Abstract
Background
Invasive group A streptococcal (iGAS) disease (caused by Streptococcus pyogenes) has been a nationally notifiable disease in Canada since 2000. This report summarizes the demographics, emm types and antimicrobial resistance of iGAS infections in Canada in 2020.
Methods
The Public Health Agency of Canada’s National Microbiology Laboratory (Winnipeg, Manitoba) collaborates with provincial and territorial public health laboratories to conduct national surveillance of invasive S. pyogenes. Emm typing was performed on all isolates using the Centers for Disease Control and Prevention emm sequencing protocol. Antimicrobial susceptibilities were determined using Kirby-Bauer disk diffusion according to Clinical and Laboratory Standards Institute guidelines. Population-based iGAS disease incidence rates up to 2019 were obtained through the Canadian Notifiable Disease Surveillance System.
Results
Overall, the incidence of iGAS disease in Canada has increased from 4.0 to 8.1 cases per 100,000 population from 2009 to 2019. The 2019 incidence represents a slight decrease from the 2018 rate of 8.6 cases per 100,000 population. A total of 2,867 invasive S. pyogenes isolates that were collected during 2020 are included in this report, representing a decrease from 2019 (n=3,194). The most common emm types in 2020 were emm49 (16.8%, n=483) and emm76 (15.0%, n=429), both increasing significantly in prevalence since 2016 (p<0.001). The former most prevalent type, emm1, decreased to 7.6% (n=217) in 2020 from 15.4% (n=325) in 2016. Antimicrobial resistance rates in 2020 included 11.5% resistance to erythromycin, 3.2% resistance to clindamycin and 1.6% nonsusceptibility to chloramphenicol.
Conclusion
Though the number of collected invasive S. pyogenes isolates decreased slightly in 2020 in comparison to previous years, iGAS disease remains an important public health concern. The emm distribution in Canada has been subtly shifting over the past five years, away from common and well-known emm1 and towards emm49 and emm76. It is important to continue surveillance of S. pyogenes in Canada to monitor expanding replacement emm types, as well as outbreak clones and antimicrobial resistance.
Keywords: iGAS, Streptococcus pyogenes, Canada, emm, surveillance, antimicrobial resistance, group A streptococcus
Introduction
Invasive group A Streptococcus (Streptococcus pyogenes) is responsible for a wide range of human diseases, the most serious of which include bacteraemia, streptococcal toxic shock syndrome, necrotizing fasciitis and endocarditis ((1)). In Canada, the incidence of invasive group A streptococcal (iGAS) infections is increasing; doubling from 4.0 cases per 100,000 population in 2009 to 8.1 cases per 100,000 in 2019 ((2)). Though iGAS disease is a global cause of morbidity and mortality ((3)), many studies have indicated that certain populations are at particular risk of disease, including those who are disadvantaged or living in overcrowded conditions ((4,5)).
The M protein, encoded by the emm gene, is an important virulence factor and an epidemiological marker used to characterize S. pyogenes isolates ((1)). A significant amount of iGAS disease is caused by a small number of emm types; however, shifts in prevalence can cause substantial temporal and geographic variability. Studies have noted frequent fluctuations in emm type prevalence in so-called “epidemic behaviour”, where new, emerging strains ultimately replace those previously circulating ((1,6)). Furthermore, the accumulation of mutations through acquisition of exogenous DNA may result in more virulent clones expanding in prevalence or causing new outbreaks of disease in vulnerable populations ((6)).
As rapid clonal spread and outbreaks of iGAS disease continue to occur in Canada ((4–6)), it has become increasingly important to monitor the constantly shifting virulence patterns associated with this organism. This report provides a summary of invasive S. pyogenes isolates collected in Canada in 2020.
Methods
Surveillance program
Surveillance of iGAS infections in Canada consists of a passive, laboratory-based system where invasive S. pyogenes isolates from all provincial and territorial public health laboratories (except Alberta) are sent to the National Microbiology Laboratory (NML) in Winnipeg for further testing. A total of 2,867 invasive S. pyogenes isolates were reported in 2020, including 1) 2,409 isolates submitted directly to NML by provincial and territorial public health laboratories and 2) data for a further 458 isolates collected and tested by the Provincial Laboratory for Public Health in Edmonton, Alberta (ProvLab Alberta) (Table 1). Isolates are collected from sterile clinical isolation sites, which include blood, cerebrospinal fluid, deep tissue, biopsy and surgical samples, bone and any clinical sources associated with necrotizing fasciitis or toxic shock syndrome.
Figure 1.
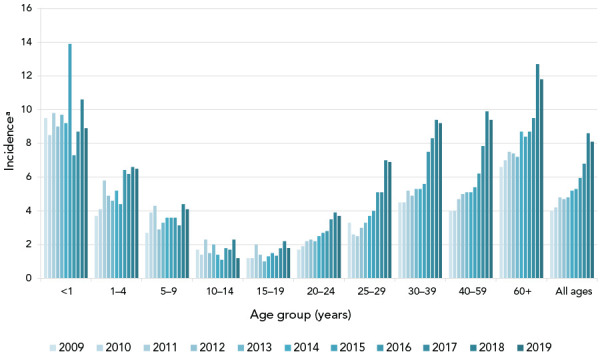
Annual incidence rates of invasive Streptococcus pyogenes cases in Canada by age group, 2010–2019
a Cases per 100,000 population
Table 1. Number of invasive Streptococcus pyogenes isolates collected by each Canadian province/region, 2020.
| Province | Age group (years) | Not given | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Younger than 2 | 2–4 | 5–14 | 15–49 | 50–64 | 65 and older | |||
| British Columbia | 2 | 1 | 4 | 159 | 104 | 67 | 1 | 338 |
| Alberta | 16 | 3 | 9 | 248 | 121 | 61 | 0 | 458 |
| Saskatchewan | 13 | 2 | 3 | 168 | 58 | 28 | 0 | 272 |
| Manitoba | 12 | 10 | 4 | 114 | 70 | 58 | 0 | 268 |
| Ontario | 10 | 6 | 18 | 458 | 274 | 294 | 3 | 1,063 |
| Quebec | 12 | 7 | 8 | 143 | 74 | 101 | 0 | 345 |
| New Brunswick | 2 | 0 | 3 | 25 | 14 | 8 | 1 | 53 |
| Atlantica | 2 | 1 | 5 | 48 | 25 | 17 | 1 | 99 |
| Northernb | 0 | 1 | 0 | 11 | 11 | 1 | 0 | 24 |
| Total | 67 | 31 | 51 | 1,349 | 737 | 627 | 5 | 2,867 |
a Includes isolates from New Brunswick, Prince Edward Island, Nova Scotia and Newfoundland and Labrador
b Includes isolates from Yukon, Northwest Territories and Nunavut
Population-based incidence of iGAS disease up to 2019 was obtained through Canadian Notifiable Disease Surveillance System (CNDSS). Population data for incidence rates were obtained from Statistics Canada’s July 1st annual population estimates.
Isolate testing
Streptococcus pyogenes isolates were confirmed by a positive PYR (pyrrolidonyl-β-naphthylamide) reaction and susceptibility to bacitracin ((7)). Emm typing was performed on all iGAS isolates submitted to NML and ProvLab Alberta using the Centers for Disease Control and Prevention (CDC) emm sequencing protocol. The sequences obtained were compared with the CDC emm database and results reported to the subtype level. Antimicrobial susceptibilities for S. pyogenes (n=2,375) were determined using Kirby-Bauer disk diffusion for chloramphenicol (30 μg), erythromycin (15 μg), clindamycin (2 μg), penicillin (10 μg), ceftriaxone (30 μg) and vancomycin (30 μg) according to Clinical and Laboratory Standards Institute (CLSI) guidelines ((8)).
Supplementary testing was performed on a subset of emm1 isolates to determine the prevalence of the novel M1UK lineage. Genotypes for M1UK isolates were determined by mapping whole genome sequencing reads against reference strain MGAS5005 and identifying 27 characteristic genomic single-nucleotide variants (SNVs), as previously described ((9,10)).
Data analysis
Data submitted with bacterial isolates included patient age, sex, clinical source, province and date of collection. Multiple isolates with the same emm type and collected from the same patient within 14 days were counted once with the most invasive isolation site assigned. Meningitis-related isolates were regarded as most invasive, followed by blood and then other sterile sites. The laboratory data were aggregated by age into younger than two, 2–4, 5–14, 15–49, 50–64 and 65 years and older age groups, and regionally into Western (British Columbia, Alberta, Saskatchewan, Manitoba), Central (Ontario and Québec), Eastern (New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador) and Northern (Yukon Territories, Northwest Territories and Nunavut) regions of Canada. Statistical significance of trends was assessed using the Cochran Armitage test of trend, with a p-value of <0.05 considered significant.
Results
The overall incidence of iGAS disease in Canada decreased slightly in 2019 after successive annual increases from 2009 to 2018. The overall incidence rate in 2019 was 8.1 cases per 100,000 population—twice the rate observed in 2009 (Figure 1, Supplemental material Table S1). In 2020, 2,867 isolates of invasive S. pyogenes were collected, representing a decrease from 2019 (n=3,194).
Of the 2,867 invasive S. pyogenes isolates tested in 2020, 2,862 (99.8%) had patient ages. Infants younger than two years of age accounted for 1.7% (n=67), toddlers aged 2–4 years for 1.1% (n=31), children aged 5–14 years for 1.8% (n=51), teens/adults aged 15–49 years for 47.1% (n=1,349), adults aged 50–64 years for 25.7% (n=737) and seniors aged 65 years and older for 21.9% (n=627). Five isolates had no ages provided. Isolates from male patients represented 58.1% (n=1,635) of the isolates for which sex information was available. Blood was the main clinical isolation site, accounting for 67.9% (n=1,947) of isolates collected. Additional information on emm types by specimen source can be found in Figures S1 to S5.
The most predominant emm types overall in 2020 were emm49 (16.8%, n=483) and emm76 (15.0%, n=429), which have increased significantly in prevalence since 2016 (from 0.6%, n=12 and 0.4%, n=8, respectively; p<0.001) (Figure 2). Other emm types that demonstrated significantly increasing trends from 2016 to 2020 include emm53 (1.2% to 2.1%, p<0.001), emm77 (1.7% to 2.4%, p=0.005), emm80 (1.0% to 3.4%, p<0.001) and emm92 (0.1% to 2.9%, p<0.001). Other emm types demonstrated significantly decreasing trends (see Figure 2), such as emm1 from 15.4% (n=325) of all invasive S. pyogenes isolates collected in 2016, to only 7.6% (n=217) in 2020 (p<0.001). Of note, 33% (n=138) of emm1 isolates sequenced in 2019 were the novel M1UK lineage; in comparison, only three M1UK isolates were identified in 2015.
Figure 2.
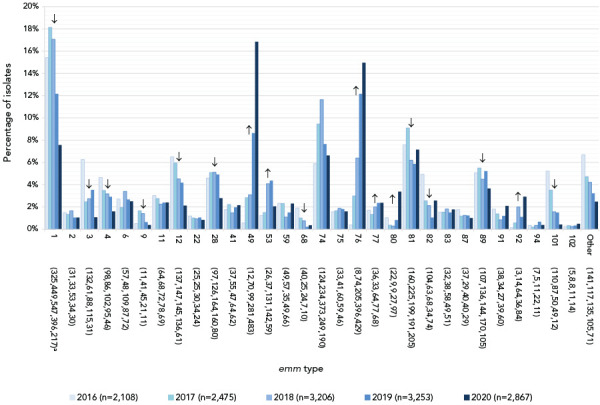
Prevalence of invasive Streptococcus pyogenes emm types in Canada, 2016–2020a,b,c
a Number of isolates for 2016, 2017, 2018, 2019 and 2020, respectively
b Increase in 2016 and 2017 totals from previous annual reports is due to inclusion of submitted data from Alberta
c For emm types with an overall (2016–2020) n≥30: up or down arrows indicate statistically significant trends toward increasing or decreasing prevalence for the 2016–2020 timespan, using the chi-squared test for trend. Emm types with no arrow either did not demonstrate a statistically significant trend, or did not have an overall n≥30
In 2020, the most common emm type from children younger than two years of age was emm49 (20.9%, n=14), while emm1 predominated for children 2–4 years (41.9%, n=13) and 5–14 years (37.3%, n=19) (Figure S6). In patients aged 15–49 and 50–64 years, emm49 was most common (17.9%, n=242; 17.9%, n=132, respectively), followed by emm76 (16.3%, n=220; 14.2%, n=105). For adults 65 years and older, emm76 (14.4%, n=90) and emm49 (14.2%, n=89) were also most common, but emm1 was also frequently identified (12.0%, n=75) (Figure S7). Emm types associated with Western Canada (Figure 3) included emm49 (16.2%, n=217), emm76 (15.9%, n=213), emm74 (11.2%, n=150) and emm81 (9.1%, n=122). In Central Canada, emm49 (17.1%, n=240) and emm76 (14.8%, n=209) were predominant, while emm49 (26.3%, n=26) and emm75 (14.1%, n=14) were most common in Eastern Canada. Isolates from Northern Canada were highly represented by emm1 at 25.0% (n=6), though only 24 isolates were submitted from this region (Figures S8 to S11).
Figure 3.
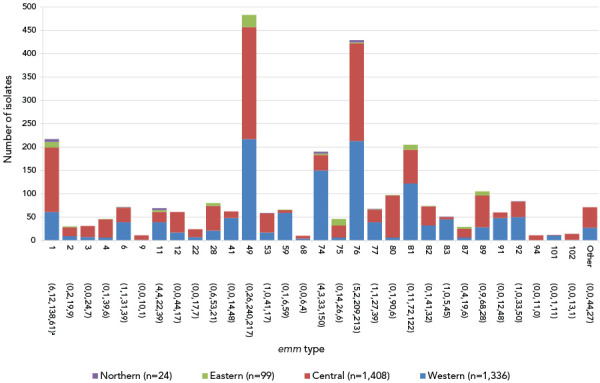
Regional distribution of invasive Streptococcus pyogenes isolates collected in 2020, by emm typea
a Number of isolates in the Northern, Eastern, Central and Western regions of Canada, respectively
Upon request, NML provides assistance to provincial and territorial public health laboratories for S. pyogenes outbreak investigations. During 2020, NML assisted in six outbreak investigations from various jurisdictions, including emm6.4 (n=224 cases), emm74 (n=3), emm81 (n=9), emm92 (n=5) and two multi-emm type outbreaks (emm1, emm74, emm76 and emm92, n=14; emm1, emm76 and emm77, n=10).
Antimicrobial resistance among invasive S. pyogenes isolates remained low in 2020 (Figure 4, Table S2). After dropping to 8.5% (n=235) in 2019, erythromycin resistance increased to 11.5% (n=273) in 2020; however, the overall increase from 2016 to 2020 was not statistically significant. Chloramphenicol nonsusceptibility decreased significantly from 4.7% in 2016 to 1.6% in 2020 (p<0.001), and clindamycin resistance has remained relatively stable over the previous three years (3.0%–3.4%). There was no resistance observed to penicillin or vancomycin. Emm types associated with erythromycin and clindamycin resistance included emm11 (88.6%, n=39; 79.5%, n=35); emm77 (80.8%. n=42; 78.8%, n=41) emm83 (45.7%, n=21; 47.8%, n=22) and emm92 (97.4%, n=74; 93.4%, n=71; respectively) (Figure S12, Table S3).
Figure 4.
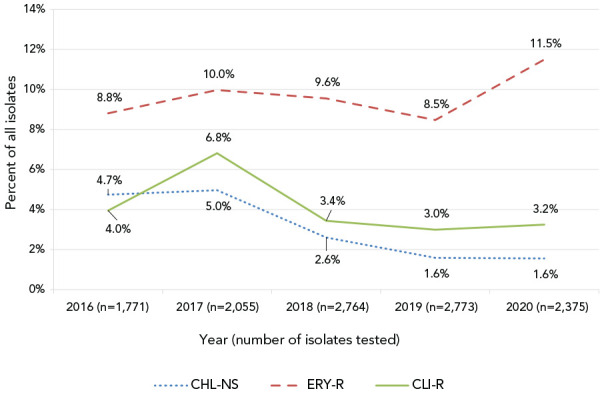
Antimicrobial resistance of invasive Streptococcus pyogenes in Canada, 2016–2020
Abbreviation: CHL-NS, chloramphenicol-nonsusceptible (resistant or intermediate); CLI-R, constitutively clindamycin-resistant; ERY-R, erythromycin-resistant
Discussion
In 2019, 3,054 cases of iGAS disease were reported to CNDSS, with a national incidence rate of 8.1 cases per 100,000 population; more than double the lowest recorded national incidence (2.7 cases per 100,000 population in 2004) since iGAS disease became notifiable in Canada in 2000. Other countries have noted similar increases in iGAS disease over time ((11–14)), and have hypothesized that the overall increase could be due to increasing molecular diversity of the M protein, or expansion of particularly virulent strains of S. pyogenes ((13,14)). Horizontal gene transfer of large regions of genetic material has resulted in a number of unusually virulent clones that have become dominant worldwide, such as the pandemic emm1 clone that resulted from acquisition of a 36kb region, resulting in increased expression of the cytotoxins nga (NADase) and slo (streptolysin O) ((15)). It has also been shown that in addition to increased toxin expression, no or low capsule production may also support the expansion of successful lineages ((16)); examples include emm89, emm28 and emm87 ((16,17)).
The number of invasive S. pyogenes isolates collected by NML decreased from 3,194 in 2019 to 2,867 in 2020. Though 2020 incidence data for iGAS disease was unavailable at the time of writing, it is likely there was a slight decrease between 2019 and 2020. This decrease may have been an indirect effect of the containment measures put in place in 2020 to prevent the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic virus. Several studies have noted that there was decreased invasive disease due to respiratory-transmitted pathogens in 2020; among other routes, S. pyogenes may also be transmitted via respiratory droplets, so the same non-pharmaceutical public health measures put in place to prevent the spread of coronavirus disease 2019 (COVID-19) may have prevented some spread of S. pyogenes, resulting in fewer cases of iGAS disease. The Invasive Respiratory Infection Surveillance Initiative noted worldwide decreases in invasive diseases caused by respiratory pathogens Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis due to containment measures and social changes caused by the pandemic ((18)). A Houston, Texas area hospital also identified a decrease in invasive pneumococcal disease and iGAS disease in 2020 due to COVID-19 containment measures ((19)).
The most prevalent emm type collected in Canada over the past decade was emm1, accounting for over 25% of reported iGAS cases in the early 2010s and reflecting levels reported in Europe and North America ((20)). Although emm1 has decreased in prevalence in Canada since 2014, an increasing number of sequenced isolates (138 isolates in 2019 in comparison to three in 2015) are the novel, hypertoxigenic M1UK lineage originally described by Lynskey et al. ((10)). Recent publications indicated that the prevalence of this lineage is variable: 64% of sequences emm1 isolates in the Netherlands grouped with M1UK, while the United States has not seen significant expansion ((21,22)). It will be crucial to monitor the expansion of this lineage in Canada and determine whether it results in increasing prevalence or outbreaks of emm1.
Despite decreasing in prevalence for a number of years, emm1 has only been surpassed by emm76 in 2019 ((2)), and by emm49 in 2020, each of which accounted for fewer than 1% of reported iGAS cases in 2016. Many outbreaks of iGAS disease across Canada in recent years have been due to emm76 and emm49 (unpublished data). Emm type replacements such as these may often be driven by low population immunity to rare emm types, and intensified transmission of disease within at-risk populations such as people experiencing homelessness (PEH), people who inject drugs (PWID) and other closed/semi-closed populations; in fact, it has been noted that the distribution of emm types varies between non-risk and at-risk groups, and even between different risk groups ((14,23)). Valenciano et al. observed that the emm distribution in the United States varied between PEH, PWID and those with both risk factors ((23)). Rapid expansion of previously uncommon emm types has been noted recently in a number of countries: emm74 in various disadvantaged groups across Canada; emm6 in a semi-closed population of military trainees in Canada; emm26.3 in PEH in the United States; and emm66 in PEH/PWID in England ((4,5,24,25)).
Streptococcus pyogenes remains susceptible to penicillin—the most commonly chosen antimicrobial treatment for iGAS infections, however, there is growing resistance to second-line agents such as macrolides and clindamycin ((1)). In 2020, common emm types in Canada that had high levels (more than 40%) of erythromycin and clindamycin resistance included emm11, emm77, emm83 and emm92, and two of these types (emm77, emm92) also demonstrated increasing prevalence from 2016 to 2020. These emm types were also found to be significant sources of macrolide/lincosamide resistance in countries such as Spain and the United States ((26,27)), with the latter study also noting increases over time of emm11, emm77 and emm92 ((27)). Importantly, all four emm types are included in an investigational 30-valent M-protein-based vaccine currently undergoing clinical trials ((28)). Further clinical development and eventual use of this vaccine worldwide could help to reduce the burden of disease associated with antimicrobial resistant emm types.
Strengths and limitations
Caution should be exercised when interpreting the data presented in this report as the overall interpretation of the results is limited to only isolates available for testing. Only a subset of laboratory isolates from each province may have been submitted for testing and therefore this report does not reflect the true incidence or rates of disease in Canada. The representativeness of the proportions of isolates submitted for testing to NML as compared to the CNDSS are presented in Table S4. Not all provinces and territories report line list data to CNDSS and therefore only aggregated data are available at the national level; therefore, CNDSS data and NML laboratory data are presented differently in terms of age grouping.
Conclusion
Although the number of isolates collected decreased in 2020 in comparison to previous years, iGAS disease remains an important public health concern. In the past five years the emm distribution in Canada has shifted away from the common and well-known emm1 and towards previously uncommon emm49 and emm76. Continued surveillance of invasive S. pyogenes in Canada is imperative to monitor these expanding replacement emm types, as well as outbreak clones and antimicrobial resistance.
Supplemental material
These documents can be accessed on the Supplemental material file.
Table S1: Annual incidence rates of invasive Streptococcus pyogenes cases in Canada by age group, 2009–2019
Figure S1: Clinical isolation sites of invasive Streptococcus pyogenes from children younger than 15 years of age in 2020 (n=149)
Figure S2: Clinical isolation sites of invasive Streptococcus pyogenes from patients 15 years of age and older in 2020 (n=2,718)
Figure S3: Percentage of invasive Streptococcus pyogenes isolates from blood in 2020, by emm type (n=1,947)
Figure S4: Percentage of invasive Streptococcus pyogenes isolates from other sterile sites in 2020, by emm type (n=910)
Figure S5: Percentage of invasive Streptococcus pyogenes isolates from cerebrospinal fluid in 2020, by emm type (n=10)
Figure S6: Prevalence of invasive Streptococcus pyogenes emm types isolated in 2020 for those younger than two, 2–4 and 5–14 years old
Figure S7: Prevalence of invasive Streptococcus pyogenes emm types isolated in 2020 for those 15–49, 50–64 and 65 years and older
Figure S8: Prevalence of the ten most common invasive Streptococcus pyogenes emm types collected from Western Canada in 2020
Figure S9: Prevalence of the ten most common invasive Streptococcus pyogenes emm types collected from Central Canada in 2020
Figure S10: Prevalence of the ten most common invasive Streptococcus pyogenes emm types collected from Eastern Canada in 2020
Figure S11: Prevalence of invasive Streptococcus pyogenes emm types collected from Northern Canada in 2020
Table S2: Antimicrobial resistant invasive Streptococcus pyogenes isolates by year, 2016–2020
Figure S12: Percentage of macrolide and lincosamide resistant invasive Streptococcus pyogenes isolates collected in 2020, by emm type
Table S3: Percentage of macrolide and lincosamide resistant invasive Streptococcus pyogenes isolates collected in 2020, by emm type
Table S4: Number of invasive Streptococcus pyogenes isolates typed by the National Microbiology Laboratory (NML) in comparison to the total number of cases reported to Canadian Notifiable Diseases Surveillance System (CNDSS) in 2019, by patient age group
Acknowledgements
We thank A Yuen, R Mallari, and G Severini from the Streptococcus and Sexually Transmitted Diseases Unit at National Microbiology Laboratory for their laboratory technical assistance, and the staff of provincial and public health laboratories in Canada for participating in the national laboratory surveillance program.
Competing interests
None.
Funding
This project was supported by internal funding from the Public Health Agency of Canada.
References
- 1.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev 2014. Apr;27(2):264–301. 10.1128/CMR.00101-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada. National laboratory aurveillance of invasive streptococcal disease in Canada - Annual Summary 2019. Ottawa (ON): PHAC; 2020. https://www.canada.ca/en/public-health/services/publications/drugs-health-products/national-laboratory-surveillance-invasive-streptococcal-disease-canada-annual-summary-2019.html
- 3.Ralph AP, Carapetis JR. Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol 2013;368:1–27. 10.1007/82_2012_280 [DOI] [PubMed] [Google Scholar]
- 4.Teatero S, McGeer A, Tyrrell GJ, Hoang L, Smadi H, Domingo MC, Levett PN, Finkelstein M, Dewar K, Plevneshi A, Athey TB, Gubbay JB, Mulvey MR, Martin I, Demczuk W, Fittipaldi N. Canada-wide epidemic of emm74 group A Streptococcus invasive disease. Open Forum Infect Dis 2018. Apr;5(5):ofy085. 10.1093/ofid/ofy085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond-Collins K, Strauss B, Barnes K, Demczuk W, Domingo MC, Lamontagne MC, Lu D, Martin I, Tepper M. Streptococcus outbreak in a Canadian Armed Forces training facility. Mil Med 2019. Mar;184(3-4):e197–204. 10.1093/milmed/usy198 [DOI] [PubMed] [Google Scholar]
- 6.DebRoy S, Sanson M, Shah B, Regmi S, Vega LA, Odo C, Sahasrabhojane P, McGeer A, Tyrrell GJ, Fittipaldi N, Shelburne SA, Flores AR. Population genomics of emm4 group A Streptococcus reveals progressive replacement with a hypervirulent clone in North America. mSystems 2021. Aug;6(4):e0049521. 10.1128/mSystems.00495-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spellerberg B, Brandt C. Streptococcus. In: Jorgensen JH, Carroll KC, Funke G, Pfaller MA, Landry M, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th ed. Washington (DC): ASM Press; 2015. p. 383–402. https://onlinelibrary.wiley.com/doi/abs/10.1128/9781555817381.ch22 [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests. M02, 13th ed. Wayne (PA): USA; 2018. https://clsi.org/standards/products/microbiology/documents/m02/
- 9.Demczuk W, Martin I, Domingo FR, MacDonald D, Mulvey MR. Identification of Streptococcus pyogenes M1UK clone in Canada. Lancet Infect Dis 2019. Dec;19(12):1284–5. 10.1016/S1473-3099(19)30622-X [DOI] [PubMed] [Google Scholar]
- 10.Lynskey NN, Jauneikaite E, Li HK, Zhi X, Turner CE, Mosavie M, Pearson M, Asai M, Lobkowicz L, Chow JY, Parkhill J, Lamagni T, Chalker VJ, Sriskandan S. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis 2019. Nov;19(11):1209–18. 10.1016/S1473-3099(19)30446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance (ABCs) report, Emerging infections program network, group A streptococcus, 2004. Atlanta, GA: CDC; 2004; [Accessed 2022 Feb 08]. https://www.cdc.gov/abcs/reports-findings/survreports/gas04.pdf
- 12.Centers for Disease Control and Prevention (CDC). Active Bacterial Core surveillance (ABCs) report, Emerging infections program network, group A streptococcus, 2019. Atlanta, GA: CDC; 2019; [Accessed 2022 Feb 08]. https://www.cdc.gov/abcs/downloads/GAS_Surveillance_Report_2019.pdf
- 13.Canetti M, Carmi A, Paret G, Goldberg L, Adler A, Amit S, Rokney A, Ron M, Grisaru-Soen G. Invasive group A Streptococcus infection in children in Central Israel in 2012–2019. Pediatr Infect Dis J 2021;40(7):612–6. 10.1097/INF.0000000000003087 [DOI] [PubMed] [Google Scholar]
- 14.Blagden S, Watts V, Verlander NQ, Pegorie M. Invasive group A streptococcal infections in North West England: epidemiology, risk factors and fatal infection. Public Health 2020;186:63–70. 10.1016/j.puhe.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 15.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, Kristinsson KG, Gottfredsson M, Vuopio J, Raisanen K, Caugant DA, Steinbakk M, Low DE, McGeer A, Darenberg J, Henriques-Normark B, Van Beneden CA, Hoffmann S, Musser JM. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci USA 2014;111(17):E1768–76. 10.1073/pnas.1403138111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner CE, Holden MT, Blane B, Horner C, Peacock SJ, Sriskandan S. The emergence of successful Streptococcus pyogenes lineages through convergent pathways of capsule loss and recombination directing high toxin expression. MBio 2019;10(6):e02521–19. 10.1128/mBio.02521-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner CE, Abbott J, Lamagni T, Holden MT, David S, Jones MD, Game L, Efstratiou A, Sriskandan S. Emergence of a new highly successful acapsular group A Streptococcus clade of genotype emm89 in the United Kingdom. MBio 2015;6(4):e00622. 10.1128/mBio.00622-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, McCarthy ND, Jolley KA, Maiden MC, van der Linden MP, Amin-Chowdhury Z, Bennett DE, Borrow R, Brandileone MC, Broughton K, Campbell R, Cao B, Casanova C, Choi EH, Chu YW, Clark SA, Claus H, Coelho J, Corcoran M, Cottrell S, Cunney RJ, Dalby T, Davies H, de Gouveia L, Deghmane AE, Demczuk W, Desmet S, Drew RJ, du Plessis M, Erlendsdottir H, Fry NK, Fuursted K, Gray SJ, Henriques-Normark B, Hale T, Hilty M, Hoffmann S, Humphreys H, Ip M, Jacobsson S, Johnston J, Kozakova J, Kristinsson KG, Krizova P, Kuch A, Ladhani SN, Lâm TT, Lebedova V, Lindholm L, Litt DJ, Martin I, Martiny D, Mattheus W, McElligott M, Meehan M, Meiring S, Mölling P, Morfeldt E, Morgan J, Mulhall RM, Muñoz-Almagro C, Murdoch DR, Murphy J, Musilek M, Mzabi A, Perez-Argüello A, Perrin M, Perry M, Redin A, Roberts R, Roberts M, Rokney A, Ron M, Scott KJ, Sheppard CL, Siira L, Skoczyńska A, Sloan M, Slotved HC, Smith AJ, Song JY, Taha MK, Toropainen M, Tsang D, Vainio A, van Sorge NM, Varon E, Vlach J, Vogel U, Vohrnova S, von Gottberg A, Zanella RC, Zhou F. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health 2021;3(6):e360–70. 10.1016/S2589-7500(21)00077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeil JC, Flores AR, Kaplan SL, Hulten KG. The indirect impact of the SARS-CoV-2 pandemic on invasive group A Streptococcus, Streptococcus pneumoniae and Staphylococcus aureus infections in Houston area children. Pediatr Infect Dis J 2021;40(8):e313–6. 10.1097/INF.0000000000003195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gherardi G, Vitali LA, Creti R. Prevalent emm types among invasive GAS in Europe and North America since year 2000. Front Public Health 2018;6:59. 10.3389/fpubh.2018.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rümke LW, de Gier B, Vestjens SM, van der Ende A, van Sorge NM, Vlaminckx BJ, Witteveen S, van Santen M, Schouls LM, Kuijper EJ. Dominance of M1UK clade among Dutch M1 Streptococcus pyogenes. Lancet Infect Dis 2020;20(5):539–40. 10.1016/S1473-3099(20)30278-4 [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Nanduri SA, Van Beneden CA, Beall BW. M1UK lineage in invasive group A streptococcus isolates from the USA. Lancet Infect Dis 2020;20(5):538–9. 10.1016/S1473-3099(20)30279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenciano SJ, Onukwube J, Spiller MW, Thomas A, Como-Sabetti K, Schaffner W, Farley M, Petit S, Watt JP, Spina N, Harrison LH, Alden NB, Torres S, Arvay ML, Beall B, Van Beneden CA. Invasive group A streptococcal infections among people who inject drugs and people experiencing homelessness in the United States, 2010-2017. Clin Infect Dis 2021;73(11):e3718–26. 10.1093/cid/ciaa787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubba L, Bundle N, Kapatai G, Daniel R, Balasegaram S, Anderson C, Chalker V, Lamagni T, Brown C, Ready D, Efstratiou A, Coelho J. Genomic sequencing of a national emm66 group A streptococci (GAS) outbreak among people who inject drugs and the homeless community in England and Wales, January 2016-May 2017. J Infect 2019;79(5):435–43. 10.1016/j.jinf.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 25.Mosites E, Frick A, Gounder P, Castrodale L, Li Y, Rudolph K, Hurlburt D, Lecy KD, Zulz T, Adebanjo T, Onukwube J, Beall B, Van Beneden CA, Hennessy T, McLaughlin J, Bruce MG. Outbreak of invasive infections from subtype emm26.3 group A Streptococcus among homeless adults-Anchorage, Alaska, 2016–2017. Clin Infect Dis 2018;66(7):1068–74. 10.1093/cid/cix921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villalón P, Sáez-Nieto JA, Rubio-López V, Medina-Pascual MJ, Garrido N, Carrasco G, Pino-Rosa S, Valdezate S. Invasive Streptococcus pyogenes disease in Spain: a microbiological and epidemiological study covering the period 2007-2019. Eur J Clin Microbiol Infect Dis 2021;40(11):2295–303. 10.1007/s10096-021-04279-2 [DOI] [PubMed] [Google Scholar]
- 27.Fay K, Onukwube J, Chochua S, Schaffner W, Cieslak P, Lynfield R, Muse A, Smelser C, Harrison LH, Farley M, Petit S, Alden N, Apostal M, Snippes Vagnone P, Nanduri S, Beall B, Van Beneden CA. Patterns of antibiotic nonsusceptibility among invasive group A Streptococcus infections—united States, 2006–2017. Clin Infect Dis 2021;73(11):1957–64. 10.1093/cid/ciab575 [DOI] [PubMed] [Google Scholar]
- 28.Pastural É, McNeil SA, MacKinnon-Cameron D, Ye L, Langley JM, Stewart R, Martin LH, Hurley GJ, Salehi S, Penfound TA, Halperin S, Dale JB. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: A randomized, controlled phase I study. Vaccine 2020;38(6):1384–92. 10.1016/j.vaccine.2019.12.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
These documents can be accessed on the Supplemental material file.
Table S1: Annual incidence rates of invasive Streptococcus pyogenes cases in Canada by age group, 2009–2019
Figure S1: Clinical isolation sites of invasive Streptococcus pyogenes from children younger than 15 years of age in 2020 (n=149)
Figure S2: Clinical isolation sites of invasive Streptococcus pyogenes from patients 15 years of age and older in 2020 (n=2,718)
Figure S3: Percentage of invasive Streptococcus pyogenes isolates from blood in 2020, by emm type (n=1,947)
Figure S4: Percentage of invasive Streptococcus pyogenes isolates from other sterile sites in 2020, by emm type (n=910)
Figure S5: Percentage of invasive Streptococcus pyogenes isolates from cerebrospinal fluid in 2020, by emm type (n=10)
Figure S6: Prevalence of invasive Streptococcus pyogenes emm types isolated in 2020 for those younger than two, 2–4 and 5–14 years old
Figure S7: Prevalence of invasive Streptococcus pyogenes emm types isolated in 2020 for those 15–49, 50–64 and 65 years and older
Figure S8: Prevalence of the ten most common invasive Streptococcus pyogenes emm types collected from Western Canada in 2020
Figure S9: Prevalence of the ten most common invasive Streptococcus pyogenes emm types collected from Central Canada in 2020
Figure S10: Prevalence of the ten most common invasive Streptococcus pyogenes emm types collected from Eastern Canada in 2020
Figure S11: Prevalence of invasive Streptococcus pyogenes emm types collected from Northern Canada in 2020
Table S2: Antimicrobial resistant invasive Streptococcus pyogenes isolates by year, 2016–2020
Figure S12: Percentage of macrolide and lincosamide resistant invasive Streptococcus pyogenes isolates collected in 2020, by emm type
Table S3: Percentage of macrolide and lincosamide resistant invasive Streptococcus pyogenes isolates collected in 2020, by emm type
Table S4: Number of invasive Streptococcus pyogenes isolates typed by the National Microbiology Laboratory (NML) in comparison to the total number of cases reported to Canadian Notifiable Diseases Surveillance System (CNDSS) in 2019, by patient age group


