Abstract
The constitutively expressed CorA Mg2+ transporter is the major Mg2+ influx system of Salmonella typhimurium and Escherichia coli. Genomic sequence data indicated the presence of a homolog in the archaeal organism Methanococcus jannaschii. The putative M. jannaschii CorA was expressed in an Mg2+-transport-deficient strain of S. typhimurium to determine its functional characteristics. The archaeal CorA homolog is a functional Mg2+ uptake system when expressed in S. typhimurium and has properties which are highly similar to those of the normal CorA transporter of S. typhimurium despite having a low level of sequence identity with the protein and being expressed in a lipid membrane of quite different composition than normal. This implies that the overall function of the proteins is the same and further suggests that their structures are very similar.
The CorA Mg2+ transporter was originally identified phenotypically in Escherichia coli (10, 12) and has been cloned, sequenced, and characterized from Salmonella typhimurium (4, 5, 13). In these organisms, a single corA locus constitutively expresses a polypeptide of 316 amino acids with a large N-terminal periplasmic domain of about 240 amino acids followed by three transmembrane domains at the C terminus. CorA mediates the influx of Mg2+ with a Ka of about 20 μM extracellular Mg2+. Influx of Ni2+ and Co2+ is also supported by CorA albeit at extracellular concentrations that are immediately toxic to the cell. At very high extracellular Mg2+ concentrations (>1 mM), CorA also mediates Mg2+ efflux. No other genetic locus has been identified as being necessary for Mg2+ influx via CorA; in contrast, the efflux seen at high extracellular Mg2+ concentrations is altered by mutations in the corB, corC, and corD loci and is abolished in a strain carrying mutations at all three loci (2). Two additional Mg2+ influx systems in S. typhimurium, MgtA and MgtB, are sibling P-type ATPases with no sequence similarities to CorA. Both are highly regulated by Mg2+ concentration in the growth medium and under normal laboratory growth conditions are almost completely repressed by the Mg2+ content of the medium (4, 16, 17).
Work by Silver and colleagues has shown that CorA-like Mg2+ uptake systems are present in E. coli, Bacillus subtilis, and Rhodobacter capsulatus (6, 11, 12). Using both genomic Southern blot analysis and PCR followed by Southern blot analysis, we showed that corA or a similar gene was present in a wide variety of gram-negative and gram-positive bacteria (14); these results suggest that CorA forms the dominant Mg2+ transport system of the domain Bacteria. Recently, several complete microbial genomic sequences have become available including examples within both the Bacteria and the domain Archaea. A CorA homolog has been shown to be present in all of these sequences to date except for those for Mycoplasma genitalium, Mycoplasma pneumoniae, and Borrelia burgdorferi, confirming the ubiquity of this class of Mg2+ transporter. However, comparison of these CorA-like proteins suggests that the major sequence conservation is within the second and third transmembrane domains (8, 13). The question therefore arises whether this sequence conservation reflects a functional similarity. We tested this possibility by comparing the Mg2+ transport properties of the putative CorA homolog of the archaeon Methanococcus jannaschii (MJ-CorA) and the S. typhimurium CorA (ST-CorA).
The M. jannaschii corA gene complements a growth defect in S. typhimurium.
The genomic sequence of M. jannaschii (1) contains an open reading frame (accession no. L77117) encoding a polypeptide with sequence similarity to the ST-CorA (13). The amino acid sequences share a low identity of about 22% and a similarity of 23% (Fig. 1). Both polypeptides are highly charged, with predicted pIs of 4.59 and 5.02 for ST-CorA and MJ-CorA, respectively. In addition, the alignment shown in Fig. 1 reveals 15 sites within the polypeptides with charge reversals. The large majority of these are changes to positive charges in MJ-CorA, accounting for the difference in pI. Optimal growth conditions for the marine archaeon M. jannaschii are reported to be 85°C at >200 atm of pressure in seawater (7). Of relevance is that the concentration of Mg2+ in seawater is generally about 55 mM. Moreover, archaeal membrane lipid composition is markedly different than that in bacteria, with phospholipids containing ether rather than fatty acyl linkages. Thus it was possible that the relatively low level of sequence similarity of these two proteins did not reflect a functional similarity.
FIG. 1.
Sequence alignment of MJ-CorA and ST-CorA. The two polypeptides were aligned by eye by using the following sets of amino acid similarities (with C and P each having no similar amino acids): G, A, S, and T; I, L, V, and M; H, R, and K; D, E, N, and Q; and F, Y, and W. The three C-terminal transmembrane domains of CorA (13) are underlined. Colons indicate identical amino acids, and periods indicate similar amino acids. S. typh., ST-CorA; M. jann., MJ-CorA.
An M. jannaschii genomic DNA clone containing the putative corA gene was obtained from the American Type Culture Collection as construct AMJFH86 carried on pUC18 in E. coli. The plasmid was transferred into S. typhimurium JR501 by transformation for restriction modification and then into strain MM281 (4), yielding strain MM1556. MM281 carries insertions in all three S. typhimurium Mg2+ transport systems, has no detectable Mg2+ transport, and requires 10 to 100 mM Mg2+ for growth. A strain carrying any one of the three S. typhimurium Mg2+ transporters requires no supplemental Mg2+ in the growth medium. Thus, MM281 is a good screen for expression of a protein capable of mediating Mg2+ uptake. The MJ-CorA in MM1556 was compared to the ST-CorA encoded by a plasmid-borne S. typhimurium corA allele (pRS170) in the same MM281 background (MM1278).
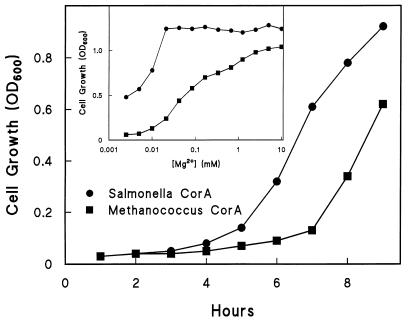
Upon transformation into MM281, the M. jannaschii corA gene complemented the Mg2+ growth defect of this strain and allowed relatively normal growth on N-minimal medium supplemented with 0.4% glucose and 0.1% Casamino Acids (4, 10) or Luria-Bertani nutrient agar plates or broth. In N-minimal medium without added Mg2+, the strain carrying MJ-CorA exhibited a significant lag period before beginning growth but had the same rate of exponential growth as a plasmid-borne ST-CorA (Fig. 2). The strain carrying MJ-CorA also exhibited a slight rightward shift in Mg2+ dependence for growth compared to the plasmid-borne ST-CorA (Fig. 2, insert). Thus at the level of growth, the archaeal MJ-CorA is functional as an Mg2+ transporter in S. typhimurium.
FIG. 2.
Time and Mg2+ dependence of growth. Overnight cultures of MM1278 (containing ST-CorA) and MM1556 (containing MJ-CorA) were grown in supplemented N-minimal medium containing 100 mM Mg2+. Cells were washed twice in the same medium without Mg2+ before being resuspended in the same medium. Cells were then aliquoted to tubes containing the same medium plus the indicated Mg2+ concentration (inset) at an initial optical density at 600 nm (OD600) of 0.05. Cell growth was measured as OD600. The inset shows the Mg2+ dependence of growth measured after 17 h, and the main panel shows the time course of growth at 0.1 mM Mg2+. The experiment was repeated, and similar results were obtained.
The M. jannaschii and S. typhimurium CorA transporters have functionally identical properties.
Cation uptake was measured by previously published procedures by using 63Ni2+ as an alternative substrate since 28Mg2+ is unavailable (3, 15). ST-CorA has an apparent affinity for Ni2+ of about 250 μM compared to an affinity for Mg2+ of 20 to 30 μM (15). The final Ni2+ concentration in the assay buffer for these experiments was 100 μM; because this is well below the apparent affinity for Ni2+, measurement of the cation concentration required for half-maximal inhibition of uptake is within a factor of 2 of the actual Ki for that cation.
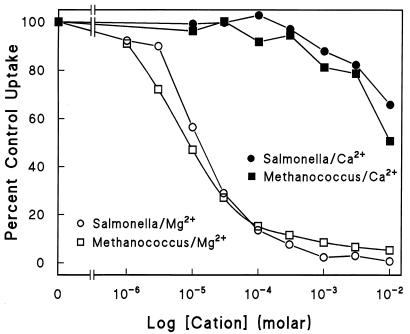
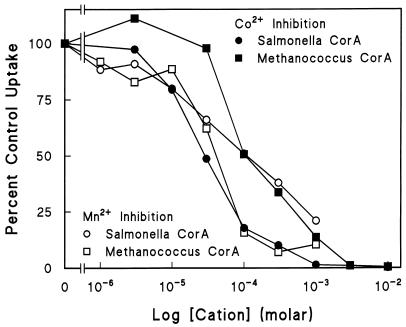
The apparent affinities of Mg2+ for the two systems as estimated by inhibition of the uptake of 63Ni2+ (Fig. 3) were virtually identical. Ca2+ did not significantly inhibit either CorA system. Co2+ is also transported by ST-CorA (5, 12). In contrast to the affinities of Ni2+ and Mg2+ for the two CorA transporters, Co2+ affinity appeared slightly less for MJ-CorA compared to that for ST-CorA (Fig. 4). Mn2+ inhibition was difficult to assess accurately because higher concentrations of Mn2+ were acutely toxic to whole cells and caused extensive clumping. Inhibition of 63Ni2+ uptake by Mn2+ in the MJ-CorA transporter system appeared to be similar to that in the ST-CorA system. Overall, these data indicate that the inhibition profile of MJ-CorA is remarkably similar to that of ST-CorA.
FIG. 3.
Mg2+ and Ca2+ inhibition of transport. Mg2+ and Ca2+ inhibitions of 63Ni2+ uptake were measured as previously described (3, 15). The data shown are normalized to the maximal uptake of each CorA. The data for Mg2+ are the averages of three independent experiments while those for Ca2+ are from a single experiment. The Ni2+ concentration used was 100 μM. Uptake was measured for 5 min with triplicates at each concentration. Uptake by the ST-CorA in the Mg2+ experiments was 1.0 nmol of 63Ni2+ min−1 unit of optical density at 600 nm (OD600)−1; uptake by the MJ-CorA was 0.14 nmol of 63Ni2+ min−1 unit of OD600−1. Uptake in the Ca2+ experiment was comparable to that in the Mg2+ experiments.
FIG. 4.
Co2+ and Mn2+ inhibition of transport. Co2+ and Mn2+ inhibitions of 63Ni2+ uptake were measured as previously described (3, 15). The data shown are normalized to the maximal uptake of each CorA. A single experiment with each cation (with triplicates at each concentration) was performed and was repeated once with similar results. The Ni2+ concentration used was 100 μM. Uptake by the ST-CorA was 0.85 nmol of 63Ni2+ min−1 unit of optical density at 600 nm (OD600)−1; uptake by the MJ-CorA was 0.09 nmol of 63Ni2+ min−1 unit of OD600−1.
Total uptake by MJ-CorA in MM281 was less than that in MM281 carrying ST-CorA on a plasmid. In various experiments, the total uptake via MJ-CorA was 5 to 15% of that via ST-CorA. Because the Ni2+ and Mg2+ affinities of the two systems were comparable, this implies that the Vmaxs for the two systems were in approximately the same ratio as total uptakes. There are several potential reasons for the apparently lower uptake capacity of the MJ-CorA. First, plasmid copy number for ST-CorA was greater than that for MJ-CorA. Further, although the apparent ribosomal binding site sequence for the archaeal gene is comparable to that of a favorable bacterial sequence, there is no recognizable S. typhimurium promoter adjacent to the M. jannaschii corA allele; transcription was presumably via read-through from a plasmid-borne promoter, likely from the antibiotic promoter. Most importantly, codon usage in the archaeal MJ-CorA is not optimal for S. typhimurium; this might greatly diminish translation compared to that of ST-CorA. For these reasons, we did not attempt to quantitate a maximal transport rate, although taken together, our results suggest that the Vmax of MJ-CorA would not be markedly different than that of ST-CorA if expressed on a per polypeptide basis. Thus, the longer lag phase before growth of MM281 carrying MJ-CorA starts is unlikely to be due to an intrinsically poor transport capacity.
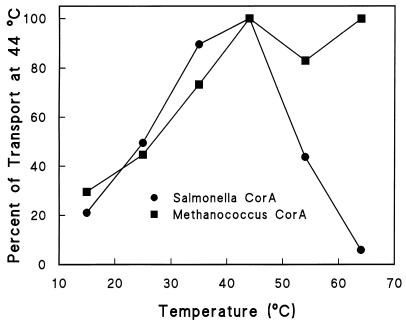
Finally, since M. jannaschii is a thermophile with optimal growth at 85°C, the temperature dependence of uptake in S. typhimurium carrying either MJ-CorA or ST-CorA was examined. Although S. typhimurium viability decreases greatly upon exposure to higher temperatures, the cells remain intact and functional for at least 15 min after heating so transport can be measured. Since transport was being measured in isogenic cells differing only in the CorA protein each was expressing, any differences in transport must be due to the stability of the transporter itself in the context of the S. typhimurium membrane. Cells were heated to the indicated temperature for 5 min before addition of 63Ni2+ for assessment of transport over the following 5 min. The ST-CorA rapidly lost activity above 44°C while the MJ-CorA was completely stable at 65°C, the highest temperature tested (Fig. 5). This result is reflective of the normal growth temperatures of the two organisms.
FIG. 5.
Temperature stability of ST-CorA and MJ-CorA. The temperature stability of uptake was measured by diluting an aliquot of cells 1:10 into medium prewarmed to the indicated temperatures. After 5 min, Ni2+ containing 63Ni2+ was added to a final concentration 100 μM. Uptake was measured for 5 min as previously described (3, 15). Uptake at 44°C by the ST-CorA was 1.5 nmol of 63Ni2+ min−1 unit of optical density at 600 nm (OD600)−1; uptake by the MJ-CorA at 44°C was 0.22 nmol of 63Ni2+ min−1 unit of OD600−1.
Conclusions.
Three conclusions arise from these results. First, despite the low sequence similarity of the MJ-CorA and ST-CorA, functionally the proteins appear to be essentially identical. Both could mediate sufficient uptake of Mg2+ to allow growth of the Mg2+-transport-deficient MM281 strain, even when, as is the case with the MJ-CorA carried in S. typhimurium, expression of the protein was not optimal. In addition, their cation inhibition profiles are very similar. This is surprising because as a marine organism M. jannaschii lives in a medium that contains not only high NaCl but >50 mM Mg2+. By the results shown in Fig. 3 and 4, uptake of Mg2+ by the MJ-CorA should be saturated in its normal environment. In contrast, S. typhimurium grows in a variety of environments, most of which are not rich in Mg2+, and thus a high Mg2+ affinity might be required. Thus, there is no obvious physiological reason why the MJ-CorA should have such a high affinity, not just for Mg2+, but also for other divalent cations.
The ST-CorA can also mediate efflux of Mg2+, but only at high extracellular Mg2+ concentrations which are saturating for influx (2). Since the MJ-CorA has evolved in an environment with a high concentration of Mg2+, it might also mediate efflux rather than influx as a primary function, particularly since it would normally be exposed to very high Mg2+ concentrations in seawater. However, if the primary function of the MJ-CorA was as an efflux system, it would likely leak Mg2+ extensively from the S. typhimurium cell, thus inhibiting growth markedly. This phenotype is seen with at least one mutant of ST-CorA (16a). In addition, the quite high affinity of MJ-CorA for the various other cations required for influx argues strongly that this protein is not a primary Mg2+ efflux system in M. jannaschii. This further suggests that M. jannaschii possesses a separate active efflux mechanism for Mg2+.
Second, even with such relatively weak sequence conservation between the two polypeptides, their highly similar functions imply that their overall tertiary structures may be very similar.
Finally, these data confirm the ubiquity of this class of Mg2+ transport system. An archaeal CorA homolog is able to function as an Mg2+ transporter in a cell type from another kingdom of life. The two other archaeal organisms whose genomes have been completely sequenced, Archeoglobus fulgidus and Pyrococcus horikoshii ot3, both carry a clear CorA homolog according to genome sequence data (8, 9). Our previous data demonstrated the ubiquity of corA-like genes in virtually all gram-positive and gram-negative bacterial species tested (14). Thus, we suggest that a CorA-like Mg2+ transporter is the major constitutive Mg2+ uptake system of both the Bacteria and the Archaea. It remains to be seen whether similar proteins will be found in eukaryotes.
Acknowledgments
This work was supported by PHS grant GM39447 to M.E.M. R.L.S. was supported by a Metabolism Training Grant (DK07319).
We also thank The Institute for Genomic Research for making available the M. jannaschii clones via the American Type Culture Collection.
REFERENCES
- 1.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidman J F, Fuhrmann J L, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Roberts K M, Hurst M A, Kaine B P, Borodovsky M, Klenk H-P, Fraser C M, Smith H O, Woese C R, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 2.Gibson M M, Bagga D A, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol Microbiol. 1991;5:2753–2762. doi: 10.1111/j.1365-2958.1991.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 3.Grubbs R D, Snavely M D, Hmiel S P, Maguire M E. Magnesium transport in eukaryotic and prokaryotic cells using magnesium-28 ion. Methods Enzymol. 1989;173:546–563. doi: 10.1016/s0076-6879(89)73038-x. [DOI] [PubMed] [Google Scholar]
- 4.Hmiel S P, Snavely M D, Florer J B, Maguire M E, Miller C G. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol. 1989;171:4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hmiel S P, Snavely M D, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986;168:1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jasper P, Silver S. Magnesium transport in microorganisms. In: Weinberg E D, editor. Microorganisms and minerals. New York, N.Y: Marcel Dekker; 1977. pp. 7–47. [Google Scholar]
- 7.Jones W J, Leigh J A, Mayer F, Woese C R, Wolfe R S. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch Microbiol. 1983;136:254–261. [Google Scholar]
- 8.Kehres, D., C. H. Lawyer, and M. E. Maguire. The CorA magnesium transporter gene family. Submitted for publication. [DOI] [PubMed]
- 9.Klenk H-P, Clayton R A, Tomb J-F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwinn M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenney K, Adams M D, Loftus B, Peterson S, Reich C I, McNeil L K, Badger J H, Glodek A, Zhou L, Overbeek R, Gocayne J D, Weidman J F, McDonald L, Utterback T R, Cotton M D, Spriggs T, Artiach P, Kaine B P, Sykes S M, Sadow P W, D’Andrea K P, Bowman C, Fujii C, Garland S A, Mason T M, Olsen G J, Fraser C M, Smith H O, Woese C R, Venter J C. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 10.Nelson D L, Kennedy E P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem. 1971;246:3042–3049. [PubMed] [Google Scholar]
- 11.Scribner H, Eisenstadt E, Silver S. Magnesium transport in Bacillus subtilis W23 during growth and sporulation. J Bacteriol. 1974;117:1224–1230. doi: 10.1128/jb.117.3.1224-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver S. Active transport of magnesium in Escherichia coli. Proc Natl Acad Sci USA. 1969;62:764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith R L, Banks J L, Snavely M D, Maguire M E. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. Identification of a new class of transport protein. J Biol Chem. 1993;268:14071–14080. [PubMed] [Google Scholar]
- 14.Smith R L, Maguire M E. Distribution of the CorA Mg2+ transport system in gram-negative bacteria. J Bacteriol. 1995;177:1638–1640. doi: 10.1128/jb.177.6.1638-1640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snavely M D, Florer J B, Miller C G, Maguire M E. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol. 1989;171:4761–4766. doi: 10.1128/jb.171.9.4761-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snavely M D, Miller C G, Maguire M E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266:815–823. [PubMed] [Google Scholar]
- 16a.Szegedy, M. A., and M. E. Maguire. Unpublished data.
- 17.Tao T, Snavely M D, Farr S G, Maguire M E. Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J Bacteriol. 1995;177:2654–2662. doi: 10.1128/jb.177.10.2654-2662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]