Abstract
Enteric hyperoxaluria is a medical condition characterized by elevated urinary oxalate excretion due to increased gastrointestinal oxalate absorption. Causative features include fat malabsorption and/or increased intestinal permeability to oxalate. Enteric hyperoxaluria has long been known to cause nephrolithiasis and nephrocalcinosis, and, more recently, an association with CKD and kidney failure has been shown. Currently, there are no US Food and Drug Administration–approved therapies for enteric hyperoxaluria, and it is unclear what end points should be used to evaluate the efficacy of new drugs and biologics for this condition. This study represents work of a multidisciplinary group convened by the Kidney Health Initiative to review the evidence supporting potential end points for clinical trials in enteric hyperoxaluria. A potential clinical outcome is symptomatic kidney stone events. Potential surrogate end points include (1) an irreversible loss of kidney function as a surrogate for progression to kidney failure, (2) asymptomatic kidney stone growth/new stone formation observed on imaging as a surrogate for symptomatic kidney stone events, (3) urinary oxalate and urinary calcium oxalate supersaturation as surrogates for the development of symptomatic kidney stone events, and (4) plasma oxalate as a surrogate for the development of the clinical manifestations of systemic oxalosis. Unfortunately, because of gaps in the data, this Kidney Health Initiative workgroup was unable to provide definitive recommendations. Work is underway to obtain robust information that can be used to inform trial design and medical product development in this space.
Keywords: CKD
Background
Enteric hyperoxaluria is a medical condition characterized by elevated urinary oxalate excretion due to increased gastrointestinal oxalate absorption. Ordinarily, <10% of dietary oxalate is absorbed by the gut, but in enteric hyperoxaluria this percentage is increased to 30% or more.1,2 Causative features include fat malabsorption and/or increased intestinal permeability to oxalate. Enteric hyperoxaluria has long been known to cause nephrolithiasis and nephrocalcinosis, and, more recently, an association with CKD and kidney failure has been shown.3–14
There are no US Food and Drug Administration–approved therapies for enteric hyperoxaluria, and it is unclear what end points should be used to evaluate the efficacy of new drugs and biologics for this condition. In 2017, the Oxalosis and Hyperoxaluria Foundation (OHF) in coordination with the Kidney Health Initiative formed a workgroup comprising scientists, clinicians, industry representatives, and patient advocacy representatives with the goal of identifying suitable end points for clinical trials in enteric hyperoxaluria. The workgroup completed a systematic review of the literature to describe the pathophysiology and clinical consequences of enteric hyperoxaluria, available therapies, and knowledge gaps.3 This study extends the discussion of the evidence supporting potential end points for clinical trials in enteric hyperoxaluria.
Diagnosis and Prevalence of Enteric Hyperoxaluria
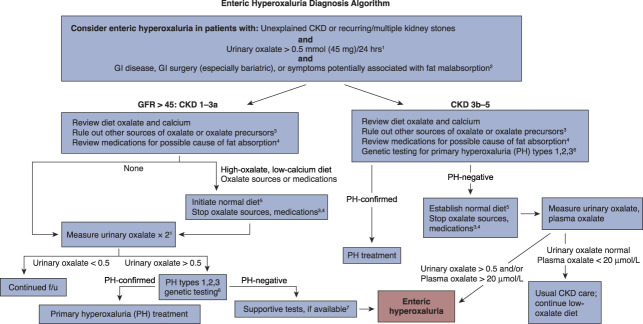
The diagnosis of enteric hyperoxaluria is based on the presence of hyperoxaluria in a patient with recurrent oxalate stones; the presence of a potential enteric etiology (gastrointestinal disease or surgery associated with fat malabsorption); and the exclusion of other etiologies, such as primary hyperoxaluria or dietary factors such as excessive intake of oxalate-rich foods. Diagnostic algorithms, such as that shown in Figure 1, are often used to identify patients who may have enteric hyperoxaluria and confirm the diagnosis. Enteric hyperoxaluria is believed to affect somewhere between 5% and 24% of patients who have diseases associated with malabsorption.3,15,16 On the basis of available data, it has been estimated that the number of patients in the United States with gastrointestinal conditions associated with malabsorption and kidney failure or stones is approximately 250,000,3 whereas approximately 40% of these will have hyperoxaluria.17
Figure 1.
Enteric hyperoxaluria diagnostic algorithm. Enteric hyperoxaluria is caused by the increased absorption of oxalate from foods in the digestive tract (also called the gut or intestines). This leads to high levels of oxalate in the urine. Enteric hyperoxaluria may occur as a result of certain weight loss surgeries or in inflammatory bowel disease, including Crohn disease, cystic fibrosis, or chronic pancreatitis. Excess oxalate in the urine may lead to recurring kidney stones, CKD, or kidney failure. Symptoms vary but may include kidney stones, loose stools, or weight loss. 124-hour urine collection is important for measurement of oxalate. In children, measurements should be corrected for BSA, with normal values <0.45 mmol/1.73 m2 per 24 hours. In very young children unable to complete a 24-hour urine collection, urine oxalate/urine creatinine ratio may be used to estimate oxalate excretion. Urinary oxalate excretion can be variable in enteric hyperoxaluria, so a normal urinary oxalate does not exclude the possibility of enteric hyperoxaluria. 2Any gastrointestinal (GI) disease that causes fat malabsorption, such as cystic fibrosis or inflammatory bowel disease, may result in enteric hyperoxaluria. Symptoms of malabsorption include diarrhea, fatty stools, or weight loss. Patients can have significant fat malabsorption and enteric hyperoxaluria without GI symptoms. 3Oxalate precursors include vitamin C, which is metabolized to oxalate and may be consumed in high doses by some patients. This is a particular problem in patients with advanced CKD who are less able to excrete resulting oxalate. Low doses of vitamin C, such as those contained in daily multivitamins, are not a concern. 4Medications that can cause fat malabsorption include orlistat, tetracycline, colchicine, acarbose, phenytoin, cholestyramine, laxatives, and ezetimibe. 5Elimination of potential oxalate sources in diet and medications should be maintained for 1–3 months before recheck of urinary oxalate. For purposes of this algorithm, a normal diet is one that avoids intake of high oxalate foods, contains the recommended daily allowance for calcium, and is low fat. If dietary changes are needed, it is recommended that patients be provided with lists of high-oxalate foods to be avoided (www.ohf.org). 6If there is an obvious source of GI hyperabsorption of oxalate, such as new onset after bariatric surgery, PH genetic testing may not be required. 7Elevated fecal fat supports the diagnosis; measurement is recommended but not essential. GI absorption of oxalate can be tested in some referral centers by oral administration and measurement in urine of 13C oxalate but is not widely available. f/u, follow-up; PH, primary hyperoxaluria.
Potential End Points for Clinical Trials in Enteric Hyperoxaluria
Approval of a product in the United States can be supported by demonstration of a clinically meaningful improvement in clinical outcome(s) that directly measure how a patient feels, functions, or survives or by a surrogate end point.18,19 As noted in the Biomarkers, Endpoints, and other Tools Resource Glossary, a surrogate end point is a marker, such as a laboratory measure or physical sign, that is used in a clinical trial as a substitute for a direct measure of how a patient feels, functions, or survives. Surrogate end points are not direct measures of clinical benefit; however, treatment effects on a surrogate end point are expected to predict the clinical benefit of a treatment.
Surrogate end points can be categorized as validated, reasonably likely, or candidate on the basis of their level of clinical validation.20 Treatment effects on validated surrogate end points can be used to support traditional approval. Treatment effects on reasonably likely surrogate end points can be used to support accelerated approval of drugs that treat a serious condition and generally provide a meaningful advantage over available therapies. Drugs approved under the accelerated approval pathway are generally required to conduct postmarketing confirmatory trial(s) to verify the anticipated clinical benefit and are subject to withdrawal if the trial(s) fail to verify the clinical benefit or do not demonstrate sufficient clinical benefit to justify the risks associated with the drug.21
In its evaluation of potential end points for clinical trials in enteric hyperoxaluria, the workgroup considered both clinical outcomes and potential surrogate end points. The importance of the clinical outcome to patients and the feasibility of showing a treatment effect in a trial of reasonable duration were the primary considerations for our evaluation of clinical outcomes. Factors considered when assessing whether a biomarker may have utility as a surrogate end point were (1) to what extent the pathophysiology of the disease is well understood and whether data suggest that the candidate surrogate is on the causal pathway of progression to the clinical outcome(s) of interest; (2) the strength and consistency of the epidemiologic data supporting the relationship between the biomarker and the clinical outcome of interest; and (3) whether treatment effects on the biomarker have been shown to be associated with treatment effects on the clinical outcome of interest, ideally with different types of interventions. Of note, when assessing whether a biomarker may have utility as a surrogate end point in clinical trials of enteric hyperoxaluria, the workgroup focused primarily on the evidence supporting its use as a surrogate end point in enteric hyperoxaluria, as opposed to other disease states associated with stone formation, given differences in disease pathophysiology and the understanding that natural history data obtained in one condition may not be readily translatable to another condition.
The most relevant candidate end points for enteric hyperoxaluria clinical trials are listed in Table 1 and discussed individually below. A potential clinical outcome was symptomatic kidney stone events. Potential surrogate end points included (1) an irreversible loss of kidney function as a surrogate for progression to kidney failure, (2) asymptomatic kidney stone growth/new stone formation observed on imaging as a surrogate for symptomatic kidney stone events, (3) urinary oxalate excretion and urinary calcium oxalate supersaturation as surrogates for the development of symptomatic kidney stone events, and (4) plasma oxalate as a surrogate for the development of the clinical manifestations of systemic oxalosis.
Table 1.
Potential end points for clinical trials in enteric hyperoxaluria
| Potential Trial End Point | Strengths | Limitations |
|---|---|---|
| Clinical outcome | ||
| Symptomatic kidney stone events | Clinical outcome that could support traditional approval | Limited available data on frequency and risk factors of symptomatic kidney stone events in patients with EH |
| Surrogate | ||
| Irreversible loss of kidney function | Validated surrogate for progression to kidney failure that could support traditional approval | Limited data on the rate of loss of kidney function and risk factors of more rapid loss of kidney function in patients with EH |
| New kidney stones or growth of kidney stones on imaging | Mechanistic rationale and observational data suggest changes on imaging could be associated with future symptomatic kidney stone events | No data available to demonstrate that treatment of asymptomatic kidney stones that are detected by imaging reduces the risk of future symptomatic kidney stones; not clear how to define this end point or the magnitude of change that would confer benefit |
| Urinary oxalate | Mechanistic rationale and observational data suggest magnitude of hyperoxaluria is associated with future symptomatic kidney stone events | Available observational data suggest substantial uncertainty regarding the relationship between urinary oxalate and symptomatic kidney stone events; available data inadequate to determine how an end point based on urinary oxalate should be defined, magnitude of urinary oxalate change that would confer benefit, and in what patient population(s) |
| Urinary calcium oxalate supersaturation | Mechanistic rationale and observational data in non-EH populations suggest an association with kidney stone formation | Limited data in EH populations to support use of calcium oxalate supersaturation as a surrogate end point |
| Plasma oxalate | Mechanistic rationale for use as a surrogate for the development of clinical manifestations of systemic oxalosis | Reports of systemic oxalosis are rare in patients with EH; hence, the role of plasma oxalate as an end point in EH trials is unclear |
EH, enteric hyperoxaluria.
Symptomatic Kidney Stones
Calcium oxalate kidney stone formation is the most recognized clinical consequence of enteric hyperoxaluria.4,22,23 Symptomatic kidney stones cause pain and can lead to work or school absences, reduced quality of life, and need for hospitalizations and surgical interventions. Symptomatic kidney stones have also been associated with loss of kidney function.24–28 In clinical studies, symptomatic kidney stone events have often been defined as a composite of symptomatic stone passage (associated with pain and/or hematuria) or the need for a surgical procedure to remove a stone.
Although symptomatic kidney stone events could be used as a clinical outcome to demonstrate the benefit of new enteric hyperoxaluria treatments, at this time, it is unclear whether it would be possible to demonstrate an effect on a kidney stone end point in a clinical trial of a feasible size and duration. There are limited data on the frequency of symptomatic kidney stone events in patients with enteric hyperoxaluria or on kidney stone risk factors for stone events in patients with enteric hyperoxaluria. In one retrospective cohort study, 27% of patients with enteric hyperoxaluria had at least one symptomatic kidney stone event during a median follow-up of 4.9 years, with 14.5% having four or more episodes.17 However, given the rarity of enteric hyperoxaluria and limited data on the frequency of symptomatic kidney stone events, conducting a clinical trial using symptomatic stone events as the primary end point to support a traditional approval would be challenging, particularly if the treatment effect is modest.
Loss of Kidney Function
Oxalate nephropathy can occur in patients with enteric hyperoxaluria and lead to loss of kidney function. The loss of kidney function is believed to be caused by precipitation of calcium oxalate crystals within renal tubules, leading to cell necrosis, inflammation, tubular interstitial scarring, and eventual tubule obstruction (oxalate nephropathy).6,11,29–31 In principle, demonstrating a clinically meaningful treatment effect on the loss of kidney function could support the efficacy of products intended to treat enteric hyperoxaluria. However, data on the rate of loss of kidney function in patients with enteric hyperoxaluria are limited, and risk factors of more rapid loss of kidney function are not well understood. Thus, additional information is needed to design clinical trials in enteric hyperoxaluria around such an end point.
Stone Burden
Asymptomatic stone growth and new stone formation are on the causal pathway to symptomatic stone events; hence, there is strong mechanistic rationale supporting the use of measures of stone burden as a surrogate for symptomatic stone events. Observational studies in patients with kidney stones of various etiologies also support an association between stone size on imaging and future clinically significant symptomatic kidney stone events, although observational studies also suggest that the location of the stone has prognostic importance. In a retrospective observational study of 550 patients with a history of kidney stones of assorted etiologies and compositions who underwent surveillance computed tomography imaging, a multivariable model found that total stone volume on imaging was associated with symptomatic stone events independent of demographics, urine chemistry findings, and stone composition. While 43% of patients experienced a stone event over a mean follow-up of 4.7 years, the hazard ratio associated with increasing total stone volume was 1.67 (confidence interval [CI], 1.38 to 2.04) over the first 2 years and 1.24 (CI, 1.06 to 1.45) for events beyond 2 years.32 In another stone clinic retrospective review, baseline stone size >4 mm was associated with higher risk while upper pole stones were associated with lower risk of future events, after a mean follow-up of 3.26 years.33 Stones larger than 15 mm were almost uniformly associated with future symptomatic stone events because 29% of these patients required intervention, 57% developed pain, 71% had stone growth, and 100% had at least one of these three kidney stone–related events. Another study also found that stones >5 mm detected on baseline ultrasound and a lower pole location were associated with a higher risk of symptomatic events over a mean follow-up of 4.2 years.34,35 When serial images over 63 months were compared, an annual stone growth of over 1 mm was associated with a greater likelihood of spontaneous stone passage over a median of 31 months.35
While mechanistic rationale and observational data suggest that stone burden could serve as a surrogate for symptomatic stone events, there are no data demonstrating that the treatment of asymptomatic kidney stones detected on imaging reduces the risk of symptomatic kidney stone events in enteric hyperoxaluria. It is also unclear at this time how such an end point should be defined and what magnitude of change would be expected to confer benefit.
Urinary Oxalate
Elevated urinary oxalate excretion and concentration are on the causal pathway for calcium oxalate stone formation because an elevated calcium oxalate relative supersaturation >1.0 is required for urinary calcium oxalate crystal formation.36 Although there may be mechanistic rationale supporting the use of urinary oxalate excretion as a surrogate for the development of symptomatic kidney stones in patients with enteric hyperoxaluria, clinical data supporting such a relationship are mixed. Multiple studies report a correlation between baseline urinary oxalate and the subsequent development of symptomatic kidney stone events, particularly in patients who have undergone bariatric surgery,5,8,37–40 although at least one older study failed to detect this association.41 A recently published retrospective study of 297 patients with enteric hyperoxaluria followed for a median of 4.9 years demonstrated an association between higher baseline urinary oxalate excretion and subsequent symptomatic stone events using a model-based approach.17 However, the CIs around the modeled estimates were large, indicating substantial uncertainty in the relationship (Table 2), perhaps because urinary factors other than oxalate that are affected by the underlying gastrointestinal malabsorptive process in patients with enteric hyperoxaluria also contribute to symptomatic kidney stone risk.4,40 Therefore, available data are not sufficient to adequately define urinary oxalate as a surrogate end point (i.e., to determine what magnitude of change in urinary oxalate would be expected to confer benefit in patients with enteric hyperoxaluria).
Table 2.
Estimated reduction in odds of a stone event after enteric hyperoxaluria diagnosis for a given decrease in urine oxalate17,42
| % Decrease in Urinary Oxalate | Odds Ratio of Stone (95% CI) | % Lower Odds (95% CI) | Mean Urinary Oxalate (mg/d) |
|---|---|---|---|
| 50 | 0.41 (0.22 to 0.75) | 59.2 (25.5 to 77.6) | 27.5 mg |
| 40 | 0.52 (0.33 to 0.81) | 48.3 (19.5 to 66.9) | 33 mg |
| 30 | 0.63 (0.46 to 0.86) | 36.9 (14.0 to 53.7) | 38.5 mg |
| 20 | 0.75 (0.62 to 0.91) | 25.1 (9.0 to 38.3) | 44 mg |
| 10 | 0.87 (0.80 to 0.96) | 12.7 (4.4 to 20.4) | 49.5 mg |
| 0 | Ref | Ref | 55 mg |
CI, confidence interval.
Urinary Calcium Oxalate Supersaturation
Urinary calcium oxalate supersaturation is the ratio of the concentration of calcium oxalate to its solubility in urine. It has been hypothesized that urinary calcium oxalate supersaturation may be a better predictor of symptomatic kidney stone formation in enteric hyperoxaluria than urinary oxalate excretion because supersaturation simultaneously accounts for hyperoxaluria, hypocitraturia, and lower urine volume, all of which can contribute to calcium oxalate stone formation.
Data from observational studies suggest an association between urinary calcium oxalate supersaturation and kidney stone formation. In an analysis from three large observational cohorts (2630 women, 1145 men) that included both enteric hyperoxaluria and nonenteric hyperoxaluria populations, the odds ratio for having had an initial diagnosis of a symptomatic kidney stone over a median 3.9–5.8 years before the urine study was approximately six- to seven-fold greater for those individuals with a calcium oxalate relative supersaturation >1.0 than for those with a relative supersaturation of <1.0.42 The odds ratio was higher with greater relative supersaturation (P < 0.001 for trend from relative supersaturation <1, 1–2, 2–3, 3–4, and >4).42 In another retrospective study of stone formers with available laboratory data, those with a history of a bariatric surgery (n=132) had an oxalate excretion two to three times higher and calcium oxalate relative supersaturation 20%–50% higher than the nonenteric hyperoxaluria stone formers (n=2048), with the first stone event among the bariatric group occurring at a mean of 3.6 (SD 5.4) years after the surgery.37 In a separate cross-sectional study of 51 patients with fat malabsorption due to diverse causes, mean urinary calcium oxalate supersaturation was higher in those with a history of stone events (n=10; relative supersaturation=8.16) compared with those without prior stones (relative supersaturation=3.94).40 While such data are interesting and suggest value in monitoring calcium oxalate supersaturation, further work is needed to determine the role of this metric as an end point in clinical enteric hyperoxaluria trials.
Plasma Oxalate
Increased systemic absorption of oxalate is generally balanced by urinary oxalate excretion in patients with enteric hyperoxaluria who have preserved kidney function.43 Thus, plasma oxalate concentrations are typically normal to modestly elevated even if urinary oxalate excretion is elevated.43 However, as eGFR declines to below approximately 45 ml/min per 1.73 m2, plasma oxalate concentrations can increase.43 Although there are reports of patients with enteric hyperoxaluria and CKD developing systemic oxalosis, such reports are rare.44 Hence, although marked reductions in plasma oxalate have been used as a surrogate end point in PH1 patients with advanced CKD, the role of plasma oxalate in clinical enteric hyperoxaluria trials remains unclear.42
In this study, we critically evaluated the evidence supporting the use of potential end points for enteric hyperoxaluria clinical trials as a follow-up to the enteric hyperoxaluria systematic review the OHF/Kidney Health Initiative workgroup previously published.3 Although we identified a number of promising end points, we are unable to provide definitive recommendations because of gaps in existing data. Fortunately, work is underway to address these gaps to inform trial design and medical product development in this space. Over the past 5 years, the OHF has held a number of focus groups to better understand patients' experiences living with enteric hyperoxaluria. An enteric hyperoxaluria patient registry, modeled after the successful primary hyperoxaluria registry, has also been developed. This enteric hyperoxaluria registry, which includes retrospective and prospective components, will greatly advance our understanding of the natural history of enteric hyperoxaluria and help us identify appropriate end points that could be used to establish the efficacy of new treatments for this serious disease.
Disclosures
D. Assimos reports serving on the Editorial Boards of the Journal of Endourology and the Journal of Urology. J. Calle reports employment with Benesch Friedlander Coplan & Aronoff LLP and Cleveland Clinic; honoraria from Travere Pharmaceuticals; advisory or leadership role for Precision BioSciences, Inc. Primary Hyperoxaluria type 1 Advisory board; and speakers bureau for Travere Pharmaceuticals. A. Grauer reports employment with Federation Bio; ownership interest in Federation Bio; and advisory or leadership role for Sirana Pharma, Scientific Advisory Board. A. Kausz reports consultancy for Allena Pharmaceuticals, Inc., Noveome Biotherapeutics, ProKidney, and Viridian and advisory or leadership role for Kidney Health Initiative Board of Directors and Noveome Biotherapeutics. F. Knauf reports consultancy for Allena Pharmaceuticals, United States; Alnylam Pharmaceutical, United States; Chinook Pharmaceuticals, United States; Oxthera Pharmaceuticals, Sweden; Zai Pharmaceuticals, China; and EcoR1, United States; research funding from Alnylam Pharmaceuticals, Deutsche Forschungsgemeinschaft, Dicerna Pharmaceuticals, and Else Kröner Fresenius Stiftung; honoraria from Advicenne, Alnylam, ECoR1, Medice, and Sanofi; royalties from PocketDoktor Medical Books; and advisory or leadership role for Oxalosis and Hyperoxaluria Foundation, NYC, Scientific Advisory Board. F. Knauf is an employee of the nonprofit institute Charité-Universitätsmedizin Berlin, which recently filed a patent application for oxalate-lowering agents in dialysis patients with F. Knauf as one of two inventors. There has been no decision yet. C.B. Langman reports consultancy for Alexion Pharmaceuticals, Allena Pharmaceuticals, and Dicerna Pharmaceuticals and honoraria from Alexion Pharmaceuticals and Horizon Pharmaceuticals. J.C. Lieske reports employment with Mayo Clinic; consultancy for Allena, Alnylam, the American Board of Internal Medicine, BioMarin, Chinook, Dicerna, Federation Bio, Intellia, Mirium, Novo Nordisk, Novobiome, Orfan, Oxidien, OxThera, Siemens, and Synlogic; research funding from Allena, Alnylam, Dicerna, Novobiome, OxThera, Retrophin, Siemens, and Synlogic; honoraria from the American Board of Internal Medicine and UpToDate; and advisory or leadership role for Kidney International and ABIM. M.A. Malley reports employment with Travere Therapeutics and ownership interest in Travere Therapeutics and Altimmune. D. Milliner reports employment with Mayo Clinic; consultancy for Alnylam, Dicerna, Mirum, Novo Nordisk, and OxThera pharmaceutical companies; research funding from Alnylam, Dicerna, Novo Nordisk, and OxThera pharmaceutical companies—all research funding is provided to Mayo Clinic and not to D. Milliner personally; advisory or leadership roles for Urolithiasis Editorial Board, Alnylam pharmaceuticals Humanitarian Program, Advisory committee for Alnylam pharmaceutical company, Data Safety Monitoring Board for clinical trials conducted by Dicerna pharmaceutical company, and ongoing work with the Oxalosis and Hyperoxaluria Foundation, a nonprofit, private foundation. This includes some research funding; there is no personal compensation. D. Milliner reports ongoing work with the OHF and KHI for a KHI-sponsored project. L. Nazzal reports consultancy for Allena, Dicerna, Federation Bio, Oxthera, Novome, and Synlogic; honoraria from Guidepoint, GLG consultancy, and Magnolia innovations; and advisory or leadership role for Scientific Advisory Board of the Oxalosis and Hyperoxaluria Foundation. G. Tasian reports consultancy for and research funding from Alnylam Pharmaceuticals and Novo Nordisk. A. Thompson reports employment with US Food and Drug Administration. K.D. Wood reports consultancy for Alnylam Pharmaceuticals, Biomarin, Chinook Therapeutics, Intellia Therapeutics, Novome Biotechnologies, Steris Corporation, and Synlogic Therapeutics; research funding from Arbor Biotechnology and Chinook Therapeutics; patents through University of Alabama; and advisory or leadership role for Scientific Advisory Board for the Oxalosis and Hyperoxaluria Foundation (not paid). E. Worcester reports patents or royalties from BMA Biomedical and advisory or leadership role for the Oxalosis and Hyperoxaluria Foundation. S. Yang reports employment with FDA and ownership interest in BABA, Facebook, FVRR, LPSN, Lyft, NVDA, OPEN, PINS, PLTR, RIVN, TWLO, UPWK, and Yelp. All remaining authors have nothing to disclose.
Acknowledgments
The views and opinions expressed in this publication are those of the authors and do not necessarily reflect the official policies of any KHI member organization, the US Department of Veterans Affairs, or the US Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the US Government.
This work was supported by the Kidney Health Initiative (KHI), a public-private partnership between the American Society of Nephrology, the US FDA, and >90-member organizations and companies to enhance patient safety and foster innovation in kidney disease. Support was also provided by the Rare Kidney Stone Consortium (U54DK083908), a part of the Rare Diseases Clinical Research Network of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences of the NIH.
We would also like to acknowledge Kim Hollander and Julie Bertarelli from the Oxalosis and Hyperoxaluria Foundation for their initiation of this project and continued support along with the Enteric Hyperoxaluria patients and families who motivated this work. We would also like to acknowledge members of the OHF Enteric Hyperoxaluria Workgroup who developed the diagnostic algorithm: Dean Assimos, MD; Juan Calle, MD; Helena Cowley, MS, MBA; Naomi Dahl, PharmD; Andreas Grauer, MD; Kim Hollander; Craig Langman, MD; John Lieske, MD; Lacy McClean, MD; Dawn Milliner, MD; Lama Nazzal, MD, MSc; Marja Puurunen, MD, PhD; Alistair Wheeler, MD; Kyle Wood, MD; Richard Yocum, MD; and Joumana Zeid, MS, MBA. KHI funds were used to defray costs incurred during the conduct of the project, including project management support, which was expertly provided by American Society of Nephrology staff members Meaghan Malley and Melissa West. There was no honorarium or other financial support provided to KHI workgroup members. The authors of this study had final review authority and are fully responsible for its content. KHI makes every effort to avoid actual, potential, or perceived conflicts of interest that may arise because of industry relationships or personal interests among the members of the workgroup. More information on KHI, the workgroup, or the conflict of interest policy can be found at www.kidneyhealthinitiative.org.
Footnotes
Oxalosis and Hyperoxaluria Foundation Enteric Hyperoxaluria Workgroup: Dean Assimos, MD; Juan Calle, MD; Helena Cowley, MS, MBA; Naomi Dahl, PharmD; Andreas Grauer, MD; Kim Hollander; Craig Langman, MD; John Lieske, MD; Lacy McClean, MD; Dawn Milliner, MD; Lama Nazzal, MD, MSc; Marja Puurunen, MD, PhD; Alistair Wheeler, MD; Kyle Wood, MD; Richard Yocum, MD; and Joumana Zeid, MS, MBA.
Contributor Information
Collaborators: Dean Assimos, Juan Calle, Helena Cowley, Naomi Dahl, Andreas Grauer, Kim Hollander, Craig Langman, John Lieske, Lacy McClean, Dawn Milliner, Lama Nazzal, Marja Puurunen, Alistair Wheeler, Kyle Wood, Richard Yocum, and Joumana Zeid
Funding
This work was supported by the Kidney Health Initiative (KHI), the Oxalosis and Hyperoxaluria Foundation, and the Rare Kidney Stone Consortium (U54DK083908).
Author Contributions
Conceptualization: Dean Assimos, Melanie Blank, Annamaria Kausz, Felix Knauf, Craig B. Langman, John C. Lieske, Meaghan A. Malley, Dawn Milliner, Kimberly Smith, Greg Tasian, Aliza Thompson, Elaine Worcester, Sixun Yang.
Data curation: Dean Assimos, Melanie Blank, Annamaria Kausz, Felix Knauf, Craig B. Langman, John C. Lieske, Meaghan A. Malley, Dawn Milliner, Kimberly Smith, Greg Tasian, Aliza Thompson, Elaine Worcester, Sixun Yang.
Funding acquisition: John C. Lieske, Dawn Milliner.
Project administration: John C. Lieske.
Writing – original draft: Dean Assimos, Melanie Blank, Annamaria Kausz, Felix Knauf, Craig B. Langman, John C. Lieske, Meaghan A. Malley, Dawn Milliner, Kimberly Smith, Greg Tasian, Aliza Thompson, Elaine Worcester, Sixun Yang.
Writing – review & editing: Dean Assimos, Melanie Blank, Juan Calle, Andreas Grauer, Annamaria Kausz, Felix Knauf, Craig B. Langman, John C. Lieske, Meaghan A. Malley, Dawn Milliner, Lama Nazzal, Kimberly Smith, Greg Tasian, Aliza Thompson, Kyle D. Wood, Elaine Worcester, Sixun Yang.
References
- 1.Stauffer JQ. Hyperoxaluria and intestinal disease. The role of steatorrhea and dietary calcium in regulating intestinal oxalate absorption. Am J Dig Dis. 1977;22(10):921–928. doi: 10.1007/bf01076170 [DOI] [PubMed] [Google Scholar]
- 2.Chadwick VS, Modha K, Dowling RH. Mechanism for hyperoxaluria in patients with ileal dysfunction. N Engl J Med. 1973;289(4):172–176. doi: 10.1056/nejm197307262890402 [DOI] [PubMed] [Google Scholar]
- 3.Witting C Langman CB Assimos D, et al. Pathophysiology and treatment of enteric hyperoxaluria. Clin J Am Soc Nephrol. 2021;16(3):487–495. doi: 10.2215/CJN.08000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nazzal L, Puri S, Goldfarb DS. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol Dial Transplant. 2016;31(3):375–382. doi: 10.1093/ndt/gfv005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1(5):481–485. doi: 10.1016/j.soard.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Lieske JC, Mehta RA, Milliner DS, Rule AD, Bergstralh EJ, Sarr MG. Kidney stones are common after bariatric surgery. Kidney Int. 2015;87(4):839–845. doi: 10.1038/ki.2014.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal V, Wilfong JB, Rich CE, Gibson PC. Reversal of gastric bypass resolves hyperoxaluria and improves oxalate nephropathy secondary to Roux-en-Y gastric bypass. Case Rep Nephrol Dial. 2016;6(3):114–119. doi: 10.1159/000449128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar NB, Grundfest S, Jones JS, Streem SB. Jejunoileal bypass reversal: effect on renal function, metabolic parameters and stone formation. J Urol. 2005;174(5):1844–1846; discussion 1846. doi: 10.1097/01.ju.0000177079.56949.1a [DOI] [PubMed] [Google Scholar]
- 9.Nasr SH D'Agati VD Said SM, et al. Oxalate nephropathy complicating Roux-en-Y gastric bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3(6):1676–1683. doi: 10.2215/CJN.02940608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cartery C Faguer S Karras A, et al. Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol. 2011;6(8):1895–1902. doi: 10.2215/CJN.00010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troxell ML, Houghton DC, Hawkey M, Batiuk TD, Bennett WM. Enteric oxalate nephropathy in the renal allograft: an underrecognized complication of bariatric surgery. Am J Transplant. 2013;13(2):501–509. doi: 10.1111/ajt.12029 [DOI] [PubMed] [Google Scholar]
- 12.Karaolanis G, Lionaki S, Moris D, Palla VV, Vernadakis S. Secondary hyperoxaluria: a risk factor for kidney stone formation and renal failure in native kidneys and renal grafts. Transplant Rev. 2014;28(4):182–187. doi: 10.1016/j.trre.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 13.Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P. Secondary oxalate nephropathy: a systematic review. Kidney Int Rep. 2018;3(6):1363–1372. doi: 10.1016/j.ekir.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wharton R, D'Agati V, Magun AM, Whitlock R, Kunis CL, Appel GB. Acute deterioration of renal function associated with enteric hyperoxaluria. Clin Nephrol. 1990;34(3):116–121. [PubMed] [Google Scholar]
- 15.Williams HE. Oxalic acid and the hyperoxaluric syndromes. Kidney Int. 1978;13(5):410–417. doi: 10.1038/ki.1978.59 [DOI] [PubMed] [Google Scholar]
- 16.Earnest DL. Enteric hyperoxaluria. Adv Intern Med. 1979;24:407–427. [PubMed] [Google Scholar]
- 17.D'Costa MR Kausz AT Carroll KJ, et al. Subsequent urinary stone events are predicted by the magnitude of urinary oxalate excretion in enteric hyperoxaluria. Nephrol Dial Transplant. 2021;36(12):2208–2215. doi: 10.1093/ndt/gfaa281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milliner DS McGregor TL Thompson A, et al. End points for clinical trials in primary hyperoxaluria. Clin J Am Soc Nephrol. 2020;15(7):1056–1065. doi: 10.2215/CJN.13821119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agyeman AS, Siegel JN, Leptak C. Establishing a public resource for acceptable surrogate endpoints to support FDA marketing applications. Front Med (Lausanne). 2022;9:820990. doi: 10.3389/fmed.2022.820990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.21st Century Cures Act. Subtitle B—Advancing New Drug Therapies. Sec 507. 2016. Accessed October 4, 2023. https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf [Google Scholar]
- 21.U.S. Department of Health and Human Services. Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER); 2014. Accessed October 4, 2023. https://www.fda.gov/media/86377/download [Google Scholar]
- 22.Park S, Pearle MS. Pathophysiology and management of calcium stones. Urol Clin North Am. 2007;34(3):323–334. doi: 10.1016/j.ucl.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 23.Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012;97(6):1847–1860. doi: 10.1210/jc.2011-3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhondup T Kittanamongkolchai W Vaughan LE, et al. Risk of ESRD and mortality in kidney and bladder stone formers. Am J Kidney Dis. 2018;72(6):790–797. doi: 10.1053/j.ajkd.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander RT Hemmelgarn BR Wiebe N, et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287. doi: 10.1136/bmj.e5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhojani N Mandeville JA Hameed TA, et al. Lithotripter outcomes in a community practice setting: comparison of the LithoGold LG-380 and Storz Modulith SLX. J Urol. 2015;193(3):875–879. doi: 10.1016/j.juro.2014.09.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jungers P, Joly D, Barbey F, Choukroun G, Daudon M. ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am J Kidney Dis. 2004;44(5):799–805. doi: 10.1016/s0272-6386(04)01131-x [DOI] [PubMed] [Google Scholar]
- 28.Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. 2011;6(8):2069–2075. doi: 10.2215/CJN.10651110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulay SR, Anders HJ. Crystal nephropathies: mechanisms of crystal-induced kidney injury. Nat Rev Nephrol. 2017;13(4):226–240. doi: 10.1038/nrneph.2017.10 [DOI] [PubMed] [Google Scholar]
- 30.Knauf F Asplin JR Granja I, et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84(5):895–901. doi: 10.1038/ki.2013.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turgeon NA Perez S Mondestin M, et al. The impact of renal function on outcomes of bariatric surgery. J Am Soc Nephrol. 2012;23(5):885–894. doi: 10.1681/ASN.2011050476 [DOI] [PubMed] [Google Scholar]
- 32.Selby MG Vrtiska TJ Krambeck AE, et al. Quantification of asymptomatic kidney stone burden by computed tomography for predicting future symptomatic stone events. Urology. 2015;85(1):45–50. doi: 10.1016/j.urology.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgher A, Beman M, Holtzman JL, Monga M. Progression of nephrolithiasis: long-term outcomes with observation of asymptomatic calculi. J Endourol. 2004;18(6):534–539. doi: 10.1089/end.2004.18.534 [DOI] [PubMed] [Google Scholar]
- 34.Li X, Zhu W, Lam W, Yue Y, Duan H, Zeng G. Outcomes of long-term follow-up of asymptomatic renal stones and prediction of stone-related events. BJU Int. 2019;123(3):485–492. doi: 10.1111/bju.14565 [DOI] [PubMed] [Google Scholar]
- 35.Darrad MP, Yallappa S, Metcalfe J, Subramonian K. The natural history of asymptomatic calyceal stones. BJU Int. 2018;122(2):263–269. doi: 10.1111/bju.14354 [DOI] [PubMed] [Google Scholar]
- 36.Werness PG, Bergert JH, Lee KE. Urinary crystal growth: effect of inhibitor mixtures. Clin Sci. 1981;61(4):487–491. doi: 10.1042/cs0610487 [DOI] [PubMed] [Google Scholar]
- 37.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177(2):565–569. doi: 10.1016/j.juro.2006.09.033 [DOI] [PubMed] [Google Scholar]
- 38.Valezi AC, Fuganti PE, Junior JM, Delfino VD. Urinary evaluation after RYGBP: a lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes Surg. 2013;23(10):1575–1580. doi: 10.1007/s11695-013-0916-0 [DOI] [PubMed] [Google Scholar]
- 39.Canales BK, Hatch M. Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis. 2014;10(4):734–742. doi: 10.1016/j.soard.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siener R, Petzold J, Bitterlich N, Alteheld B, Metzner C. Determinants of urolithiasis in patients with intestinal fat malabsorption. Urology. 2013;81(1):17–24. doi: 10.1016/j.urology.2012.07.107 [DOI] [PubMed] [Google Scholar]
- 41.Gregory JG, Park KY, Schoenberg HW. Oxalate stone disease after intestinal resection. J Urol. 1977;117(5):631–634. doi: 10.1016/s0022-5347(17)58564-x [DOI] [PubMed] [Google Scholar]
- 42.Prochaska M, Taylor E, Ferraro PM, Curhan G. Relative supersaturation of 24-hour urine and likelihood of kidney stones. J Urol. 2018;199(5):1262–1266. doi: 10.1016/j.juro.2017.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perinpam M Enders FT Mara KC, et al. Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem. 2017;50(18):1014–1019. doi: 10.1016/j.clinbiochem.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hueppelshaeuser R von Unruh GE Habbig S, et al. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn's disease. Pediatr Nephrol. 2012;27(7):1103–1109. doi: 10.1007/s00467-012-2126-8 [DOI] [PubMed] [Google Scholar]