Abstract
Background:
Numerous studies characterized how resting-state functional connectivities (rsFCs) of the amygdala were disrupted in emotional disorders and varied with emotional traits, including anxiety. With trait anxiety known to diminish with age, a critical issue concerns disambiguating the effects of age and anxiety on amygdala rsFCs in studying the neural bases of individual differences in anxiety.
Methods:
Two-hundred adults (83 women) 19–85 years of age underwent fMRI and assessment for trait anxiety. Amygdala rsFC correlates were identified using multiple regression with age and anxiety in the same model for all and separately in men and women. The rsFC correlates were examined for age-anxiety interaction.
Results:
Anxiety was negatively correlated with amygdala-temporooccipital gyri rsFC in all and in men alone. In women, amgydala rsFC with the thalamus/pallidum, angular/supramarginal gyri, inferior temporal gyrus, and posterior insula correlated positively and rsFC with calcarine cortex and caudate correlated negatively with anxiety. We also observed sex differences in age correlation of amgydala-posterior cingulate cortex/precuneus and -insula/temporoparietal rsFCs, with stronger associations in women. In women alone, anxiety and age interacted to determine amygdala rsFC with the thalamus/pallidum, calcarine cortex, and caudate, with older age associated with stronger correlation between anxiety and the rsFCs.
Limitations:
The findings need to be validated in an independent sample and further explored using task-based data.
Conclusion:
Highlighting anxiety- and age- specific as well as interacting correlates of amygdala rsFCs and sex differences in the correlates, the findings may shed light on the neural markers of anxiety.
Keywords: Amygdala, Age, Trait-anxiety, fMRI, rsFC, Sex difference
1. Introduction
Anxiety is an emotional state with complex neural mechanisms. In health, anxiety signals a state of vigilance essential for survival (Craske and Stein, 2016). However, dysregulated anxiety may lead to emotional disorders, which affect ~28% of adults during their lifetime and almost twice as many women as men (Babaev et al., 2018). Reflecting dysregulated emotion (Liu et al., 2018) and a dimension of neuroticism (Wiebe et al., 2018), trait anxiety represents a relatively stable personality trait of individuals who tend to respond fearfully and anxiously to a wide variety of situations and to experience anxiety-relevant thoughts/feeling and/or exhibit anxiety-related behaviors (Elwood et al., 2012). High trait-anxiety individuals appraise situations, including those that are non-threatening, with negative valence and attend to negatively perceived stimuli (Elwood et al., 2012). Trait anxiety increases the risk of developing anxiety disorders (Knowles and Olatunji, 2020; Weger and Sandi, 2018). Hence, elucidation of the neural underpinnings of trait anxiety is essential to understanding anxiety in health and illness.
The amygdala circuit plays a central role in the manifestation and regulation of emotion and emotion-related behavior. The amygdala receives inputs from many subcortical structures, including the thalamus, and cortical areas, including the prefrontal cortex (PFC), and projects to the periaqueductal gray, bed nucleus of stria terminalis, hypothalamus, and dorsal vagal complex to support autonomic and motor responses of avoidance, freezing, and other anxiety-related behaviors (Babaev et al., 2018; Martin et al., 2009; Ressler, 2010). Both structural and functional changes in the amygdala are noted in individuals with higher levels of anxiety. For instance, high trait anxiety and anxiety disorders were associated with lower amygdala connectivity with fronto-parietal networks and default mode networks (DMN) and higher connectivity with ventral attention network, both during rest and task challenges (Sylvester et al., 2012). Greater trait anxiety correlated positively with amygdala-anterior cingulate cortex (ACC)/ventromedial PFC (vmPFC) resting-sate functional connectivity (rsFC) during stress exposure in men (Nanni-Zepeda et al., 2022). Threat bias – a behavioral index of anxiety – as assessed by a dot-probe task, was positively correlated with amygdala-thalamus rsFC (Jenks et al., 2020). Reduced amygdala-rostral ACC rsFC was correlated with greater trait anxiety and illness severity in generalized anxiety disorder (GAD) (Du et al., 2021). Amygdala-superior temporal gyrus/insula rsFCs were positively correlated with trait anxiety in individuals with GAD and across those with GAD and healthy people (Wang et al., 2021b). A meta-analysis of anxiety disorders showed hypo-connectivity of the amygdala with executive control network (ECN) and DMN during rest (Xu et al., 2019). Another meta-analysis noted hyperconnectivity of limbic-DMN, primary sensorimotor, and salience network in anxiety disorders (Brandl et al., 2022). A systematic review reported reduced amygdala rsFC with the dorso-lateral PFC and ACC in GAD (Kolesar et al., 2019). People with social anxiety showed greater amygdala activation during emotion processing (Brühl et al., 2014) and altered rsFC of the amygdala with frontal, parietal and temporal regions (Brühl et al., 2014; Mizzi et al., 2022), relative to healthy people. Thus, higher levels of anxiety are associated with reduced amygdala-frontoparietal/ECN and altered DMN and amygdala-temporal/occipital cortical rsFCs.
Women seem to experience more severe and longer-lasting symptoms of anxiety than men (Albert, 2015; Domes et al., 2010; Farhane--Medina et al., 2022; Hallers-Haalboom et al., 2020; Kelly et al., 2008; Kogler et al., 2015; Wu et al., 2016b), and many imaging studies investigated the neural bases of sex differences (Li et al., 2020; Lungu et al., 2015; Mak et al., 2009; Stevens and Hamann, 2012). For instance, a meta-analysis showed greater activation of the amygdala, hypothalamus, thalamus, mammillary bodies, caudate and medial frontal cortex in women than in men during negative emotion processing (Stevens and Hamann, 2012). Another study reported significant effective amygdala connectivity with dorsomedial PFC in men but not in women during negative emotion processing, with men experiencing less negative emotion (Lungu et al., 2015). Women vs. men showed a more significant positive correlation between amygdala-thalamus rsFC and threat bias in a dot-probe task (Jenks et al., 2020). In young adults, women with more severe trait anxiety demonstrated higher and lower left amygdala rsFC each with the supplementary motor area and visual network; in contrast, higher trait anxiety was associated with reduced bilateral amygdala rsFC with the salience network and ECN in men (Wang et al., 2021a). A study of participants of a wider age range (18–84 years) showed weaker amygdala rsFC with the ventral attention network in correlation with higher trait anxiety in women but not in men (He et al., 2016). Thus, how amygdala connectivities are altered in link with anxiety may depend on sex.
Age is known to influence amygdala connectivities, including rsFCs (Xiao et al., 2018) as well as connectivities in response to negative emotion exposure (St. Jacques et al., 2010; Wu et al., 2016a) and emotional memory (St Jacques et al., 2009). In fact, age may have pervasive impacts on functional connectivities of the entire brain, likely concurrent with age-related structural changes (Tomasi and Volkow, 2012). Importantly, age also significantly impacts one’s emotional state. Elderly as compared to young people tend to experience more positive and less negative affect (Carstensen et al., 2011; Charles et al., 2003; Mather, 2003; Reed et al., 2014), and previous studies have specifically shown age-related reduction in trait anxiety (Chaudhary et al., 2023; Kim and Kim, 2022; Machado et al., 2019) and prevalence of anxiety disorders (Byers et al., 2010; Rutter et al., 2019). Further, age-associated changes in rsFCs seem to vary with sex (Alarcón et al., 2015; Xiao et al., 2018; Zonneveld et al., 2019). Thus, studies of the connectivity correlates of anxiety would need to consider the effects of age, and vice versa, and how sex influences the connectivity markers.
Here, we examined how sex influences anxiety- and age-related differences in amygdala rsFC. We included both age and trait anxiety in the same regression model to characterize age- and anxiety-specific differences in amygdala rsFCs and performed post-hoc analyses to explore the interacting effects of age and anxiety. We examined the findings across men and women as well as in men and women separately. To confirm sex differences, for the findings revealed in men or in women only, we extracted the β estimates of rsFC and performed a slope test of men vs. women. Based on the literature, we broadly hypothesized reduction in amygdala-frontoparietal and altered temporo-occipital rsFC with higher levels of anxiety, with women more sensitive to the changes. Further, men and women would differ in the interactive effects of age and anxiety on amygdala rsFC. Elucidation of sex differences in the effects of age and anxiety on amygdala rsFC may help clinical research of the pathophysiology of anxiety disorders.
2. Materials and methods
2.1. Subjects and assessments
Recruited from the greater New Haven, Connecticut, area, 200 healthy adults (83 women) 19–85 years of age volunteered for the study. All participants were physically healthy and cognitively intact (Mini Mental State Examination score ≥27) with no major medical conditions. Those with current use of prescription medications or with a history of head injury or neurological illness were excluded. Other exclusion criteria included current or history of Axis I disorders according to the Structured Clinical Interview for DSM-IV (First et al., 1996). Candidates who reported current use of illicit substances or tested positive for cocaine, methamphetamine, opioids, marijuana, barbiturates, or benzodiazepines were not invited to participate. All participants were assessed with the State-Trait Anxiety Inventory (STAI). The trait anxiety scale (O scale or Form 2-M) contains 20 statements about how one feels in general (Spielberger, 1989). The STAI trait score ranged from 20 to 64 with a mean±SD of 35.5 ± 11.6 in the current sample. The Human Investigation Committee at Yale School of Medicine approved the study procedures. All participants signed an informed consent prior to the study.
2.2. MRI protocol and data analyses
Participants were scanned on a 3-Tesla Siemens Trio TIM with a 32-channel head coil. Conventional T1-weighted spin echo sagittal anatomical images were collected for slice localization. Anatomical images for functional slice localization were acquired using spin echo imaging in the axial plane parallel to the AC–PC line with TR = 2530 ms, TE = 3.66 ms, bandwidth = 181 Hz/pixel, flip angle = 7°, field of view = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm in isotropic voxels and no gap. With participants’ eyes closed, resting-state blood oxygen level-dependent (BOLD) signals were acquired for 10 min with a single-shot gradient echo echoplanar imaging sequence in 51 axial slices parallel to the AC–PC line covering the whole brain using TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62°, field of view = 210 × 210 mm, matrix = 84 × 84, slice thickness = 2.5 mm without gap.
Data were analyzed with Statistical Parametric Mapping (SPM12). We followed and applied a published preprocessing pipeline to functional images after first discarding the images of the initial five TRs so only BOLD signals in steady state equilibrium were included in the analyses (Chaudhary et al., 2022; Zhang et al., 2021). Next, the functional images of each subject were slice-time corrected for the temporal offset and motion corrected (realigned). Realignment produced a mean functional image which was co-registered with high resolution structural image and segmented for normalization with affine registration followed by nonlinear transformation. The estimated normalization parameters were subsequently applied to the corresponding functional volumes for each subject. The functional images were then normalized to Montreal Neurological Institute (MNI) space with resampled voxel size of 2.5 × 2.5 × 2.5 mm3 and smoothed with a Gaussian kernel of 6-mm FWHM.
Nuisance signals unlikely to reflect neural activity were removed using linear regression by including the six motion parameters from realignment, signals from whole brain, ventricular system, white matter, and their first-order derivatives (Fair et al., 2007; Fox et al., 2005; Fox and Raichle, 2007; Rombouts et al., 2003; Zhang et al., 2018). Next, functional images were checked for micro-head motion (>0.1 mm) as this may lead to spurious correlations in rsFC analysis, followed by “scrubbing” to remove time points affected by head motions, using the thresholds of FD(t)>0.5 mm (Power et al., 2012; Zhang et al., 2018) or DVARS(t)>75 (Li et al., 2019a). Before computing the correlation maps to estimate rsFC, we applied a temporal band-pass filter (0.009 Hz<f<0.08 Hz) to the time course to obtain low-frequency fluctuations (Cordes et al., 2001; Fair et al., 2007; Fox et al., 2005; Fox and Raichle, 2007).
We employed the amygdala mask from the WFU Pick-Atlas (Maldjian et al., 2003) as the seed. The correlation coefficients r’s between the averaged time course of the seed and time courses of all other brain voxels were computed for each participant. The correlation maps were converted into z-score maps by Fisher’s Z transform: z = 0.5loge [1 + r/1—r].
In group analyses we assessed the correlations between amygdala rsFC and anxiety and age across all participants and separately in men and women, using multiple regression with STAI score and age in the same model. We evaluated the results at cluster p < 0.05 family-wise error (FWE)-corrected with a cluster-forming voxel p < 0.001, uncorrected (Le et al., 2020; Zhang et al., 2018) implemented in SPM. Clusters were identified in MNI coordinates and mean amygdala-cluster rsFCs were extracted for post-hoc analyses. We used slope test (Zar, 1999) to assess sex differences in the correlation of amygdala rsFCs and age or anxiety for those clusters identified in men and women alone. Note that this analysis did not represent “double-dipping” as we identified the clusters in whole-brain analysis with a threshold and the clusters with rsFC showing a significant correlation in men, for instance, may have just missed the threshold in women, and vice versa. Thus, slope tests were needed to confirm sex differences.
Of note, we also evaluated amygdala rsFC and age/anxiety correlates of the same model using non-parametric method implemented in SnPM (SnPM13.1.09, http://nisox.org/Software/SnPM13/) at voxel p < 0.05 FWE-corrected. We highlighted the significant findings in Tables (significant clusters marked with ‘*’).
3. Results
3.1. Age and trait anxiety
Men and women did not differ in age (mean±SD: 51.1 ± 15.1 vs. 47.3 ± 18.6; t = 1.60, p = 0.110). Women relative to men showed higher trait anxiety (STAI-trait score) (37.8 ± 10.9 vs. 33.9 ± 11.8; t = 2.35, p = 0.019). Trait anxiety and age were correlated significantly and negatively in all (r = −0.32, p < 0.001), men (r = −0.36, p < 0.001), and women (r = −0.25, p = 0.028). Slope test showed no significant sex difference in age-anxiety correlation (t = 1.5, p = 0.135).
3.2. Whole-brain amygdala connectivity
One sample t-test in all participants showed positive rsFC of amygdala with subcortical areas including the putamen, ventral caudate, pallidum, and insula, middle/inferior temporal gyrus, anterior/mid-cingulate gyrus, pre-/post-central gyrus, medial and posterior lateral orbitofrontal cortices (OFC), and negative rsFC with posterior parietal and occipital regions, precuneus, posterior cingulate gyrus, superior frontal gyri, including the pre-supplementary motor area, anterior lateral OFC, as well as a wide swath of the cerebellum (Fig. 1). In Supplementary Figs. S1.1 and S1.2 we showed the results of R and L amygdala seeds for men and women combined and separately. The connectivity patterns were consistent with those of the R + L amygdala.
Fig. 1.
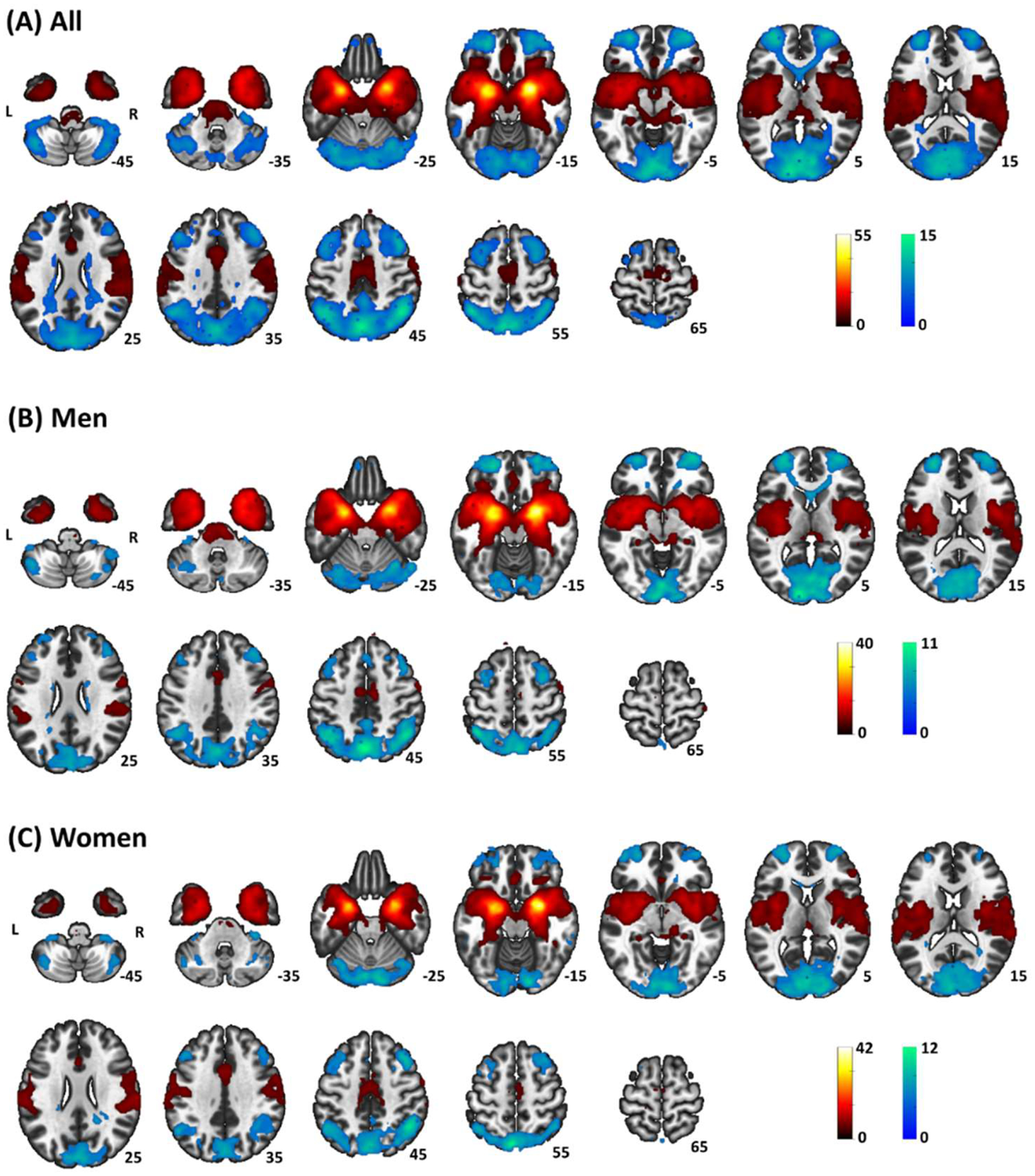
Whole-brain rsFC of bilateral amygdala in (A) all, (B) men, and (C) women – one-sample t-test evaluated at voxel p < 0.05 corrected for family-wise error of multiple comparisons. Clusters with positive and negative connectivity are shown in warm and cool color, respectively. Color bar shows T-value of voxels. Neurological orientation: right=right.
3.3. Trait anxiety and amygdala connectivity
Whole-brain anxiety correlates of amygdala rsFC were evaluated for men and women together as well as separately, accounting for the effect of age (Fig. 2, Table 1). At voxel p < 0.001, uncorrected and cluster p < 0.05 FWE, trait anxiety correlated negatively with amygdala-rsFC with the right MTG/occipital gyrus (OG) across all participants. The analysis in men alone also showed a cluster in the right MTG. In women, trait anxiety correlated positively with amygdala-rsFC with left inferior temporal gyrus (ITG), right angular gyrus (AG)/SMG, right posterior insula and bilateral thalamus/caudate/pallidum and negatively with left calcarine cortex and bilateral caudate.
Fig. 2.
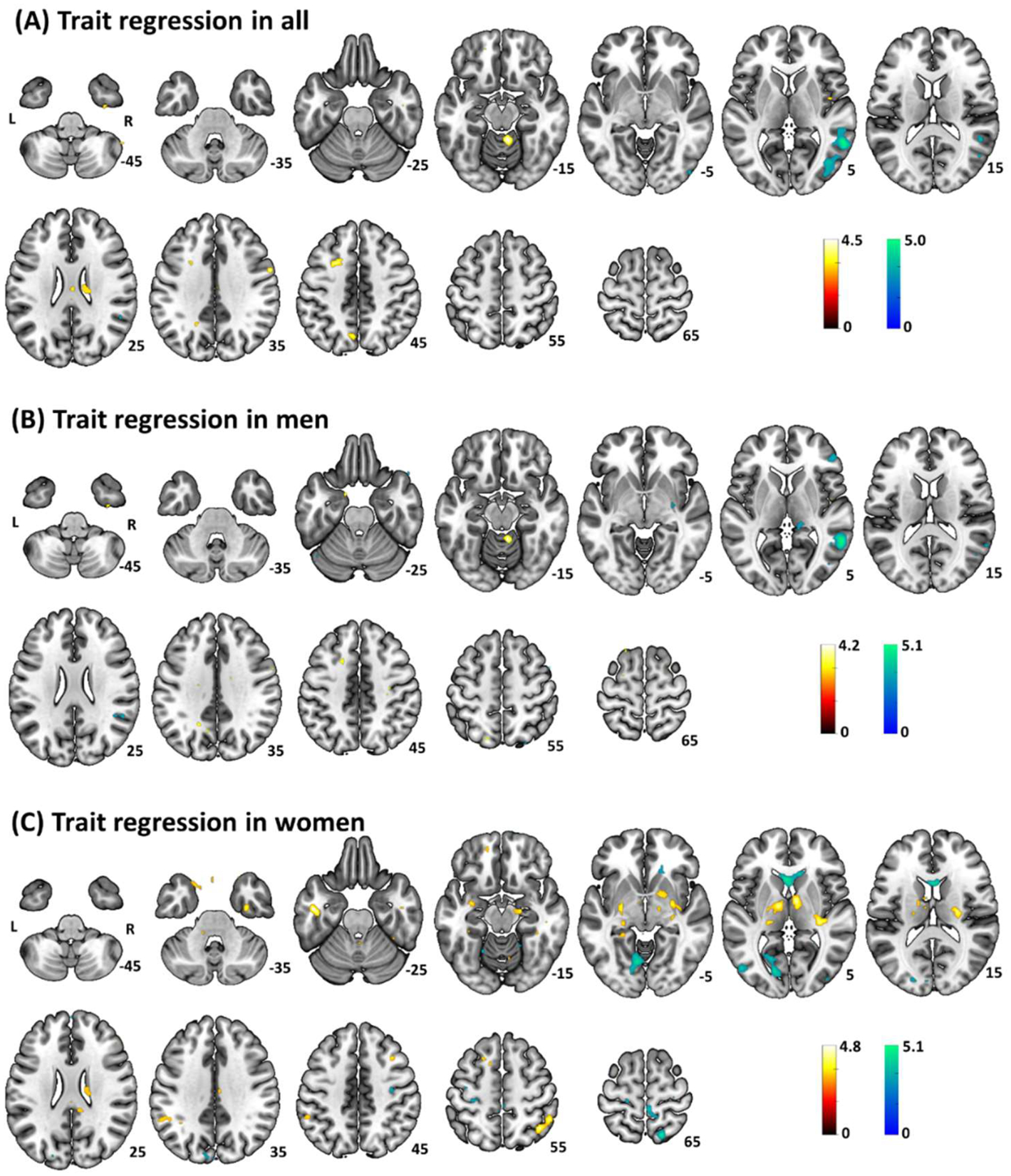
Whole-brain STAI-trait correlates of bilateral amygdala resting state functional connectivity in (A) all, (B) men, and (C) women, at voxel p < 0.001 uncorrected and with ‘age’ as a covariate. Clusters with positive and negative correlation are shown in warm and cool color, respectively. Color bar shows T-value of voxels. Neurological orientation: right=right.
Table 1.
Whole-brain STAI-trait correlates of bilateral amygdala rsFC in all, men and women.
| Cluster size (k) | x | y | z | Z value | Region |
|---|---|---|---|---|---|
| All | |||||
| Trait positive | |||||
| Ns | |||||
| Trait negative | |||||
| 336 | 60 | −54 | 8 | 4.89 | R MTG/Occipital gyrus* |
| 43 | −74 | 8 | 4.02 | ||
| 50 | −46 | 23 | 3.80 | ||
| Men | |||||
| Trait positive | |||||
| ns | |||||
| Trait negative | |||||
| 179 | 58 | −49 | 6 | 4.90 | R MTG* |
| 55 | −64 | 11 | 3.77 | ||
| Women | |||||
| Trait positive | |||||
| 71 | −48 | −11 | −25 | 4.59 | L ITG |
| −43 | −11 | −17 | 3.73 | ||
| −45 | −4 | −32 | 3.51 | ||
| 89 | 40 | −56 | 56 | 4.37 | R AG/SMG |
| 50 | −49 | 56 | 3.97 | ||
| 131 | 8 | −4 | 6 | 4.34 | R Thalamus |
| 18 | −16 | −15 | 4.15 | ||
| 15 | −11 | −5 | 3.37 | ||
| 97 | 33 | −26 | 3 | 4.34 | R pIC |
| 28 | −16 | 16 | 4.00 | ||
| 15 | −16 | 26 | 3.41 | ||
| 182 | −10 | −9 | 6 | 4.31 | L Thalamus/pallidum |
| −33 | −6 | −15 | 4.03 | ||
| −25 | −9 | −10 | 4.01 | ||
| Trait negative | |||||
| 195 | −15 | −79 | 1 | 4.83 | L Lingual gyrus/calcarine |
| −8 | −69 | −5 | 4.46 | cortex | |
| −20 | −64 | 6 | 3.67 | ||
| 185 | −3 | 19 | 8 | 4.65 | L/R Caudate* |
| 3 | 17 | 1 | 4.56 | ||
| 13 | 27 | 3 | 4.25 |
Note: cluster p < 0.05 FWE-corrected and with age as a covariate;
voxel non-parametric p < 0.05 FWE-corrected; R: right, L: left, MTG: middle temporal gyrus, ITG: inferior temporal gyrus, AG: angular gyrus, SMG: supramarginal gyrus, pIC: posterior insular cortex.
We extracted the β estimates (rsFCs) of the clusters identified in men or in women alone and performed a slope test to assess slope differences between the sexes. Men and women differed significantly in all except the correlation of trait anxiety and amygdala-right MTG rsFCs (Table 2).
Table 2.
Slope tests of STAI trait correlates in men vs. women with age as a covariate.
| Trait correlated clusters | Correlation in men (r, p-value) |
Correlation in women (r, p-value) |
Slope test (t-, p-value) |
|---|---|---|---|
| R MTG | −0.46, <0.001 | −0.22, 0.044 | 1.08, 0.283 |
| L ITG | −0.14, 0.131 | 0.52, <0.001 | 4.99, <0.001* |
| R AG/SMG | −0.08, 0.409 | 0.60, <0.001 | 4.78, <0.001* |
| R Thalamus | −0.15, 0.101 | 0.55, <0.001 | 5.02, <0.001* |
| R pIC | −0.12, 0.215 | 0.62, <0.001 | 5.35, <0.001* |
| L Thalamus/pallidum | 0.02, 0.794 | −0.51, <0.001 | −3.87, <0.001* |
| L Lingual G/CAL | −0.004, 0.952 | −0.56, <0.001 | −4.23, <0.001* |
| L/R Caudate | 0.02, 0.819 | −0.47, <0.001 | −3.65, <0.001* |
Note:
p < 0.001;
R: right, L: left, MTG: middle temporal gyrus, ITG: inferior temporal gyrus, AG: angular gyrus, SMG: supramarginal gyrus, pIC: posterior insular cortex, CAL: calcarine cortex.
Whole-brain anxiety correlates of right and left amygdala-rsFC, evaluated separately in all, men, and women and adjusted for the effects of age are shown in Supplementary Fig. S2.
3.4. Age and amygdala connectivity
Whole-brain age correlates of amygdala rsFC were evaluated separately in all, men and women, correcting for the effect of trait anxiety (Fig. 3, Table 3). Evaluated at voxel p < 0.001, uncorrected and cluster p < 0.05 FWE-corrected, in all participants, age correlated positively with bilateral posterior cingulate cortex (PCC)/precuneus and left middle frontal gyrus rsFC, and negatively with clusters in bilateral temporoparietal regions including the posterior insula (pINS)/ temporoparietal junction (TPJ)/supramarginal gyrus (SMG)/sensorimotor cortex (SMC)/superior temporal gyrus (STG), bilateral middle cingulate cortex (MCC) and left amygdala/hippocampus. Age correlates in women followed the pattern of the whole sample with positive associations in bilateral PCC/precuneus, left MFG and left angular gyrus/SMG and negative associations in bilateral pINS/TPJ/SMG/SMC/STG. In men, we observed significant negative age correlates in bilateral middle temporal gyrus (MTG)/superior temporal gyrus (STG) but no positive age correlates.
Fig. 3.
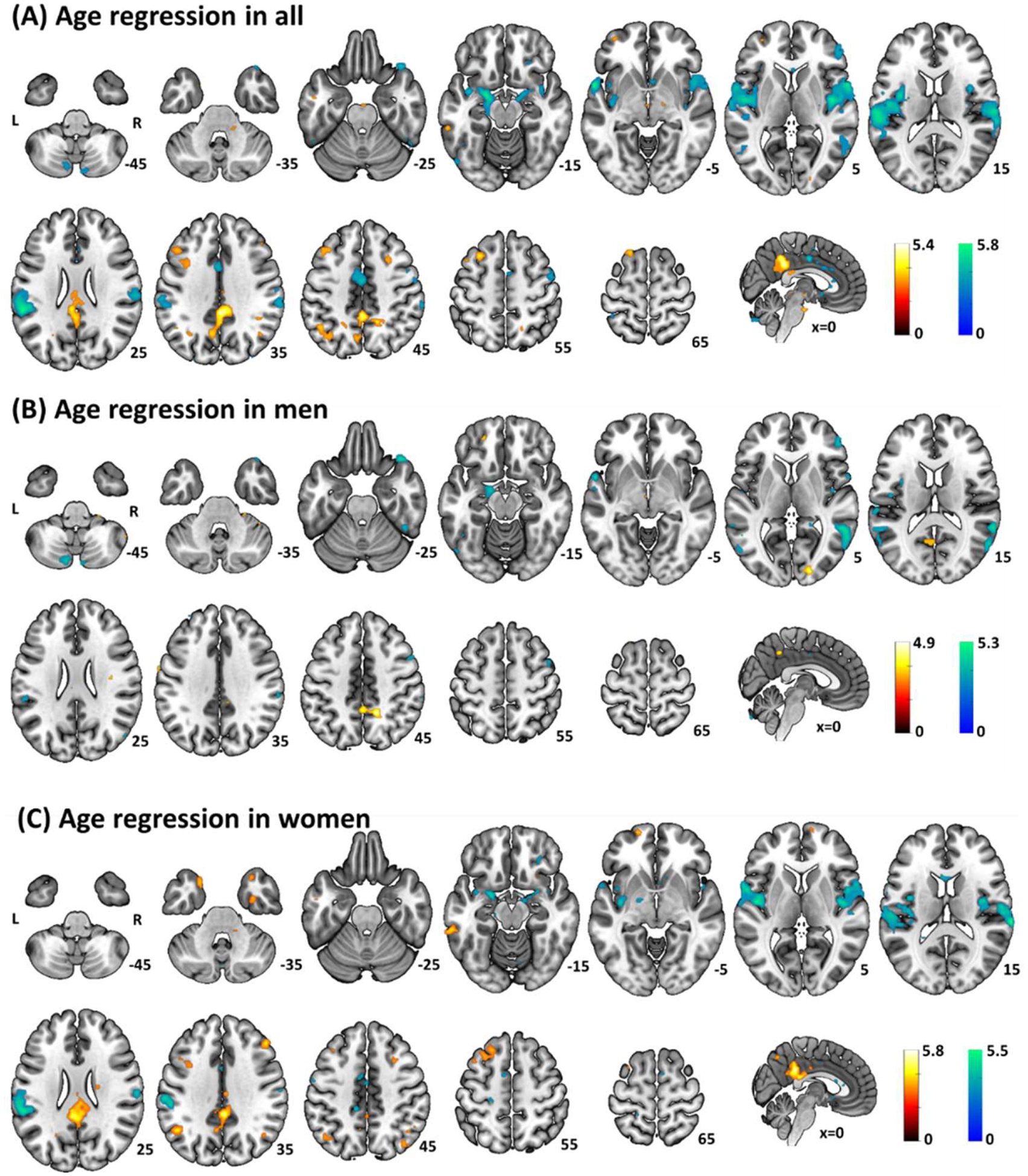
Whole-brain age correlates of bilateral amygdala resting state functional connectivity in (A) All, (B) Men and (C) Women at p < 0.001 uncorrected voxel threshold with ‘trait’ as a covariate. Clusters with positive and negative correlation are shown in warm and cool color, respectively. Color bar shows T-value of voxels. Neurological orientation: right=right.
Table 3.
Whole-brain age correlates of amygdala rsFC in all, men, and women.
| Cluster (k) | x | y | z | Z-value | Region |
|---|---|---|---|---|---|
| All | |||||
| Age positive | |||||
| 713 | 0 | −44 | 43 | 5.35 | R/L PCC/Precuneus* |
| 3 | −41 | 36 | 5.11 | ||
| −5 | −36 | 28 | 4.35 | ||
| 133 | −45 | 24 | 38 | 4.58 | L MFG |
| −35 | 12 | 36 | 3.90 | ||
| −43 | 14 | 38 | 3.34 | ||
| Age negative | |||||
| 1063 | 45 | −9 | 8 | 5.64 | R pINS/SMC/STG/TPJ/ |
| 60 | 9 | 3 | 5.51 | SMG* | |
| 63 | −21 | 18 | 5.10 | ||
| 1501 | −58 | −39 | 26 | 5.62 | L pINS/SMC/STG/TPJ/ |
| −58 | 7 | −5 | 5.54 | SMG* | |
| −63 | −21 | 13 | 5.33 | ||
| 130 | −20 | −4 | −15 | 5.53 | L AMG/Hippocampus* |
| −8 | −1 | −7 | 3.52 | ||
| −20 | −24 | −15 | 3.51 | ||
| 179 | 0 | −6 | 43 | 4.86 | R/L MCC* |
| −3 | 7 | 36 | 3.99 | ||
| 0 | 14 | 31 | 3.95 | ||
| Men | |||||
| Age positive | |||||
| Ns | |||||
| Age negative | |||||
| 281 | 58 | −64 | 11 | 5.12 | R MTG/STG* |
| 63 | −46 | 13 | 4.76 | ||
| 60 | −56 | 8 | 4.74 | ||
| 171 | −58 | −59 | 11 | 4.24 | L MTG/STG |
| −48 | −41 | 11 | 3.82 | ||
| −60 | −49 | 18 | 3.73 | ||
| Women | |||||
| Age positive | |||||
| 395 | 3 | −41 | 33 | 5.38 | R/L PCC/Precuneus* |
| −5 | −44 | 28 | 4.95 | ||
| 3 | −34 | 23 | 4.64 | ||
| 104 | −48 | −56 | 36 | 4.21 | L AG/SMG |
| −43 | −61 | 41 | 3.88 | ||
| 105 | −20 | 27 | 58 | 4.06 | L MFG/SFG |
| −30 | 19 | 58 | 4.06 | ||
| −15 | 17 | 58 | 3.29 | ||
| Age negative | |||||
| 553 | 43 | −9 | 8 | 5.16 | R pINS/SMC/STG/TPJ* |
| 70 | −29 | 16 | 4.99 | ||
| 58 | 12 | 8 | 4.57 | ||
| 975 | −48 | −9 | 6 | 5.03 | L pINS/SMC/STG/TPJ/ |
| −58 | 4 | 3 | 4.93 | SMG* | |
| −58 | −36 | 23 | 4.84 |
Note: cluster p < 0.05 FWE-corrected and with STAI-trait as a covariate;
voxel non-parametric p < 0.05 FWE-corrected; PCC: posterior cingulate cortex, MFG: middle frontal gyrus, pINS: posterior insula, SMC: sensorimotor cortex, STG: superior temporal gyrus, TPJ: temporoparietal junction, SMG: supramarginal gyrus, AMG: amygdala, vCN: ventral caudate nucleus, MCC: middle cingulate cortex, MTG: middle temporal gyrus, SFG: superior frontal gyrus.
We extracted the β estimates (rsFCs) of the clusters identified in men or women alone and examined sex differences in the correlation of regional connectivity and age. Men and women significantly differed in all correlations except for amygdala- left MTG/STG connectivity (Table 4).
Table 4.
Slope tests of age correlates in men vs. women with trait anxiety as a covariate.
| Age correlates | Men (r, p-value) |
Women (r, p-value) |
Slope test (t-, p-value) |
|---|---|---|---|
| R MTG/STG | −0.51, <0.001 | −0.05, 0.632 | 2.21, 0.028* |
| L MTG/STG | −0.44, <0.001 | −0.06, 0.586 | 1.66, 0.098 |
| R/L PCC/Precuneus | 0.19, 0.042 | 0.57, <0.001 | 3.88, <0.001** |
| L AG/SMG | 0.06, 0.484 | 0.43, <0.001 | 3.00, 0.003* |
| L MFG/SFG | 0.12, 0.211 | 0.47, <0.001 | 3.38, 0.001* |
| R pINS/SMC/STG/TPJ | −0.28, 0.002 | −0.62, <0.001 | −4.11, <0.001** |
| L pINS/SMC/STG/TPJ/SMG | −0.29, 0.002 | −0.64, <0.001 | −4.06, <0.001** |
p < 0.05,
p < 0.001;
PCC: posterior cingulate cortex, MFG: middle frontal gyrus, pINS: posterior insula, SMC: sensorimotor cortex, STG: superior temporal gyrus, TPJ: temporoparietal junction, SMG: supramarginal gyrus, AMG: amygdala, vCN: ventral caudate nucleus, MCC: middle cingulate cortex, MTG: middle temporal gyrus, SFG: superior frontal gyrus.
Whole-brain age correlates of right and left amygdala-rsFC evaluated separately in all, men and women and adjusted for the effects of trait anxiety are shown in Supplementary Fig. S3.
3.5. Amygdala connectivity – interaction of age and trait anxiety
For clusters identified from whole brain regression on age and on trait anxiety, we performed post-hoc regressions on the β’s to examine whether the interactions between age and anxiety were significant, using β = b1 × age + b2 × anxiety + b3 × age × anxiety, for all, men, and women. The results are shown in Supplementary Table S1 with significant interactions (b3) highlighted in bold. The clusters showing significant interactions included amygdala-bilateral thalamus/pallidum, left lingual gyrus/calcarine cortex, and bilateral caudate in women. None of the clusters identified in all and in men showed a significant interaction.
We visualized the interaction effects of age and anxiety on amygdala rsFCs in two ways. First, we showed the scatter plots and regression lines for three age groups: 18–33 (n = 26), 34–55 (n = 27), and 56–88 (n = 30) years in women. As shown in Fig. 4, the slope of positive association between trait anxiety and amygdala rsFC with bilateral thalamus/pallidum increased with each group of increasing age with coefficients (b’s) of right thalamus: b18–33 = 0.003, b34–55 = 0.004, b56–88 = 0.007; and of the left thalamus b18–33 = 0.004, b34–55 = 0.005, b56–88 = 0.009. Likewise, the slope of negative association between trait anxiety and amygdala rsFCs with left lingual gyrus/calcarine cortex (b18–33 = −0.004, b34–55 = −0.004, b56–88 = −0.010) and with bilateral caudate (b18–33 = −0.003, b34–55 = −0.006, b56–88 = −0.010) increased with each group of increasing age. Second, we showed the interactions by computing the predicted regression in amygdala rsFC vs. anxiety for each unit increase in age (Fig. 5). Thus, for the R/L thalamus cluster identified in women, for 1 unit increase in age, the positive slope of amygdala- R/L thalamus rsFC vs. anxiety increased by 0.00011 and 0.00012, respectively. Likewise, for 1 unit increase in age, the negative slope of amygdala-L lingual gyrus/calcarine cortex rsFC vs. anxiety increased by 0.00012 and the slope of amygdala-bilateral caudate rsFC vs. anxiety increased by 0.00014. The regression plots for all clusters are shown in Supplementary Fig. S4.
Fig. 4.
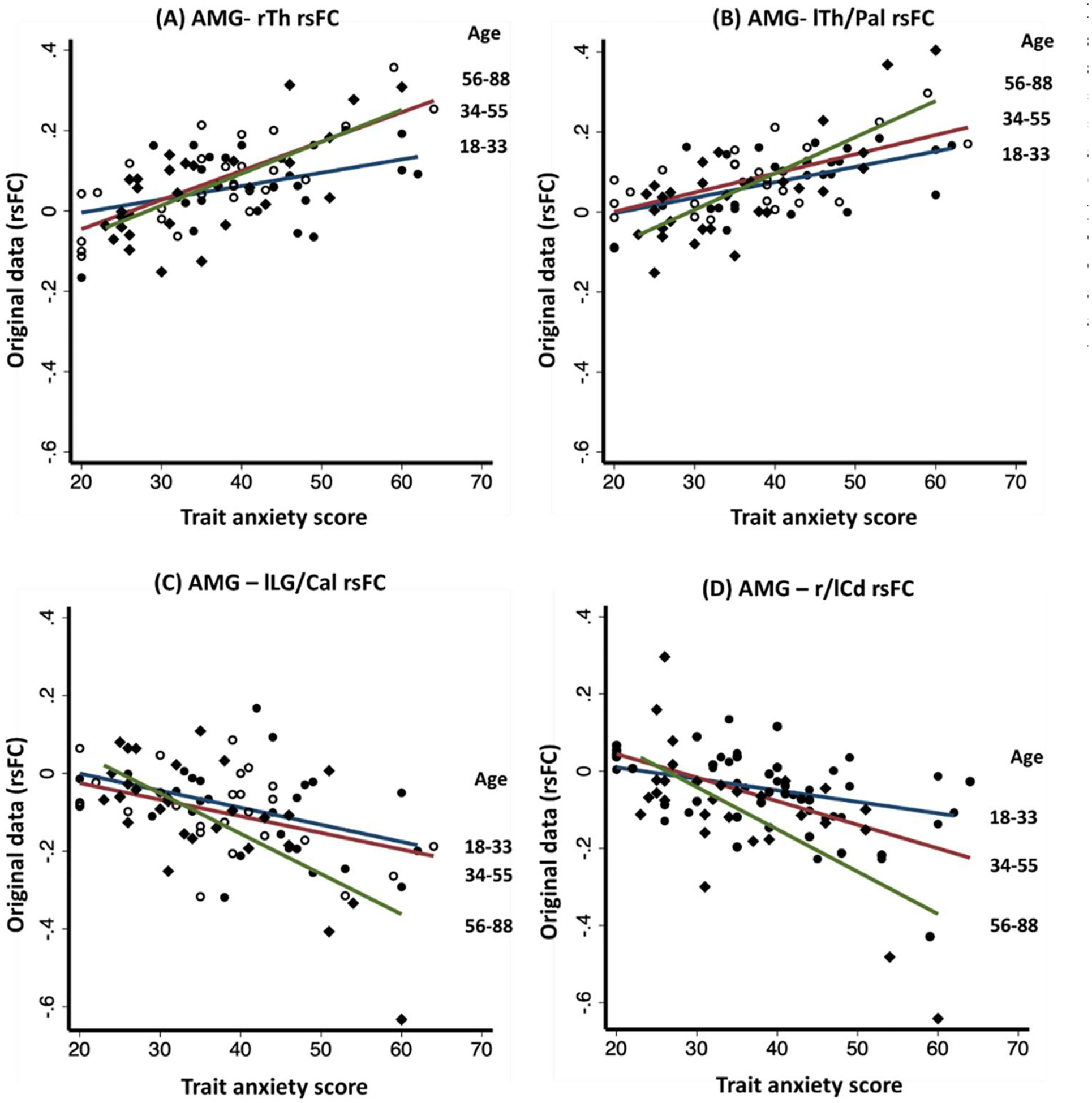
Scatterplot of amygdala (AMG) resting state functional connectivity (rsFC) vs. anxiety score in women for age groups 18–33 years (n = 26), 34–55 years (n = 27) and 56–88 years (n = 30). The clusters showing significant age × anxiety interaction in amygdala rsFC included (A) right thalamus (rTh), (B) left thalamus/pallidum (lTh/Pal), (C) left lingual gyrus/calcarine cortex (lLG/Cal), and (D) bilateral caudate (r/lCd). The slope of positive (A & B) and negative (C & D) correlations between amygdala rsFCs and trait anxiety were steeper with increasing age.
Fig. 5.
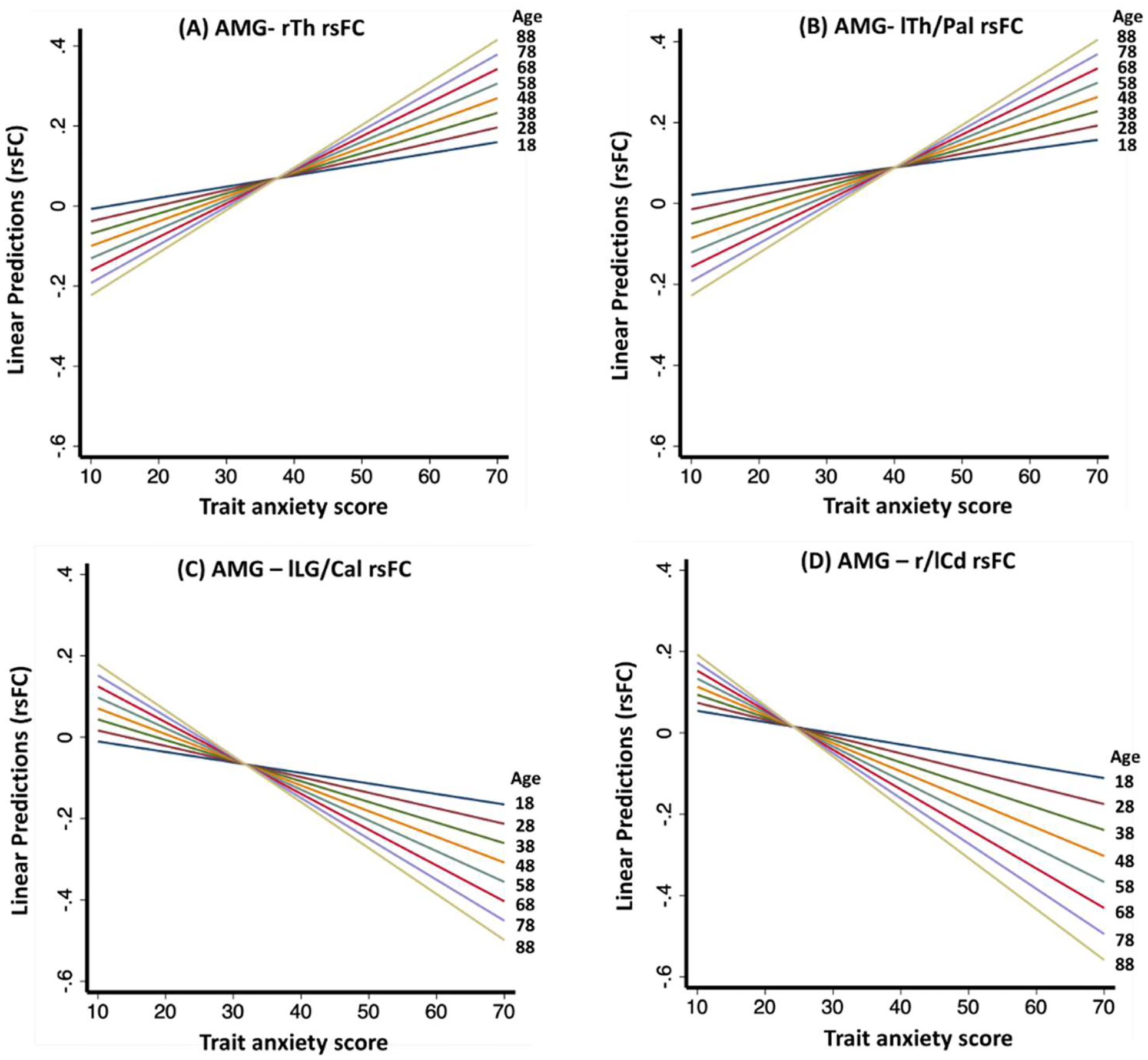
Regression of predicted amygdala rsFCs against trait anxiety score with 10 unit increases in years of age, depicting significant interactive effects of age and trait anxiety score on amygdala rsFC with the (A) right thalamus (rTh), (B) left thalamus/pallidum (lTh/Pal), (C) left lingual gyrus/calcarine cortex (lLG/Cal), and (D) bilateral caudate (r/lCd) in women. For each 10 years increase in age, the positive regression slope of amygdala rsFC on trait anxiety increased by 0.0011 and 0.0012 for the rTh and lTh/Pal, respectively; and the negative slope became more negative by 0.0012 and 0.0014 in lLG/Cal and r/lCd, respectively. The linear predictions of amygdala rsFCs were calculated using the regression equation: β (amygdala rsFC) = b1 × age + b2 × anxiety + b3 × age × anxiety.
4. Discussion
Women compared to men reported higher levels of anxiety, consistent with the literature (Albert, 2015; Domes et al., 2010; Farhane-Medina et al., 2022; Hallers-Haalboom et al., 2020; Kelly et al., 2008; Kogler et al., 2015; Wu et al., 2016b). Also consistent with previous reports of age-related positivity in affect (Carstensen et al., 2011; Charles et al., 2003; Mather, 2003; Reed et al., 2014) and reduction in anxiety (Machado et al., 2019; Rutter et al., 2019), age was correlated negatively with anxiety, both in men and women equally. The latter findings add to literature by showing that, although women tend to be more anxious than men, age-related decline in anxiety is consistent across sexes. In the below, we highlighted anxiety- and age-related changes in amygdala rsFC and sex differences in these changes. As anxiety represents the primary symptom (Knowles and Olatunji, 2020) and increases the risk of anxiety disorders (Weger and Sandi, 2018), we discussed our findings with reference to anxiety disorders, wherever appropriate.
4.1. Validation of amygdala rsFC
In accord with earlier studies (Roy et al., 2009; Xiao et al., 2018), we showed that, at rest, the amygdala showed positive connectivities with the mid-cingulate cortex, medial orbitofrontal cortex (OFC), inferior frontal cortex, middle/inferior temporal cortex, insula, pre-/post-central gyri, as well as subcortical areas, including the lentiform nucleus and thalamus, and negative connectivities with middle/lateral frontal cortical regions, lateral OFC, posterior parietal areas, posterior cingulate cortex, precuneus, and occipital cortex.
4.2. Anxiety and amygdala rsFC
Adjusting for age, we showed significant negative associations of anxiety and amygdala rsFC with the middle temporal/occipital gyri in all participants and in men. In women, amygdala rsFC with thalamus/pallidum, angular/supramarginal gyri (AG/SMG), inferior temporal gyrus (ITG), and posterior insula correlated positively and rsFC with calcarine cortex/lingual gyrus and caudate correlated negatively with anxiety. Further, the latter correlations significantly differed between men and women in slope tests. Thus, women with higher anxiety showed amygdala hyperconnectivity with the subregions of the DMN and salience network (SN) and altered connectivity with subcortical and temporo-occipital regions.
The thalamus integrates inputs from the cortex, hypothalamus, and brain stem, and projects to the amygdala, nucleus accumbens, and bed nucleus stria terminalis, to support emotional behaviors, such as defensive responses (Kirouac, 2021), fear formation and learning (Lee and Shin, 2016). The pallidum controls both approach and avoidance behaviors (Ipser et al., 2013). Animal studies implicated the amygdala-pallidum circuit in fear learning (Giovanniello et al., 2020). In humans, individuals with high trait anxiety frequently appraised situations as threatening (Elwood et al., 2012) and demonstrated faster fear acquisition (Wong and Lovibond, 2018) and impaired extinction (Soeter and Kindt, 2013). In people with social anxiety disorder (SAD), the thalamus showed higher rsFC with the temporal lobe, including the amygdala, relative to healthy controls (Zhang et al., 2022). People with SAD showed hyperactivated globus pallidus, amygdala, and parahippocampus during facial emotion processing (Binelli et al., 2014). In response to fear-eliciting stimuli, individuals with phobic disorders showed higher activations in the thalamus, globus pallidus, amygdala, and insula (Ipser et al., 2013).
Here, we not only demonstrated higher amygdala-thalamus/pallidum rsFC in link with anxiety, broadly consistent with the literature, but also highlighted sex differences, with women but not men showing a positive correlation of the rsFCs with anxiety. Another subcortical cluster with anxiety-associated hypoconnectivity with amygdala in women, but not in men, was identified in the caudate. The caudate nucleus receives inputs from frontal lobe and projects to amygdala, hippocampus, thalamus, and pallidum (Driscoll et al., 2023; Robinson et al., 2012), and caudate connectivity with amygdala supports emotion/reward processing and motivated behaviors (Liu et al., 2011; Robinson et al., 2012). Anxiety is often observed in patients with caudate lesions (Caplan et al., 1990; Mendez et al., 1989). Thus, amygdala-caudate hypoconnectivity may suggest impaired emotion processing and regulation in highly anxious individuals, particularly women. In support, men and women showed differences in caudate recruitment during emotional tasks as well as during rest. In a meta-analysis women exhibited greater caudate activations than men during negative emotion processing (Stevens and Hamann, 2012). On the other hand, caudate responses to emotional pictures correlated with subjective arousal rating more strongly in men than in women (Moriguchi et al., 2014). Caudate responses to emotional scenes correlated with recognition memory in men but not in women (Canli et al., 2002). During rest, amygdala-caudate rsFC correlated positively with cortisol levels in men but negatively in women (Kogler et al., 2016). Thus, anxiety in women appears be characterized by opposing subcortical amygdala connectivities, with caudate hypoconnectivity in link with reduced emotion regulation and thalamus/pallidum hyperconnectivity with heightened threat response. The pathophysiological pathways of the sex differences remain to be clarified.
Altered amygdala-DMN/SN connectivity are noted in individuals with higher levels of anxiety and anxiety disorders (Brandl et al., 2022; Du et al., 2021; Nanni-Zepeda et al., 2022; Wang et al., 2021b; Xu et al., 2019). We observed that amygdala rsFC with the AG/SMG correlated positively with trait anxiety in women but not in men, broadly consistent with a recent meta-analysis that noted limbic network hyperconnectivity with the AG/SMG in anxiety disorders (Brandl et al., 2022). As part of the DMN, AG/SMG supports self-referential thoughts (Ionta et al., 2011; Seghier, 2013) and the hyperconnectivity with amygdala may indicate emotionally, possibly negatively biased, self-referential processes, a trait often noted along with anxiety (Tracy et al., 2021). Further, women vs. men demonstrated stronger activations of the right AG during exposure to stress (Kogler et al., 2015), broadly consistent with the present observation of women being more sensitive to amygdala-AG/SMG rsFC changes in anxiety. Within the SN, we observed greater amygdala-insula rsFC in correlation with higher anxiety score, as reported in patients with generalized anxiety disorders (Kolesar et al., 2019). A meta-analysis of task-based studies of affective, cognitive, social processing noted hypoactive insula across anxiety disorders (Janiri et al., 2020). Earlier studies associated insula response with exposure to emotional scenes and with recognition memory in women only (Canli et al., 2002) and higher insula activity during autobiographical memory recall in women, relative to men (Young et al., 2013). The current results are broadly consistent with these findings and others of higher stress responses in the insula in women vs. men (Goldfarb et al., 2019; Whittle et al., 2011). Of note, investigators reported a shift in activity from the DMN to SN, with DMN intra-/inter-network connectivity decreasing and SN connectivity increasing, during stress exposure, implying a reduction in self-related processing and augmented attention to stress (Zhang et al., 2019). Here, we observed heightened anxiety-linked amygdala connectivity both with DMN and SN regions during rest, likely suggesting a more entrenched state of stress (Lanius et al., 2020).
Within the temporo-occipital network, we observed hyper- (ITG) and hypo- (occipital lobe) connectivity with amygdala, mainly in women. The ITG represents an end node of the ventral visual pathway processing objects, faces, and scenes (Conway, 2018). Reciprocally connected with the amygdala and OFC (Iidaka et al., 2001), the ITG responds to processing, including regulation and memory, of negative emotional stimuli (Lin et al., 2020; Min et al., 2022). A study associated the severity of anxiety with higher spontaneous activity, as reflected in the amplitude of low-frequency fluctuations, of the ITG (Li et al., 2019b). Considering sex differences, earlier studies have noted higher ITG activation in women than men during various emotion tasks (Whittle et al., 2011). In contrast, ITG activation during emotional scene processing correlated with recognition performance in men but not in women (Canli et al., 2002). Another study noted higher ITG activation in men vs. women during negative emotion regulation (Domes et al., 2010). Thus, as a higher-order visual area, the roles of the ITG in processing emotional stimuli appear to vary with both sex and behavioral task. Studies on emotion processing and trait anxiety have implicated the occipital cortex in anticipatory anxiety, face recognition, sensitivity to fearful stimuli, and self-regulation (Lai, 2019). A core symptom of anticipatory anxiety, worry was associated with lower regional blood flow (Hoehn-Saric et al., 2005) and with activity of the occipital cortex in individuals high on neuroticism, perhaps as a coping mechanism to reduce negative emotional imagery during worry (Servaas et al., 2014). Thus, resting-state amygdala-occipital cortical hypoconnectivity may reflect a mechanism to avoid negative emotions in individuals with high anxiety. Previous studies also noted sex differences in visual cortical activities both during task challenges and rest, including higher lingual gyrus activation in men vs. women during negative emotion regulation (Domes et al., 2010). Men relative to women showed higher and lower occipital cortical activations, respectively, each during exposure to positive and negative emotional stimuli that were equally arousing (Whittle et al., 2011). Further, during rest, women vs. men showed weaker occipital cortical connectivity (Weis et al., 2020). In dynamic resting connectivity, females mainly engaged the occipital/sensory-motor and dorsal attention networks, while males engaged the salience network (Murray et al., 2021). Although not directly comparable with these previous reports, the current findings highlight sex differences of neural markers of trait anxiety.
4.3. Age and amygdala rsFC
Men and women differed in age-related changes in amygdala rsFC. Most importantly, stronger amygdala-PCC/precuneus and weaker amygdala-pINS/STG/TPJ/SMC rsFCs were associated with older age, and these age-related differences were more prominent in women than in men. PCC/precuneus is part of the default mode network (DMN) and negatively connected with the amygdala (note that other DMN regions, e.g., medial OFC, are positively connected with the amygdala, suggesting heterogeneity of DMN regional connectivities with the amygdala; see Fig. 1). Thus, age is associated with less negative amygdala PCC/precuneus connectivity. Previous studies noted age-related decline in DMN connectivity (Jockwitz and Caspers, 2021), with some highlighting a more complex pattern of changes (Ferreira et al., 2016; Staffaroni et al., 2018). For instance, Staffaroni et al. reported DMN connectivity increasing from age 50, plateauing at 70, and unergoinog a rapid decline afterwards (Staffaroni et al., 2018). Another work emphasized ubiquitous elevation in positive inter-network correlations and losses of focal inter-network (inlcuding the DMN) anti-correlations during aging (Ferreira et al., 2016). Other studies noted age-related decline in the connectivities of the salience network, involving the amygdala, insula, and TPJ (He et al., 2014; Onoda et al., 2012). In accord, we observed that the positive rsFC of the amygdala with pINS/STG/TPJ/SMC diminished with age. Thus, the age-related changes observed for amygdala intrinsic connectivity are largely consistent with these literure, with sex differences highlighted here for the changes.
4.4. The interacting effects of age and anxiety on amygdala rsFC
We observed significant interacting effects of age and anxiety on amygdala connectivity in women but not in men or in the full sample. We noted a positive interaction between age and trait anxiety in the effects on amaygdala rsFC with the thalamus/pallidum and negative interaction in the effects on rsFC with the lingual gyrus/calcarine cortex and caudate nuleus. Trait anxiety is positively correlated with higher amygdala rsFC with the thalamus and pallidum; thus, older age renders the correlation even more positive. Conversely, anxiety is negatively correlated with amygdala rsFC with the lingual gyrus/calcarine cortex and caudate nuleus, and older age renders the negative correlation even more negative. To our knowedge, these are the first findings to show the interacting effects of age and anxiety on amygdala rsFCs. Of note, extant literature did show age- and anxiety-related differences in amygdala rsFC, though most examined selected regions of interest and none directly characterized their interacting effects. For instance, in adults 18 to 83 years of age, amygdala rsFC with the dorsal attention network correlated positively and negatively with age and anxiety, respectively, whereas amygdala rsFC with the ventral attention network increased with both age and anxiety (He et al., 2016). In older vs. younger adults, greater heart rate variability – a physiological marker of efficient emotion regulation – was associated with reduced amygdala-ventrolateral PFC connectivity (Sakaki et al., 2016). During threat appraisal, amygdala-ventromedial PFC showed more negative and positive coupling each in anxious youth and adults (Gold et al., 2016).
Here, we showed how age may affect the inter-relationship between anxiety and amygdala connectivity over a life span from 18 to 88 years. Importantly, these interacting effects were observed only for women, suggesting sex-specific mechanisms of the neural processes of anxiety as one ages. Age is known to affect emotional states, including anxiety and depression, differently between women and men (Moulinet et al., 2022; Shi et al., 2021). In a population study of 2500 participants (mean age = 49.9 ± 18.5 years, 54.6% women), women were twice as likely to have anxiety disorders than men; however, the incidence of anxiety disorders between 14 and 19 years as well as after menopause (and corresponding age in men) did not differ between men and women, suggesting the importance of age and potentially age-related hormonal changes on affective functioning (Faravelli et al., 2013). Along with this literature, the current findings suggest potentially sex-specific age effects on emotional function and dysfunction that may manifest in link with amygdala connectivities. These findings may help research of therapeutics, e.g., brain stimulation, to target the sex-specific cirucits in the management of anxiety and of animal models to examine the sex-specific neurobiology of anxiety.
4.5. Limitations and conclusion
A few limitations should be considered. First, we did not examine the connectivity of amygdala sub-regions that may involve distinct connectivities. Second, rsFCs reflect functional organization of the amygdala; however, task-based studies, particularly those with exposure to emotional stimuli and reguation of emotions, are needed to fully reveal the inter-relationship between age, anxiety, and amygdala connectivities. Third, anxiety is comorbid with many other psychiatric symptoms, including depression. More studies are needed to distigunish the effects of age on amygdala and limbic circuit connectivities in anxiety vs. depression and sex differences in these effects. Finally, although obtained of a relatively large sample with roughly equal numbers of men and women, the current findings need to be validated in an independent sample. Further, it remains to be seen whether or how the current findings can be extended to clinical populations with anxiety disorders.
To conclude, the study demonstrates sex differences in trait anxiety and age correlates of intrinsic amygdala connectivity, including those with the thalamus/pallidum, angular/supramarginal gyri, inferior temporal gyrus, posterior insula, calcarine cortex/lingual gyrus and caudate in association with anxiety, and posterior cingulate cortex/precuneus and insula/temporo-parietal cortex in association with age. The associations were stronger in women than in men and may represent neural markers of sex differences in emotional reactivity and regulation and potentially in the pathophysiological processes of anxiety disorders. Age moderated the association of amygdala rsFC with anxiety in women but not in men, suggesting sex-specific neural processes that may be altered in the development of anxiety disorders across the lifespan.
Supplementary Material
Role of funding source
The current study was supported by NIH grants R21AG067024 (Chiang-Shan R. Li), R01AG072893 (Chiang-Shan R. Li), R01AG078266 (Sien Hu), R01CA218501 (Herta H. Chao), as well as a VA Merit Award CX001301 (Herta H. Chao). The NIH and VA are otherwise not responsible for the design of the study or data analyses and interpretation or in the decision to publish these findings.
Footnotes
Declaration of Competing Interest
The authors report no conflict of interests.
CRediT authorship contribution statement
Shefali Chaudhary: Data curation, Writing – original draft, Writing – review & editing. Sien Hu: Conceptualization, Methodology, Writing – review & editing. Kesong Hu: Writing – review & editing. Jacqueline C. Dominguez: Writing – review & editing. Herta H. Chao: Conceptualization, Methodology, Writing – review & editing. Chiang-Shan R. Li: Conceptualization, Methodology, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jadr.2023.100646.
References
- Alarcón G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ, 2015. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage 115, 235–244. 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, 2015. Why is depression more prevalent in women? J. Psychiatry. Neurosci 40, 219–221. 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev O, Piletti Chatain C, Krueger-Burg D, 2018. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med 50, 1–16. 10.1038/s12276-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binelli C, Subirà S, Batalla A, Muñiz A, Sugranyés G, Crippa JA, Farré M, Pérez-Jurado L, Martín-Santos R, 2014. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: a systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia 64, 205–217. 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Brandl F, Weise B, Mulej Bratec S, Jassim N, Hoffmann Ayala D, Bertram T, Ploner M, Sorg C, 2022. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: a transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology 47, 1071–1080. 10.1038/s41386-022-01271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S, 2014. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev 47, 260–280. 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML, 2010. High occurrence of mood and anxiety disorders among older adults: the national comorbidity survey replication. Arch. Gen. Psychiatry 67, 489–496. 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JDE, 2002. Sex differences in the neural basis of emotional memories. Proc. Natl. Acad. Sci 99, 10789–10794. 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, Gorelick PB, Helgason C, Hier DB, 1990. Caudate infarcts. Arch. Neurol 47, 133–143. 10.1001/archneur.1990.00530020029011. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin GR, Brooks KP, Nesselroade JR, 2011. Emotional experience improves with age: evidence based on over 10 years of experience sampling. Psychol. Aging. 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL, 2003. Aging and emotional memory: the forgettable nature of negative images for older adults. J. Exp. Psychol. Gen 132, 310–324. 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Chaudhary S, Zhang S, Zhornitsky S, Chen Y, Chao HH, Li C-SR, 2023. Age-related reduction in trait anxiety: behavioral and neural evidence of automaticity in negative facial emotion processing. Neuroimage 276, 120207. 10.1016/j.neuroimage.2023.120207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S, Zhornitsky S, Roy A, Summers C, Ahles T, Li C-SR, Chao HH, 2022. The effects of androgen deprivation on working memory and quality of life in prostate cancer patients: the roles of hypothalamic connectivity. Cancer Med. 10.1002/cam4.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, 2018. The organization and operation of inferior temporal cortex. Annu. Rev. Vis. Sci 4, 381–402. 10.1146/annurev-vision-091517-034202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME, 2001. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR. Am. J. Neuroradiol 22, 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, 2016. Anxiety. Lancet 388, 3048–3059. 10.1016/S0140-6736(16)30381-6. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Böttger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC, 2010. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum. Brain Mapp 31, 758–769. 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll ME, Bollu PC, Tadi P, 2023. Neuroanatomy, Nucleus Caudate. StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
- Du Y, Li H, Xiao H, Wang M, Zhang W, Gong Q, Qiu C, Huang X, 2021. Illness severity moderated association between trait anxiety and amygdala-based functional connectivity in generalized anxiety disorder. Front. Behav. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood LS, Wolitzky-Taylor K, Olatunji BO, 2012. Measurement of anxious traits: a contemporary review and synthesis. Anxiety Stress Coping 25, 647–666. 10.1080/10615806.2011.582949. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE, 2007. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage 35, 396–405. 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Alessandra Scarpato M, Castellini G, Lo Sauro C, 2013. Gender differences in depression and anxiety: the role of age. Psychiatry Res. 210, 1301–1303. 10.1016/j.psychres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Farhane-Medina NZ, Luque B, Tabernero C, Castillo-Mayén R, 2022. Factors associated with gender and sex differences in anxiety prevalence and comorbidity: a systematic review. Sci. Prog 105, 368504221135469 10.1177/00368504221135469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Regina ACB, Kovacevic N, Martin M, da GM, Santos PP, Carneiro C, de G, Kerr DS, Amaro E Jr, McIntosh AR, Busatto GF, 2016. Aging effects on whole-brain functional connectivity in adults free of cognitive and psychiatric disorders. Cereb. Cortex 26, 3851–3865. 10.1093/cercor/bhv190. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, Williams J, 1996. Structured Clinical Interview for DSM-IV Axis I disorders. American Psychiatric Press., Washington, DC. [Google Scholar]
- Fox MD, Raichle ME, 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8, 700–711. 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME, 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A 102, 9673–9678. 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanniello J, Yu K, Furlan A, Nachtrab GT, Sharma R, Chen X, Li B, 2020. A central amygdala-globus pallidus circuit conveys unconditioned stimulus-related information and controls fear learning. J. Neurosci. Off. J. Soc. Neurosci 40, 9043–9054. 10.1523/JNEUROSCI.2090-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Shechner T, Farber MJ, Spiro CN, Leibenluft E, Pine DS, Britton JC, 2016. Amygdala-cortical connectivity: associations with anxiety, development, and threat. Depress. Anxiety 33, 917–926. 10.1002/da.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb EV, Seo D, Sinha R, 2019. Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol. Stress 11, 100177. 10.1016/j.ynstr.2019.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallers-Haalboom ET, Maas J, Kunst LE, Bekker MHJ, 2020. The role of sex and gender in anxiety disorders: being scared “like a girl”? Handb. Clin. Neurol 175, 359–368. 10.1016/B978-0-444-64123-6.00024-2. [DOI] [PubMed] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, Li K, Jiang T, Yu C, 2014. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp 35, 3446–3464. 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Xu T, Zhang W, Zuo XN, 2016. Lifespan anxiety is reflected in human amygdala cortical connectivity. Hum. Brain Mapp 37, 1178–1193. 10.1002/hbm.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, Lee JS, McLeod DR, Wong DF, 2005. Effect of worry on regional cerebral blood flow in nonanxious subjects. Psychiatry Res. 140, 259–269. 10.1016/j.pscychresns.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, Sadato N, 2001. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J. Cogn. Neurosci 13, 1035–1047. 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Ionta S, Heydrich L, Lenggenhager B, Mouthon M, Fornari E, Chapuis D, Gassert R, Blanke O, 2011. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 70, 363–374. 10.1016/j.neuron.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Ipser JC, Singh L, Stein DJ, 2013. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin. Neurosci 67, 311–322. 10.1111/pcn.12055. [DOI] [PubMed] [Google Scholar]
- Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, Murrough JW, Sani G, Eickhoff SB, Frangou S, 2020. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry 77, 172–179. 10.1001/jamapsychiatry.2019.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks SK, Zhang S, Li CSR, Hu S, 2020. Threat bias and resting state functional connectivity of the amygdala and bed nucleus stria terminalis. J. Psychiatr. Res 122, 54–63. 10.1016/j.jpsychires.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockwitz C, Caspers S, 2021. Resting-state networks in the course of aging—differential insights from studies across the lifespan vs. amongst the old. Pflügers Arch. Eur. J. Physiol 473, 793–803. 10.1007/s00424-021-02520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Price LH, Carpenter LL, 2008. Sex differences in the use of coping strategies: predictors of anxiety and depressive symptoms. Depress. Anxiety 25, 839–846. 10.1002/da.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Kim MJ, 2022. Morphological similarity of amygdala-ventral prefrontal pathways represents trait anxiety in younger and older adults. Proc. Natl. Acad. Sci 119, e2205162119 10.1073/pnas.2205162119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, 2021. The paraventricular nucleus of the thalamus as an integrating and relay node in the brain anxiety network. Front. Behav. Neurosci 15, 627633 10.3389/fnbeh.2021.627633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles KA, Olatunji BO, 2020. Specificity of trait anxiety in anxiety and depression: meta-analysis of the state-trait anxiety inventory. Clin. Psychol. Rev 82, 101928 10.1016/j.cpr.2020.101928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Gur RC, Derntl B, 2015. Sex differences in cognitive regulation of psychosocial achievement stress: brain and behavior. Hum. Brain Mapp 36, 1028–1042. 10.1002/hbm.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Müller VI, Seidel E-M, Boubela R, Kalcher K, Moser E, Habel U, Gur RC, Eickhoff SB, Derntl B, 2016. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage 134, 410–423. 10.1016/j.neuroimage.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesar TA, Bilevicius E, Wilson AD, Kornelsen J, 2019. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. NeuroImage. Clin 24, 102016 10.1016/j.nicl.2019.102016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-H, 2019. Fear network model in panic disorder: the past and the future. Psychiatry Investig. 16, 16–26. 10.30773/pi.2018.05.04.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Terpou BA, McKinnon MC, 2020. The sense of self in the aftermath of trauma: lessons from the default mode network in posttraumatic stress disorder. Eur. J. Psychotraumatol 11, 1807703 10.1080/20008198.2020.1807703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Liao D-L, Ide J, Zhang S, Zhornitsky S, Wang W, Li C-SR, 2020. The interrelationship of body mass index with gray matter volume and resting-state functional connectivity of the hypothalamus. Int. J. Obes 44, 1097–1107. 10.1038/s41366-019-0496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Shin H-S, 2016. The role of mediodorsal thalamic nucleus in fear extinction. J. Anal. Sci. Technol 7, 13. 10.1186/s40543-016-0093-6. [DOI] [Google Scholar]
- Li G, Zhang S, Le TM, Tang X, Li C-SR, 2020. Neural responses to negative facial emotions: sex differences in the correlates of individual anger and fear traits. Neuroimage 221, 117171. 10.1016/j.neuroimage.2020.117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kong R, Liégeois R, Orban C, Tan Y, Sun N, Holmes AJ, Sabuncu MR, Ge T, Yeo BTT, 2019a. Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage 196, 126–141. 10.1016/j.neuroimage.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang M, Li K, Zou F, Wang Y, Wu X, Zhang H, 2019b. The altered somatic brain network in state anxiety. Front. Psychiatry 10, 465. 10.3389/fpsyt.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-H, Young IM, Conner AK, Glenn CA, Chakraborty AR, Nix CE, Bai MY, Dhanaraj V, Fonseka RD, Hormovas J, Tanglay O, Briggs RG, Sughrue ME, 2020. Anatomy and white matter connections of the inferior temporal gyrus. World Neurosurg. 143, e656–e666. 10.1016/j.wneu.2020.08.058. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang Y, Li X, 2018. Implicit emotion regulation deficits in trait anxiety: an ERP study. Front. Hum. Neurosci 12, 382. 10.3389/fnhum.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J, 2011. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev 35, 1219–1236. 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Potvin S, Tikàsz A, Mendrek A, 2015. Sex differences in effective frontolimbic connectivity during negative emotion processing. Psychoneuroendocrinology 62, 180–188. 10.1016/j.psyneuen.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Machado L, Thompson LM, Brett CHR, 2019. Visual analogue mood scale scores in healthy young versus older adults. Int. Psychogeriatr 31, 417–424. 10.1017/S1041610218000996. [DOI] [PubMed] [Google Scholar]
- Mak AKY, Hu Z, Zhang JXX, Xiao Z, Lee TMC, 2009. Sex-related differences in neural activity during emotion regulation. Neuropsychologia 47, 2900–2908. 10.1016/j.neuropsychologia.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB, 2009. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr. Clin. North Am 32, 549–575. 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, 2003. Aging and Emotional Memory. Oxford University Press, London. [Google Scholar]
- Mendez MF, Adams NL, Lewandowski KS, 1989. Neurobehavioral changes associated with caudate lesions. Neurology 39, 349–354. 10.1212/wnl.39.3.349. [DOI] [PubMed] [Google Scholar]
- Min J, Nashiro K, Yoo HJ, Cho C, Nasseri P, Bachman SL, Porat S, Thayer JF, Chang C, Lee T-H, Mather M, 2022. Emotion downregulation targets interoceptive brain regions while emotion upregulation targets other affective brain regions. J. Neurosci 42 10.1523/JNEUROSCI.1865-21.2022, 2973 LP–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzi S, Pedersen M, Lorenzetti V, Heinrichs M, Labuschagne I, 2022. Resting-state neuroimaging in social anxiety disorder: a systematic review. Mol. Psychiatry 27, 164–179. 10.1038/s41380-021-01154-6. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Touroutoglou A, Dickerson BC, Barrett LF, 2014. Sex differences in the neural correlates of affective experience. Soc. Cogn. Affect. Neurosci 9, 591–600. 10.1093/scan/nst030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulinet I, Landeau B, Touron E, De La Sayette V, Desgranges B, Vivien D, Marchant N, Poisnel G, Chételat G, 2022. Sex-specificities in anxiety and depressive symptoms across the lifespan and their links with multimodal neuroimaging. J. Affect. Disord 296, 593–602. 10.1016/j.jad.2021.10.004. [DOI] [PubMed] [Google Scholar]
- Murray L, Maurer JM, Peechatka AL, Frederick BB, Kaiser RH, Janes AC, 2021. Sex differences in functional network dynamics observed using coactivation pattern analysis. Cogn. Neurosci 12, 120–130. 10.1080/17588928.2021.1880383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni-Zepeda M, Alizadeh S, Chand T, Kasties V, Fan Y, van der Meer J, Herrmann L, Vester JC, Schulz M, Naschold B, Walter M, 2022. Trait anxiety is related to Nx4’s efficacy on stress-induced changes in amygdala-centered resting state functional connectivity: a placebo-controlled cross-over trial in mildly to moderately stressed healthy volunteers. BMC Neurosci. 23, 68. 10.1186/s12868-022-00754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S, 2012. Decreased functional connectivity by aging is associated with cognitive decline. J. Cogn. Neurosci 24, 2186–2198. 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AE, Chan L, Mikels JA, 2014. Meta-analysis of the age-related positivity effect: age differences in preferences for positive over negative information. Psychol. Aging 29, 1–15. 10.1037/a0035194. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, 2010. Amygdala activity, fear, and anxiety: modulation by stress. Biol. Psychiatry 67, 1117–1119. 10.1016/j.biopsych.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, Fox PT, 2012. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage 60, 117–129. 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SARB, Stam CJ, Kuijer JPA, Scheltens P, Barkhof F, 2003. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage 20, 1236–1245. 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP, 2009. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45, 614–626. 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter LA, Scheuer L, Vahia IV, Forester BP, Smoller JW, Germine L, 2019. Emotion sensitivity and self-reported symptoms of generalized anxiety disorder across the lifespan: a population-based sample approach. Brain Behav. 9, e01282 10.1002/brb3.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Yoo HJ, Nga L, Lee T-H, Thayer JF, Mather M, 2016. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage 139, 44–52. 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, 2013. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61. 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, Riese H, Ormel J, Aleman A, 2014. The neural correlates of worry in association with individual differences in neuroticism. Hum. Brain Mapp 35, 4303–4315. 10.1002/hbm.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Yang A, Zhao Q, Chen Z, Ren X, Dai Q, 2021. A hypothesis of gender differences in self-reporting symptom of depression: implications to solve under-diagnosis and under-treatment of depression in males. Front Psychiatry. 12, 589687. 10.3389/fpsyt.2021.589687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeter M, Kindt M, 2013. High trait anxiety: a challenge for disrupting fear memory reconsolidation. PLoS One 8, e75239. 10.1371/journal.pone.0075239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, 1989. State–Trait Anxiety Inventory: A Comprehensive Bibliography. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- St. Jacques P, Dolcos F, Cabeza R, 2010. Effects of aging on functional connectivity of the amygdala during negative evaluation: a network analysis of fMRI data. Neurobiol. Aging 31, 315–327. 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R, 2009. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol. Sci 20, 74–84. 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Brown JA, Casaletto KB, Elahi FM, Deng J, Neuhaus J, Cobigo Y, Mumford PS, Walters S, Saloner R, Karydas A, Coppola G, Rosen HJ, Miller BL, Seeley WW, Kramer JH, 2018. The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J. Neurosci 38 10.1523/JNEUROSCI.3067-17.2018, 2809 LP–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S, 2012. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia 50, 1578–1593. 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ, 2012. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 35, 527–535. 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, 2012. Aging and functional brain networks. Mol. Psychiatry 17, 549–558. 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy A, Jopling E, LeMoult J, 2021. The effect of self-referential processing on anxiety in response to naturalistic and laboratory stressors. Cogn. Emot 35, 1320–1333. 10.1080/02699931.2021.1951675. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang Y, Lau WKW, Wei X, Feng X, Zhang C, Liu Y, Huang R, Zhang R, 2021a. Anomalous static and dynamic functional connectivity of amygdala subregions in individuals with high trait anxiety. Depress. Anxiety 38, 860–873. 10.1002/da.23195. [DOI] [PubMed] [Google Scholar]
- Wang M, Cao L, Li H, Xiao H, Ma Y, Liu S, Zhu H, Yuan M, Qiu C, Huang X, 2021b. Dysfunction of resting-state functional connectivity of amygdala subregions in drug-naïve patients with generalized anxiety disorder. Front. Psychiatry 12, 758978. 10.3389/fpsyt.2021.758978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger M, Sandi C, 2018. High anxiety trait: a vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev 87, 27–37. 10.1016/j.neubiorev.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Weis S, Patil KR, Hoffstaedter F, Nostro A, Yeo BTT, Eickhoff SB, 2020. Sex classification by resting state brain connectivity. Cereb. Cortex 30, 824–835. 10.1093/cercor/bhz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Yap MBH, Allen NB, 2011. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biol. Psychol 87, 319–333. 10.1016/j.biopsycho.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Wiebe DJ, Song A, Ramirez Loyola MD, 2018. What mechanisms explain the links between personality and health?*. In: Personality and Disease. Academic Press, San Diego, pp. 223–245. [Google Scholar]
- Wong AHK, Lovibond PF, 2018. Excessive generalisation of conditioned fear in trait anxious individuals under ambiguity. Behav. Res. Ther 107, 53–63. 10.1016/j.brat.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS, Phan KL, 2016a. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp 37, 1684–1695. 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li H, Zhou Y, Yu J, Zhang Y, Song M, Qin W, Yu C, Jiang T, 2016b. Sexspecific neural circuits of emotion regulation in the centromedial amygdala. Sci. Rep 6, 23112 10.1038/srep23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Zhang S, Lee LE, Chao HH, van Dyck C, Li C-SR, 2018. Exploring age-related changes in resting state functional connectivity of the amygdala: from young to middle adulthood. Front. Aging Neurosci 10, 209. 10.3389/fnagi.2018.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Van Dam NT, Feng C, Luo Y, Ai H, Gu R, Xu P, 2019. Anxious brain networks: a coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci. Biobehav. Rev 96, 21–30. 10.1016/j.neubiorev.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Young KD, Bellgowan PSF, Bodurka J, Drevets WC, 2013. Functional neuroimaging of sex differences in autobiographical memory recall. Hum. Brain Mapp 34, 3320–3332. 10.1002/hbm.22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH, 1999. Biostatistical Analysis, 4th ed. Prentice Hall, Upper Saddle River. [Google Scholar]
- Zhang S, Wang W, Zhornitsky S, Li C-SR, 2018. Resting state functional connectivity of the lateral and medial hypothalamus in cocaine dependence: an exploratory study. Front. Psychiatry 9, 344. 10.3389/fpsyt.2018.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Wang W, Le TM, Dhingra I, Chen Y, Li C-SR, 2021. Resting state hypothalamic and dorsomedial prefrontal cortical connectivity of the periaqueductal gray in cocaine addiction. Addict. Biol 26, e12989 10.1111/adb.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hashemi MM, Kaldewaij R, Koch SBJ, Beckmann C, Klumpers F, Roelofs K, 2019. Acute stress alters the ‘default’ brain processing. Neuroimage 189, 870–877. 10.1016/j.neuroimage.2019.01.063. [DOI] [PubMed] [Google Scholar]
- Zhang X, Suo X, Yang X, Lai H, Pan N, He M, Li Q, Kuang W, Wang S, Gong Q, 2022. Structural and functional deficits and couplings in the corticostriato-thalamo-cerebellar circuitry in social anxiety disorder. Transl. Psychiatry 12, 26. 10.1038/s41398-022-01791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld HI, Pruim RHR, Bos D, Vrooman HA, Muetzel RL, Hofman A, Rombouts SARB, van der Lugt A, Niessen WJ, Ikram MA, Vernooij MW, 2019. Patterns of functional connectivity in an aging population: the Rotterdam study. Neuroimage 189, 432–444. 10.1016/j.neuroimage.2019.01.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


