Abstract
Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is an aggressive subtype of inflammatory myofibroblastic tumour which rarely affects the chest cavity. We, for the first time, report a case of mediastinal EIMS with the EML4‐ALK fusion. A young woman presented to our hospital with cough, chest tightness and shortness of breath. Computed tomography (CT) showed a mixed attenuation soft‐tissue mass in the right middle and upper mediastinum. Negative results were obtained from bronchoscopy forceps biopsy and endobronchial ultrasound‐guided transbronchial fine needle aspiration. CT‐guided percutaneous biopsy was finally performed. However, due to the rapidly progressed EIMS that compressed the trachea and right main bronchus, the patient died of respiratory failure 1 day before diagnosis. EIMS progresses rapidly, and an early diagnosis is important. For mediastinal EIMS, CT‐guided percutaneous biopsy may be useful. Next‐generation sequencing of blood may be instructive to EIMS patients who are intolerant to invasive biopsy.
Keywords: EML4‐ALK fusion, epithelioid inflammatory myofibroblastic sarcoma, inflammatory myofibroblastic tumour, mediastinal tumour, percutaneous mediastinal biopsy
Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is a highly aggressive, recurrent, and metastatic subtype of inflammatory myofibroblastic tumour (IMT). Here, we for the first time reported a case of mediastinal EIMS with the EML4‐ALK fusion. Unfortunately, the patient died of respiratory failure due to the rapidly progressing EIMS that compressed the trachea and right main bronchus, 1 day before diagnosis.
INTRODUCTION
Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is a highly aggressive, recurrent, and metastatic subtype of inflammatory myofibroblastic tumour (IMT). It is characterized by round‐to‐epithelioid cells, interstitial edema, mucoid degeneration, and neutrophil infiltration pathologically. EIMS mostly occurs in the abdominal cavity, and only a few cases have been reported in the thoracic cavity (Table 1). ALK rearrangements have been detected in most cases of EIMS, with the fusion gene consisting of RANB2, 1 RRBP1 2 and EML4, 3 among others. A complete surgical resection and oral administration of ALK inhibitors are first‐line treatments of EIMS. Here, we for the first time reported a case of mediastinal EIMS with the EML4‐ALK fusion. Unfortunately, the patient died of respiratory failure due to the rapidly progressing EIMS that compressed the trachea and right main bronchus, 1 day before diagnosis.
TABLE 1.
Clinical manifestations of reported cases of primary EIMS in the thoracic cavity.
| Authors (yr) | Age (yr)/sex | Tumour location | Size (cm) | Clinical manifestations | Diagnosis method | Immuno‐phenotype | Molecular change (detection method) | Treatment | Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Fu et al. (2015) 5 | 21/M | Left lower lobe lung | 10 | General fatigue and rapid weight loss within 3 months | Postoperative lung biopsy | Vimentin (+), Desmin (+), ALK (c) a , CD30 (−), SMA (−) | ALK rearrangement (FISH) | Lobectomy, followed by Crizotinib, 250 mg, b.i.d. | Pelvis and vertebrae metastases at 3 months after pulmonary surgery | Died 4 months after laminectomy |
| Sarmiento et al. (2015) 6 | 71/M | Pleura, left lower lobe | 12.5 | Dyspnea and weight loss for 3 months, and pleural effusion | Postoperative lung biopsy | Unknown | ALK rearrangement (FISH) | Lobectomy, adjuvant therapy with Crizotinib, and second‐line ALK inhibitors after disease progression | Absence | Remission at 1 yr of the last follow‐up visit |
| Kozu et al. (2014) 8 | 57/M | Pleura or chest wall | Not available | Dyspnea and pleural effusion | Thoracoscopic tumour biopsy | Desmin (+), cytokeratin (+), ALK (+, c), CD30 (−) | RANBP2‐ALK fusion (PCR) | Crizotinib | A short follow‐up period | A short follow‐up period |
| Singh et al. (2022) 9 | 25/M | Lung Right lower lobe | 7 | Cough, loss of weight, fever, dyspnea | Surgical lung biopsy | Desmin (+), focal cytokeratin (+), ALK (+, c), CD30 (−) | ALK rearrangement (FISH) | Crizotinib, lobectomy, palliative radiotherapy | Liver, bone | Asymptomatic at 4 months postoperatively |
| Azad et al. (2022) 7 | 53/F | Pericardium, pleura | Not available | Progressive fatigue and shortness of breath, bilateral pleural effusions and a large circumferential pericardial effusion | Postoperative lung biopsy | Desmin (+), CD30 (+), ALK (−) | No gene fusions (NGS) | Emergent sternotomy, chemotherapy | Absence | Acute hypoxic respiratory failure and transfer to comfort care |
| Present case | 33/F | Upper middle mediastinum | 7.2 | Cough, anterior chest pain, shortness of breath | CT‐guided percutaneous lung biopsy | Vimentin (+++), ALK (+), CD30 (+ in individual cells), CK‐P (−), SMA (focal +), Desmin (focal +) | ALK rearrangement (NGS) | Symptomatic supportive treatment | Lung | Died of respiratory failure |
c, cytoplasmic pattern.
CASE REPORT
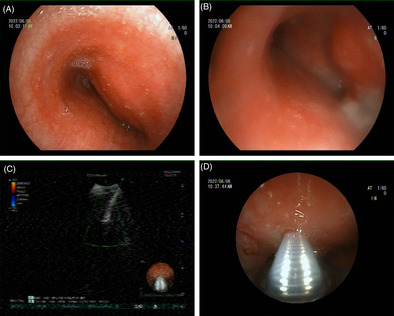
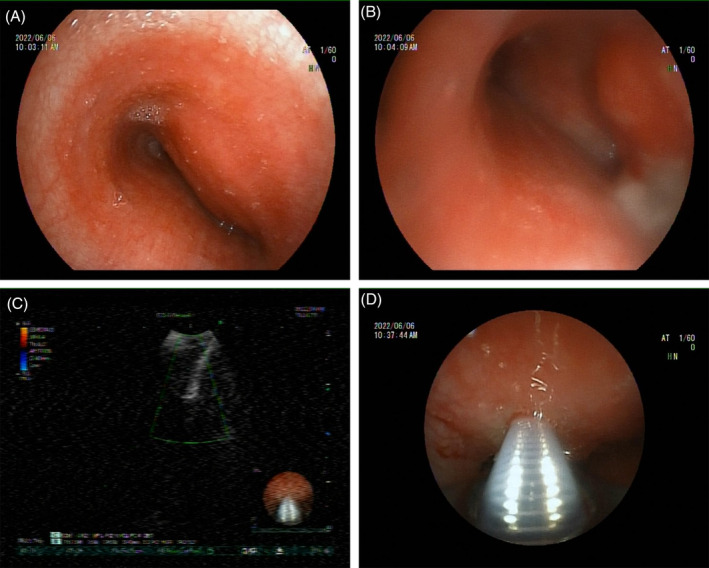
A 33‐year‐old woman had been experiencing a dry cough and throat discomfort for 1 month, which had not been relieved by intravenous antibiotics at a local community hospital. The patient presented aggravated cough, pain in the right anterior chest, chest tightness, wheezing, expectoration of a small amount of white and sticky sputum with blood. Enhanced CT scans of the chest revealed occupying lesions in the anterior middle mediastinum, enlarged lymph nodes in both axillas and under carina, and multiple nodules in both lungs. The patient was admitted for further diagnostic tests and treatment. On admission, she had a difficult breathing with the oxygen saturation (SpO2) of 89.0%. Under local anaesthesia, fiberoptic bronchoscopy performed on 1st day showed an obvious external pressure at a stenosis (Figure 1A). The bronchoscope was barely able to enter the left main bronchus due to the narrowing (Figure 1B). The swollen mucosae were easy to bleed, which were resected for biopsy. The endobronchial bleeding was then stopped by administering iced saline and topical epinephrine through the bronchus, and the bronchoscope was extubated after haemostasis. The tracheal carina was not detectable. Endobronchial ultrasound (EBUS) bronchoscopy was then performed, which revealed a lesion localized in the trachea (Figure 1C). Under the guidance of ultrasound, transbronchial needle aspiration (TBNA) was performed to obtain lesion tissues through 4 aspirations (Figure 1D). Pathological examinations on the 2nd day showed epithelial hyperplasia in mucosal tissues from the left main bronchus, with local interstitial haemorrhage, infiltration of a small amount of acute and chronic inflammatory cells and amorphous cellulose. Blood clots, inflammatory exudates and ciliated columnar epithelium were observed in lesions aspirated from the main bronchus, and tumour cells were not detectable (Figure 2). In massive blood clots, small flakes of fibrous tissue enriched with blood vessels were observed, surrounded by infiltration of scattered lymphocytes and plasma cells. Focal granuloma formation was also observed. Special stains were used to examine the granulomatous inflammation, which showed negative results for periodic acid‐Schiff (PAS), acid‐fast, and silver stains.
FIGURE 1.
Representative images of fiberoptic bronchoscopy (A, B) and EBUS‐TBNA (C, D). (A) An obvious external pressure at a stenosis. (B) Swollen mucosae at the opening of the left main trachea were easy to bleed, which were resected for biopsy. (C, D) EBUS bronchoscopy visualized a lesion localized in the trachea, followed by ultrasound‐guided TBNA.
FIGURE 2.
Representative images of biopsy near the opening of the left main bronchus and the lesion in the main trachea collected by EBUS‐TBNA. H&E staining revealed blood clots, inflammatory exudates and absence of tumour cells (A), and blood clots, inflammatory exudates, ciliated columnar epithelium and absence of tumour cells (B). Scale bar = 50 μm.
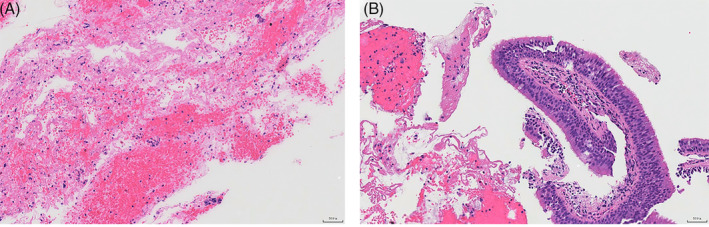
On the 4th day, the patient coughed up 10 mL of blood, and suffered pain in the right anterior chest, and aggravated asthma when coughing, which was managed with hemostatic treatment. An enhanced CT scan of the chest was performed (Figure 3), revealing (1) a mixed attenuation soft‐tissue mass (7.2 × 7.1 cm) in the right middle and upper mediastinum that was adjacent to the hilum, which was in the heterogeneous enhancement pattern, considering a malignant tumour of suspicious mediastinal mass; (2) narrowed, and partially occluded right main bronchus and some branches; (3) lymph node metastases in the mediastinum and right hilar lung, and multiple lung metastases. The patient experienced aggravated dyspnea on the 8th day, with inspiratory wheezing and positive three concave sign. After a multidisciplinary team discussion, CT‐guided percutaneous biopsy was performed. The patient was placed in a supine position, and the optimal puncture site was determined by CT scans (Figure 4A). Lesions were collected a total of 6 times using a needle puncture of 10 cm in length. CT scans after biopsy showed a small amount of haemorrhage, but pneumothorax was not detectable (Figure 4B). In the early morning of the 10th day, the patient experienced aggravated shortness of breath, difficulty in expectoration, an apathetic expression, and sweaty, cold skin. The arterial blood gas (ABG) test indicated type II respiratory failure and respiratory acidosis. A series of corresponding interventions were given, including electrocardiogram (ECG) monitoring, pulse oximeter monitoring, non‐invasive mechanical ventilation, respiratory support and sodium bicarbonate administration. At 05:30, the patient's heart rate and blood pressure rapidly dropped, despite receiving mechanical ventilation through oral intubation, vasoactive drugs, and volume expansion. The patient finally succumbed to respiratory failure.
FIGURE 3.
Representative images of enhanced CT of the chest. (A) A mixed attenuation soft‐tissue mass (7.2 × 7.1 cm) in the right middle and upper mediastinum that was adjacent to the hilum. (B) Involvement of the narrowed, and partially occluded right main bronchus and some branches. Lymph node enlargement below the tracheal eminence. (C) An obvious heterogeneous enhancement pattern of the mass. (D) Sagittal images of CT.
FIGURE 4.
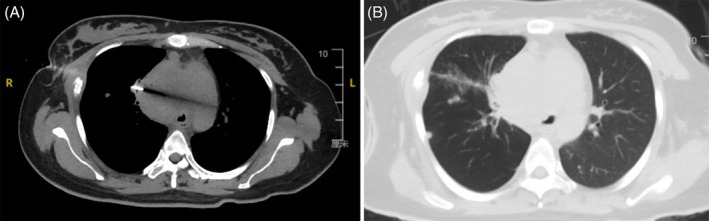
Representative images of pathological examinations following CT‐guided percutaneous biopsy. (A) A 10 cm length of inserted aspiration needle. (B) CT scans after biopsy showed a small amount of haemorrhage, and pneumothorax was not detectable.
On the 11th day, mediastinoscopy with biopsy revealed spindle cells and epithelioid cells in the tumour mass, with heterotypic nucleus, and mucoid changes in the interstitium. Infiltration of lymphocytes and local necrosis were also present. Immunohistochemical staining of the mass showed: Vimentin (+++), ALK (5A4) (++), SMA (+ in focal lesions), Desmin (+ in focal lesions), CD30 (+ in individual cells), TFE3 (weak + in focal lesions), negative (−) for CK‐P, CD2, CD7, CD5, CD20, CD3, S‐100, CD34, MyD1, and TdT (Figure 5). The patient was finally diagnosed with EIMS. High‐throughput sequencing revealed: (1) the EML4‐ALK fusion: exon 6 of the EML4 fused to exon 20 of the ALK, with the tissue abundance of 17.9%; (2) the NF2 (c.810 + 1G > A) gene mutation, with the tissue abundance of 12.7%; (3) the CYP2D6 (p.R201C) gene mutation, with the tissue abundance of 25.7%. The results from whole‐transcriptome analysis using total RNA sequencing revealed exon 6 of the EML4 fused to exon 20 of the ALK, with the tissue abundance of 20.0%.
FIGURE 5.
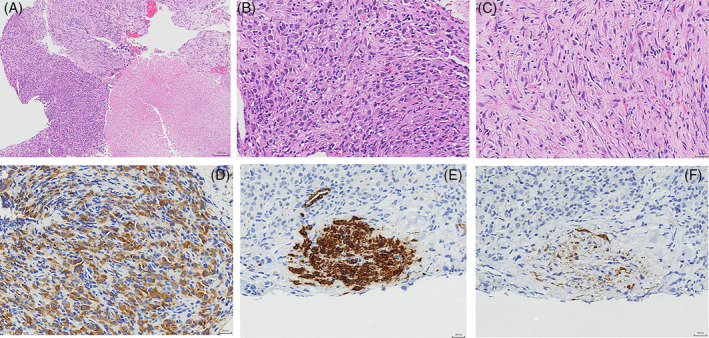
Histological and immunohistochemical characteristics of the EIMS. (A) H&E staining of the EIMS consisted of spindle cells and epithelioid cells, with local necrosis (lower right area). Magnification = 100×. (B) H&E staining showing an epithelial morphology, nuclear hyperchromasia, and lymphocyte infiltration. Magnification = 400×. (C) H&E staining showing spindle cells, mucoid degeneration and scattered inflammatory cells. Magnification = 400×. (D) Positive expressions of ALK in the cytoplasm and cell membrane of epithelioid tumour cells. (E) Positive expressions of Desmin in some of tumour cells. (F) Positive expressions of SMA in some of tumour cells.
DISCUSSION
Inflammatory myofibroblastic tumour (IMT) is a rare type of metastatic tumour composed of myofibroblasts and fibroblast‐like spindle cells, along with an inflammatory infiltration of plasma cells, lymphocytes, and/or eosinophils. EIMS is an aggressive subtype of IMT with rapid local recurrence, which was first described by Mariño‐Enríquez. It affects all age groups ranging from 4 months to 76 years, which is more common in men. 4 Since the first case of EIMS was reported in 2011, about 50 cases have been reported, with most affecting the abdominal cavity, including the mesentery and omentum, liver, pancreas, transverse colon and rectum, and ovary. Only 5 English‐language articles have reported EIMS in the thoracic cavity, which is an extremely rare site for this type of tumour. 5 , 6 , 7 , 8 , 9 Clinical symptoms of EIMS are linked to the site of primary lesions and the extent of invasion. In cases of EIMS in the thoracic cavity, symptoms may include fatigue, weight loss, cough, dyspnea, and pleural effusion if the pleura is involved. These symptoms can rapidly develop within months. Morphologically, EIMS in the thoracic cavity usually presents as a solitary solid mass with a well‐defined border and rapid growth.
IMT is a tumour composed of fibroblasts, myofibroblasts and inflammatory cells, which consists of three basic histological patterns, including myxoid, vascular and inflammatory areas resembling nodular fasciitis; compact spindle cells with intermingled inflammatory cells resembling fibrous histiocytoma; and dense plate‐like collagen resembling a desmoid or scar. One or more patterns exist in IMT. 10 It encompasses plump spindle cells along with varying amount of lymphoplasmacytic infiltrate. In IMTs, spindle cells lack the cytologic atypia and nuclear hyperchromasia of sarcomas. On the contrary, EIMS encompasses large epithelioid cells along with myxoid stroma, inflammatory infiltration of neutrophils, and vascular network. It possesses highly malignant manifestations, including highly atypia polygonal cells, round nuclei, cystic chromatin, obvious nucleolus, nuclear hyperchromasia, and the presence of ganglion‐like cells and Reed‐Sternberg cells. Immunologically, IMT is positively reactive to myogenic markers SMA, muscle‐specific actin (MSA), calponin, and desmin, as well as ALK (cytoplasm or nucleoplasm). Unlikely, EIMS is typically reactive to ALK (nuclear membrane) and desmin, and some cases of EIMS are positively stained with CK (AE1/AE3) and CD30. SMA is usually stained negative or weakly positive in cases of EIMS. The promoter for the rearrangement and overexpression of ALK can be provided by its fusion gene, which determines the ALK staining pattern in IMT. For example, diffuse cytoplasmic staining is visualized when cytoplasmic proteins like TPM3, TPM4, and SEC31L1 fuse to ALK. CLTC is located on the vesicle surface of protein transport, the fusion of which to ALK leads to a granular cytoplasmic staining. Nuclear membrane staining is observed in the case of the ALK‐RANBP2 fusion, the latter of which is a protein found at nuclear pores. Cytoplasmic staining with perinuclear enhancement is visualized when the fusion involves RRBP1, which contains an endoplasmic reticulum transmembrane domain and a ribosomal receptor domain. 11 Amplification of the ALK gene can be detected in almost all cases of EIMS, and the ALK‐RANBP2 gene fusion is the most common one. 4 The ALK‐EML4 gene fusion detected by next‐generation sequencing in this patient was previously reported in a case of abdominal EIMS. 3 Similarly, cytoplasmic staining below the cell membrane is dominant in the ALK staining pattern, the distribution of which is consistent with that of EML4.
The prognosis for EIMS is typically poor, with local recurrence and distant metastasis rates exceeding 80% and 25%, respectively. 1 Effective therapeutic strategies for EIMS include complete surgical resection and administration of ALK inhibitors, while radiotherapy and chemotherapy can also be used as options. According to a literature review, 12 out of 30 IMT/EIMS patients treated with Crizotinib achieved complete or partial remission. 12 During ALK‐tyrosine kinase inhibitor (TKI) therapy, timely recognition of drug‐resistance genes is necessary to control disease progression. The ALK‐R1192P, ALK‐L1196M and ALK‐G1269A mutations have been reported as the driving factors for drug resistance during ALK‐TKI therapy for EIMS. 13 , 14
EIMS progresses rapidly, and an early diagnosis is extremely important, especially for cases with the respiratory and circulatory system affected that may be fatal. In our case report, the patient died of respiratory failure due to tracheal compression, with only 1.5 months between the initial complaint and the diagnosis. Notably, our report is the first to describe a case of mediastinal EIMS with an ALK gene rearrangement. We have summarized some key points regarding the clinical management of this rare case. First, an optimal biopsy is essential for enhancing diagnostic accuracy. CT‐guided percutaneous biopsy is characterized as a high diagnostic rate and high safety. A retrospective study showed that the diagnostic rate of CT‐guided percutaneous biopsy for such lesions is as high as 77% (40/52), with a low incidence of complications at 3.8%. 15 Neither EBUS‐TBNA nor CT‐guided percutaneous biopsy had been performed in cases of mediastinal EIMS prior to our report. In our case, we used a 21‐gauge aspiration needle in EBUS‐TBNA procedures, but no positive results were obtained from the small amount of tissue aspirated for biopsy. We later performed CT‐guided percutaneous biopsy using an 18‐gauge aspiration needle to collect more tissue for the final diagnosis. Therefore, obtaining a histological diagnosis of EIMS can be challenging, and a negative false result can occur during biopsy using an aspiration needle under 18‐gauge. Another consideration is the optimal timing for placing a metal stent in cases like this. While metallic tracheal stenting can rapidly alleviate respiratory dysfunction, the U.S. Food and Drug Administration (FDA) has issued a warning against their use in patients with benign airway stenosis due to the difficulties associated with removal and the potential for severe complications. 16 Moreover, anaesthesia in patients with mediastinal masses is a risk event for cardiopulmonary events, which may be fatal. 17 Due to the unclear diagnosis and the high risk of anaesthesia, we did not opt for metallic stent implantation in this case. However, considering the rapid deterioration and unfortunate outcome in this patient, the question of optimal timing for stent placement necessitates further deliberation and may warrant careful consideration in future cases. Thirdly, ALK translocation is involved in many types of tumours, and some ALK inhibitors have been shown to be effective in treating tumours carrying ALK gene fusions, which are referred to as ALKomas. Immunohistochemical staining of ALK, especially the highly sensitive anti‐ALK antibody [5A4] (ALK p80) or anti‐ALK (D5F3), is recommended to young people with rapidly‐growing, suspicious of malignant tumours. 18
In conclusion, we present a case report of mediastinal epithelioid inflammatory myofibroblastic tumour (EIMS) with EML4‐ALK fusion and summarize some clinical experiences for the management of intrathoracic EIMS based on our case and literature review. Firstly, EIMS located close to the mediastinum is more likely to affect the respiratory system, and a timely biopsy is crucial for an accurate diagnosis. Secondly, a needle thinner than 18‐gauge may not be suitable for pathological diagnosis of EIMS due to the small amount of tissues collected. Thirdly, additional immunohistochemical staining of ALK or next‐generation sequencing of blood samples may be useful for patients with limited tissues for pathological examination and those who are intolerant to invasive biopsy. If ALK is positively stained, ALK‐TKI therapy may be a viable treatment option for EIMS patients who are not suitable for surgery.
AUTHOR CONTRIBUTIONS
Xianmei Zhou, Qian Wang, and Shi Chen conceived and designed the work. Tingyu Pan, Xinyu Sun, Xiao Wu, Qian Wang, and Shi Chen collected the clinical data and wrote the original draft. Futing Tang collected the pathological images of the case. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported in part by a grant from Natural Science Foundation of Jiangsu Science and Technology Department (No: BK20201504) and a grant from Intra‐hospital Fund of Jiangsu Provincial Hospital of Traditional Chinese Medicine Development (No: Y21020).
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
Written informed consent was obtained from the patient ‘s next of kin for publication of this case report and any accompanying images.
Pan T, Sun X, Wu X, Tang F, Zhou X, Wang Q, et al. Mediastinal epithelioid inflammatory myofibroblastic sarcoma with the EML4‐ALK fusion: A case report and literature review. Respirology Case Reports. 2023;12:e01267. 10.1002/rcr2.1267
Tingyu Pan and Xinyu Sun have contributed equally to this article.
Associate Editor: Johnny Chan
Contributor Information
Qian Wang, Email: 1604224946@qq.com.
Shi Chen, Email: sun1604224946@163.com.
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Marino‐Enriquez A, Wang WL, Roy A, Lopez‐Terrada D, Lazar AJ, Fletcher CD, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra‐abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35(1):135–144. [DOI] [PubMed] [Google Scholar]
- 2. Lee JC, Li CF, Huang HY, Zhu MJ, Marino‐Enriquez A, Lee CT, et al. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1‐ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol. 2017;241(3):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang Q, Tong HX, Hou YY, Zhang Y, Li JL, Zhou YH, et al. Identification of EML4‐ALK as an alternative fusion gene in epithelioid inflammatory myofibroblastic sarcoma. Orphanet J Rare Dis. 2017;12(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chopra S, Maloney N, Wang WL. Correction to: epithelioid inflammatory myofibroblastic sarcoma with VCL‐ALK fusion of central nervous system: case report and brief review of the literature. Brain Tumor Pathol. 2022;39(1):43. [DOI] [PubMed] [Google Scholar]
- 5. Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol. 2015;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarmiento DE, Clevenger JA, Masters GA, Bauer TL, Nam BT. Epithelioid inflammatory myofibroblastic sarcoma: a case report. J Thorac Dis. 2015;7(10):E513–E516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azad M, Oye M, Torrente N, Mirsaeidi M. Pericardial epithelioid inflammatory Myofibroblastic sarcoma: an atypical presentation. Cureus. 2022;14(7):e26827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kozu Y, Isaka M, Ohde Y, Takeuchi K, Nakajima T. Epithelioid inflammatory myofibroblastic sarcoma arising in the pleural cavity. Gen Thorac Cardiovasc Surg. 2014;62(3):191–194. [DOI] [PubMed] [Google Scholar]
- 9. Singh P, Nambirajan A, Gaur MK, Raj R, Kumar S, Malik PS, et al. Primary pulmonary epithelioid inflammatory myofibroblastic sarcoma: a rare entity and a literature review. J Pathol Transl Med. 2022;56(4):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19(8):859–872. [DOI] [PubMed] [Google Scholar]
- 11. Doyle LA. Soft tissue tumor pathology: new diagnostic immunohistochemical markers. Semin Diagn Pathol. 2015;32(5):370–380. [DOI] [PubMed] [Google Scholar]
- 12. Theilen TM, Soerensen J, Bochennek K, Becker M, Schwabe D, Rolle U, et al. Crizotinib in ALK+ inflammatory myofibroblastic tumors‐current experience and future perspectives. Pediatr Blood Cancer. 2018;65(4):e26920. [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Geng Y, Yuan LY, Wang MM, Ye CY, Sun L, et al. Durable clinical response to ALK tyrosine kinase inhibitors in epithelioid inflammatory Myofibroblastic sarcoma harboring PRRC2B‐ALK rearrangement: a case report. Front Oncol. 2022;12:761558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Li H, Peng K, Yu Y, Chen L, Fang Y, et al. ALK‐G1269A mutation in epithelioid inflammatory myofibroblastic sarcoma after progression on crizotinib: a case report. Oncol Lett. 2019;17(2):2370–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petranovic M, Gilman MD, Muniappan A, Hasserjian RP, Digumarthy SR, Muse VV, et al. Diagnostic yield of CT‐guided percutaneous transthoracic needle biopsy for diagnosis of anterior mediastinal masses. Am J Roentgenol. 2015;205(4):774–779. [DOI] [PubMed] [Google Scholar]
- 16. Tian S, Huang H, Hu Z, Dong Y, Bai C. A narrative review of progress in airway stents. J Thorac Dis. 2022;14(5):1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narang S, Harte BH, Body SC. Anesthesia for patients with a mediastinal mass. Anesthesiol Clin North America. 2001;19(3):559–579. [DOI] [PubMed] [Google Scholar]
- 18. Taheri D, Zahavi DJ, Del Carmen RM, Meliti A, Rezaee N, Yonescu R, et al. For staining of ALK protein, the novel D5F3 antibody demonstrates superior overall performance in terms of intensity and extent of staining in comparison to the currently used ALK1 antibody. Virchows Arch. 2016;469(3):345–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


