Abstract
Background
Whether increased BMI is associated with an increased risk of venous thromboembolism (VTE) is controversial. Despite this, BMI > 40 kg/m2 remains a common cutoff for lower limb arthroplasty eligibility. Current United Kingdom national guidelines list obesity as a risk factor for VTE, but these are based on evidence that has largely failed to differentiate between potentially minor (distal deep vein thrombosis [DVT]), and more harmful (pulmonary embolism [PE] and proximal DVT) diagnoses. Determining the association between BMI and the risk of clinically important VTE is needed to improve the utility of national risk stratification tools.
Questions/purposes
(1) In patients undergoing lower limb arthroplasty, is BMI 40 kg/m2 or higher (morbid obesity) associated with an increased risk of PE or proximal DVT within 90 days of surgery, compared with patients with BMI less than 40 kg/m2? (2) What proportion of investigations ordered for PE and proximal DVT were positive in patients with morbid obesity who underwent lower limb arthroplasty compared with those with BMI less than 40 kg/m2?
Methods
Data were collected retrospectively from the Northern Ireland Electronic Care Record, a national database recording patient demographics, diagnoses, encounters, and clinical correspondence. Between January 2016 and December 2020, 10,217 primary joint arthroplasties were performed. Of those, 21% (2184 joints) were excluded; 2183 were in patients with multiple arthroplasties and one had no recorded BMI. All 8033 remaining joints were eligible for inclusion, 52% of which (4184) were THAs, 44% (3494) were TKAs, and 4% (355) were unicompartmental knee arthroplasties; all patients had 90 days of follow-up. The Wells score was used to guide the investigations. Indications for CT pulmonary angiography for suspected PE included pleuritic chest pain, reduced oxygen saturations, dyspnea, or hemoptysis. Indications for ultrasound scans for suspected proximal DVT included leg swelling, pain, warmth, or erythema. Distal DVTs were recorded as negative scans because we do not treat them with modified anticoagulation. The division of categories was set at BMI 40 kg/m2, a common clinical cutoff used in surgical eligibility algorithms. Patients were grouped according to WHO BMI categories to assess for the following confounding variables: sex, age, American Society of Anesthesiologists grade, joint replaced, VTE prophylaxis, grade of operative surgeon, and implant cement status.
Results
We found no increase in the odds of PE or proximal DVT in any WHO BMI category. When comparing patients with BMI less than 40 kg/m2 with those with a BMI of 40 kg/m2 or higher, there was no difference in the odds of PE (0.8% [58 of 7506] versus 0.8% [four of 527]; OR 1.0 [95% CI 0.4 to 2.8]; p > 0.99) or proximal DVT (0.4% [33 of 7506] versus 0.2% [one of 527]; OR 2.3 [95% CI 0.3 to 17.0]; p = 0.72). Of those who received diagnostic imaging, 21% (59 of 276) of CT pulmonary angiograms and 4% (34 of 718) of ultrasounds were positive for patients with BMI less than 40 kg/m2 compared with 14% (four of 29; OR 1.6 [95% CI 0.6 to 4.5]; p = 0.47) and 2% (one of 57; OR 2.7 [95% CI 0.4 to 18.6]; p = 0.51) for patients with BMI 40 kg/m2 or higher. There was no difference in the percentage of CT pulmonary angiograms ordered (4% [276 of 7506] versus 5% [29 of 527]; OR 0.7 [95% CI 0.5 to 1.0]; p = 0.07) or ultrasounds ordered (10% [718 of 7506] versus 11% [57 of 527]; OR 0.9 [95% CI 0.7 to 1.2]; p = 0.49) for BMI less than 40 kg/m2 and BMI 40 kg/m2 or higher.
Conclusion
Increased BMI should not preclude individuals from lower limb arthroplasty based on suspected risk of clinically important VTE. National VTE risk stratification tools should be based on evidence assessing clinically relevant VTE (specifically, proximal DVT, PE, or death of thromboembolism) only.
Level of Evidence
Level III, therapeutic study.
Introduction
Venous thromboembolism (VTE), defined as pulmonary embolism (PE) or deep vein thrombosis (DVT), is a widely studied complication in patients who undergo lower extremity arthroplasty. VTE can be a serious complication, sometimes leading to extended hospital stays, unplanned readmissions, and additional costs for health services [21]. However, the term “VTE” fails to differentiate potentially minor diagnoses (asymptomatic distal DVT) from more harmful ones (PE and proximal DVT). To complicate matters, widely used national guidelines for VTE risk assessment are derived from research that includes clinically unimportant distal DVTs.
VTE risk factors include lower limb arthroplasty, reduced mobility, age older than 60 years, and obesity [12]. Obesity, defined as BMI 30 kg/m2 or more, has become a global epidemic, with its prevalence tripling between 1975 and 2016 [24]. Patients with higher BMI are at increased risk of infection and revision surgery [9], and perhaps extended length of hospital stay [19]. Further, orthopaedic surgeons commonly use the arbitrary BMI cutoff of 40 kg/m2 or higher to disqualify individuals from surgery, with the general belief that these patients are at increased risk of postoperative complications [20]. Determining the risk of PE and proximal DVT in patients with increased BMI is therefore important.
Interpreting the available evidence about the association between increased BMI and VTE risk is complex, because most studies on the topic did not assess clinically relevant diagnoses alone but included distal DVTs as part of the definition of VTE. Because many thromboembolic events are distal DVTs, which have little clinical importance, it is therefore difficult to interpret most of what has been published on this topic. With this caveat in mind, many studies have reported an association between increased BMI and increased risk of VTE [3, 6, 10, 14, 16, 18, 23]; however, a few studies refute this [4, 17, 18]. More recently, the 2022 International Consensus Meeting examining this question found the evidence inconclusive [7]. Further, there is an almost unquestioned assumption in the orthopaedic community that increased BMI is associated with an increased risk of PE and proximal DVT after lower limb arthroplasty, despite insufficient evidence to support this. These assumptions may lead to excessive imaging and detection of clinically unimportant PE and proximal DVT, which can result in prolonged anticoagulation and put patients at risk of side effects associated with these medications. Research assessing only clinically relevant complications in this domain, therefore, is paramount.
In this retrospective observational study, we asked: (1) In patients undergoing lower limb arthroplasty, is BMI 40 kg/m2 or higher (morbid obesity) associated with an increased risk of PE or proximal DVT within 90 days of surgery, compared with patients with BMI less than 40 kg/m2? (2) What proportion of investigations ordered for PE and proximal DVT were positive in patients with morbid obesity who underwent lower limb arthroplasty compared with those with BMI less than 40 kg/m2?
Patients and Methods
Study Design and Setting
This retrospective, comparative, large-database study was performed at a single-center, regional elective orthopaedic hospital. Patient demographics and surgical data were obtained from the Northern Ireland Electronic Care Record. The Northern Ireland Electronic Care Record provides healthcare professionals with a single view of key patient information, including demographics, laboratory results, medications, allergies, diagnoses, encounters, and clinical correspondence. It robustly captures all out-of-hours general practitioner visits, emergency department visits to any hospital, and readmissions to any hospital across the region. We interrogated the Northern Ireland Electronic Care Record for any complications, including all VTE investigations, up to 90 days postoperatively.
Participants
Between January 2016 and December 2020, we performed 10,217 primary joint arthroplasties: primary THAs, TKAs, and unicompartmental knee arthroplasties (UKAs). Thus, emergency reconstructions for fractures of the femoral neck, revision arthroplasties, and other complex reconstructions (such as surgeries to treat tumors and bone defects) were not included. Of those, 21% (2184 joints) were excluded; 2183 joints were in patients with multiple arthroplasties and therefore would not provide independent datapoints for analysis, while one patient did not have BMI recorded at the time of surgery (Fig. 1). All remaining joints (79% [8033 of 10,217]) were eligible for inclusion in this study; 52% (4184) were THAs, 44% (3494) were TKAs, and 4% (355) were UKAs. Of the patients reviewed, 89% (7123 of 8033) did not attend a clinic for any VTE investigation in Northern Ireland during the 90-day study period. Because the record uses a unique National Health Service Health and Care number for each patient, we could account for all patients during the 90-day study period; however, we cannot ensure that all patients had complete follow-up. The accuracy and reliability of the Northern Ireland Electronic Care Record were not assessed during this study; as such, it is possible that VTE investigations were performed in Northern Ireland but not recorded. Additionally, investigations occurring outside Northern Ireland would not have been captured in the database.
Fig. 1.
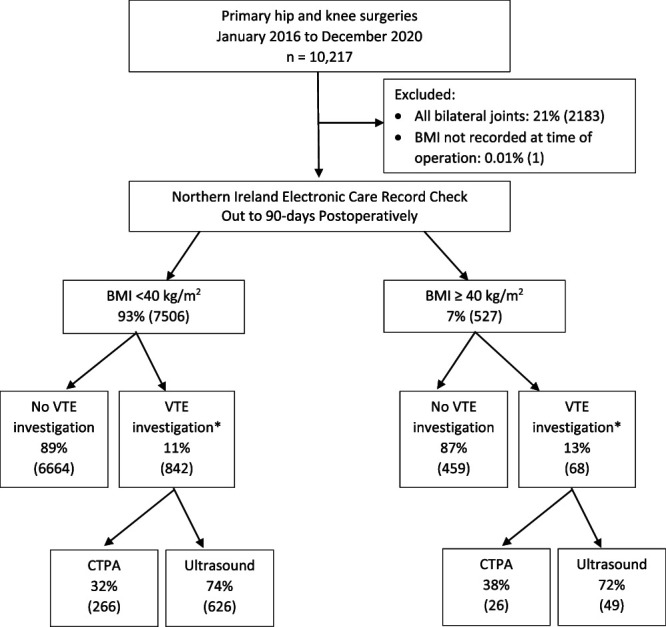
This flowchart outlines the patient exclusion criteria and group selection for the study. Data are presented as % (n). *VTE investigation includes patients who had both CT pulmonary angiography and ultrasound scans.
Patients were dichotomized into two groups: BMI less than 40 kg/m2 (93% [7506 of 8033]) and BMI of 40 kg/m2 or higher (7% [527 of 8033]). We set the division of categories at BMI 40 kg/m2 because this is the most frequently used clinical cutoff for surgeons whose guidelines include BMI in their algorithms for offering surgery.
Descriptive Data
In the two BMI groups, there was a preponderance of female patients with a BMI of 40 kg/m2 or higher (80% [363 of 4556]) compared with male patients (47% [164 of 3477]); OR 1.7 [95% CI 1.4 to 2.0]; p < 0.001). The median age difference between the two groups was 4.5 years; patients with BMI of 40 kg/m2 or higher were younger than those with a smaller BMI (median 65 years [IQR 58 to 71] versus median 70 years [IQR 62 to 76]; p < 0.001). Patients with a BMI of 40 kg/m2 or higher had higher odds of having a TKA than those with a smaller BMI (57% [299 of 527] versus 43% [3195 of 7506]; OR 1.8 [95% CI 1.5 to 2.1]; p < 0.001). The distribution of American Society of Anesthesiologists (ASA) grades also differed between the groups; patients with BMI of 40 kg/m2 or higher had higher odds of having ASA grade 3 or 4 than those with a smaller BMI (33% [174 of 527] versus 14% [1072 of 7506]; OR 3.0 [95% CI 2.4 to 3.6]; p < 0.001). There was no difference in the number of patients who had CT pulmonary angiography (CTPA) or ultrasound ordered between the two groups (13% [68 of 527] versus 11% [842 of 7506]; OR 1.2 [95% CI 0.9 to 1.5]; p = 0.24). There was also no difference in the number of patients who had a PE or proximal DVT (0.9% [five of 527] versus 1% [90 of 7506]; OR 1.3 [95% CI 0.5 to 2.9]; p = 0.61 (Table 1).
Table 1.
Demographics of patients in each BMI category
| Variable | BMI < 40 kg/m2 (n = 7506) | BMI ≥ 40 kg/m2 (n = 527) | p value |
| Male | 44% (3313) | 31% (164) | < 0.001 |
| Age in years | 70 (16-97) | 65 (16-86) | < 0.001 |
| Joint replaced | |||
| THA | 53% (3977) | 39% (207) | < 0.001 |
| TKA | 43% (3195) | 57% (299) | |
| UKA | 4.4% (334) | 4.0% (21) | |
| ASA Grade 1 and 2 | 86% (6434) | 67% (353) | < 0.001 |
| Operation performed by consultant | 81% (6050) | 81% (425) | 0.98 |
| VTE prophylaxis | |||
| Aspirin | 20% (1491) | 22% (114) | 0.49 |
| Enoxaparin | 80% (6008) | 78% (413) | |
| Mechanical only | 0.1% (7) | 0% (0) | |
| Cement status | |||
| Cementless | 63% (4750) | 74% (379) | < 0.001 |
| Cemented | 7.2% (538) | 6.1% (32) | |
| Hybrid | 29% (2172) | 22% (116) | |
| Reverse hybrid | 0.6% (46) | 0% (0) | |
| CTPA or ultrasound examination | 11% (842) | 13% (68) | 0.24 |
| Positive CTPA or ultrasound examination | 11% (90 of 842) | 7.4% (5 of 68) | 0.54 |
Data are presented as % (n) or median (range).
In the multivariate analysis, described below, the differences in demographics between the BMI groups (more female patients, more TKAs, and more patients with ASA grade 3 or 4 in the BMI 40 kg/m2 or higher group) were considered as potential confounding factors.
Procedures were performed under the care of 33 surgeons using cementless and cemented implants. For VTE prophylaxis, aspirin was given to 20% (1605 of 8033) of patients, all of whom had a low risk, while the remaining received enoxaparin (80% [6421 of 8033]) or mechanical prophylaxis alone (0.09% [seven of 8033]).
Description of Experiment, Treatment, or Surgery
Generally, practitioners ordering investigations were guided by the Wells score, a widely used clinical decision tool for suspected PE and DVT [13]. Indications for CTPA for PE included pleuritic chest pain, reduced oxygen saturations, dyspnea, or hemoptysis. Indications for ultrasound for DVT included leg swelling, pain, warmth, or erythema beyond what would be expected after lower limb arthroplasty. There was no documentation of Wells scores in patient records; as such, we could not ensure adherence to this scoring system. However, we believe that orthopaedic surgical teams are astute in monitoring for the clinical features of PE and DVT because of their education about and familiarity with such complications, along with a relatively higher suspicion in this cohort of patients.
Variables, Outcome Measures, Data Sources, and Bias
The proportion of patients with PE and proximal DVT in two BMI categories was determined. Data captured included age, sex, BMI, ASA grade, cement status, surgeon experience (consultant, staff surgeon, or trainee), and data for 90-day postoperative PE and proximal DVT investigations, including the number of CTPAs ordered for suspected PE, the number of ultrasounds for suspected proximal DVT, and the number of confirmed PEs and proximal DVTs. BMI was calculated and recorded for each patient by independent nursing staff before surgery. ASA grade was recorded in the operative notes by independent anesthetists. Cement status and surgeon grade were obtained from operative notes. CTPAs and ultrasound investigations were performed by radiographers and sonographers. All CTPAs were reported by independent radiologists. Any central filling defects in any vessel, including subsegmental PEs, were recorded as positive CTPA results. Positive ultrasound scan results were those that showed a thrombus or noncompressibility of the popliteal, femoral, or iliac veins. Although noted, distal DVTs were recorded as negative results because generally they are not treated with additional anticoagulation.
We obtained data from a concurrent audit in our institution that began in January 2016, investigating 90-day postoperative complications and nonscheduled care in all patients undergoing primary THA, TKA, or UKA. As such, study size was determined by the total number of patients enrolled in the audit up to and including December 31, 2020. By obtaining data in this way, selection bias was minimized because data were initially collected for all patients.
Ethical Approval
Institutional review board approval was not sought because the information used in this study was recorded in such a manner that patients cannot be identified directly or through identifiers linked to the patients. Although ethical approval was not sought, this study was registered with the Standard, Quality, and Audit department in our institution (audit number: 6090).
Statistical Analysis
The statistical analysis was performed with SPSS version 27 software (IBM Corp). Data were assessed for normality using the Shapiro-Wilk test. Categorical variables were compared and ORs were calculated using the chi-square or Fisher exact test. Nonparametric continuous variables were assessed with the Mann-Whitney U test or the Kruskal-Wallis test where appropriate. A multivariate regression analysis was performed using the following variables: WHO categories of BMI (reference category: BMI less than 24.9 kg/m2), sex (reference category: female), age (in years, continuous), ASA grade (reference category: ASA grades 1 and 2), joint replaced (reference category: THA), VTE prophylaxis (reference category: enoxaparin), grade of operative surgeon (reference category: consultant surgeon), and implant cement status (reference category: cementless). To avoid data sparsity in the regression analysis, the following steps were taken: seven patients treated with only mechanical prophylaxis were excluded, two WHO BMI categories were combined (underweight [BMI less than 18.5 kg/m2] and healthy weight [BMI between 18.5 and 24.9 kg/m2]), all hybrid cemented implants were combined, and to assess DVT only TKA and UKA were combined. Unless otherwise stated, statistical significance was set at p < 0.05.
Results
Odds of PE or Proximal DVT as a Function of BMI
After controlling for potentially confounding variables (including WHO categories of BMI, sex, age, joint replaced, ASA grade, VTE prophylaxis, grade of operative surgeon, and implant cement status) we found no increase in the odds of PE or proximal DVT in any BMI category. Patients undergoing TKA had a higher odds of experiencing PE than those with THA (OR 2.9 [95% CI 1.3 to 6.5]; p < 0.001), as did patients with hybrid cemented implants (OR 3.5 [95% CI 1.5 to 8.2]; p < 0.001). Patients receiving enoxaparin, however, had lower odds of PE than patients receiving aspirin (OR 0.5 [95% CI 0.3 to 0.8]; p = 0.01) (Table 2).
Table 2.
Multivariate analysis
| Adjusted OR (95% CI) | p value | |
| Positive CTPA (0.8%, 61 of 8026) | ||
| BMI < 24.9 kg/m2 (reference group) | 1.0 | |
| BMI 25 to 29.9 kg/m2 | 1.0 (0.4 to 2.5) | 0.94 |
| BMI 30 to 34.9 kg/m2 | 1.3 (0.5 to 3.0) | 0.61 |
| BMI 35 to 39.9 kg/m2 | 0.8 (0.3 to 2.4) | 0.70 |
| BMI ≥ 40 kg/m2 | 1.2 (0.3 to 4.3) | 0.77 |
| THA (reference group) | 1.0 | |
| TKA | 2.9 (1.3 to 6.5) | < 0.001 |
| UKA | 2.1 (0.5 to 8.3) | 0.28 |
| Aspirin (reference group) | 1.0 | |
| Enoxaparin | 0.5 (0.3 to 0.8) | 0.01 |
| ASA Grade 1 and 2 (reference group) | 1.0 | |
| ASA Grade 3 and 4 | 0.5 (0.2 to 1.2) | 0.11 |
| Consultant surgeon (reference group) | 1.0 | |
| Trainee surgeon | 0.9 (0.5 to 1.7) | 0.71 |
| Cementless implant (reference group) | 1.0 | |
| Cemented implant | 0.6 (0.1 to 2.5) | 0.47 |
| Hybrid implant | 3.5 (1.5 to 8.2) | < 0.001 |
| Female (reference group) | 1.0 | |
| Male | 1.0 (0.6 to 1.7) | 0.96 |
| Age per year | 1.0 (0.99 to 1.0) | 0.24 |
| Positive ultrasound (0.4%, 34 of 8026) | ||
| BMI < 24.9 kg/m2 (reference group) | 1.0 | |
| BMI 25 to 29.9 kg/m2 | 1.7 (0.5 to 6.3) | 0.42 |
| BMI 30 to 34.9 kg/m2 | 3.6 (1.0 to 12.6) | 0.046 |
| BMI 35 to 39.9 kg/m2 | 1.5 (0.3 to 7.7) | 0.62 |
| BMI ≥ 40 kg/m2 | 1.5 (0.2 to 14.9) | 0.74 |
| THA (reference group) | 1.0 | |
| TKA | 0.3 (0.1 to 0.7) | 0.03 |
| Aspirin (reference group) | 1.0 | |
| Enoxaparin | 0.8 (0.3 to 1.8) | 0.52 |
| ASA Grade 1 and 2 (reference group) | 1.0 | |
| ASA Grade 3 and 4 | 0.5 (0.2 to 1.6) | 0.26 |
| Consultant surgeon (reference group) | 1.0 | |
| Trainee surgeon | 0.7 (0.3 to 1.7) | 0.41 |
| Cementless implant (reference group) | 1.0 | |
| Cemented implant | 1.3 (0.4 to 3.9) | 0.66 |
| Hybrid implant | 0.5 (0.2 to 1.2) | 0.11 |
| Female (reference group) | 1.0 | |
| Male | 0.7 (0.4 to 1.5) | 0.41 |
| Age per year | 1.1 (1.0 to 1.1) | < 0.001 |
Again, after controlling for potential confounding factors, the odds of a proximal DVT appeared to be greater in patients with BMI 30 to 34.9 kg/m2; however, the CIs were too wide to draw an adequate conclusion (OR 3.6 [95% CI 1.0 to 12.6]; p = 0.046). We found no difference in the association between BMI categories for the development of a proximal DVT (BMI 25 to 29.9 kg/m2: OR 1.7 [95% CI 0.5 to 6.3]; p = 0.42, BMI 35 to 39.9 kg/m2: OR 1.5 [95% CI 0.3 to 7.7]; p = 0.62, and BMI of 40 kg/m2 or higher: OR 1.5 [95% CI 0.2 to 14.9]; p = 0.74).We found that for every increasing year of age, there was a 6% increased odds of proximal DVT (OR 1.1 [95% CI 1.0 to 1.1]; p < 0.001). Patients undergoing TKA were less likely to experience a proximal DVT than those undergoing THA (OR 0.3 [95% CI 0.1 to 0.7]; p = 0.03) (Table 2). In this study, no patients who underwent UKA had a proximal DVT.
Odds of a Positive Diagnostic Study Finding as a Function of BMI
Of the CTPAs ordered, we observed no difference in the proportion of positive scan results between patients with BMI less than 40 kg/m2 and those with BMI 40 kg/m2 or greater (21% [59 of 276] versus 14% [four of 29]; OR 1.6 [95% CI 0.6 to 4.5]; p = 0.47) (Fig. 2). This remained unchanged when separate joints were assessed: THA (22% [25 of 114] versus 14% [one of seven]; OR 1.6 [95% CI 0.2 to 13.0]; p > 0.99), TKA (21% [31 of 146] versus 14% [three of 21]; OR 1.5 [95% CI 0.5 to 4.9]; p = 0.57), and UKA (19% [three of 16] versus 0% [0 of one]; p = 0.82) (Fig. 2). Of the CTPAs ordered, 79% (242 of 305) of scan results were negative.
Fig. 2.
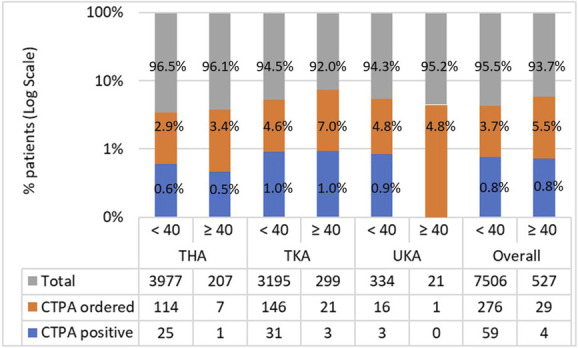
This bar chart shows the number of CTPAs ordered and number of positive scans, expressed as logarithmic proportions of the total number of operations, according to BMI category and joint replaced. Absolute numbers are given in the table below. A color image accompanies the online version of this article.
Of the ultrasounds ordered, we observed no difference in the proportion of positive results between patients with BMI less than 40 kg/m2 and those with BMI 40 kg/m2 or greater (5% [34 of 718] versus 2% [one of 57]; OR 2.7 [95% CI 0.4 to 18.6]; p = 0.51) (Fig. 3). This remained unchanged when separate joints were assessed: THAs (7% [22 of 341] versus 6% [one of 18]; OR 1.2 [95% CI 0.2 to 8.4]; p > 0.99), TKA (3% [12 of 359] versus 0% [0 of 36]; p = 0.61) (Fig. 3). Of the patients who had ultrasounds ordered, 96% (740 of 775) of scan results were negative.
Fig. 3.
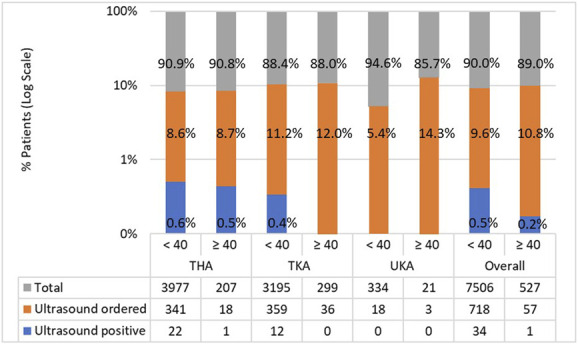
This bar chart shows the number of ultrasounds ordered and number of positive scans, expressed as logarithmic proportions of the total number of operations, according to BMI category and joint replaced. Absolute numbers are given in the table below. Only proximal DVTs were considered as positive results. A color image accompanies the online version of this article.
Discussion
When studies pool clinically unimportant distal DVTs with more-harmful diagnoses (proximal DVT, PE, and death from thromboembolism), those studies risk misleading clinicians and causing overtreatment of a condition that seldom harms patients. Additionally, current risk stratification tools based on the available evidence list obesity as a risk factor. Because BMI 40 kg/m2 or greater is often used to preclude patients from lower limb arthroplasty, we sought to determine whether patients with morbid obesity had higher odds of experiencing clinically important VTE within 90 days of primary THA and TKA. We found that patients with a BMI of 40 kg/m2 or greater did not have higher odds of PE or proximal DVT than those with a BMI less than 40 kg/m2. This suggests that this arbitrary cutoff should not prevent patients from having these operations because of concerns about VTE. Further, guidelines for VTE should be formulated from evidence on clinically relevant diagnoses (proximal DVT, PE, and death of thromboembolism). Improving risk stratification systems is important to identify those at risk early, enabling early surveillance and initiation of preventative measures such as anticoagulation and mechanical prophylaxis.
Limitations
First, dichotomizing BMI is a widely used approach, but it is inappropriate for assessing relationships between a continuous predictor and a clinical outcome [1]. Categorizing a continuous variable assumes that the risk is constant for all values in the same category, leading to potentially biased results. Assessing BMI as a continuous variable is also problematic, imposing a linear relationship between the variable in question (BMI) and the outcome (PE or proximal DVT). Modeling continuous data as a spline would avoid arbitrary cutoffs for BMI and provide a more accurate analysis of the risk of PE and proximal DVT. The expertise to achieve this was not available at the time of our data analysis. Therefore, our results from analyzing BMI as categorized variables provide an informative analysis (because the cutoff BMI of 40 kg/m2 that we used is commonly used clinically) but should be considered within the limitations of our statistical methods. Second, data on patient comorbidities, presurgery mobility, or medical history of VTE were not available. We recognize that these factors are potentially confounding. By accounting for factors associated with an increased VTE risk that other studies often have not included, such as use of bone cement and chemoprophylaxis, we hope to limit any effect that missing data may have had on our results [26]. Third, because this study was performed in a single center, we reported a small number of patients with BMI 40 kg/m2 or greater who had positive CTPA and ultrasound results. A multicenter study would have had a larger number of patients with morbid obesity and positive results, but that approach would have resulted in limitations of its own, including differences in surgical technique, postoperative care (such as early mobilization, VTE chemoprophylaxis, and mechanical prevention measures), ordering of CTPAs, and sonographer experience.
Importantly, we could not control the accuracy of investigation reports. A recent systematic review of 61 international studies reported that CTPA sensitivity and specificity were 94% and 98%, respectively [15]. We infer that our reporting would be comparable. The accuracy of ultrasound reporting is more difficult to control for because this relies on the experience of the sonographer. All sonographers in our institution undergo in-depth training and are permitted to sign off on reports only when they have established the experience to do so. Additionally, the reporting of any false-positive CTPA or ultrasound results is likely to be comparable to the number of false-negative findings. Lastly, we did not assess the accuracy or reliability of the Northern Ireland Electronic Care Record database used in this study. As such, it is possible that VTE investigations were performed in Northern Ireland and not recorded or occurred in other countries. Because this database is a longstanding record collating patient data from all healthcare trusts across the country and records encounters in primary and secondary care, we anticipate that the number of investigations that have potentially been missed in the database is minimal. Further, it is likely that very few patients underwent imaging outside Northern Ireland during the 90-day follow-up period.
Odds of PE or Proximal DVT as a Function of BMI
We found that patients with morbid obesity did not have higher odds of experiencing proximal DVT or PE within 90 days of lower limb arthroplasty than those who did not have morbid obesity. Our findings suggest that individuals with morbid obesity should not be disqualified from primary lower limb arthroplasty based on a presumed risk of clinically important VTE. This is important because lower limb arthroplasties are associated with improved mobility, reduced pain, and increased quality of life [2, 11]. Further, we reported no increase in the odds of PE or proximal DVT with increasing BMI category, referenced to BMI of 25 kg/m2 or less. Current guidelines state obesity is a risk factor for VTE; patients with morbid obesity might more readily meet the threshold for investigation when risk stratification tools are used. As mentioned earlier, the available evidence about the association between increased BMI and the risk of VTE is controversial. Appropriate comparison of studies is also challenging because of differences in BMI grouping, joint that was operated on, duration of follow-up, and thromboembolic event assessed (Supplemental Table 1; http://links.lww.com/CORR/B158). Regardless, our findings suggest that removing obesity as a risk factor from the risk stratification tools that surgeons use would be reasonable, and likely would lead to fewer individuals meeting the investigation criteria, preserving resources and reducing the number of patients with a diagnosis of distal DVT. DVT is not generally a clinically important finding in this context, but sometimes it is inappropriately and unnecessarily treated with strong anticoagulants.
Odds of a Positive Diagnostic Study Finding as a Function of BMI
We found that patients with morbid obesity were not more likely to have a positive investigation than those without morbid obesity. By assessing total positive and negative investigations, we reported the proportion of positive results rather than simply the incidence of PE and proximal DVT. Studies that do not report negative investigations cannot exclude a bias toward ordering more investigations in patients with morbid obesity, and potentially thereby inflating their recorded risk of VTE. Further, we report that patients with morbid obesity did not undergo more CTPAs or ultrasounds than patients with a BMI of less than 40 kg/m2, suggesting this bias was not a factor in our institution during the study period. One explanation for why we observed no difference in the number of scans ordered between BMI groups is because we used the Wells score to guide investigations, particularly when ordering ultrasound. Because of lower limb arthroplasty, symptoms similar to those caused by DVT (swelling, pain, and erythema) are expected. Therefore, using the Wells score to direct investigations is perhaps unreasonable in the early period after surgery. We suggest that VTE assessment criteria specifically for patients who undergo arthroplasty could be developed to prevent overinvestigation and reduce the number of clinically unimportant diagnoses that are made. This would reduce the number of patients receiving unnecessary anticoagulation and the risk of side effects associated with these medications.
Other Relevant Findings
We reported high proportions of negative investigations. Studies with lower investigation thresholds will likely report higher proportions of patients testing positive for VTE, because detection and diagnoses of clinically unimportant VTE is more likely. This is apparent in prior studies in which imaging in all patients resulted in higher percentages of patients with a diagnosis of VTE [5, 19] than in studies that obtained diagnostic imaging in patients with symptoms of DVT [8, 14, 22, 25]. Therefore, for this reason, we suggest that the proportion of negative imaging studies needs to be reported to appropriately compare studies.
Conclusion
We found that patients in our institution with morbid obesity (BMI of 40 kg/m2 or higher) were not at increased odds of experiencing PE or proximal DVT after lower limb arthroplasty. This remained unchanged when assessing BMI in WHO categories, referenced against a BMI less than 25 kg/m2. This is contrary to much of the published evidence on which current national risk stratification tools are based, and so those may need to be revised. Our findings suggest that morbid obesity should not disqualify patients from undergoing elective hip or knee arthroplasty out of concern for clinically important VTE. We suggest that risk stratification tools guiding the use of diagnostic imaging studies should be developed specifically for patients undergoing lower limb arthroplasty, because using current scoring systems results in patients being screened too frequently with ultrasound after arthroplasty. Future studies assessing the risk of VTE and BMI should assess only clinically important complications (proximal DVT, PE, and death from thromboembolism) and report the percentage of negative imaging studies to facilitate fair comparisons across studies on the topic.
Supplementary Material
Acknowledgment
We thank the Trauma and Orthopaedics Research Charity for providing their expertise in the design of this study and manuscript preparation. TORC charity number; NIC105791.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval was not sought for the present study; however, permission was requested from and granted by our institution’s Standards, Quality, and Audit Department (Audit number: 6090).
Contributor Information
Roslyn Cassidy, Email: roslyn.cassidy@belfasttrust.hscni.net.
Janet Hill, Email: janet.hill@belfasttrust.hscni.net.
Leeann Bryce, Email: leeann.bryce@belfasttrust.hscni.net.
Richard Napier, Email: Richard.napier@belfasttrust.hscni.net.
David Beverland, Email: david.beverland@belfasttrust.hscni.net.
References
- 1.Dawson NV, Weiss R. Dichotomizing continuous variables in statistical analysis: a practice to avoid. Med Decis Making . 2012;32:225-226. [DOI] [PubMed] [Google Scholar]
- 2.Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JV. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86:963-74. [DOI] [PubMed] [Google Scholar]
- 3.Feng B, Lin J, Jin J, Qian W-W, Wang W, Weng X-S. Thirty-day postoperative complications following primary total knee arthroplasty: a retrospective study of incidence and risk factors at a single center in China. Chin Med J (Engl). 2017;130:2551-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman RJ, Hess S, Berkowitz SD, Homering M. Complication rates after hip or knee arthroplasty in morbidly obese patients. Clin Orthop Relat Res. 2013;471:3358-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi R, Salonen D, Geerts WH, Khanna M, McSweeney S, Mahomed NN. A pilot study of computed tomography-detected asymptomatic pulmonary filling defects after hip and knee arthroplasties. J Arthroplasty. 2012;27:730-735. [DOI] [PubMed] [Google Scholar]
- 6.Haverkamp D, Klinkenbijl MN, Somford MP, Rob Albers GH, Van der Vis HM. Obesity in total hip arthroplasty-does it really matter? A meta-analysis. Acta Orthop. 2011;82:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ICM-VTE Hip & Knee Delegates. Recommendations from the ICM-VTE: hip & knee. J Bone Joint Surg Am. 2022;104;180-231. [DOI] [PubMed] [Google Scholar]
- 8.Januel JM, Chen G, Ruffieux G, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307:294-303. [DOI] [PubMed] [Google Scholar]
- 9.Kerkhoffs GM, Servien E, Dunn W, Dahm D, Bramer JAM, Haverkamp D. The influence of obesity on the complication rate and outcome of total knee arthroplasty: a meta-analysis and systematic literature review. J Bone Joint Surg Am. 2012;94:1839-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology. 2003;99:552-560. [DOI] [PubMed] [Google Scholar]
- 11.Meding J, Meding LK, Ritter M, Keating M. Pain relief and functional improvement remain 20 years after knee arthroplasty. Clin Orthop Relat Res. 2012;470:144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. Risk assessment for venous thromboembolism. Available at: https://www.nice.org.uk/guidance/ng89/resources/department-of-health-vte-risk-assessment-tool-pdf-4787149213. Accessed July 26, 2022.
- 13.National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Available at: https://www.nice.org.uk/guidance/ng158/resources/venous-thromboembolic-diseases-diagnosis-management-and-thrombophilia-testing-pdf-66141847001797. Accessed September 26, 2022.
- 14.Parvizi J, Huang R, Raphael IJ, Arnold WV, Richard RH. Symptomatic pulmonary embolus after joint arthroplasty: stratification of risk factors. Clin Orthop Relat Res. 2014;472:903-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel P, Patel P, Bhatt M, et al. Systematic review and meta-analysis of test accuracy for the diagnosis of suspected pulmonary embolism. Blood Adv. 2020;4:4296-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen PB, Jørgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-track Hip Knee Replacement Collaborative Group. Venous thromboembolism despite ongoing prophylaxis after fast-track hip and knee arthroplasty: a prospective multicenter study of 34,397 procedures. Thromb Haemost. 2019;119:1877-1885. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi B, Romano PS, Maynard G, et al. Mechanical and suboptimal pharmacologic prophylaxis and delayed mobilization but not morbid obesity are associated with venous thromboembolism after total knee arthroplasty: a case-control study. J Hosp Med. 2012;7:665-671. [DOI] [PubMed] [Google Scholar]
- 18.Sloan M, Sheth N, Lee GC. Is obesity associated with increased risk of deep vein thrombosis or pulmonary embolism after hip and knee arthroplasty? A large database study. Clin Orthop Relat Res. 2019;477:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song K, Xu Z, Rong Z, et al. The incidence of venous thromboembolism following total knee arthroplasty: a prospective study by using computed tomographic pulmonary angiography in combination with bilateral lower limb venography. Blood Coagul Fibrinolysis . 2016;27:266-269. [DOI] [PubMed] [Google Scholar]
- 20.Sherman WF, Patel AH, Kale NN, Freiberger CM, Barnes CL, Lee OC. Surgeon decision-making for individuals with obesity when indicating total joint arthroplasty. J Arthroplasty. 2021;36:2708-2715. [DOI] [PubMed] [Google Scholar]
- 21.Sykes PK, Walsh K, Darcey CM, et al. Prevention of venous thromboembolism amongst patients in an acute tertiary referral teaching public hospital: a best practice implementation project. Int J Evid Based Healthc. 2016;14:64-73. [DOI] [PubMed] [Google Scholar]
- 22.Tay K, Bin Abd Razak HR, Tan AHC. Obesity and venous thromboembolism in total knee arthroplasty patients in an Asian population. J Arthroplasty. 2016;31:2880-2883. [DOI] [PubMed] [Google Scholar]
- 23.Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage. 2014;22:918-927. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Obesity and overweight. Available at: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed July 26, 2022.
- 25.Zeng Y, Si H, Wu Y, et al. The incidence of symptomatic in-hospital VTEs in Asian patients undergoing joint arthroplasty was low: a prospective, multicenter, 17,660-patient-enrolled cohort study. Knee Surg Sports Traumatol Arthrosc. 2019;27:1075-1082. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZH, Shen B, Yang J, Zhou ZK, Kang PD, Pei FX. Risk factors for venous thromboembolism of total hip arthroplasty and total knee arthroplasty: a systematic review of evidences in ten years. BMC Musculoskelet Disord . 2015;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]


