Abstract
Background and Objectives
The factors that drive progression in multiple sclerosis (MS) remain obscure. Identification of key properties of meningeal inflammation will contribute to a better understanding of the mechanisms of progression and how to prevent it.
Methods
Applying single-cell RNA sequencing, we compared gene expression profiles in immune cells from meningeal ectopic lymphoid tissue (mELT) with those from secondary lymphoid organs (SLOs) in spontaneous chronic experimental autoimmune encephalomyelitis (EAE), an animal model of MS.
Results
Generally, mELT contained the same immune cell types as SLOs, suggesting a close relationship. Preponderance of B cells over T cells, an increase in regulatory T cells and granulocytes, and a decrease in naïve CD4+ T cells characterize mELT compared with SLOs. Differential gene expression analysis revealed that immune cells in mELT show a more activated and proinflammatory phenotype compared with their counterparts in SLOs. However, the increase in regulatory T cells and upregulation of immunosuppressive genes in most immune cell types indicate that there are mechanisms in place to counter-regulate the inflammatory events, keeping the immune response emanating from mELT in check.
Discussion
Common features in immune cell composition and gene expression indicate that mELT resembles SLOs and may be regarded as a tertiary lymphoid tissue. Distinct differences in expression profiles suggest that mELT rather than SLOs is a key driver of CNS inflammation in spontaneous EAE. Our data provide a starting point for further exploration of molecules or pathways that could be targeted to disrupt mELT formation.
Introduction
Despite the success of various immunomodulatory therapies in the treatment of multiple sclerosis (MS), progressive forms of MS remain difficult to treat. Our understanding of the pathophysiologic mechanisms responsible for progressive MS is still limited. The occurrence of meningeal ectopic lymphoid tissue (mELT), which tends to be more prevalent in progressive MS,1-3 may be a key element relevant for this stage of MS, in which inflammation may smolder in the CNS behind an increasingly closed blood-brain barrier (BBB).
In general, ectopic lymphoid tissue (ELT), also called tertiary lymphoid tissue, is frequently observed in tissues affected by chronic inflammation because of an infection, autoimmune disease, cancer, or allograft rejection. The cellular composition of ELT resembles that of secondary lymphoid organs (SLOs), such as the spleen and lymph nodes (LN).4 ELT within tissues can show varying levels of organization, ranging from unorganized collections of immune cells to well-organized structures, with segregated T-cell and B-cell zones. They frequently display evidence of germinal center activity.5 ELT directs various B-cell and T-cell responses, including the induction of effector functions, antibody generation, affinity maturation, isotype switching, and clonal expansion. In this way, ELT contributes to local immune activation, which in case of autoimmune diseases, promotes tissue destruction through autoantibody production, complement activation, and release of proinflammatory cytokines.1,3-11 mELT is found in up to 2/3 of patients with progressive MS.2,12 Inflammatory aggregates are also found in relapsing-remitting MS, however, less frequent. The presence of leptomeningeal inflammation in MS is linked to greater cortical damage, an earlier disease onset, and more rapid disease progression.9,10,13 This renders mELT a potential therapeutic target in MS.3
mELT shares cellular and organizational similarities with SLOs.5,14,15 However, differences between mELT and SLOs exist, most obviously in development, location, and morphology. How large the intersection between mELT and SLOs is is the subject of debate.4 The goal of this study was to use single-cell sequencing to explore differences and similarities between mELT and SLOs in a spontaneous form of experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. Our group and others have previously used the spontaneous chronic EAE that occurs in approximately half of all 2D2xTh mice to study mELT because mELT forms abundantly in the meningeal space around the spinal cord. While mELT is present in all 2D2xTh mice that develop EAE during EAE onset, approximately one-third of those 2D2xTh mice that do not develop EAE still present with mELT histologically, although mELT in these mice tends to be much smaller.16 This suggests that mELT formation is linked to EAE immunopathology. Our data elaborate similarities and differences between mELT and SLOs, most importantly demonstrating a proinflammatory role for mELT in EAE.
Methods
Mice and Spontaneous EAE
2D2xTh EAE mice were generated, held and clinically assessed as previously described.16,17
Experimental Procedures and Data Analysis
See eMethods (links.lww.com/NXI/A951).
Standard Protocol Approvals, Registrations, and Patient Consents
All animal experiments were approved by the competent authority, Regierung von Oberbayern, Munich, Germany (ROB-55.2-2532.Vet_02-16-100).
Data Availability
The raw datasets generated and analyzed for this study can be found in the GEO repository, as records GSE181526 (experiment 1) and GSE182774 (experiment 2). All in-house scripts and R code were submitted to a Github repository.
Results
Single-Cell Transcriptomics of Cells in mELT, Lumbar and Inguinal Lymph Nodes, and Spleen
We aimed to identify differences in cell composition and gene expression between mELT and SLOs using single-cell sequencing. mELT from the spinal cord meninges and draining lumbar lymph nodes were collected from 2D2xTh mice with spontaneous EAE (score ≥3) in 2 independent experiments. In one of these experiments, spleen and inguinal (nondraining) lymph nodes were also collected, and hashtag oligomers were used to multiplex cells from different tissues (for experimental setup, see eMethods, links.lww.com/NXI/A951 and Figure 1A, for characteristics of the mice, see eTable 1, links.lww.com/NXI/A942). Concordance between datasets enabled pooled analysis of both experiments.
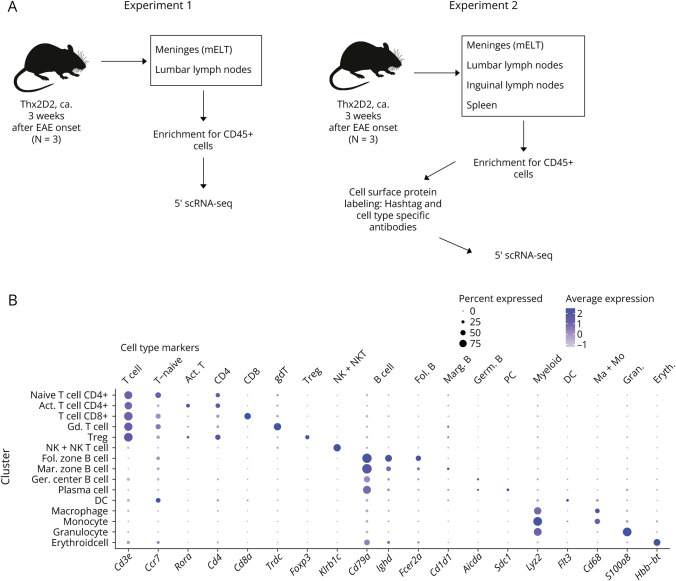
Figure 1. Study Design and Cell Cluster Definition.
(A) overview of the study design, (B) Dotplot depicting expression of selected marker genes for specific cell types in cell clusters. Dot size encodes percentage of cells expressing the gene, color encodes the average gene expression level per cell. The Y-axis names the 15 specific clusters. The genes named on the lower X-axis define the cell types in the upper X-axis (e.g., expression of FoxP3 defines Tregs).
After filtering out low-quality cells and doublets, we obtained a transcriptome from a total of 65,939 single cells: 17,470 cells from mELT, 19,111 cells from spleen, 19,230 cells from lumbar LN, and 10,128 from inguinal LN. The average amount of genes detected per cell was 1,270 for mELT, 1,185 for lumbar LN, 886 for inguinal LN, and 944 for spleen. After harmony correction, we performed clustering of the merged cell dataset. Five ambiguous clusters, which we could not confidently assign with cell identities, were excluded (see methods, and eFigure 1, links.lww.com/NXI/A949). In the end, we classified 63,263 single cells into 15 final cell clusters (Figure 2 and eFigure 1). Based on a combination of automatic cluster annotation and marker gene expression, we identified the following 15 cell types (Figure 1B): T cells (Cd3e), more specifically naïve CD4+ T cells (Cd4, Ccr7, Sell), activated CD4+ T cells (Cd4, Rora, and Pdcd1), CD8+ T cells (Cd8a), gd T cells (Trdc, Tcrg-C2), and regulatory T cells (Foxp3); B cells (Cd79a), separated into follicular zone (FZ) (Fcer2a, IghD) and marginal zone (MZ) (-like) B cells (Cd1d1, Cd9, and S1pr3), germinal center (GC) B cells (Aicda, Rgs13), and plasma cells (Sdc1, Tnfrsf17); a cluster containing natural killer (NK) cells and NK T cells (Klrb1c, Klrk1); and myeloid lineage cells, separated into dendritic cells (DC) (Flt3), macrophages (Cd68, Adgre1), monocytes (Cd68), and granulocytes (S100a8 and S100a9). In addition, we also found a small erythroid cell cluster in all samples, characterized by expression of hemoglobin genes (e.g., Hbb-bt), probably coming from blood contamination, despite thorough perfusion.
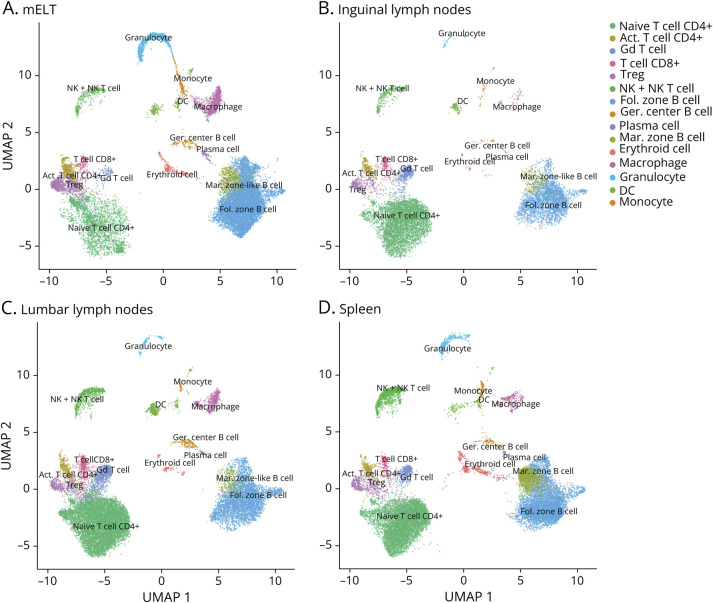
Figure 2. Meningeal Ectopic Lymphoid Tissue Strongly Resembles Secondary Lymphoid Organs.
Uniform Manifold Approximation and Projection (UMAP) plot representing 15 color-coded cell clusters identified in using single-cell sequencing, split per tissue: mELT (A), inguinal (B) and lumbar (C) lymph nodes, and spleen (D).
mELT and SLOs Are Composed of the Same Cell Types, but Contain Relatively Fewer T Cells and More B Cells, Treg, and Granulocytes
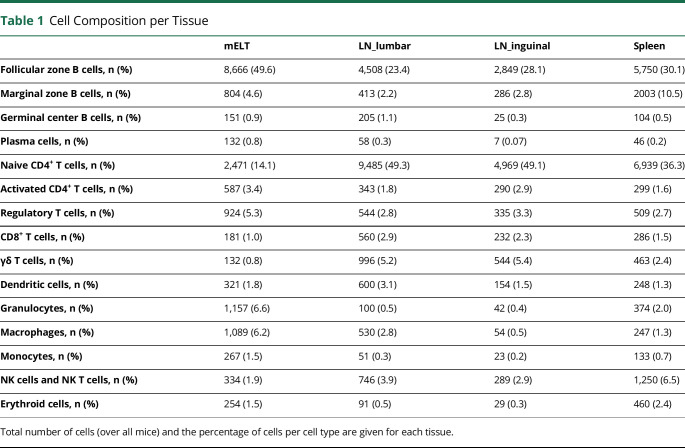
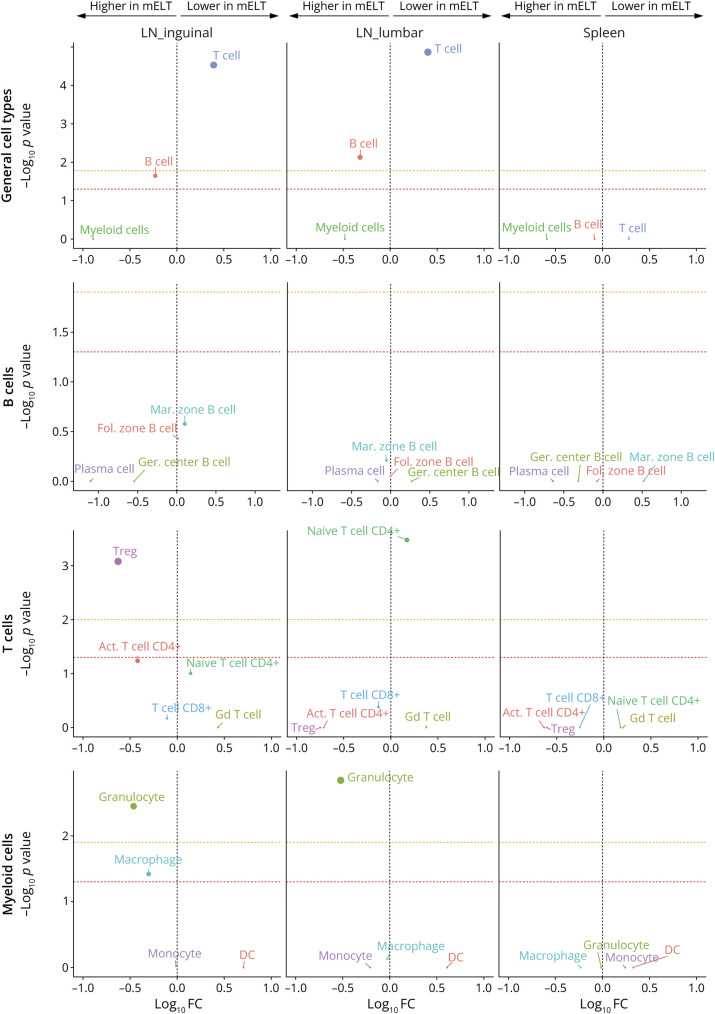
First, we analyzed the proportions of CD45+ cells in mELT when compared with the proportions in spleen and lymph nodes (lumbar and inguinal). The number and percentage of cells per cell type for every tissue are summarized in Table 1, and the UMAP plots per tissue are shown in Figure 2. To compare the cell composition between tissues, the Welch t test was used, applied first on the 3 general cell types (B cells, T cells, and myeloid cells) and second on specific cell subtypes (Figure 3). We found significant differences in cell composition between mELT and SLOs, despite the limited sample number (n = 6 for mELT and LNlumbar, n = 3 for LNinguinal, n = 2 for spleen). mELT contains significantly less T cells (LNlumbar: p = 1.4 × 10−5; LNinguinal: p = 2.9 × 10−5) and more B cells (LNlumbar: p = 7.4 × 10−3; LNinguinal: p = 2.2 × 10−2) compared with both lymph node subsets. Within T-cell subtypes, we found a higher proportion of Tregs in mELT compared with that in SLOs (significant for mELT compared with LNinguinal, p = 8.3 × 10−4). Moreover, CD4+ T cells tend to be more activated in mELT compared with those in SLOs (significantly less naïve CD4+ T cells in mELT compared with those in LNlumbar, p = 3.3 × 10−4). In addition, we found significantly more granulocytes in mELT compared with those in both lymph node subsets (LNlumbar: p = 1.4 × 10−3; LNinguinal: p = 3.5 × 10−3).
Table 1.
Cell Composition per Tissue
| mELT | LN_lumbar | LN_inguinal | Spleen | |
| Follicular zone B cells, n (%) | 8,666 (49.6) | 4,508 (23.4) | 2,849 (28.1) | 5,750 (30.1) |
| Marginal zone B cells, n (%) | 804 (4.6) | 413 (2.2) | 286 (2.8) | 2003 (10.5) |
| Germinal center B cells, n (%) | 151 (0.9) | 205 (1.1) | 25 (0.3) | 104 (0.5) |
| Plasma cells, n (%) | 132 (0.8) | 58 (0.3) | 7 (0.07) | 46 (0.2) |
| Naive CD4+ T cells, n (%) | 2,471 (14.1) | 9,485 (49.3) | 4,969 (49.1) | 6,939 (36.3) |
| Activated CD4+ T cells, n (%) | 587 (3.4) | 343 (1.8) | 290 (2.9) | 299 (1.6) |
| Regulatory T cells, n (%) | 924 (5.3) | 544 (2.8) | 335 (3.3) | 509 (2.7) |
| CD8+ T cells, n (%) | 181 (1.0) | 560 (2.9) | 232 (2.3) | 286 (1.5) |
| γδ T cells, n (%) | 132 (0.8) | 996 (5.2) | 544 (5.4) | 463 (2.4) |
| Dendritic cells, n (%) | 321 (1.8) | 600 (3.1) | 154 (1.5) | 248 (1.3) |
| Granulocytes, n (%) | 1,157 (6.6) | 100 (0.5) | 42 (0.4) | 374 (2.0) |
| Macrophages, n (%) | 1,089 (6.2) | 530 (2.8) | 54 (0.5) | 247 (1.3) |
| Monocytes, n (%) | 267 (1.5) | 51 (0.3) | 23 (0.2) | 133 (0.7) |
| NK cells and NK T cells, n (%) | 334 (1.9) | 746 (3.9) | 289 (2.9) | 1,250 (6.5) |
| Erythroid cells, n (%) | 254 (1.5) | 91 (0.5) | 29 (0.3) | 460 (2.4) |
Total number of cells (over all mice) and the percentage of cells per cell type are given for each tissue.
Figure 3. Distinct Changes in Cell Composition Distinguish Meningeal Ectopic Lymphoid Tissue From Secondary Lymphoid Organs.
Plot of differences in cluster abundance in mELT compared with inguinal lymph nodes, lumbar lymph nodes, and spleen, plotting fold change (log10) against p value (-log10) based on the Welch t test (see methods for details). Horizontal dotted lines indicate significance threshold (p = 0.05), before (red) and after (orange) Bonferroni correction for multiple testing. n = 6 for mELT and lumbar LN, n = 3 for inguinal LN, and n = 2 for spleen.
Immune Cells in mELT Are in a More Activated and Proinflammatory State Than Their Counterparts in SLOs
To identify key gene expression differences between mELT and SLOs, we performed differential gene expression analysis for each of the cell clusters per tissue comparison. Lists of differentially expressed genes between mELT and each of the SLOs are summarized in eTables 2–5 (links.lww.com/NXI/A943, links.lww.com/NXI/A944, links.lww.com/NXI/A945, links.lww.com/NXI/A946), and the top 10 genes per cell type are shown in Figure 6 for LNlumbar and in eFigure 2 (links.lww.com/NXI/A950) for LNlinguinal and spleen. The total number of upregulated and downregulated genes per cell type and per tissue is shown in Figure 4A. Overall, most of the differentially expressed genes were upregulated in mELT compared with those in SLOs.
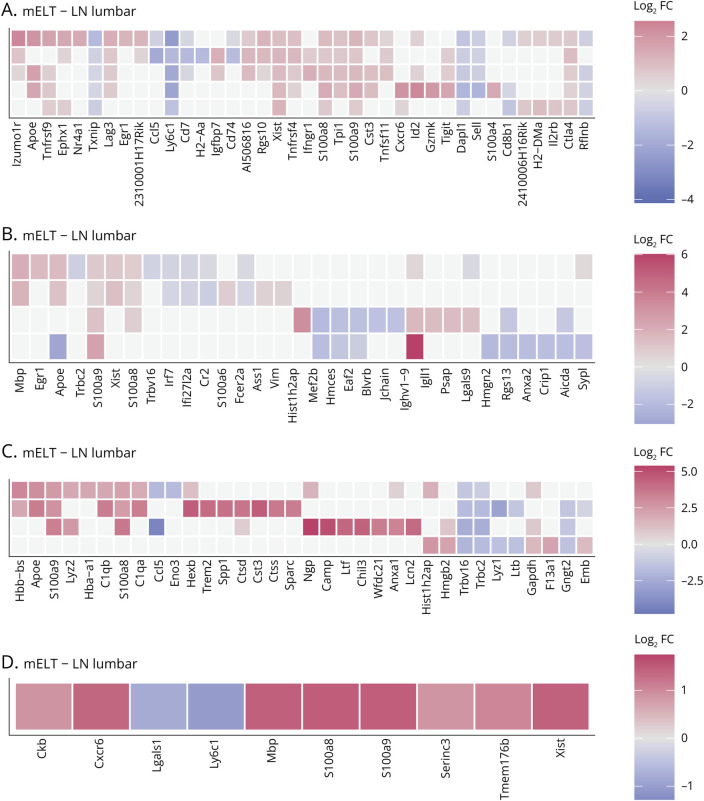
Figure 6. Differential Gene Expression in Meningeal Ectopic Lymphoid Tissue Compared With Lumbar Lymph Nodes.
Top 10 differentially expressed genes per cell cluster between mELT and lumbar lymph nodes for T cells (A), B cells (B), myeloid cells (C), and the NK- and NKT-cell cluster (D). Heatmaps showing average log2 fold change (FC) for the top 10 genes (highest FC) for each cell cluster. Genes are ordered by the cluster in which they were selected; if they were in the top 10 of multiple cell clusters, they were shown only once. Genes that show higher expression in mELT are shown in red (positive FC) and genes showing lower expression in mELT (negative FC) in blue. Genes that were not significantly differentially expressed are shown in gray. Cell clusters that are missing for specific tissue comparisons were not analyzed because too little cells were picked up in that particular tissue (<50 cells). Ribosomal protein genes were not considered. A similar figure for the top 10 differentially expressed genes between meningeal ectopic lymphoid tissue and inguinal lymph nodes, or spleen, can be found in eFigure 2 (links.lww.com/NXI/A950).
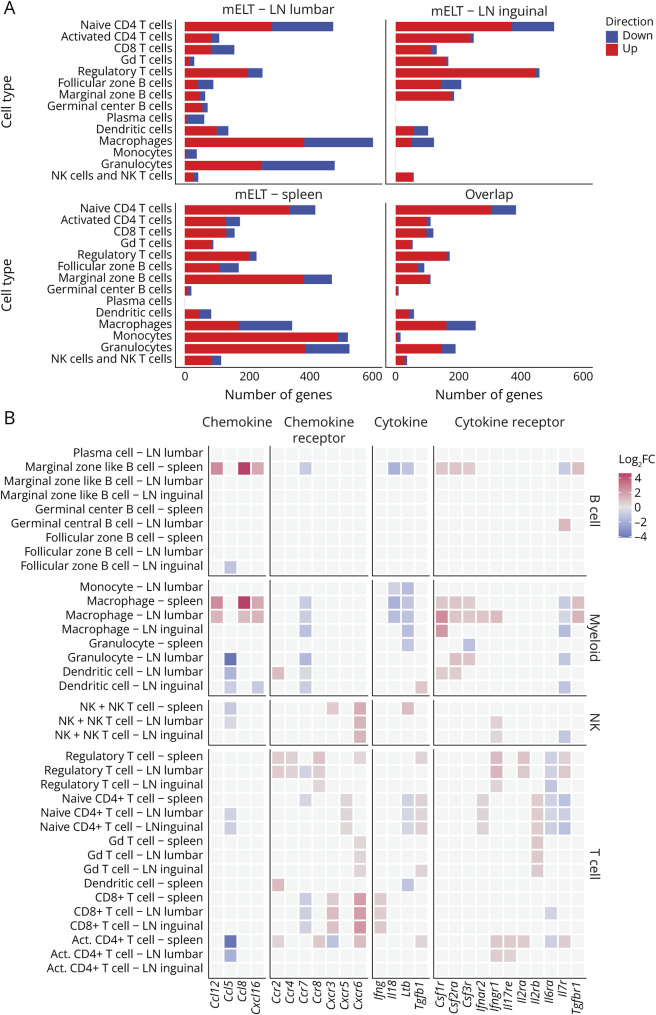
Figure 4. Gene Expression Analysis Defines the Pro- and Anti-inflammatory Profile of Meningeal Ectopic Lymphoid Tissue.
(A) Number of differentially expressed genes (DEG) between mELT and SLOs per cell cluster. x-axis represents the number of DEGs between mELT and lumbar lymph nodes (LN), inguinal LN, and spleen. The overlap contains all genes significantly differentially expressed between mELT and at least 2 of 3 of the SLOs. Downregulated genes are shown in blue; upregulated genes are shown in red. The y-axis shows the cell clusters. Ribosomal protein genes were not counted. (B) Cytokine-related gene expression differences between mELT and SLOs. Color reflects log2 fold change (FC) between mELT and the indicated SLOs (LN lumbar, LN inguinal, or spleen). Gray squares mean there was no significant difference (significance level p = 0.05). Cytokine genes shown are the ones for which the authors found significant differences in at least 2 of 3 tissue comparisons for at least one of the cell types.
To get an overview of the major functional differences between mELT and SLOs, we created a gene list containing all genes that were significantly differentially expressed between mELT and at least 2 of 3 of the SLOs (lumbar LN, inguinal LN, and spleen) (eTable 5, links.lww.com/NXI/A946), and performed functional enrichment analysis on this gene list using Metascape (Figure 5, eTable 6, links.lww.com/NXI/A947).18 Functional enrichment analysis was only performed on the differentially expressed genes that showed higher expression in mELT, compared with SLOs, as the number of genes downregulated in mELT compared with SLOs were mostly too low (<25 genes). The overarching theme resulting from this analysis, was that immune cells in mELT were in a more activated and pro-inflammatory state compared with their counterparts in SLOs.
Figure 5. Functional Enrichment Analysis for Genes Differentially Expressed Between mELT and SLOs Using Metascape.
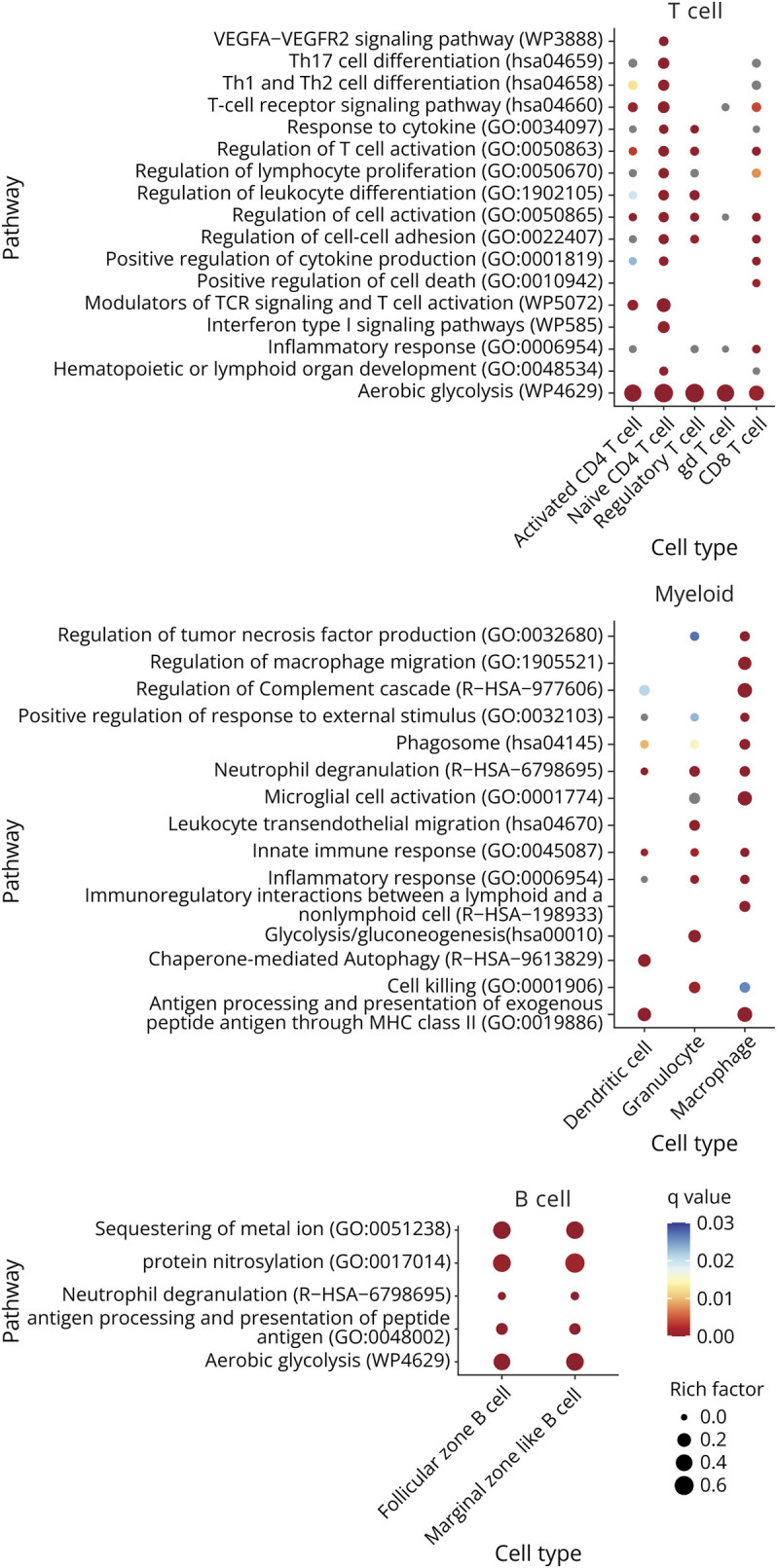
The overlap gene list (see Figure 4A) was used as input for the functional enrichment analysis. Figure shows a summary of the statistically most enriched pathways (adjusted p value<0.001 for at least 1 cell type) for T-cell, B-cell, and myeloid cell subtypes. Redundant pathways were collapsed into a single biological theme. The dot size reflects the rich factor (ratio of the DEG number and the total number of genes annotated in this GO term) and its color the corresponding statistical significance (q value, Bonferroni corrected). For gray dots, the pathway was significant based on p value, but not on q value. Absence of a dot indicates the annotation was not significantly enriched for this cell type. Cell clusters that are missing for specific tissue comparisons were not analyzed because either too little cells were picked up (<50 cells), or the amount of differentially expressed genes was too small (<20). Ribosomal protein genes were not considered for functional enrichment analysis. Complete functional enrichment analysis results are summarized in eTable 6 (links.lww.com/NXI/A947).
First, we found that genes upregulated in mELT compared with those in SLOs showed enrichment for pathways related to glycolysis, especially in B and T cells. Indeed, glycolytic enzymes (Gapdh, Aldoa, Eno1, Pkm, Gpi1, and Tpi1) were highly upregulated in mELT in all cell clusters. In addition, mELT granulocytes expressed more ADP-dependent glucokinase (Adpgk), involved in shifting metabolism to a highly glycolytic phenotype, typical for activated immune cells to provide energy to fuel inflammation.19 Besides, the macrophage cell cluster showed enrichment for genes related to microglia activation.
In T cells specifically, we found enrichment of pathways related to T-cell activation, proliferation, and differentiation and for T-cell receptor signaling, cell-cell adhesion, and response to cytokines. In addition, naïve CD4+ T cells were enriched for genes related to VEGFA-VEGFR2 and interferon type I signaling pathways and to Th1, Th2, and Th17 cell differentiation. In line with this, the naive CD4+ T-cell cluster showed higher expression of Ifnar2, and the activated CD4+ T-cell cluster showed higher expression of Il17re than SLOs, which is typically expressed on Th17 cells. CD8+ T cells in mELT were found to express higher levels of interferon gamma (Ifng) compared with the ones in SLOs. Interferon gamma is traditionally associated with CD8+ T-cell effector function and cytotoxicity.20 In addition, the cytotoxic protease Granzyme K (Gzmk) was found to be upregulated in mELT.
Next, antigen processing and presentation, which is essential for T-cell activation, was found to be enriched in B cells and myeloid cells in mELT. This was mainly driven by an upregulation of both classical and nonclassical MHCII genes in mELT compared with SLOs in antigen presenting cells (FZ and MZ B cells, DCs, and macrophages) (eTables 2–4, links.lww.com/NXI/A943, links.lww.com/NXI/A944, links.lww.com/NXI/A945). Myellowed cells showed additional enrichment for pathways related to effector functions, including neutrophil degranulation, phagocytosis, and cell killing (granulocytes and macrophages). Accordingly, granulocytes in mELT expressed higher levels of granule genes such as Camp, Ltf, Lcn2, Mmp8, Ngp, and Chil3 than the ones in SLOs. Granulocytes in mELT also showed higher expression of activation marker Cd177, compared with the ones in SLOs.21,22 Similarly, also in macrophages, we found evidence for enhanced effector function, including upregulation in mELT of genes encoding hydrolytic enzymes (Hexb, Ctsd, Ctss, Ctsl) and cathepsins (Ctsb, Ctsbz, Ctsd, and Ctss), which degrade ingested particles in the phagolysosome after macrophage activation.
Moreover, both T cells and myeloid cells showed enrichment for genes related to inflammatory response. Indeed, we found that monocytes in mELT are in a more proinflammatory state (higher expression of F13a1 and Ly6c2) compared with the ones in SLOs. In T cells (except for gd T cells) mELT showed elevated expression of Bhlhe40, which promotes the expression of proinflammatory genes, while inhibiting the expression of anti-inflammatory factors. Furthermore, the proinflammatory genes S100a8 and S100a9 (calprotectin) were upregulated in mELT in practically all cell clusters. Calprotectin is known to be abundantly expressed by neutrophils but can be induced in nonmyeloid cells on inflammation.23 In macrophages, the colony-stimulating factor 1 receptor (Csf1r) and colony-stimulating factor 2 receptor subunit alpha (Csf2ra) show higher expression in mELT compared with SLOs. Csfr2 is the receptor for the proinflammatory cytokine GM-CSF, and under inflammatory conditions, also Csf1r has a proinflammatory function, by sustaining the survival and expansion of inflammatory myeloid cells.24
Finally, T and B cells in mELT showed higher expression of activation markers and activity-induced genes than the ones in SLOs (eTables 2–4, links.lww.com/NXI/A943, links.lww.com/NXI/A944, links.lww.com/NXI/A945, Figure 6, eFigure 2, links.lww.com/NXI/A950). In B cells, immediate early genes (IEG) (Egr1, Ier2, Jun, Junb, and Fos), which are rapidly induced as response to stimulation through antigen receptor or cytokines,25 showed higher expression in mELT compared with those in SLOs (Jun and Fos in FZ B cells only; others in both FZ and MZ B cells). Along the same line, the top overrepresented transcription factor (TF) for genes upregulated in mELT in FZ and MZ B cells (eTable 7, links.lww.com/NXI/A948) included several immediate early response TFs (incl. Jun, Junb, Jund, Klf2, Atf3, Fosb, and Nr4a1 for both FZ and MZ B cells and Egr2 specifically for FZ B cells). In addition, BAFF-receptor (Tnfrsf13c)—one of the main prosurvival factors in B cells—showed higher expression in mELT in both FZ and MZ B cells, as did the activation marker Cd83. Finally, complement receptor 2 (Cr2/Cd21), an activating coreceptor for B cells, which is known to be downregulated following B-cell activation, was expressed at a lower level in mELT compared with that in SLOs in both FZ and MZ B-cell clusters. Ighv1-9, which is the immunoglobulin heavy variable gene of the knock-in in 2D2xTh mice, is significantly higher expressed in mELT compared with that in SLOs in follicular zone B cells. Specifically, in mELT vs lumbar lymph nodes, we find higher levels of the knock-in in mELT in germinal center B cells and plasma cells. This implies that potentially MOG-specific B-cell receptors are expressed at higher levels in mELT than in SLOs. Together, this suggests B cells in mELT show a higher level of activation than the ones in SLOs, and elevated IEG expression suggests recent antigen stimulation. Activation-induced cytidine deaminase (Aicda), which is specifically expressed in germinal center B cells, where it is involved in somatic hypermutation, gene conversion, and class-switch recombination, was expressed in mELT and SLOs. This suggests the presence of active germinal centers in both tissues. Expression levels in mELT were similar to the ones found in spleen, but lower compared with Aicda levels in lumbar lymph nodes.
In T cells, mELT showed higher expression of genes that are typically induced on T-cell stimulation (eTables 2–4, links.lww.com/NXI/A943, links.lww.com/NXI/A944, links.lww.com/NXI/A945, Figure 6A, eFigure 2A, links.lww.com/NXI/A950). One such example is Cish, which was found to be upregulated in mELT in all T-cell clusters except for naïve CD4+ T cells. In the naïve T-cell cluster, several markers that are transiently induced on T-cell activation (Nr4a1, Egr1, Myb, and Dusp2) were upregulated in mELT compared with that in SLOs. In addition, several TNF superfamily ligands and receptors that are known to be induced on T-cell activation showed higher expression in mELT compared with that in SLOs. The ligands Tnfsf8 (Cd30l) and Tnfsf11 (Rankl) were increased in mELT in naïve CD4+ T cells and in all CD4+ T-cell clusters (including Tregs), respectively. The TNF receptors Tnfrsf4 (O × 40), Tnfrsf18 (Gitr), and Tnfrsf9 (4-1bb) were increased in all T-cell clusters in mELT compared with those in SLOs (Tnfrsf9 was not found to be upregulated in activated CD4+ T cells, Tnfrsf18 only in CD4+ T cells). Tnfrsf4, Tnfrsf18, and Tnfrsf9 are all costimulatory receptors for T-cell activation. Moreover, TF target overrepresentation analysis using Chea3 showed that genes upregulated in T cells in mELT are enriched for target genes of Foxp3, Stat4, Tbx21 (Tbet), and Irf4 in all T-cell subclusters (eTable 7, links.lww.com/NXI/A948). All these transcription factors are typically induced on T-cell stimulation. In conclusion, the upregulation of genes known to be induced on T-cell activation in mELT shows that T cells in mELT are in a more activated state than their counterparts in SLOs, suggesting that, among the tissues examined in our study, mELT is the lymphoid tissue most relevant for T-cell autoimmunity in spontaneous EAE at the investigated time point in disease.
Regulatory Mechanisms Are in Place to Keep Inflammation in Check
Besides the costimulatory receptors described earlier, T cells in mELT also showed upregulation of several coinhibitory receptors, including Pdcd1, cytotoxic T lymphocyte antigen-4 (Ctla4), Lag3, and Tigit. These inhibitory receptors have been associated with T-cell exhaustion. They are, however, also known to be strongly upregulated on T-cell activation, and their upregulation could therefore as well reflect T-cell activation and differentiation. Pdcd1 and Ctla4 were upregulated in mELT in all T-cell clusters, while Lag3 and Tigit were not upregulated in gd T cells (both) and naïve CD4+ T cells (Tigit). Several of these coinhibitory receptors, including Ctla4 and Lag3, are also known to be involved in Treg suppressive activity. Along the same line, the expression of other Treg signature genes including Ikzf2, Izumo1r, and Il2ra were found to be upregulated in mELT compared with that in SLOs. Of these, Izumo1r was upregulated in mELT in all T-cell clusters. Transcription factor Helios (Ikzf2) was found to be upregulated in mELT in naïve CD4+ T cells and to a lesser extent also in CD8+ T cells, while Il2ra was upregulated in Tregs only. Furthermore, the genome organizer Satb1 was downregulated in mELT in the naïve CD4+ T-cell and CD8+ T-cell clusters. Satb1 downregulation in T cells has been linked to a heightened susceptibility to suppression by Tregs.26 As mentioned earlier, TF target overrepresentation analysis showed enrichment for target genes of Foxp3 in the genes upregulated in mELT T cells compared with their counterparts in SLOs (eTable 7, links.lww.com/NXI/A948). Foxp3 is best known as maker for Tregs, but is also transiently induced after T-cell stimulation in different T-cell subsets, and is associated with hyporesponsiveness of activated T cells.27 In addition, there was also enrichment for target genes of Ikzf3, which is associated with IL-10 expression. These findings indicate that the continuous T-cell stimulation occurring in mELT may render T cells hyporesponsive because many activity-induced genes have a suppressive function.
In addition, apolipoprotein E (Apoe), one of the most differentially expressed genes overall in our data, exerts anti-inflammatory and neuroprotective effects in the CNS 2,28 in addition to its lipid-related properties. Apoe showed higher expression in mELT compared with that in SLOs in all T-cell subsets and FZ and MZ B cells, DCs, and macrophages as well. In the NK- and NKT-cell cluster, many target genes of the anti-inflammatory transcription factor Nr1h3 (LXRα) were found to be expressed higher in mELT compared with that in SLOs (including e.g., Tmem176a, Tmem176b, Ctsd, Cited4, and Ckb) (eTables 2–4, links.lww.com/NXI/A943, links.lww.com/NXI/A944, links.lww.com/NXI/A945). Accordingly, Nr1h3 was the top overrepresented TF for the list of genes upregulated in mELT in the NK and NKT-cell cluster (eTable 7, links.lww.com/NXI/A948). Furthermore, the naïve CD4+ T cell and Treg clusters showed lower levels of expression of the IL6 receptor (Il6ra) compared with SLOs (Figure 4B, eTables 2–4) and are therefore less responsive for classical signaling of the proinflammatory cytokine IL6. Finally, naïve CD4+ T cells in mELT showed higher expression of the regulatory cytokine gene-transforming growth factor beta 1 (Tgfb1) compared with the ones in SLOs (Figure 4B, eTables 2–4), and the macrophage cluster showed lower expression of the inflammatory cytokine IL18.
Together with the fact that mELT contains relatively more regulatory T cells than SLOs, these findings indicate that there are mechanisms in place to mitigate the inflammatory response.
mELT Macrophages Can Stimulate the Recruitment of Other Immune Cells, While DCs and T Cells Upregulate Chemokine Receptors That Direct Their Migration Into the Inflamed CNS
Functional enrichment analysis indicated that pathways related to migration were differentially regulated between mELT and SLOs in myeloid cells (granulocytes, macrophages, and DCs) (eTable 6, links.lww.com/NXI/A947, Figure 5). Along the same line, we found that several chemokines and chemokine receptors were differentially expressed between mELT and SLOs (Figure 4B, eTables 2–4, links.lww.com/NXI/A943, links.lww.com/NXI/A944, links.lww.com/NXI/A945). Macrophages in mELT show higher expression of chemokines Ccl8, Ccl12, and Cxcl16, all of which can stimulate the recruitment of immune cells. In addition, macrophages in mELT express more Sparc. Sparc enhances immune cell migration and was shown to promote recruitment of antigen-specific T cells to the inflamed CNS.29 In T cells and DCs, by contrast, we found differential expression of chemokine receptor genes: naïve CD4+ T cells in mELT showed higher expression of Cxcr5. Cxcr5 is known to be expressed on follicular helper T (TFH) cells and makes these cells sensitive to recruitment through Cxcl13 signaling, which was found to be produced by stromal cells in the leptomeninges of secondary progressive MS and in actively demyelinating lesions.30 Treg in mELT express more Ccr2, CCr4 and Ccr8, and CD8+ T cells in mELT express higher levels of Cxcr6 and Cxcr3, but less Ccr7. Moreover, VLA-4 (Itga4), an integrin subunit that is essential for the migration of T cells into the CNS,31 showed upregulation in mELT CD8+ T cells. DCs in mELT have higher Ccr2 expression compared with SLOs, which play a role in DC recruitment into the CNS,32 but lower Ccr7 expression, which is necessary to direct DCs to secondary lymphoid nodes.33
These results suggest that macrophages in mELT can stimulate the recruitment of other immune cells to the inflamed CNS and in this way possibly contribute to ectopic lymphoid tissue formation. In addition, T cells and DCs in mELT have enhanced expression of chemokine receptors that enable their recruitment into the inflamed CNS.
Discussion
Comparing mELT with SLOs in a model of chronic CNS autoimmunity by single-cell RNA sequencing, we confirmed that all major immune cell types prevalent in SLOs could also be found in mELT, clearly identifying mELT as tertiary lymphoid tissue. However, the proportion of the 2 major cell types, T and B lymphocytes, was characteristically shifted toward B cells. This has been a consistent finding in the literature both in MS and the specific EAE model we applied.1,34 This led other authors to refer to mELT as B-cell rich meningeal follicles or B-cell aggregates. Within the T-cell compartment, a shift was detectable away from naïve T cells, which dominate SLOs, toward activated T cells in mELT. Our data did not allow further subclustering of activated T cells to identify distinct Th1, Th2, or Th17 subpopulations.
To date, less is known about B-cell and T-cell subsets and other immune cell types in mELT. Small numbers of antibody-producing plasma cells have been described in meningeal niches in progressive MS and murine and primate EAE.1,35,36 Our study identified a small fraction of plasma cells in mELT. Their frequency seemed to be higher compared with SLOs, although this was not statistically significant. By contrast, the CSF of patients with MS is characterized by a high proportion of mostly short-lived plasma blasts.37 Controversial results exist regarding the pathologic relevance of myelin-reactive antibodies, which may originate from those plasma cells, in spontaneous EAE.38,39 Tregs have important implications in controlling GC reactions.40 While one study found hardly any T follicular regulatory cells in human postmortem meninges,41 mELT contained approximately 5% Treg in our model, almost twice as much as in SLOs.
Innate immune cells are also present in meningeal inflammation, yet much less frequent than lymphocytes. Our single-cell sequencing data showed that neutrophils are enriched in mELT compared with lymph nodes. While our data cannot clarify the biological relevance of the relative neutrophil abundance, it is noteworthy that neutrophil granulocytes may be critical for the formation of mELT by coordinating meningeal B-cell accumulation.42
Differential gene expression analysis and functional enrichment analysis revealed an overarching theme in mELT immune cells: a more activated and proinflammatory phenotype compared with their counterparts in SLOs, especially in T cells, B cells, and granulocytes. These 3 cell types similarly had upregulated expression of genes related to glycolysis. A relationship between glycolytic metabolites and the proinflammatory phenotype has been established because cells involved in the proinflammatory response rapidly provide energy by glycolysis.43 Both B and T cells were more activated in mELT compared with that in SLOs because they feature higher expression of immediate early genes, suggesting recent antigen stimulation. T cells, particularly, showed marked signs of activation and inflammatory response in mELT. Many genes typically upregulated on TCR activation were upregulated, including costimulatory and coinhibitory receptors. Moreover, various antigen-presenting cells in mELT, including B cells, DCs, and macrophages, exhibited higher expression of MHCII genes, potentially facilitating antigen-induced T-cell activation.
While immune cells in mELT were generally in a more activated and proinflammatory state, features of immune regulation were present in mELT at the same time. Not only did we observe more Treg in mELT compared with SLOs but also an increased expression of Treg signature genes, coinhibitory receptors, and T-cell exhaustion genes. Apoe, a gene known to have anti-inflammatory and neuroprotective effects, was one of the most differentially expressed genes in our dataset, showing upregulation. These examples highlight that mELT, in principle, inherently possess the capacity to keep its immune response in check.
Many of the top differentially expressed genes between mELT and SLOs have previously been linked to EAE, MS, or autoimmune disease in general. For example, in T cells, we found upregulation of Bhlhe40 in mELT, while mice deficient for Bhlhe40 were found to be resistant to the induction of EAE.44 In naïve CD4+ T-cell and CD8+ T-cell clusters, Satb1 expression was downregulated in mELT. Mice with conditional knockout of Satb1 in T cells were found to develop autoimmune disease, and interestingly, they also showed infiltrations of inflammatory cells in various organs.45 Itga4, which we found to be upregulated in CD8+ T cells in mELT, is a therapeutic target used in the treatment of MS (natalizumab) because it mediates the ability of leukocytes to cross the BBB.46 In addition, Cr2, which we found to be downregulated in mELT B cells, is believed to provide protection against autoimmunity.47 Moreover, expression of Csf1r, which we found to be upregulated in mELT macrophages, was also upregulated in CNS tissue of patients with MS compared with that in healthy controls.48 Finally, in our study, Apoe showed higher expression in mELT compared with that in SLOs in almost all cell clusters. An association was found between Apoe genotype and the severity and progression of MS.28 Similarly, genetic variants of Nr1h3 are associated with primary progressive MS.49 Several target genes of this TF were upregulated in mELT in the NK and NKT cell cluster. Nr1h3 itself was not upregulated in mELT, suggesting differential activation of this TF between mELT and SLOs.
In summary, many genes previously linked to autoimmune disease were differentially expressed between mELT and SLOs. This makes them interesting potential targets for therapy because targeting these genes could result in disruption of mELT formation, with lesser effects on SLOs. In addition, this further emphasizes that mELT is more relevant for autoimmunity in spontaneous EAE than SLOs.
Overall, these differences in cellular composition and gene expression between mELT and SLOs point to a crucial role for mELT in spontaneous EAE. Compared with SLOs, immune cells in mELT are more activated and proinflammatory. This is in contrast to the outside-in hypothesis in MS, which postulates an autoimmune attack against CNS tissue driven by the periphery through an impaired BBB. However, because we only studied 1 time point in fully established EAE, we cannot exclude that this occurred earlier in the course of the disease. Yet, mELT may represent the critical lymphoid tissue for initiation and/or maintenance of CNS inflammation, making it a therapeutic target. Because B cells predominate in mELT and given their efficacy in treating relapsing MS, they are obvious targets. Although anti-CD20 depletes B cells from mELT, it failed to ameliorate spontaneous EAE or prevent the formation of mELT altogether.16 As reported in the Ocrelizumab Biomarker Outcome Evaluation trial, B cell-depleting ocrelizumab significantly decreased CSF B cells in progressive and relapsing MS.50 Refining B-cell modulating strategies by Bruton tyrosine kinase (BTK) inhibitors may be a valid approach. As small molecules, BTK inhibitors readily reach the CNS. Strategies that target the immune response more broadly beyond the B-cell compartment, such as S1P receptor modulation by siponimod, have demonstrated promising results, both ameliorating spontaneous EAE and reducing mELT formation.17 The notion that mELT contains more activated immune cells compared with SLOs in spontaneous EAE suggests that BBB penetration will be crucial, favoring small molecules. Gene expression data of the most differentially expressed genes between mELT and SLOs, as provided in this study, may be helpful to identify novel potential targets.
Further studies will be required to determine to which degree insights from this study in a genetically determined EAE model can be applied to MS. The heterogeneity of meningeal inflammation in MS, ranging from loose B-cell aggregates to highly organized follicle-like structures, has to be taken into account.1,9,10 Furthermore, it would be informative to investigate single-cell gene expression in mELT at different time points, such as early in its formation, at various time points of the disease course, and under certain therapeutic interventions, such as B-cell depletion or modulation. While existing data suggest that affinity maturation of MOG-specific B cells occurs in mELT,5 future studies, which investigate the time course of proinflammatory and anti-inflammatory properties of immune cells in mELT, should include analyses of B-cell and T-cell receptor evolution in mELT in more detail.
In conclusion, mELT comprises all cellular components of a lymphoid organ and should be considered tertiary lymphoid tissue. Differences in cellular composition and particularly in gene expression compared with SLOs in spontaneous EAE show that immune cells in mELT are more activated compared with those in SLOs, suggesting that mELT is a main driver of chronic CNS inflammation. At the same time, we observed an increase in regulatory T cells and upregulation of immunosuppressive genes in various cell types. This suggests that regulatory mechanisms are in place, which limit the autoimmune response in mELT. Our data provide a starting point for future studies of the immune response in mELT in EAE and potentially MS and how to interrupt it.
Acknowledgment
The authors thank Francesca Romana de Frachis, Melina Pekic Hajdarbasic, and Beatrix Lunk (all TUM) for excellent technical assistance and Katja Steiger and Olga Seelbach (Comparative Experimental Pathology (CEP), TUM) for histologic analysis. Sequencing was performed at the Helmholtz Zentrum München (HMGU) by the Genomics Core Facility.
Glossary
- BBB
blood-brain barrier
- BTK
Bruton tyrosine kinase
- EAE
experimental autoimmune encephalomyelitis
- ELT
ectopic lymphoid tissue
- LN
lymph nodes
- mELT
meningeal ectopic lymphoid tissue
- MS
multiple sclerosis
- SLOs
secondary lymphoid organs
Appendix. Authors
| Name | Location | Contribution |
| Jolien Diddens, MSc | Department of Neurology, School of Medicine, Technical University of Munich, Germany | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Gildas Lepennetier, PhD | Department of Neurology, School of Medicine, Technical University of Munich, Germany | Analysis or interpretation of data |
| Verena Friedrich, PhD | Department of Neurology, School of Medicine, Technical University of Munich, Germany | Major role in the acquisition of data |
| Monika Schmidt, BSc | Department of Neurology, School of Medicine, Technical University of Munich, Germany | Major role in the acquisition of data |
| Rosa M. Brand, MD | Department of Neurology, School of Medicine, Technical University of Munich, Germany | Major role in the acquisition of data |
| Tanya Georgieva, MD | Department of Neurology, School of Medicine, Technical University of Munich, Germany | Major role in the acquisition of data |
| Bernhard Hemmer, MD | Munich Cluster of Systems Neurology (SyNergy), Germany | Drafting/revision of the article for content, including medical writing for content |
| Klaus Lehmann-Horn, MD | Department of Neurology, School of Medicine, Technical University of Munich, Germany | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
Study Funding
B. Hemmer received funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology [EXC 2145 SyNergy - ID 390857198] and the European commission (MultipleMS). K. Lehmann-Horn received research support from the Deutsche Forschungsgemeinschaft (SFB-TR-128, projects A4 and A12, and LE 3079/3-1), the Hertie Foundation (MyLab program), and the US National Multiple Sclerosis Society (NMSS) (G-1508-07064).
Disclosure
J. Diddens, G. Lepennetier, V. Friedrich, M. Pfaller, and T. Georgieva report no disclosures relevant to the manuscript. R.M. Brand is a fellow of the Hertie Foundation (medMS Doctoral Program). B. Hemmer has served on scientific advisory boards for Novartis and Sandoz; he has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon, and TG therapeutics; his institution received research grants from Roche for multiple sclerosis research. He has received honoraria for counseling (Gerson Lehrmann Group). He holds part of 2 patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and the other for genetic determinants of neutralizing antibodies to interferon. All conflicts are not relevant to the topic of the study. K. Lehmann-Horn has received research support (to TUM) from Novartis and honoraria and compensation for travel expenses from Novartis, F. Hoffmann-La Roche, Biogen, Teva, Hexal, and Merck Serono. Go to Neurology.org/NN for full disclosures.
References
- 1.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14(2):164-174. doi: 10.1111/j.1750-3639.2004.tb00049.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhan J, Kipp M, Han W, Kaddatz H. Ectopic lymphoid follicles in progressive multiple sclerosis: from patients to animal models. Immunology. 2021;164(3):450-466. doi: 10.1111/imm.13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negron A, Stüve O, Forsthuber TG. Ectopic lymphoid follicles in multiple sclerosis: centers for disease control? Front Neurol. 2020;11:607766. doi: 10.3389/fneur.2020.607766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones GW, Hill DG, Jones SA. Understanding immune cells in tertiary lymphoid organ development: it is all starting to come together. Front Immunol. 2016;7:401. doi: 10.3389/fimmu.2016.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann-Horn K, Wang SZ, Sagan SA, Zamvil SS, von Büdingen HC. B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue. JCI Insight. 2016;1(20):e87234. doi: 10.1172/jci.insight.87234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447-462. doi: 10.1038/nri3700 [DOI] [PubMed] [Google Scholar]
- 7.Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic lymphoid structures: powerhouse of autoimmunity. Front Immunol. 2016;7:430. doi: 10.3389/fimmu.2016.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SR, Howell OW, Carassiti D, et al. . Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain. 2012;135(Pt 10):2925-2937. doi: 10.1093/brain/aws189 [DOI] [PubMed] [Google Scholar]
- 9.Lucchinetti CF, Popescu BF, Bunyan RF, et al. . Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365(23):2188-2197. doi: 10.1056/NEJMoa1100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magliozzi R, Howell O, Vora A, et al. . Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089-1104. doi: 10.1093/brain/awm038 [DOI] [PubMed] [Google Scholar]
- 11.Reali C, Magliozzi R, Roncaroli F, Nicholas R, Howell OW, Reynolds R. B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis. Brain Pathol. 2020;30(4):779-793. doi: 10.1111/bpa.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haugen M, Frederiksen JL, Degn M. B cell follicle-like structures in multiple sclerosis-with focus on the role of B cell activating factor. J Neuroimmunol. 2014;273(1-2):1-7. doi: 10.1016/j.jneuroim.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 13.Howell OW, Reeves CA, Nicholas R, et al. . Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755-2771. doi: 10.1093/brain/awr182 [DOI] [PubMed] [Google Scholar]
- 14.Pikor NB, Prat A, Bar-Or A, Gommerman JL. Meningeal tertiary lymphoid tissues and multiple sclerosis: a gathering place for diverse types of immune cells during CNS autoimmunity. Front Immunol. 2015;6:657. doi: 10.3389/fimmu.2015.00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafflick D, Wolbert J, Heming M, et al. . Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci. 2021;24(9):1225-1234. doi: 10.1038/s41593-021-00880-y [DOI] [PubMed] [Google Scholar]
- 16.Brand RM, Friedrich V, Diddens J, et al. . Anti-CD20 depletes meningeal B cells but does not halt the formation of meningeal ectopic lymphoid tissue. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1012. doi: 10.1212/NXI.0000000000001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brand RM, Diddens J, Friedrich V, et al. . Siponimod inhibits the formation of meningeal ectopic lymphoid tissue in experimental autoimmune encephalomyelitis. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1117. doi: 10.1212/NXI.0000000000001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Zhou B, Pache L, et al. . Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imle R, Wang BT, Stützenberger N, et al. . ADP-dependent glucokinase regulates energy metabolism via ER-localized glucose sensing. Sci Rep. 2019;9(1):14248. doi: 10.1038/s41598-019-50566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krummel MF, Mahale JN, Uhl LFK, et al. . Paracrine costimulation of IFN-γ signaling by integrins modulates CD8 T cell differentiation. Proc Natl Acad Sci USA. 2018;115(45):11585-11590. doi: 10.1073/pnas.1804556115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou G, Yu L, Fang L, et al. . CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67(6):1052-1063. doi: 10.1136/gutjnl-2016-313535 [DOI] [PubMed] [Google Scholar]
- 22.Lévy Y, Wiedemann A, Hejblum BP, et al. . CD177, a specific marker of neutrophil activation, is associated with coronavirus disease 2019 severity and death. iScience. 2021;24(7):102711. doi: 10.1016/j.isci.2021.102711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang D, Seyedsadr MS, Ishikawa LLW, et al. . CSF-1 maintains pathogenic but not homeostatic myeloid cells in the central nervous system during autoimmune neuroinflammation. Proc Natl Acad Sci USA. 2022;119(14):e2111804119. doi: 10.1073/pnas.2111804119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gururajan M, Simmons A, Dasu T, et al. . Early growth response genes regulate B cell development, proliferation, and immune response. J Immunol. 2008;181(7):4590-4602. doi: 10.4049/jimmunol.181.7.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta PK, Allocco JB, Fraipont JM, et al. . Reduced Satb1 expression predisposes CD4+ T conventional cells to Treg suppression and promotes transplant survival. Proc Natl Acad Sci USA. 2022;119(40):e2205062119. doi: 10.1073/pnas.2205062119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129-138. doi: 10.1002/eji.200636435 [DOI] [PubMed] [Google Scholar]
- 28.Li FQ, Sempowski GD, McKenna SE, Laskowitz DT, Colton CA, Vitek MP. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J Pharmacol Exp Ther. 2006;318(3):956-965. doi: 10.1124/jpet.106.103671 [DOI] [PubMed] [Google Scholar]
- 29.McGovern KE, Nance JP, David CN, et al. . SPARC coordinates extracellular matrix remodeling and efficient recruitment to and migration of antigen-specific T cells in the brain following infection. Scientific Rep. 2021;11(1):4549. doi: 10.1038/s41598-021-83952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrer C, Otto F, Pilz G, et al. . The CXCL13/CXCR5-chemokine axis in neuroinflammation: evidence of CXCR5+CD4 T cell recruitment to CSF. Fluids Barriers CNS. 2021;18(1):40. doi: 10.1186/s12987-021-00272-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendirli A, de la Rosa C, Lämmle KF, et al. . A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model. Nature Neuroscience. 2023;26:1713-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarkson BD, Walker A, Harris MG, Rayasam A, Sandor M, Fabry Z. CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. J Immunol. 2015;194(2):531-541. doi: 10.4049/jimmunol.1401320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riol-Blanco L, Sánchez-Sánchez N, Torres A, et al. . The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol. 2005;174(7):4070-4080. doi: 10.4049/jimmunol.174.7.4070 [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, Baeten D, Jäger A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116(9):2393-2402. doi: 10.1172/JCI28334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramann N, Neid K, Menken L, et al. . Increased meningeal T and plasma cell infiltration is associated with early subpial cortical demyelination in common marmosets with experimental autoimmune encephalomyelitis. Brain Pathol. 2015;25(3):276-286. doi: 10.1111/bpa.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollok K, Mothes R, Ulbricht C, et al. . The chronically inflamed central nervous system provides niches for long-lived plasma cells. Acta Neuropathol Commun. 2017;5(1):88. doi: 10.1186/s40478-017-0487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cepok S, Rosche B, Grummel V, et al. . Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128(Pt 7):1667-1676. doi: 10.1093/brain/awh486 [DOI] [PubMed] [Google Scholar]
- 38.Molnarfi N, Schulze-Topphoff U, Weber MS, et al. . MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210(13):2921-2937. doi: 10.1084/jem.20130699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinzel S, Lehmann-Horn K, Torke S, et al. . Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta Neuropathol. 2016;132(1):43-58. doi: 10.1007/s00401-016-1559-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderleyden I, Linterman MA, Smith KG. Regulatory T cells and control of the germinal centre response. Arthritis Res Ther. 2014;16(5):471. doi: 10.1186/s13075-014-0471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell L, Lenhart A, Rosenwald A, Monoranu CM, Berberich-Siebelt F. Lymphoid aggregates in the CNS of progressive multiple sclerosis patients lack regulatory T cells. Front Immunol. 2019;10:3090. doi: 10.3389/fimmu.2019.03090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker Harp CR, Archambault AS, Cheung M, et al. . Neutrophils promote VLA-4-dependent B cell antigen presentation and accumulation within the meninges during neuroinflammation. Proc Natl Acad Sci USA. 2019;116(48):24221-24230. doi: 10.1073/pnas.1909098116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soto-Heredero G, Gómez de Las Heras MM, Gabandé-Rodríguez E, Oller J, Mittelbrunn M. Glycolysis—a key player in the inflammatory response. FEBS J. 2020;287(16):3350-3369. doi: 10.1111/febs.15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin CC, Bradstreet TR, Schwarzkopf EA, et al. . Bhlhe40 controls cytokine production by T cells and is essential for pathogenicity in autoimmune neuroinflammation. Nat Commun. 2014;5:3551. doi: 10.1038/ncomms4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondo M, Tanaka Y, Kuwabara T, Naito T, Kohwi-Shigematsu T, Watanabe A. SATB1 plays a critical role in establishment of immune tolerance. J Immunol. 2016;196(2):563-572. doi: 10.4049/jimmunol.1501429 [DOI] [PubMed] [Google Scholar]
- 46.Schwab N, Schneider-Hohendorf T, Wiendl H. Therapeutic uses of anti-α4-integrin (anti-VLA-4) antibodies in multiple sclerosis. Int Immunol. 2015;27(1):47-53. doi: 10.1093/intimm/dxu096 [DOI] [PubMed] [Google Scholar]
- 47.Isaák A, Prechl J, Gergely J, Erdei A. The role of CR2 in autoimmunity. Autoimmunity. 2006;39(5):357-366. doi: 10.1080/08916930600739001 [DOI] [PubMed] [Google Scholar]
- 48.Hagan N, Kane JL, Grover D, et al. . CSF1R signaling is a regulator of pathogenesis in progressive MS. Cell Death Dis. 2020;11(10):904. doi: 10.1038/s41419-020-03084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Sadovnick AD, Traboulsee AL, et al. . Nuclear receptor NR1H3 in familial multiple sclerosis. Neuron. 2016;90(5):948-954. doi: 10.1016/j.neuron.2016.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bar-Or A, Bennett J, Budingen HV, et al. . B cells, T cells and inflammatory CSF biomarkers in primary progressive MS and relapsing MS in the OBOE (Ocrelizumab Biomarker Outcome Evaluation) trial (1635). Neurology. 2020;94:1635. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw datasets generated and analyzed for this study can be found in the GEO repository, as records GSE181526 (experiment 1) and GSE182774 (experiment 2). All in-house scripts and R code were submitted to a Github repository.