130 (51.5%) | 0.130 |
| Hydrochlorothiazide | 5/35 (14.3%) | 26/130 (20.0%) | 0.442 |
| Loop diuretics | 12/35 (34.3%) | 23/130 (17.7%) | 0.033 |
| Calcium antagonist | 5/35 (14.3%) | 28/130 (21.5%) | 0.341 |
| Aldosterone antagonist | 2/35 (5.7%) | 6/130 (4.6%) | 0.678 |
| Alpha‐receptor blocker | 0/35 (0.0%) | 5/130 (3.8%) | 0.585 |
| Stroke characteristics | |||
| Admission NIHSS | 15 (11–17) (n = 35) | 14 (8–18) (n = 128) | 0.197 |
| Location of occlusion | |||
| Internal carotid artery | 7/35 (20.0%) | 24/130 (18.5%) | 0.836 |
| Anterior cerebral artery | 1/35 (2.9%) | 4/130 (3.1%) | 0.946 |
| Middle cerebral artery (M1) | 18/35 (51.4%) | 62/130 (47.7%) | 0.695 |
| Middle cerebral artery (M2) | 10/35 (28.6%) | 35/130 (26.9%) | 0.846 |
| Posterior cerebral artery | 2/35 (5.7%) | 3/130 (2.3%) | 0.297 |
| Basilar artery | 5/35 (14.3%) | 16/130 (12.3%) | 0.755 |
| Vertebral artery | 1/35 (2.9%) | 1/130 (0.8%) | 0.380 |
| ASPECTS pre‐intervention | 10 (8–10) (n = 33) | 10 (9–10) (n = 128) | 0.168 |
| Treatment parameters | |||
| Intravenous thrombolysis | 15/35 (42.9%) | 73/130 (56.2%) | 0.162 |
| Admission to IVT (minutes) | 32 (16–55) (n = 6) | 27 (19–40) (n = 46) | 0.886 |
| Symptom onset to groin puncture (minutes) | 183 (130–321) (n = 26) | 192 (131–245) (n = 92) | 0.790 |
| Symptom onset to recanalization (minutes) | 248 (180–348) (n = 24) | 245 (189–318) (n = 87) | 0.753 |
| General anesthesia | 15/35 (42.9%) | 42/129 (32.6%) | 0.256 |
| Outcome parameters | |||
| Final infarct volume [mm3] | 13.7 (0.4–30.1) (n = 20) | 4.9 (0–20.2) (n = 71) | 0.297 |
| sNfL 3 days after MT [pg/mL] | 239.7 (168.4–303.4) (n = 13) | 118.8 (52.5–220.5) (n = 63) | 0.026 |
| Discharge mRS | 4 (3–5) (n = 35) | 2 (1–5) (n = 130) | 0.032 |
| Discharge NIHSS | 6 (2–14) (n = 30) | 2 (1–8) (n = 113) | 0.016 |
| 90‐day mRS | 4 (3–6) (n = 35) | 2 (0–5) (n = 130) | 0.002 |
| 90‐day mRS ≤ 2 | 7/35 (20.0%) | 70/130 (53.8%) | <0.001 |
| 90‐day MoCA | 18 (14–21) (n = 6) | 19 (15–21) (n = 65) | 0.909 |
| 90‐day EQ‐5D‐3L index | 0.45 ± 0.28 (n = 24) | 0.63 ± 0.31 (n = 106) | 0.009 |
Data is displayed as absolute number/number of available data (%) or mean ± SD or median (IQR) (number of observations available). Significant differences are marked bold.
ASPECTS, Alberta stroke program early CT score; BP, blood pressure; EQ‐5D‐3L, European quality of life index version 5D‐3L; MAP, mean arterial pressure; MT, mechanical thrombectomy; M1, M1 segment; M2, M2 segment; NIHSS, National Institutes of Health Stroke Scale; MoCA, Montreal Cognitive Assessment; mRS, modified Rankin Scale score; sNfL, serum neurofilament light chain.
Table 2.
Aggregated blood pressure parameters within 24 h after MT.
| Variable | BP ≤ 160/90 mmHg “low‐BP” (n = 35) | BP > 160/90 mmHg “high BP” (n = 130) | p‐value |
|---|---|---|---|
| Mean MAP | 80.6 ± 9.0 | 93.5 ± 11.1 | <0.001 |
| Mean systolic BP | 126.6 ± 16.1 | 132.1 ± 15.0 | 0.057 |
| Mean diastolic BP | 56.5 ± 11.1 | 73.0 ± 11.4 | <0.001 |
| Maximum MAP | 120.6 ± 49.1 | 137.0 ± 32.0 | 0.018 |
| Maximum systolic BP | 146.7 ± 12.1 | 168.5 ± 23.3 | <0.001 |
| Maximum diastolic BP | 76.1 ± 11.6 | 116.0 ± 20.7 | <0.001 |
| Minimum MAP | 59.3 ± 9.9 | 66.6 ± 14.2 | 0.005 |
| Minimum systolic BP | 92.5 ± 21.7 | 92.9 ± 22.3 | 0.925 |
| Minimum diastolic BP | 39.3 ± 10.4 | 48.4 ± 12.8 | <0.001 |
| MAP drops < 60 mmHg | 17 (48.6%) | 38 (29.2%) | 0.031 |
| Median duration of MAP < 60 mmHg (min) in patients with MAP drops | 60 [30–195] | 30 [30–60] | 0.072 |
Data are displayed as mean ± SD in mmHg. Except for MAP drops: absolute number (percentage) and Number of MAP drops: median (IQR). Significant differences are marked bold.
BP, blood pressure; MAP, mean arterial pressure.
Association of BP target groups and functional outcome parameters
Patients in low‐BP group had higher NIHSS at discharge (median NIHSS [IQR] 6 [2–14] vs. 2 [1–8], p = 0.016) and worse mRS‐based functional outcome (median mRS [IQR] 4 [3–5] vs. 2 [1–5], p = 0.032) compared to patients in high‐BP group. Worse functional outcome in low‐BP group persisted at three months follow‐up (median mRS [IQR] 4 [3–6] vs. 2 [0–5], p = 0.002). For details on mRS distribution, see also Fig. 2A. In multiple logistic regression analysis low‐BP group (adjusted Odds Ratio (aOR [95%CI]): 5.88 [1.88–18.32], p = 0.002), as well as higher NIHSS on admission (aOR [95%CI]: 1.15 [1.07–1.23], p < 0.001) and older age (aOR [95%CI]: 1.08 [1.04–1.12], p < 0.001) remained independent predictors for poor functional outcome (mRS > 2) three months after MT (R 2[Nagelkerke] = 0.44, p < 0.001, for details, see Table 3). We did not observe any difference with regard to cognitive performance as assessed by MoCA score three months after MT between the two different BP target groups with cognitive assessment being available in a subset of only n = 71 patients (see also Table 1). Health‐related quality of life at 90‐day follow‐up as measured by EQ‐5D‐index was worse in patients of low‐BP group (EQ‐5D‐3L index ± SD: 0.45 ± 0.28 vs. 0.63 ± 0.31, p = 0.009). This finding persisted in multiple linear regression analysis where low‐BP group was an independent predictor of lower EQ‐5D index (β = −0.138, 95%CI: −0.263−(−)0.012, p = 0.032). Further predictors of health‐related quality of life were older age (β = −0.008, 95%CI −0.012−(−)0.004, p < 0.001) and higher NIHSS on admission (β = −0.014, 95%CI −0.022−(−)0.006, p < 0.001) (R 2(corrected) = 0.25, p < 0.001, for details, see Table 3).
Figure 2.
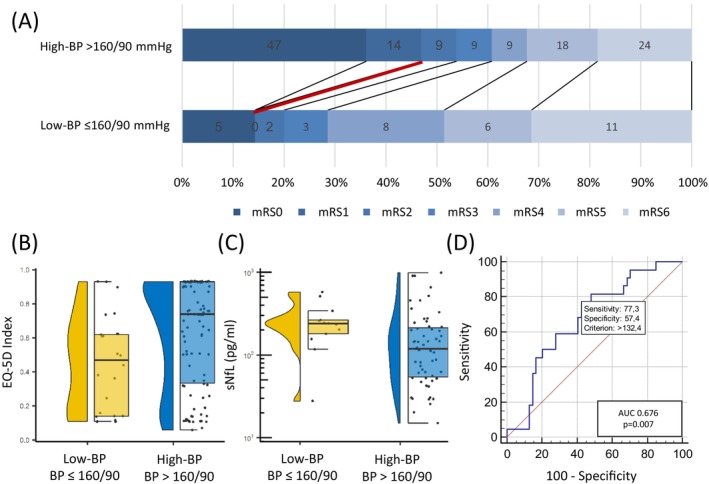
low‐BP is associated with unfavorable functional outcome and increased sNfL level. (A) Patients achieving BP target ≤ 160/90 mmHg have worse functional outcome at 90‐day follow‐up (low‐BP ≤ 160/90 mmHg: median [IQR] mRS: 4 [3–6] vs. high BP > 190/90 mmHg: median [IQR] mRS: 2 [0–5], p = 0.002). (B) low‐BP is associated with worse health‐related quality of life as assessed by EQ‐5D index value 90 days after MT (BP ≤ 160/90 mmHg: mean EQ‐5D index value: 0.045 ± 0.28 vs. high BP > 160/90 mmHg mean EQ‐5D index value: 0.63 ± 0.31, p = 0.009). (C) low‐BP is associated with increased sNfL level (BP ≤ 160/90 mmHg median [IQR] sNfL: 239.7 pg/mL [168.4–303.4] vs. high BP > 160/90 mmHg median [IQR] sNfL: 118.8 pg/mL [52.5–220.5], p = 0.026). (D) Receiver operating characteristic (ROC) curve for identification of patients with MAP drops < 60 mmHg by sNfL levels revealed an Area under the ROC(AUC) of 0.68 (95%CI: 0.56–0.78. p = 0.007). The optimal cut‐off for discrimination of patients with MAP drops below 60 mmHg by sNfL was sNfL > 132.4 pg/mL. BP, blood pressure; EQ‐5D, European quality of life index version 5D; sNfL, serum neurofilament light chain.
Table 3.
Multiple regression analyses of independent predictors of functional outcome at 90‐day follow‐up and sNfL level 3 days after mechanical thrombectomy.
| Variable | Adjusted Odds Ratio (aOR)/beta‐coefficient (β) | 95%CI | p‐value |
|---|---|---|---|
| Multiple logistic regression analysis of independent predictors of unfavorable functional outcome (mRS > 2) at 90‐day follow‐up | |||
| Age | aOR = 1.08 | 1.04–1.12 | <0.001 |
| Admission NIHSS | aOR = 1.15 | 1.07–1.23 | <0.001 |
| CAD | aOR = 1.24 | 0.40–3.83 | 0.713 |
| Admission MAP | aOR = 1.00 | 0.97–1.02 | 0.783 |
| BP target ≤ 160/90 mmHg achieved | aOR = 5.88 | 1.88–18.32 | 0.002 |
| Multiple linear regression analysis of independent predictors of health‐related quality of life (EQ‐5D‐3L) at 90‐day follow‐up | |||
| Age | β = −0.008 | −0.012−(−)0.004 | <0.001 |
| Admission NIHSS | β = −0.014 | −0.022−(−)0.006 | <0.001 |
| CAD | β = −0.060 | −0.201−0.080 | 0.480 |
| Admission MAP | β = 0.001 | −0.002−0.004 | 0.409 |
| BP target ≤ 160/90 mmHg achieved | β = −0.138 | −0.263−(−)0.012 | 0.032 |
| Multiple linear regression analysis of independent predictors of log sNfL 3 days after MT | |||
| Age | β = 0.010 | 0.003–0.018 | 0.009 |
| Admission NIHSS | β = 0.004 | −0.012−0.020 | 0.604 |
| Admission MAP | β = 0.005 | −0.001−0.011 | 0.105 |
| Infarct volume | β = 0.004 | 0.003–0.006 | <0.001 |
| MAP max. 24 h post‐MT | β = 0.000 | −0.003−0.002 | 0.980 |
| MAP min 24 h post‐MT | β = −0.010 | −0.017−(−)0.003 | 0.007 |
| BP target ≤ 160/90 mmHg achieved | β = 0.106 | −0.144−0.357 | 0.399 |
Model parameters: mRS > 2 regression model: R 2(Nagelkerke) = 0.44, p < 0.001. EQ‐5D regression model: R 2(corrected) = 0.25, p < 0.001. sNfL regression model: R 2(corrected) = 0.42, p < 0.001. Significant predictors are marked bold.
BP, blood pressure; CAD, coronary artery disease; MAP, mean arterial pressure; NIHSS, National Institutes of Health Stroke Scale; 24 h post‐MT, first day following mechanical thrombectomy.
Association of BP parameters, sNfL and functional outcome
sNfL collected 3 days after MT was increased in patients of low‐BP group compared to patients in high‐BP group (median [IQR]: 239.7 pg/mL [168.4–303.4] vs. 118.8 pg/mL [52.5–220.5], p = 0.026, Fig. 2C). In multiple linear regression analysis, predictors of increased sNfL levels at day three after MT were older age (β = 0.010, 95%CI: 0.003–0.018, p = 0.009), increased infarct volume (β = 0.004, 95%CI 0.003–0.006, p < 0.001) and lower minimum MAP within 24 h after MT (β = −0.010, 95%CI: −0.017−(−)0.003, p = 0.007, R 2(corrected) = 0.42, p < 0.001, for details, see Table 3).
Correlation analyses showed significant correlation of sNfL with minimum MAP values (r 2 = −0.32, p = 0.005). In line with this, minimal MAP during first 24 h after thrombectomy predicts poor functional outcome with an AUC of 0.601 (95%CI: 0.522–0.677; p = 0.022, Fig. S1A). We also report significant correlation of sNfL with minimum diastolic BP values (r 2 = −0.41, p < 0.001). Accordingly, minimal, maximal and mean diastolic BP values yielded significant AUC for prediction of poor outcome (Fig. S1G–I). sNfL levels showed a trend towards correlation with duration of hypotensive episodes (MAP drops < 60 mmHg) (r 2 = 0.20, p = 0.083). In AUC analyses, longer duration of hypotensive episodes during first 24 h after thrombectomy predicted poor functional outcome with an AUC of 0.590 (95% CI: 0.511–0.666; p = 0.013, Fig. S2). No correlation could be observed between sNfL values and maximum systolic BP (r 2 = 0.13, p = 0.278), minimum systolic BP (r 2 = −0.11, p = 0.336), maximum diastolic BP (r 2 = −0.16, p = 0.178) and maximum MAP (r 2 = 0.01, p = 0.914). Also admission BP was not significantly correlated with sNfL levels (r 2 = −0.09, p = 0.489). For details, see Fig. 3.
Figure 3.
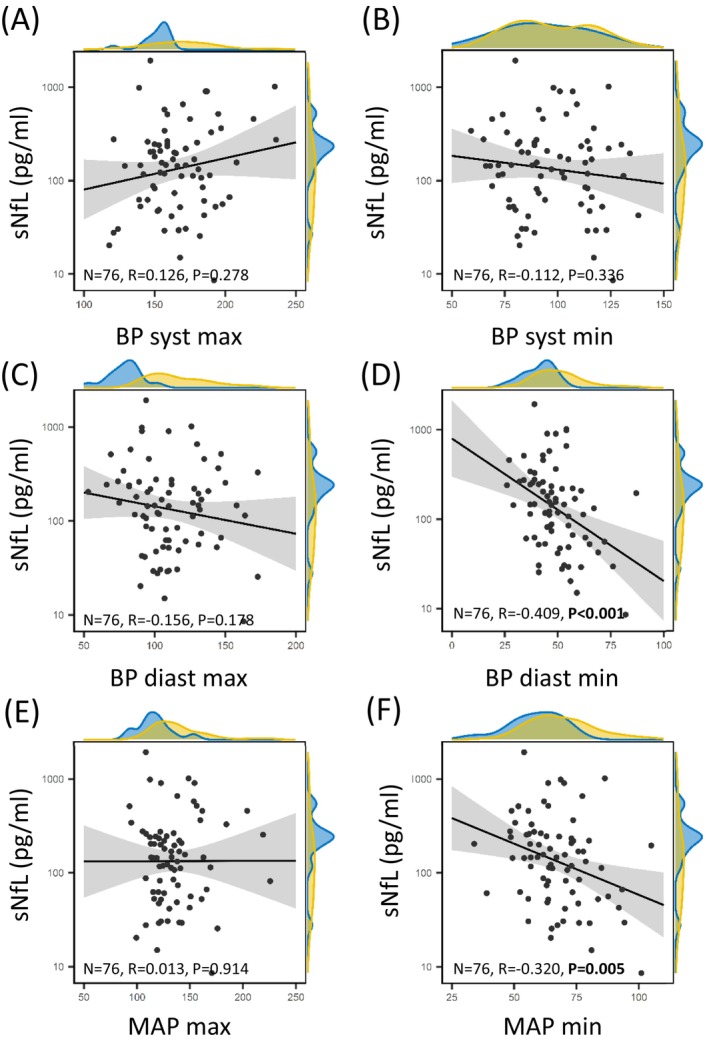
Increased sNfL level inversely correlate with minimal MAP and minimal diastolic BP. Scatter‐Plot displaying the correlation between log sNfL and BP parameters. A density plot of sNfL and BP parameters is displayed at the margins highlighting “low‐BP” patients strictly achieving BP target ≤ 160/90 mmHg in blue and “high‐BP” patients (>160/90 mmHg) in yellow. BP, blood pressure; diast, diastolic; MAP, mean arterial pressure; max, maximum; min, minimum; sNfL, serum neurofilament light chain; syst, systolic.
Resulting from AUC analysis, patients who had experienced hypotensive episodes by means of MAP drops < 60 mmHg within 24 h following MT could be identified by sNfL collected 3 days following MT with high discriminative ability (AUC [95%CI] = 0.68 [0.56–0.78], p = 0.007, Fig. 2D). Youden‐Index calculation suggested a sNfL cut‐off ≥132.4 pg/mL for optimal discrimination of patients with MAP drops below 60 mmHg, with a resulting sensitivity of 77.3% (95%CI: 54.6–92.2) and a specificity of 57.4% (95%CI: 43.2–70.8).
Discussion
Within this study, we provide evidence that strict BP control of ≤160/90 mmHg within the first 24 h after successful recanalization of LVO by MT is associated with poor functional outcome, potentially attributable to hypotensive neuronal loss. We furthermore report that a lower minimum MAP post‐thrombectomy is independently associated with increased sNfL levels, potentially displaying increased neuronal injury in these patients. Notably, these findings were independent of age and stroke severity, displayed by NIHSS on admission and infarct volume in follow‐up CT imaging. Additionally, we report that sNfL level could well identify patients who had experienced hypotensive episodes by means of MAP < 60 mmHg, supporting the hypothesis of hypotension‐induced neuronal damage following recanalization of major ischemic stroke by MT.
Current guidelines recommend post‐procedural BP targets of <180/105 mmHg for the first 24 h following successful recanalization of LVO by MT. The objective to avoid excessively increased BP following MT is supported by a recently conducted meta‐analysis of observational studies. In this, Katsanos et al., report worse functional outcome, higher mortality and an increased symptomatic intracranial hemorrhage rate with higher MAP following MT. 5 At the same time, effects and safety of intensified post‐recanalization BP targets on functional outcome are unclear and under intense evaluation in randomized controlled trials. Up to date, controlled trials in patients treated with intravenous thrombolysis (IVT) have failed to demonstrate superiority of intensified BP lowering. 13 However, only a minority of these trial cohorts were treated by MT (e.g., less than 2% of the ENCHANTED cohort investigating intensive blood pressure reduction with IVT therapy for acute ischemic stroke). Intensified BP targets in patients treated with MT are being investigated in ongoing randomized controlled trials, of which the first one to be recently completed comparing systolic BP target groups of <130 mmHg versus <185 mmHg also could not delineate a difference in clinical or imaging endpoints. 14 As yet, the authors of the BP TARGET study report that intensified blood pressure lowering intervention resulted in a tendency of more frequent hypotensive events in the intensive systolic BP target group. Furthermore, the ENCHANTED2 randomized controlled trial, investigating intensified BP targets following successful reperfusion by MT with a target systolic BP of less than 120 mmHg in the intensified treatment group, has recently even reported worse functional outcome in the more intensive treatment group. 15 In conclusion, targeting systolic BP as low as <120 mmHg should be avoided after MT of LVO.
Hypotensive episodes and BP drops have already been linked to larger infarct volume and worse functional outcome in observational studies investigating peri‐interventional BP levels. 9 Especially peri‐interventional MAP drops < 60 mmHg have been shown to be strongly associated with unfavorable outcome 16 and also within the early post‐MT phase, lower minimum BP has been reported to be associated with worse outcomes. 17 We expand these findings and hypothesize, that strictly meeting BP targets during the first 24 h following MT, might contribute to hypotension‐associated neuronal damage following major ischemic stroke. Consequences of hypotensive episodes on infarct volume and functional outcome in the context of post‐procedural BP targets are widely understudied. Blood based biomarkers might contribute to further understanding of pathophysiology and mechanisms behind hypo‐ and hyperperfusion related effects. sNfL has been identified as a marker reflecting neuroaxonal damage resulting from stroke, potentially serving as a biomarker in stroke work up. 18 Up to date, higher sNfL levels have been shown to be associated with both increased infarct volume 10 and lower rates of functional independence 90 days after ischemic stroke. 12 As a marker of neuronal injury, sNfL might potentially serve to quantify differences in neuronal damage associated with varying BP regimens. Our finding of increased sNfL levels in the patients strictly meeting low‐BP targets may depict higher neuronal damage in these patients as a potential mediator of the observed association of strictly achieving low‐BP targets of ≤160/90 mmHg with worse functional outcome. This is also supported by the fact that in our multiple linear regression analysis, sNfL level was independently associated with lower minimum MAP following MT, notably also adjusted for infarct volume. Also the observation of a significant correlation between sNfL with minimum MAP in the context of an absent correlation with maximum MAP, fosters our hypothesis of hypotension‐induced neuronal damage following successful recanalization. Vice versa, patients in our cohort who had experienced hypotensive episodes within 24 h following MT could be identified by sNfL collected 3 days following MT with high discriminative ability.
Current standard of care following MT prevents prolonged excessively elevated blood pressure following successful recanalization. This is also displayed within our data, since mean systolic BP did not significantly differ between both BP level groups. This might well address the intent to decrease bleeding complications and reperfusion injury. However, the optimal extent of BP reduction has yet to be investigated. The optimal BP target might even depend on further individualized patient characteristics, such as pre‐existing arterial hypertension and pre‐stroke BP control, collateral vessel status, application of bridging IVT and further factors yet to be determined. Furthermore, hypoperfusion‐related effects may also depend on specific infarct location, which might not be captured by crude infarct volume and should be subject to further investigation. Eventually, BP targets might not be applicable to every MT patient in the same way and strictly achieving a systolic BP target of ≤160 mmHg might result in insufficient perfusion of parenchymal areas affected by ischemia, thereby promoting neuronal damage. Future studies will have to further investigate patient and stroke characteristics that might moderate the effects of post‐thrombectomy BP on clinical outcome in order to allow for individualizing BP targets and identify patients that might benefit from higher BP targets as are generally recommended. Especially in the context of patient‐individualized approaches of post‐thrombectomy BP management, further integration of sNfL as a real‐time biomarker in clinical prospective studies might help to better understand hypo‐ and hyperperfusion related mechanisms contributing to maximize patient benefit by definition of individualized BP targets following MT.
We are aware of the need for further prospective studies to investigate and confirm our observations that were made in a retrospective analysis of prospectively collected data. Therefore, due to the nature of our dataset, validity and generalizability of our findings are limited. Also, we analyzed observational data resulting from routine care, which depicts achieved BP targets in a non‐interventional, non‐randomized setting. In the same way, we cannot depict and control for further patient‐individual factors, which might have contributed to treatment decisions with regard to BP management following MT. We are also limited with regard to the availability of additional imaging characteristics, such as infarct growth, which may help to further differentiate BP‐associated effects on biomarkers and functional outcome and should be integrated in further studies.
At the same time, our analysis is based on an extensive dataset of patient‐individual post‐procedural BP data, enabling to investigate associations of BP parameters and long‐term functional outcome. We increased transferability by analyzing only patients with successful recanalization, a cohort with distinct characteristics and needs as opposed to the heterogenous MT cohorts in clinical trials. Also, we regard our approach of comparing groups depending on actually achieved BP targets especially valuable with regard to mechanistic analyses. This is due to the fact that in randomized interventional clinical trials, the time a patient actually achieves the BP target range is far from complete but, as for example, recently reported for the BP target trial, less than 70% of the study period. 14 With further integration of sNfL, we were able to address a pathophysiologic perspective of BP management following MT. This potentially contributes to improved mechanistic explanation of the complexity of optimal BP management following MT, which cannot be depicted by currently ongoing randomized clinical trials applying a simplified intervention strategy in a “one size fits all approach”.
To conclude, neuronal survival in stroke patients seems to be dependent on a fine‐tuned balance of factors influencing neuronal activity. 8 , 9 , 19 Our findings suggest that strict adherence to BP targets ≤160/90 mmHg within the first 24 h following successful recanalization of LVO by MT is associated with worse functional outcome. We suggest that this might be attributable to hypotension‐induced neuronal loss, as is suggested by increased sNfL levels in these patients and the observed independent association of sNfL with minimum MAP values. Further prospective studies are needed to better understand the complex mechanisms behind optimal patient‐individual BP management following successful recanalization of LVO by MT.
Author Contributions
KG and TU involved in conception of the study. EH and TU involved in the acquisition of data. EH, MH, and TU involved in analysis and interpretation of data. EH, FS, MH, and TU involved in drafting a significant proportion of the manuscript or figures. AO, FZ, KG, LB, MH, MP, SB, SG, and TU involved in critical revision of the manuscript and figures.
Conflict of Interest
FZ reports Research grants and/or consultation funds from Biogen, Max Planck Society (MPG), Ministry of Education and Research (BMBF), Bristol‐Meyers‐Squibb, Celgene, German Research Foundation (DFG), Hexal, Horizon, Janssen, Merck Serono, Novartis, Progressive MS Alliance (PMSA), Roche, Sanofi Genzyme, Sandoz. KG reports personal fees and non‐financial support from Bayer AG, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo and Pfizer, outside the submitted work. MH reports personal fees from Bristol‐Myers Squibb, outside the submitted work. SB reports personal fees from Biogen Idec, Bristol Meyer Squibbs, Merck Healthcare, Novartis, Roche, Sanofi Genzyme and TEVA, outside the submitted work. TU reports personal fees from Merck Serono and Pfizer, outside the submitted work. AO, EH, FS, LB, MP and SG report no disclosures.
Funding Information
No funding information provided.
Supporting information
Supplemental Figure S1.
Acknowledgements
This work was supported by the Else Kröner‐Fresenius‐Foundation (EKFS) grant 2022_EKCS.10 to TU. and grant SFB‐TR‐128 to TU. Open Access funding enabled and organized by Projekt DEAL.
Funding Statement
This work was funded by Else Kröner‐Fresenius Stiftung grant SFB‐TR‐128.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344‐e418. [DOI] [PubMed] [Google Scholar]
- 2. Novak V, Chowdhary A, Farrar B, et al. Altered cerebral vasoregulation in hypertension and stroke. Neurology. 2003;60:1657‐1663. [DOI] [PubMed] [Google Scholar]
- 3. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021;6:XLVIII‐LXXXIX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ringleb P. S2e Leitlinie zur Akuttherapie des ischämischen Schlaganfalls: Kurzfassung. AWMF‐Registernummer 030‐046, 2021.
- 5. Katsanos AH, Malhotra K, Ahmed N, et al. Blood pressure after endovascular thrombectomy and outcomes in patients with acute ischemic stroke: an individual patient data meta‐analysis. Neurology. 2022;98:e291‐e301. [DOI] [PubMed] [Google Scholar]
- 6. Maïer B, Gory B, Chabanne R, et al. Effect of an individualized versus standard blood pressure management during mechanical thrombectomy for anterior ischemic stroke: the DETERMINE randomized controlled trial. Trials. 2022;23:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martins AI, Sargento‐Freitas J, Silva F, et al. Recanalization modulates association between blood pressure and functional outcome in acute ischemic stroke. Stroke. 2016;47:1571‐1576. [DOI] [PubMed] [Google Scholar]
- 8. Nepal G, Shrestha GS, Shing YK, Muha A, Bhagat R. Systolic blood pressure variability following endovascular thrombectomy and clinical outcome in acute ischemic stroke: a meta‐analysis. Acta Neurol Scand. 2021;144:343‐354. [DOI] [PubMed] [Google Scholar]
- 9. Petersen NH, Ortega‐Gutierrez S, Wang A, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke. 2019;50:1797‐1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onatsu J, Vanninen R, Jäkälä P, et al. Serum neurofilament light chain concentration correlates with infarct volume but not prognosis in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:2242‐2249. [DOI] [PubMed] [Google Scholar]
- 11. Greiner W, Claes C, Busschbach JJV, Graf von der Schulenburg JM. Validating the EQ‐5D with time trade off for the German population. Eur J Health Econ. 2005;6:124‐130. [DOI] [PubMed] [Google Scholar]
- 12. Uphaus T, Bittner S, Gröschel S, et al. NfL (neurofilament light chain) levels as a predictive marker for Long‐term outcome after ischemic stroke. Stroke. 2019;50:3077‐3084. [DOI] [PubMed] [Google Scholar]
- 13. Anderson CS, Huang Y, Lindley RI, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open‐label, blinded‐endpoint, phase 3 trial. Lancet (London, England). 2019;393:877‐888. [DOI] [PubMed] [Google Scholar]
- 14. Mazighi M, Richard S, Lapergue B, et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP‐TARGET): a multicentre, open‐label, randomised controlled trial. Lancet Neurol. 2021;20:265‐274. [DOI] [PubMed] [Google Scholar]
- 15. Yang P, Song L, Zhang Y, et al. Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open‐label, blinded‐endpoint, randomised controlled trial. Lancet (London, England). 2022;400:1585‐1596. [DOI] [PubMed] [Google Scholar]
- 16. Fandler‐Höfler S, Heschl S, Argüelles‐Delgado P, et al. Single mean arterial blood pressure drops during stroke thrombectomy under general anaesthesia are associated with poor outcome. J Neurol. 2020;267:1331‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samuels N, van de Graaf RA, van den Berg CAL, et al. Blood pressure in the first 6 hours following endovascular treatment for ischemic stroke is associated with outcome. Stroke. 2021;52:3514‐3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchez JD, Martirosian RA, Mun KT, et al. Temporal patterning of neurofilament light as a blood‐based biomarker for stroke: a systematic review and meta‐analysis. Front Neurol. 2022;13:841898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bitar L, Uphaus T, Thalman C, et al. Inhibition of the enzyme autotaxin reduces cortical excitability and ameliorates the outcome in stroke. Sci Transl Med. 2022;14:eabk0135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1.


