Summary
Quiescent cells require a continuous supply of proteins to maintain protein homeostasis. In fission yeast, entry into quiescence is triggered by nitrogen stress, leading to the inactivation of TORC1 and the activation of TORC2. Here, we report that the Greatwall-Endosulfine-PPA/B55 pathway connects the downregulation of TORC1 with the upregulation of TORC2, resulting in the activation of Elongator-dependent tRNA modifications essential for sustaining the translation programme during entry into quiescence. This process promotes U34 and A37 tRNA modifications at the anticodon stem loop, enhancing translation efficiency and fidelity of mRNAs enriched for AAA versus AAG lysine codons. Notably, some of these mRNAs encode inhibitors of TORC1, activators of TORC2, tRNA modifiers, and proteins necessary for telomeric and subtelomeric functions. Therefore, we propose a novel mechanism by which cells respond to nitrogen stress at the level of translation, involving a coordinated interplay between the tRNA epitranscriptome and biased codon usage.
Keywords: Quiescence, Nitrogen starvation, TORC1, TORC2, Greatwall, Endosulfine, PP2A/B55, tRNA modifications, Elongator, translation
Introduction
Most cells in living organisms rest in a non-dividing state called the G0 phase, also known as quiescence. Quiescent cells can re-enter the cell cycle with full viability when provided with the appropriate signals. Examples of quiescent cells include stem cells, neuronal progenitor cells, memory T cells, eggs, and spores. Despite their importance, the molecular mechanisms governing quiescence entry, its maintenance, and exit are not yet fully understood.
In nature, unicellular organisms continuously enter and exit quiescence depending on nutrient availability. In fission yeast, entry into quiescence is induced by nitrogen starvation, resulting in the inactivation of TOR complex 1 (TORC1) and the activation of TOR complex 2 (TORC2). TORC1 promotes cell growth in response to nutrients, growth factors, or cellular energy 1,2, while TORC2 is required for the nutrient stress response, cell survival during quiescence, and cell differentiation 3–5. The activation of the Greatwall-Endosulfine switch upon TORC1 inactivation leads to inhibition of the PP2A/B55 protein phosphatase, which is necessary for switching on TORC2 activity by increasing Gad8 phosphorylation 4–7.
TORC2 activation also regulates protein translation by controlling tRNA modifications through the Elongator complex 8. Elongator is a multiprotein complex that modifies the anticodon stem loop of tRNALysUUU, tRNAGluUUC and tRNAGlnUUG by introducing an acetyl group at position 5 of U34 (cm5U34), which is further modified by Trm112-Trm9, a methyltransferase complex involved in the formation of mcm5U34, and by Ctu1-Ctu2 complex, which catalyses the thiolation at carbon 2 of U34 (mcm5s2U34). These U34 modifications counteract codon misreading resulting from low effective stacking interactions between A-U bases 9–11. They also play a crucial role in maintaining translational fidelity under stress conditions 12–15. Thus, Elongator is necessary for the efficient translation of mRNAs with a high AAA codon usage.
Previous studies have reported a feedback loop between the TORC1-TORC2 signalling cascade and the Elongator complex. In this loop, Elongator plays an essential role in the translation of key components of TORC2 and repressors of TORC1. Additionally, the TORC2 pathway functions as an activator of Elongator by down-regulating Gsk3, a glycogen synthase kinase that inhibits Elongator by phosphorylating the Elp4 subunit at Serine114 8,16,17.
In this study, we report that elevated PP2A/B55 phosphatase activity, resulting from the deletion of Endosulfine (igo1Δ), impairs the translation efficiency of mRNAs enriched in AAA codons during entry into quiescence. Additionally, we demonstrate a physical and functional interaction between PP2A/B55 and Gad8, Trm112, Ctu1, and the Elongator complex. Furthermore, hyperactivation of PP2A/B55 protein phosphatase reduces the function of the Elongator complex and the amount of Trm112, Ctu1 and Cgi121 proteins, which are essential for U34 and A37 tRNA modifications at the anticodon stem loop. This reduction in translational efficiency leads to decreased protein levels from transcripts containing high AAA codon usage, such as rap1, sgo2, clr2, or clr3, all of which are crucial for telomeric and subtelomeric organisation. This induces telomeric detachment, upregulation of subtelomeric gene expression, and eventually, cell death. Our work suggests that the Greatwall-Endosulfine-PP2A/B55 pathway governs the translational programme during entry into quiescence through the control of U34 and A37 tRNA modifications. We propose that the implementation of an alternative gene expression programme in response to nitrogen starvation is based on translation of mRNAs enriched in sub-optimal AAA codons by activation of tRNA-modifying complexes.
Results
The Greatwall-Endosulfine switch regulates telomere silencing and telomere attachment to the nuclear envelope
To understand the function of the Greatwall-Endosulfine-PP2A/B55 pathway during entry into quiescence we compared the transcriptome of the wild-type (WT) and the Endosulfine mutant (igo1Δ) by RNAseq after shifting cells from nitrogen-rich (EMM2) to nitrogen-free (EMM2-N) media at times 0 and 4 hours. In nitrogen-rich medium (EMM2, time 0) the transcriptome was almost identical between the two strains. However, after 4 hours of nitrogen starvation, we found significant changes in subtelomeric gene expression, as igo1Δ cells showed a high expression level (more than 10-fold) of a group of subtelomeric genes in chromosomes I and II compared to WT cells (Fig. 1a; Supplementary Table 1). Similar results were obtained when we analysed the transcriptome of the Greatwall (ppk18Δ cek1Δ) mutant (Supplementary Fig. 1a; Supplementary Table 2), consistent with the fact that the Greatwall-Endosulfine-PP2A/B55 is a linear pathway 4. These results suggest that downregulation of PP2A/B55 plays a key role in transcriptional silencing of subtelomeric genes during quiescence entry.
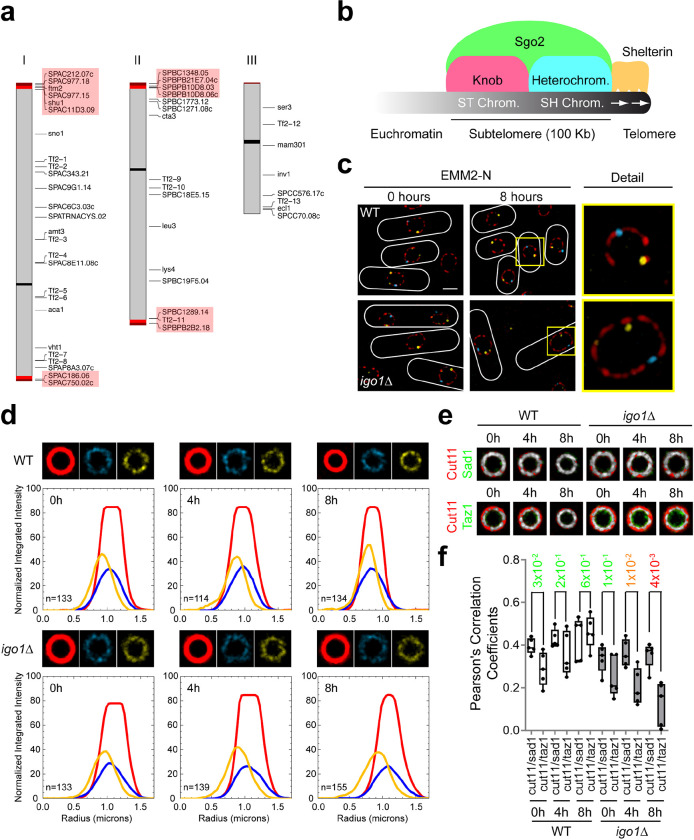
Fig. 1. The Greatwall-Endosulfine switch regulates subtelomeric gene silencing and telomeric anchoring to the nuclear envelope.
a Schematic representation of transcriptionally upregulated genes in the Endosulfine (igo1Δ) mutant. Genes overexpressed more than 10-fold in igo1Δ cells compared to the wild-type after 4 hours in nitrogen-free EMM2 medium. Subtelomeric genes are highlighted in red. b Schematic illustration of S. pombe subtelomeric chromatin structure (modified from 84). c Representative Super-Resolution Radial Fluctuations (SRRF) micrographs of wild-type (WT) and igo1Δ cells expressing Cut11:mCherry, Sad1:CFP and Taz1:YFP in nitrogen-rich EMM2 media (0 hours) and after 8 hours of nitrogen starvation in EMM2-N. The merged image and a detail view are shown. Bar: 2 μm. d Radial Profile Analysis for WT and igo1Δ cells after 0, 4 or 8 hours of nitrogen deprivation (see details in Supplementary Fig. 1b). The average projection signals for the NE (in red), the SPB (in cyan) and the telomeres (in yellow) are shown. The graphs represent the normalized integrated intensity as a function of distance in microns. The red lines correspond to the NE signal, the cyan lines correspond to the SPB signal and the yellow lines correspond to the telomeric signal. Over 100 nuclei were analysed at each time point. e Overlay between the average projection signals for Cut11/Sad1 or Cut11/Taz1 in WT and igo1Δ cells during entry into quiescence. The images were generated by projecting at least 100 nuclei. f Co-localization between Cut11/Sad1 and Cut11/Taz1 signals was quantified as Pearson correlation coefficients using ImageJ software. Student’s t-test p-values are indicated, significant differences are in orange or red.
The ends of chromosomes I and II are composed of telomeric repeats and the subtelomeric regions. While the telomeric repeats extend approximately 300 bp, the subtelomeric regions consist of about 100 kilobases between the telomeric repeats and the euchromatin (Fig. 1b). The heterochromatin present in the subtelomeric regions can be divided into SH chromatin, characterised by highly methylated histone H3K9, and ST chromatin, in which histone modifications are kept at low levels, but exhibit highly condensed chromosome structures called knobs 18–22. Several protein complexes essential for maintaining the telomeric and subtelomeric structure have been identified. For example, Rap1 (a component of the shelterin complex) and Bqt4 (a component of the bouquet complex) create a molecular link between telomeres and the nuclear envelope 23–26. Proteins such as Swi6, the SHREC complex or the CLRC complex play a role in H3K9 methylation, control nucleosome maintenance and genome stability 27,28. Finally, shugoshin 2, Sgo2, is an essential protein for condensation of ST chromatin and knob stability 21,22 (Fig. 1b).
To study the role of the Greatwall-Endosulfine-PP2A/B55 pathway in telomeric organisation during quiescence, we analysed nuclear-telomeric attachment in the wild-type (WT) and in the Endosulfine (igo1Δ) mutant in nitrogen-rich media (EMM2) and after 8 hours of nitrogen starvation (EMM2-N) using Super-Resolution Radial Fluctuations (SRRF) microscopy. Wild-type and igo1Δ cells tagged with Cut11-mCherry (a nuclear envelope -NE- marker), Sad1-CFP (a spindle pole body -SPB- marker) and Taz1-YFP (a telomeric marker), showed no significant differences in nitrogen-rich media. In contrast, after 8 hours of nitrogen starvation, the igo1Δ mutant showed telomeric detachment from the NE (Fig. 1c). To analyse the defect of the igo1Δ mutant in more detail, we combined SRRF microscopy with Radial Profile Analysis (see details in Supplementary Fig. 1b and Methods). The wild-type and igo1Δ mutant showed a perfect overlap between the NE signal (red line) and the SPB signal (blue line). However, we detected differences in the telomeric signals (yellow line) between strains. While in the wild-type strain the three signals overlapped more with time (4 and 8 hours of nitrogen starvation), in the igo1Δ mutant, the telomeric signal separated from the NE and the SPB signals (Fig. 1d). A similar result was obtained when we analysed the overlap between the mean NE signal and the mean SPB signal or the mean NE signal and the mean telomeric signal at different time points (Fig. 1e). Pearsońs correlation coefficients allowed us to identify significant differences between Cut11/Sad1 and Cut11/Taz1 signals in the wild-type and the igo1Δ mutant (Fig. 1f). These results indicate that the interaction between the NE and telomeres is lost in the igo1Δ mutant.
Telomeric detachment is mediated by reduced levels of the Rap1 protein, a component of the shelterin complex
A high level of PP2A/B55 activity, caused by deleting igo1, delays entry into mitosis during vegetative growth when fission yeast cells are shifted from a nitrogen-rich to a nitrogen-poor medium, or during entry into quiescence 4. Therefore, it seemed possible that elevated PP2A/B55 activity caused telomeric detachment during entry into quiescence. In S. pombe, two different complexes have been described as essential for maintaining telomeric-NE attachment, the bouquet complex and the shelterin complex. Interestingly, two subunits of the shelterin complex, Rap1 and Ccq1, have been described as heavily phosphorylated proteins 25,26,29,30. Thus, we considered that the phosphorylation state of these proteins might be affected by the high PP2A/B55 phosphatase activity in the igo1Δ mutant, triggering telomeric detachment. However, we did not detect changes in the phosphorylation state of either Rap1 or Ccq1. Surprisingly, we detected a dramatic reduction in the amount of Rap1 protein levels in the igo1Δ mutant, while the Ccq1 levels remained constant during the experiment (Fig. 2a). To confirm this data and improve our temporal resolution, we repeated the experiment taking samples every 30 minutes during the first 2 hours and then after 4 hours of nitrogen starvation. Once more, we detected a very significant decrease in Rap1 protein levels after 2–4 hours of nitrogen deprivation in the igo1Δ mutant (Fig. 2b; Fig. 2c, left panel).
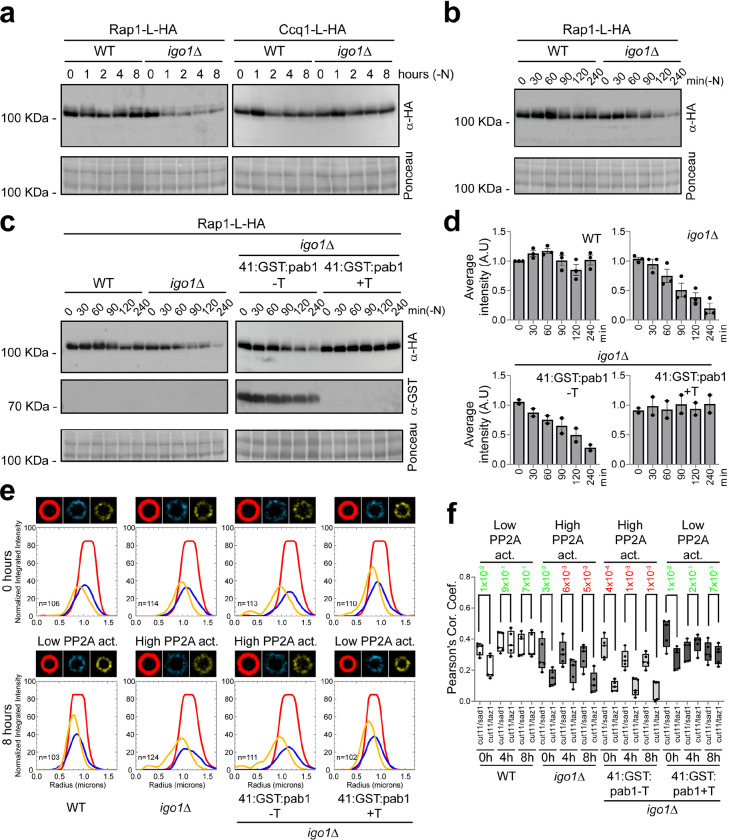
Fig. 2. Telomeric detachment from the nuclear envelope in igo1Δ cells is mediated by reduced Rap1 protein levels.
a Extracts from rap1:L:HA and ccq1:L:HA cells in a WT and igo1Δ background were collected at 0, 1, 2, 4 and 8 hours of nitrogen starvation. These extracts were analysed by SDS-PAGE and western blotting using anti-HA antibodies. Ponceau staining was used as the loading control. b Extracts from rap1:L:HA cells in a WT and igo1Δ background, collected every 30 minutes during the first 2 hours and then at 4 hours of nitrogen starvation. These extracts were analysed by SDS-PAGE and western blotting using anti-HA antibodies. Ponceau staining was used as the loading control. c Extracts from rap1:L:HA and rap1:L:HA Pnmt41x:GST:pab1 cells in a WT and igo1Δ background were collected during nitrogen starvation and analysed by SDS-PAGE and western blot using anti-HA and anti-GST antibodies. Strains were grown with or without thiamine (+T or −T) to repress or induce the pab1 gene, encoding the B55 regulatory subunit of PP2A. Ponceau staining was used as the loading control. d Immunoblot quantification of c was performed with Image Studio Lite software from at least two independent experiments. e Radial Profile Analysis of WT, igo1Δ and igo1Δ Pnmt41x:GST:pab1 cells bearing Cut11 (in red), Sad1 (in cyan) or Taz1 (in yellow) in EMM2 (0 hours) and after 8 hours of nitrogen starvation. The igo1Δ Pnmt41x:GST:pab1 cells were grown with or without thiamine (+T or −T) to repress or induce the expression of pab1. Over 100 nuclei were projected to generate the images and graphics. f Co-localization between Cut11/Sad1 and Cut11/Taz1 signals of e was quantified as Pearson correlation coefficients using ImageJ software. Student’s t-test p-values are indicated, significant differences are in red.
Previous results from our lab have shown that igo1Δ phenotypes could be restored by decreasing PP2A/B55 activity 31, including the reduction in viability during quiescence (Supplementary Fig. 2a). This prompted us to investigate whether a reduction of PP2A/B55 activity could restore Rap1 protein levels. To modulate PP2A/B55 activity, we placed the pab1 open reading frame, encoding the PP2A B55 regulatory subunit, under the control of the thiamine-repressible nmt41 promoter at its chromosomal locus. We found that repressing Pab1 production and therefore PP2A/B55 activity reinstated Rap1 protein levels (Fig. 2c). Data quantification further confirmed the restoration of Rap1 protein levels when PP2A/B55 activity was reduced (Fig. 2d).
To investigate whether reducing PP2A/B55 activity could also prevent telomeric detachment in vivo, we examined the localization of Taz1-YFP in strains exhibiting low PP2A/B55 activity (wild-type and igo1Δ nmt41:GST:pab1 + Thiamine) in comparison to strains with elevated PP2A/B55 activity (igo1Δ and igo1Δ nmt41:GST:pab1 − Thiamine) during nitrogen starvation. Our analysis showed that low PP2A/B55 activity restored the telomeric detachment phenotype, whereas high PP2A/B55 activity maintained the telomeric attachment defect (Fig. 2e; Supplementary Fig. 2b). Statistical analysis of the data confirmed that reduced PP2A/B55 activity during nitrogen starvation was necessary for preserving telomeric organisation during quiescence (Fig. 2f). In summary, these findings suggest that the downregulation of PP2A/B55 activity during entry into quiescence is crucial for maintaining Rap1 protein levels and for anchoring telomeres to the NE.
Sgo2, Clr2 and Clr3 protein levels are reduced in quiescent igo1Δ mutant cells
Different protein complexes coordinately maintain chromatin silencing in subtelomeric regions during quiescence in fission yeast. One of the critical factors for this regulation is shugoshin 2, Sgo2. Sgo2 is essential for the formation of knobs, highly condensed chromatin structures organised close to the ends of chromosomes I and II 21. Lack of Sgo2 (sgo2Δ) induces transcription of genes located at subtelomeric regions on chromosomes I and II 22, similar to what we observed in cells lacking Endosulfine (igo1Δ) or Greatwall (ppk18Δ cek1Δ) after nitrogen starvation (Fig. 1a; Supplementary Fig. 1a; Supplementary Tables 1, 2). This correlation prompted us to investigate whether Sgo2 levels might be altered in the igo1Δ mutant. Western-blot analysis revealed that igo1-deleted cells show a severe reduction of Sgo2 levels during entry into quiescence (Fig. 3a). As in the case of Rap1, reducing Pab1 levels in the igo1Δ mutant restored Sgo2 levels (Fig. 3b). To confirm the role of Igo1 in maintenance of Sgo2 levels and knob formation, we studied the localisation of Sgo2 during quiescence entry. Sgo2 protein was tagged with GFP and its localisation during nitrogen starvation was examined. As previously described, in the wild-type strain, Sgo2-GFP localised as nuclear dots in most cells, ranging from 1 to 3 dots per cell (Fig. 3c). In the igo1Δ mutant, we detected no significant differences with the wild-type in nitrogen-rich media (t=0 hours), only a slight decrease in dot size and brightness. However, when we shifted the cells to nitrogen-free media we observed a clear decrease in the number of dots per cell in the igo1Δ mutant (Fig. 3c). These data indicate that Igo1 is required for maintaining Sgo2 protein levels and for the formation of knobs, a structure essential for maintaining the transcriptional repression of subtelomeric genes.
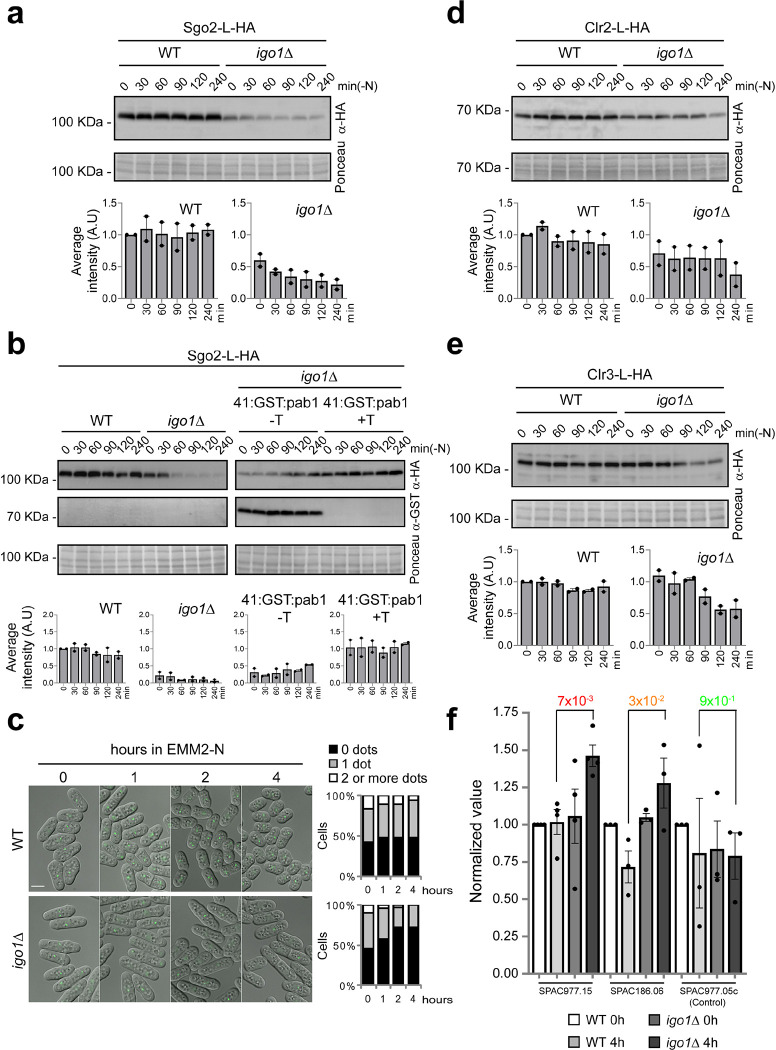
Fig. 3. Crucial proteins required for silencing subtelomeric gene expression are downregulated in igo1Δ cells.
a Extracts from sgo2:L:HA cells in a WT and igo1Δ backgrounds were collected every 30 minutes during the first 2 hours and then at 4 hours of nitrogen starvation. These extracts were analysed by SDS-PAGE and western blotting using anti-HA antibodies. Ponceau staining was used as the loading control. Immunoblot quantification was performed using Image Studio Lite software from at least two independent experiments. b Extracts from strains bearing sgo2:L:HA or sgo2:L:HA Pnmt41x:GST:pab1, were analysed by SDS-PAGE and western blotting with anti-HA and anti-GST antibodies. The igo1Δ Pnmt41x:GST:pab1 cells were grown with or without thiamine (+T or −T) to repress or induce the expression of pab1. Ponceau staining was used as the loading control. Immunoblot quantification was performed with Image Studio Lite software from at least two independent experiments. c Representative micrographs of WT or igo1Δ cells expressing sgo2:L:GFP during entry into quiescence. The overlay of fluorescence and DIC images is shown. Quantification was carried out using ImageJ software from two independent experiments involving more than 150 cells. Bar: 5 μm. d Similar to (a), clr2:L:HA protein was analysed in both WT and igo1Δ backgrounds. e Similar to (a), clr3:L:HA protein was analysed in both WT and igo1Δ backgrounds. f ChIP-qPCR was performed with anti-H3K14-acetyl antibodies and quantified with primer pairs at the indicated ORFs. WT and igo1Δ cells grown in nitrogen-rich media (EMM2) or after 4 hours of nitrogen starvation were analysed. The graphs represent normalized values, and error bars (SD) for all ChIP-qPCR experiments were calculated from biological triplicates.
Another key protein complex required for silencing subtelomeric regions is the heterochromatic repressor complex SHREC (Snf2-like/HDAC-containing repressor complex) 32,33, composed of Mit1, Clr1, Clr2, and Clr3. The SHREC complex plays regulatory roles in histone acetylation, as a chromatin remodeller and in the stability of subtelomeric nucleosomes 27,32,33. To determine if SHREC was also affected by PP2A activity, we examined Clr2 and Clr3 levels. In both cases, we detected a modest but reproducible decrease in protein levels during nitrogen starvation in the igo1Δ mutant (Fig. 3d,e). ChIP analysis showed that lack of Igo1 caused an increase in histone H3-K14 acetylation in the overexpressed subtelomeric genes SPCC977.15 and SPAC186.06 after 4 hours of nitrogen starvation consistent with loss of SHREC function (Fig. 3f). These results suggest that the Greatwall-Endosulfine-PP2A/B55 pathway modulates SH chromatin organisation and subtelomeric gene silencing.
PP2A/B55 regulates translation through its physical and functional interaction with protein complexes involved in tRNA modification
Our data indicate that the igo1Δ mutant exhibits reduced levels of proteins essential for maintaining telomeric and subtelomeric organisation. However, what is the molecular mechanism underlying these phenotypes? We explored three possibilities: reduced protein stability, reduced transcription, or reduced translation.
To examine protein stability, we treated cells with cycloheximide and monitored Rap1 protein levels over time. After treatment with cycloheximide, Rap1 was degraded with similar kinetics in wild-type and igo1Δ cells (Supplementary Fig. 3a). Similarly, rap1 mRNA levels were not reduced in igo1Δ cells; on the contrary, the rap1 gene exhibited higher transcript levels in the igo1Δ mutant compared to wild-type cells (Supplementary Fig. 3b).
Evidence of a translation defect in the igo1Δ mutant was obtained by mass-spectrometry analysis of proteins co-purifying with the PP2A/Pab1 protein phosphatase. Paa1, the structural subunit of the PP2A complex, was tagged with YFP and expressed from its endogenous promoter at its chromosomal locus. After one hour of nitrogen starvation, Paa1-YFP was pulled down and co-purifying proteins were analysed by mass-spectrometry. Our results revealed the presence of all components of PP2A protein complexes including Paa1, the catalytic subunits Ppa1, Ppa2, and Ppa3, and the regulatory subunits Pab1, Par1 and Par2 (Supplementary Table 3). Additionally, several PP2A regulators (Igo1, Zds1, Dis2, Ppe1 and Ekc1) and components of the PP2A SIP/STRIPAK complex 34 were also detected, confirming that the pull-down approach was successful.
The mass-spectrometry analysis also showed an over-representation of proteins related to ribosome structure, translation initiation, aminoacylation, and tRNA modification in the Paa1 interactome (Fig. 4a). We focused on Trm112, a widely conserved protein with a crucial role in translation. Specifically, Trm112 regulates methyltransferase enzymes (Trm9, Trm11, Mtq2 and Bud23) during ribosome biogenesis, tRNA modification and stop codon recognition 35,36. We confirmed an interaction between Trm112 and the PP2A-Pab1 complex by repeating the mass-spectrometry analysis using Pab1, the B55 regulatory subunit of the PP2A complex, as bait. Trm112 was pulled down as an interacting partner of PP2A/Pab1 (Supplementary Fig. 4a,b; Supplementary Table 4). The Trm112-Paa1 interaction was further validated by co-immunoprecipitation, showing a stronger association in nitrogen-depleted than in nitrogen-rich media (Fig. 4b). Interestingly, several subunits of the Elongator complex (Elp1, Elp2 and Elp3) were also pulled down as interacting partners of PP2A/Pab1 when Pab1 was slightly overexpressed from the nmt41 promoter (Supplementary Fig. 4b,c; Supplementary Table 5).
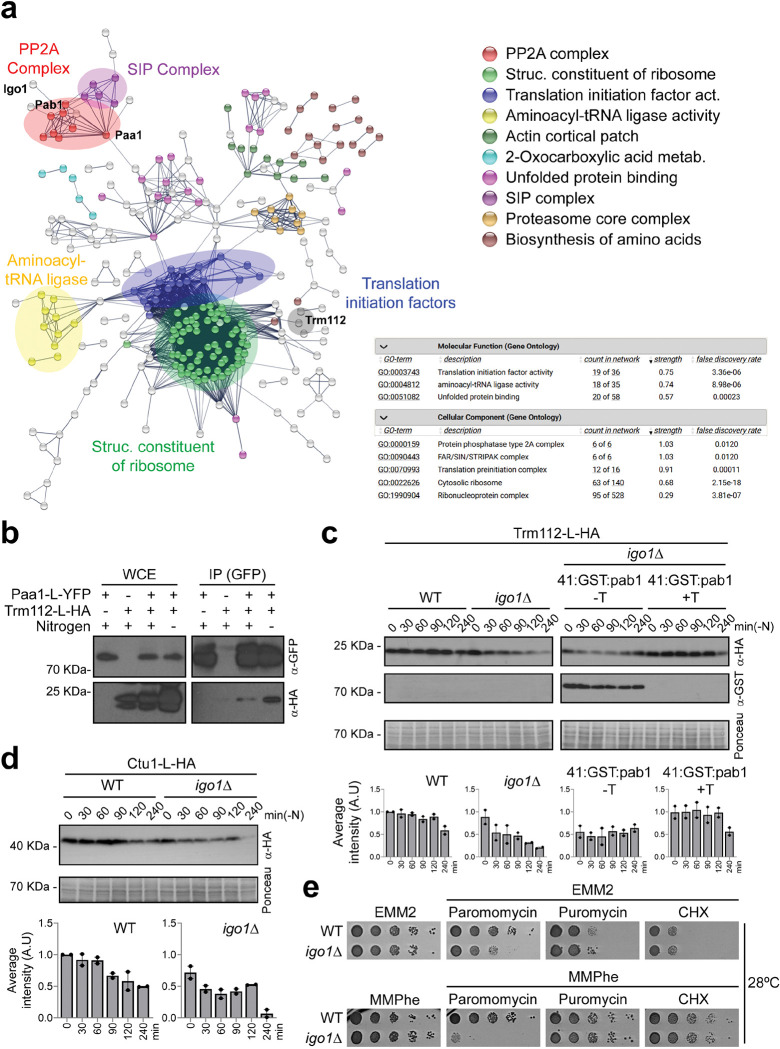
Fig. 4. PP2A interacts with proteins involved in tRNA modification.
a Interacting network resulting from mass-spectrometry analysis for paa1:L:YFP in EMM-N. Cellular processes or protein complexes with a significant enrichment are colour-coded. b Interaction between paa1:L:YFP and trm112:L:HA. Protein extracts from cells expressing paa1:L:YFP, trm112:L:HA or paa1:L:YFP trm112:L:HA were immunoprecipitated in nitrogen-rich or nitrogen-depleted media with anti-GFP beads and probed with anti-GFP and anti-HA antibodies. Extracts (WCE) were assayed for levels of paa1:L:YFP and trm112:L:HA by western blot. c Extracts from cells expressing trm112:L:HA or trm112:L:HA Pnmt41x:GST:pab1, were analysed by SDS-PAGE followed by immunoblotting with anti-HA and anti-GST antibodies. The igo1Δ Pnmt41x:GST:pab1 cells were grown in EMM2 with or without thiamine (+T or −T) to repress or induce the expression of pab1. Ponceau staining was used as the loading control. Immunoblot quantification was performed with Image Studio Lite software from at least two independent experiments. d Extracts from Ctu1:L:HA cells in a WT and igo1Δ backgrounds were collected every 30 minutes during the first 2 hours and then at 4 hours of nitrogen starvation. These extracts were analysed by SDS-PAGE and immunoblotting using anti-HA antibodies. Ponceau staining was used as the loading control. Immunoblot quantification was performed using Image Studio Lite software from at least two independent experiments. e Serial dilutions from WT and igo1Δ cultures were spotted onto EMM2 (Minimal Media containing NH4Cl) or MMPhe (Minimal Media containing Phenylalanine) plates without or with paromomycin (0.5 mg/ml), puromycin (0.5 mg/ml) or cycloheximide (CHX, 2.5 μg/ml).
The Elongator complex, along with Trm112/Trm9 and the Ctu1/Ctu2 complexes, plays a critical role in the formation of the 5-methoxycarbonylmethyl (mcm5) and 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2) side chains on uridine 34 (U34) at the tRNA wobble position during vegetative growth and under stress conditions 8,12,13,37–40. We conducted an analysis of Trm112 protein levels during nitrogen starvation in both wild-type and igo1 deleted cells. In the wild-type, Trm112 levels remained constant during the first two hours and then exhibited a slight decrease after four hours. In contrast, in the igo1Δ mutant, we observed a more pronounced reduction in Trm112 proteins levels during entry into quiescence (Fig. 4c, left panel). Interestingly, as shown previously for other proteins, the reduction of PP2A/B55 activity restored Trm112 protein levels (Fig. 4c, right panel). We also found that the levels of Ctu1 protein, which cooperates with Ctu2 in tRNA U34 thiolation 41,42, were also diminished in the igo1Δ background (Fig. 4d).
Collectively, these data suggest potential impairment of tRNA modifications in the igo1Δ mutant. To assess this possibility, we tested the sensitivity of the igo1Δ mutant to drugs that affect translation, such as paromomycin, puromycin or cycloheximide, in nitrogen-rich (EMM2) and nitrogen-poor (MMPhe) media. Among all the drugs tested, only paromomycin, which induces codon misreading 43, exhibited an effect on the igo1Δ mutant (Fig. 4e), particularly in MMPhe.
In summary, our data indicate a potential role for the Greatwall-Endosulfine-PP2A/B55 pathway in translation during the onset of quiescence. This role likely involves tRNA modifications that enhance codon-anticodon recognition.
The igo1Δ mutant is defective in U34 and A37 tRNA modifications
In all organisms, modifications of uridine 34 at the wobble position (U34) of certain tRNAs are necessary to enhance codon-anticodon recognition 37. These modifications are mediated by the Elongator complex, which introduces an acetyl group at position 5 of U34 (cm5U34), the Trm112/Trm9 methyltransferase complex involved in the formation of mcm5U34, and the Ctu1-Ctu2 complex, which catalyses the thiolation at carbon 2 of U34 (mcm5s2U34) (Supplementary Fig. 5a).
Previous studies in S. pombe have reported that differences in codon usage and tRNA modifications play a crucial role in regulating translation efficiency during the cell cycle and under oxidative stress. The mRNAs of the cell cycle regulator Cdr2 39 and of the stress-responsive transcription factors Atf1 and Pcr1 12,13 exhibit a high usage of lysine AAA codons compared to AAG codons, and their translational rate is particularity sensitive to deficiencies in tRNA modifications mediated by the Elongator, Trm112/Trm9 and Ctu1/Ctu2 complexes 8,12,13,39. Therefore, we hypothesised that differences in AAAlys codon usage might be responsible for the translation phenotype observed in the igo1Δ mutant. To test this hypothesis, we examined the use of AAAlys versus AAGlys codons for some of the proteins analysed in our study and found that all the proteins defective in the igo1Δ mutant (Rap1, Sgo2, Clr3, Clr2, and Ctu1) primarily utilise the AAAlys codon (Supplementary Fig. 5b). Proteins with reduced AAAlys codon usage, such as Ccq1, Pgk1, Krs1, or Swi6, did not exhibit translation deficiencies during nitrogen starvation in the igo1Δ mutant (Fig. 2a; Supplementary Fig. 5b,c).
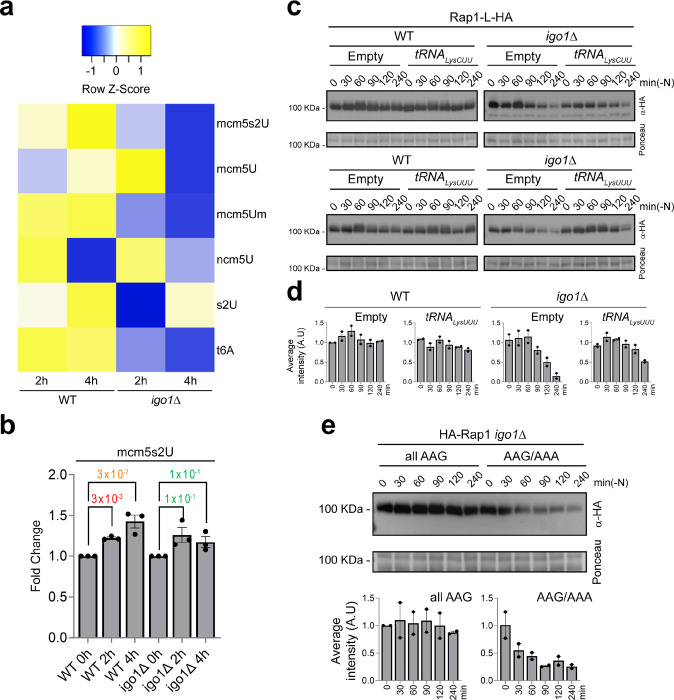
To confirm the potential defect in U34 tRNA modification in the igo1Δ mutant, we employed quantitative liquid chromatography coupled to mass spectrometry (LC-MS) 8 to analyse tRNAs extracted from wild-type and igo1Δ cells. Samples were collected during exponential growth and at 2 and 4 hours of nitrogen starvation. This analysis revealed an increase in mcm5s2U34 levels in wild-type cells upon entry into quiescence. However, in the igo1Δ mutant, the levels of mcm5s2U34 diminished after 4 hours of nitrogen starvation (Fig. 5a,b). Furthermore, this analysis also indicated a reduction in A37 N6-threonylcarbamoyladenosine (t6) modification in the igo1Δ mutant (Fig. 5a; Supplementary Fig. 6a). The t6A37 tRNA modification, present in Archaea and Eukarya, mediated by the protein Sua5 and the KEOPS/EKC complex, is essential for cell growth and accurate translation 44–46. Once again, we hypothesised that differences in AAAlys codon usage might be responsible for the reduction in t6 A37 modification in the igo1Δ mutant. When we examined the use of AAAlys versus AAGlys codons for Sua5 and the KEOPS/EKC subunits, we found that Pcc1 and Cgi121 (two components of KEOPS/EKC complex) primarily use the AAAlys codons (Supplementary Fig. 6b). Taking Cgi121 as an example, we detected a significant reduction in Cgi121 level in the igo1 mutant during nitrogen starvation (Supplementary Fig. 6c). These data suggest that a defect in the translation of Cgi121 protein could be responsible of the reduction in t6A37 tRNA modification in the igo1Δ mutant. Both mcm5s2U34 and t6A37 modifications are involved in decoding codons that start with adenosine, promoting codon-anticodon pairing and enhancing translation fidelity 47,48. These findings provide a molecular explanation for the paromomycin hypersensitivity and translation defect observed in the igo1Δ mutant under nitrogen-stress conditions (Fig. 4e).
Fig. 5. Igo1 regulates some tRNA modifications.
a Heatmap analysis of changes in the relative levels of tRNA ribonucleoside modifications in the WT and the igo1Δ mutant. The side colour bar displays the range of z-score change values. The z-score was calculated as the value for each time point minus the average value for the modification, and the resulting value was divided by the standard deviation. b Fold-change of mcm5S2U34 modification in WT and igo1Δ mutant cells. Student’s t-test p-values were calculated from biological triplicates. c Extracts from strains bearing rap1:L:HA protein transformed with episomal plasmids ptRNACUULys, ptRNAUUULys or the empty vector pREP42x were analysed by SDS-PAGE and western blotting with anti-HA antibodies during nitrogen starvation. Ponceau stain was used as a loading control. d Immunoblot quantification performed with Image Studio Lite software from at least three independent experiments. e Extracts from igo1Δ mutant transformed with episomal plasmids Pnmt41x:HA:rap1 (AAA/AAG) or mutated version Pnmt41x:HA:rap1 (all AAG) were analysed by SDS-PAGE followed by immunoblotting with anti-HA antibodies during nitrogen starvation. Ponceau staining was used as a loading control. Immunoblot quantification were performed with Image Studio Lite software from at least two independent experiments.
As cells enter quiescence, a notable reduction in tRNALysUUU levels was observed in both wild-type and igo1Δ cells (Supplementary Fig. 5d), suggesting that this tRNA becomes limiting in quiescent cells. Previous studies in yeast and worms have shown that over-expression of tRNAs can effectively restore translation rates and protein homeostasis in mutants defective in tRNA modification 8,12,13,39,47. Using Rap1 as an example, we assessed whether over-expression of tRNALysUUU would lead to recovery of Rap1 protein levels in the igo1Δ mutant, with tRNALysCUU over-expression serving as a control. As anticipated, tRNALysCUU overexpression had no impact on Rap1 levels, while overexpression of tRNALysUUU partially restored Rap1 protein levels (Fig. 5c, d). To confirm that the reduced levels of Rap1 protein in igo1Δ cells resulted from defective translation of its mRNA, we engineered a mutant version of the rap1 gene in which the 40 AAA codons were substituted with AAG, making all lysine codons independent of tRNA modification. Consistent with previous findings for other proteins, the translation deficiency of Rap1 in the igo1Δ mutant was completely rescued by expressing the rap1-allAAG allele (Fig. 5e).
In summary, considering all the data, we conclude that during the transition into quiescence, the Endosulfine Igo1 is necessary for facilitating U34 and A37 tRNA modifications, which are critical for enhancing the translation efficiency and fidelity of proteins encoded by mRNAs with high AAAlys codon usage.
Gad8 phosphorylation is required to enhance translation during quiescence entry
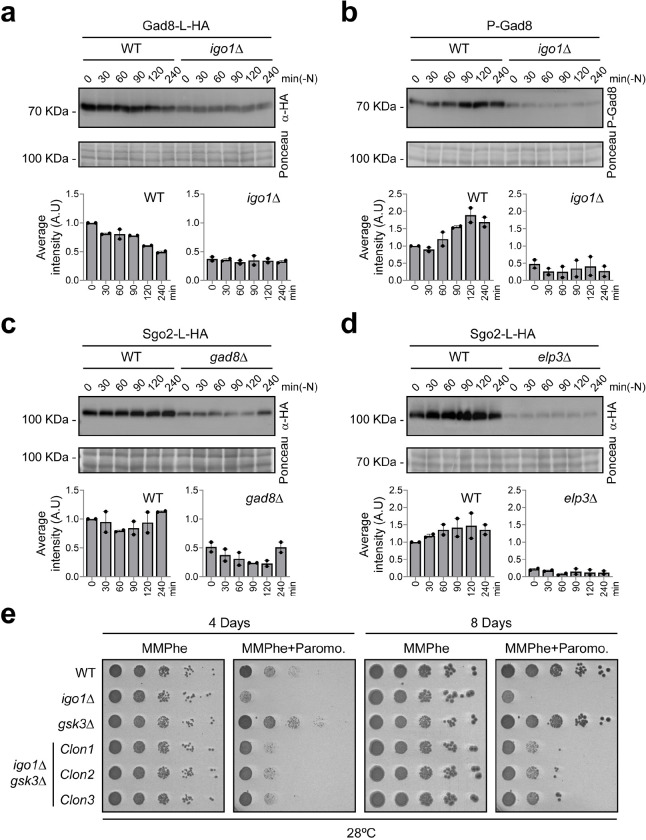
We then investigated the underlying molecular mechanism that triggers the translational defect due to a failure in tRNA modification. We identified Gad8 as an interactor of PP2A/Pab1 (Supplementary Fig. 4c). Previous studies demonstrated that PP2A/Pab1 counteracts the phosphorylation of Gad8 by TORC2 at serine 546, suggesting that Gad8 is a direct target of PP2A/Pab1 6,7. Additionally, active Gad8 phosphorylated at S546 activates Elongator by inhibiting Gsk3, a glycogen synthase kinase that inhibits Elongator by phosphorylating the Elp4 subunit at serine 114 8.
These findings prompted us to investigate the potential role of Igo1 in Gad8 phosphorylation during quiescence entry. Notably, it has previously been reported that deletion of elp3, which encodes the tRNA acetyltransferase subunit of the Elongator complex, leads to a reduction in Gad8 protein levels 8. A sequence analysis of the gad8 mRNA revealed a high usage of AAAlys codons, like rap1 (Supplementary Fig. 5b, gad8 z-scoreAAA/AAG = 0.73/−0.73 vs. rap1 z-scoreAAA/AAG = 0.72/−0.71). This observation led us to investigate whether high PP2A/B55 activity, in the absence of Igo1, could be relevant for maintaining Gad8 protein levels during nitrogen starvation. Western blot analysis clearly showed a decrease in Gad8 protein levels in the igo1Δ mutant (Fig. 6a). Furthermore, the phosphorylation status of Gad8 at S546 was also reduced in the absence of Igo1, compared to wild-type cells (Fig. 6b). These results suggest that during nitrogen starvation, inhibition of PP2A/Pab1 leads to the accumulation of phosphorylated Gad8 at S546 by TORC2 and consequent activation of the Elongator complex. This mechanism generates a positive feedback loop that enhances translation of Gad8 and promotes more U34 tRNA modifications (see Fig. 7).
Fig. 6. Endosulfine, Gad8 and Elongator are required for efficient translation of certain mRNAs during quiescence entry.
a Extracts from WT and igo1Δ cells expressing gad8:L:HA, were analysed by SDS-PAGE and immunoblotting with anti-HA antibodies during nitrogen starvation. Ponceau stain was used as a loading control. Immunoblot quantification were performed with Image Studio Lite software from at least two independent experiments. b Same as in (a), Gad8 phosphorylation state was analysed in WT and igo1Δ cell extracts. c Same as in (a), sgo2:L:HA protein was analysed in a WT and gad8Δ cell extracts. d Same as in (a), sgo2:L:HA protein was analysed in a WT and elp3Δ cell extracts. e Serial dilutions from cultures of WT, igo1Δ, gsk3Δ and igo1Δ gsk3Δ were spotted onto MMPhe (Minimal Media with Phenylalanine) plates without or with paromomycin (0.5 mg/ml).
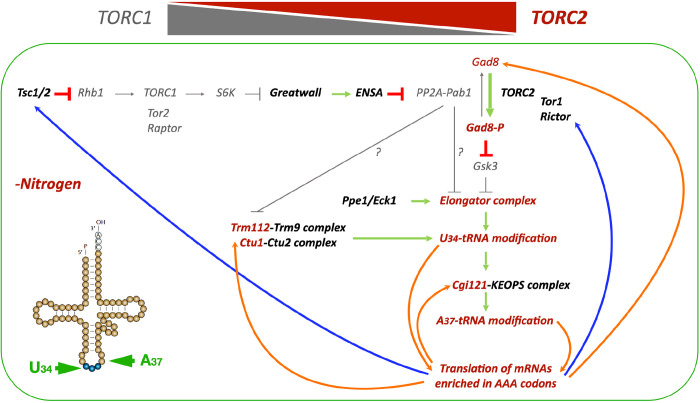
Fig. 7. Activation of TORC2-Gad8 signalling in quiescent cells promotes the translation of mRNAs with a high AAALys codon usage.
This model is based on previous work in fission yeast, demonstrating that nitrogen starvation induces the inactivation of TORC1 and the activation of TORC2 signalling through the Greatwall-Endosulfine-PP2A/B55 pathway 4–7. Phosphorylation of Gad8 at S546 leads to the inhibition of Gsk3 and the activation of Elongator, which promotes U34 tRNA modification and translation of Tsc1, an inhibitor of TORC1, as well as activators of TORC2, such as Tor1 and Rictor (depicted by blue arrows) 8. In this study, we present additional feedback loops (indicated by orange arrows) that enhance the translation of Gad8, Trm112, Ctu1 and Cgi121, further increasing the U34 and A37 tRNA modifications necessary for the efficient translation of mRNAs enriched in AAA codons. Such mRNAs include rap1, clr2, clr3 and sgo2, which encode proteins required for the correct attachment of telomeres to the NE.
If our model is correct, and PP2A/B55 indeed regulates Elongator activity through Gad8 protein homeostasis, the deletion of the gad8 gene should lead to a substantial decrease in proteins with high AAAlys codon usage. To test this hypothesis, we examined the levels of Sgo2, a protein with a very high AAAlys codon usage (z-scoreAAA/AAG = 1.28/−1.28), in a gad8Δ mutant background. Western blot analysis showed a reduction in Sgo2 protein levels in the gad8Δ mutant (Fig. 6c). As a positive control, we used the elp3Δ mutant, where the reduction in Sgo2 levels was even greater (Fig. 6d). Moreover, both gad8Δ and elp3Δ mutants displayed sensitivity to paromomycin in both nitrogen-rich and nitrogen-poor media (Supplementary Fig. 6d), although the sensitivity to paromomycin in nitrogen-poor medium was more pronounced in the igo1Δ mutant (Figs. 6e; Supplementary Fig. 6d). Thus, our data strongly suggests that defects in the Elongator activation pathway led to decreased translation efficiency of mRNAs with high AAAlys codon usage.
Finally, to confirm the connection between Igo1 and the Elongator activation pathway, we generated a double mutant, igo1Δ gsk3Δ. Gsk3 is an Elongator inhibitor in S. pombe 8. As mentioned earlier, the igo1Δ mutant exhibited sensitivity to paromomycin, particularly in nitrogen-poor media containing phenylalanine (Fig. 4e). If this phenotype is indeed related to reduced Elongator activity, the igo1Δ gsk3Δ double deletion should rescue this defect. Wild-type, igo1Δ and gsk3Δ single mutants, along with the igo1Δ gsk3Δ double mutant, were cultivated in nitrogen-poor (MMPhe) medium with or without paromomycin, and their growth phenotype was assessed. The igo1Δ mutant displayed hypersensitivity to paromomycin, while the gsk3Δ mutant exhibited mild resistance compared to the wild-type strain. A partial improvement in cell growth in the presence of paromomycin was detected in the igo1Δ gsk3Δ double mutant clones compared to the igo1Δ mutant (Fig. 6e). These findings strongly suggest that proper Igo1-mediated activation of Elongator is crucial for maintaining the rate of translation during quiescence entry.
Discussion
Entry into quiescence in yeasts is regulated by diverse signalling cascades that converge at the Greatwall-Endosulfine-PP2A/B55 pathway 3,5,49–51. In S. pombe, the primary signal regulating entry into quiescence is nitrogen starvation, which reduces the activity of TORC1 and increases the activity of TORC2 4,6,7,52–58. Inactivation of TORC1 results in reduced protein synthesis and the activation of protein degradation through autophagy. However, quiescent cells must maintain a continuous supply of specific proteins to remain viable.
In this study, we demonstrate that the Greatwall-Endosulfine-PP2A/B55 pathway links the inactivation of TORC1 with the activation of TORC2 signalling to promote the activation of the Elongator complex and other tRNA modification complexes essential for sustaining the translation programme during quiescence. This is achieved by facilitating U34 and A37 tRNA modifications, which increase translation efficiency and fidelity of critical proteins, including those necessary for telomeric and subtelomeric functions.
The reduction of PP2A/B55 activity, achieved through the activation of Greatwall and Endosulfine, is required to accumulate phosphorylated Gad8 at S546 7. This phosphorylation event increases the activity of the Elongator complex by inhibiting glycogen synthase kinase, Gsk3 8. The increased Elongator activity promotes the efficient translation of mRNAs containing high AAAlys codon usage, such as tsc2 (an inhibitor of TORC1) 8, gad8 (a positive effector of TORC2), trm112, ctu1 and cgi121 (involved in U34 and A37 tRNA modifications). All of these facilitate the switch from high TORC1 to high TORC2 activity as cells enter quiescence. Furthermore, the synthesis of key proteins with roles in telomeric and subtelomeric organisation, such as Rap1, Sgo2, Clr2 or Clr3, which also exhibit a high AAAlys codon usage, is dependent on the correct activation of Elongator (Fig. 7).
Previous studies have demonstrated that the deletion of rap1 (encoding a component of the shelterin complex) or bqt4 (encoding a component of the bouquet complex) causes telomeric detachment from the NE 23,25,26. Our findings suggest that the telomeric detachment defect in the igo1Δ mutant is probably caused by a reduction in Rap1 protein levels (Fig. 2a–d). Interestingly, Bqt4, the other protein that creates a molecular link between telomeres and the NE, has a low AAAlys codon usage (z-scoreAAA/AAG = −0.94/0.95), making it unlikely to be responsible for the telomeric detachment phenotype in the igo1Δ mutant. However, we cannot rule out the possibility that other proteins may be involved in the telomeric attachment to the NE, such as Lem2, a member of the Lap2/Emerin/Man1 (LEM) family of lamin-associated proteins, which is known to be involved in telomere anchoring and heterochromatic gene silencing 59–61. Lem2 mRNA has a high AAAlys codon usage (z-scoreAAA/AAG = 0.88/−0.88), suggesting that it may also be subject to translation defects in the igo1Δ mutant, which could potentially affect telomere attachment to the NE.
In addition to the telomeric anchoring defect, the igo1Δ mutant exhibited upregulation of genes located in the subtelomeric regions of chromosomes I and II (Fig. 1a; Supplementary Fig. 1a). Several proteins related to subtelomeric organisation were defective in the igo1Δ background, particularly Sgo2, whose protein levels were significantly reduced (Fig. 3a). Sgo2 plays a role in knobs assembly, and its deletion leads to derepression of genes located in subtelomeric regions 21,22. Our data demonstrate that Igo1 is involved in knobs assembly, and its deletion results in defects in the regulation of subtelomeric genes. The overlapping roles and phenotypes between Sgo2 and Igo1 suggest that the reduction in Sgo2 protein levels is responsible for the derepression of subtelomeric genes in the igo1Δ mutant. However, other proteins, such as Clr2 or Clr3, which are involved in silencing could also contribute to this phenotype.
All the proteins tested in our study had their levels restored after reducing PP2A/B55 activity, indicating that low PP2A/B55 activity was necessary to maintain protein homeostasis during quiescence entry. But what is the link between PP2A/B55 activity, translation and the telomeric/subtelomeric organisation?
Previous research has shown that deleting specific components of TORC2 signalling, such as tor1 or gad8, leads to a significant derepression of genes located in subtelomeric regions 62. However, the molecular mechanism linking these processes remained unclear. Our data reveal that not only components of the TORC2 complex but also elements acting downstream of the TORC1 complex, such as Greatwall (Ppk18 and Cek1) or Endosulfine (Igo1), play a crucial role in regulating subtelomeric gene expression. These findings establish a connection between the Greatwall-Endosulfine-PP2A/B55 pathway and telomeric/subtelomeric organisation.
A link between the TORC2 signalling pathway, the Elongator complex, and tRNA modifications has been demonstrated 8. Our data further demonstrate that this connection is particularly relevant during entry into quiescence by elucidating the molecular details linking nitrogen starvation, TORC1 inactivation, activation of Greatwall-Endosulfine, inactivation of PP2A/Pab1, activation of Gad8, and upregulation of tRNA-modifying complexes (Fig. 7). Indeed, these molecular events affect not only the Elongator complex but also other tRNA modifiers like Trm112 or Ctu1. These interactions create positive feedback loops that are responsible for the transition from high TORC1 to high TORC2 activity, subsequently leading to further tRNA modifications. Mutations in Greatwall (Ppk18 and Cek1) or Endosulfine (igo1) result in a defect in this transition, causing a failure in tRNA modifications that affect the translation of mRNAs with a high AAAlys codon usage encoding for proteins such as Rap1, Sgo2, Clr2 and Clr3. This, in turn, triggers telomeric detachment and the derepression of subtelomeric genes.
In addition to the mcm5s2U34 defect, we also observed a reduction in t6A37 in the Endosulfine (igo1Δ) mutant (Fig. 5a). These two closely located tRNA modifications play a crucial role in ensuring accurate codon-anticodon interactions by stabilising codon-anticodon pairings 63–66. Interestingly, tRNALysUUU carries both the mcm5s2U34 and t6A37 modifications, both of which are defective in the Endosulfine (igo1Δ) mutant. This finding provides an explanation for the strong sensitivity of igo1Δ cells to paromomycin compared to mutants in Elongator (elp3Δ) or Gad8 (gad8Δ). The defect in the t6A37 modification could be explained by a reduction in Cgi121 protein levels (Supplementary Fig. 6c), one of the subunits of the KEOPS complex responsible for this modification 48,64, which is encoded by an mRNA with a high AAAlys codon usage.
Finally, an interesting concept has emerged over the last few years on tRNA modifications and stress, known as tRNA modification tuneable transcripts (MoTTs). These transcripts are characterised by a specific use of degenerate codons and codon biases to encode essential stress response proteins. Translation of these transcripts is affected by modifications at the wobble position of the tRNAs 14,15. Our work supports the idea that mRNAs encoding proteins involved in nutrient starvation, stress response or even the translation of viral RNA genomes present MoTTs, which allow for an increase in translation efficiency under stress conditions 8,12,13,67–70. One of these transcripts, the Hif1α mRNA, is translated in drug-resistant melanomas through a mechanism involving the activation of Elp1 by the PI3 kinase signalling pathway. The activation of Hif1α promotes drug resistance by inducing anaerobic glycolysis 71. Therefore, the mcm5s2U34 modification in the tRNA anticodon promotes the translation of mRNAs enriched in AAA codons, including Hif1α mRNA. This discovery opens new avenues for identifying inhibitors of Elongator and other tRNA modifiers to treat drug-resistant tumours and combat viral infections.
Methods
Strains and Growth Conditions
Yeast strains are listed in Supplementary Table 6. Fission yeast cells were cultured and genetically manipulated according to standard protocols 72. Genetic crosses were performed on malt extract agar plates. Cells were typically cultured overnight at the appropriate temperatures in yeast extract supplemented with adenine, leucine, histidine, lysine, and uracil (YES), or in Edinburgh minimal medium containing 93.5 mM ammonium chloride (EMM2) as a nitrogen source. For nitrogen starvation experiments, exponentially growing cells were shifted from Edinburgh minimal medium (EMM2) at 28°C to minimal medium without nitrogen (EMM2-N) at 25°C.
In overexpression experiments using the nmt1+ promoter, cells were grown to the logarithmic phase in EMM2 containing 15 μM thiamine. Then, the cells were harvested and inoculated in fresh EMM2 medium without thiamine.
DNA Techniques and Plasmid Construction
DNA manipulations were performed as described in Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Enzymes for molecular biology were obtained from Fermentas and Thermo Fisher. PCRs were performed with using Velocity DNA polymerase (Bioline). Oligonucleotides employed for strain and plasmid construction are listed in Supplementary Table 7. Information regarding construction strategies is available upon request. Plasmids used in this study carry the ampicillin resistance gene for selection in E. coli and are listed in Supplementary Table 7.
RNA Isolation, RNAseq and RT-qPCR
Wild type (2666), igo1Δ (2727) and ppk18Δ cek1Δ (2883) cells were grown to mid-exponential phase in EMM2, centrifuged and washed three times in EMM2-N, and cultured in EMM2-N at 25°C. For RNAseq, 2×108 cells were harvested at times 0 and 4 hours, washed with cold DEPC-H20, and snap frozen. RNA extraction was carried out by disrupting the cells with glass beads using RNAeasy Mini kit (Qiagen) and following the manufacturer’s instructions. RNA quality was evaluated using the Bioanalyzer 2100 (Agilent). Library preparation, using the Illumina Ribo Zero and TruSeq Stranded kits, and subsequent NGS sequencing were performed by Macrogen. Sequencing quality was assessed with FastQC (v 0.11.8, Babraham Bioinformatics). If necessary, adaptors were trimmed using Trimmomatic (v 0.38) 73. Alignment was performed with HISAT2 v 2.1.0 (CCB in John Hopkins University) 74 using S. pombe reference genome from Pombase (downloaded on 30/11/2018). Samtools (v 1.9) and deepTools (v 3.3.0) were used to obtain bigWig files to visualize in IGV (v 2.4.16) and JBrowse (v 1.15.4) browsers. Read counts were obtained with featureCounts (Subread package v 1.6.3, Walter+Eliza Hall Bioinformatics) 75. DESeq2 (v1.22.2) 76 was used for the differential expression analysis. Plots representing upregulated genes in Endosulfine (igo1Δ) and Greatwall (cek1Δ ppk18Δ) mutants shown in Fig. 1a and Supplementary Fig. 1a were generated with karyoploteR (v 1.12.4) 77.
For RT-qPCR, total RNA was isolated from 2×108 S. pombe cells in exponential phase by disrupting the cells with glass beads in TRIzol® Reagent (Invitrogen) and following the manufacturer’s instructions. The integrity of the RNA was verified through 1% agarose gel electrophoresis, and its quality and quantity were determined using a microspectrophotometer. RNA was treated with RNase-free DNAse I (Invitrogen) at 25°C for 30 minutes, following the manufacturer’s instructions. Each RNA sample (1.2–1.5 μg) was then reverse transcribed with the SuperScript™ First-Strand Synthesis System (Invitrogen) using the oligo(dT) primer supplied with the kit or the tRNALysUUU specific reverse primer in combination with the act1 gene reverse primer (Supplementary Table 7) at 50°C for 30 minutes in a 20-μl total volume. Quantitative PCR amplification of cDNA (1 μl) was carried out using TB Green Premix Ex Taq™ (TaKaRa) and the primer pairs indicated in the Supplementary Table 7, in a 20-μl total volume with the following cycling parameters: 95°C for 45 seconds, 40 cycles of 95°C for 5 seconds and 60°C for 31 seconds, followed by a dissociation step at 95°C for 15 seconds, 60°C for 1 minute and 95°C for 15 seconds. The reactions were run in duplicate or triplicate in an Applied Biosystems 7300 Real-Time PCR System. Negative controls without reverse transcriptase, without RT-primer and without cDNA were included to control for DNA contaminations. Fold changes in the expression levels relative to the wild-type strain grown in EMM2 were calculated according to the mathematic model described by 78, with normalization to act1 expression levels. The experiments were performed at least twice with cDNA from different biological repeats.
SRFF microscopy
Samples were observed using a Confocal Andor Dragonfly 200 microscope, equipped with a 100x/1.45 Oil Plan Apo objective, an Andor sCMOS Sona 4.2B-11 camera and controlled by Fusion (SRRF-STREAM) software. Image J software was used for general image and movie manipulation. Radial Profile Analysis and the calculations of Pearsońs Correlation Coefficients were performed using ImageJ. More than 100 nuclei were measured for each strain.
S. pombe protein extracts and western blot
TCA extraction was performed as previously described 79. For immunoblotting, PVDF membranes were probed with anti-HA (12CA5, Roche), anti-GFP (3H9, Chromotek), anti-GST (RPN1236V, Cytiva) or anti-P-Gad8 (kindly provided by José Cansado, University of Murcia, Spain). Standard procedures were employed for protein transfer, blotting and chemiluminescence detection. Protein detection was performed using the ECL kit (BioRad).
Chromatin immunoprecipitation (ChIP)
Chromatin isolation and immunoprecipitation was performed as previously described 80. S. pombe cell cultures were grown in EMM2 or after 4 hours of nitrogen starvation in EMM2-N to OD600 of 0.5–0.6 and crosslinked with 1% formaldehyde for 10 min at room temperature. To terminate crosslinking, 2.5M glycine was added to a final concentration of 125 mM for 5 min. Cells were pelleted by centrifugation, washed twice with 10 ml of cold PBS, frozen on dry ice and stored at −80°C. Cell pellets from 50 ml cultures were resuspended in 0.25 ml of Breaking buffer (0.1M Tris-HCl pH 8.0, 20% glycerol, 1 mM PMSF) and lysed in a Fast-prep (2 cycles of 45 s) in the presence of glass beads (50 micron; Sigma) at 4°C. Lysates were centrifuged at 14,000 g for 1 min at 4°C. Pellets were washed with 1 ml of Lysis buffer (50 mM HEPES pH 7.6, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF). Pellets containing chromatin were resuspended in 0.25 ml of Lysis buffer. Lysates were sonicated for 6 min at 4°C (30 seconds on, 30 seconds off), using a water bath sonicator (Diagenode Bioruptor Plus), transferred to new 1.5-ml Eppendorf tubes, added 0.75 ml of Lysis Buffer and centrifuged at 14,000 g for 30 min at 4°C. 50 μl of supernatant was kept as ‘input’ and the remainder (~950 μl) was subjected to immunoprecipitation with antibodies against K14-acetylated histone H3 (07–353, Upstate Biotechnology) and 20 μl of protein G agarose beads (100.04D, Dynabeads Protein G, Thermo Fisher). After an overnight incubation at 4°C with mixing, beads were washed sequentially with 1 ml of Lysis buffer once, Lysis + 500 mM NaCl twice, Wash buffer (10 mM Tris pH 8.0, 1 mM EDTA, 250 mM LiCl, 0.5% sodium deoxycholate, 0.5% NP-40, 1 mM PMSF) twice, and TE buffer (10 mM Tris pH 7.5, 1 mM EDTA) once. Each wash was for 5 min with mixing at room temperature. Immune complexes were eluted in 100 μl elution buffer (50 mM Tris pH 8.0, 10 mM EDTA, 1% SDS) at 65°C for 20 min. Beads were washed with 150 μl TE + 0.67% SDS, which was combined with the eluate. 150 μl TE + 0.67% SDS was also added to the input samples, and both IP and input samples were incubated at 65°C overnight to reverse protein–DNA crosslinks. DNA was purified by phenol/chloroform extraction. Analysis by qPCR was carried out using a Bio-Rad CFX96 instrument, Takara TB Green premix Ex-Taq, and primers listed in the Key resources table. ChIP signals were calculated as IP/input and normalized to WT 0h with an assigned value of 1.
Immunoprecipitations and mass-spectrometry analysis
Immunoprecipitation was performed following previously established protocols 81. For the immunoprecipitation of Paa1-L-YFP (strain JED62) and Pab1-L-YFP (strain JED56), 1 l of cells was grown to mid-log phase in EMM2, then shifted to EMM2-N for 1 hour and crosslinked with 1% formaldehyde for 10 min at 25 °C. The reaction was quenched by adding glycine to 250 mM and incubating for 5 min on ice. Cells were collected by centrifugation, washed with PBS 1x, frozen in liquid nitrogen and broken with a Freezer/Mill in lysis buffer (25 mM Tris HCl pH 7.5, 150 mM NaCl, 0.5% SDS, 1% NP40, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin). Cell lysates were then slowly diluted to 0.1% SDS final concentration for immunoprecipitation in lysis buffer without SDS at 4°C for 30 min. Clarified extracts were immunoprecipitated by adding 40 μl of GFP-Trap beads (gta-20, Chromotek) for 1 hour at 4 °C. The beads were washed six times with lysis buffer containing 500 mM NaCl. Finally, the beads were sent to the Proteomics Facility of the Salamanca Cancer Research Center for Mass-spectrometry analysis. Analysis and interpretation of the results were carried out using the String Database.
For the immunoprecipitation of GFP-Pab1 (strain 2895), 16 l of cells were grown to mid-log phase in EMM2 at 25°C. After one day, half of the culture was shifted to EMM2-N for 1 hour. Subsequently, cells were harvested by centrifugation and frozen at −80°C. Cells were lysed using glass beads and a bead beater in 100 ml of NP-40 buffer (6 mM Na2HPO4, 4 mM NaH2PO4, 1% NP-40, 150 mM NaCl, 2 mM EDTA, 50 mM NaF and 0.1 mM Na3VO4) supplemented with complete EDTA-free protease inhibitor cocktail (Roche), 1.3 mM benzamidine (Sigma) and 1 mM PMSF (Sigma). Lysates were cleared by centrifugation, and supernatants were mixed with 60 μl of 50 % slurry GFP-TRAP magnetic agarose beads (GFP-Trap® magnetic agarose, ChromoTek) equilibrated with NP-40 buffer. After 90 minutes of incubation at 4°C, beads were magnetically separated from lysates and washed twice with 5 ml of NP-40 buffer. Samples were washed with 5 ml of low-NP-40 buffer (0.02% NP-40) to reduce total detergent in purified proteins and subsequently resuspended in 1 ml of low-NP-40 buffer. Proteins were eluted twice with 150 μl of elution buffer (200 mM glycine-HCl pH 2.5) and precipitated for 30 minutes on ice using 100 μl of 100% TCA. Samples were then spun down for 30 minutes at 13000 rpm and 4°C, washed with 1 ml of cold acetone containing 0.05 N HCl and 1 ml of cold acetone. Finally, pellets were dried at room temperature and stored at 4°C for mass spectrometry analysis. A small amount of each sample was used to confirmed proper purification of GFP-tagged proteins. For that purpose, the Plus One Silver Staining protein kit (GE Healthcare) was employed following manufacturer instructions. TCA-precipitated proteins were digested with trypsin and analyzed by two-dimensional liquid chromatography tandem MS (2D-LC-MS/MS) as previously described 82. MS2 and MS3 spectra were extracted separately from RAW files, and converted to DTA files using Scansifter software 83 (v2.1.25). Spectra with less than 20 peaks were excluded and the remaining spectra were searched using the SEQUEST algorithm (Thermo Fisher Scientific, San Jose, CA, USA; version 27, rev. 12). Sequest was set up to search the S. pombe protein database (pombe_contams_20151012_rev database, created in October 2015 from pombase.org). Common contaminants were added, and all sequences were reversed to estimate the false discovery rate (FDR), yielding 10390 total entries. Variable modifications (C+57, M+16, [STY]+80 for all spectra and [STY]-18 for MS3), strict trypsin cleavage, <10 missed cleavages, fragment mass tolerance: 0.00 Da (because of rounding in SEQUEST, this results in 0.5 Da tolerance), and parent mass tolerance: 2.5 Da were allowed. Peptide identifications were assembled and filtered in Scaffold (v4.8.4, Proteome Software, Portland, OR) using the following criteria: minimum of 99.0% protein identification probability; minimum of two unique peptides; minimum of 95% peptide identification probability. FDRs were estimated in Scaffold based on the percentage of decoy sequences identified after using the above filtering criteria; the protein level FDR was 0.7% and the peptide level FDR was 0.3%. Proteins containing the same or similar peptides that could not be differentiated based on MS/MS alone were grouped to satisfy the principles of parsimony. Mass spectrometry identified proteins were exported from Scaffold to Excel for further analysis. Further analysis and interpretation of the results was carried out using the String Database (https://string-db.org/).
tRNA purification
tRNA purification assay was performed following established procedures 8.
Quantification of tRNA modifications
The purified tRNAs (500 ng per sample) were subjected to hydrolysis in a 40 μL digestion cocktail containing 10 U benzonase, 4 U calf intestinal alkaline phosphatase, 0.12 U phosphodiesterase I, 0.1 mM deferoxamine, 0.1 mM butylated hydroxytoluene, 4 ng pentosatin, 2.5 mM MgCl2 and 5 mM tris buffer (pH 8.0). The digestion mixture was incubated at 37 °C for 6 h. For the verification of HPLC retention times of RNA modifications, synthetic standards were employed. Analytical separation was facilitated by a Thermo Hypersil Gold aQ C18 column (100 × 2.1 mm, 1.9 μm), which was interfaced with an Agilent 1290 HPLC system and an Agilent 6495 triple quadrupole mass spectrometer. The employed LC system operated at 35 °C, maintaining a flow rate of 0.35 mL/min. The gradient starts with 100% solution A (0.1% formic acid in water) for 4 min, followed by a 4–15 min phase involving a transition from 0% to 20% solution B (0.1% formic acid in acetonitrile). The HPLC column was coupled with an Agilent 6495 triple quadrupole mass spectrometer, utilizing an electrospray ionization source in positive ion mode. Operational parameters were set as follows: gas temperature at 120 °C; gas flow rate at 11 L/min; nebulizer pressure at 20 psi; sheath gas temperature at 400 °C; sheath gas flow rate at 12 L/min; capillary voltage at 1500 V; and nozzle voltage maintained at 0 V. The dynamic multiple reaction monitoring mode was used for detection of product ions derived from their respective precursor ions for all the RNA modifications. The collision energy was optimized to ensure maximal detection sensitivity for each modification. To ensure the same sample input, the MS signal intensity for each ribonucleoside was normalized with the UV signal intensity of canonical ribonucleosides. The fold change of the modified ribonucleosides in experiment group was calculated relative to the control group.
Drug sensitivity assays
For survival on agar plates, S. pombe strains were cultured in YES, diluted and the cells were spotted onto plates with minimal medium containing 93.5 mM NH4Cl (EMM2) or 20 mM phenylalanine (MMPhe) without or with paromomycin (0.5 mg/ml), puromycin (0.5 mg/ml) or cycloheximide (2.5 μg/ml). The plates were then incubated at the indicated temperatures for 4–8 days.
Statistical methods
Average, standard deviation, and P values for the two-sided Student’s t test of statistically significant differences were calculated with Microsoft Excel. Data distribution was assumed to be normal, but this was not formally tested.
Data availability
Further information and requests for reagents may be directed to Sergio Moreno (smo@usal.es) or Javier Encinar del Dedo (jedel_dedo@usal.es).
Acknowledgements
We thank Rosa Degano and Nieves Ibarrola from the Salamanca Cancer Research Institute Proteomic Facility for their technical assistance in mass spectrometry analyses, Jorge Fernández-Vázquez, José Cansado, Yolanda Sánchez, Carlos R. Vázquez and Mercedes Tamame for sharing plasmids, antibodies and reagents, and Pilar Pérez and members of the Moreno lab for their valuable discussions and comments on the manuscript. This work was funded by the Spanish Ministry of Science and Innovation-MCIN (grants BFU2017–88335-R and PID2020–115929RB-I00) and from the Castile and Leon government (grants CSI259P20, CSI010P23 and IBFG Unit of Excellence programmes CLU-2017–03 and CL-EI-2021–08 co-funded by the P.O. Feder of Castile and Leon 14–20 and European Union ERDF “Europe drives our growth”), and by the National Research Foundation of Singapore under its Singapore-MIT Alliance for Research and Technology Antimicrobial Resistance Interdisciplinary Research Group (J.S., P.D.). The work in K.L.G.ś lab was supported by the National Institutes of Health R35GM131799, and in D.H.ś lab by PDR T.0012.14, CDR J.0066.16 and PDR T.0112.21 grants. D.H. is a FNRS Director of Research. N.G.-B. and A.V.-B. were funded by XFPU15/03654, BES-2015–073171, predoctoral training contracts. R.L.-S.S. was funded by a predoctoral fellowship from the Castile and Leon government.
Footnotes
Competing interests
The authors declare no competing interests.
Additional information
Supplementary information can be found online at:
Supplementary Figs. 1-6.
Supplementary Table 1: List of the top 50 genes overexpressed in igo1Δ at 4 hours in EMM2-N.
Supplementary Table 2: List of the top 50 genes overexpressed in ppk18Δ cek1Δ at 4 hours in EMM2-N.
Supplementary Table 3: List of the proteins interacting with Paa1:L:GFP.
Supplementary Table 4: List of the proteins interacting with Pab1:L:GFP.
Supplementary Table 5: List of the proteins interacting with GFP:Pab1
Supplementary Table 6: List of fission yeast strains used in this work.
Supplementary Table 7: List of oligonucleotides and plasmids used in this work.
Data Availability Statement:
The RNAseq data in this study has been deposited in GEO database with the following accession number GSE217398.
References
- 1.Gonzalez A. & Hall M. N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J 36, 397–408, doi: 10.15252/embj.201696010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez A., Hall M. N., Lin S. C. & Hardie D. G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab 31, 472–492, doi: 10.1016/j.cmet.2020.01.015 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Bontron S. et al. Yeast endosulfines control entry into quiescence and chronological life span by inhibiting protein phosphatase 2A. Cell Rep 3, 16–22, doi: 10.1016/j.celrep.2012.11.025 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Chica N. et al. Nutritional Control of Cell Size by the Greatwall-Endosulfine-PP2A.B55 Pathway. Curr Biol 26, 319–330, doi: 10.1016/j.cub.2015.12.035 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Aono S., Haruna Y., Watanabe Y. H., Mochida S. & Takeda K. The fission yeast Greatwall-Endosulfine pathway is required for proper quiescence/G(0) phase entry and maintenance. Genes Cells 24, 172–186, doi: 10.1111/gtc.12665 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Laboucarie T. et al. TORC1 and TORC2 converge to regulate the SAGA co-activator in response to nutrient availability. EMBO Rep 18, 2197–2218, doi: 10.15252/embr.201744942 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin R. et al. A PP2A-B55-Mediated Crosstalk between TORC1 and TORC2 Regulates the Differentiation Response in Fission Yeast. Curr Biol 27, 175–188, doi: 10.1016/j.cub.2016.11.037 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candiracci J. et al. Reciprocal regulation of TORC signaling and tRNA modifications by Elongator enforces nutrient-dependent cell fate. Sci Adv 5, eaav0184, doi: 10.1126/sciadv.aav0184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarian C. et al. Modified nucleoside dependent Watson-Crick and wobble codon binding by tRNALysUUU species. Biochemistry 39, 13390–13395, doi: 10.1021/bi001302g (2000). [DOI] [PubMed] [Google Scholar]
- 10.Murphy F. V. t., Ramakrishnan V., Malkiewicz A. & Agris P. F. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat Struct Mol Biol 11, 1186–1191, doi: 10.1038/nsmb861 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Kruger M. K., Pedersen S., Hagervall T. G. & Sorensen M. A. The modification of the wobble base of tRNAGlu modulates the translation rate of glutamic acid codons in vivo. J Mol Biol 284, 621–631, doi: 10.1006/jmbi.1998.2196 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Garcia P., Encinar Del Dedo J., Ayte J. & Hidalgo E. Genome-wide Screening of Regulators of Catalase Expression: role of a transcription complex and histone and tRNA modification complexes on adaptation to stress. J Biol Chem 291, 790–799, doi: 10.1074/jbc.M115.696658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Vazquez J. et al. Modification of tRNA(Lys) UUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet 9, e1003647, doi: 10.1371/journal.pgen.1003647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endres L., Dedon P. C. & Begley T. J. Codon-biased translation can be regulated by wobble-base tRNA modification systems during cellular stress responses. RNA Biol 12, 603–614, doi: 10.1080/15476286.2015.1031947 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu C., Begley T. J. & Dedon P. C. tRNA modifications regulate translation during cellular stress. FEBS Lett 588, 4287–4296, doi: 10.1016/j.febslet.2014.09.038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermand D. Anticodon Wobble Uridine Modification by Elongator at the Crossroad of Cell Signaling, Differentiation, and Diseases. Epigenomes 4, doi: 10.3390/epigenomes4020007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi A. et al. Chemical genomics approach to identify genes associated with sensitivity to rapamycin in the fission yeast Schizosaccharomyces pombe. Genes Cells 20, 292–309, doi: 10.1111/gtc.12223 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Yadav R. K., Matsuda A., Lowe B. R., Hiraoka Y. & Partridge J. F. Subtelomeric Chromatin in the Fission Yeast S. pombe. Microorganisms 9, doi: 10.3390/microorganisms9091977 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanoh J. Unexpected roles of a shugoshin protein at subtelomeres. Genes Genet Syst 92, 127–133, doi: 10.1266/ggs.17-00016 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Hirano Y., Asakawa H., Sakuno T., Haraguchi T. & Hiraoka Y. Nuclear Envelope Proteins Modulating the Heterochromatin Formation and Functions in Fission Yeast. Cells 9, doi: 10.3390/cells9081908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda A. et al. Highly condensed chromatins are formed adjacent to subtelomeric and decondensed silent chromatin in fission yeast. Nat Commun 6, 7753, doi: 10.1038/ncomms8753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tashiro S. et al. Shugoshin forms a specialized chromatin domain at subtelomeres that regulates transcription and replication timing. Nat Commun 7, 10393, doi: 10.1038/ncomms10393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maestroni L. et al. Nuclear envelope attachment of telomeres limits TERRA and telomeric rearrangements in quiescent fission yeast cells. Nucleic Acids Res 48, 3029–3041, doi: 10.1093/nar/gkaa043 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chikashige Y. et al. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J Cell Biol 187, 413–427, doi: 10.1083/jcb.200902122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue H., Horiguchi M., Ono K. & Kanoh J. Casein kinase 2 regulates telomere protein complex formation through Rap1 phosphorylation. Nucleic Acids Res 47, 6871–6884, doi: 10.1093/nar/gkz458 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita I. et al. Telomere-nuclear envelope dissociation promoted by Rap1 phosphorylation ensures faithful chromosome segregation. Curr Biol 22, 1932–1937, doi: 10.1016/j.cub.2012.08.019 (2012). [DOI] [PubMed] [Google Scholar]
- 27.van Emden T. S. et al. Shelterin and subtelomeric DNA sequences control nucleosome maintenance and genome stability. EMBO Rep 20, doi: 10.15252/embr.201847181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanoh J., Sadaie M., Urano T. & Ishikawa F. Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr Biol 15, 1808–1819, doi: 10.1016/j.cub.2005.09.041 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Harland J. L., Chang Y. T., Moser B. A. & Nakamura T. M. Tpz1-Ccq1 and Tpz1-Poz1 interactions within fission yeast shelterin modulate Ccq1 Thr93 phosphorylation and telomerase recruitment. PLoS Genet 10, e1004708, doi: 10.1371/journal.pgen.1004708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X., Liu J., Jun H. I., Kim J. K. & Qiao F. Multi-step coordination of telomerase recruitment in fission yeast through two coupled telomere-telomerase interfaces. Elife 5, doi: 10.7554/eLife.15470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Bolado A. et al. The Greatwall-Endosulfine Switch Accelerates Autophagic Flux during the Cell Divisions Leading to G1 Arrest and Entry into Quiescence in Fission Yeast. Int J Mol Sci 24, doi: 10.3390/ijms24010148 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiyama T. et al. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128, 491–504, doi: 10.1016/j.cell.2006.12.035 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Job G. et al. SHREC Silences Heterochromatin via Distinct Remodeling and Deacetylation Modules. Mol Cell 62, 207–221, doi: 10.1016/j.molcel.2016.03.016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh N. S. et al. SIN-inhibitory phosphatase complex promotes Cdc11p dephosphorylation and propagates SIN asymmetry in fission yeast. Curr Biol 21, 1968–1978, doi: 10.1016/j.cub.2011.10.051 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourgeois G., Letoquart J., van Tran N. & Graille M. Trm112, a Protein Activator of Methyltransferases Modifying Actors of the Eukaryotic Translational Apparatus. Biomolecules 7, doi: 10.3390/biom7010007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liger D. et al. Mechanism of activation of methyltransferases involved in translation by the Trm112 ‘hub’ protein. Nucleic Acids Res 39, 6249–6259, doi: 10.1093/nar/gkr176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agris P. F., Vendeix F. A. & Graham W. D. tRNA’s wobble decoding of the genome: 40 years of modification. J Mol Biol 366, 1–13, doi: 10.1016/j.jmb.2006.11.046 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Karlsborn T. et al. Elongator, a conserved complex required for wobble uridine modifications in eukaryotes. RNA Biol 11, 1519–1528, doi: 10.4161/15476286.2014.992276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer F. et al. Translational control of cell division by Elongator. Cell Rep 1, 424–433, doi: 10.1016/j.celrep.2012.04.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang B., Johansson M. J. & Bystrom A. S. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11, 424–436, doi: 10.1261/rna.7247705 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjork G. R., Huang B., Persson O. P. & Bystrom A. S. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA 13, 1245–1255, doi: 10.1261/rna.558707 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewez M. et al. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc Natl Acad Sci U S A 105, 5459–5464, doi: 10.1073/pnas.0709404105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer E., Wilhelm J. M. & Sherman F. Variation of phenotypic suppression due to the psi+ and psi-extrachromosomal determinants in yeast. J Mol Biol 128, 107–110, doi: 10.1016/0022-2836(79)90311-5 (1979). [DOI] [PubMed] [Google Scholar]
- 44.Deutsch C., El Yacoubi B., de Crecy-Lagard V. & Iwata-Reuyl D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J Biol Chem 287, 13666–13673, doi: 10.1074/jbc.M112.344028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Yacoubi B. et al. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J 30, 882–893, doi: 10.1038/emboj.2010.363 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Yacoubi B. et al. The universal YrdC/Sua5 family is required for the formation of threonylcarbamoyladenosine in tRNA. Nucleic Acids Res 37, 2894–2909, doi: 10.1093/nar/gkp152 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nedialkova D. D. & Leidel S. A. Optimization of Codon Translation Rates via tRNA Modifications Maintains Proteome Integrity. Cell 161, 1606–1618, doi: 10.1016/j.cell.2015.05.022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su C., Jin M. & Zhang W. Conservation and Diversification of tRNA t(6)A-Modifying Enzymes across the Three Domains of Life. Int J Mol Sci 23, doi: 10.3390/ijms232113600 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar S., Dalgaard J. Z., Millar J. B. & Arumugam P. The Rim15-endosulfine-PP2ACdc55 signalling module regulates entry into gametogenesis and quiescence via distinct mechanisms in budding yeast. PLoS Genet 10, e1004456, doi: 10.1371/journal.pgen.1004456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo X., Talarek N. & De Virgilio C. Initiation of the yeast G0 program requires Igo1 and Igo2, which antagonize activation of decapping of specific nutrient-regulated mRNAs. RNA Biol 8, 14–17, doi: 10.4161/rna.8.1.13483 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Talarek N. et al. Initiation of the TORC1-regulated G0 program requires Igo1/2, which license specific mRNAs to evade degradation via the 5’–3’ mRNA decay pathway. Mol Cell 38, 345–355, doi: 10.1016/j.molcel.2010.02.039 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez B. & Moreno S. Fission yeast Tor2 promotes cell growth and represses cell differentiation. J Cell Sci 119, 4475–4485, doi: 10.1242/jcs.03241 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Hayashi T. et al. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12, 1357–1370, doi: 10.1111/j.1365-2443.2007.01141.x (2007). [DOI] [PubMed] [Google Scholar]
- 54.Ikai N., Nakazawa N., Hayashi T. & Yanagida M. The reverse, but coordinated, roles of Tor2 (TORC1) and Tor1 (TORC2) kinases for growth, cell cycle and separase-mediated mitosis in Schizosaccharomyces pombe. Open Biol 1, 110007, doi: 10.1098/rsob.110007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuo T., Otsubo Y., Urano J., Tamanoi F. & Yamamoto M. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol 27, 3154–3164, doi: 10.1128/MCB.01039-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uritani M. et al. Fission yeast Tor2 links nitrogen signals to cell proliferation and acts downstream of the Rheb GTPase. Genes Cells 11, 1367–1379, doi: 10.1111/j.1365-2443.2006.01025.x (2006). [DOI] [PubMed] [Google Scholar]
- 57.Weisman R. & Choder M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J Biol Chem 276, 7027–7032, doi: 10.1074/jbc.M010446200 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Weisman R., Roitburg I., Schonbrun M., Harari R. & Kupiec M. Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 175, 1153–1162, doi: 10.1534/genetics.106.064170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebrahimi H., Masuda H., Jain D. & Cooper J. P. Distinct ‘safe zones’ at the nuclear envelope ensure robust replication of heterochromatic chromosome regions. Elife 7, doi: 10.7554/eLife.32911 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banday S., Farooq Z., Rashid R., Abdullah E. & Altaf M. Role of Inner Nuclear Membrane Protein Complex Lem2-Nur1 in Heterochromatic Gene Silencing. J Biol Chem 291, 20021–20029, doi: 10.1074/jbc.M116.743211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrales R. R., Forn M., Georgescu P. R., Sarkadi Z. & Braun S. Control of heterochromatin localization and silencing by the nuclear membrane protein Lem2. Genes Dev 30, 133–148, doi: 10.1101/gad.271288.115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen A. et al. TOR complex 2 in fission yeast is required for chromatin-mediated gene silencing and assembly of heterochromatic domains at subtelomeres. J Biol Chem 293, 8138–8150, doi: 10.1074/jbc.RA118.002270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atkins J. F. & Bjork G. R. A gripping tale of ribosomal frameshifting: extragenic suppressors of frameshift mutations spotlight P-site realignment. Microbiol Mol Biol Rev 73, 178–210, doi: 10.1128/MMBR.00010-08 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El Yacoubi B., Bailly M. & de Crecy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet 46, 69–95, doi: 10.1146/annurev-genet-110711-155641 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Gustilo E. M., Vendeix F. A. & Agris P. F. tRNA’s modifications bring order to gene expression. Curr Opin Microbiol 11, 134–140, doi: 10.1016/j.mib.2008.02.003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jenner L. B., Demeshkina N., Yusupova G. & Yusupov M. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat Struct Mol Biol 17, 555–560, doi: 10.1038/nsmb.1790 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Chan C. T. et al. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun 3, 937, doi: 10.1038/ncomms1938 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dedon P. C. & Begley T. J. A system of RNA modifications and biased codon use controls cellular stress response at the level of translation. Chem Res Toxicol 27, 330–337, doi: 10.1021/tx400438d (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jungfleisch J. et al. CHIKV infection reprograms codon optimality to favor viral RNA translation by altering the tRNA epitranscriptome. Nat Commun 13, 4725, doi: 10.1038/s41467-022-31835-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patil A. et al. Translational infidelity-induced protein stress results from a deficiency in Trm9-catalyzed tRNA modifications. RNA Biol 9, 990–1001, doi: 10.4161/rna.20531 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rapino F. et al. Codon-specific translation reprogramming promotes resistance to targeted therapy. Nature 558, 605–609, doi: 10.1038/s41586-018-0243-7 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Moreno S., Klar A. & Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194, 795–823, doi: 10.1016/0076-6879(91)94059-l (1991). [DOI] [PubMed] [Google Scholar]
- 73.Bolger A. M., Lohse M. & Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120, doi: 10.1093/bioinformatics/btu170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim D., Langmead B. & Salzberg S. L. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12, 357–360, doi: 10.1038/nmeth.3317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao Y., Smyth G. K. & Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930, doi: 10.1093/bioinformatics/btt656 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550, doi: 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gel B. & Serra E. karyoploteR: an R/Bioconductor package to plot customizable genomes displaying arbitrary data. Bioinformatics 33, 3088–3090, doi: 10.1093/bioinformatics/btx346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29, e45, doi: 10.1093/nar/29.9.e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanso M., Gogol M., Ayte J., Seidel C. & Hidalgo E. Transcription factors Pcr1 and Atf1 have distinct roles in stress- and Sty1-dependent gene regulation. Eukaryot Cell 7, 826–835, doi: 10.1128/EC.00465-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanso M. et al. Cdk9 and H2Bub1 signal to Clr6-CII/Rpd3S to suppress aberrant antisense transcription. Nucleic Acids Res 48, 7154–7168, doi: 10.1093/nar/gkaa474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia P. et al. Eng2, a new player involved in feedback loop regulation of Cdc42 activity in fission yeast. Sci Rep 11, 17872, doi: 10.1038/s41598-021-97311-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts-Galbraith R. H., Chen J. S., Wang J. & Gould K. L. The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring. J Cell Biol 184, 113–127, doi: 10.1083/jcb.200806044 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Z. Q. et al. Supporting tool suite for production proteomics. Bioinformatics 27, 3214–3215, doi: 10.1093/bioinformatics/btr544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanoh J. Telomeres and subtelomeres: new insights into the chromatin structures and functions of chromosome ends. Genes Genet Syst 92, 105, doi: 10.1266/ggs.17-10001 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further information and requests for reagents may be directed to Sergio Moreno (smo@usal.es) or Javier Encinar del Dedo (jedel_dedo@usal.es).
The RNAseq data in this study has been deposited in GEO database with the following accession number GSE217398.