Abstract
The phosphate-binding loop of ς54-dependent activators is thought to participate in ATP binding and/or hydrolysis. Alanine substitutions at positions 3, 4, 6, 7, and 8 of this motif in Rhizobium meliloti DctD disrupted transcriptional activation and ATP hydrolysis. Interestingly, substitution of alanine at position 7 also affected DNA binding.
Transcription by ς54-RNA polymerase holoenzyme (ς54-holoenzyme) requires an activator protein (16, 20). The activator binds to upstream activation sequences (UAS) that are generally located 100 to 200 bp upstream of the transcriptional start site and contacts ς54-holoenzyme bound to the promoter in a closed complex through DNA looping (3, 17, 24, 28). The activator catalyzes the isomerization of the closed complex to an open complex that is transcriptionally active in a reaction that requires ATP hydrolysis by the activator (10, 12, 13, 16, 20, 30). The barrier to open complex formation by ς54-holoenzyme is thought to be both kinetic and thermodynamic, and the activator is believed to act as a simple molecular machine that couples the energy from ATP hydrolysis to open complex formation (29).
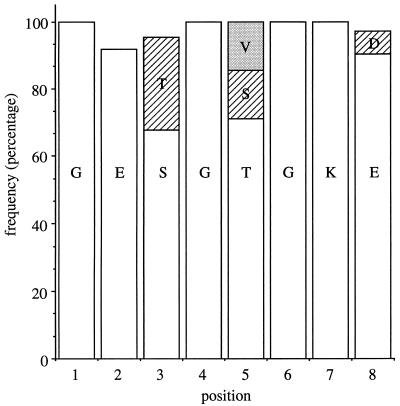
ς54-dependent activators contain a phosphate-binding loop (also referred to as P loop or Walker A sequence) which occurs in other GTP- and ATP-binding proteins and binds the phosphate moiety of the nucleotide (19, 23, 25). The consensus sequence for the P loop is GXXXXGK(T/S), where X denotes various amino acids and the parentheses enclose alternative amino acids at one position (19, 25). The P loop often has distinctive features within protein families. Sequence comparisons of over 60 ς54-dependent activators indicates the consensus sequence GE(S/T)G(T/S/V)GK(E/D) for the P loop of this family of proteins (Fig. 1).
FIG. 1.
Consensus sequence for P-loop motif of ς54-dependent activators. The frequency at which a given amino acid occurs at each position within the P-loop motif was calculated after comparisons of the amino acid sequences of 62 ς54-dependent activators. The amino acid sequence of the P-loop motif of R. meliloti DctD is GETGSGKE and spans positions 173 through 180.
Substitution of asparagine for glycine at position 6 in the P loops of the ς54-dependent activators NtrC of Salmonella typhimurium and XylR of Pseudomonas putida interfered with the abilities of these proteins to hydrolyze ATP and activate transcription (14, 15). It is unclear from these studies, however, if other amino acid residues are critical for ATP hydrolysis or if amino acid substitutions in the P loop affect other functions of the activator, such as DNA binding or interaction with ς54-holoenzyme. To understand better the function of the P loop in ς54-dependent activators, we systematically changed each amino acid residue in the P loop of Rhizobium meliloti DctD (C4-dicarboxylic acid transport protein D) by site-directed mutagenesis and biochemically characterized several of the mutant proteins. DctD activates transcription from dctA, which encodes a permease for C4-dicarboxylic acids. It forms a two-component regulatory system with DctB that positively regulates expression from dctA (2, 7, 8, 18). In this study, we used DctD(Δ1-142), which is a truncated, constitutively active form of DctD that lacks the N-terminal regulatory domain (12). Unlike the full-length DctD protein, this truncated protein does not need to be phosphorylated to hydrolyze ATP or activate transcription (12).
Alanine substitutions at five of the eight positions in the P loop of DctD result in loss of transcriptional activation.
The P loop of R. meliloti DctD has the amino acid sequence 173GETGSGKE180 (8). Alanine substitutions were introduced into this motif by site-directed mutagenesis, as described previously for DctD(Δ1-142) (27). Alanine was chosen because it is generally thought to have minimal effects on protein structure (6).
Alanine substitutions at positions 3 (Thr-175), 4 (Gly-176), 6 (Gly-178), 7 (Lys-179), and 8 (Glu-180) resulted in loss of the ability of DctD(Δ1-142) to activate transcription from a dctA′-′lacZ reporter gene in Escherichia coli (Table 1). The eighth amino acid of the P loop is usually a hydroxyl-containing residue that serves as one of the protein ligands for the divalent metal cation associated with the nucleotide (23). Substitution of a threonine for Glu-180 resulted in a protein that was unable to activate transcription (Table 1). If Glu-180 is a ligand to the divalent cation associated with ATP, then the failure of threonine to replace Glu-180 suggests that it may not be properly positioned to ligate the cation. Alternatively, Glu-180 may have other roles that cannot be accomplished by threonine.
TABLE 1.
Transcriptional activation from a dctA′-′lacZ reporter gene by mutant forms of DctD(Δ1-142)
| Protein | β-Galactosidase activity (Miller units) | % Activity of DctD(Δ1-142) |
|---|---|---|
| None | 10 | |
| DctD(Δ1-142) | 4,210 | 100 |
| DctD(Δ1-142, G173A) | 3,630 | 86 |
| DctD(Δ1-142, E174A) | 2,970 | 70 |
| DctD(Δ1-142, T175A) | 14 | <0.1 |
| DctD(Δ1-142, G176A) | 13 | <0.1 |
| DctD(Δ1-142, S177A) | 3,800 | 90 |
| DctD(Δ1-142, G178A) | 11 | <0.1 |
| DctD(Δ1-142, K179A) | 14 | <0.1 |
| DctD(Δ1-142, E180A) | 12 | <0.1 |
| DctD(Δ1-142, E180T) | 14 | <0.1 |
Introducing alanine at position 5 (Ser-177) did not affect the ability of DctD(Δ1-142) to activate transcription, but this position is not well conserved in ς54-dependent activators (Fig. 1). Alanine substitutions at positions 1 and 2 of the P loop (Gly-173 and Glu-174, respectively) did not seriously affect the ability of the protein to activate transcription either, despite the fact that these amino acids are well conserved in ς54-dependent activators.
Mutant proteins that fail to activate transcription are deficient in the ability to hydrolyze ATP.
Mutant forms of DctD(Δ1-142) were purified as described previously (26) so that we could characterize their activities in vitro. We were unable to purify two of the mutant proteins, DctD(Δ1-142, G176A) and DctD(Δ1-142, G178A), both of which behaved differently in the purification protocol than DctD(Δ1-142). All of the other mutant proteins behaved similarly to DctD(Δ1-142) in the purification protocol, suggesting that the amino acid substitutions in these proteins did not cause major structural changes.
Mutant proteins that failed to activate transcription from the dctA′-′lacZ reporter gene in vivo also failed to activate transcription from the dctA promoter regulatory region in an in vitro transcription assay (data not shown). No transcripts were produced with these mutant proteins even when the concentration of ATP in the assay was increased from 3 to 10 mM.
For the mutant proteins that were purified, we examined their ATPase activities as described previously (26). ATPase activities were determined in the presence of a plasmid that carries the dctA UAS, which was shown to stimulate ATP hydrolysis by DctD(Δ1-142) (12). The mutant proteins that activated transcription in vivo, DctD(Δ1-142, G173A), DctD(Δ1-142, E174A), and DctD(Δ1-142, S177A), exhibited ATPase activities that ranged from 54 to 140% of that observed with DctD(Δ1-142) (Table 2). In contrast, the mutant proteins that failed to activate transcription were severely affected in their abilities to hydrolyze ATP, which likely accounted for their failure to activate transcription.
TABLE 2.
ATPase activities of mutant forms of DctD(Δ1-142)
| Protein | Phosphate releaseda (pmol) | Incubation period (min) | % Activity of DctD(Δ1-142)b |
|---|---|---|---|
| DctD(Δ1-142) | 4,010 | 10 | 100 |
| DctD(Δ1-142, G173A) | 2,160 | 10 | 54 |
| DctD(Δ1-142, E174A) | 3,070 | 10 | 77 |
| DctD(Δ1-142, S177A) | 5,650 | 10 | 140 |
| DctD(Δ1-142) | 7,760 | 15 | 100 |
| DctD(Δ1-142, T175A) | 50 | 15 | 0.6 |
| DctD(Δ1-142, K179A) | <15 | 15 | <0.2 |
| DctD(Δ1-142, E180A) | 150 | 15 | 1.9 |
| DctD(Δ1-142, E180T) | 160 | 15 | 2.1 |
Assay mixtures contained 1.5 μM DctD(Δ1-142) protein (dimer) and 50 nM pJHL2, which carries the dctA UAS. Each value is the amount of phosphate released over the indicated incubation period.
ATPase activities of the mutant proteins are compared to the activities observed for DctD(Δ1-142) for the corresponding incubation periods.
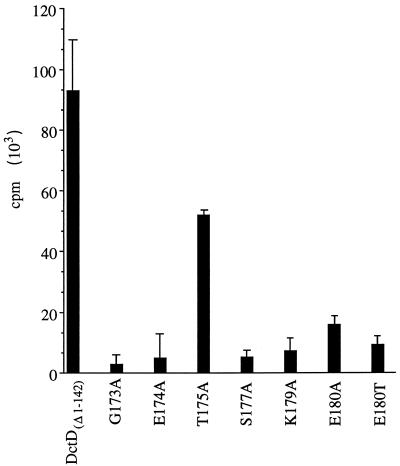
We predicted that some substitutions in the P loop might affect the affinity of the protein for ATP, so we examined the abilities of the purified proteins to bind ATP. ATP binding assays were carried out essentially as described previously (5). DctD proteins were spotted onto 5-mm-diameter nitrocellulose discs and dried at room temperature for 20 min. The nitrocellulose discs were incubated for 5 min in a solution of 0.5% (wt/vol) bovine serum albumin in the transcription assay buffer (29) and then transferred to tubes that contained 0.5 ml of the same buffer with 2 μCi of [γ-32P]ATP (∼3,000 Ci/mmol; 1.3 nM ATP) and incubated on ice for 50 min. The buffer was removed, and the discs were quickly washed with 1 ml of buffer and then analyzed for radioactivity by liquid scintillation counting. Controls in which no DctD protein was applied to the nitrocellulose disc were included. For reasons that we do not understand, the background radioactivity from these control discs was unchanged when unlabelled ATP was included in the binding assay. Cold ATP did, however, reduce the amount of radioactivity associated with bound DctD, indicating that the proteins were specifically binding ATP (data not shown). Because the background counts were unaffected by cold ATP, we had to use ATP with a high specific activity. As indicated in Fig. 2, most of the mutant proteins bound ATP less efficiently than did DctD(Δ1-142).
FIG. 2.
ATP binding assays for DctD(Δ1-142) and mutant forms of the protein. Purified proteins were spotted onto nitrocellulose discs and incubated with [γ-32P]ATP for 50 min on ice. After washing of the discs, the amount of radioactivity associated with each disc was determined by liquid scintillation counting. Background counts were determined from discs on which no DctD had been applied, and these counts were subtracted from the counts determined for the filters with the various DctD(Δ1-142) proteins. The data are averages of four independent assays; error bars show the 95% confidence limits for these averages.
Despite the fact that the ATPase activity of DctD(Δ1-142, T175A) was severely diminished, it appeared to bind ATP almost as well as did DctD(Δ1-142). These data indicate that Thr-175 may have a direct role in ATP hydrolysis. Like DctD, substitution of an alanine at the corresponding position (Ser-170) in Klebsiella pneumoniae NtrC resulted in loss of transcriptional activation (1). In contrast, mutant forms of K. pneumoniae NifA with either glycine or alanine substitutions at this position (Ser-242) retained activity in vivo (4).
It seemed unusual that DctD(Δ1-142, G173A), DctD(Δ1-142, E174A), and DctD(Δ1-142, S177A) hydrolyzed ATP but showed reduced affinities for ATP in the binding assay. These binding assays, however, were done at nanomolar ATP concentrations, whereas millimolar concentrations of ATP were present in the ATPase assays. We infer that these mutant proteins have reduced affinities for ATP but that the affinities are not reduced enough to affect ATP hydrolysis at physiological ATP concentrations.
Mutant proteins appear to interact normally with ς54-holoenzyme.
DctD(Δ1-142) can be cross-linked to both ς54 and the β subunit of RNA polymerase, suggesting that it interacts with these subunits to activate transcription (11). We had previously isolated mutant forms of DctD(Δ1-142) that failed to activate transcription and were also deficient in their abilities to cross-link to ς54 and the β subunit (27). To determine if any of the mutant proteins generated in this study were similarly defective in interaction with ς54-holoenzyme, mutant proteins were cross-linked to either S. typhimurium ς54 or the β subunit of E. coli core RNA polymerase with succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate, as described previously (11). All of the mutant proteins that we purified in this study cross-linked normally to ς54 and the β subunit, suggesting that the amino acid substitutions did not significantly interfere with interactions between DctD(Δ1-142) and ς54-holoenzyme (data not shown).
Substitution of alanine for Lys-179 affects DNA binding.
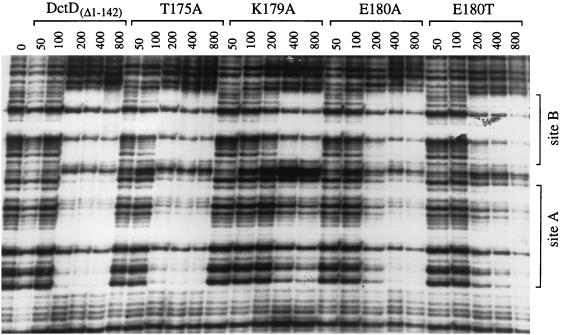
The UAS of R. meliloti dctA contains two DctD-binding sites, referred to as sites A and B. DctD has a 50- to 100-fold-higher affinity for site B (the site that is proximal to the dctA promoter) than for site A when the sites are separated (9), but it binds cooperatively to these sites when they are together in the native UAS (21). We compared the DNA-binding activities of the mutant proteins that failed to activate transcription with that of DctD(Δ1-142) in a DNase I footprinting assay.
DNase I footprinting patterns observed with three of the four mutant proteins, DctD(Δ1-142, T175A), DctD(Δ1-142, E180A), and DctD(Δ1-142, E180T), were very similar to that observed with DctD(Δ1-142) (Fig. 3). For each of these proteins, sites A and B appeared to be equally protected from DNase I digestion at the various protein concentrations, suggesting that these proteins bound cooperatively to the UAS. Binding of DctD(Δ1-142, K179A) to the native UAS, however, was somewhat different. Site A was not fully protected from DNase I digestion even though site B was fully protected. These data indicate that substitution of alanine for Lys-179 interferes with binding of DctD(Δ1-142) to the low-affinity site of the UAS, either by reducing the affinity of the protein for this site or by disrupting cooperative binding to the UAS.
FIG. 3.
DNase I footprinting of DctD(Δ1-142) and mutant forms of the protein at the R. meliloti dctA UAS. DNase I footprinting assays were performed with a 5′-end-labelled DNA fragment carrying the dctA UAS and various purified proteins, as indicated at the top of the figure. Final protein concentrations were varied from 50 to 800 nM (dimer), as indicated for each lane. The two DctD binding sites (sites A and B) are labelled at the side of the figure. Footprints were visualized by subjecting the reactions to denaturing gel electrophoresis and then exposing X-ray film to the resulting gel.
Conclusions.
We have shown that Thr-175, Gly-176, Gly-178, Lys-179, and Glu-180 are important for the structural or functional integrity of the P loop of DctD. It is surprising that Gly-173 and Glu-174 (positions 1 and 2, respectively) are not essential for activity given the high degree to which these residues are conserved among ς54-dependent activators. Moreover, examination of the P loops of diverse protein families reveals that glycine at position 1 is essentially invariant (19). In general, conserved glycines help to maintain the structure of the loop and allow main-chain hydrogen bonds between adjacent amino acids and the β and γ phosphates of the nucleotide (23). If Gly-173 has a similar function, it appears that substitutions of at least small amino acids, such as alanine, are tolerated at this position. Like ς54-dependent activators, other protein families often show conservation at position 2. For example, position 2 in the β subunit of the F1-ATPase protein family is glycine. This glycine in the F1 β subunit from yeast, however, can be replaced with virtually any amino acid and still produce an active enzyme (22). While the alanine substitutions at Gly-173 and Glu-174 of DctD appeared to affect the affinity of the protein for ATP, this was not enough to interfere with ATP hydrolysis or transcriptional activation at physiological ATP concentrations. Taken together, our data suggest that Gly-173 and Glu-174 play only a minor role in the structure and function of the P loop of DctD.
Acknowledgments
We thank Sydney Kustu and Konstantin Severinov for providing antibodies. We also thank Elliot Altman and Mary Kelly for helpful comments on the manuscript.
This work was funded by award MCB-9630454 from the National Science Foundation.
REFERENCES
- 1.Austin S, Kundrot C, Dixon R. Influence of a mutation in the putative nucleotide binding site of the nitrogen regulatory protein NTRC on its positive control function. Nucleic Acids Res. 1991;19:2281–2287. doi: 10.1093/nar/19.9.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolton E, Higgisson B, Harrington A, O’Gara F. Dicarboxylic acid transport in Rhizobium meliloti: isolation of mutants and cloning of dicarboxylic acid transport genes. Arch Microbiol. 1986;144:142–146. [Google Scholar]
- 3.Buck M, Cannon W, Woodcock J. Transcriptional activation of the Klebsiella pneumoniae nitrogenase promoter may involve DNA loop formation. Mol Microbiol. 1987;1:243–249. doi: 10.1111/j.1365-2958.1987.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 4.Cannon W, Buck M. Central domain of the positive control protein NifA and its role in transcriptional activation. J Mol Biol. 1992;225:271–286. doi: 10.1016/0022-2836(92)90921-6. [DOI] [PubMed] [Google Scholar]
- 5.Cronet P, Bellsolell L, Sander C, Coll M, Serrano L. Investigating the structural determinants of the p21-like triphosphate and Mg2+ binding site. J Mol Biol. 1995;249:654–664. doi: 10.1006/jmbi.1995.0326. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham B, Wells J. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 7.Engelke T, Jording D, Kapp D, Puhler A. Identification and sequence analysis of the Rhizobium meliloti dctA gene encoding the C4-dicarboxylate carrier. J Bacteriol. 1989;171:5551–5560. doi: 10.1128/jb.171.10.5551-5560.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang J, Gu B, Albright L M, Nixon B T. Conservation between coding and regulatory elements of Rhizobium meliloti and Rhizobium leguminosarum dct genes. J Bacteriol. 1989;171:5244–5253. doi: 10.1128/jb.171.10.5244-5253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledebur H, Nixon B T. Tandem DctD-binding sites of the Rhizobium meliloti dctA upstream activating sequence are essential for optimal function despite a 50- to 100-fold difference in affinity for DctD. Mol Microbiol. 1992;6:3479–3492. doi: 10.1111/j.1365-2958.1992.tb01783.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee H-S, Berger D K, Kustu S. Activity of purified NIFA, a transcriptional activator of nitrogen fixation genes. Proc Natl Acad Sci USA. 1993;90:2266–2270. doi: 10.1073/pnas.90.6.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J H, Hoover T R. Protein crosslinking studies suggest that Rhizobium meliloti C4-dicarboxylic acid transport protein D, a ς54-dependent transcriptional activator, interacts with ς54 and the β subunit of RNA polymerase. Proc Natl Acad Sci USA. 1995;92:9702–9706. doi: 10.1073/pnas.92.21.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J H, Scholl D, Nixon B T, Hoover T R. Constitutive ATP hydrolysis and transcriptional activation by a stable, truncated form of Rhizobium meliloti DCTD, a ς54-dependent transcriptional activator. J Biol Chem. 1994;269:20401–20409. [PubMed] [Google Scholar]
- 13.Morett E, Buck M. In vivo studies on the interaction of RNA polymerase-ς54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters: the role of NIFA in the formation of an open promoter complex. J Mol Biol. 1989;210:65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- 14.North A K, Weiss D S, Suzuki H, Flashner Y, Kustu S. Repressor forms of the enhancer-binding protein NtrC: some fail in coupling ATP hydrolysis to open complex formation by ς54-holoenzyme. J Mol Biol. 1996;260:317–331. doi: 10.1006/jmbi.1996.0403. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Martin J, de Lorenzo V. ATP binding to the ς54-dependent activator XylR triggers a protein multimerization cycle catalyzed by UAS DNA. Cell. 1996;86:331–339. doi: 10.1016/s0092-8674(00)80104-x. [DOI] [PubMed] [Google Scholar]
- 16.Popham D, Szeto D, Keener J, Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989;243:629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- 17.Rippe K, Guthold M, von Hippel P H, Bustamante C. Transcriptional activation via DNA-looping: visualization of intermediates in the activation pathway of E. coli RNA polymerase ς54 holoenzyme by scanning force microscopy. J Mol Biol. 1997;270:125–138. doi: 10.1006/jmbi.1997.1079. [DOI] [PubMed] [Google Scholar]
- 18.Ronson C W, Astwood P M, Nixon B T, Ausubel F M. Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucleic Acids Res. 1987;15:7921–7934. doi: 10.1093/nar/15.19.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saraste M, Sibbald P R, Wittinghofer A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biol Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 20.Sasse-Dwight S, Gralla J D. Probing the Escherichia coli glnALG upstream activation mechanism in vivo. Proc Natl Acad Sci USA. 1988;85:8934–8938. doi: 10.1073/pnas.85.23.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholl D, Nixon B T. Cooperative binding of DctD to the dctA UAS of Rhizobium meliloti is enhanced in a constitutively active truncated mutant. J Biol Chem. 1996;271:26435–26442. doi: 10.1074/jbc.271.42.26435. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Yao B-Y, Mueller D M. Primary structural constraints of P-loop of mitochondrial F1-ATPase from yeast. J Biol Chem. 1994;269:9424–9428. [PubMed] [Google Scholar]
- 23.Smith C A, Rayment I. Active site comparisons highlight structural similarities between myosin and other P-loop proteins. Biophys J. 1996;70:1590–1602. doi: 10.1016/S0006-3495(96)79745-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su W, Porter S, Kustu S, Echols H. DNA-looping and enhancer activity: association between DNA-bound NTRC activator and RNA polymerase at the bacterial glnA promoter. Proc Natl Acad Sci USA. 1990;87:5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y-K, Hoover T R. Alterations within the activation domain of the ς54-dependent activator DctD that prevent transcriptional activation. J Bacteriol. 1997;179:5812–5819. doi: 10.1128/jb.179.18.5812-5819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y-K, Lee J H, Brewer J M, Hoover T R. A conserved region in the ς54-dependent activator DctD is involved in both binding to RNA polymerase and coupling ATP hydrolysis to activation. Mol Microbiol. 1997;26:373–386. doi: 10.1046/j.1365-2958.1997.5851955.x. [DOI] [PubMed] [Google Scholar]
- 28.Wedel A, Weiss D S, Popham D, Droge P, Kustu S. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science. 1990;248:486–490. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- 29.Wedel A B, Kustu S. The bacterial enhancer-binding protein NtrC is a molecular machine: ATP hydrolysis is coupled to transcriptional activation. Genes Dev. 1995;9:2042–2052. doi: 10.1101/gad.9.16.2042. [DOI] [PubMed] [Google Scholar]
- 30.Weiss D S, Batut J, Klose K E, Keener J, Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991;67:155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]