Abstract
Background
Cataract is a blinding disease worldwide. It is an age-related disease that mainly occurs in people over 65 years old. Cataract is also prevalent in patients with diabetes mellites (DM). The pathological mechanisms underlying diabetic cataract (DC) are more complex than that of age-related cataract. Studies have identified that polyol pathway, advanced glycation end products (AGEs) and oxidative stress are the primary pathogenesis of DC. In recent years, molecular-level regulations and pathological processes of lens epithelial cells (LECs) have been confirmed to play roles in the initiation and progression of DC. A comprehensive understanding and elucidation of how chronic hyperglycemia drives molecular-level regulations and cytopathological processes in the lens will shed lights on the prevention, delay and treatment of DC.
Main text
Excessive glucose in the lens enhances polyol pathway and AGEs formation. Polyol pathway causes imbalance in the ratio of NADPH/NADP+ and NADH/NAD+. Decrease in NADPH/NADP+ ratio compromises antioxidant enzymes, while increase in NADH/NAD+ ratio promotes reactive oxygen species (ROS) overproduction in mitochondria, resulting in oxidative stress. Oxidative stress in the lens causes oxidation of DNA, proteins and lipids, leading to abnormalities in their structure and functions. Glycation of proteins by AGEs decreases solubility of proteins. High glucose triggered epigenetic regulations directly or indirectly affect expressions of genes and proteins in LECs. Changes in autophagic activity, increases in fibrosis and apoptosis of LECs destroy the morphological structure and physiological functions of the lens epithelium, disrupting lens homeostasis.
Conclusions
In both diabetic animal models and diabetics, oxidative stress plays crucial roles in the formation of cataract. Epigenetic regulations, include lncRNA, circRNA, microRNA, methylation of RNA and DNA, histone acetylation and pathological processes, include autophagy, fibrosis and apoptosis of LECs also involved in DC.
Keywords: Oxidative stress, Epigenetic regulation, Autophagy, Fibrosis, Apoptosis, Diabetic cataract
1. Introduction
Cataract is the leading cause of blindness, accounting for about 51 % of all blind people worldwide.1 It is mainly prevalent in people over 65 years old and patients with diabetes mellitus (DM).2 Both Type-1 and Type-2 diabetics are susceptible to develop cataract at an earlier age.3 Epidemiological investigations have shown that as the rise of diabetics, so does the patients with diabetic cataract (DC).4 Implantation of an intraocular lens (IOL) instead of cloudy lens by surgery is the only effective way to treat cataract, however, postoperative complications remain unavoidable problems. Some researches indicate that diabetics are at a higher risk of postoperative complications. Herein, understanding the pathogenesis of DC is essential to prevent, delay and treat cataract in diabetics. The purpose of this review is to discuss molecular-level regulations and cytopathological activities of lens epithelial cells (LECs) triggered by high glucose, and how they drive and facilitate the development of cataract.
The lens is an avascular and transparent tissue that is composed of LECs, lens fiber cells differentiated from LECs at the equatorial region and mature fiber cells. Although the metabolic activity is different between LECs and lens fiber cells, glucose metabolism occurs in both LECs and lens fiber cells. Studies have shown that excess glucose in LECs and lens fiber cells results in different outcomes. Chronic hyperglycemia compromises antioxidant capacity and initiates apoptosis in the LECs,5,6 while causes swelling and liquefaction of lens fiber cells as a result of sorbitol accumulation and osmotic stress.7 A large number of studies have demonstrated that polyol pathway, advanced glycation end products (AGEs) and oxidative stress are the primary pathological mechanisms underlying DC.8 Polyol pathway and AGEs compromise antioxidant defense system, leading to reactive oxygen species (ROS) accumulation and oxidative stress.9 In some cells (e.g., endothelial cells), ROS also enhances polyol pathway and AGEs formation under hyperglycemic condition.10 In LECs of diabetics11,12 and diabetic animal models,13 oxidative stress plays important roles in the development of cataract according to literatures summarized in Table 1 and Table 2.
Table 1.
Oxidative stress and involvement of oxidative stress underlying diabetic cataract in human.
| Materials | Alteration of redox homeostasis | Involvement of oxidative stress | References |
|---|---|---|---|
| Treatment of SRA01/04 with high glucose (50 mM, 24 h) | Decrease in GSH/GSSG ratio, Total-SOD, CAT, GPx | Lipids peroxidation | 81 |
| Human LECs line cultured with high glucose (25.6 mM, 7 d) | ROS production | Increases in intracellular Ca2+ and apoptosis mediated by TRPV2 upregulation | 78 |
| Human LECs cultured with high glucose (125 mM, 24 h) | ROS production | Activation of unfolded protein response (UPR) | 82 |
| LECs from lenses of diabetic cataract | Increases in AKR1B1 expression and SOD2 acetylation | Protein oxidation with an increase in 3-nitrotyrosine level | 83,5 |
| Lenses of diabetic cataract | Decrease in GSH, GR, GPx | Protein oxidation | 11,84 |
| Plasma and serum of patients with diabetic cataract | Increases in ALR2 and AGEs; decreases in GSH and GPX-3 | Lipids peroxidation | 42,85 |
| Lenses of diabetic cataract | Increases in RAGE and RAC1(regulatory subunit of NADPH oxidase) | / | 86 |
MDA: malondialdehyde; GR: Glutathione reductase; GPx: Glutathione peroxidase; EMT: epithelial-to-mesenchymal transition; ALR2: aldose reductase 2; AKR1B1: aldo-keto reductases1B1.
Table 2.
Oxidative stress and involvement of oxidative stress underlying diabetic cataract in animal models.
| Materials | Alteration of redox homeostasis | Involvement of oxidative stress | References |
|---|---|---|---|
| Lens epithelium of diabetic rats induced by fructose | Increases in ROS and SOD2 acetylation | Protein oxidation with increase in 3-nitrotyrosine (3-NT) level | 83 |
| LECs of diabetic rats induced by fructose | ROS production (p47-phox, p67-phox subunits of NOXs and NOX4 upregulation) | / | 86,87 |
| LECs of diabetic rats induced by high glucose | AR overexpression (decrease in NADPH/NADP+) | LECs apoptosis | 88 |
| LECs of diabetic rats induced by high glucose | ROS generation | Increases in intracellular Ca2+ and apoptosis mediated byTRPV2 upregulation | 78 |
| Rat lenses cultured with high glucose, Lens of diabetic rats induced by STZ | Decreases in GSH, Total-SOD, GSH-Px, CAT, α-Klotho and Nrf2 | Lipids peroxidation | 81,43,13 |
| Lenses of diabetic rats fed with galactose | GSH decrease | UPR activation and LECs apoptosis | 82 |
| Lenses of diabetic cataract of STZ induced rats | / | Protein oxidation | 32,89 |
| Cataractous lenses of SDH deficient mice | GSH decrease | Lipids oxidation, Na+/K+-ATPase activity reduction, osmoregulatory machinery impairment | 90 |
| Lenses of diabetic rats induced by STZ | GSH decrease | Protein and lipids oxidation | 91 |
LECs: lens epithelial cells; STZ: streptozotocin; RAGE: receptor for AGEs; SGLT2: sodium-glucose cotransporter 2; SDH: dehydrogenase; UPR: unfolded protein response; MDA: malondialdehyde.
In addition to the three pathways mentioned above, lncRNA, circRNA, mircoRNA, RNA and DNA methylation, histone acetylation affected by high glucose are regulatory mechanisms mediating survival and apoptosis of LECs. Pathological processes of LECs, include autophagy, fibrosis and apoptosis are also involved in the initiation and progression of DC. This review comprehensively discusses the mechanisms underlying DC and aims to provide theoretical supports for prevention, delay and treatment of DC.
2. Glucose metabolisms and oxidative stress
2.1. Polyol pathway and oxidative stress
Under physiological conditions, glucose is mainly metabolized by glycolysis and citric acid cycle to provide ATP for survival and growth of the lens. About 10 % of glucose is metabolized through pentose phosphate pathway to provide sugar residues for nucleotide synthesis, and some of glucose is utilized to synthesize glycogen.14 Only less than 5 % of glucose shunts into polyol pathway due to the low affinity of aldose reductase (AR) to glucose.15 Under the condition of hyperglycemia, increased glucose levels in aqueous humor prompt excess glucose to be transported into the lens by glucose transporters (e.g., GLUT1, GLUT3 and GLUT5) and sodium-glucose cotransporter (SGLT) on the cell membrane through an insulin-independent manner.14 Elevation of glucose levels in LECs leads to saturation of hexokinase and restriction of glycolytic pathway. However, both the activity and expression of AR are increased under such condition, approximately 33 % of glucose in lens cells shunts into polyol pathway.16 Glucose is oxidated to sorbitol by AR and sorbitol is reduced to fructose by sorbitol dehydrogenase (SDH), which leads to an reduction in NADPH/NADP+ ratio and an increase in NADH/NAD+ ratio, respectively (Fig. 1). NADPH is a coenzyme of several antioxidant enzymes, such as glutathione reductase (GR), glutathione peroxidase (GPx) and catalase (CAT). Decrease in NADPH/NADP+ ratio impairs the antioxidant capacity of cells, leading to ROS accumulation.17 Increase in NADH/NAD+ ratio promotes ROS overproduction in mitochondria.15
Fig. 1.
Schematic diagram of polyol pathway and oxidative stress. Reduction of glucose to sorbitol by AR leads to decrease in NADPH/NADP+ ratio, this compromises generation of GSH from GSSG and the ability of CAT to reduce H2O2 to H2O. Oxidation of sorbitol to fructose by SDH results in increase in NADH/NAD+ ratio, NADH is a donor of electron and a higher ratio of NADH/NAD+ in the mitochondrial matrix promotes ROS generation.
In vitro, ROS also facilitates polyol pathway by impacting AR under certain conditions.18 It has been found that superoxide anion radical () is the first ROS produced in mitochondria in bovine aortic endothelial cells cultured with high glucose. Normalizing mitochondrial prevents high glucose induced ROS generation, indicating is a key contributor to ROS induced by high glucose.19 Enhancement of polyol pathway by ROS may be attributed to the increases in activity and transcription of AR. It has been identified that has the ability to quench nitric oxide (NO), which inhibits AR activity by S-thiolation of cystine 298 at the active site.20 Although we discuss the impact of ROS on AR according to findings in endothelial cells, this mechanism may be dependent on the cellular context.
2.2. Advanced glycation end products (AGEs) and oxidative stress
Accumulation of AGEs is one of the major risk factors for diabetic complications (e.g., diabetic nephropathy, diabetic retinopathy, diabetic cataract) due to the ability of AGEs to induce oxidative stress and initiate inflammation by binding to their receptor RAGE.21 In vitro studies have shown that ROS also enhances AGEs formation in certain conditions.22
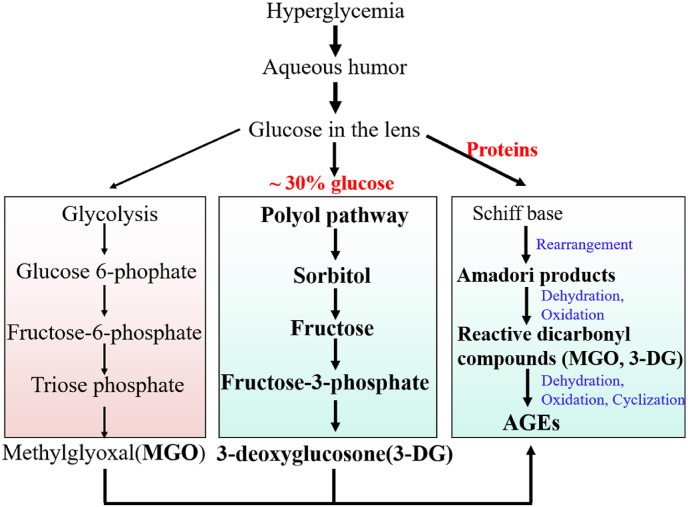
AGEs are a class of heterogeneous compounds that primarily generated by the reactions between carbonyl groups of reducing sugar (e.g., glucose, fructose, galactose) and amino groups of proteins, lipids and nucleic acids, known as Maillard reaction. The processes of AGEs formation are spontaneous that do not require catalysis of enzyme. Under the condition of high glucose, AGEs formation is enhanced due to increases in glucose availability and dicarbonly compounds (e.g., methylglyoxal, 3-deoxyglucosone, glyoxal). Intermediates or byproducts from glycolysis, polyol pathway and lipids peroxidation are also contributor to AGEs (Fig. 2).23 Studies have shown that AGEs levels in the cataractous lens of diabetics are higher than that without diabetes.3 AGEs induce oxidative stress not only by activating NADPH oxidases (NOXs) through AGEs/RAGE signaling,24,25 but also reducing activity of antioxidant enzymes by glycation (e.g., site specific or random fragmentation of Cu/ZnSOD).26 In vitro, ROS also enhances AGEs formation through different mechanisms in several types of cells (e.g., retinal vascular endothelial cells).18 Firstly, inactivates glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by oxidation of cysteine residue (Cys-149) at the active site.27 Secondly, ROS caused DNA strand breakage activates poly (ADP-ribose) polymerase-1 (PARP-1), which inactivates GAPDH by mediating GAPDH poly (ADP-ribosyl)ation.28 Thirdly, high glucose also inhibits GAPDH transcription.29 GAPDH is the enzyme that catalyzes the conversion of glyceraldehyde-3-phosphate (GAP) to 1, 3-biphospgoglycerate in the processes of glycolysis. Reduction in GAPDH causes accumulation of GAP, which can be converted into methylglyoxal (MGO), a major precursor of AGEs.30 ROS also enhances AGEs formation by promoting binding of carbonyl groups to amino groups.31 Although we discuss the interactions between AGEs and ROS, their interplays in the lens still need to be furtherly explored, especially in the context of high glucose.
Fig. 2.
Pathways for AGEs formation under the condition of high glucose. Intermediates from glycolysis and polyol pathway, such as MGO and 3-DG are primary precursors for AGEs. Higher glucose inside cells enhances Maillard reaction.
3. Molecular-level regulations and cellular pathological processes
3.1. Impairment of biomacromolecules
3.1.1. Protein modifications and insolubility
Proteins in the lens account for 33 % of the total weight of the lens, and the high content of proteins is essential for maintaining refractive property and transparency of the lens. Oxidation of proteins changes the structure and function and contributes to lens opacity (Fig. 3). In the development of DC in streptozotocin (STZ) induced rats, the amount of protein carbonyl groups in the lens increases in a time-dependent manner,32 indicating the degree of proteins oxidation is positively correlated with the time of high glucose exposure. Oxidation of proteins alters surface charge and native conformation, causing partial unfolding and exposure of hydrophobic groups. The exposed hydrophobic groups interact with other exposed hydrophobic groups and form aberrant protein-protein interactions, leading to aggregation and insolubility.
Fig. 3.
Pathological mechanisms involved in diabetic cataract. A. Polyol pathway, AGEs, oxidative stress. B. Epigenetic regulations: includes lncRNA, CircRNA, microRNA, methylation of DNA and RNA, histone acetylation. C. Cellular pathological processes: includes autophagy, fibrosis, apoptosis of lens epithelial cells.
Glycation of proteins is also a cause of partial unfolding, exposure of sulfhydryl groups and hydrophobic groups, resulting in formation of disulfide bonds and hydrophobic interactions. Both glycated modifications and abnormal interactions cause cross-linking, high-molecular-weight aggregation and insolubility.33 Proteins in both intracellular and extracellular matrix (ECM) can be modified by glycation. Glycation of proteins in ECM (e.g., collagen) causes abnormal interactions between collagens and other components or receptors on cell membrane, impairing cellular structure.34
α-Crystallin is the protein with molecular chaperone and anti-apoptotic activity. It is essential to protect other proteins from oxidative damage and apoptosis of LECs. High glucose impairs the molecular chaperone and anti-apoptotic activity of α-crystallin via different modifications, including glycation,35 oxidation,36 and phosphorylation. Glycation of α-crystallin damages the integrity of fiber cells.37 It has been shown that glycation of α-crystallin by MGO causes partial unfolding and decrease in the stability, making it easily degrade.38 In cataractous lens of diabetic rats, phosphorylated level of αB-crystallin is increased, which causes an increase in the fraction of insolubility and formation of cataract.39 Studies have indicated that p38 and ERK1/2 phosphorylate αB-crystallin at Ser59 and Ser45, respectively. Both p38 and ERK1/2 kinases are activated by high glucose and may be responsible for proteins phosphorylation in the development of DC. In vitro, low concentrations (5 and 50 mM) of sorbitol subtly changes the secondary and tertiary structure of α-crystallin, reducing the chaperone activity.40
3.1.2. Lipids peroxidation
Cell membrane is an important barrier to maintain osmotic balance. Lipids are key components of cell membrane. Although the composition and structure of lipids in lens cell membrane confer them resistance to oxidation, lipids peroxidation also occurs in aging and cataractous lens.41 In cataractous lenses of diabetics and diabetic rats, the content of malondialdehyde (MDA) is obviously elevated and its concentration is positively correlated with the duration and severity of hyperglycemia,42,43 indicating high glucose induces lipids peroxidation in the lens.
Lipids peroxidation disrupts the phospholipid bilayer structure of cell membrane, leading to impairment of membrane structure and selective permeability. Lipids peroxidation also causes uncoupling of membrane-bound Na+/K+-ATPase and oxidative inhibition of Ca2+-ATPase in lens cells.44 Decrease in Ca2+-ATPase activity increases intracellular Ca2+ concentration and disturbs osmotic balance.45 Besides, lipids peroxides (e.g., MDA, HNE) also damage proteins and DNA due to their longer half-life and the ability to diffuse to distant sites.46 Thus, elevation of intracellular Ca2+ level and secondary damages to DNA and proteins may be responsible for DC as a consequence of lipids peroxidation (Fig. 3).
3.1.3. Oxidative damages to DNA
Oxidation of DNA influences genome stability, transcription, protein misfolding and cellular damages. Oxidation of DNA causes DNA strand breakage and activation of PARP-1. PARP-1 is the best characterized DNA repair enzyme and plays double roles in H2O2 treated lens cells. Medium activity of PARP repairs damaged DNA strand, while prolonged activation of PARP-1 promotes cell death in the lens.47 Treatment of human LECs with 30 mM glucose for 48 h significantly increases poly(ADP-ribosyl)ated protein, indicating PARP-1 activation.48 PARP-1 activation results in NAD+ decrease and polymers of ADP-ribose (PAR) increase, leading to energy imbalance of cells. In STZ-induced diabetic cataract rats, DNA oxidation marker 8-Hydroxydeoexyguanosine (8-OHdG) in serum is significantly increased.49 DNA oxidation contributes to DC through direct or indirect roles (e.g., overactivation of PARP-1) (Fig. 3).
3.2. Epigenetic regulations
3.2.1. LncRNA and miRNA
LncRNA are crucial regulatory molecules related to physical development and multiple diseases, including DC. LncRNA NEAT1 is downregulated in the LECs of patients with DC. It regulates microRNA-205-3p/MMP16 axis in the development of DC.50 In high glucose treated HLE-B3 cells, lncRNA PVT1 is upregulated. This inhibits proliferation and promotes apoptosis of LECs by regulating miR-214-3p/MMP2 axis.51 Circular RNAs (circRNAs) is the covalently closed loop long non-coding RNAs and act as inhibitors (‘sponges’) of microRNA or protein.52 In the LECs of patients with DC and high glucose cultured HLE-B3 cells, downregulation of circPAG1 caused by high glucose promotes oxidative stress and apoptosis. It has been identified that circPAG1 acts as a ‘sponge’ of miR-630, which targets to EPHA2. Upregulation of circPAG1 protects high glucose induced oxidative damage to LECs by regulating miR-630/EPHA2 axis.53 In cataractous lenses of diabetics, high glucose induced upregulation of circKMT2E promotes DC by downregulating miR-204-5p. miR-204 inhibits LECs EMT by binding to the 3’-UTR of Smad4.54 Upregulation of circKMT2E involves in EMT processes of LECs in the context of high glucose.
MicroRNAs (miRNAs) are key regulatory molecules in physical development and pathological processes by target a majority of mRNAs.55 In aqueous humor of patients with DC, miR-551b is significantly upregulated. And this reduces the viability and increases apoptosis of LECs by downregulating CRYAA expression.56 Downregulation of miR-30a implicates in DC by targeting SNAL1, an important transcription factor regulating EMT processes.57 Both in vivo and in vitro studies in human LECs have indicated that miRNA-199a-5p is downregulated by high glucose. And this induces EMT by regulating SP1.58
3.2.2. N6-methyladenosine (m6A)
m6A modification involves in various of RNA biological processes, including transport, splicing, stability, degradation and translation. Treatment of human LECs with high glucose increases RNA methyltransferase like 3 (METTL3) and total m6A levels. Upregulation of METTL3 represses proliferation and promotes apoptosis by targeting intercellular adhesion molecule-1 (ICAM-1),59 indicating METTL3 not only regulates methylation of mRNA, but also affects fate of LECs under the condition of high glucose.
3.2.3. DNA methylation
Methylation and demethylation of CpG islands in the promoter of DNA are regulatory mechanisms of gene expression by influencing the accessibility of the functional factors in transcriptional machinery.60 Kelch-like ECH associated protein 1 (Keap1) is a protein negatively regulates Nrf2, a key nuclear transcriptional factor regulating expression of several antioxidant enzymes in the lens. Studies have shown that high glucose regulates methylation levels in the promoter region of Keap1. In the lenses of patients with DC, methylation level in the promoter of Keap1 is significantly decreased, and this increases its expression of mRNA and protein. Increase in Keap1 promotes Nrf2 degradation that downregulates transcription of several antioxidant enzymes. Thus, demethylation of Keap1 implicates in DC by aggravating oxidative stress and proteins aggregation.61 It has been revealed that increase of MGO may be responsible for keap1 demethylation under the condition of high glucose.62
3.2.4. Histone acetylation
Histone acetylation enhances gene expression by relaxing chromatin. Histone deacetylation suppresses gene expression due to the unmodified histones possess a positive charge. In this situation, histones interact more closely with the negatively charged DNA backbone, leading to condensation of chromatin and restriction of transcriptional machinery.63 In galactose induced rats, the formation of cataract is attributed to upregulation of Polo-like kinase 3 (Plk3) mRNA. Mechanically, overexpression of Polo-like kinase 3 (Plk3) mRNA is regulated by histone acetylation.64 This implies that acetylation and deacetylation are one of the regulatory mechanisms underlying galactose induced cataract in rats.
3.3. Cellular pathological processes
3.3.1. Autophagy of LECs
Autophagy is a catabolic process implicated in both cellular survival and death. It degrades defective or aggregated proteins, lipids, toxic debris, as well as disused organelles and membrane. It has been shown that alternations in autophagic activity are associated with cataract.65 Exposure of HLE-B3 cells to high glucose results in a decrease in autophagic activity and activation of EMT.66 In high glucose treated HLE-B3 cells, inhibition of autophagic activity is correlated with EMT processes. NICD/ULK1 signaling is confirmed to mediate the crosstalk between autophagy and EMT processes in the development of DC.67 In high glucose cultured SRA01/04 cells and lens epithelium of patients with DC, autophagic activity is significantly suppressed. Downregulations of AMPK-dependent FOXO3 and TFEB are the regulatory mechanisms of autophagy inhibition induced by high glucose.68 Both in vivo and in vitro studies in LECs of mice demonstrate inhibition of autophagy induced by high glucose is related to oxidative damage.69
3.3.2. Fibrosis of LECs
Fibrosis of LECs destroys the morphological structure and causes abnormal deposition of ECM (e.g., collagen), leading to LECs hardness and loss of elasticity. Both TGFβ1 and TGFβ2 are highly expressed in aqueous humor and lens epithelium of patients with DC. Moreover, the expression of TGFβ is positively correlated with the level of glycosylated haemoglobin.70 TGFβ is the most effective inductor of fibrosis, suggesting high glucose may promote LECs fibrosis by stimulating TGFβs expression. In lens epithelium of diabetics, LECs EMT is also associated with the increase in RAGE.5 AGEs/RAGE activates or participates in signaling pathways to regulate EMT. It has been found that AGEs/RAGE also involves in TGF-β signaling in the processes of EMT in LECs.71 In diabetic animal models, fibrosis of LECs is also indueced by AR.72
3.3.3. Apoptosis of LECs
In the lenses of patients with DC, apoptosis of LECs is obviously observed.73 In lens epithelium of diabetics, apoptosis of LECs is evidenced by expression of Bax/Bcl-2,74 p53 and caspase-8.75 Oxidative stress induced by high glucose is also an initiator of apoptosis in human LECs.76, 77, 78 Elevation of intracellular Ca2+ induced by high glucose also mediates apoptosis of LECs. Both in vivo and in vitro experiments suggested that disruption of Ca2+ homeostasis enhances apoptosis of human LECs and contributes to lens opacity.73 Exposing ex vivo lenses of rats to galactose aggravates apoptosis of LECs, indicating accumulation of sugar alcohols is also a risk factor for LECs apoptosis and DC.79 In STZ induced diabetic rats, apoptosis of LECs at the central of anterior capsule and equatorial zones is remarkably observed. And this is related to the reduction of NGF level.80
4. Conclusions
Oxidative stress plays key functions in the formation of cataract in both diabetics and diabetic animal models. Additionally, epigenetic regulations, autophagy, fibrosis and apoptosis of LECs also implicated in the initiation and progression of DC according to findings, which are mainly derived from in vitro studies of human cell lines.
Study approval
The study obtains approval of Shaanxi Eye Hospital, Xi'an People's Hospital (Xi'an Fourth Hospital), Affiliated People's Hospital of Northwest University.
Author contributions
The authors confirm contribution to the paper as follows: Conception and design of study: HY; Drafting the manuscript: ZG; Figures and Tables set up: XM; Revision of Manuscript: RZ. All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81873674 and 82070947).
Declaration of competing interest
The authors declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to all the peer reviewers for their opinions and suggestions.
Contributor Information
Zaoxia Guo, Email: guozaoxia2012@126.com.
Xiaopan Ma, Email: maxiaopan2023@163.com.
Rui Xue Zhang, Email: zhangruixue@nwpu.edu.cn.
Hong Yan, Email: yan2128ts@med.nwu.edu.cn.
Abbreviations
- DC
Diabetic cataract
- LECs
Lens epithelial cells
- AGEs
Advanced glycation end products
- RAGE
Receptor for advanced glycation end products
- ROS
Reactive oxygen species
Superoxide anion radical
- NOXs
NADPH oxidases
- GSH
Glutathione
- GSSG
Glutathione disulfide
- H2O2
Hydrogen peroxide
- CAT
Catalase
- SOD
Superoxide dismutase
- GPx
Glutathione peroxidases
- NAD+
Oxidized nicotinamide adenine dinucleotide
- NADH
Reduced nicotinamide adenine dinucleotide
- NADP+
Nicotinamide adenine dinucleotide phosphate
- NADPH
Nicotinamide adenine dinucleotide phosphate hydrogen
References
- 1.Lo K., Kloek C. In: Managing Diabetic Eye Disease in Clinical Practice. 1 ed. Singh R.P., editor. Adis Cham; Switzerland: 2015. Diabetes and cataracts; pp. 49–57. [Google Scholar]
- 2.Haddad N.M., Sun J.K., Abujaber S., et al. Cataract surgery and its complications in diabetic patients. Semin Ophthalmol. 2014;29(5-6):329–337. doi: 10.3109/08820538.2014.959197. [DOI] [PubMed] [Google Scholar]
- 3.Hashim Z., Zarina S. Advanced glycation end products in diabetic and non-diabetic human subjects suffering from cataract. Age (Dordr). 2011;33(3):377–384. doi: 10.1007/s11357-010-9177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiziltoprak H., Tekin K., Inanc M., et al. Cataract in diabetes mellitus. World J Diabetes. 2019;10(3):140–153. doi: 10.4239/wjd.v10.i3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T.T., Chen Y.Y., Chang H.Y., et al. AKR1B1-Induced epithelial-mesenchymal transition mediated by RAGE-oxidative stress in diabetic cataract lens. Antioxidants. 2020;9(4) doi: 10.3390/antiox9040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takamura Y., Sugimoto Y., Kubo E., et al. Immunohistochemical study of apoptosis of lens epithelial cells in human and diabetic rat cataracts. Jpn J Ophthalmol. 2001;45:559–563. doi: 10.1016/s0021-5155(01)00418-x. [DOI] [PubMed] [Google Scholar]
- 7.Mathebula S.D. Polyol pathway: a possible mechanism of diabetes complications in the eye. African Vis Eye Health. 2015;74(1) doi: 10.4102/aveh.v74i1.13. [DOI] [Google Scholar]
- 8.Obrosova I.G., Chung S.S., Kador P.F. Diabetic cataracts: mechanisms and management. Diabetes Metab Res Rev. 2010;26(3):172–180. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 9.Araki E., Nishikawa T. Oxidative stress: a cause and therapeutic target of diabetic complications. J Diabetes Investig. 2010;1(3):90–96. doi: 10.1111/j.2040-1124.2010.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikawa T., Edelstein D., Du X.L., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 11.Hashim Z., Zarina S. Osmotic stress induced oxidative damage: possible mechanism of cataract formation in diabetes. J Diabetes Complications. 2012;26(4):275–279. doi: 10.1016/j.jdiacomp.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Haykin V., Oscar A., Dimitrova V., et al. Bioimage analysis of cell physiology of primary lens epithelial cells from diabetic and non-diabetic cataract patients. Biotechnol Biotechnol Equip. 2020;35(1):170–178. doi: 10.1080/13102818.2020.1861978. [DOI] [Google Scholar]
- 13.Ma Z., Liu J., Li J., et al. Klotho ameliorates the onset and progression of cataract via suppressing oxidative stress and inflammation in the lens in streptozotocin-induced diabetic rats. Int Immunopharm. 2020;85 doi: 10.1016/j.intimp.2020.106582. [DOI] [PubMed] [Google Scholar]
- 14.Zahraei A., Guo G., Varnava K.G., et al. Mapping glucose uptake, transport and metabolism in the bovine lens cortex. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.901407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakur S., Gupta S.K., Ali V., et al. Aldose Reductase: a cause and a potential target for the treatment of diabetic complications. Arch Pharm Res (Seoul) 2021;44(7):655–667. doi: 10.1007/s12272-021-01343-5. [DOI] [PubMed] [Google Scholar]
- 16.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 17.Ighodaro O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 18.Kyselova Z., Stefek M., Bauer V. Pharmacological prevention of diabetic cataract. J Diabetes Complications. 2004;18(2):129–140. doi: 10.1016/s1056-8727(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa T., Edelstein D., Du X.L., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 20.Chandra D., Jackson E.B., Ramana K.V., et al. Nitric oxide prevents aldose reductase activation and sorbitol accumulation during diabetes. Diabetes. 2002;51(10):3095–3101. doi: 10.2337/diabetes.51.10.3095. [DOI] [PubMed] [Google Scholar]
- 21.Singh V.P., Bali A., Singh N., et al. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014;18(1):1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowotny K., Jung T., Hohn A., et al. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guimarães E.L., Empsen C., Geerts A., et al. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J Hepatol. 2009;52(3):389–397. doi: 10.1016/j.jhep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M., Kho A.L., Anilkumar N., et al. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113(9):1235–1243. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- 26.Ookawara T., Kawamura N., Kitagawa Y., et al. Site-specific and random fragmentation of Cu,Zn-superoxide dismutase by glycation reaction. Implication of reactive oxygen species. J Biol Chem. 1992;267(26):18505–18510. [PubMed] [Google Scholar]
- 27.Rodacka A., Serafin E., Puchala M. Efficiency of superoxide anions in the inactivation of selected dehydrogenases. Radiat Phys Chem. 2010;79(9):960–965. doi: 10.1016/j.radphyschem.2010.04.001. [DOI] [Google Scholar]
- 28.Du X., Matsumura T., Edelstein D., et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112(7):1049–1057. doi: 10.1172/jci18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanwar M., Kowluru R.A. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58(1):227–234. doi: 10.2337/db08-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 31.Peppa M., Uribarri J., Vlassara H. The role of advanced glycation end products in the development of atherosclerosis. Curr Diabetes Rep. 2004;4(1):31–36. doi: 10.1007/s11892-004-0008-6. [DOI] [PubMed] [Google Scholar]
- 32.Kyselová Z., Garcia S.J., Gajdosíková A., et al. Temporal relationship between lens protein oxidation and cataract development in streptozotocin-induced diabetic rats. Physiol Res. 2005;54(1):49–56. doi: 10.33549/physiolres.930613. [DOI] [PubMed] [Google Scholar]
- 33.Nagaraj R.H., Linetsky M., Stitt A.W. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2012;42(4):1205–1220. doi: 10.1007/s00726-010-0778-x. [DOI] [PubMed] [Google Scholar]
- 34.Ho M.-C., Peng Y.-J., Chen S.-J., et al. Senile cataracts and oxidative stress. J Clin Gerontol Geriatr. 2010;1(1):17–21. doi: 10.1016/j.jcgg.2010.10.006. [DOI] [Google Scholar]
- 35.Stitt A.W. The maillard reaction in eye diseases. Ann N Y Acad Sci. 2005;1043(1):582–597. doi: 10.1196/annals.1338.066. [DOI] [PubMed] [Google Scholar]
- 36.Jain A.K., Lim G., Langford M., et al. Effect of high-glucose levels on protein oxidation in cultured lens cells, and in crystalline and albumin solution and its inhibition by vitamin B6 and N-acetylcysteine its possible relevance to cataract formation in diabetes. Free Radic Biol Med. 2002;33:1615–1621. doi: 10.1016/s0891-5849(02)01109-7. [DOI] [PubMed] [Google Scholar]
- 37.Bejarano E., Taylor A. Too sweet: problems of protein glycation in the eye. Exp Eye Res. 2019;178:255–262. doi: 10.1016/j.exer.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satish Kumar M., Mrudula T., Mitra N., et al. Enhanced degradation and decreased stability of eye lens alpha-crystallin upon methylglyoxal modification. Exp Eye Res. 2004;79(4):577–583. doi: 10.1016/j.exer.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Reddy V.S., Kumar C.U., Reddy G.B. Effect of chronic hyperglycemia on crystallin levels in rat lens. Biochem Biophys Res Commun. 2014;446(2):602–607. doi: 10.1016/j.bbrc.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Kumar C.U., Suryavanshi U., Sontake V., et al. Effect of sorbitol on alpha-crystallin structure and function. Biochemistry (Mosc) 2022;87(2):131–140. doi: 10.1134/S0006297922020055. [DOI] [PubMed] [Google Scholar]
- 41.Borchman D., Yappert M.C. Lipids and the ocular lens. J Lipid Res. 2010;51(9):2473–2488. doi: 10.1194/jlr.R004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chitra P.S., Chaki D., Boiroju N.K., et al. Status of oxidative stress markers, advanced glycation index, and polyol pathway in age-related cataract subjects with and without diabetes. Exp Eye Res. 2020;200 doi: 10.1016/j.exer.2020.108230. [DOI] [PubMed] [Google Scholar]
- 43.Ma Z., Li J., Jiang H., et al. Expression of alpha-Klotho is downregulated and associated with oxidative stress in the lens in streptozotocin-induced diabetic rats. Curr Eye Res. 2021;46(4):482–489. doi: 10.1080/02713683.2020.1805768. [DOI] [PubMed] [Google Scholar]
- 44.Kisic Bojana, Miric Dijana, Zoric Lepsa, et al. Lipid Peroxidation; 2012. Role of Lipid Peroxidation in the Pathogenesis of Age-Related Cataract. [Google Scholar]
- 45.Tang D., Borchman D., Yappert M.C., et al. Influence of age, diabetes, and cataract on calcium, lipid-calcium, and protein-calcium relationships in human lenses. Invest Ophthalmol Vis Sci. 2003;44(5):2059–2066. doi: 10.1167/iovs.02-0345. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W., Xiao S., Ahn D.U. Protein oxidation: basic principles and implications for meat quality. Crit Rev Food Sci Nutr. 2013;53(11):1191–1201. doi: 10.1080/10408398.2011.577540. [DOI] [PubMed] [Google Scholar]
- 47.Smith A.J., Ball S.S., Bowater R.P., et al. PARP-1 inhibition influences the oxidative stress response of the human lens. Redox Biol. 2016;8:354–362. doi: 10.1016/j.redox.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drel V.R., Xu W., Zhang J., et al. Poly(ADP-ribose)polymerase inhibition counteracts cataract formation and early retinal changes in streptozotocin-diabetic rats. Invest Ophthalmol Vis Sci. 2009;50(4):1778–1790. doi: 10.1167/iovs.08-2191. [DOI] [PubMed] [Google Scholar]
- 49.Wang F., Ma J., Han F., et al. DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci Rep. 2016;6(1) doi: 10.1038/srep19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y., Jiang S.H., Liu S., et al. Role of lncRNA NEAT1 mediated by YY1 in the development of diabetic cataract via targeting the microRNA-205-3p MMP16 axis. Eur Rev Med Pharmacol Sci. 2020;24(11):5863–5870. doi: 10.26355/eurrev_202006_21478. [DOI] [PubMed] [Google Scholar]
- 51.Yang J., Zhao S., Tian F. SP1-mediated lncRNA PVT1 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract via miR-214-3p/MMP2 axis. J Cell Mol Med. 2020;24(1):554–561. doi: 10.1111/jcmm.14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kristensen L.S., Andersen M.S., Stagsted L.V.W., et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y., Li Q., Zhang X., et al. CircPAG1 inhibits the high glucose-induced lens epithelial cell injury by sponging miR-630 and upregulating EPHA2. Curr Eye Res. 2021:1–10. doi: 10.1080/02713683.2021.1933058. [DOI] [PubMed] [Google Scholar]
- 54.Fan C., Liu X., Li W., et al. Circular RNA circ KMT2E is up-regulated in diabetic cataract lenses and is associated with miR-204-5p sponge function. Gene. 2019;710:170–177. doi: 10.1016/j.gene.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 55.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao C., Fan F., Liu X., et al. Exosomal miRNA analysis of aqueous humour of diabetes and cataract patients. Curr Eye Res. 2021;46(3):324–332. doi: 10.1080/02713683.2020.1797107. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L., Wang Y., Li W., et al. MicroRNA-30a regulation of epithelial-mesenchymal transition in diabetic cataracts through targeting SNAI1. Sci Rep. 2017;7(1):1117. doi: 10.1038/s41598-017-01320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X., Gong Q., Yang L., et al. microRNA-199a-5p regulates epithelial-to-mesenchymal transition in diabetic cataract by targeting SP1 gene. Mol Med. 2020;26(1):122. doi: 10.1186/s10020-020-00250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J., Liu J., Zhao S., et al. N(6)-Methyladenosine METTL3 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract. Mol Ther Nucleic Acids. 2020;20:111–116. doi: 10.1016/j.omtn.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kato M.N.R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15(6):327–345. doi: 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palsamy P., Ayaki M., Elanchezhian R., et al. Promoter demethylation of Keap1 gene in human diabetic cataractous lenses. Biochem Biophys Res Commun. 2012;423(3):542–548. doi: 10.1016/j.bbrc.2012.05.164. [DOI] [PubMed] [Google Scholar]
- 62.Palsamy P., Bidasee K.R., Ayaki M., et al. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free Radical Biol Med. 2014;72:134–148. doi: 10.1016/j.freeradbiomed.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadden Mja A. Histone deacetylase inhibitors and diabetic kidney disease. Int J Mol Sci. 2018;19(9):2630. doi: 10.3390/ijms19092630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanada F., Takamura Y., Miyake S., et al. Histone acetyltransferase and Polo-like kinase 3 inhibitors prevent rat galactose-induced cataract. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-56414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang J., Yu W., He Q., et al. Autophagy facilitates age-related cell apoptosis-a new insight from senile cataract. Cell Death Dis. 2022;13(1):37. doi: 10.1038/s41419-021-04489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J., Ye W., Xu W., et al. Activation of autophagy inhibits epithelial to mesenchymal transition process of human lens epithelial cells induced by high glucose conditions. Cell Signal. 2020;75 doi: 10.1016/j.cellsig.2020.109768. [DOI] [PubMed] [Google Scholar]
- 67.Ma J., Ye W., Yang Y., et al. The interaction between autophagy and the epithelial-mesenchymal transition mediated by NICD/ULK1 is involved in the formation of diabetic cataracts. Mol Med. 2022;28(1):116. doi: 10.1186/s10020-022-00540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J., Sun Q., Qiu X., et al. Downregulation of AMPK dependent FOXO3 and TFEB involves in the inhibition of autophagy in diabetic cataract. Curr Eye Res. 2022;47(4):555–564. doi: 10.1080/02713683.2021.2009516. [DOI] [PubMed] [Google Scholar]
- 69.Liu X., Zhao X., Cheng R., et al. Autophagy attenuates high glucose-induced oxidative injury to lens epithelial cells. Biosci Rep. 2020;40(4) doi: 10.1042/BSR20193006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao C., Lin X., Fan F., et al. Status of higher TGF-beta1 and TGF-beta2 levels in the aqueous humour of patients with diabetes and cataracts. BMC Ophthalmol. 2022;22(1):156. doi: 10.1186/s12886-022-02317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raghavan C.T., Nagaraj R.H. AGE-RAGE interaction in the TGFβ2-mediated epithelial to mesenchymal transition of human lens epithelial cells. Glycoconj J. 2016;33(4):631–643. doi: 10.1007/s10719-016-9686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zablocki G.J., Ruzycki P.A., Overturf M.A., et al. Aldose reductase-mediated induction of epithelium-to-mesenchymal transition (EMT) in lens. Chem Biol Interact. 2011;191(1-3):351–356. doi: 10.1016/j.cbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y., Bai S., Zhang R., et al. Orai3 exacerbates apoptosis of lens epithelial cells by disrupting Ca(2+) homeostasis in diabetic cataract. Clin Transl Med. 2021;11(3):e327. doi: 10.1002/ctm2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim B., Kim S.Y., Chung S.K. Changes in apoptosis factors in lens epithelial cells of cataract patients with diabetes mellitus. J Cataract Refract Surg. 2012;38(8):1376–1381. doi: 10.1016/j.jcrs.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 75.Lim S.A., Joo C.K., Kim M.S., et al. Expression of p53 and caspase-8 in lens epithelial cells of diabetic cataract. J Cataract Refract Surg. 2014;40(7):1102–1108. doi: 10.1016/j.jcrs.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 76.Liu S., Jin Z., Xia R., et al. Protection of human lens epithelial cells from oxidative stress damage and cell apoptosis by KGF-2 through the Akt/Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/6933812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu X., Liang Y., Zhao B., et al. Oxyresveratrol protects human lens epithelial cells against hydrogen peroxide-induced oxidative stress and apoptosis by activation of Akt/HO-1 pathway. J Pharmacol Sci. 2019;139(3):166–173. doi: 10.1016/j.jphs.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Chen L., Chen Y., Ding W., et al. Oxidative stress-induced TRPV2 expression increase is involved in diabetic cataracts and apoptosis of lens epithelial cells in a high-glucose environment. Cells. 2022;11(7) doi: 10.3390/cells11071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takamura Y., Kubo E., Tsuzuki S., et al. Apoptotic cell death in the lens epithelium of rat sugar cataract. Exp Eye Res. 2003;77(1):51–57. doi: 10.1016/s0014-4835(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 80.Kim J.Y., Park J.H., Kang S.S., et al. Topical nerve growth factor attenuates streptozotocin-induced diabetic cataracts via polyol pathway inhibition and Na(+)/K(+)-ATPase upregulation. Exp Eye Res. 2021;202 doi: 10.1016/j.exer.2020.108319. [DOI] [PubMed] [Google Scholar]
- 81.Du L., Hao M., Li C., et al. Quercetin inhibited epithelial mesenchymal transition in diabetic rats, high-glucose-cultured lens, and SRA01/04 cells through transforming growth factor-beta2/phosphoinositide 3-kinase/Akt pathway. Mol Cell Endocrinol. 2017;452:44–56. doi: 10.1016/j.mce.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Mulhern M.L., Madson C.J., Danford A., et al. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci. 2006;47(9):3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- 83.Wu T.-T., Chen Y.-Y., Ho C.-Y., et al. 3H-1,2-Dithiole-3-Thione protects lens epithelial cells against fructose-induced epithelial-mesenchymal transition via activation of AMPK to eliminate AKR1B1-induced oxidative stress in diabetes mellitus. Antioxidants. 2021;10(7) doi: 10.3390/antiox10071086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boscia F., Grattagliano I., Vendemiale G., et al. Protein oxidation and lens opacity in humans. Invest Ophthalmol Vis Sci. 2000;41(9):5. [PubMed] [Google Scholar]
- 85.Kaliaperumal R., Venkatachalam R., Nagarajan P., et al. Association of serum magnesium with oxidative stress in the pathogenesis of diabetic cataract. Biol Trace Elem Res. 2021;199(8):2869–2873. doi: 10.1007/s12011-020-02429-9. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y.Y., Wu T.T., Ho C.Y., et al. Dapagliflozin prevents NOX- and SGLT2-dependent oxidative stress in lens cells exposed to fructose-induced diabetes mellitus. Int J Mol Sci. 2019;20(18) doi: 10.3390/ijms20184357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y.Y., Wu T.T., Ho C.Y., et al. Blocking of SGLT2 to eliminate NADPH-induced oxidative stress in lenses of animals with fructose-induced diabetes mellitus. Int J Mol Sci. 2022;23(13) doi: 10.3390/ijms23137142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nambu H., Kubo E., Takamura Y., et al. Attenuation of aldose reductase gene suppresses high-glucose-induced apoptosis and oxidative stress in rat lens epithelial cells. Diabetes Res Clin Pract. 2008;82(1):18–24. doi: 10.1016/j.diabres.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 89.Higashi Y., Higashi K., Mori A., et al. Anti-cataract effect of resveratrol in high-glucose-treated streptozotocin-induced diabetic rats. Biol Pharm Bull. 2018;41(10):1586–1592. doi: 10.1248/bpb.b18-00328. [DOI] [PubMed] [Google Scholar]
- 90.Chan A.W., Ho Y.S., Chung S.K., et al. Synergistic effect of osmotic and oxidative stress in slow-developing cataract formation. Exp Eye Res. 2008;87(5):454–461. doi: 10.1016/j.exer.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Zhou Y., Li L., Li S., et al. Autoregenerative redox nanoparticles as an antioxidant and glycation inhibitor for palliation of diabetic cataracts. Nanoscale. 2019;11(27):13126–13138. doi: 10.1039/c9nr02350j. [DOI] [PubMed] [Google Scholar]