Lipoprotein(a) [Lp(a)] is an independent risk factor for atherogenesis and has been postulated to exert a prothrombotic effect.1,2 Studies have suggested that antiplatelet therapy may attenuate the risk conferred by higher levels of Lp(a).3,4 An analysis of the Women’s Health study suggested greater benefit with low-dose aspirin vs placebo in carriers harboring variants associated with elevated Lp(a) concentrations.3 A smaller study from the ASPREE trial also suggested greater benefit with aspirin use in carriers harboring the same variant, but a similar interaction was not seen when applying a more comprehensive Lp(a) genetic risk score.4 Whether prolongation or intensification of dual antiplatelet therapy is especially beneficial in a secondary prevention population with higher Lp(a) concentrations remains uncertain. We assessed the benefit of prolonged ticagrelor vs placebo on a background of low-dose aspirin as a function of baseline Lp(a) concentrations in PEGASUS-TIMI 54,5 a randomized trial of stable patients enrolled 1 to 3 years after a myocardial infarction (NCT01225562).
This analysis includes patients consenting to the biomarker substudy (conducted in a subset of countries participating in the parent trial) with available Lp(a) at randomization (n = 8,967), measured using an isoform-independent assay (Randox) on the Cobas 6000 analyzer (Roche). The outcome was major adverse cardiovascular events (MACE) (cardiovascular death, myocardial infarction, or stroke) at a median follow-up of 2.7 years, assessed as time-to-event. The ticagrelor 90 and 60 mg twice daily dosing arms were pooled for this analysis given nearly identical treatment effect on MACE observed in the primary trial results. Event rates are Kaplan-Meier estimates at 3 years. HRs were derived using a Cox model with adjustment for age, sex, race, hypertension, diabetes, smoking, creatinine clearance <60 mL/min, and apolipoprotein-B (apoB) at baseline. Lp(a) was categorized as high vs low using 200 nmol/L as a previously proposed threshold of risk2 and modeled continuously. The prognostic associations of Lp(a) with MACE and treatment effect of ticagrelor vs placebo was assessed using 3 separate analyses. First, the prognostic associations of high vs low Lp(a) were evaluated separately in the placebo and the ticagrelor arm. Second, the treatment effect for ticagrelor vs placebo was evaluated separately in the high- and low-Lp(a) groups. Third, the treatment effect of ticagrelor vs placebo was evaluated across Lp(a) as a continuous variable using restricted cubic splines. Interaction testing was performed for treatment allocation by Lp(a) concentration.
The median Lp(a) was 29 (IQR: 12-137) nmol/L. Those with Lp(a) ≥200 (11.7%) vs <200 nmol/L were less likely to be White (94.6% vs 96.8%) or male (63.7% vs 78.2%), with higher prevalence of hyperlipidemia (87.0% vs 83.2%), lower prevalence of diabetes (25.5% vs 30.4%), and higher baseline apoB (median: 0.8 [IQR: 0.7-1.0] mg/dL vs 0.7 [IQR: 0.6-0.9] mg/dL) (P < 0.01 for each). No significant differences were observed in baseline characteristics between treatment allocation arms within Lp(a) groups (P ≥ 0.05 for each).
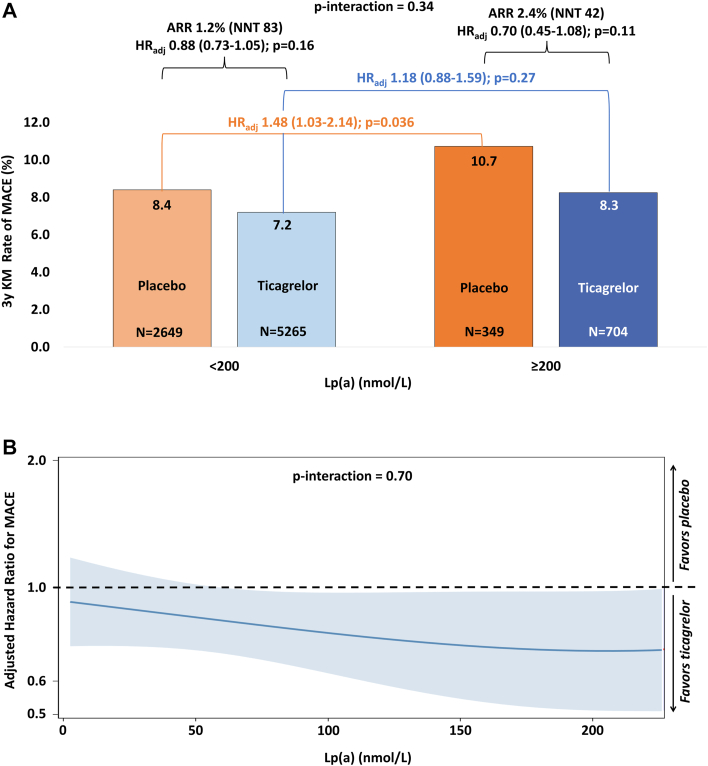
A total of 621 MACE events occurred during follow-up. In the complete trial population, ticagrelor (pooled) vs placebo reduced the risk of MACE (HR: 0.84 [95% CI: 0.76-0.94]) overall, with consistent treatment effect in this subset of patients with available Lp(a) (HRadj: 0.85 [95% CI: 0.72-1.004]). After multivariable adjustment, patients with high Lp(a) concentration randomized to placebo had 48% greater risk of MACE compared with those with low Lp(a) (≥200 vs <200 nmol/L: HRadj: 1.48 [95% CI: 1.03-2.14]; P = 0.036) (Figure 1A). In contrast, the risk conferred by high vs low Lp(a) for MACE tended to be attenuated in those randomized to ticagrelor (HRadj: 1.18, 95% CI: 0.88-1.59; P = 0.27; P interaction = 0.34) (Figure 1A).
Figure 1.
Treatment Benefit of Ticagrelor vs Placebo Across Lipoprotein(a) Concentrations
(A) depicts the prognostic associations and the treatment effect of ticagrelor compared with placebo across lipoprotein(a) analyzed dichotomously. (B) depicts the treatment effect of ticagrelor vs placebo across lipoprotein(a) analyzed continuously. ARR = absolute risk reduction; MACE = major adverse cardiovascular event composite of cardiovascular death, myocardial infarction or stroke; NNT = number needed to treat.
The treatment effect (HRadj) of ticagrelor vs placebo on MACE was 0.70 (95% CI: 0.45-1.08; P = 0.11) in those with high Lp(a) compared with 0.88 (95% CI: 0.73-1.05; P = 0.16) in those with low Lp(a) (P interaction = 0.34) (Figure 1A), with absolute risk reductions of 2.4% and 1.2%, respectively. The effect of ticagrelor on MACE by continuous Lp(a) is shown in Figure 1B.
These data reaffirm the prognostic associations of Lp(a) with cardiovascular events in a secondary prevention population, highlighting a potential role for emerging Lp(a)-lowering therapies. Moreover, these findings suggest ticagrelor may partially mitigate the risk conferred by higher Lp(a), and that Lp(a) may potentially help identify those who derive greater absolute benefit from prolonged dual antiplatelet therapy. Despite numerically greater absolute risk reduction with ticagrelor vs placebo in those with higher Lp(a), it should be noted that treatment interaction by Lp(a) did not achieve statistical significance, which may be due to limited power for interaction testing. As such, our findings should be considered hypothesis-generating. In conclusion, long-term secondary preventive therapy with ticagrelor reduces MACE across the range of Lp(a), with a potential for greater benefit in those with higher Lp(a) that warrants further investigation in larger studies.
Footnotes
Dr Patel is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32HL007604). PEGASUS-TIMI 54 was funded by AstraZeneca. Lp(a) assessment was funded by an institutional grant from Amgen to the TIMI Study group. Dr Bonaca has received consulting fees from AstraZeneca, Merck, Bayer, and Roche Diagnostics. Dr Morrow has received grants to the Brigham and Women’s Hospital from Abbott Laboratories, Abiomed, Amgen, Anthos Therapeutics, AstraZeneca, Eisai, Medicines Co, Merck, Novartis, Pfizer, Roche Diagnostics, and Siemens; and consultant fees from Abbott Laboratories, InCardia, Inflammatix, Merck & Co, Novartis, and Roche Diagnostics; and is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Anthos Therapeutics, ARCA Biopharma, AstraZeneca, Bayer, Daiichi-Sankyo, Eisai, Intarcia, Ionis Pharmaceuticals, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, The Medicines Company, and Zora Biosciences. Dr Steg has received significant research grants from Sanofi and Servier; other personal fees and non-financial support from AstraZeneca, Sanofi, Servier; personal fees from Amarin, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CSL-Behring, Daiichi-Sankyo, Lilly, Merck, Janssen, Novartis, Medtronic, Pfizer, The Medicines Company, and GSK. Dr Bhatt has the following relationships—Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Angiowave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda. Dr Storey has received research grants and personal fees from AstraZeneca, Cytosorbents, GlyCardial Diagnostics and Thromboserin; and personal fees from Alnylam, Bayer, Bristol Myers Squibb/Pfizer, Chiesi, CSL Behring, Daiichi Sankyo, HengRui, Idorsia, Intas Pharmaceuticals, Medscape, Novartis, PhaseBio, and Sanofi Aventis. Dr Sabatine has received research grant support through Brigham and Women's Hospital from Abbott Laboratories, Accumetrics, Amgen, Anthos Therapeutics, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Critical Diagnostics, Daiichi-Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, Ionis, Merck. Nanosphere; Novartis, Pfizer, Roche Diagnostics; Sanofi-aventis; and Takeda, as well as consulting for: Aegerion; Alnylam; Amgen; AstraZeneca; Beren Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb; Cubist; CVS Caremark; Fibrogen, Intarcia; Merck; MyoKardia; Novo Nordisk, Pfizer; Precision BioSciences, Quest Diagnostics; Sanofi-Aventis; Silence Therapeutics, Vertex; and Zeus Scientific. Dr O’Donoghue has received grant support through Brigham and Women’s Hospital from Amgen, Novartis, AstraZeneca, Janssen, Intarcia, Merck, and Pfizer; and honararia from Novartis, AstraZeneca, Amgen, and Janssen. Dr Palazzolo has reported that he has no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Kronenberg F., Mora S., Stroes E.S.G., et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society Consensus Statement. Eur Heart J. 2022;43:3925–3946. doi: 10.1093/eurheartj/ehac361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cegla J., Neely R.D.G., France M., et al. HEART UK consensus statement on Lipoprotein(a): a call to action. Atherosclerosis. 2019;291:62–70. [Google Scholar]
- 3.Chasman D.I., Shiffman D., Zee R.Y., et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203:371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacaze P., Bakshi A., Riaz M., et al. Aspirin for primary prevention of cardiovascular events in relation to lipoprotein(a) Genotypes. J Am Coll Cardiol. 2022;80:1287–1298. doi: 10.1016/j.jacc.2022.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaca M.P., Bhatt D.L., Cohen M., et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]